
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 23 May 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.853012
Background: Jidabokuippo (JDI) (治打撲一方) has been used in Japan to alleviate contusion-induced swelling and pain since medieval times.
Method: This review investigated the effects of JDI on various symptoms in patients with trauma or static blood[TM1]. The PubMed and Igaku Chuo Zasshi databases were searched until 24 December 2021. We summarize the benefits of applying JDI to inflammatory conditions, including bruises.
Results: JDI has been used to resolve blood [TM1] stasis, regulate qi in trauma patients, and treat inflammatory swelling and pain caused by rheumatoid arthritis and cellulitis. As the adverse event rate associated with JDI is low (1.3%), JDI is considered a safe drug.
Conclusion: JDI can be used to resolve blood[TM1] stasis in trauma patients without adverse events associated with nonsteroidal anti-inflammatory drugs.
Jidabokuippo (JDI) (治打撲一方) is an herbal mixture used in Japan to alleviate contusion-induced swelling and pain. It is composed of Nuphar japonica DC., Quercus acutissima Carruth., Ligusticum officinale (Makino) Kitag., Neolitsea cassia (L.) Kosterm., Syzygium aromaticum (L.) Merr. and L.M.Perry, Rheum palmatum L., and Glycyrrhiza glabra L. (Table 1; Figure 1) (Department of Pharmacognosy and DPPN, 2018; Sakakibara, 2008; Nakae and Irie, 2020).
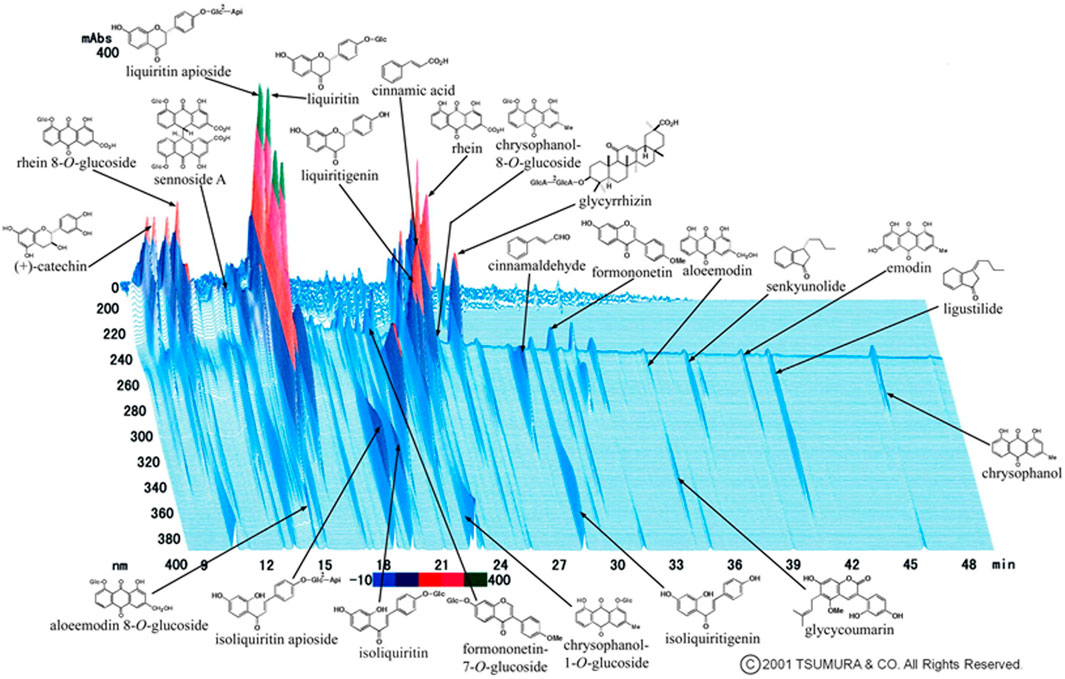
FIGURE 1. Three-dimensional high-performance liquid chromatography profile of jidabokuippo. Nuphar japonica DC. contains major ingredients: nupharidine, deoxynupharidine, nupharamine, and nupharin. Quercus acutissima Carruth. contains quercitrin, scopoline, fraxin, and tannic acid. Ligusticum officinale (Makino) Kitag. contains cnidilide, neocnidilide, ligustilide*, senkyunolide*, butylphthalide, butylidenephthalide, pregnenolone, vanillin, coniferyl ferulate, ferulic acid, and scopoletin. Neolitsea cassia (L.) Kosterm. contains cinnamaldehyde*, cinnamyl acetate, phenylpropyl acetate, cinnamic acid*, and salicylaldehyde. Syzygium aromaticum (L.) Merr. and L.M.Perry contains acetyleugenol, chavicol, caryophyllene, humulene, caryophylla, eugenocide, eugeniin, higenamine, rhamnetin, and kaempferol. Rheum palmatum L. contains sennoside A*–F, rhein*, aloe emodin*, emodin*, chrysophanol*, naphthalene, catechin*, epicatechin, and cinnamic acid*. Glycyrrhiza glabra L. contains glycyrrhizin*, glabric acid, liquiritin*, liquiritin apioside*, liquiritigenin*, isoliquiritin*, licoricidin, licoricone, licoflavone, formononetin*, glycerol, and glycycoumarin*.*Shown in Panel 1).
