
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 24 October 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1022053
This article is part of the Research Topic Regulation of PI3K/Akt Signaling Pathway: A Feasible Approach for Natural Neuroprotective Agents to Treat Various Neuron Injury-Related Diseases View all 8 articles
Neurological diseases impose a tremendous and increasing burden on global health, and there is currently no curative agent. Puerarin, a natural isoflavone extracted from the dried root of Pueraria montana var. Lobata (Willd.) Sanjappa and Predeep, is an active ingredient with anti-inflammatory, antioxidant, anti-apoptotic, and autophagy-regulating effects. It has great potential in the treatment of neurological and other diseases. Phosphatidylinositol 3-kinases/protein kinase B (PI3K/Akt) signal pathway is a crucial signal transduction mechanism that regulates biological processes such as cell regeneration, apoptosis, and cognitive memory in the central nervous system, and is closely related to the pathogenesis of nervous system diseases. Accumulating evidence suggests that the excellent neuroprotective effect of puerarin may be related to the regulation of the PI3K/Akt signal pathway. Here, we summarized the main biological functions and neuroprotective effects of puerarin via activating PI3K/Akt signal pathway in neurological diseases. This paper illustrates that puerarin, as a neuroprotective agent, can protect nerve cells and delay the progression of neurological diseases through the PI3K/Akt signal pathway.
With the aging of the population and the increase in life expectancy, the prevalence of neurological diseases is increasing, causing a huge health burden worldwide (Charlson et al., 2019). Including Alzheimer’s disease (AD), Parkinson’s disease (PD), traumatic brain injury (TBI), stroke, intracerebral hemorrhage (ICH), epilepsy, and other neurological diseases, affecting about one billion people worldwide, involving people of all ages, races and socioeconomic status (Misra et al., 2018). Neurological disorders have become the second leading cause of death in the world (Evans-Lacko et al., 2018), and it is also an important cause of disability and premature death. At present, there are few drugs to treat and prevent these diseases, which makes neurological diseases have become an obvious challenge (Zhou et al., 2019). Therefore, there is an urgent need to develop new drugs for the treatment of various nervous system diseases. The variety of neurological diseases with a wide range of neuropathological features, including neurological damage, cognitive dysfunction, and motor coordination disorders, has led to complex and diverse pathogenesis and involved signaling pathways, hindering the development of new drugs. Phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signal pathway participates in many cellular processes such as cell proliferation, differentiation, protein synthesis, and apoptosis, and is the key mechanism for the occurrence and development of central nervous system diseases (Matsuda et al., 2019). Focusing on the PI3K/Akt pathway, it is of great significance to seek reliable treatments and new drugs for neurological diseases.
Chinese herbal medicine and its natural products have the advantages of multiple targets and few adverse reactions, so they have a good prospect in the treatment of nervous system diseases (Yang et al., 2017). Puerarin is an isoflavone with high bioactivity. It is a key active substance extracted from the traditional Chinese medicine Kudzu root (Gegen in Chinese), the dried root of Pueraria montana var. Lobata (Willd.) Maesen & S. M. Almeida ex Sanjappa & Predeep. Experimental and clinical studies have reported that puerarin has been widely used in the treatment of cardiovascular disease (Zhou et al., 2021), diabetes mellitus and its complications (Bai et al., 2021), cancer (Ahmad et al., 2020), osteoporosis (Cao et al., 2022), nonalcoholic fatty liver (Wang B. et al., 2019) and endometriosis (Meresman et al., 2021). In recent years, puerarin has attracted much attention because of its excellent neuroprotective effect in AD, PD, and other central nervous system diseases. The activation of the PI3K/Akt pathway is an important way for puerarin to play a neuroprotective role. In this article, we summarized the research on puerarin protecting the nervous system by regulating PI3K/Akt pathway, in order to provide significant implications for elucidating the mechanism of puerarin in treating nervous system diseases and get further promotion in clinical application.
PI3K/Akt signal pathway is one of the classical pathways to regulate the cell cycle, which plays an important role in cell division, differentiation, and survival. PI3K is an intracellular phosphatidylinositol kinase and an important anti-apoptotic regulatory factor. Protein kinase B (PKB), also known as Akt, is an intracellular serine/threonine protein kinase. A cascade reaction pathway involving multiple signaling molecules centered on PI3K and Akt is called the PI3K/Akt signal pathway, and its activation and signal transduction regulates various physiological responses of the body. In response to various growth factors and neurotrophic factors, transmembrane receptors such as receptor tyrosine kinases (RTKs) undergo autophosphorylation, causing PI3K to be activated on the plasma membrane. Activated PI3K catalyzes the production of the second messenger phosphatidylinositol-3,4,5-trisphosphate (PIP3), which in turn recruits Akt for phosphorylation (p-Akt) at the plasma membrane. p-Akt initiates downstream effectors including mammalian target of rapamycin (mTOR), glycogensynthasekinase3β (GSK3β), and actin-related proteins, and exerts a variety of mechanisms to significantly affect cell growth and survival. Among them, mTOR, as a highly conserved serine/threonine kinase, is the core downstream factor of the PI3K/Akt signal pathway, which can be activated directly or indirectly by Akt. Meanwhile, mTOR is also an activator of Akt (Long et al., 2021). After activation, mTOR can directly act on p70 ribosomal S6 protein kinases one and 2 (p70S6K1/2), eukaryotic initiation factor 4 E-binding proteins (4 E-BPs), and other proteins involved in translation regulation, thereby initiating the protein synthesis, transcription, autophagy, metabolism, cell growth and proliferation (Lipton and Sahin, 2014).
PI3K/Akt signal pathway has been proven to play an important role in the pathogenesis of neurological diseases (Matsuda et al., 2019) and is one of the most important regulatory pathways for neuronal survival, capable of regulating neurogenesis and synaptic plasticity (Manning and Toker, 2017). Activation of the PI3K/Akt pathway promotes neuronal survival in the presence of in vitro hypoxia, excitotoxic neuronal death, and in vivo ischemic neuronal death (Lai, Zhang, and Wang, 2014). Multiple studies have demonstrated significantly reduced levels of p-PI3K and p-Akt in injured brain cells. Inhibition of the PI3K/Akt pathway exacerbates ischemic neuronal death (Endo et al., 2006). Activation of PI3K/Akt signal pathway can significantly improve acute brain injury such as cerebral hemorrhage (Krafft et al., 2013; Cui et al., 2017) and subarachnoid hemorrhage (Hao et al., 2014; Xie et al., 2018). Akt is considered a therapeutic target in neurological diseases that impact neuronal survival. Excitotoxicity is a critical link between ischemia and neuronal death (Molina et al., 2021). Akt is able to inhibit the death signal BAD (Noshita et al., 2002), the forkhead transcription factor (Trotman et al., 2006), and the proline-rich Akt substrate (Saito et al., 2004) in excitotoxicity and stroke models. Akt phosphorylates and inhibits the death-signaling protein GSK-3β, which is implicated in the pathogenesis of several neurological diseases (Chigusa et al., 2017). GSK-3β is one of the direct substrates of Akt, involved in synapse formation and microglia activation, and is able to regulate a variety of biological activities such as cell metabolism, apoptosis, inflammation, and oxidative stress. Over-activated GSK-3β leads to excitotoxic neuronal damage (French and Heberlein, 2009). In addition, its over-activation can cause synaptic defects, promote the hyperphosphorylation of tau to form neurofibrillary tangles, ultimately leading to diseases such as AD (Golpich et al., 2015; Abdallah et al., 2021). p-Akt inhibits its activity by phosphorylating GSK-3β, and phosphorylation GSK-3β (p-GSK-3β) is neuroprotective. p-GSK-3β induces the accumulation of MCL-1 protein of the Bcl-2 family, and exert an anti-apoptotic effect to protect neuronal cells (Banach et al., 2022). Studies have reported that Akt is a key mediator of several aspects of neurite growth, including elongation, branching, and caliber (Read and Gorman, 2009). Akt is able to phosphorylate GSK-3β to promote neurite growth (Ooms et al., 2006). The PI3K/Akt/mTOR pathway has been shown to promote the growth and branching of hippocampal neurons and plays an important role in the development of brain structures (Chen et al., 2003). Akt is a major upstream regulator of mTOR. mTOR is involved in the entire brain function, regulating neuronal cell synaptic plasticity, myelin formation, memory and retention (Lipton and Sahin, 2014). Studies have shown that mTOR is able to increase the length and complexity of dendrites (Jin et al., 2012). mTOR also promotes axonal regeneration in the adult central nervous system (Abe et al., 2010). In addition, mTOR can regulate a variety of downstream factors involved in neuroprotection. The sterol-response binding proteins (SREBPs) are one of the transcription factors regulated by mTOR and are involved in nutrient sensing, excitotoxicity, myelination and neurodegenerative diseases (Lebrun-Julien et al., 2014). mTOR regulates the transcription and translation of hypoxia inducible factor 1α (HIF1α), which mediates mechanisms associated with stroke and neurodegenerative diseases. Recent studies have reported that Eukaryotic Elongation Factor-2 Kinase (eEF2K) is essential for the physiopathology of the nervous system. eEF2K is able to influence synaptic plasticity, memory, and regulate protein translation in dendrites. Increased eEF2K expression is observed in AD, PD and epilepsy, and its phosphorylation is reduced as a key downstream effector of mTOR (Ballard et al., 2021). In conclusion, targeting PI3K/Akt is of great value for studying the pathogenesis of neurological diseases and new drug development.
Puerarin has the chemical structure of 7,4'-dihydroxy-8-C-glucosylisoflavone (Figure 1), molecular formula C21H20O9, molecular weight 416.38 (Zhou Y. X. et al., 2014). Studies have shown that puerarin can be detected rapidly and extensively in most organs such as the hippocampus, heart, and lungs after intravenous administration (Anukunwithaya et al., 2018; Zhang, 2019). Also, puerarin can be quickly eliminated. Puerarin also crosses the blood-brain barrier and is widely distributed in the hippocampus, cerebral cortex, striatum, and other regions of the brain, but at low levels (Kong et al., 2017). The gastrointestinal absorption and bioavailability of puerarin are poor. The absorption in both the stomach and intestine of rats was 20% (Chen et al., 2018), and the absolute oral bioavailability in rats (5 mg/kg, 10 mg/kg) was 7% (Anukunwithaya et al., 2018). Daidzein is the major hydrolytic metabolite of puerarin (Jung et al., 2014), which was formed by cytochrome P450 proteins in the liver microsomes (Wen et al., 2008). Glucuronoside is the main metabolite of puerarin, which is excreted in the urine and feces (Anukunwithaya et al., 2018). UDP-glucuronosyltransferase 1A1 is the principal enzyme responsible for puerarin metabolism in human liver microsomes (Luo et al., 2012). The toxicity of puerarin in experimental animals is low, but its toxicity has yet to be re-evaluated in clinical studies (Zhang, 2019). Although puerarin is easy to extract, its chemical structural properties result in poor solubility and permeability, resulting in its low oral bioavailability (Yu et al., 2022). Therefore, it is not feasible to increase the bioavailability by increasing the dose alone in clinical application, but it will increase the toxic side effects of the drug. At present, injection is the main mode of administration of puerarin (China Food and Drug Administration, http://app1.sfda.gov.cn/datasearchcnda/face3/dir.html). In order to improve the bioavailability of puerarin injections, the formulation must be supplemented with polyvinylpyrrolidone (PVP) or high concentrations of propylene glycol. However, this has consequently led to adverse effects such as fever and vascular irritation, limiting its widespread clinical use. To overcome this limitation, microemulsions and self-microemulsifying drug delivery systems (Zhang, 2019), dendrimers (Gu et al., 2013), nanoparticle carriers (Chen et al., 2019), nanocrystals (Xiong et al., 2019) and other drug delivery systems were used to improve the bioavailability and brain targeting of puerarin. In addition, structural biotransformation of puerarin using sucrose amylosucrase (Ding et al., 2022), microbial and free enzymes (Liu B. et al., 2016) was also able to improve the bioavailability of puerarin. All of the above are of great value in advancing the research and application of puerarin.
