
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 29 October 2019
Sec. Ethnopharmacology
Volume 10 - 2019 | https://doi.org/10.3389/fphar.2019.01223
 Farzana Khan1,2
Farzana Khan1,2 Md. Moklesur Rahman Sarker1,2*
Md. Moklesur Rahman Sarker1,2* Long Chiau Ming3,4
Long Chiau Ming3,4 Isa Naina Mohamed5*
Isa Naina Mohamed5* Chao Zhao6
Chao Zhao6 Bassem Y. Sheikh7
Bassem Y. Sheikh7 Hiew Fei Tsong8
Hiew Fei Tsong8 Mohammad A. Rashid9
Mohammad A. Rashid9Gymnema sylvestre is a plant included in Apocynaceae family and is located in many regions of Asia, Africa and Australia. This plant is widely used as a traditional therapy for different purposes. Even now it is being used as a dietary supplement due to its numerous therapeutic uses. It is known to have blood glucose lowering potential and, thus, is widely used in traditional and Ayurvedic systems of medicine. It renders glucose lowering activity due to the presence of phytochemicals, such as gurmarin, gymnemic acid as well as gymnemasaponins. Gymnema sylvestre is also known to have anti-oxidant, antibiotic, anti-inflammatory, antiviral, gastro and hepatoprotective, anticancer and lipid-lowering activities. This review discusses in details on different pharmacological and clinical potentials of Gymnema sylvestre and its chemical constituents associated with its therapeutic potentials.
Plants are a great concern for drug discovery exploration and a major source of our modern medicine. About 25% of modern medicines are derived from a plant source and merely 5-15% of plants have been investigated for their medicinal use (Gurnani et al., 2014). Nowadays, natural plants, herbal medicines, phytomedicines, and functional foods are extensively studied by scientists all over the world which resulted with the lucrative therapeutic potentials such as antidiabetic (Sarker, 2015; Shah et al., 2016; Rouhi et al., 2017; Chen et al., 2018), anticancer (Sheikh et al., 2017a; Sheikh et al., 2017b), immunomodulating (Goto et al., 2010; Sarker et al., 2011; Sarker et al., 2012a; Sarker et al., 2012b; Sarker and Gohda, 2013), antiobesity and lipid lowering (Kazemipoor et al., 2015; Sarker, 2015), anti-inflammatory (Imam et al., 2013) and anti-bacterial (Yasmin et al., 2009) activies. Among the potential medicinal plants, Gymnema sylvestre, belongs to the family of Apocynaceae, and is traditionally used for the treatment of various dieseases. It is a wild herb found in India, Africa, Australia, and China (Christopoulos et al., 2010). It is known as Meshashringi, Merasingi, Kavali, Kalikardori, Vakundi, Dhuleti, Mardashingi, Podapatri, Adigam, Cherukurinja, Sannagerasehambu, Chigengteng or Australian Cowplant, Waldschlinge in German, Periploca of the woods in English (Kanetkar et al., 2007). This plant is also recognized as ‘Gurmur’, due to having sugar lowering property (Tiwari et al., 2014). Gymnema sylvestre was considered as one of the major botanicals to treat diabetes in the Ayurvedic system of medicine and also is included in Indian Pharmacopoeia as an anti-diabetic plant (Singh et al., 2008). As it is useful against major diseases such as cardiovascular diseases, asthma, cancer, diabetes and obesity, different formulation of this plant is found in a number of preparations such as tea bags, health tablets, and food supplements. In various studies, Gymnema sylvestre is reported to be effective against arthritis, diuretic, anemia, osteoporosis, hypercholesterolemia, cardiopathy, asthma, constipation, microbial infections, indigestion, and as an anti-inflammatory agent (Tiwari et al., 2014). Although this plant has been proven valuable through its numerous useful properties, not many studies especially clinical studies on this plant are available. We aim to extensively review the therapeutic potential and phytochemical compounds present in this plant based on the published reports so far.
