
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pharmacol., 17 January 2022
Sec. Pharmacology of Anti-Cancer Drugs
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.825475
This article is part of the Research TopicCombating Cancer with Natural Products: non-coding RNA and RNA modificationView all 20 articles
Gastric cancer and colorectal cancer are malignant tumors found in the human gastrointestinal tract. Bidirectional communication between tumor cells and their microenvironment can be realized through the transmission of exosomes—small, cell-derived vesicles containing complex RNA and proteins. Exosomes play an important role in the proliferation, metastasis, immune response, and drug resistance of cancer cells. In this review, we focus on the role and application of exosomes in gastric and colorectal cancer. We also summarize the role of exosomes secreted by different types of cells in tumor development and as drug carriers in cancer treatment.
Gastric and colorectal cancer are globally-important diseases with high molecular and phenotypic heterogeneity (Smyth et al., 2020). Gastric cancer can be caused by a variety of genetic and epigenetic mutations, and Helicobacter pylori is also an important pathogenic factor (Uemura et al., 2001). The tumor microenvironment has a strong influence on the survival and treatment response of gastric cancer patients (Quail and Joyce, 2013). At present, early diagnosis of gastric cancer remains problematic because clinical symptoms often only appear in the late stages of cancer development, which significantly limits treatment options (Maconi et al., 2008). Colorectal cancer is the fourth most deadly cancer in the world; its etiologies include eating habits, old age, and smoking (Dekker et al., 2019). Colorectal cancer is normally treated with adjuvant therapy after surgical resection. However, the risk of subsequent cancer recurrence and metastasis remains high, and it is often related to resistance to traditional therapies such as chemotherapy and radiation (Jänne and Mayer, 2000). As gastric and colorectal cancers are associated with high morbidity and mortality, research into new targeted therapies is urgent.
Recent studies have shown that exosomes can be used as targeted drug carriers. Exosomes are small endocytic vesicles secreted by most cells (Théry et al., 2002), the diameters of which range between 40 and 100 nm. Exosomes have been found to be able to deliver bioactive molecules or other substances to specific recipient cells for intercellular communication (Figure 1). Increasing numbers of studies have indicated that exosomes are important nanomaterials that can regulate essential biological behaviors through intercellular transmission (Yang et al., 2019). They also play an important role in the pathogenesis of many diseases, including cancer, as they are involved in the apoptosis of tumor cells, the proliferation and migration of cancer cells, regulation of the tumor microenvironment, and angiogenesis (Nabariya et al., 2020). Because of these characteristics, exosomes can also be used as an efficient targeted drug delivery system in cancer treatment.
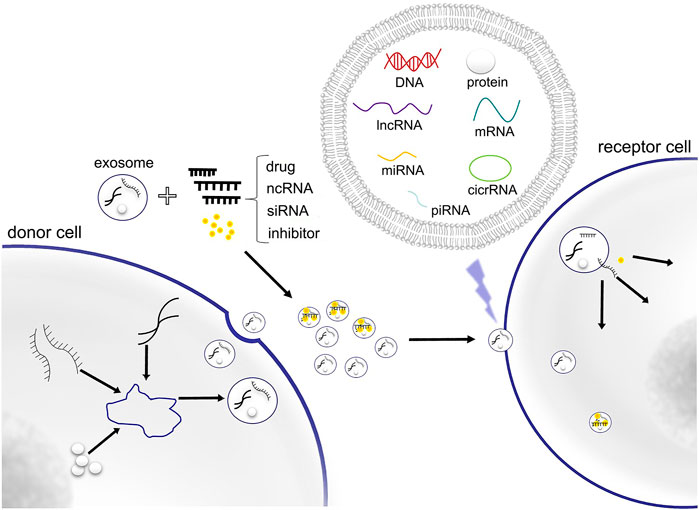
FIGURE 1. Exosome contains DNA, RNA, protein and other substances for cellular component communication. Exosomes can also be used as delivery vectors of drugs, ncRNA, siRNA and inhibitor.
Exosomes play an important role in the communication between tumor cells themselves and between the tumor and its microenvironment. Exosomes can transfer many types of biomolecules, including DNA, RNA, and protein. Exosomes derived from cancer cells affect the biological characteristics of recipient cells and change the tumor microenvironment by transmitting these bioactive molecules to regulate the tumor’s development process. Noncoding RNA (ncRNA), including microRNA, circRNA, and lncRNA, is one of the most important bioactive molecules which does not encode protein and perform their biological functions at the RNA level.