FIGURE 1. The chemical structure of puerarin. In the ball and stick model, grey, red, and white balls represent carbon, oxygen, and hydrogen atoms, respectively. (National Center for Biotechnology Information (2022). PubChem Compound Summary for CID 5281807, Puerarin. Retrieved 17 August 2022 from (https://pubchem.ncbi.nlm.nih.gov/compound/5281807).
Up to now, puerarin has been widely proven to have pharmacological activities such as vasodilator, anti-inflammatory, antioxidant, anti-apoptotic, regulating autophagy, anti-insulin resistance, anti-cancer, etc. It has protective effects on cell damage caused by pathological factors and has shown good efficacy in the treatment of various diseases (Ma et al., 2022). Clinically, puerarin has been approved by China Food and Drug Administration as a vasodilator in the treatment of patients with coronary heart disease and hypertension (Huang et al., 2021). Puerarin has significant anti-inflammatory and antioxidant effects. It has been shown that puerarin reduced the mRNA and protein levels of Tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6), and IL-1β in a mouse model of myocardial ischemia/reperfusion (MI/R) injury, inhibited NLRP3 inflammasome and protected the heart (Wang et al., 2020). Puerarin can significantly reduce reactive oxygen species (ROS) production, while excessive ROS can activate downstream apoptotic factors such as cytochrome C and apoptosis-inducing factors, and induce apoptosis (Liang et al., 2019). Puerarin can reduce ROS by increasing the gene expression of MnSod and Gpx-1, which are scavengers of ROS. It is also possible that puerarin can reduce the levels of lipid peroxidation product mitochondrial malondialdehyde (MDA) and increase superoxide dismutase (SOD) by reducing the production of ROS. Atherosclerosis is a pathological process characterized by a chronic inflammatory response, which is closely related to the inflammatory response of vascular endothelial cells. Puerarin modulates mitochondrial function in lipopolysaccharide-stimulated human umbilical vein endothelial cells, inhibits the expression of inflammatory factors and oxidative stress damage, increases autophagy and mitochondrial antioxidant capacity, and is considered to be a natural antioxidant for the treatment of atherosclerosis (Liang et al., 2019). Puerarin significantly increased the levels of antioxidant markers such as superoxide dismutase, glutathione peroxidase, and catalase in serum and liver of NAFLD, and decreased the levels of TNF-α, IL-18, and IL-1β (Zhou J. F. et al., 2022). Puerarin also mediates hepatoprotective effects by inhibiting oxidative stress and inflammation through the microRNA (miR)-34a-5p/Sirt1 axis (Hao et al., 2022). The efficacy of puerarin against preeclampsia is also related to its anti-inflammatory effect (Guo et al., 2022). In addition, puerarin also reduces pro-inflammatory factors in the chronic unpredictable mild stress (CUMS) rat model of depression via the gut-brain axis, and increases the level of anti-inflammatory factors, thereby achieving an antidepressant effect (Song et al., 2022). Recently, Zeng et al. (2022) also explored the anti-inflammatory mechanism of puerarin, suggesting that the anti-inflammatory effect of puerarin may be achieved by regulating multiple metabolic pathways and metabolites related to ferroptosis. Puerarin can improve diabetes and a series of complications by anti-inflammation and reducing oxidative stress. At the same time, it can also lower blood glucose, improve insulin resistance, and protect pancreatic β-cells through anti-apoptotic effects (Chen et al., 2018; Xu et al., 2021). Based on these diverse pharmacological effects, puerarin has been confirmed to have good anticancer potential and can exert therapeutic effects on cancer by blocking the cell cycle, inhibiting cell migration, inducing apoptosis, and regulating autophagy (Ahmad et al., 2020; Murahari et al., 2020). Puerarin also promotes the proliferation and differentiation of rat primary osteoblasts and human osteoblastic MG-63 cells by altering cell cycle distribution (Zhang et al., 2007; Wang et al., 2013), In contrast, puerarin inhibits vascular smooth muscle cell proliferation in the diabetic setting (Zhu et al., 2010).
Nevertheless, the potential mechanisms and direct targets for the diverse therapeutic effects of puerarin remain unclear. Many studies have pointed out that the activation of the PI3K/Akt signal pathway may be a key pathway for puerarin to protect the survival of various cells. Embryonic stem cells are the best platform for the research and screening of new drugs (Xi et al., 2010). Yin et al. (2015) found that puerarin could down-regulate the transcription level of RE1 silencing transcription factor (rest), a key factor for maintaining the self-renewal of embryonic stem cells, and up-regulate miR-21, a downstream target of rest, by activating PI3K/Akt pathway, thereby inhibiting the self-renewal of murine embryonic stem cells and turning them into endoderm and ectoderm differentiation. Endothelial-mesenchymal transition (EndMT) is closely related to cardiac fibrosis. Puerarin can inhibit EndMT and improve cardiac fibrosis. It was shown (Li et al., 2020) that puerarin alleviates HO-stimulated EndMT in human coronary artery endothelial cells (HCAECs) by inhibiting ROS and activating PI3K/Akt pathway. Chen et al. (2022) conducted a study using two animal models of pulmonary arterial hypertension (PAH), a rat model of monocrotaline (MCT)-induced PAH and a mouse model of hypoxia-induced hypoxic pulmonary hypertension (HPH). They confirmed that puerarin can effectively improve the structural and functional abnormalities of the pulmonary artery and right ventricle, and restore the PI3K/Akt pathway downregulated by MCT and hypoxia. Wang et al. (2022) treated cigarette smoke extract (CSE)-stimulated human bronchial epithelial cells (HBECs) with different concentrations of puerarin. The significant efficacy of puerarin in reducing ROS content and apoptosis was confirmed. It was also found that puerarin inhibited mitochondrial autophagy and bronchial epithelial cell apoptosis by activating PI3K/Akt/mTOR pathway and down-regulating DRP1 and FUN14 domain protein 1 (FUNDC1) expression. In diabetes, apoptosis is the predominant form of pancreatic β-cell death. Puerarin significantly inhibited CoCl2-induced pancreatic β-cell apoptosis and reduced ROS production and pro-apoptotic Bcl-2-associated X protein (Bax), and upregulated Sod2 and Gpx1. It is suggested that puerarin can act directly on pancreatic β-cell through anti-apoptotic and antioxidant effects by activating the PI3K/Akt pathway (Li et al., 2014). Puerarin rapidly activated Akt in mouse insulinoma β-cells (MIN6) and also restored the diminished p-Akt induced by CoCl2. Puerarin improved insulin resistance also by activating PI3K/Akt pathway (Xu et al., 2021). In terms of anti-osteoporosis, the mechanism by which puerarin promotes proliferation and differentiation of rat primary osteoblasts and human osteoblastic MG-63 cells is also achieved by increasing Akt basal phosphorylation levels in a PI3K-dependent manner (Zhang et al., 2007; Wang et al., 2013). Anti-osteoclast apoptosis is usually a key target for the prevention of glucocorticoid-induced osteoporosis. The PI3K/Akt pathway also mediates the inhibition of glucocorticoid-induced apoptosis in human fetal osteoblastic cells (hFOB1.19) by puerarin Yu et al. (2015). The PI3K/Akt pathway can be activated in a variety of human cancers, affecting the activity of transcriptional regulators. Elevated expression of its downstream factor mTOR often suggests a poor prognosis of cancer (Ahmad et al., 2020). In cancer diseases including non-small cell lung cancer (Hu et al., 2018), bladder cancer (Jiang et al., 2018), mantle cell lymphoma (Gan and Yin, 2015), chondrosarcoma (Huang et al., 2017), and other cancer diseases, puerarin was able to downregulate their PI3K, p-Akt, and mTOR levels, induce autophagy, inhibit tumor cell proliferation, and increase apoptosis. It can be seen that puerarin may have a bidirectional regulation of the PI3K/Akt pathway. In addition, the mechanisms by which puerarin inhibited apoptosis in human papillomavirus-positive HeLa cervical cancer cells (Jia et al., 2019) and ameliorated NAFLD induced by high fat and high sucrose diet in mice (Wang S. et al., 2019) were also closely related to PI3K/Akt/mTOR pathway.
Puerarin is also a natural phytoestrogen. Because of its estrogenic activity, puerarin can regulate the apoptosis of MG-63 cells through the estrogen receptor-dependent PI3K/Akt pathway (Wang et al., 2013) and inhibit the oxidative injury of Mouse hepatoma Hepa1c1c7 and HepG2 cells (Hwang and Jeong, 2008). In addition, puerarin also phosphorylated eNOS at the Ser1177 site of the reductase structural domain in EA. hy926 human endothelial cells to produce NO, which in turn protected endothelial cells (Hwang et al., 2011).
In conclusion, PI3K/Akt signal pathway is the key mechanism by which puerarin exerts protective effects on various cells. A review of the research progress of puerarin protection against neurological diseases using the PI3K/Akt signal pathway as the target is valuable to elucidate the mechanism of puerarin protection against neuronal cells.
Puerarin has potent neuroprotective effects due to its excellent anti-inflammatory, antioxidant, and anti-apoptotic properties (Yu et al., 2022). Recent studies have provided much evidence suggesting that puerarin may ameliorate neurological disorders by activating the PI3K/Akt signal pathway (Table 1 and Figure 2).
