- 1Department of Anesthesiology, Haikou Women and Children’s Hospital, Haikou Maternity and Child Health Hospital, Haikou, China
- 2Department of Anesthesiology, Hainan General Hospital, Haikou, China
Objective: This prospective study evaluated the 50% effective dose (ED50) and 95% effective dose (ED95) of nalbuphine combined with propofol during painless gastroscopy.
Methods: Seventy-five patients who underwent painless gastroscopy were randomly divided into five groups (group N0, N0.05, N0.1, N0.15, and N0.2), with doses of 0, 0.05, 0.1, 0.15, or 0.2 mg/kg nalbuphine in each group. Propofol was given to all groups as the sedative. The bispectral index (BIS) value, propofol dose, examination time, and awakening time were recorded. The number of patients with intolerance indexes (coughing, retching, swallowing, or limb movement) was recorded in each group. The ED50/ED95 of nalbuphine combined with propofol for gastroscopy were calculated.
Results: Compared with those of groups N0, N0.05, or N0.1, the propofol dose and awakening time were significantly reduced in group N0.15 or N0.2 (p < 0.05). The successful rate of painless gastroscopy in group N0.15 or N0.2 significantly increased compared to that of group N0 or N0.05 (p < 0.05). When combined with propofol, nalbuphine had an ED50 and ED95 for painless gastroscopy of 0.078 mg/kg (95% CI, 0.056–0.098 mg/kg) and 0.162 mg/kg (95% CI, 0.134–0.217 mg/kg), respectively.
Conclusion: The ED50/ED95 of nalbuphine combined with propofol are 0.078 and 0.162 mg/kg, respectively, for painless gastroscopy. Nalbuphine at 0.162 mg/kg combined with propofol is effective and safe for painless gastroscopy in adults.
1 Introduction
Gastroscopy is an efficient method for the diagnosis and treatment of digestive system diseases, including those of the throat, esophagus, stomach, and duodenum (Liu et al., 2015). Gastroscopy is a noninvasive operation, but gastroscopy without sedation/anesthesia often causes adverse reactions such as nausea, vomiting, and coughing (Meng et al., 2016). To provide patients with comfortable (i.e., painless) gastroscopy, propofol is considered the best sedation anesthetic according to many studies (Smith et al., 1994; Ellett, 2010; Olofsen et al., 2010; LaPierre et al., 2012; Zhang et al., 2014; Kılıc et al., 2016; Ma et al., 2016). Propofol has no analgesic effect; so, propofol combined with opioids can alleviate the cardiovascular response, reduce noxious stimuli, and reduce the dose of propofol (LaPierre et al., 2012; Anesthesiology Branch of Chinese Medical Association, Digestive Endoscopy Branch of Chinese Medical Association, 2014; Zhang et al., 2014; Kılıc et al., 2016). Too large a dose of opioid drugs could inhibit the patients’ respiratory and circulatory systems, and too small a dose of opioid drugs could not help patients to finish a gastroscope placement. Thus, exploration of the 50% effective dose (ED50) and 95% effective dose (ED95) of opioid drugs is useful to achieve a comfortable and safe gastroscopy for patients.
Nalbuphine is a κ-receptor agonist and partial μ-receptor antagonist. As a κ-receptor agonist, nalbuphine has a unique analgesic effect on visceral pain (Riviere, 2004), which is suitable for gastroscopy. As a partial antagonist of the μ-receptor, nalbuphine use may avoid a series of adverse reactions related to the activation of the μ-receptor (e.g., respiratory depression, addiction, euphoria, bradycardia, itching, immunosuppression, nausea and vomiting, intestinal peristalsis, and impaired bladder muscle function) (Misiołek et al., 2014). At the same time, the activity of nalbuphine on the δ-receptor is very weak; so, the drug does not produce irritability and anxiety (Misiołek et al., 2014). Low-dose nalbuphine has almost no effect on hemodynamics and respiration; so, it is very suitable for outpatient surgery (Lake et al., 1982). The application of nalbuphine in painless gastroscopy has a certain advantage over other opioids: nalbuphine acts quickly and safely (Liu, 2017). Finding the ED50/ED95 of nalbuphine combined with propofol will help patients have a safe, painless gastroscopy.