Studies have shown that miR-15b-3p is highly expressed in exosomes secreted by gastric cancer cells and miR-15b-3p can be transferred by exosomes to enhance migration, invasion, and proliferation; it also inhibit the apoptosis of gastric cancer through the DYNLT1/caspase-3/caspase-9 signaling pathway (Wei et al., 2020). Exosomal miR-25-3p from colorectal cancer promotes cancer development by inducing vascular permeability and angiogenesis (Zeng et al., 2018). In addition, exosomal circSHKBP1 regulates the miR-582-3p/HUR/VEGF pathway, thereby promoting the progression of gastric cancer (Xie M. et al., 2020). Studies have also shown that exosomal lncRNA 91H promotes the proliferation of colorectal cancer by changing HNRNPK expression (Gao et al., 2018).
Exosomes also deliver cancer-related molecules that can influence the chemotherapeutic resistance of recipient cells. Cisplatin is one of the most effective and commonly used of the basic chemotherapy drugs for advanced gastric cancer treatment (Keehn and Higgins, 1981). Studies have shown that exosomes from cisplatin-resistant gastric cancer can enhance gastric cancer cells resistance to cisplatin by delivering miR-500a-3p. Therefore, exosomal miR-500a-3p could potentially be used to predict and eliminate chemotherapy-resistant gastric cancer cells (Lin et al., 2020). Research has also shown that p-STAT3-containing exosomes contribute to 5-FU resistance in colorectal cancer cells (Zhang et al., 2019).
Protein is an important component of exosomes, and exosomes have been shown to regulate the liver microenvironment by delivering EGFR to promote liver metastasis of gastric cancer (Zhang et al., 2017). Furthermore, exosomal ANGPTL1 has been shown to alleviate liver metastasis in colorectal cancer by reprogramming Kupffer cells and reducing MMP9 expression (Jiang et al., 2021).
In addition to acting as cell-to-cell transporters, cancer-derived exosomes have also been widely used as biomarkers in cancer diagnosis. Tumors of unknown primary origin can be classified by extracting tumor-type specific proteins that are contained in exosomes from tissues and plasma. Therefore, exosomal proteins can be used as reliable biomarkers for cancer detection and classification (Hoshino et al., 2020). Other research has revealed that exosomal piRNAs in serum may be a biomarker for the diagnosis and monitoring of gastric cancer metastasis (Ge et al., 2020). LncRNA HOTTIP has been found to be significantly up-regulated in gastric cancer cell exosomes; it could be used as a new biomarker for determining the diagnosis and prognosis of gastric cancer (Zhao et al., 2018). Further to this, studies have indicated that exosomal circRNA-PNN is a potential biomarker of colorectal cancer (Xie Y. et al., 2020).
These results suggest that tumor-derived exosomes can promote cancer progression and influence drug resistance through ncRNA transmission. Moreover, tumor-derived exosomes can be used as biomarkers for cancer; this indicates that tumor-derived exosomes may be used to diagnose and treat gastric and colorectal cancer.
Exosomes derived from cancer cells play an important role in intracellular communication, treatment and diagnosis of gastric and colorectal cancers. Similarly, other types of cell-derived exosomes in the tumor microenvironment are important for cancer progression (Figure 2). Cancer-associated fibroblasts (CAFs) are the main components of tumor stroma; they can influence tumor development and drug resistance by secreting exosomes. Studies have shown that exosomal miR-139 from CAFs can inhibit the progression and metastasis of gastric cancer by reducing MMP11 (Xu et al., 2019). Furthermore, miR-34 in exosomes secreted by CAFs can inhibit gastric cancer cell proliferation and invasion both in vivo and in vitro (Shi et al., 2020). Other research has found that exosomes delivery of miR-590-3p by CAFs can improve radioresistance of colorectal cancer by regulating the CLCA4-dependent PI3K/Akt signaling pathway (Chen X. et al., 2021). Moreover, CAFs can also enhance colorectal cancer drug resistance through exosomes delivery of lncRNA H19 (Ren et al., 2018).
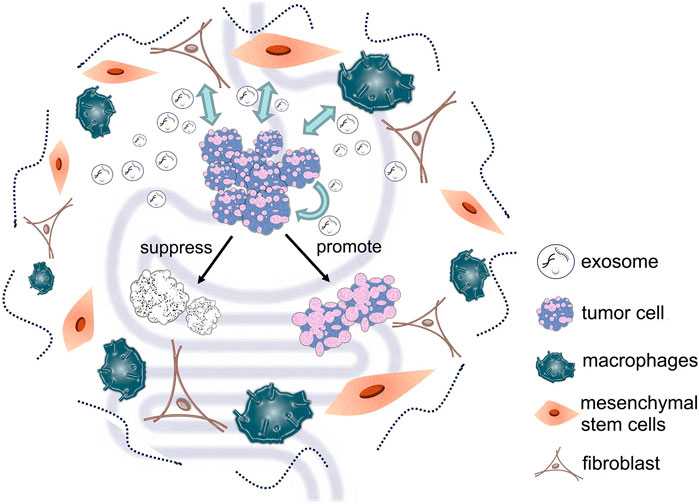
FIGURE 2. Different types of cell-derived exosomes in the tumor microenvironment can deliver various substances to tumor cells, thereby affecting tumor cell development.