Herein, we document that JDI treatments have been applied to bruises and various inflammation conditions since medieval Japanese society. Potentially relevant articles were identified through a PubMed and Igaku Chuo Zasshi (ICHUSHI) literature search using the keywords (jidabokuppo OR jidabokuippou) for articles published until 24 December 2021. ICHUSHI contains bibliographic citations and abstracts from more than 2,500 biomedical journals and other serial publications published in Japan. Since Kampo medicine targets many intractable and rare diseases and the course of treatment differs in each case, it is difficult to conduct large-scale randomized controlled trials and secure high-quality evidence. Therefore, a case report and case series are also included.
Kampo prescriptions developed by Japanese expert clinicians in the Edo era were called “honchokeikenho” and are thought to include JDI. In the Sengoku era, the age of provincial wars (1467–1615), some traumatologists called “kinsoi” used drugs that resembled JDI for sword wounds. Shuan Kagawa, who lived from 1683 to 1755, finalized JDI and collected information on treating bruises. It was originally named “ippo (一方)” for “bruise” in “Ippondo-iji-setsuyaku.” Sohaku Asada, a well-known Kampo medicine expert who practiced during the late 19th century (between the end of the Edo era and the early Meiji era), was the first to call it JDI in “Futsugo-yakushitsu-hokan-kuketsu” published in 1878. He reported that Shuan Kagawa developed JDI (Asada, 1981; Morikubo, 1999; Nakae and Irie, 2020).
Shuan Kagawa reported that Quercus bark has the potential to resolve blood[TM1] stasis and improve fluid congestion found in bruises in “Ippondoyakusen (Ippondoyakusen, 2021).” Contusion and pain caused by trauma are considered static blood[TM1], a sign of a microcirculatory disorder, and JDI alleviates blood[TM1] stasis patterns (Morikubo, 1999). Gentatsu Matsuoka, who lived from 1668 to 1746, reported that Nuphar japonica should be used for bruises. Prescriptions that included it were especially effective for bruises in “Yoyakusuchi (Yoyakusuchi, 2021).” Sohaku Asada explained that JDI improved myalgia and ostealgia caused by trauma; Nuphar japonica improved blood flow, and Quercus acutissima alleviated ostealgia. These two crude elements were the principal agents. Aconitum carmichaelii Debeaux with warm meridian is added in the chronic stage in “Futsugo-yakushitsu-hokan-kuketsu” (Asada, 1981). He also explained that dokoppito (土骨皮湯), composed of Quercus acutissima, Carthamus tinctorius L., Glycyrrhiza glabra, Bupleurum falcatum L., and Curcuma zedoaria (Christm.) Roscoe improved eczema capitis and ostealgia. In dokoppi, also known as Bokusoku, Quercus acutissima has strong potential of releasing exterior in “Futsugo-yakushitsu-hokan-kuketsu.”
Rheum palmatum has sedative effects in addition to resolving blood[TM1] stasis (Sumida et al., 1988). Ligusticum officinale, Neolitsea cassia, and Syzygium aromaticum have the potential to regulate qi (Table 1). Wada Tokaku, who lived from 1742 to 1803, stated “It is not good resolving blood[TM1] stasis using Carthamus tinctorius and Biancaea sappan (L.) Tod. for bruises. The regulating qi method should be chosen for this purpose. Provide sedation using shigyakusan (四逆散) or jinkokokito (沈香降気湯)” (Shosozatsuwa, 2021). Since both resolving blood[TM1] stasis and regulating qi should be performed for the treatment of bruises, JDI is thought to have the ideal composition of crude drugs.
Kampo formulations are made from several crude drugs, with each crude drug having several constituents. Therefore, Kampo prescriptions are considered interaction-based multicomponent medicines. The blending effect of crude drugs in JDI is shown in Table 2.
The relevance of JDI in modern society is the same as that in its classical use. In short, swelling caused by trauma is diagnosed as blood[TM1] stasis, and JDI is applied to resolve it (Table 3) (Yamamoto, 1975; Hijikata et al., 2007; Futenma et al., 2014; Irie and Nakae, 2019; Yoshinaga et al., 2020).
Yamamoto reported that JDI was effective for bruises in acute and chronic settings. JDI was much more effective than keishibukuryogan (桂枝茯苓丸), and aconite tuber should be added to JDI in the chronic stage. He also recommended treatment-induced diarrhea using JDI and Rheum palmatum in acute severely injured patients, regardless of stool consistency (Yamamoto, 1975).
Plants contain various antioxidants that protect organisms from injury caused by ultraviolet radiation. Kampo formulations have antioxidant and multiple bioactive properties (Table 1) (Nakae, 2011; Hirayama et al., 2018). Yamane evaluated the radical scavenging potentials of seven herbs [Rheum palmatum, Uncaria gambir (W.Hunter) Roxb., Syzygium aromaticum, Paeonia lactiflora Pallas, Glycyrrhiza glabra, Polyporus umbellatus Fries, and Prunus persica (L.) Batsch] and reported that the scavenging potential of diphenylpicrylhydrazyl (DPPH) was the highest in Rheum palmatum, followed by Syzygium aromaticum (Yamane et al., 2000). Tani suggested that polyphenol is closely involved in antioxidant effects based on a positive correlation between the polyphenol content and DPPH radical scavenging potential of herbs (Tani et al., 2004). They investigated 25 herbs. The polyphenol content was highest in Rheum palmatum, followed by Quercus acutissima, Nuphar japonica, Glycyrrhiza glabra, Syzygium aromaticum, and Neolitsea cassia. The DPPH radical scavenging potential was high in Rheum palmatum, followed by Quercus acutissima, Nuphar japonica, Syzygium aromaticum, and Neolitsea cassia. In addition, Ligusticum officinale have anti-inflammatory and antioxidant effects. A study designed to evaluate the effect of herbal extracts in suppressing reactive oxygen formation in human neutrophils showed a suppressive action by Ligusticum officinale (Luo et al., 1993). In addition, this herb protects organisms from radiation-induced damage (Ohta et al., 1987; Shinoda, 1995) and protects against edema (Tahara et al., 1998). Neolitsea cassia suppresses the formation of reactive oxygen in aqueous extracts (Toda et al., 1991), inhibits O2 formation in macrophages (Imamichi et al., 1990), and protects against radiation disorders (Ohta et al., 1987). Rheum palmatum, containing anthraquinones, suppresses lipid peroxide formation in human neutrophils (Mian et al., 1987), and condensed tannins have radical scavenging activity (Uchida et al., 1988). Glycyrrhiza glabra has anti-inflammatory and edema-suppressing activities (Kumagai, 1982; Amagaya et al., 1984). In addition, Glycyrrhiza glabra protects organisms from radiation (Ohta et al., 1987). Thus, JDI includes herbs with antioxidant effects; these herbs may act synergistically to exert antioxidant effects.