A comprehensive, electronic search was conducted for studies published before April 2019 using PubMed, SCOPUS, Web of Science, EMBASE, Elsevier, ScienceDirect, Researchgate, Google, and Google Scholar databases. Keywords related to, `Pharmacology’, ‘Antioxidant’, ‘Anti-diabetic’, ‘Anticancer’, ‘Immunomodulatory’, ‘Anti-arthritis’, Weight loss’, ‘Lipid lowering’, ‘Antimicrobial’, ‘Anti-inflammatory’, ‘Hepatoprotective’, ‘Gastroprotective’, ‘Traditional’, ‘Phytochemicals’ combined with ‘Gymnema sylvestre’ were used.
Gymnema sylvestre (Retz.) R.Br. ex Sm. is a vulnerable and slow growing species. It appears as highly branched, woody and can climb up to the top of the tree that grows in the dry forests of central and southern India and in other regions of Asia (Wu et al., 2012; Kapoor, 2017). This is a shrub of pubescent type which has young stems and branches (Kanetkar et al., 2007). Its root system is of tap root type (Najafi and Deokule, 2011). Stems are cylindrical, branched, hard, twining, internodes terete, 0.7-17.2 cm long and 2 -10 mm in diameter (Najafi and Deokule, 2011; Pramanick, 2016). The leaves have distichous phyllotactic opposite arrangement pattern, are 2.5–6 cm long, usually ovate or elliptical and simple (Kanetkar et al., 2007). Leaves are acute or shortly acuminate, have petioles of 1- to 2-cm long, are smooth above, with a rounded base, a densely velvety pubescent beneath, and ciliate along margins, especially on the nerves. Venation is of transverse and reticulate type with a marginal vein (Kirtikar and Basu, 1975; Pramanick, 2016). Seeds are 1.3 cm long, flat with a thin marginal wing and narrowly ovoid-oblong (Chopra et al., 2002; Kirtikar and Basu, 1975). Flowers are small and yellow in color, in axillary and lateral umbel in cymes. Follicles are terete, lanceolate and of up to 3 inches in length (Kanetkar et al., 2007). Calyx is 5-lobed, ovate, obtuse, ciliated where corolla is campanulated, yellow, 5-lobed (Pramanick, 2016). Flowering of the plant occurs during August to March. Propagation through seed is difficult due to a low viability of seeds and, thus, plantation of root cuttings in June and July or plantation of terminal cuttings in February and March is done as an alternative approach (Kirtikar and Basu, 1975).
Gymnema sylvestre (Retz.) R.Br. ex Sm. is from Gymnema genus which belongs to Apocynaceae family. This genus has 49 other approved species which includes Gymnema acuminatum Wall., Gymnema brevifolium Benth., Gymnema chalmersii Schltr., Gymnema hirsutum Wight and Arn. etc. (The Plant List, 2013). The Taxonomy of the plant is presented in Table 1.
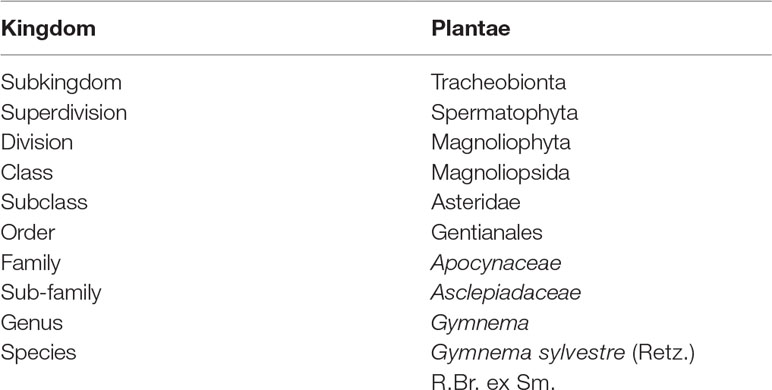
Table 1 Taxonomy of Gymnema sylvestre (Kirtikar and Basu, 1987).