Tumor-associated macrophages (TAMs) are macrophages that infiltrate tumor tissue; they are the most abundant immune cells within the tumor microenvironment (Zhang Y. et al., 2018). Studies have shown that exosomes down-regulate PTEN and inhibit apoptosis by delivering TAMs-derived miR-21; they also improve cisplatin resistance in gastric cancer cells (Zheng et al., 2017). Research has also indicated that exosomes derived from M1 macrophages carry miR-16-5p; this activates the T-cell immune response through PD-L1 and inhibits gastric cancer proliferation (Li et al., 2020). In addition, exosomes derived from mouse TAMs have been shown to be associated with Th1/M1 polarization, inflammation, and enhancement of immune response in colorectal tumors (Cianciaruso et al., 2019). In M2 macrophage-derived exosomes, miR-21-5p and miR-155-5p were highly expressed, which mediated the migration and invasion of colorectal cancer cells (Lan et al., 2019).
Mesenchymal stem cells (MSCs) have the ability to self-replicate and they have strong differentiation potential, which means they can contribute to the formation of the tumor microenvironment and they can interact with cancer cells (Poggi and Giuliani, 2016). Exosomes derived from human umbilical cord mesenchymal stem cells (hUMSCs) that contain miR-6785-5p inhibit both gastric cancer angiogenesis and metastasis by inhibiting INHBA expression (Chen Z. et al., 2021). Moreover, exosomes derived from MSCs in gastric cancer tissues can deliver miR-221 to gastric cancer cells, thereby promoting their proliferation and migration (Wang et al., 2014). Studies have revealed that GARP knockdown MSCs inhibit the proliferation and invasion of mouse colorectal cancer cells through exosomes (Xing et al., 2020). It has also been shown that exosomes from bone marrow-derived mesenchymal stem cells (BMSCs) can promote stem cell-like characteristics of colorectal cancer through miR-142-3p (Li and Li, 2018).
Proteins delivered by CAFs, TAMs, and MSCs through exosomes are also important mediators of tumor and tumor microenvironment regulation. Studies that used proteomic analysis of CAFs and serum-derived exosomes have identified QSOX1 as a marker for non-invasive colorectal cancer detection (Ganig et al., 2021). TAM-derived exosomes have also been found to promote migration of gastric cancer cells by delivering functional apolipoprotein E (Zheng et al., 2018), and BMSCs exosomes with p53 deletion can regulate the Wnt/β-catenin pathway by transferring UBR2 to target cells, which promotes the growth and metastasis of gastric cancer (Mao et al., 2017). As can be seen from the above studies, CAFs, TAMs, and MSCs are important components of the tumor microenvironment; they cooperate with the cancer cells to regulate the tumor’s growth environment.
Recent studies have indicated that exosomes are the promising carrier of anticancer drugs and that exosome-based therapy may be an efficient and beneficial approach during cancer treatment. Exosomes have good biocompatibility, specificity, and low immunogenicity, and since they can be easily internalized by cells, they can effectively deliver drugs to cancer cells. The proteins and lipid compositions of tumor-derived exosomes are similar to those of the cells that secret them. (Sun W. et al., 2018), and report shows that exosomes from cancer cells are specifically taken up by the same type of tumor tissue or cell. If the tumor-derived exosomes are injected into the body, the exosomes will eventually return to the original tumor tissue. The tumor-targeting ability of tumor cell-derived exosomes may be related to the expression of integrin (Qiao et al., 2020). Because of this characteristic, tumor-derived exosomes are natural vectors that have great potential for targeted delivery of antitumor drugs. Studies have constructed nano aspirin exosome drug delivery systems that can effectively deliver aspirin to the tumor site in vivo; this induces apoptosis and autophagy of colorectal cancer cells, thus producing a good tumor treatment effect (Tran et al., 2019). In other study, exosomes were isolated from A33-positive LIM1215 cells and loaded with doxorubicin (DOX) to target A33-positive colorectal cancer cells. The results revealed that DOX-loaded exosomes have good tumor-targeting ability; they could also inhibit colorectal cancer growth (Li et al., 2018). In addition, researchers have loaded DOX into exosomes secreted by MSCs, which effectively inhibited colorectal tumor growth (Bagheri et al., 2020). Furthermore, there is evidence shown that exosomes coated with oxaliplatin and PGM5-AS1 can reverse drug resistance in colorectal cancer cells and inhibit their growth (Hui et al., 2021).