We have previously demonstrated the antioxidant activity of JDI in a clinical setting (Nakae, 2010a). Swelling related to trauma occurs due to the enhanced permeability caused by the overproduction of chemical mediators such as free radicals. JDI may improve the pathological condition through these antioxidant properties.
In the clinical setting, Kampo prescriptions should be first administered in doses two to three times greater than the common starting doses in patients with severe symptoms (Nakae and Irie, 2020; Nakae et al., 2021).
The hypothetical mechanisms of JDI are shown in Figure 2. A patient’s signs and symptoms are diagnosed based on theories of Kampo medicine such as yin and yang, deficiency and excess, cold[TM1] and heat[TM1], exterior[TM1] and interior[TM1], six-stage patterns, qi, blood[TM1], fluid[TM1], and zang-fu organs. The patient is to be treated based on those patterns. When a patient’s pattern is in static blood[TM1] and qi depression, JDI is applied to the pattern, regardless the patient’s condition being acute or chronic inflammation.
As for the effectiveness of JDI as compared with Western drugs, there are only two randomized controlled studies (Table 3). Takeda compared the efficacy of JDI and nonsteroidal anti-inflammatory drugs (NSAIDs), loxoprofen, in patients with anterior tibiofibular ligament injuries by analyzing the treatment duration using a visual analog scale and girth (Takeda, 2010). The results showed that compared to loxoprofen, JDI could shorten the swelling duration 2 weeks after the administration. We compared the efficacy of JDI and NSAIDs in patients with rib fractures by analyzing the treatment duration. Our results suggest that compared to NSAIDs, JDI could shorten the treatment duration and may be a promising analgesic agent for both medical and economic reasons (Nakae et al., 2012.).
We have used JDI for various trauma such as rib fractures, fractures of extremities, abdominal wall hematoma, and traumatic asphyxia (Nakae et al., 2012; Nakae et al., 2015a; Nakae et al., 2016; Kitamura et al., 2022; Nakae et al., 2015b; Nakae et al., 2020.).
Suzuki reported that the JDI group had a significantly more robust remission effect than the non-JDI group in postoperative finger swelling (Suzuki and Yoshida, 2016). Nagashima reported that a 35-year-old man with massive subcutaneous swelling after decompressive craniectomy for head trauma showed a rapid reduction of swelling after JDI administration (Nagashima et al., 2018). Furthermore, JDI was applied for chronic subdural hematoma, puncture hematoma after angiography, and subgaleal hematoma with skull fractures (Tsugane et al., 2011; Yoshida et al., 2018; Nakao and Kaneko, 2019; Yamada et al., 2019). The use of JDI has also been applied to treat rheumatoid arthritis and cellulitis (Kita et al., 1995; Nogami et al., 2003; Yoshinaga et al., 2021). Since JDI has antioxidant activity and also inhibits prostaglandin production, its indications may be broader. In Kampo medicine, peripheral neuropathy is often diagnosed as related to blood[TM1] deficiency, static blood[TM1], fluid[TM1] retention, and kidney[TM1] deficiency (Shimada, 2005). Therefore, JDI can resolve blood[TM1] stasis in these indications (Uemura et al., 2013; Narai et al., 2014; Okamoto, 2015; Yabe et al., 2018). Furthermore, JDI has been used for unexplained perineal pain and wasp stings (Nakae, 2013a; Ogata et al., 2019).
Concomitant use of NSAIDs or other Kampo medicines may be necessary for multiple injuries should severe inflammatory reactions occur and severe pain persist (Table 4) (Yonemitsu, 2017; Iwata, 2020). Aconitum carmichaelii should be added to JDI when swelling and pain persist. In Kampo medicine, Aconitum carmichaelii can move old blood[TM1] stasis (Yamamoto, 1975; Takamura et al., 2018). We previously reported that the Aconitum carmichaelii had analgesic and hyperthermic activity and increased blood flow (Nakae, 2008; Nakae et al., 2008; Nakae, 2010b; Nakae, 2010c; Nakae, 2010d; Nakae, 2013b; Nakae et al., 2014). Drug treatment with carbamazepine and pregabalin, nerve block injection, acupuncture, and moxibustion treatment combined with JDI were administered to treat neuropathic pain (Kase et al., 2009; Imaizumi et al., 2016; Suzuki et al., 2017; Okuno and Gi, 2019; Yano et al., 2020).
Takagi reported that JDI was effective in patients with abdominal tenderness at the right side of the paraumbilical site before treatment (Figure 3) (Takagi, 1995). This tender point is considered to indicate blood[TM1] stasis (Morikubo, 1999; Sudo and Oribe, 2005; Suzuki et al., 2017). It is difficult to confirm whether Takagi’s suggestion could be used in the absence of this tender point.