In this study, we evaluated the ED50/ED95 of nalbuphine combined with propofol during painless gastroscopy, and we determined the feasibility of nalbuphine combined with propofol for painless gastroscopy in adults.
2 Materials and Methods
This experiment has been approved by the Medical Ethics Committee of Hainan General Hospital (China, Reference No. 2018-103). Informed consent forms were signed by patients.
2.1 Patients
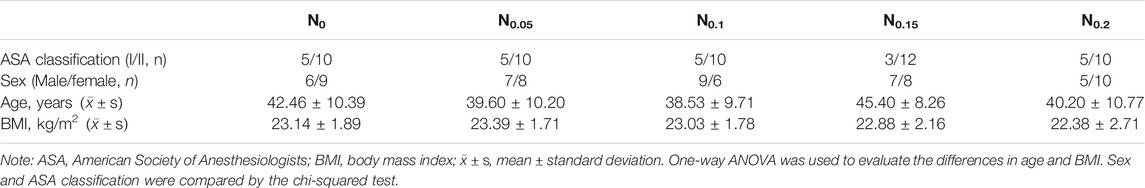
Overall, 75 patients who underwent painless gastroscopy in Hainan General Hospital from April 2020 to July 2020 were selected. The inclusion criteria were as follows: age 18–60 years, body mass index of 18–25 kg/m2, and American Society of Anesthesiologists classification I–II. The exclusion criteria included hepatitis and renal failure, habitual sedative or analgesic use, mental illness, and allergy to nalbuphine or propofol.
2.2 Clinical Protocol
The patients were randomly divided into five groups of nalbuphine according to dose, as follows: 0 mg/kg (group N0), 0.05 mg/kg (group N0.05), 0.1 mg/kg (group N0.1), 0.15 mg/kg (group N0.15), and 0.2 mg/kg (group N0.2). All patients fasted from solids for 8 h and from liquids for 2 h. All patients took the left recumbent position in the operating room, with standard monitoring that included oxygen saturation (SpO2), noninvasive blood pressure, and electrocardiogram. All patients inhaled oxygen using the nasal catheter (3 L/min) for 5 min before anesthesia.
Nalbuphine (batch number: 1189503; Yichang Renfu Pharmaceutical Co., Ltd. Yichang, Hubei province, China) was injected intravenously at the onset; at 3 min after the onset, propofol (Batch number: RA170; AstraZeneca UK Limited, Macclesfield, Cheshire, SK10 2NA, United Kingdom) was administrated intravenously at a rate of 50–150 μg/kg/min until the patient lost consciousness, as reflected by a bispectral index (BIS) (BIS Complete Monitoring System, Covidien) value between 50 and 65. Then, the gastroscopy was performed. If the gastroscopy failed (defined as any coughing, swallowing, retching, or limb movement by the patient), the propofol dose was increased to facilitate completion of the examination. When the heart rate decreased to 50 beats per min, 0.2–0.5 mg atropine was injected. When the mean arterial pressure was less than 60 mmHg, 5–10 mg ephedrine was applied. When SpO2 decreased to 90%, assisted ventilation with oxygen via a facial mask was applied.
Observation indexes included the following: the BIS value at baseline (before the beginning of anesthesia) and at the beginning of gastroscopy, the effect-site concentration of propofol at the beginning of gastroscopy and at the end of gastroscopy, the propofol dose (the amount of propofol), the examination time (the time of gastroscopy), and the awakening time (the time between the end of the gastroscopy and when the patients to awake) were recorded. The number of patients with any coughing, swallowing, retching, or limb movement was recorded in each group. The number of patients who experienced a successful gastroscopy (defined as having none of the intolerance of coughing, swallowing, retching, or limb movement) was recorded in each group. The number of patients with apnea, postoperative pruritus, postoperative nausea/vomiting, or postoperative anxiety/irritability was recorded.
The effect index was the number of patients who experienced a successful gastroscopy, and the index was used to calculate the ED50/ED95 of nalbuphine combined with propofol. The patients who required increasing propofol doses were regarded as having a failed gastroscopy.
2.3 Statistical Analysis
IBM SPSS Statistics 23.0 (IBM Corporation, version 19; Armonk, NY, United States) was used for statistical analyses of data. The median effective dose (i.e., the ED50) and the ED95 as well as the 95% CIs of nalbuphine when combined with propofol were determined by binary regression (probit) (Gorges et al., 2017).