In addition to being used to package drugs, exosomes can also be used to deliver biological molecules for RNA-based therapeutic strategies. Hepatocyte growth factor (HGF) can promote the growth of tumor cells and blood vessels. HGF siRNA can be transported to cancer cells using exosomes, which can down-regulate expression of HGF, thereby inhibiting the proliferation and migration of gastric cancer cells (Zhang H. et al., 2018). Furthermore, exosomes can be used as nanoparticles to deliver anti-miR-214 and down-regulate the expression of miR-214 in gastric cancer cells, which can reduce cisplatin chemotherapy resistance in gastric cancer and inhibit tumor growth (Wang et al., 2018). Other research has shown that delivering c-Met siRNA via exosomes can promote cell apoptosis, inhibit tumor growth in vivo, and reduce gastric cancer cisplatin resistance (Zhang et al., 2020). It has also been reported that exosomes can be used to simultaneously deliver 5-FU and miR-21 inhibitors to colorectal cancer cells; researchers found that the constructed exosomes had significant anti-tumor effects both in vivo and in vitro (Liang et al., 2020). Furthermore, exosomes loaded with miR-128-3p from normal intestinal FHC cells have been found to be able to inhibit the expression of MRP5, thereby improving the colorectal cancer cells’ sensitivity to oxaliplatin (both in vivo and in vitro) (Liu et al., 2019).
Tumor-derived exosomes have been shown to be able to target tumors, and exosomes can be used to directly package anticancer drugs and deliver them specifically to gastric and colorectal tumors to inhibit tumor development. They can also use regulatory mechanisms to inhibit the growth of cancer cells or reverse drug resistance by deliver biomolecules such as siRNA.
Gastric and colorectal cancer are common and significant cancers worldwide that cause high morbidity and mortality rate (Athauda et al., 2019). Therefore, it is urgent to develop new diagnostic and therapeutic methods. Increasing numbers of studies indicate that exosomes are involved in various stages of cancer progression and can influence its overall course (Bebelman et al., 2018). For example, exosomes regulate tumor progression due to their ability to communicate between cells and deliver various substances. The contents related to exosomes and cancer regulation introduced in this review mainly include ncRNAs (miRNA, lncRNA, circRNA, piRNA) and proteins, and the role of exosomes includes regulating tumor microenvironments and affecting tumor proliferation, metastasis, and drug resistance (Mashouri et al., 2019). Exosomes secreted by different cells also have different functions and characteristics. In the case of gastric and colorectal cancer cells, they have been shown to promote the proliferation and invasion of their own cancer cells through exosomes and to make the surrounding environment more suitable for tumor growth. Moreover, exosomes secreted by tumor cells can be better ingested by the same type of tumor cells, which indicates that they can be used to carry treatment drugs. In addition, other cells in the tumor microenvironment, such as CAFs, TAMs, and MSCs, have a bidirectional molecular transfer process with tumor cells. In some cases, these cells can promote tumor growth, while in other cases they can deliver substances that inhibit tumor growth. This suggests that the tumor-inhibiting effects can be produced by regulating the tumor microenvironment.
Depending on exosome specificity, exosomes can selectively deliver drugs to specific tumor cells with the advantages of high efficiency and low toxicity (Wu et al., 2021). However, only a few studies have examined the application of exosomal delivery drugs for gastric cancer and colorectal cancer. Moreover, exosomes are rich in miRNAs, which can alter the fate of tumor cells by influencing the expression of related miRNAs in tumor cells (Sun Z. et al., 2018). Studies have found new types of RNA, such as piRNA and tsRNA, in addition to miRNA, lncRNA, and cicrRNA. There have only been a few investigations into the existence and function of these types of ncRNA in gastric cancer and colorectal cancer exosomes. Therefore, although the treatment of exosomes has shown great application prospects in gastric and colorectal cancers, many challenges remain before we can use exosomes in the clinical treatment of cancer. For example, information is still needed regarding the detailed mechanisms of exosomes in cancer cells, the isolation of a large number of exosomes, their precise detection, and the drug loading efficiency and preservation methods of exosomes. In an attempt to improve the safety and quality of exosome engineering in the clinical application of treatments for both gastric and colorectal cancer, it is imperative that further studies be carried out to determine how to improve drug delivery methods and the targeting effect of exosomes.