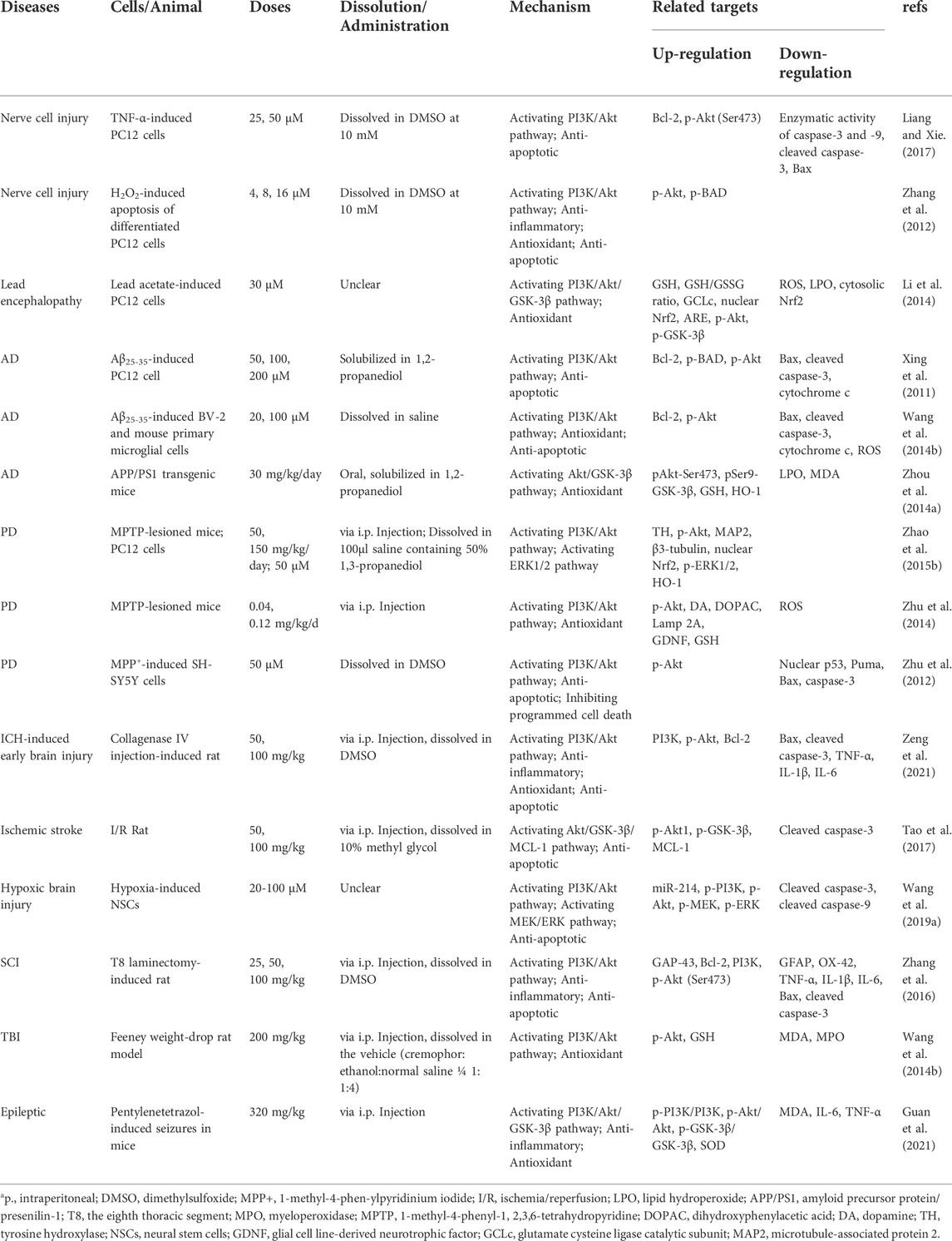
TABLE 1. Neuroprotective effects of puerarin on neurological diseases via modulating PI3K/Akt signal pathway.
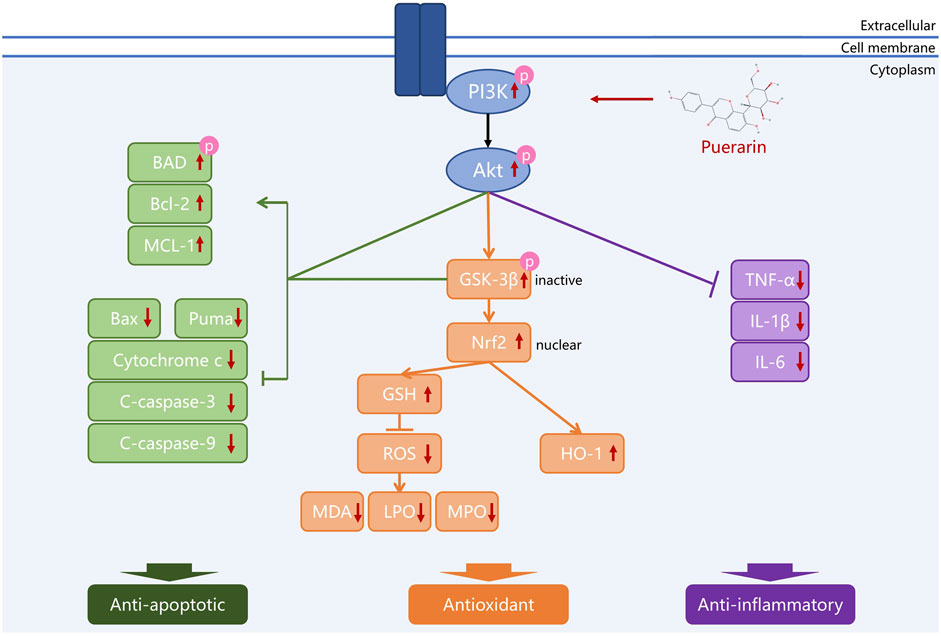
FIGURE 2. Puerarin protects neurons by regulating the PI3K/Akt pathway. Different colors are used to represent that puerarin protects neurons through anti-apoptosis, antioxidation, and anti-inflammation, which are closely related to the regulation of the PI3K/Akt pathway by puerarin.
PC12 cells are a differentiated cell line of rat adrenal medullary pheochromocytoma with general characteristics of neuroendocrine cells. It has been widely used in neurophysiological and neuropharmacological studies (Wang et al., 2015). Liang and Xie (Liang and Xie, 2017) used TNF-α to induce neurotoxicity in PC12 cells. It was found that puerarin significantly promoted Akt (Ser473) phosphorylation and inhibited TNF-α-induced apoptosis. Application of PI3K inhibitor LY294002 attenuated the effect of puerarin, indicating that puerarin inhibited TNF-α-induced apoptosis in PC12 cells by activating the PI3K/Akt pathway. Zhang et al. (2012) reported the effect and mechanism of puerarin on oxidative stress-induced neurotoxicity in PC12 cells. The results showed that puerarin was able to dose-dependently inhibit H2O2-induced PC12 cells from apoptosis under oxidative stress by increasing the production of p-Akt and apoptotic factor p-BAD (Bcl-2/Bcl-XL-antagonist). These protective effects were reversed by the highly specific PI3K inhibitor wortmannin, suggesting that puerarin may exert antioxidant and anti-apoptotic effects by activating the PI3K/Akt pathway. The central nervous system is the main target of lead poisoning, and neuronal oxidative stress is the key mechanism (Lu et al., 2013). Puerarin has great potential to treat lead neurotoxicity-related diseases due to its excellent antioxidant effect. It was noted (Li et al., 2014) that puerarin significantly attenuated ROS and lipid hydroperoxide levels in lead acetate-induced PC12 cells, and induced phosphorylation of Akt and GSK-3β. In addition, puerarin also elevated the level of glutathione (GSH), a cellular antioxidant, and upregulated the expression of glutamate cysteine ligase catalytic subunit (GCLc), a major rate-limiting enzyme for GSH synthesis. Puerarin induces nuclear factor erythroid 2-related factor 2 (Nrf2) nuclear accumulation, thus exerting a role in regulating GSH biosynthetic enzymes. The above protective effect of puerarin could be blocked after using LY294002. It indicates that puerarin exerts an antioxidant mechanism to ameliorate lead poisoning injury in neuronal cells, mainly through activation of the PI3K/Akt/GSK-3β pathway. In summary, puerarin exerts anti-apoptotic, antioxidant, and anti-inflammatory effects to ameliorate neuronal cell injury through activation of the PI3K/Akt pathway.
AD is a neurodegenerative disease characterized by progressive memory and cognitive impairment, and behavioral and motor disorders. The main pathological features are plaques of β-amyloid (Aβ) and neurofibrillary tangles composed of hyperphosphorylated tau. As well as extensive synaptic and neuronal loss, oxidative stress and neuroinflammation, for which there is no effective treatment (Knopman et al., 2021). More and more studies have confirmed that puerarin can effectively improve AD cognitive dysfunction through multiple mechanisms (Yu et al., 2022). Puerarin reduces Aβ deposition, decreases protein levels of hyperphosphorylated tau and its phosphorylation levels, alleviates neuroinflammation, and prevents neuronal loss (Huang et al., 2019). Puerarin can improve synaptic plasticity impairment in AD by regulating the p38 MAPK-CREB signal pathway (Liu C. et al., 2021). Increased ROS production can directly impair synaptic plasticity and cause cognitive dysfunction (Butterfield and Halliwell, 2019). Puerarin can increase the activity of oxidative stress markers glutathione peroxidase (GSH-Px) and superoxide dismutases (SOD) in brain tissue, and reduce ROS generation and the level of lipid peroxidation product malondialdehyde (MDA) (Zhao J. et al., 2015). Puerarin also alleviated Aβ25-35-induced PC12 cell apoptosis and increased the Bcl-2/Bax ratio (Zhang et al., 2008). PI3K/Akt pathway is one of the important mechanisms by which puerarin ameliorates AD cognitive impairment. It was shown Xing et al. (2011) that puerarin was able to activate Akt phosphorylation in Aβ25-35-induced PC12 cells. Meanwhile, puerarin up-regulated the levels of Bcl-2 and p-BAD, and down-regulated Bax and cleaved caspase-3. It is suggested that puerarin improves Aβ25-35-induced PC12 cell apoptosis depending on PI3K/Akt pathway. Wang C. et al. (2014) used Aβ25-35-induced BV-2 and mouse primary microglial cells as AD models and further confirmed that the anti-apoptotic effect of puerarin on AD is dependent on PI3K/Akt signal pathway. In addition, they found that Aβ25-35-induced primary microglial cells exhibited disruption of mitochondrial membrane potential and increased ROS production, which was inhibited by puerarin. Zhou Y. et al. (2014) also confirmed that puerarin can activate the Akt/GSK-3β signal pathway in amyloid precursor protein/presenilin-1 (APP/PS1) mice and induce Nrf2 nuclear translocation in the hippocampus. Puerarin also down-regulated lipid hydroperoxide and MDA, and up-regulated the levels of GSH and antioxidant enzyme heme oxygenase-1 (HO-1). These results suggest that puerarin improves AD cognitive dysfunction by exerting antioxidant effects, rather than by altering soluble or insoluble Aβ1-42 levels. In addition, puerarin can activate PI3K/Akt/eNOS pathway to reduce ROS production and avoid neuronal death (Lu et al., 2014; Liu et al., 2019). It can be seen that puerarin has a good therapeutic prospect in improving AD cognitive dysfunction, and its mechanism is closely related to PI3K/Akt-mediated anti-apoptosis and antioxidant. However, there are still few studies on the mechanism of improvement of AD by puerarin, and more studies targeting PI3K/Akt pathway are needed in the future. This is of great interest to elucidate the mechanism of action of puerarin in improving AD.