The required sample size was calculated using PASS 11.0 (Power Analysis and Sample Size; NCSS, LLC, Englewood, NJ, United States). The main indicator considered was the awakening time. The pilot study had 5 cases in each group. The mean ± standard deviation of awakening time in group N0 and group N0.2 were 726.13 ± 123.46 (s) and 245.00 ± 68.74 (s), respectively. A sample size of 15 in each group was required for a beta value of 0.10 and an alpha value of 0.05.
Normally distributed statistics were analyzed as the mean ± standard deviation and by one-way analysis of variance. Categorical variables were presented as proportions (%) and were compared using Fisher’s exact test or the chi-squared test. Trends in the nalbuphine doses were evaluated using the chi-squared test for trend. A p value of <0.05 was considered to be statistically significant.
3 Results
3.1 Included Patients Information
Overall, 77 patients were enrolled in the study, and 75 patients were assessed for eligibility. Two patients were excluded (one from group N0 and one from group N0.15) because gastric mucosa tissues were taken for observation by pathology and the duration of gastroscopy was more than 10 min. No significant demographic differences were noted among the five groups (p > 0.05). Results are shown in Table 1.
3.2 Intolerance Indexes: Coughing, Retching, Swallowing, and Limb Movement
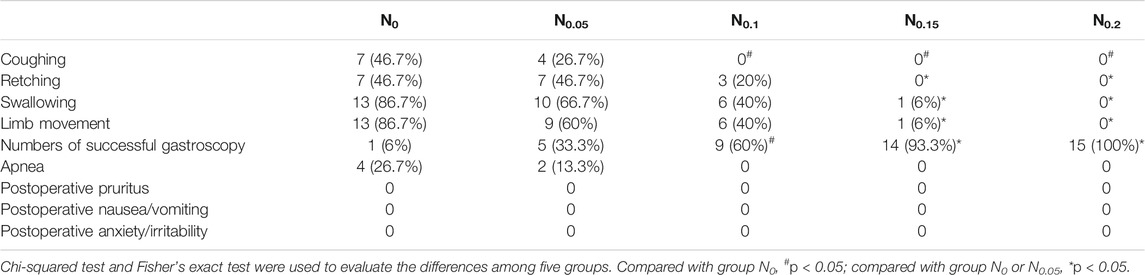
Compared with group N0, group N0.1 had significantly decreased incidences of coughing (p < 0.05); groups N0.15 and N0.2 had significantly reduced incidences of coughing, retching, swallowing, and limb movement (p < 0.05). Compared with group N0.05, groups N0.15 and N0.2 had significantly reduced incidences of retching, swallowing, and limb movement (p < 0.05). Results are shown in Table 2.
3.3 Adverse Effects
There was no significant difference in the incidence of apnea, postoperative pruritus, postoperative nausea/vomiting, or postoperative anxiety/irritability among the five groups (p > 0.05). Results are shown in Table 2.
3.4 Incidences of Successful Gastroscopy
Forty-four patients experienced successful gastroscopies (i.e., without any coughing, retching, swallowing, or limb movement). The incidences of successful gastroscopy in groups N0.1, N0.15, and N0.2 were significantly higher than those in group N0 (p < 0.01); compared with group N0.05, groups N0.15 and N0.2 had significantly increased incidences of successful gastroscopy (p < 0.01). No significant differences in success were noted among the other groups (p > 0.05). Results are shown in Table 2.
3.5 ED50 and ED95 of Nalbuphine Combined With Propofol
The statistics were analyzed by binary regression (probit). The ED50 of nalbuphine combined with propofol for painless gastroscopy was 0.078 mg/kg (95% CI, 0.056–0.098 mg/kg), and the ED95 of nalbuphine was 0.162 mg/kg (95% CI, 0.134–0.217 mg/kg) for the same procedure. Results are shown in Figure 1.
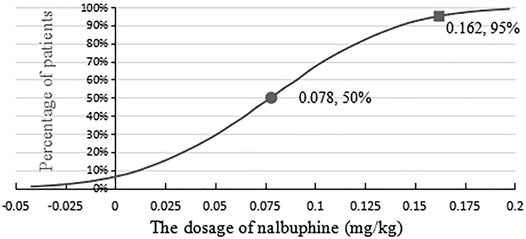
FIGURE 1. Effective dose of nalbuphine being combined with propofol anesthesia for gastroscopy in adults.