Exosomes play an important role in the occurrence and development of gastric and colorectal cancer, and substances such as miRNA that are contained in exosomes can affect tumor progression. Due to their biological characteristics, exosomes can be used for targeted transport of tumor drugs. Therefore, it is reasonable to presume that exosomes can be modified for use as biomarkers, vaccines, and drug carriers to develop more effective clinical diagnosis and treatment strategies for cancer.
QL and DW wrote the manuscript. QL, DD, YF, RH, DL, CL, and YG collected the references and prepared figures. All authors reviewed the manuscript.
This work was supported by the Jilin Health Commission Program under Grant 2020J05S, the Fundamental Research Funds for the Central Universities under Grant 2019JCKT-70, the Jilin Education Department Program under Grant JJKH20200950KJ, and the Jilin Scientific and Technological Development Program under Grant 20190103071JH, 202002006JC and 20210101010JC.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Athauda, A., Segelov, E., Ali, Z., and Chau, I. (2019). Integrative Molecular Analysis of Colorectal Cancer and Gastric Cancer: What Have We Learnt? Cancer Treat. Rev. 73, 31–40. doi:10.1016/j.ctrv.2018.12.004
Bagheri, E., Abnous, K., Farzad, S. A., Taghdisi, S. M., Ramezani, M., and Alibolandi, M. (2020). Targeted Doxorubicin-Loaded Mesenchymal Stem Cells-Derived Exosomes as a Versatile Platform for Fighting against Colorectal Cancer. Life Sci. 261, 118369. doi:10.1016/j.lfs.2020.118369
Bebelman, M. P., Smit, M. J., Pegtel, D. M., and Baglio, S. R. (2018). Biogenesis and Function of Extracellular Vesicles in Cancer. Pharmacol. Ther. 188, 1–11. doi:10.1016/j.pharmthera.2018.02.013
Chen, X., Liu, Y., Zhang, Q., Liu, B., Cheng, Y., Zhang, Y., et al. (2021a). Exosomal miR-590-3p Derived from Cancer-Associated Fibroblasts Confers Radioresistance in Colorectal Cancer. Mol. Ther. Nucleic Acids 24, 113–126. doi:10.1016/j.omtn.2020.11.003
Chen, Z., Xie, Y., Chen, W., Li, T., Chen, X., and Liu, B. (2021b). microRNA-6785-5p-loaded Human Umbilical Cord Mesenchymal Stem Cells-Derived Exosomes Suppress Angiogenesis and Metastasis in Gastric Cancer via INHBA. Life Sci. 284, 119222. doi:10.1016/j.lfs.2021.119222
Cianciaruso, C., Beltraminelli, T., Duval, F., Nassiri, S., Hamelin, R., Mozes, A., et al. (2019). Molecular Profiling and Functional Analysis of Macrophage-Derived Tumor Extracellular Vesicles. Cell Rep 27 (10), 3062–e11. doi:10.1016/j.celrep.2019.05.008
Dekker, E., Tanis, P. J., Vleugels, J. L. A., Kasi, P. M., and Wallace, M. B. (2019). Colorectal Cancer. Lancet 394 (10207), 1467–1480. doi:10.1016/S0140-6736(19)32319-0
Ganig, N., Baenke, F., Thepkaysone, M. L., Lin, K., Rao, V. S., Wong, F. C., et al. (2021). Proteomic Analyses of Fibroblast- and Serum-Derived Exosomes Identify QSOX1 as a Marker for Non-invasive Detection of Colorectal Cancer. Cancers (Basel) 13 (6), 1351. doi:10.3390/cancers13061351
Gao, T., Liu, X., He, B., Nie, Z., Zhu, C., Zhang, P., et al. (2018). Exosomal lncRNA 91H Is Associated with Poor Development in Colorectal Cancer by Modifying HNRNPK Expression. Cancer Cell Int 18, 11. doi:10.1186/s12935-018-0506-2
Ge, L., Zhang, N., Li, D., Wu, Y., Wang, H., and Wang, J. (2020). Circulating Exosomal Small RNAs Are Promising Non-invasive Diagnostic Biomarkers for Gastric Cancer. J. Cell Mol Med 24 (24), 14502–14513. doi:10.1111/jcmm.16077