PD is the second major neurodegenerative disorder characterized by progressive loss of nigrostriatal dopaminergic neurons, manifested by bradykinesia, rigidity, or resting tremor. Like AD, there is still no effective treatment (Aborode et al., 2022). Several studies have reported that puerarin can improve PD through neuroprotection and antioxidant effects, but the specific mechanism is unclear. Nerve growth factor (NGF), a neurotrophic factor, is significantly downregulated in the substantia nigra of PD patients and inhibits neuronal function, growth, and differentiation (Ubhi et al., 2010). The PI3K/Akt signal pathway is an important mechanism regulating the neurotrophic activity of NGF (Tzeng et al., 2021). A study by Zhao J. et al. (2015) noted that puerarin significantly improved tyrosine hydroxylase levels and motor dysfunction in dopaminergic neurons of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned mice. In PC12 cells, the combination of NGF (2 ng/ml) with puerarin (50 μM) greatly enhanced NGF-promoted neurite formation and length, as well as the levels of microtubule-associated protein 2 (MAP2) and β3-tubulin. Further studies confirmed that puerarin activates PI3K/Akt and ERK1/2 pathways to promote Nrf2 into the nucleus, upregulates HO-1, and subsequently enhances NGF-induced neurogenesis. ROS is thought to be critical in mediating dopaminergic neuronal cell death. It has been suggested (Zhu et al., 2014) that puerarin can exert antioxidant effects by activating PI3K/Akt pathway, which in turn reduces ROS production, and upregulates the expression of GSH and glial cell line-derived neurotrophic factor (GDNF), thereby alleviating MPTP-induced of motor dysfunction and degeneration of dopaminergic neurons. Puerarin also ameliorated oxidative stress-induced reduction in chaperone-mediated autophagy (CMA) activity, a hallmark of PD pathogenesis (Zhu et al., 2014; Kuo et al., 2022). In addition, puerarin was able to activate the PI3K/Akt pathway in 1-methyl-4-phen-ylpyridinium iodide (MPP+)-induced dopaminergic SH-SY5Y cells and inhibit p53-mediated caspase-3-dependent programmed cell death (Zhu et al., 2012). In conclusion, puerarin is able to restore neurotrophic factor levels and improve motor dysfunction and dopaminergic neuronal degeneration in PD by activating the PI3K/Akt pathway. It is necessary to conduct more experimental and clinical studies.
ICH refers to primary cerebral parenchymal hemorrhage, a cerebrovascular disease with high disability and mortality rates worldwide, for which there is no specific and effective treatment (Zhou J. et al., 2022). A growing number of studies have shown that high-intensity oxidative stress, neuroinflammation, and neuronal apoptosis are important pathogenic mechanisms of secondary brain injury after ICH (Hu et al., 2016; Zheng et al., 2016; Lan et al., 2017). Therefore, targeting the regulation of oxidative stress, inflammatory response, and apoptosis in brain tissue is the key to the treatment of secondary brain injury after ICH. Zeng et al. (2021) showed that puerarin was able to activate the PI3K/Akt pathway after ICH, which in turn inhibited the activation of apoptotic signaling, reduced oxidative stress, and downregulated the levels of pro-inflammatory factors, ultimately relieving the early brain injury after ICH. The results showed that in an ICH rat model induced by collagenase IV injection, both 50 mg/kg and 100 mg/kg doses of puerarin significantly upregulated the expression levels of PI3K and p-Akt, thereby reducing the histological damage, cerebral edema, cerebral hematoma volume, and blood-brain barrier damage. In addition, apoptosis of brain cells was significantly increased after ICH. The production of ROS around the hematoma increased, and the activation of the NF-κB pathway caused a significant increase in the levels of various inflammatory factors. Puerarin can regulate the levels of B-cell lymphoma-2 (Bcl-2), Bax, and cleaved caspase-3, inhibit cell apoptosis, and reduce the levels of ROS and various inflammatory factors. These results confirmed that puerarin can exert anti-apoptotic, antioxidant, and anti-inflammatory effects by activating PI3K/Akt pathway to alleviate early brain injury caused by ICH. However, there are still relatively few studies on the effects and mechanisms of puerarin in improving cerebral hemorrhage, and more evidence is needed to support this.
Ischemic stroke, caused by rupture or occlusion of blood vessels, can cause irreversible damage to the brain. It is a common cause of death and severe disability, with millions of people worldwide suffering from ischemic stroke each year (Benjamin et al., 2019). Previous studies suggest that puerarin can exert cerebral protective effects by inhibiting apoptosis after cerebral ischemia (Xu et al., 2005). In China, puerarin injections have been widely used in the clinical treatment of patients with acute ischemic stroke. By evaluating 20 randomized controlled trials (RCTs) with 1574 participants as of 2015, Liu G. et al. (2016) suggested that puerarin improves neurological impairment after ischemic stroke. Hu et al. (2012) evaluated 15 RCTs and 1603 patients, and the results showed that puerarin was more effective than the control treatment for acute cerebral infarction. Tao et al. (2017) used a rat model of cerebral ischemia/reperfusion (I/R) as the experimental subject to investigate the mechanism of puerarin in improving ischemic stroke. The results showed that puerarin could upregulate p-Akt1 (Ser473), p-GSK-3β (Ser9), and myeloid cell leukemia-1 (MCL-1), and downregulate cleaved caspase-3, thereby improving the survival rate of neurons in cerebral cortex and hippocampus. PI3K inhibitor LY294002 was able to counteract the neuroprotective effects of puerarin mentioned above. This study provides a new idea to reveal the mechanism of puerarin in the treatment of ischemic stroke, but the way of activation of the PI3K/Akt pathway by puerarin remains to be further elucidated. In addition, the combination of puerarin and catalpol (an active monomer isolated from Rehmannia glutinosa) could protect neurovascular units (neurons, astrocytes, and brain vascular endothelial cells) by improving edema, anti-inflammation, antioxidant, and anti-apoptosis, and this protective effect was dependent on the regulation of PI3K/Akt/mTOR/HIF-1α and ERK/HIF-1α pathways (Liu et al., 2017). In conclusion, although puerarin has been widely used in the clinical treatment of acute ischemic stroke patients, its mechanism of action is still unclear. PI3K/Akt pathway may be the key mechanism for puerarin to improve ischemic stroke, which needs to be further elucidated by numerous studies in the future.
Hypoxia can cause different degrees of brain damage, especially in neonates. It is a major cause of neonatal death and disability (Kennedy et al., 2022). MiR-214 is involved in cell differentiation and apoptosis and is associated with myocardial damage (Liu S. et al., 2021; Xiao et al., 2021), hepatocellular carcinoma cell invasion (Zhao et al., 2022), and rectal cancer (Yang et al., 2021). It was shown that PI3K/Akt/mTOR pathway mediates hypoxia-induced apoptosis and autophagy (Gong et al., 2019), and regulation of miR-214 could activate PI3K/Akt/mTOR pathway to alleviate hypoxia-induced apoptosis and autophagy. To explore the role and mechanism of puerarin in ameliorating hypoxic brain injury, Wang B. et al. (2019) isolated neural stem cells (NSCs) from the hippocampus of E14 rats treated with hypoxia (an anaerobic gas mixture of 94% N2, 5% CO2, and 1% O2 for 8 h), and administered puerarin (20–100 μM) pretreatment. The results showed that puerarin (60 μM) significantly increased the cell viability of hypoxia-induced NSCs, and inhibited the expression of cleaved caspase-3 and -9. Puerarin relieved PI3K and Akt phosphorylation inhibited by hypoxia, while miR-214 deficiency treatment inhibited the effect of puerarin on PI3K and Akt phosphorylation. In conclusion, puerarin can activate PI3K/Akt pathway through upregulation of miR-214 and exert a protective effect against hypoxic NSCs injury through anti-apoptosis. The above study suggests that miR-214 may be a target for geranium to regulate the PI3K/Akt pathway to protect neuronal cells, but further studies are needed.
Puerarin has neuroprotective effects against secondary injury in acute spinal cord injury (SCI) and TBI. SCI is a traumatic injury caused by mechanical damage to the spinal cord tissue, which can undergo secondary injury such as neuronal apoptosis, glial cell activation, axonal degeneration, and ultimately lead to neurological dysfunction (Noristani, 2022). Zhang et al. (2016) found that puerarin was able to upregulate PI3K and p-Akt (Ser473), and significantly restore motor function in a rat model of SCI by exerting anti-inflammatory and anti-apoptotic effects. Puerarin can also maintain the survival and cell morphology of neurons and promote the regeneration of neurons. In addition, puerarin significantly inhibited the activation of astrocytes and microglia. It is evident that PI3K/Akt is an important mechanism by which puerarin protects against secondary injury in SCI. Primary brain injury in TBI is difficult to intervene in, but reversible secondary brain injury has critical therapeutic implications (Bramlett and Dietrich, 2007). Studies have shown that the degree of oxidative stress is closely related to the severity of secondary brain injury in TBI, and protection against secondary brain injury in TBI through antioxidants is of interest (Wang J. W. et al., 2014). Wang C. et al. (2014) demonstrated that puerarin can down-regulate MDA, Myeloperoxidase (MPO), and up-regulate GSH by activating the PI3K/Akt pathway and improve TBI-induced neurodegeneration by exerting antioxidant and anti-inflammatory effects. It can be seen that puerarin has a good therapeutic prospect in the treatment of SCI and TBI secondary injury. The PI3K/Akt pathway may be the key mechanism for this effect of puerarin in promoting neuronal survival and regeneration.
Epilepsy is caused by excessive abnormal discharge of nerve cells in the brain. Persistent seizures can lead to oxidative stress and inflammation in the brain, damage brain tissue, and cause cognitive dysfunction (Knowles et al., 2022). Puerarin is able to improve epilepsy-induced brain damage through anti-inflammatory, antioxidant, and anti-apoptotic effects, but the exact mechanism is unclear (Xie et al., 2014). Guan et al. (2021) found that puerarin significantly reduced the number, duration, and mean escape latency of pentylenetetrazol-induced seizures in mice. Further research found that puerarin improved epileptic seizures and cognitive function in mice by activating the PI3K/Akt/GSK-3β signal pathway. Although there are still few studies on the mechanism of puerarin to ameliorate epilepsy-induced brain injury, a related study targeting the PI3K/Akt pathway provides us with new ideas to understand the antiepileptic effects of puerarin.