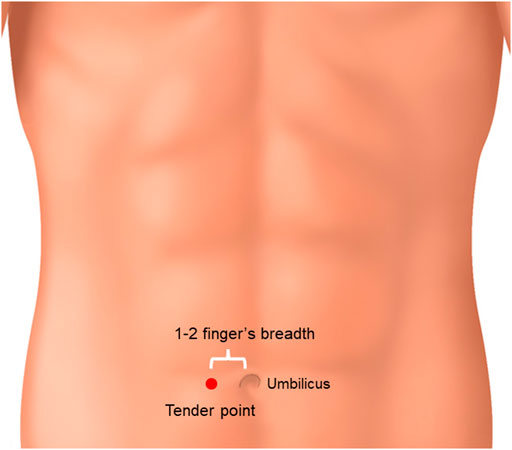
FIGURE 3. Tender point of jidabokuippo. When abdominal tenderness at the right side of the paraumbilical site was observed, JDI might have been effective.
The incidence of adverse events associated with Kampo formulations remains unclear. Kitamura et al. studied the adverse events in 1,104 patients who had JDI prescribed (Kitamura et al., 2022). The reported adverse event rate was 1.3%, falling within a low rate of previous reports (0–6.4%) (Ikeda et al., 1986; Kita et al., 1995; Takagi, 1995; Sudo and Oribe, 2005; Sudo, 2005; Sakurai et al., 2006; Takeda, 2010; Nakae et al., 2012; Minamitani, 2014; Nakae et al., 2015a; Yoshida, 2015; Nakae et al., 2016; Hasegawa et al., 2016; Suzuki and Yoshida, 2016; Saito et al., 2019; Akiyama et al., 2020). The most common adverse event was digestive symptoms (0.9%), with diarrhea caused by Rheum palmatum being the most common. The adverse event rate of glycyrrhiza-induced pseudoaldosteronism was 0.33% (Table 5). The adverse event rate associated with JDI use is low, and the onset is relatively rapid. Kon reported that the laxative action that accompanies decreased aquaporin-3 expression due to sennoside A in Rheum palmatum was mitigated by the anti-inflammatory effects of glycyrrhizin (Kon et al., 2018). Glycyrrhizin is considered to attenuate the adverse events caused by sennoside A. However, we need to pay attention to the pharmacological action of Rheum palmatum and Glycyrrhiza glabra before concluding that JDI is a safe drug.
NSAIDs are often used to treat pain associated with trauma. However, NSAIDs intake often induces gastrointestinal symptoms. In addition, the use of selective cyclooxygenase-2 inhibitors poses a risk of ischemic heart disease (Hippisley-Cox and Coupland, 2005), and physicians hesitate to use them in patients with a history of cardiovascular disease. In recent years, proton pump inhibitors (PPIs) have been used to prevent NSAID-induced ulcers. However, PPIs pertain to medical economics, fractures, community-acquired pneumonia, watery stools, etc. (Bombardier et al., 2000; Dalton et al., 2009). JDI can be used as an alternative drug under such conditions. Moreover, JDI may be applied to non-trauma patients with blood[TM1] stasis. A large randomized controlled trial is necessary to establish JDI treatment for various diseases with blood[TM1] stasis.
All authors contributed to the writing of this review. HN conceived the idea for the article, drafted the methods and results, and developed it in collaboration with YI, TK, and MO. HN wrote the first draft of the manuscript. YI and TK contributed to the article and edited the manuscript. All authors contributed to the revisions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The basic terms of Kampo medicine are based on the Dictionary of Kampo Medicine (Basic terms) of the Japanese Society of Oriental Medicine (The Editing Committee for Dictionary of Kampo Medicine, 2019). We acknowledge Editage (https://www.editage.jp) for proofreading the text in English.
Akiyama, O., Harada, Y., Akiyama, I., Suzuki, M., Shimizu, Y., and Kondo, A. (2020). Therapeutic Effects of Jidabokuippo for Painful Swelling from Head Injury. J. Neurosurg. Kampo Med. 6, 12–17. (in Japanese).
Amagaya, S., Sugishita, E., Ogihara, Y., Ogawa, S., Okada, K., and Aizawa, T. (1984). Comparative Studies of the Stereoisomers of Glycyrrhetinic Acid on Anti-inflammatory Activities. J. Pharmacobiodyn. 7, 923–928. doi:10.1248/bpb1978.7.923
Bombardier, C., Laine, L., Reicin, A., Shapiro, D., Burgos-Vargas, R., Davis, B., et al. (2000). Comparison of Upper Gastrointestinal Toxicity of Rofecoxib and Naproxen in Patients with Rheumatoid Arthritis. VIGOR Study Group. N. Engl. J. Med. 343 (1520–8), 1520–1528. 2 p following 1528. doi:10.1056/NEJM200011233432103
Dalton, B. R., Lye-Maccannell, T., Henderson, E. A., Maccannell, D. R., and Louie, T. J. (2009). Proton Pump Inhibitors Increase Significantly the Risk of Clostridium difficile Infection in a Low-Endemicity, Non-outbreak Hospital Setting. Aliment. Pharmacol. Ther. 29, 626–634. doi:10.1111/j.1365-2036.2008.03924.x
Department of Pharmacognosy and DPPN, (2018). Phytochemistry and Narcotics(DPPN). National Institute of Health Sciences (NIHS) of Japan and Research Center for Medicinal Plant Resources (RCMPR), National Institute of Biomedical Innovation (NIBIO) of Japan, STORK. http://mpdb.nibiohn.go.jp/stork (Accessed December 16, 2021).