Hoshino, A., Kim, H. S., Bojmar, L., Gyan, K. E., Cioffi, M., Hernandez, J., et al. (2020). Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell 182 (4), 1044–e18. doi:10.1016/j.cell.2020.07.009
Hui, B., Lu, C., Wang, J., Xu, Y., Yang, Y., Ji, H., et al. (2021). Engineered Exosomes for Co‐delivery of PGM5‐AS1 and Oxaliplatin to Reverse Drug Resistance in colon Cancer. J. Cell Physiol. doi:10.1002/jcp.30566
Jänne, P. A., and Mayer, R. J. (2000). Chemoprevention of Colorectal Cancer. N. Engl. J. Med. 342 (26), 1960–1968. doi:10.1056/NEJM200006293422606
Jiang, K., Chen, H., Fang, Y., Chen, L., Zhong, C., Bu, T., et al. (2021). Exosomal ANGPTL1 Attenuates Colorectal Cancer Liver Metastasis by Regulating Kupffer Cell Secretion Pattern and Impeding MMP9 Induced Vascular Leakiness. J. Exp. Clin. Cancer Res. 40 (1), 21. doi:10.1186/s13046-020-01816-3
Keehn, R. J., and Higgins, G. A. (1981). Chemotherapy for Gastric Cancer. Lancet 1 (8215), 323. doi:10.1016/s0140-6736(81)91928-0
Lan, J., Sun, L., Xu, F., Liu, L., Hu, F., Song, D., et al. (2019). M2 Macrophage-Derived Exosomes Promote Cell Migration and Invasion in Colon Cancer. Cancer Res. 79 (1), 146–158. doi:10.1158/0008-5472.CAN-18-0014
Li, H., and Li, F. (2018). Exosomes from BM-MSCs Increase the Population of CSCs via Transfer of miR-142-3p. Br. J. Cancer 119 (6), 744–755. doi:10.1038/s41416-018-0254-z
Li, Y., Gao, Y., Gong, C., Wang, Z., Xia, Q., Gu, F., et al. (2018). A33 Antibody-Functionalized Exosomes for Targeted Delivery of Doxorubicin against Colorectal Cancer. Nanomedicine 14 (7), 1973–1985. doi:10.1016/j.nano.2018.05.020
Li, Z., Suo, B., Long, G., Gao, Y., Song, J., Zhang, M., et al. (2020). Exosomal miRNA-16-5p Derived from M1 Macrophages Enhances T Cell-dependent Immune Response by Regulating PD-L1 in Gastric Cancer. Front Cell Dev Biol 8, 572689. doi:10.3389/fcell.2020.572689
Liang, G., Zhu, Y., Ali, D. J., Tian, T., Xu, H., Si, K., et al. (2020). Engineered Exosomes for Targeted Co-delivery of miR-21 Inhibitor and Chemotherapeutics to Reverse Drug Resistance in colon Cancer. J. Nanobiotechnology 18 (1), 10. doi:10.1186/s12951-019-0563-2
Lin, H., Zhang, L., Zhang, C., and Liu, P. (2020). Exosomal MiR-500a-3p Promotes Cisplatin Resistance and Stemness via Negatively Regulating FBXW7 in Gastric Cancer. J. Cell Mol Med 24 (16), 8930–8941. doi:10.1111/jcmm.15524
Liu, T., Zhang, X., Du, L., Wang, Y., Liu, X., Tian, H., et al. (2019). Exosome-transmitted miR-128-3p Increase Chemosensitivity of Oxaliplatin-Resistant Colorectal Cancer. Mol. Cancer 18 (1), 43. doi:10.1186/s12943-019-0981-7
Maconi, G., Manes, G., and Porro, G. B. (2008). Role of Symptoms in Diagnosis and Outcome of Gastric Cancer. World J. Gastroenterol. 14 (8), 1149–1155. doi:10.3748/wjg.14.1149
Mao, J., Liang, Z., Zhang, B., Yang, H., Li, X., Fu, H., et al. (2017). UBR2 Enriched in P53 Deficient Mouse Bone Marrow Mesenchymal Stem Cell-Exosome Promoted Gastric Cancer Progression via Wnt/β-Catenin Pathway. Stem Cells 35 (11), 2267–2279. doi:10.1002/stem.2702
Mashouri, L., Yousefi, H., Aref, A. R., Ahadi, A. M., Molaei, F., and Alahari, S. K. (2019). Exosomes: Composition, Biogenesis, and Mechanisms in Cancer Metastasis and Drug Resistance. Mol. Cancer 18 (1), 75. doi:10.1186/s12943-019-0991-5
Nabariya, D. K., Pallu, R., and Yenuganti, V. R. (2020). Exosomes: The Protagonists in the Tale of Colorectal Cancer? Biochim. Biophys. Acta Rev. Cancer 1874 (2), 188426. doi:10.1016/j.bbcan.2020.188426