The activation of the PI3K/Akt pathway may be the key pathway for puerarin to ameliorate a variety of diseases and cells; therefore, it is important to review the research progress of puerarin in protecting neurological diseases by targeting the PI3K/Akt pathway. In summary, the mechanism of action of puerarin in regulating the PI3K/Akt pathway to protect neuronal cells is complex and mutual, mainly related to antioxidant, anti-apoptotic and anti-inflammatory, but the specific molecular mechanisms involved are still unclear. Among them, the antioxidant effect of puerarin is considered the central mechanism of action and investigated deeply by many studies. Although most of the biological effects of puerarin on different cells have been shown to be related to the regulation of the PI3K/Akt pathway, there are still many limitations of the existing studies. For example, current evidence does not explain how puerarin targets activation of the PI3K/Akt pathway and the direct target of puerarin action remains unclear. Some arguments suggest that puerarin (Hwang and Jeong, 2008; Wang et al., 2013), because of its natural estrogenic activity, may interact directly with the estrogen receptor, binding to the p85 regulatory subunit of PI3K in a ligand-dependent manner, which in turn causes an Akt phosphorylation hierarchical response. Further studies on the direct interaction between puerarin and estrogen receptors are needed in the future to better explain this issue. This is a key question that needs to be addressed for many natural drugs. Further mechanistic studies might help in understanding the significance at the molecular level. Furthermore, although most studies suggest that puerarin exerts its biological activity by activating the PI3K/Akt pathway, in diseases such as cancer, hepatic fibrosis (Guo et al., 2013), and cardiac hypertrophy (Yuan et al., 2014), the therapeutic effects of puerarin are mediated by blocking the PI3K/Akt pathway. These results suggest that puerarin may be a bidirectional regulator of the PI3K/Akt pathway. This also requires more research to demonstrate and analyze. Notwithstanding the continued progress made in the treatment of neurological diseases of this natural agent, the development and application of puerarin as a novel drug still require more experimental. According to the available Pharmacokinetics studies on Puerarin, there is still room for improvement in its solubility, permeability, and bioavailability of Puerarin. To improve the bioavailability of puerarin, a number of approaches have been developed in recent years to improve the brain targeting of the drug, including the use of drug delivery systems and structural biotransformation. However, puerarin is still mainly administered by intraperitoneal injection in the current in vivo studies. Further research on puerarin in combination with new techniques of drug delivery is necessary. Although puerarin has been used clinically in the treatment of ischemic stroke in China, the quality of clinical studies needs to continue to improve. Also, the efficacy and mechanisms of puerarin in the treatment of other neurological disorders need to be supported by clinical studies. Finally, although the toxicity of puerarin to experimental animals is low, the existing toxicology studies are far from adequate. More and higher quality studies are needed, especially in human populations.
QW completed the manuscript. Z-NS, S-JZ, and YS contributed to searching for information or preparing figures or tables. F-JZ and Y-HL contributed to the conception and design of the review. All authors contributed to the article and approved the final manuscript.
This work was supported by National Natural Science Foundation of China grants (82074325 and 82104814) and China Postdoctoral Science Foundation funded project (2020M680469).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdallah, H. M., El Sayed, N. S., Sirwi, A., Ibrahim, S. R. M., Mohamed, G. A., and Abdel Rasheed, N. O. (2021). Mangostanaxanthone IV ameliorates streptozotocin-induced neuro-inflammation, amyloid deposition, and tau hyperphosphorylation via modulating PI3K/Akt/GSK-3β pathway. Biol. (Basel) 10 (12), 1298. doi:10.3390/biology10121298
Abe, N., Borson, S. H., Gambello, M. J., Wang, F., and Cavalli, V. (2010). Mammalian target of rapamycin (mTOR) activation increases axonal growth capacity of injured peripheral nerves. J. Biol. Chem. 285 (36), 28034–28043. doi:10.1074/jbc.M110.125336
Aborode, A. T., Pustake, M., Awuah, W. A., Alwerdani, M., Shah, P., Yarlagadda, R., et al. (2022). Targeting oxidative stress mechanisms to treat alzheimer's and Parkinson's disease: A critical review. Oxid. Med. Cell. Longev. 2022, 7934442. doi:10.1155/2022/7934442
Ahmad, B., Khan, S., Liu, Y., Xue, M., Nabi, G., Kumar, S., et al. (2020). Molecular mechanisms of anticancer activities of puerarin. Cancer Manag. Res. 12, 79–90. doi:10.2147/cmar.S233567
Anukunwithaya, T., Poo, P., Hunsakunachai, N., Rodsiri, R., Malaivijitnond, S., and Khemawoot, P. (2018). Absolute oral bioavailability and disposition kinetics of puerarin in female rats. BMC Pharmacol. Toxicol. 19 (1), 25. doi:10.1186/s40360-018-0216-3
Bai, Y. L., Han, L. L., Qian, J. H., and Wang, H. Z. (2021). Molecular mechanism of puerarin against diabetes and its complications. Front. Pharmacol. 12, 780419. doi:10.3389/fphar.2021.780419
Ballard, D. J., Peng, H. Y., Das, J. K., Kumar, A., Wang, L., Ren, Y., et al. (2021). Insights into the pathologic roles and regulation of eukaryotic elongation factor-2 kinase. Front. Mol. Biosci. 8, 727863. doi:10.3389/fmolb.2021.727863
Banach, E., Jaworski, T., and Urban-Ciećko, J. (2022). Early synaptic deficits in GSK-3β overexpressing mice. Neurosci. Lett. 784, 136744. doi:10.1016/j.neulet.2022.136744
Benjamin, E. J., Muntner, P., Alonso, A., Bittencourt, M. S., Callaway, C. W., Carson, A. P., et al. (2019). Heart disease and stroke statistics-2019 update: A report from the American heart association. Circulation 139 (10), e56–e528. doi:10.1161/cir.0000000000000659
Bramlett, H. M., and Dietrich, W. D. (2007). Progressive damage after brain and spinal cord injury: Pathomechanisms and treatment strategies. Prog. Brain Res. 161, 125–141. doi:10.1016/s0079-6123(06)61009-1
Butterfield, D. A., and Halliwell, B. (2019). Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 20 (3), 148–160. doi:10.1038/s41583-019-0132-6
Cao, L., Wang, J., Zhang, Y., Tian, F., and Wang, C. (2022). Osteoprotective effects of flavonoids: Evidence from in vivo and in vitro studies (Review). Mol. Med. Rep. 25 (6), 200. doi:10.3892/mmr.2022.12716
Charlson, F., van Ommeren, M., Flaxman, A., Cornett, J., Whiteford, H., and Saxena, S. (2019). New WHO prevalence estimates of mental disorders in conflict settings: A systematic review and meta-analysis. Lancet 394 (10194), 240–248. doi:10.1016/s0140-6736(19)30934-1
Chen, D., Zhang, H. F., Yuan, T. Y., Sun, S. C., Wang, R. R., Wang, S. B., et al. (2022). Puerarin-V prevents the progression of hypoxia- and monocrotaline-induced pulmonary hypertension in rodent models. Acta Pharmacol. Sin. 43, 2325–2339. doi:10.1038/s41401-022-00865-y
Chen, J., Zhang, Z. G., Li, Y., Wang, Y., Wang, L., Jiang, H., et al. (2003). Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann. Neurol. 53 (6), 743–751. doi:10.1002/ana.10555
Chen, T., Liu, W., Xiong, S., Li, D., Fang, S., Wu, Z., et al. (2019). Nanoparticles mediating the sustained puerarin release facilitate improved brain delivery to treat Parkinson's disease. ACS Appl. Mat. Interfaces 11 (48), 45276–45289. doi:10.1021/acsami.9b16047
Chen, X., Yu, J., and Shi, J. (2018). Management of diabetes mellitus with puerarin, a natural isoflavone from Pueraria lobata. Am. J. Chin. Med. 46 (8), 1771–1789. doi:10.1142/s0192415x18500891
Chigusa, S., Moroi, T., and Shoji, Y. (2017). State-of-the-Art calculation of the decay rate of electroweak vacuum in the standard model. Phys. Rev. Lett. 119 (21), 211801. doi:10.1103/PhysRevLett.119.211801
Cui, J., Cui, C., Cui, Y., Li, R., Sheng, H., Jiang, X., et al. (2017). Bone marrow mesenchymal stem cell transplantation increases GAP-43 expression via ERK1/2 and PI3K/Akt pathways in intracerebral hemorrhage. Cell. Physiol. biochem. 42 (1), 137–144. doi:10.1159/000477122
Ding, H. Y., Wang, T. Y., Wu, J. Y., Tsai, Y. L., and Chang, T. S. (2022). Enzymatic synthesis of novel and highly soluble puerarin glucoside by deinococcus geothermalis amylosucrase. Molecules 27 (13), 4074. doi:10.3390/molecules27134074
Endo, H., Nito, C., Kamada, H., Nishi, T., and Chan, P. H. (2006). Activation of the Akt/GSK3beta signaling pathway mediates survival of vulnerable hippocampal neurons after transient global cerebral ischemia in rats. J. Cereb. Blood Flow. Metab. 26 (12), 1479–1489. doi:10.1038/sj.jcbfm.9600303
Evans-Lacko, S., Aguilar-Gaxiola, S., Al-Hamzawi, A., Alonso, J., Benjet, C., Bruffaerts, R., et al. (2018). Socio-economic variations in the mental health treatment gap for people with anxiety, mood, and substance use disorders: Results from the WHO world mental health (WMH) surveys. Psychol. Med. 48 (9), 1560–1571. doi:10.1017/s0033291717003336
French, R. L., and Heberlein, U. (2009). On the Glycogen synthase kinase-3/Shaggy mediates ethanol-induced excitotoxic cell death of Drosophila olfactory neurons. Proc. Natl. Acad. Sci. U S. 106 (49), 20924–20929. doi:10.1073/pnas.0910813106
Gan, M., and Yin, X. (2015). Puerarin induced in mantle cell lymphoma apoptosis and its possible mechanisms involving multi-signaling pathway. Cell biochem. Biophys. 71 (1), 367–373. doi:10.1007/s12013-014-0207-y
Golpich, M., Amini, E., Hemmati, F., Ibrahim, N. M., Rahmani, B., Mohamed, Z., et al. (2015). Glycogen synthase kinase-3 beta (GSK-3β) signaling: Implications for Parkinson's disease. Pharmacol. Res. 97, 16–26. doi:10.1016/j.phrs.2015.03.010
Gong, L., Xu, H., Zhang, X., Zhang, T., Shi, J., and Chang, H. (2019). Oridonin relieves hypoxia-evoked apoptosis and autophagy via modulating microRNA-214 in H9c2 cells. Artif. Cells Nanomed. Biotechnol. 47 (1), 2585–2592. doi:10.1080/21691401.2019.1628037
Gu, L., Wu, Z., Qi, X., He, H., Ma, X., Chou, X., et al. (2013). Polyamidomine dendrimers: An excellent drug carrier for improving the solubility and bioavailability of puerarin. Pharm. Dev. Technol. 18 (5), 1051–1057. doi:10.3109/10837450.2011.653822
Guan, P., Liu, W., Lu, P., and Xu, M. (2021). Exploring the mechanism of puerarin alleviating symptoms in epileptic mice based on PI3K/Akt/GSK-3β pathway. Chin. J. Immunol. 14, 1706–1710+1716.