3.6 Comparison of the BIS Value, Effect-Site Concentration and Dose of Propofol, Examination Time, and Awakening Time
No statistically significant differences were observed in the BIS value at baseline or at the beginning of gastroscopy among the five groups (p > 0.05). After the nalbuphine dose was increased, the effect-site concentration of propofol, the propofol dose, and the awakening time decreased gradually in groups N0, N0.1, and N0.15 (p < 0.05), whereas no statistically significant differences were noted in these indexes between groups N0.2 and N0.15 (p > 0.05). The examination time for group N0 was significantly longer than that for any other group (p < 0.05), but no significant difference was noted among the other four groups (p > 0.05). All results are shown in Table 3.
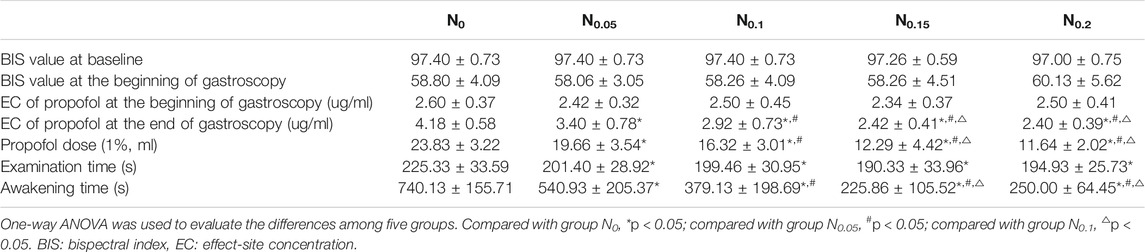
TABLE 3. Comparison of the BIS value, propofol dose, examination time, and awakening time in five groups.
4 Discussion
Although the surgery time for gastroscopy is short, it has a great impact on the physical and mental health of the patient. The anesthesia for gastroscopy requires a short time to the onset, adequate sedation and analgesia, quick recovery time after surgery, and low adverse effects. Gastroscopy often uses propofol combined with opioid analgesics. Nalbuphine, used in this study, is a κ-receptor agonist with a high concentration in the spinal cord. It produces mild respiratory depression, has hemodynamic stability, has a rapid onset, and is widely used in the clinic.
The basic requirement of anesthesia for gastroscopy is loss of consciousness and no body movement; so, this experiment set the BIS value at the ideal sedation level of 50–65 (Hao et al., 2014). According to the pharmacokinetic characteristics of nalbuphine, the time to the onset of effect is 2–3 min after intravenous injection (Cai et al., 2011); so, gastroscopy begins 2–3 min after nalbuphine injection to ensure that the analgesia of nalbuphine has taken effect.
The probit method (Cai et al., 2011) has been used to calculate the ED50 and ED95 of drugs. According to the requirements for the probit method, an arithmetic design of the 5 nalbuphine dose groups in this experiment was adopted. Dose selection was calculated according to results by Chen et al. (2018), who achieved an ED50 and an ED95 of nalbuphine during painless abortion of 0.068 mg/kg and 0.128 mg/kg, respectively. The probit method requires that the range of the selected dose in groups should cover the predicted value, and the result is more accurate when the arithmetic difference between groups is smaller. Therefore, intravenous doses of 0, 0.05, 0.1, 0.15, and 0.2 mg/kg nalbuphine were selected for this study.
In this study, six patients had apnea (4 in group N0 and 2 in group N0.05). If apnea lasts for more than 30 s, artificial respiration with a hand-controlled balloon was used to maintain blood oxygen saturation greater than 95% until spontaneous breathing was restored. The momentary apnea did not cause adverse effects for the patients. No patients had adverse cardiovascular reactions after receiving the anesthetic drugs; fluctuations of blood pressure and heart rate were within the physiological ranges, and cardiovascular drugs were not used.
In this study, as the dose of nalbuphine is increased, the total propofol dose is decreased; so, the awakening time of patients is shortened accordingly. It has shown that nalbuphine could be applied for patients safely in gastroscopy; no obvious respiratory depression, postoperative pruritus, postoperative nausea/vomiting, or postoperative anxiety/irritability occurred. Chen et al. (2018) compared nalbuphine with sufentanil—both in combination with propofol as anesthesia for abortion—and found that the awakening time in the nalbuphine group was significantly shorter than that in the sufentanil group. The incidence of dizziness (10%) in the nalbuphine group was significantly lower than that in the sufentanil group (33%) (Chen et al., 2018).