Poggi, A., and Giuliani, M. (2016). Mesenchymal Stromal Cells Can Regulate the Immune Response in the Tumor Microenvironment. Vaccines (Basel) 4 (4). doi:10.3390/vaccines4040041
Qiao, L., Hu, S., Huang, K., Su, T., Li, Z., Vandergriff, A., et al. (2020). Tumor Cell-Derived Exosomes home to Their Cells of Origin and Can Be Used as Trojan Horses to Deliver Cancer Drugs. Theranostics 10 (8), 3474–3487. doi:10.7150/thno.39434
Quail, D. F., and Joyce, J. A. (2013). Microenvironmental Regulation of Tumor Progression and Metastasis. Nat. Med. 19 (11), 1423–1437. doi:10.1038/nm.3394
Ren, J., Ding, L., Zhang, D., Shi, G., Xu, Q., Shen, S., et al. (2018). Carcinoma-associated Fibroblasts Promote the Stemness and Chemoresistance of Colorectal Cancer by Transferring Exosomal lncRNA H19. Theranostics 8 (14), 3932–3948. doi:10.7150/thno.25541
Shi, L., Wang, Z., Geng, X., Zhang, Y., and Xue, Z. (2020). Exosomal miRNA-34 from Cancer-Associated Fibroblasts Inhibits Growth and Invasion of Gastric Cancer Cells In Vitro and In Vivo. Aging (Albany NY) 12 (9), 8549–8564. doi:10.18632/aging.103157
Smyth, E. C., Nilsson, M., Grabsch, H. I., van Grieken, N. C., and Lordick, F. (2020). Gastric Cancer. Lancet 396 (10251), 635–648. doi:10.1016/S0140-6736(20)31288-5
Sun, W., Luo, J. D., Jiang, H., and Duan, D. D. (2018a). Tumor Exosomes: a Double-Edged Sword in Cancer Therapy. Acta Pharmacol. Sin 39 (4), 534–541. doi:10.1038/aps.2018.17
Sun, Z., Shi, K., Yang, S., Liu, J., Zhou, Q., Wang, G., et al. (2018b). Effect of Exosomal miRNA on Cancer Biology and Clinical Applications. Mol. Cancer 17 (1), 147. doi:10.1186/s12943-018-0897-7
Théry, C., Zitvogel, L., and Amigorena, S. (2002). Exosomes: Composition, Biogenesis and Function. Nat. Rev. Immunol. 2 (8), 569–579. doi:10.1038/nri855
Tran, P. H. L., Wang, T., Yin, W., Tran, T. T. D., Nguyen, T. N. G., Lee, B. J., et al. (2019). Aspirin-loaded Nanoexosomes as Cancer Therapeutics. Int. J. Pharm. 572, 118786. doi:10.1016/j.ijpharm.2019.118786
Uemura, N., Okamoto, S., Yamamoto, S., Matsumura, N., Yamaguchi, S., Yamakido, M., et al. (2001). Helicobacter pylori Infection and the Development of Gastric Cancer. N. Engl. J. Med. 345 (11), 784–789. doi:10.1056/NEJMoa001999
Wang, M., Zhao, C., Shi, H., Zhang, B., Zhang, L., Zhang, X., et al. (2014). Deregulated microRNAs in Gastric Cancer Tissue-Derived Mesenchymal Stem Cells: Novel Biomarkers and a Mechanism for Gastric Cancer. Br. J. Cancer 110 (5), 1199–1210. doi:10.1038/bjc.2014.14
Wang, X., Zhang, H., Bai, M., Ning, T., Ge, S., Deng, T., et al. (2018). Exosomes Serve as Nanoparticles to Deliver Anti-miR-214 to Reverse Chemoresistance to Cisplatin in Gastric Cancer. Mol. Ther. 26 (3), 774–783. doi:10.1016/j.ymthe.2018.01.001
Wei, S., Peng, L., Yang, J., Sang, H., Jin, D., Li, X., et al. (2020). Exosomal Transfer of miR-15b-3p Enhances Tumorigenesis and Malignant Transformation through the DYNLT1/Caspase-3/Caspase-9 Signaling Pathway in Gastric Cancer. J. Exp. Clin. Cancer Res. 39 (1), 32. doi:10.1186/s13046-019-1511-6
Wu, S., Li, T., Liu, W., and Huang, Y. (2021). Ferroptosis and Cancer: Complex Relationship and Potential Application of Exosomes. Front. Cell Dev Biol 9, 733751. doi:10.3389/fcell.2021.733751
Xie, M., Yu, T., Jing, X., Ma, L., Fan, Y., Yang, F., et al. (2020a). Exosomal circSHKBP1 Promotes Gastric Cancer Progression via Regulating the miR-582-3p/HUR/VEGF axis and Suppressing HSP90 Degradation. Mol. Cancer 19 (1), 112. doi:10.1186/s12943-020-01208-3