Guo, C., Xu, L., He, Q., Liang, T., Duan, X., and Li, R. (2013). Anti-fibrotic effects of puerarin on CCl4-induced hepatic fibrosis in rats possibly through the regulation of PPAR-γ expression and inhibition of PI3K/Akt pathway. Food Chem. Toxicol. 56, 436–442. doi:10.1016/j.fct.2013.02.051
Guo, J., Bian, W., and Jiang, H. (2022). Puerarin attenuates preeclampsia-induced trophoblast mobility loss and inflammation by modulating miR-181b-5p/RBAK axis. Am. J. Reprod. Immunol. 87 (2), e13510. doi:10.1111/aji.13510
Hao, R., Ge, J., Li, F., Jiang, Y., Sun-Waterhouse, D., and Li, D. (2022). MiR-34a-5p/Sirt1 axis: A novel pathway for puerarin-mediated hepatoprotection against benzo(a)pyrene. Free Radic. Biol. Med. 186, 53–65. doi:10.1016/j.freeradbiomed.2022.05.006
Hao, X. K., Wu, W., Wang, C. X., Xie, G. B., Li, T., Wu, H. M., et al. (2014). Ghrelin alleviates early brain injury after subarachnoid hemorrhage via the PI3K/Akt signaling pathway. Brain Res. 1587, 15–22. doi:10.1016/j.brainres.2014.08.069
Hu, R., Lei, J., Zhao, Q., and Lu, Y. (2012). Meta-analysis of puerarin injection on acute cerebral infarction. Pharmacol. Clin. Chin. Materia Medica 28 (3), 111–113.
Hu, X., Tao, C., Gan, Q., Zheng, J., Li, H., and andYou, C. (2016). Oxidative stress in intracerebral hemorrhage: Sources, mechanisms, and therapeutic targets. Oxid. Med. Cell. Longev. 2016, 3215391. doi:10.1155/2016/3215391
Hu, Y., Li, X., Lin, L., Liang, S., and andYan, J. (2018). Puerarin inhibits non-small cell lung cancer cell growth via the induction of apoptosis. Oncol. Rep. 39 (4), 1731–1738. doi:10.3892/or.2018.6234
Huang, H. J., Huang, C. Y., Lee, M., Lin, J. Y., and Hsieh-Li, H. M. (2019). Puerariae radix prevents anxiety and cognitive deficits in mice under oligomeric aβ-induced stress. Am. J. Chin. Med. 47 (7), 1459–1481. doi:10.1142/s0192415x19500757
Huang, L., Cao, J., Cao, L., Gao, L., Yang, Y., and Xu, L. (2017). Puerarin induces cell apoptosis in human chondrosarcoma cell line SW1353 via inhibition of the PI3K/Akt signaling pathway. Oncol. Lett. 14 (5), 5585–5590. doi:10.3892/ol.2017.6901
Huang, S., Wang, F. J., Lin, H., Liu, T., Zhao, C. X., and Chen, L. G. (2021). Affinity-based protein profiling to reveal targets of puerarin involved in its protective effect on cardiomyocytes. Biomed. Pharmacother. 134, 111160. doi:10.1016/j.biopha.2020.111160
Hwang, Y. P., and Jeong, H. G. (2008). Mechanism of phytoestrogen puerarin-mediated cytoprotection following oxidative injury: Estrogen receptor-dependent up-regulation of PI3K/Akt and HO-1. Toxicol. Appl. Pharmacol. 233 (3), 371–381. doi:10.1016/j.taap.2008.09.006
Hwang, Y. P., Kim, H. G., Hien, T. T., Jeong, M. H., Jeong, T. C., and Jeong, H. G. (2011). Puerarin activates endothelial nitric oxide synthase through estrogen receptor-dependent PI3-kinase and calcium-dependent AMP-activated protein kinase. Toxicol. Appl. Pharmacol. 257 (1), 48–58. doi:10.1016/j.taap.2011.08.017
Jia, L., Hu, Y., Yang, G., and Li, P. (2019). Puerarin suppresses cell growth and migration in HPV-positive cervical cancer cells by inhibiting the PI3K/mTOR signaling pathway. Exp. Ther. Med. 18 (1), 543–549. doi:10.3892/etm.2019.7589
Jiang, K., Chen, H., Tang, K., Guan, W., Zhou, H., Guo, X., et al. (2018). Puerarin inhibits bladder cancer cell proliferation through the mTOR/p70S6K signaling pathway. Oncol. Lett. 15 (1), 167–174. doi:10.3892/ol.2017.7298
Jin, Y., Sui, H. J., Dong, Y., Ding, Q., Qu, W. H., Yu, S. X., et al. (2012). Atorvastatin enhances neurite outgrowth in cortical neurons in vitro via up-regulating the Akt/mTOR and Akt/GSK-3β signaling pathways. Acta Pharmacol. Sin. 33 (7), 861–872. doi:10.1038/aps.2012.59
Jung, H. R., Kim, S. J., Ham, S. H., Cho, J. H., Lee, Y. B., and Cho, H. Y. (2014). Simultaneous determination of puerarin and its active metabolite in human plasma by UPLC-MS/MS: Application to a pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 971, 64–71. doi:10.1016/j.jchromb.2014.09.015
Kennedy, L., Glesaaen, E. R., Palibrk, V., Pannone, M., Wang, W., Al-Jabri, A., et al. (2022). Lactate receptor HCAR1 regulates neurogenesis and microglia activation after neonatal hypoxia-ischemia. Elife 11, e76451. doi:10.7554/eLife.76451
Knopman, D. S., Amieva, H., Petersen, R. C., Chételat, G., Holtzman, D. M., Hyman, B. T., et al. (2021). Alzheimer disease. Nat. Rev. Dis. Prim. 7 (1), 33. doi:10.1038/s41572-021-00269-y
Knowles, J. K., Helbig, I., Metcalf, C. S., Lubbers, L. S., Isom, L. L., Demarest, S., et al. (2022). Precision medicine for genetic epilepsy on the horizon: Recent advances, present challenges, and suggestions for continued progress. Epilepsia 63 (10), 2461–2475. doi:10.1111/epi.17332
Kong, H., Wang, X., Shi, R., Zhao, Y., Cheng, J., Yan, X., et al. (2017). Pharmacokinetics and tissue distribution kinetics of puerarin in rats using indirect competitive ELISA. Mol. (Basel, Switz. 22 (6), E939. doi:10.3390/molecules22060939
Krafft, P. R., Caner, B., Klebe, D., Rolland, W. B., Tang, J., and Zhang, J. H. (2013). PHA-543613 preserves blood-brain barrier integrity after intracerebral hemorrhage in mice. Stroke 44 (6), 1743–1747. doi:10.1161/strokeaha.111.000427
Kuo, S. H., Tasset, I., Cuervo, A. M., and Sulzer, D. (2022). Misfolded GBA/β-glucocerebrosidase impairs ER-quality control by chaperone-mediated autophagy in Parkinson disease. Autophagy, 1, 3. doi:10.1080/15548627.2022.2071383
Lai, T. W., Zhang, S., and Wang, Y. T. (2014). Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. Neurobiol. 115, 157–188. doi:10.1016/j.pneurobio.2013.11.006
Lan, X., Han, X., Li, Q., Yang, Q. W., and Wang, J. (2017). Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat. Rev. Neurol. 13 (7), 420–433. doi:10.1038/nrneurol.2017.69
Lebrun-Julien, F., Bachmann, L., Norrmén, C., Trötzmüller, M., Köfeler, H., Rüegg, M. A., et al. (2014). Balanced mTORC1 activity in oligodendrocytes is required for accurate CNS myelination. J. Neurosci. 34 (25), 8432–8448. doi:10.1523/jneurosci.1105-14.2014
Li, C., Pan, Z., Xu, T., Zhang, C., Wu, Q., and Niu, Y. (2014). Puerarin induces the upregulation of glutathione levels and nuclear translocation of Nrf2 through PI3K/Akt/GSK-3β signaling events in PC12 cells exposed to lead. Neurotoxicol. Teratol. 46, 1–9. doi:10.1016/j.ntt.2014.08.007
Li, X., Sun, S., Chen, D., Yuan, T., Chen, Y., Wang, D., et al. (2020). Puerarin attenuates the endothelial-mesenchymal transition induced by oxidative stress in human coronary artery endothelial cells through PI3K/AKT pathway. Eur. J. Pharmacol. 886, 173472. doi:10.1016/j.ejphar.2020.173472
Liang, F., and Xie, S. (2017). Puerarin prevents tumor necrosis factor-α-induced apoptosis of PC12 cells via activation of the PI3K/Akt signaling pathway. Exp. Ther. Med. 14 (1), 813–818. doi:10.3892/etm.2017.4545
Liang, T., Xu, X., Ye, D., Chen, W., Gao, B., and Huang, Y. (2019). Caspase/AIF/apoptosis pathway: A new target of puerarin for diabetes mellitus therapy. Mol. Biol. Rep. 46 (5), 4787–4797. doi:10.1007/s11033-019-04925-1
Lipton, J. O., and Sahin, M. (2014). The neurology of mTOR. Neuron 84 (2), 275–291. doi:10.1016/j.neuron.2014.09.034
Liu, B., Tan, Y., Wang, D., and Liu, M. (2016a). Puerarin for ischaemic stroke. Cochrane Database Syst. Rev. 2, 004955. doi:10.1002/14651858.CD004955.pub3
Liu, C., Zhang, J., Lun, X., and Li, L. (2021a). LncRNA PVT1 promotes hypoxia-induced cardiomyocyte injury by inhibiting miR-214-3p. Biomed. Res. Int. 2021, 4604883. doi:10.1155/2021/4604883
Liu, G., Liu, Z., and Yuan, S. (2016b). Recent advances in methods of puerarin biotransformation. Mini Rev. Med. Chem. 16 (17), 1392–1402. doi:10.2174/1389557516666160505114456
Liu, S., Cao, X. L., Liu, G. Q., Zhou, T., Yang, X. L., and Ma, B. X. (2019). The in silico and in vivo evaluation of puerarin against Alzheimer's disease. Food Funct. 10 (2), 799–813. doi:10.1039/c8fo01696h