Gymnema sylvestre is mentioned in Shushruta, an ancient book on medicine as a remedy for glycosuria and urinary disorder (Nadkarni, 1986). It is a therapeutic herb having multiple potentials as mentioned in folk medicine, Ayurveda, and Homeopathic systems of medicine (Kanetkar et al., 2007). Traditionally, it has been used to treat diabetes, malaria and snake bites as well as to treat diseases caused by phlegm and piles in the Ayurvedic system of medicine (Kirtikar and Basu, 1975; Singh et al., 2008). In Ayurveda, the plant is prescribed for the treatment of dyspepsia, constipation, jaundice, hemorrhoids, renal and vesicle calculi, cardiopathy, asthma, bronchitis, amenorrhea, and leucoderma (Sastry, 1994; Nadkarni, 1996; Anis et al., 2000; Mathew, 2004). Furthermore, different parts of the plant such as the roots, stem, and leaves have been used as cardiotonic, digestive, diuretic, laxative, stimulant, stomachic, and uterine tonic in traditional medicine systems (Mathew, 2004). Various parts of this plant are used by different tribes in India such as the Sahariya tribe of Madhya Pradesh, Junglee Irulas of Nilgiri hills, Kol tribe of Chhattisgarh, and the Nayaks of Karnataka, to treat mainly asthma, eye and gastric problems, parkinsonism, urinary problems, and diabetes (Chopra et al., 2002; Anis et al., 2000; Potawale et al., 2008).
Stems of Gymnema sylvestre were investigated using chromatographic techniques and were found to have several therapeutically important chemical compounds such as stigmasterol and triterpenoid saponin. Stigmasterol compounds have multiple therapeutic potentials including antidiabetic, hypoglycemic, antioxidant, anticancer activities. Triterpenoid saponins also exhibited anti-tumor, anti-fungal, hepatoprotective and antidiabetic potential in several studies (Matsuda et al., 1997; Kaur et al., 2011; Garai, 2014; Liu et al., 2014). Gymnemic acids and gymnemasaponins are major chemical constituents of this plant and are classified as oleanane saponins. Oleanane and dammarane type of saponins are found in the leaves of Gymnema sylvestre (Khramov et al., 2008). The leaves of this plant also have saponins, anthraquinones, cardiac glycosides etc. (Patel, 2017). Moreover, this plant was also observed to have tannin, quinones, flavonoids, and phenols. (Senthilkumar, 2015). The phytochemical compounds found in the analysis of Gymnema sylvestre is listed in Table 2.
In vitro and In vivo investigation of the therapeutic importance of Gymnema sylvestre revealed multifarious pharmacological potentials including anti-cancer, immunosuppressive, gastro-protective, hypoglycemic, anti-inflammatory, anti-infectious, and most importantly anti-diabetic activities. The pharmacological activities of phytochemicals derived from Gymnema sylvestre have been presented in Figure 1 and its reports are summarized in Table 3.