Xie, Y., Li, J., Li, P., Li, N., Zhang, Y., Binang, H., et al. (2020b). RNA-seq Profiling of Serum Exosomal Circular RNAs Reveals Circ-PNN as a Potential Biomarker for Human Colorectal Cancer. Front. Oncol. 10, 982. doi:10.3389/fonc.2020.00982
Xing, H., Liang, C., Xu, X., Sun, H., Ma, X., and Jiang, Z. (2020). Mesenchymal Stroma/stem-like Cells of GARP Knockdown Inhibits Cell Proliferation and Invasion of Mouse colon Cancer Cells (MC38) through Exosomes. J. Cell Mol Med 24 (23), 13984–13990. doi:10.1111/jcmm.16008
Xu, G., Zhang, B., Ye, J., Cao, S., Shi, J., Zhao, Y., et al. (2019). Exosomal miRNA-139 in Cancer-Associated Fibroblasts Inhibits Gastric Cancer Progression by Repressing MMP11 Expression. Int. J. Biol. Sci. 15 (11), 2320–2329. doi:10.7150/ijbs.33750
Yang, B., Chen, Y., and Shi, J. (2019). Exosome Biochemistry and Advanced Nanotechnology for Next-Generation Theranostic Platforms. Adv. Mater. 31 (2), e1802896. doi:10.1002/adma.201802896
Zeng, Z., Li, Y., Pan, Y., Lan, X., Song, F., Sun, J., et al. (2018). Cancer-derived Exosomal miR-25-3p Promotes Pre-metastatic Niche Formation by Inducing Vascular Permeability and Angiogenesis. Nat. Commun. 9 (1), 5395. doi:10.1038/s41467-018-07810-w
Zhang, H., Deng, T., Liu, R., Bai, M., Zhou, L., Wang, X., et al. (2017). Exosome-delivered EGFR Regulates Liver Microenvironment to Promote Gastric Cancer Liver Metastasis. Nat. Commun. 8, 15016. doi:10.1038/ncomms15016
Zhang, H., Wang, Y., Bai, M., Wang, J., Zhu, K., Liu, R., et al. (2018a). Exosomes Serve as Nanoparticles to Suppress Tumor Growth and Angiogenesis in Gastric Cancer by Delivering Hepatocyte Growth Factor siRNA. Cancer Sci. 109 (3), 629–641. doi:10.1111/cas.13488
Zhang, Q., Liu, R. X., Chan, K. W., Hu, J., Zhang, J., Wei, L., et al. (2019). Exosomal Transfer of P-STAT3 Promotes Acquired 5-FU Resistance in Colorectal Cancer Cells. J. Exp. Clin. Cancer Res. 38 (1), 320. doi:10.1186/s13046-019-1314-9
Zhang, Q., Zhang, H., Ning, T., Liu, D., Deng, T., Liu, R., et al. (2020). Exosome-Delivered C-Met siRNA Could Reverse Chemoresistance to Cisplatin in Gastric Cancer. Int. J. Nanomedicine 15, 2323–2335. doi:10.2147/IJN.S231214
Zhang, Y., Yu, G., Chu, H., Wang, X., Xiong, L., Cai, G., et al. (2018b). Macrophage-Associated PGK1 Phosphorylation Promotes Aerobic Glycolysis and Tumorigenesis. Mol. Cell 71 (2), 201–e7. doi:10.1016/j.molcel.2018.06.023
Zhao, R., Zhang, Y., Zhang, X., Yang, Y., Zheng, X., Li, X., et al. (2018). Exosomal Long Noncoding RNA HOTTIP as Potential Novel Diagnostic and Prognostic Biomarker Test for Gastric Cancer. Mol. Cancer 17 (1), 68. doi:10.1186/s12943-018-0817-x
Zheng, P., Chen, L., Yuan, X., Luo, Q., Liu, Y., Xie, G., et al. (2017). Exosomal Transfer of Tumor-Associated Macrophage-Derived miR-21 Confers Cisplatin Resistance in Gastric Cancer Cells. J. Exp. Clin. Cancer Res. 36 (1), 53. doi:10.1186/s13046-017-0528-y
Keywords: gastric cancer, colorectal cancer, ncRNA, exosomes, cancer therapy
Citation: Li Q, Wang D, Ding D, Feng Y, Hou R, Liu D, Lin C and Gao Y (2022) The Role and Application of Exosomes in Gastric and Colorectal Cancer. Front. Pharmacol. 12:825475. doi: 10.3389/fphar.2021.825475
Received: 30 November 2021; Accepted: 27 December 2021;
Published: 17 January 2022.
Edited by:
Yue Hou, Northeastern University, ChinaReviewed by:
Wan Zhuo, Fourth Military Medical University, ChinaCopyright © 2022 Li, Wang, Ding, Feng, Hou, Liu, Lin and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongjian Gao, Z2FveWoxMjNAamx1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.