Futenma, C., Kikuzato, N., Ikema, M., and Uehara, T. (2014). Treatment with Kampo Medicines in the Acute Care of Orthopedic Service. Pain Kampo Med. 24, 165–168. (in Japanese).
Hasegawa, S., Okumoto, K., Ohta, K., Ueda, Y., Itouyama, T., and Miura, M. (2016). Jidabokuippo Is Effective for the Treatment of Swelling after Trauma and Surgery. J. Neurosurg. Kampo Med. 2, 25–29. (in Japanese).
Hijikata, Y., Miyamae, Y., Takatsu, H., and Sentoh, S. (2007). Two Kampo Medicines, Jidabokuippo and Hachimijiogan Alleviate Sprains, Bruises and Arthritis. Evid. Based Complement. Alternat. Med. 4, 463–467. doi:10.1093/ecam/nel105
Hippisley-Cox, J., and Coupland, C. (2005). Risk of Myocardial Infarction in Patients Taking Cyclo-Oxygenase-2 Inhibitors or Conventional Non-steroidal Anti-inflammatory Drugs: Population Based Nested Case-Control Analysis. BMJ 330, 1366. doi:10.1136/bmj.330.7504.1366
Hirayama, A., Oowada, S., Ito, H., Matsui, H., Ueda, A., and Aoyagi, K. (2018). Clinical Significance of Redox Effects of Kampo Formulae, a Traditional Japanese Herbal Medicine: Comprehensive Estimation of Multiple Antioxidative Activities. J. Clin. Biochem. Nutr. 62, 39–48. doi:10.3164/jcbn.17-59
Ikeda, M., Nakajima, H., and Tsutsumi, Y. (1986). Jidabokuippo for Various Injuries. Kampo Shinryo 5, 35–45. (in Japanese).
Imaizumi, U., Beppu, S., Mitsuhashi, A., and Yoshida, K. (2016). A Case of a Comprehensive Medicine in a Patient Suffering from Neuropathic Pain after a Facial Injury by a Traffic Accident. Jpn. J. Orofac. Pain. 9, 75–80. (in Japanese). doi:10.11264/jjop.9.75
Imamichi, T., Nakamura, T., Hayashi, K., Kaneko, K., and Koyama, J. (1990). Different Effects of Cinnamic Acid on the O2- Generation by guinea Pig Macrophages Stimulated with a Chemotactic Peptide and Immune Complex. J. Pharmacobiodyn. 13, 344–352. doi:10.1248/bpb1978.13.344
Ippondoyakusen, K. S. (2021). National Diet Library Digital Collections. Available at: https://dl.ndl.go.jp/info:ndljp/pid/2556221 (Accessed December 16, 2021).
Irie, Y., and Nakae, H. (2019). Jidabokuippo Use in Patients with Trauma. Kampo Newest Ther. 28, 151–155. (in Japanese).
Iwata, Y. (2020). The Efficacy of Kampo in Patients with Neck Sprain under the Automobile Liability Insurance. Kampo Newest Ther. 29, 279–285. (in Japanese).
Kase, S., Shimazaki, M., Arai, T., and Okuda, Y. (2009). A Case of Complex Regional Pain Syndrome Caused by Radioulnar Fractures Treated with Kampo Medicine. J. Jpn. Soc. Study Chronic Pain 28, 189–191. (in Japanese) https://mol.medicalonline.jp/library/journal/download?GoodsID=dc1chron/2009/002801/030&name=0189-0191j&UserID=158.215.8.21&base=jamas_pdf.
Kita, T., Ito, T., Imadaya, A., Takahashi, K., and Terasawa, K. (1995). The Effects of Supplemental Administration of Ji-Daboku-Ippo on Rheumatoid Arthritis. Kampo Med. Nihon Toyo Igaku Zasshi. 46, 447–451. (in Japanese). doi:10.3937/kampomed.46.447
Kitamura, T., Nakae, H., Irie, Y., Satoh, K., Hirasawa, N., Kameyama, K., et al. (2022). Safety of Jidabokuippo Administration Based on Adverse Event Rate. Traditional Kampo Med. 9, 18–24. doi:10.1002/tkm2.1305
Kon, R., Yamamura, M., Fujikawa, T., Uemura, T., Kusunoki, Y., Ikarashi, N., et al. (2018). Laxative Action of Sennoside A, Which Causes Decreased Colonic Aquaporin-3 Expression, Is Controlled by the Anti-inflammatory Effect of Glycyrrhizin. Traditional Kampo Med. 5, 45–50. doi:10.1002/tkm2.1090
Kumagai, A. (1982). Clinical Pharmacology in Japanese Traditional Medicines-Glycyrrhizae Radix and Glycyrrhizin. Rinsho Yakuri 13, 185–188. (in Japanese). doi:10.3999/jscpt.13.185
Luo, X. X., Midorikawa, Y., and Ogata, H. (1993). Antioxidant Activities of Injectable Crude Drugs on Human Leukocyte. Jpn. J. Clin. Physiol. 23, 571–575. (in Japanese) https://mol.medicalonline.jp/library/journal/download?GoodsID=cc8jjapf/1993/002306/012&name=0571-0575j&UserID=158.215.8.21&base=jamas_pdf.