The most widely known effect of Gymnema sylvestre is anti-diabetic activity. Ethanol extract of this plant is reported to reduce glucose level by 46% where the water extract reduced glucose level by 26% and methanol extract by 12% (Mcburney and Gent, 1978; Luo et al., 2006; Kosta and Tiwari, 2009; Shah et al., 2011; Shah et al., 2012). In dexamethasone-induced insulin resistance rats, aqueous extract of this plant was found to be improving the altered glucose, insulin and lipid profile (Kumar S, et al., 2015). Administration of this plant in a diabetic animal model resulted in reductions in the blood levels of insulin, protein, triglycerides, cholesterol, and glucose, as well as a reduction in body weight and was found to improve liver histopathology (Sujin, 2008). In another study where alloxan-induced diabetic rats were used, this plant extract significantly (p < 0.05) reduced fasting blood glucose level, total cholesterol, serum triglycerides and increase HDL-cholesterol level and is also described to significantly alter (p < 0.05) the elevated level of urea, uric acid and creatinine levels in diabetic rats to nearly normal levels (Sathya et al., 2008; Mall et al., 2009). Gymnema sylvestre reduced the level of blood glucose levels after both acute and chronic administration of methanolic extract of this plant on Wister rats (Dholi and Raparla, 2014). In the case of Streptozotocin-induced diabetic rats, it has been shown that treatment using this plant significantly (p < 0.05) decreased the elevated blood glucose, ALT, AST, triglycerides, total cholesterol, LDL-cholesterol, and malondialdehyde, and significantly (p < 0.05) increased insulin, HDL-cholesterol, and erythrocyte superoxide dismutase levels in diabetic rats and also is capable of regenerating insulin producing β-cells (Aralelimath and Bhise, 2012; Shafey et al., 2013; Kumar et al., 2017; Ahmed et al., 2017). Gymnemic acids (a type of triterpene saponin compounds) are the class of active constituents isolated form Gymnema sylvestre. It was found that gymnemic acid IV given at a dose of 3.4/13.4 mg/kg adaministered for 6 hours decreased blood glucose levels by 14.0–60.0% as compared to glibenclamide. Also, gymnemic acid IV increased plasma insulin levels in STZ-diabetic mice when administered at a concentration of 13.4 mg/kg (Sugihara et al., 2000). In a study, oral administration of small concentrations (0.2 g/kg) of this plant produced a reduction in the elevated levels of blood sugar induced by sucrose (Kang et al., 1990). However, Galletto et al. (2004) also informed an absence of anti-diabetic activity of Gymnema sylvestre in an alloxan treated animal model (Galletto et al., 2004).
Several mechanisms have been proposed to explain the anti-diabetic activity of Gymnema sylvestre (Figure 2). Gymnemic acids can prevent absorption of sugar molecules by the intestine, which leads to a reduction in blood sugar levels (Tiwari et al., 2014). One of the constituents of Gymnema sylvestre is gymnemic acid which is a mixture of saponins (Yoshikawa et al., 1993). The atomic arrangement of gymnemic acid molecules is similar to that of glucose molecules and it blocks the receptor site for sugar in the intestines, preventing the absorption of sugar which reduces blood sugar level (Sahu et al., 1996). Rapid screening by Affinity Ultrafiltration-HPLC-MS shows that it contains α-glucosidase inhibitors (Chen and Guo, 2017). It is reported to increase the activity of enzymes which are insulin dependent including hexokinase, glycogen synthetase, glyceraldehydes 3-phosphate dehydrogenase, and glucose 6-phosphate dehydrogenase, and to decrease the activity of insulin-independent enzymes such as glycogen phosphorylase, gluconeogenic enzymes, glucose 6-phosphatase, fructose 1,6- diphosphatase, and sorbitol dehydrogenase, which also increases phosphorylase activity. Gymnema sylvestre was also found to increase the secretion of insulin and the possible role in regenerating insulin as well as β-cell was suggested (Shanmugasundaram et al., 1990a; Nakamura et al., 1999; Aralelimath and Bhise, 2012). In a study, methanol extract of this plant showed increased effect on β-cell regeneration and was extrapolated that this plant might be able to completely recover pancreatic-cells function and thus treating type I diabetes (Ahmed et al., 2010).