The incidences of coughing, retching, swallowing, and limb movement in patients decreased gradually with an increasing dose of nalbuphine. The reduction of these stress responses is conducive to successful gastroscopy. These results showed that the incidences of coughing, retching, swallowing, and limb movement in groups N0.15 and N0.2, with high-dose nalbuphine, were lower than those in groups N0.05 and N0.1, with low-dose nalbuphine. The number of successful gastroscopies in each group also increased with increasing nalbuphine dose: 1 case in group N0, 5 cases in group N0.05, 9 cases in group N0.1, 14 cases in N0.15, and 15 cases in group N0.2. Groups N0.15 and N0.2 had higher rates of successful gastroscopy than groups N0.05 and N0.1 had.
The probit regression method showed that the ED50 and ED95 of nalbuphine combined with propofol anesthesia for gastroscopy were 0.078 mg/kg (95% CI, 0.056–0.098 mg/kg) and 0.162 mg/kg (95% CI, 0.134–0.217 mg/kg), respectively. Therefore, 0.162 mg/kg of nalbuphine combined with propofol anesthesia is safe and effective for gastroscopy in adults, and it could provide the best conditions for gastroscopy, without any adverse reactions.
5 Conclusion
The ED50/ED95 of nalbuphine combined with propofol for patients with painless gastroscopy are 0.078 and 0.162 mg/kg, respectively. So, nalbuphine at 0.162 mg/kg combined with propofol is an effective and safe way for painless gastroscopy in adults.
This study had some limitations. First, no serum nalbuphine/propofol assays were used. Second, clinical examinations of outpatients were usually incomplete. Last, the hidden diseases and different sensitivities to drugs in individuals might have affected the results.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Hainan General Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SL and NL conceived and designed the experiments; YW and XC performed the experiments; YW and TH analyzed the data and wrote the manuscript.
Funding
This work was supported by the Hainan Health Science and Education Project of China (grant number: 20A200107).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anesthesiology Branch of Chinese Medical Association, Digestive Endoscopy Branch of Chinese Medical Association (2014). Expert Consensus on Sedation/anesthesia of Gastroenterology in China. J. Clin. Anesthesiol. (China) 30 (9), 920–927. doi:10.3760/cma.j.issn.0254-1432.2014.08.001
Cai, L. J., Zhang, J., Peng, W. X., Zhu, R. H., and Zhang, Q. Z. (2011). Pharmacokinetics of Intravenous Nalbuphine in Healthy Volunteers. Chin. Pharm. J. (China). 46 (20), 1597–1600.
Chen, L., Zhou, Y., Cai, Y., Bao, N., Xu, X., and Shi, B. (2018). The ED95 of Nalbuphine in Outpatient-Induced Abortion Compared to Equivalent Sufentanil. Basic Clin. Pharmacol. Toxicol. 123 (2), 202–206. doi:10.1111/bcpt.13022
Ellett, M. L. (2010). A Literature Review of the Safety and Efficacy of Using Propofol for Sedation in Endoscopy. Gastroenterol. Nurs. 33 (2), 111–117. doi:10.1097/SGA.0b013e3181d601fb
Görges, M., Zhou, G., Brant, R., and Ansermino, J. M. (2017). Sequential Allocation Trial Design in Anesthesia: an Introduction to Methods, Modeling, and Clinical Applications. Paediatr. Anaesth. 27, 240–247. doi:10.1111/pan.13088
Hao, G. T., Zhou, H. Y., Gao, H. Z., Qu, H. Y., Liang, Y. G., Li, Y. Y., et al. (2014). Pharmacokinetics of Oxycodone Hydrochloride and Three of its Metabolites after Intravenous Administration in Chinese Patients with Pain. Pharmacol. Rep. 66 (1), 153–158. doi:10.1016/j.pharep.2013.08.012