Mian, M., Brunelleschi, S., Tarli, S., Rubino, A., Benetti, D., Fantozzi, R., et al. (1987). Rhein: an Anthraquinone that Modulates Superoxide Anion Production from Human Neutrophils. J. Pharm. Pharmacol. 39, 845–847. doi:10.1111/j.2042-7158.1987.tb05131.x
Minamitani, T. (2014). Effect of Jidabokuippo to Improve Prolonged Swelling and Pain Caused by Fracture or Severe Contusion. Pain Kampo Med. 24, 110–116. (in Japanese).
Morikubo, H. (1999). Historic Investigation and Clinical Use of Jidabokuippo. Kampo, J. Med. 46, 1935–1957. (in Japanese).
Nagashima, T., Hayakawa, T., Yatsushige, H., et al. (2018). Treatment with Jidabokuippo for a Case of Massive Subcutaneous Hematoma and Swelling after Decompressive Craniectomy for Severe Head Trauma. J. Neurosurg. Kampo Med. 4, 44–48. (in Japanese).
Nakae, H., Fujita, Y., Igarashi, T., Tajimi, K., and Endo, S. (2008). Serum Aconitine Concentrations after Taking Powdered Processed Aconiti Tuber. Biomed. Res. 29, 225–231. doi:10.2220/biomedres.29.225
Nakae, H., Yokoi, A., Kodama, H., and Horikawa, A. (2012). Comparison of the Effects on Rib Fracture between the Traditional Japanese Medicine Jidabokuippo and Nonsteroidal Anti-inflammatory Drugs: A Randomized Controlled Trial. Evid. Based Complement. Alternat. Med. 2012, 837958. doi:10.1155/2012/837958
Nakae, H. (2013b). Clinical Evaluation of High-Sensitive C-Reactive Protein during Bushi Powder Administration. Pers (Med. Univers) (Japanese Edition) 1, 37–41.
Nakae, H. (2010a). Clinical Evaluation of Oxidative Stress after Taking Jidabokuippo. Nihon Toyo igaku zasshi 61, 847–852. (in Japanese). doi:10.3937/kampomed.61.847
Nakae, H. (2010b). Clinical Evaluation of Oxidative Stress after Taking Powdered Processed Aconiti Tuber. Nihon Toyo igaku zasshi 61, 15–18. (in Japanese). doi:10.3937/kampomed.61.15
Nakae, H. (2008). Clinical Evaluation of the finger Tissue Blood Volume during Shuchi-Bushi Powder Administration. Nihon Toyo igaku zasshi 59, 809–812. (in Japanese). doi:10.3937/kampomed.59.809
Nakae, H. (2011). Determination of the Total Antioxidant Capacity for Comparing Different Types of Suspensions of Kampo Extract. Int. J. Integr. Med. 3, 62–66. (in Japanese).
Nakae, H. (2010c). Efficacy of Powdered Processed Aconiti Tuber in Patients with Arthralgia and Somatic Pain. Jpn. J. Occup. Med. Traumatol. 58, 150–154. (in Japanese) https://mol.medicalonline.jp/library/journal/download?GoodsID=dr7jjomt/2010/005803/008&name=0150-0154j&UserID=158.215.8.27&base=jamas_pdf.
Nakae, H. (2013a). Four Cases of Hymenoptera Stings Successfully Treated with Traditional Japanese Medicine. Pers (Med. Univers) (Japanese Edition) 1, 53–58.
Nakae, H., Fujita, Y., and Endo, S. (2014). Serum Concentrations of Diterpenoid Alkaloids after Oral Administration of Powdered Processed Aconite Root. Personalized Med. Universe 3, 54–56. doi:10.1016/j.pmu.2014.04.001
Nakae, H., Hebiguchi, M., Hiroshima, Y., Okuyama, M., and Igarashi, T. (2016). Jidabokuippo Use in Patients with Trauma. Kampo Newest Ther. 25, 245–251. (in Japanese).
Nakae, H., Hebiguchi, M., and Okuyama, M. (2015a). Jidabokuippo Use in Patients with Fractures of the Extremities. Personalized Med. Universe 4, 66–69. doi:10.1016/j.pmu.2014.10.002
Nakae, H., and Irie, Y. (2020). Relevance of Kampo in Emergency and Critical Care Medicine. Traditional Kampo Med. 7, 63–68. doi:10.1002/tkm2.1252
Nakae, H., Irie, Y., Satoh, K., Kitamura, T., Kameyama, K., Furuya, T., et al. (2020). Traumatic Asphyxia Successfully Treated with Jidabokuippo. Traditional Kampo Med. 7, 183–185. doi:10.1002/tkm2.1260
Nakae, H., Irie, Y., Satoh, K., Kitamura, T., Kameyama, K., Nara, T., et al. (2021). Treatment for Tetanus Applying Kampo Medicine: Administration of Shakuyakukanzoto. Traditional Kampo Med. 8, 130–137. doi:10.1002/tkm2.1283
Nakae, H., Okuyama, M., and Igarashi, T. (2015b). Traumatic Lateral Abdominal wall Hematoma Treated with Kampo Medicines. Traditional Kampo Med. 2, 102–104. doi:10.1002/tkm2.1022
Nakae, H. (2010d). Plasma Serotonin and Interleukin 18 Levels after Taking Powdered Processed Aconiti Tuber. J. Complement. Integr. Med. 7. article 34. doi:10.2202/1553-3840.1353
Nakao, K., and Kaneko, Y. (2019). A Case Report of Recurrent Chronic Subdural Hematoma Successfully Treated with Jidabokuippo. Nihon Toyo igaku zasshi 70, 290–293. (in Japanese). doi:10.3937/kampomed.70.290
Narai, Y., Nakatani, T., Miyamoto, T., Hashimoto, T., and Saito, Y. (2014). A Case of Successful Treatment with Jidabokuippo for Refractory Pain in Lower Leg and Low Back Due to Lumbar Disc Herniation, Which Were Not Enable to Withdrawal from Opioid Patch. Pain Kampo Med. 24, 128–131. (in Japanese).