Gymnema sylvestre was found to have anticancer activity in various investigations. Its constituent gymnemagenol (C30H50O4) showed positive anticancer activity against HeLa cells (Khanna, 2010). The ethanolic, ethyl and chloroform extract were tested for anticancer activity against A549 (human lung adenocarcinoma) and MCF7 (human breast carcinsoma) cell lines. Theses extract revealed anticancer activity with a similar IC50 value against MCF cell lines where in the case of A549, ethyl and chloroform extract were more active than the ethanol extract (Srikanth et al., 2010). Ethanolic extract of Gymnema sylvestre showed anticancer activity in A375 cells (human skin melanoma). It showed cytotoxic activity against A375 cell and antitumor activity in skin Papilloma model where, in the case of normal liver cells WRL-68, it showed no cytotoxic activity (Chakraborty et al., 2013). It was revealed to have significant (p < 0.0001) inhibitory effect against intestinal breast cancer resistance protein (BCRP) (Tamaki et al., 2010). In a study, the administration of flavonoids was found to be inhibiting BCRP and subsequently, improving multi-drug resistance of BCRP substrates that was induced by it (Imai et al., 2004). Thus, it can be suggested that inhibition of this protein by Gymnema sylvestre may improve the activity of BCRP substrates methotrexate, daunorubicin, topotecan, epirubicin, flavopiridol, and so on by increasing systemic availability and absorption (Mao, 2005). Ethanolic extract of this plant exhibited antiproliferative effects in mice with two-stage carcinogenesis with a 50% inhibitory dose of 50–555 nmol/ear (Yasukawa et al., 2014). Polysaccharides (GSP11, GSP22, GSP33, GSP44 and GSP55) isolated from Gymnema sylvestre was reported to have anticancer activity by improving immunological function through increasing phagocytic function, enhanced serum hemolysin levels, thymus, and spleen indexes. GSP11 and GSP33 showed inhibitory rates of 78.6% and 83.8%, respectively, against U937 cells and GSP22 showed activity against SGC cells with an inhibitory rate of 78.2%. (Wu et al., 2012). In another study, antitumor potential of this plant was observed when methanolic extract of Gymnema sylvestre was administered on Swiss albino mice where papillomagenesis was induced using carcinogen 7, 12 - dimethylbenz (a) anthracene (DMBA). Decreased tumor incidence, tumor burden and the cumulative number of papillomas were observed after the treatment with the plant extract (Agrawal et al., 2016).
Gymnema sylvestre leaf extract was observed to possess very potent hypolipidaemic activity. In a study, Gymnema sylvestre leaf extract was administered to Wister female rats. These rats were introduced to hyperlipidemia by high-fat diet. It was detected that this extract significantly lowered the level of cholesterol (p< 0.01), low-density lipoprotein (LDL) (p< 0.01), and triglyceride (p< 0.01) as well as increased the level of high-density lipoprotein (HDL) (p< 0.001) effectively (Singh et al., 2017). Furthermore, hydro-alcoholic leaf extract of Gymnema sylvestre was also observed to have lipid-lowering potential. In this study, rats were given high cholesterol for seven days and a higher level of cholesterol, triglyceride, LDL, and a lower level of HDL was observed. After seven days, these rats were treated with Gymnema sylvestre extract and it was reported to lower the elevated cholesterol, triglyceride, LDL level and increase the HDL level. It was suggested that this plant renders lipid-lowering potential due to the presence of acidic constituents such as flavonoids, saponins, tannins etc. (Rachh et al., 2010; Dholi and Raparla, 2014). Similarly, in several other studies, it was reported to reduce triglyceride, cholesterol, very low‐density lipoprotein (VLDL) and low‐density lipoprotein (LDL) in diabetic rats (Bishayee and Chatterjee, 1994; Kumar et al., 2013).