Liu, S., Zhou, T., Chen, D., Liu, R., Qin, H. H., Min, Z. L., et al. (2021b). In silico-determined compound from the root of Pueraria lobate alleviates synaptic plasticity injury induced by Alzheimer's disease via the p38MAPK-CREB signaling pathway. Food Funct. 12 (3), 1039–1050. doi:10.1039/d0fo02388d
Liu, Y., Tang, Q., Shao, S., Chen, Y., Chen, W., and Xu, X. (2017). Lyophilized powder of catalpol and puerarin protected cerebral vessels from ischemia by its anti-apoptosis on endothelial cells. Int. J. Biol. Sci. 13 (3), 327–338. doi:10.7150/ijbs.17751
Long, H. Z., Cheng, Y., Zhou, Z. W., Luo, H. Y., Wen, D. D., and Gao, L. C. (2021). PI3K/AKT signal pathway: A target of natural products in the prevention and treatment of alzheimer's disease and Parkinson's disease. Front. Pharmacol. 12, 648636. doi:10.3389/fphar.2021.648636
Lu, X., Jin, C., Yang, J., Liu, Q., Wu, S., Li, D., et al. (2013). Prenatal and lactational lead exposure enhanced oxidative stress and altered apoptosis status in offspring rats' hippocampus. Biol. Trace Elem. Res. 151 (1), 75–84. doi:10.1007/s12011-012-9531-5
Lu, X. L., Liu, J. X., Wu, Q., Long, S. M., Zheng, M. Y., Yao, X. L., et al. (2014). Protective effects of puerarin against Aß40-induced vascular dysfunction in zebrafish and human endothelial cells. Eur. J. Pharmacol. 732, 76–85. doi:10.1016/j.ejphar.2014.03.030
Luo, C. F., Cai, B., Hou, N., Yuan, M., Liu, S. M., Ji, H., et al. (2012). UDP-glucuronosyltransferase 1A1 is the principal enzyme responsible for puerarin metabolism in human liver microsomes. Arch. Toxicol. 86 (11), 1681–1690. doi:10.1007/s00204-012-0874-7
Ma, R., Zhao, L., Zhao, Y., and Li, Y. (2022). Puerarin action on stem cell proliferation, differentiation and apoptosis: Therapeutic implications for geriatric diseases. Phytomedicine. 96, 153915. doi:10.1016/j.phymed.2021.153915
Manning, B. D., and Toker, A. (2017). AKT/PKB signaling: Navigating the network. Cell 169 (3), 381–405. doi:10.1016/j.cell.2017.04.001
Matsuda, S., Ikeda, Y., Murakami, M., Nakagawa, Y., Tsuji, A., and Kitagishi, Y. (2019). Roles of PI3K/AKT/GSK3 pathway involved in psychiatric illnesses. Diseases 7 (1), E22. doi:10.3390/diseases7010022
Meresman, G. F., Götte, M., and Laschke, M. W. (2021). Plants as source of new therapies for endometriosis: A review of preclinical and clinical studies. Hum. Reprod. Update 27 (2), 367–392. doi:10.1093/humupd/dmaa039
Misra, M. K., Damotte, V., and Hollenbach, J. A. (2018). The immunogenetics of neurological disease. Immunology 153 (4), 399–414. doi:10.1111/imm.12869
Molina, S., Rivero, S., Cabrera, R., Rodríguez, C., Langley, E., and Cerbon, M. (2021). Decoding signaling pathways involved in prolactin-induced neuroprotection: A review. Front. Neuroendocrinol. 61, 100913. doi:10.1016/j.yfrne.2021.100913
Murahari, M., Singh, V., Chaubey, P., and Suvarna, V. (2020). A critical review on anticancer mechanisms of natural flavonoid puerarin. Anticancer. Agents Med. Chem. 20 (6), 678–686. doi:10.2174/1871520620666200227091811
Noristani, H. N. (2022). Intrinsic regulation of axon regeneration after spinal cord injury: Recent advances and remaining challenges. Exp. Neurol. 357, 114198. doi:10.1016/j.expneurol.2022.114198
Noshita, N., Lewén, A., Sugawara, T., and Chan, P. H. (2002). Akt phosphorylation and neuronal survival after traumatic brain injury in mice. Neurobiol. Dis. 9 (3), 294–304. doi:10.1006/nbdi.2002.0482
Ooms, L. M., Fedele, C. G., Astle, M. V., Ivetac, I., Cheung, V., Pearson, R. B., et al. (2006). The inositol polyphosphate 5-phosphatase, PIPP, Is a novel regulator of phosphoinositide 3-kinase-dependent neurite elongation. Mol. Biol. Cell 17 (2), 607–622. doi:10.1091/mbc.e05-05-0469
Read, D. E., and Gorman, A. M. (2009). Involvement of Akt in neurite outgrowth. Cell. Mol. Life Sci. 66 (18), 2975–2984. doi:10.1007/s00018-009-0057-8
Saito, A., Narasimhan, P., Hayashi, T., Okuno, S., Ferrand-Drake, M., and Chan, P. H. (2004). Neuroprotective role of a proline-rich Akt substrate in apoptotic neuronal cell death after stroke: Relationships with nerve growth factor. J. Neurosci. 24 (7), 1584–1593. doi:10.1523/jneurosci.5209-03.2004
Song, X., Wang, W., Ding, S., Wang, Y., Ye, L., Chen, X., et al. (2022). Exploring the potential antidepressant mechanisms of puerarin: Anti-inflammatory response via the gut-brain axis. J. Affect. Disord. 310, 459–471. doi:10.1016/j.jad.2022.05.044
Tao, J., Cui, Y., Duan, Y., Zhang, N., Wang, C., and Zhang, F. (2017). Puerarin attenuates locomotor and cognitive deficits as well as hippocampal neuronal injury through the PI3K/Akt1/GSK-3β signaling pathway in an in vivo model of cerebral ischemia. Oncotarget 8 (63), 106283–106295. doi:10.18632/oncotarget.22290
Trotman, L. C., Alimonti, A., Scaglioni, P. P., Koutcher, J. A., Cordon-Cardo, C., and Pandolfi, P. P. (2006). Identification of a tumour suppressor network opposing nuclear Akt function. Nature 441 (7092), 523–527. doi:10.1038/nature04809
Tzeng, H. E., Lin, S. L., Thadevoos, L. A., Lien, M. Y., Yang, W. H., Ko, C. Y., et al. (2021). Nerve growth factor promotes lysyl oxidase-dependent chondrosarcoma cell metastasis by suppressing miR-149-5p synthesis. Cell Death Dis. 12 (12), 1101. doi:10.1038/s41419-021-04392-2
Ubhi, K., Rockenstein, E., Mante, M., Inglis, C., Adame, A., Patrick, C., et al. (2010). Neurodegeneration in a transgenic mouse model of multiple system atrophy is associated with altered expression of oligodendroglial-derived neurotrophic factors. J. Neurosci. 30 (18), 6236–6246. doi:10.1523/jneurosci.0567-10.2010
Wang, B., Ma, W., and Yang, H. (2019a). Puerarin attenuates hypoxia-resulted damages in neural stem cells by up-regulating microRNA-214. Artif. Cells Nanomed. Biotechnol. 47 (1), 2746–2753. doi:10.1080/21691401.2019.1628040
Wang, C., Xie, N., Zhang, H., Li, Y., and Wang, Y. (2014a). Puerarin protects against β-amyloid-induced microglia apoptosis via a PI3K-dependent signaling pathway. Neurochem. Res. 39 (11), 2189–2196. doi:10.1007/s11064-014-1420-1
Wang, J. W., Wang, H. D., Cong, Z. X., Zhou, X. M., Xu, J. G., Jia, Y., et al. (2014b). Puerarin ameliorates oxidative stress in a rodent model of traumatic brain injury. J. Surg. Res. 186 (1), 328–337. doi:10.1016/j.jss.2013.08.027
Wang, L., Jiang, W., Wang, J., Xie, Y., and Wang, W. (2022). Puerarin inhibits FUNDC1-mediated mitochondrial autophagy and CSE-induced apoptosis of human bronchial epithelial cells by activating the PI3K/AKT/mTOR signaling pathway. Aging (Albany NY) 14 (3), 1253–1264. doi:10.18632/aging.203317
Wang, S., Yang, F. J., Shang, L. C., Zhang, Y. H., Zhou, Y., and Shi, X. L. (2019b). Puerarin protects against high-fat high-sucrose diet-induced non-alcoholic fatty liver disease by modulating PARP-1/PI3K/AKT signaling pathway and facilitating mitochondrial homeostasis. Phytother. Res. 33 (9), 2347–2359. doi:10.1002/ptr.6417
Wang, X., Liu, J., Jin, N. A., Xu, D., Wang, J., Han, Y., et al. (2015). Fructus Corni extract-induced neuritogenesis in PC12 cells is associated with the suppression of stromal interaction molecule 1 expression and inhibition of Ca(2+) influx. Exp. Ther. Med. 9 (5), 1773–1779. doi:10.3892/etm.2015.2316
Wang, Y., Wang, W. L., Xie, W. L., Li, L. Z., Sun, J., Sun, W. J., et al. (2013). Puerarin stimulates proliferation and differentiation and protects against cell death in human osteoblastic MG-63 cells via ER-dependent MEK/ERK and PI3K/Akt activation. Phytomedicine 20 (10), 787–796. doi:10.1016/j.phymed.2013.03.005
Wang, Z. K., Chen, R. R., Li, J. H., Chen, J. Y., Li, W., Niu, X. L., et al. (2020). Puerarin protects against myocardial ischemia/reperfusion injury by inhibiting inflammation and the NLRP3 inflammasome: The role of the SIRT1/NF-κB pathway. Int. Immunopharmacol. 89, 107086. doi:10.1016/j.intimp.2020.107086
Wen, B. Y., Li, H., Wang, L., and Wang, S. c. (2008). Metabolic kinetic of puerarin in beagle liver microsomal by HPLC-ESI-MS. Zhongguo Zhong Yao Za Zhi 33, 2834–2837.