Kılıc, E., Demiriz, B., Isıkay, N., Yıldırım, A. E., Can, S., and Basmacı, C. (2016). Alfentanil versus Ketamine Combined with Propofol for Sedation during Upper Gastrointestinal System Endoscopy in Morbidly Obese Patients. Saudi. Med. J. 37 (11), 1191–1195. doi:10.15537/smj.2016.11.14557
Lake, C. L., Duckworth, E. N., Difazio, C. A., Durbin, C. G., and Magruder, M. R. (1982). Cardiovascular Effects of Nalbuphine in Patients with Coronary or Valvular Heart Disease. Anesthesiology 57 (6), 498–503. doi:10.1097/00000542-198212000-00012
LaPierre, C. D., Johnson, K. B., Randall, B. R., and Egan, T. D. (2012). A Simulation Study of Common Propofol and Propofol-Opioid Dosing Regimens for Upper Endoscopy: Implications on the Time Course of Recovery. Anesthesiology 117, 252–262. doi:10.1097/ALN.0b013e31825fb1b2
Liu, J., Wang, B., Hu, W., Sun, P., Li, J., Duan, H., et al. (2015). Global and Local Panoramic Views for Gastroscopy: An Assisted Method of Gastroscopic Lesion Surveillance. IEEE. Trans. Biomed. Eng. 62, 2296–2307. doi:10.1109/TBME.2015.2424438
Liu, Z. W. (2017). Anesthetic Effect and Safety on Propofol Combined with Nabufen Hydrochloride, Dezocine or Sufentanil Respectively for Painless Gastroscopy. J. Clin. Med. Lit. (China). 4 (29), 5708.
Ma, J., Zhang, P., Zhang, Y., Chen, Z., Xin, W., Zhang, D., et al. (2016). Effect of Dezocine Combined with Propofol on Painless Gastroscopy in Patients with Suspect Gastric Carcinoma. J. Cancer Res. Ther. 12, C271–C273. doi:10.4103/0973-1482.200755
Meng, Q. T., Cao, C., Liu, H. M., Xia, Z. Y., Li, W., Tang, L. H., et al. (2016). Safety and Efficacy of Etomidate and Propofol Anesthesia in Elderly Patients Undergoing Gastroscopy: A Double-Blind Randomized Clinical Study. Exp. Ther. Med. 12, 1515–1524. doi:10.3892/etm.2016.3475
Misiołek, H., Cettler, M., Woroń, J., Wordliczek, J., Dobrogowski, J., and Mayzner-Zawadzka, E. (2014). The 2014 Guidelines for post-operative Pain Management. Anaesthesiol. Intensive Ther. 46 (46), 221–244. doi:10.5603/AIT.2014.0041
Olofsen, E., Boom, M., Nieuwenhuijs, D., Sarton, E., Teppema, L., Aarts, L., et al. (2010). Modeling the Non-steady State Respiratory Effects of Remifentanil in Awake and Propofol-Sedated Healthy Volunteers. Anesthesiology 112, 1382–1395. doi:10.1097/ALN.0b013e3181d69087
Rivière, P. J. (2004). Peripheral Kappa-Opioid Agonists for Visceral Pain. Br. J. Pharmacol. 141 (8), 1331–1334. doi:10.1038/sj.bjp.0705763
Smith, I., White, P. F., Nathanson, M., and Gouldson, R. (1994). Propofol. An Update on its Clinical Use. Anesthesiology 81 (4), 1005–1043.
Keywords: nalbuphine, propofol, gastroscopy, dose–effect relationship, ED50, ED95, effective dose
Citation: Li S, Wang Y, Chen X, Huang T and Li N (2021) Effective Doses of Nalbuphine Combined With Propofol for Painless Gastroscopy in Adults: A Randomized Controlled Trial. Front. Pharmacol. 12:673550. doi: 10.3389/fphar.2021.673550
Received: 25 July 2021; Accepted: 03 November 2021;
Published: 01 December 2021.
Edited by:
Guoxun Chen, The University of Tennessee, Knoxville, United StatesReviewed by:
Jianye Yuan, Shanghai University of Traditional Chinese Medicine, ChinaSheikh Raisuddin, Jamia Hamdard University, India
Copyright © 2021 Li, Wang, Chen, Huang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Li, dGFuZ3hpbjE5ODFAZm94bWFpbC5jb20=
†These authors share first authorship
 Shuangfeng Li
Shuangfeng Li Ying Wang
Ying Wang Xiaojian Chen
Xiaojian Chen Tingwan Huang
Tingwan Huang Na Li
Na Li