Nogami, T., Sekiya, N., Kita, T., Shibahara, N., Shimada, Y., and Terasawa, K. (2003). Case Report of Successfully Treated Cellulitis of the Lower Extremity with Ji-Daboku-Ippo. Kampo Med. Nihon Toyo Igaku Zasshi. 54, 781–784. (in Japanese). doi:10.3937/kampomed.54.781
Ogata, M., Hayashi, T., and Kawasaki, T. (2019). Two Cases of Vulvodynia in Which Kampo Medicine Responded. Pain Kampo Med. 29, 58–61. (in Japanese).
Ohta, S., Sakurai, N., Inoue, T., and Shinoda, M. (1987). Studies on Chemical Protectors against Radiation. XXV. Radioprotective Activities of Various Crude Drugs. Yakugaku Zasshi 107, 70–75. (in Japanese). doi:10.1248/yakushi1947.107.1_70
Okamoto, I. (2015). Effect of “Jidabokuippo” on Both Shoulder Hand Syndrome and Shoulder Pain after Stroke. Pain Kampo Med. 25, 43–46. (in Japanese).
Okuno, S., and Gi, E. (2019). A Case of Meralgia Paresthetica with Spinocerebellar Degeneration Treated Using Ji-Da-Boku-Ippou, Kampo Medicine and Lumber Epidural Block. Pain Clin. 40, 707–710. (in Japanese).
Saito, H., Osaga, S., Uyama, K., et al. (2019). Clinical Effectiveness of Jidabokuippo in the Obstetrics and Gynecology Department. Recent Prog. Kampo Med. Obstet. Gynecol. 36, 44–49. (in Japanese) https://mol.medicalonline.jp/library/journal/download?GoodsID=ev4kanpo/2019/000036/010&name=0044-0049j&UserID=158.215.8.21&base=jamas_pdf.
Sakakibara, I. (2008). 3D-HPLC Fingerprints of Kampo Medicine and Botanical Raw Materials. Nihon Yakurigaku Zasshi 132, 265–269. (in Japanese). doi:10.1254/fpj.132.265
Sakurai, T., Ueda, M., and Samejima, H. (2006). Jidabokuippo for Swelling and Pain in Facial Trauma. Sci. Kampo Med. 30, 104–105. (in Japanese).
Shimada, Y. (2005). “Neuromuscular System,” in Introduction to Kampo, Japanese Traditional MedicineThe Japan Society for Oriental Medicine (TokyoJapan K.K.): Elsevier), 317–141.
Shinoda, M. (1995). Studies on Chemical Radioprotectors against X-Irradiation Used by Soft X-ray Accelerator. Yakugaku Zasshi 115, 24–41. (in Japanese). doi:10.1248/yakushi1947.115.1_24
Shosozatsuwa, W. T. (2021). Main Library. Kyoto City, Japan: Kyoto University. https://rmda.kulib.kyoto-u.ac.jp/item/rb00000250 (Accessed December 16, 2021).
Sudo, T. (2005). Effect of Jidabokuippo for Traumatic Spinal Compression Fractures: Concomitant Use of Blood Stasis-Resolving Formulas. Prog. Med. 25, 1933–1936. (in Japanese) https://mol.medicalonline.jp/library/journal/download?GoodsID=ai5prmda/2005/002507/035&name=1933-1936j&UserID=158.215.8.21&base=jamas_pdf.
Sudo, T., and Oribe, K. (2005). Application of Jidabokuippo -Takagi’s Tender point-. J. Kampo Med. Herb. 9, 198–208. (in Japanese).
Sumida, T., Sugimoto, H., Fuwa, T., Yamasaki, K., Takeda, O., Kohda, H., et al. (1988). Studies on Active Constituents in Medicinal Plants Using the Receptor Binding Assay. II. Dopamine 2 Receptor. Yakugaku Zasshi 108, 450–453. (in Japanese). doi:10.1248/yakushi1947.108.5_450
Suzuki, A., Oka, H., Mantani, N., Watanabe, T., Kamiyama, H., and Nagasaki, N. (2017). A Case of Trigeminal Neuralgia Effectively Treated with Jidabokuippo: Hint from Past Injury and Tender Point. Nihon Toyo igaku zasshi 68, 250–254. (in Japanese). doi:10.3937/kampomed.68.250
Suzuki, T., and Yoshida, H. (2016). Effect of Jidabokuippo for Postoperative Finger Swelling. Nihon Toyo igaku zasshi 67, 221–224. (in Japanese). doi:10.3937/kampomed.67.221
Tahara, E., Satoh, T., Toriizuka, K., Nagai, H., Saiki, I., and Terasawa, K. (1998). Effect of the Extracts of Cnidium Rhizoma (Senkyu) on IgE-Mediated Skin Reaction in Passively Sensitized Mice. J. Tradit. Med. 15, 294–295. (in Japanese).
Takagi, Y. (1995). Trigger Acupoint of Jidabokuippou. Kampo Med. Nihon Toyo Igaku Zasshi. 45, 541–545. (in Japanese). doi:10.3937/kampomed.45.541
Takamura, M., Yokochi, A., and Maruyama, K. (2018). Kampo Medicine and Acupuncture Therapy Relieved Postoperative Pain of Patient with Buttock Soft Tissue Tumor: a Case Report. Pain Kampo Med. 28, 44–47. (in Japanese).