Xi, J., Khalil, M., Shishechian, N., Hannes, T., Pfannkuche, K., Liang, H., et al. (2010). Comparison of contractile behavior of native murine ventricular tissue and cardiomyocytes derived from embryonic or induced pluripotent stem cells. Faseb J. 24 (8), 2739–2751. doi:10.1096/fj.09-145177
Xiao, W., Zheng, D., Chen, X., Yu, B., Deng, K., Ma, J., et al. (2021). Long non-coding RNA MIAT is involved in the regulation of pyroptosis in diabetic cardiomyopathy via targeting miR-214-3p. iScience 24 (12), 103518. doi:10.1016/j.isci.2021.103518
Xie, N., Wang, C., Lian, Y., Wu, C., Zhang, H., and Zhang, Q. (2014). Puerarin protects hippocampal neurons against cell death in pilocarpine-induced seizures through antioxidant and anti-apoptotic mechanisms. Cell. Mol. Neurobiol. 34 (8), 1175–1182. doi:10.1007/s10571-014-0093-2
Xie, Z., Enkhjargal, B., Wu, L., Zhou, K., Sun, C., Hu, X., et al. (2018). Exendin-4 attenuates neuronal death via GLP-1R/PI3K/Akt pathway in early brain injury after subarachnoid hemorrhage in rats. Neuropharmacology 128, 142–151. doi:10.1016/j.neuropharm.2017.09.040
Xing, G., Dong, M., Li, X., Zou, Y., Fan, L., Wang, X., et al. (2011). Neuroprotective effects of puerarin against beta-amyloid-induced neurotoxicity in PC12 cells via a PI3K-dependent signaling pathway. Brain Res. Bull. 85 (3-4), 212–218. doi:10.1016/j.brainresbull.2011.03.024
Xiong, S., Liu, W., Li, D., Chen, X., Liu, F., Yuan, D., et al. (2019). Oral delivery of puerarin nanocrystals to improve brain accumulation and anti-parkinsonian efficacy. Mol. Pharm. 16 (4), 1444–1455. doi:10.1021/acs.molpharmaceut.8b01012
Xu, D. X., Guo, X. X., Zeng, Z., Wang, Y., and Qiu, J. (2021). Puerarin improves hepatic glucose and lipid homeostasis in vitro and in vivo by regulating the AMPK pathway. Food Funct. 12 (6), 2726–2740. doi:10.1039/d0fo02761h
Xu, X., Zhang, S., Zhang, L., Yan, W., and Zheng, X. (2005). The Neuroprotection of puerarin against cerebral ischemia is associated with the prevention of apoptosis in rats. Planta Med. 71 (7), 585–591. doi:10.1055/s-2005-871261
Yang, K., Zhang, J., and Bao, C. (2021). Exosomal circEIF3K from cancer-associated fibroblast promotes colorectal cancer (CRC) progression via miR-214/PD-L1 axis. BMC Cancer 21 (1), 933. doi:10.1186/s12885-021-08669-9
Yang, W. T., Zheng, X. W., Chen, S., Shan, C. S., Xu, Q. Q., Zhu, J. Z., et al. (2017). Chinese herbal medicine for Alzheimer's disease: Clinical evidence and possible mechanism of neurogenesis. Biochem. Pharmacol. 141, 143–155. doi:10.1016/j.bcp.2017.07.002
Yin, M., Yuan, Y., Cui, Y., Hong, X., Luo, H., Hu, X., et al. (2015). Puerarin suppresses the self-renewal of murine embryonic stem cells by inhibition of REST-MiR-21 regulatory pathway. Cell. Physiol. biochem. 37 (2), 527–536. doi:10.1159/000430374
Yu, C. C., Du, Y. J., Li, J., Li, Y., Wang, L., Kong, L. H., et al. (2022). Neuroprotective mechanisms of puerarin in central nervous system diseases: Update. Aging Dis. 13 (4), 1092–1105. doi:10.14336/ad.2021.1205
Yu, D., Mu, S., Zhao, D., Wang, G., Chen, Z., Ren, H., et al. (2015). Puerarin attenuates glucocorticoid-induced apoptosis of hFOB1.19 cells through the JNK- and Akt-mediated mitochondrial apoptotic pathways. Int. J. Mol. Med. 36 (2), 345–354. doi:10.3892/ijmm.2015.2258
Yuan, Y., Zong, J., Zhou, H., Bian, Z. Y., Deng, W., Dai, J., et al. (2014). Puerarin attenuates pressure overload-induced cardiac hypertrophy. J. Cardiol. 63 (1), 73–81. doi:10.1016/j.jjcc.2013.06.008
Zeng, J., Zhao, N., Yang, J., Kuang, W., Xia, X., Chen, X., et al. (2022). Puerarin induces molecular details of ferroptosis-associated anti-inflammatory on RAW264.7 macrophages. Metabolites 12 (7), 653. doi:10.3390/metabo12070653
Zeng, J., Zheng, S., Chen, Y., Qu, Y., Xie, J., Hong, E., et al. (2021). Puerarin attenuates intracerebral hemorrhage-induced early brain injury possibly by PI3K/Akt signal activation-mediated suppression of NF-κB pathway. J. Cell. Mol. Med. 25 (16), 7809–7824. doi:10.1111/jcmm.16679
Zhang, D., Ma, G., Hou, M., Zhang, T., Chen, L., and Zhao, C. (2016). The neuroprotective effect of puerarin in acute spinal cord injury rats. Cell. Physiol. biochem. 39 (3), 1152–1164. doi:10.1159/000447822
Zhang, H. Y., Liu, Y. H., Wang, H. Q., Xu, J. H., and Hu, H. T. (2008). Puerarin protects PC12 cells against beta-amyloid-induced cell injury. Cell Biol. Int. 32 (10), 1230–1237. doi:10.1016/j.cellbi.2008.07.006
Zhang, L. (2019). Pharmacokinetics and drug delivery systems for puerarin, a bioactive flavone from traditional Chinese medicine. Drug Deliv. 26 (1), 860–869. doi:10.1080/10717544.2019.1660732
Zhang, Q., Huang, W. D., Lv, X. Y., and Yang, Y. M. (2012). Puerarin protects differentiated PC12 cells from H₂O₂-induced apoptosis through the PI3K/Akt signalling pathway. Cell Biol. Int. 36 (5), 419–426. doi:10.1042/cbi20100900
Zhang, Y., Zeng, X., Zhang, L., and Zheng, X. (2007). Stimulatory effect of puerarin on bone formation through activation of PI3K/Akt pathway in rat calvaria osteoblasts. Planta Med. 73 (4), 341–347. doi:10.1055/s-2007-967168
Zhao, J., Cheng, Y. Y., Fan, W., Yang, C. B., Ye, S. F., Cui, W., et al. (2015a). Botanical drug puerarin coordinates with nerve growth factor in the regulation of neuronal survival and neuritogenesis via activating ERK1/2 and PI3K/Akt signaling pathways in the neurite extension process. CNS Neurosci. Ther. 21 (1), 61–70. doi:10.1111/cns.12334
Zhao, S. S., Yang, W. N., Jin, H., Ma, K. G., and Feng, G. F. (2015b). Puerarin attenuates learning and memory impairments and inhibits oxidative stress in STZ-induced SAD mice. Neurotoxicology 51, 166–171. doi:10.1016/j.neuro.2015.10.010
Zhao, X., Liu, W., Liu, B., Zeng, Q., Cui, Z., Wang, Y., et al. (2022). Exploring the underlying molecular mechanism of liver cancer cells under hypoxia based on RNA sequencing. BMC Genom. Data 23 (1), 38. doi:10.1186/s12863-022-01055-9
Zheng, H., Chen, C., Zhang, J., and Hu, Z. (2016). Mechanism and therapy of brain edema after intracerebral hemorrhage. Cerebrovasc. Dis. 42 (3-4), 155–169. doi:10.1159/000445170
Zhou, J. F., Xiong, Y., Kang, X., Pan, Z., Zhu, Q., Goldbrunner, R., et al. (2022a). Application of stem cells and exosomes in the treatment of intracerebral hemorrhage: An update. Stem Cell Res. Ther. 13 (1), 281. doi:10.1186/s13287-022-02965-2
Zhou, J., Zhang, N., Aldhahrani, A., Soliman, M. M., Zhang, L., and Zhou, F. (2022b). Puerarin ameliorates nonalcoholic fatty liver in rats by regulating hepatic lipid accumulation, oxidative stress, and inflammation. Front. Immunol. 13, 956688. doi:10.3389/fimmu.2022.956688
Zhou, Y. Q., Liu, D. Q., Chen, S. P., Sun, J., Zhou, X. R., Xing, C., et al. (2019). The role of CXCR3 in neurological diseases. Curr. Neuropharmacol. 17 (2), 142–150. doi:10.2174/1570159x15666171109161140
Zhou, Y., Xie, N., Li, L., Zou, Y., Zhang, X., and Dong, M. (2014b). Puerarin alleviates cognitive impairment and oxidative stress in APP/PS1 transgenic mice. Int. J. Neuropsychopharmacol. 17 (4), 635–644. doi:10.1017/s146114571300148x
Zhou, Y. X., Zhang, H., and Peng, C. (2021). Effects of puerarin on the prevention and treatment of cardiovascular diseases. Front. Pharmacol. 12, 771793. doi:10.3389/fphar.2021.771793
Zhou, Y. X., Zhang, H., and Peng, C. (2014a). Puerarin: A review of pharmacological effects. Phytother. Res. 28 (7), 961–975. doi:10.1002/ptr.5083
Zhu, G., Wang, X., Wu, S., and Li, Q. (2012). Involvement of activation of PI3K/Akt pathway in the protective effects of puerarin against MPP+-induced human neuroblastoma SH-SY5Y cell death. Neurochem. Int. 60 (4), 400–408. doi:10.1016/j.neuint.2012.01.003
Zhu, G., Wang, X., Wu, S., Li, X., and Li, Q. (2014). Neuroprotective effects of puerarin on 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine induced Parkinson's disease model in mice. Phytother. Res. 28 (2), 179–186. doi:10.1002/ptr.4975
Zhu, L. H., Wang, L., Wang, D., Jiang, H., Tang, Q. Z., Yan, L., et al. (2010). Puerarin attenuates high-glucose-and diabetes-induced vascular smooth muscle cell proliferation by blocking PKCbeta2/Rac1-dependent signaling. Free Radic. Biol. Med. 48 (4), 471–482. doi:10.1016/j.freeradbiomed.2009.10.040
Keywords: PI3K/Akt signal pathway, puerarin, neurological diseases, natural products, nerve protection
Citation: Wang Q, Shen Z-N, Zhang S-J, Sun Y, Zheng F-J and Li Y-H (2022) Protective effects and mechanism of puerarin targeting PI3K/Akt signal pathway on neurological diseases. Front. Pharmacol. 13:1022053. doi: 10.3389/fphar.2022.1022053
Received: 18 August 2022; Accepted: 10 October 2022;
Published: 24 October 2022.
Edited by:
Wei Peng, Chengdu University of Traditional Chinese Medicine, ChinaReviewed by:
Wang Li, Hubei University of Chinese Medicine, ChinaCopyright © 2022 Wang, Shen, Zhang, Sun, Zheng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng-Jie Zheng, emhlbmdmZW5namllQDE2My5jb20=; Yu-Hang Li, bGl5dWhhbmdAYnVjbS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.