Takeda, N. (2010). Conservative Treatment of Fresh Anterior Tibiofibular Ligament Injury: Comparison of Pain and Swelling between the Western and Kampo Medicines. Kampo Pract. J. 1, 128–132. (in Japanese).
Tani, M., Sakurai, C., Tanago, M., et al. (2004). Inhibition of Oxidation of Low-Density Lipoprotein with Crude Drugs. J. Jpn. Mibyou Syst. Assoc. 9, 243–246. (in Japanese).
The editing committee for dictionary of Kampo medicine, (2019). “The Editing Committee for Dictionary of Kampo Medicine,” in The Dictionary of Kampo Medicine (Basic Terms) (Tokyo: The Japan Society for Oriental Medicine).
Toda, S., Ohnishi, M., and Kimura, M. (1991). Inhibitory Effects of Aromatic Herbs on Generation of Active Oxygen. J. Tradit. Med. 8, 55–58. (in Japanese).
Tsugane, S., Fukuoka, T., Yokoyama, K., et al. (2011). Effect of “Jidabokuippo” for Subgaleal Hematoma with Skull Fractures: a Case Report. Neurotraumatol 34, 192–195. (in Japanese). doi:10.32187/neurotraumatology.34.2_192
Uchida, S., Niwa, M., Ozaki, M., et al. (1988). Radical Scavenging Action of Condensed Tannins. Neurosci 14, 243–245. (in Japanese).
Uemura, Y., Suzuki, Y., Sasaguri, T., Hirata, M., and Hirakawa, N. (2013). A Case Report of Successful Treatment for Temporomandibular Joint Disorder with Jidabokuippo and Yokukansan. Pain Kampo Med. 23, 44–48. (in Japanese).
Yabe, M., Kawai, S., Yamasaki, H., Funao, T., and Mishikawa, K. (2018). Jidabokuippo Was Effective for Chronic Pain after Resection of a Retroperitoneal Tumor: a Report of Two Cases. Pain Kampo Med. 28, 109–112. (in Japanese).
Yamada, T., Natori, Y., Mori, M., Kai, Y., Miki, K., and Noguchi, N. (2019). Two Cases of Organized Chronic Subdural Hematoma in Which Hematoma Shrinks with Administration of Jidabokuippo. J. Neurosurg. Kampo Med. 5, 53–57. (in Japanese).
Yamamoto, I. (1975). Effect of Chlorella Ingestion on Oxidative Stress and Fatigue Symptoms in Healthy Men. Jidabokuippo. Kampo, J. Med. 22, 317–324. (in Japanese). doi:10.2739/kurumemedj.22.71
Yamane, T., Takashima, S., Yamane, K., and Tashiro, S. (2000). Radical Scavenging Activities of Herbal Medicines by Electron Spin Resonance Assay. Jpn. J. Instrum. 70, 489–490. (in Japanese) https://mol.medicalonline.jp/library/journal/download?GoodsID=ca6instr/2000/007010/020&name=0489-0490j&UserID=158.215.8.21&base=jamas_pdf.
Yano, H., Maki, T., Goto, Y., et al. (2020). A Case of Severe Nocturnal Pain of the Right Hand after Injury Amputation of Fingers. 2,4,5, Successfully Treated with Kampo Medicine. Kampo, J. Med. 67, 811–816. (in Japanese).
Yonemitsu, T. (2017). Jidabokuippo and Sokeikakketsuto for Prolonged Pain after Bruising : A Case Report. Nihon Toyo igaku zasshi 68, 148–151. (in Japanese). doi:10.3937/kampomed.68.148
Yoshida, A. (2015). Therapeutic Effect of Jidabokuippo on Facial Contusions in Elderly Individuals. J. Neurosurg. Kampo Med. 1, 17–22. (in Japanese).
Yoshida, K., Harada, Y., and Arai, H. (2018). Two Case Reports Prescribing Jidabokuippo for the Puncture Hematoma after the Angiography. J. Neurosurg. Kampo Med. 4, 34–38. (in Japanese).
Yoshinaga, R., Nakayasu, K., and Tahara, E. (2020). An Ankle Sprain with Long-Term Swelling and Pain Successfully Treated with the Traditional Japanese Herbal Medicine Jidabokuippo: a Case Report. J. Gen. Fam. Med. 21, 261–263. doi:10.1002/jgf2.354
Yoshinaga, R., Goto, Y., Maki, T., Inoue, H., Yano, H., and Tahara, E. (2021). The Role of Kampo Therapy to Treat Cellulitis - Three Cases of Lower Limb Cellulitis-. Kampo Med. Nihon Toyo Igaku Zasshi. 72, 135–143. (in Japanese).
Yoyakusuchi, M. G. (2021). National Diet Library Digital Collections. Available at: https://dl.ndl.go.jp/info:ndljp/pid/2536607 (Accessed December 16, 2021).
Keywords: trauma, inflammatory swelling, made-in-Japan, static blood, adverse event
Citation: Nakae H, Irie Y, Kitamura T and Okuyama M (2022) Application of Traditional Japanese Drug Jidabokuippo in a Modern Society. Front. Pharmacol. 13:853012. doi: 10.3389/fphar.2022.853012
Received: 12 January 2022; Accepted: 11 April 2022;
Published: 23 May 2022.
Edited by:
Kenny Kuchta, University Medical Center Göttingen, GermanyReviewed by:
Masahiro Ohsawa, Nagoya City University, JapanCopyright © 2022 Nakae, Irie, Kitamura and Okuyama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hajime Nakae, bmFrYWVoQGRvYy5tZWQuYWtpdGEtdS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.