Different extracts and isolated bioactive compounds of Gymnema sylvestre were reported to have anti-microbial potential against several microorganisms. Methanolic extract of the leaves of this plant was reported to show antimicrobial activity against E. coli, B. cereus, C. albicans, and C. kefyr. The aqueous extract showed moderately anti-microbial activity against S. aureus, C. krusei, C. perfringens type-A and C. kefyr where the hexane extract showed activity against S. aureus, B. cereus, S. enterica, H. paragallinarum and C. perfringens type-A (David and Sudarsanam, 2013; Tahir et al., 2017). Both aqueous and ethanol extract is active against pathogenic Salmonella species (Salmonella typhi, S. Typhimurium, and S. paratyphi). Ethanolic, chloroform, and ethyl acetate extracts were reported to be active against P. vulgaris, E. coli, P. aeroginosa, K. pneumoniae, and S. aureus (Pasha et al., 2009; Paul and Jayapriya, 2009). Swami and Prabakaran (2012) observed that this plant was effective against several gram positive and negative bacteria such as S. aureus, E. Coli, K. pneumoniae and P. aeruginosa. In a study where antimicrobial activity was measured using a disk diffusion method, gymnemic acid, isolated from this plant, also showed antimicrobial activity against E. coli, V. cholera, S. mutans, S. aureus, A. niger and C. albicans with zone of inhibition of 8.65 mm, 6.00 mm, 7.12 mm, 9.25 mm, 6.43 mm and 8.60 mm, respectively (Gupta and Singh, 2014). It has anti-microbial potential against a wide range of microorganisms including E. coli, P. aeruginosa, B. subtilis, E. hirae, M. luteus, S. aureus and C. albicans (Thanwar et al., 2016; Arora and Sood, 2017; Gunasekaran et al., 2019). Antibacterial activity of gymnemic acid, a triterpene saponin, isolated from Gymnema sylvestre was also studied against E. coli and B. cereus and it was found active against the microbes (Shivanna and Raveesha, 2009). Recently, antimicrobial properties of Gymnema sylvestre leaf extract have been enhanced using poly-ε-caprolactone nanofibers (Ramalingam et al., 2019) or by using ZnO nanoparticles (Karthikeyan et al., 2019). Gymnema sylvestre with ZnO nanoparticles was found to be effective against gram positive Staphylococcus aureus and Streptococcus pneumoniae bacteria and gram negative Klebsiella pneumoniae, Shigella dysenteriae, Escherichia coli, Pseudomonas aeruginosa and Proteus vulgaris bacterial strains (Karthikeyan et al., 2019). Using poly-ε-caprolactone nanofibers with this plant was potently active against methicillin-resistant Staphylococcus aureus, Staphylococcus aureus, Pseudomonas aeruginosa, Staphylococcus epidermidis and Escherichia coli (Ramalingam et al., 2019).
Ethanol extract of this plant revealed significant (p < 0.05) 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity and showed better antioxidant potential than A. bilimbi and C. frutescens (Rahman et al., 2014). Anti-oxidant activity of Gymnema sylvestre against DPPH was also observed in an investigation by Rupanar et al. (2012). This plant was found to have better DPPH radical scavenging than butylated hydroxyl toluene (BHT) and in another study it was also found to reduce LDL oxidation (Ohmori et al., 2005; Rupanar et al., 2012). Recently, in another study, hydroxyl free radical scavenging activity and significant antioxidative potential of this plant against DPPH was reported where DPPH inhibition was at the level of 87.3% and hydroxyl free radical inhibition was 59.8% (Gunasekaran et al., 2019). It was also found to have significant radical scavenging activity against ferric (p < 0.05), super oxide (p < 0.05) and also against hydrogen peroxide (p < 0.05) (Rachh et al., 2009). Gymnema sylvestre showed antioxidant activity in several conditions such as against high fat diets, hydrogen peroxide, nitric oxide, and superoxide radically induced oxidative stress in rats (Arun et al., 2014; Kishore and Singh, 2015; Chakrapani and Periandavan, 2018).
Aqueous and petroleum extract of Gymnema sylvestre revealed significant (p < 0.01) antiarthritic activity (Malik et al., 2010). It was suggested that Gymnema sylvestre may have reduced the release of inflammatory mediators which is necessary to reduce bone destruction in anti-arthritic condition (Malik et al., 2010). In another study, ethanolic extract of the root of Gymnema sylvestre reduced carrageenan rat paw oedema significantly (p < 0.01) and inhibited 39-75% of histamine induced rat paw oedema (Shankar and Rao, 2008)
Methanolic leaf extract of Gymnema sylvestre (MLEGS) showed immunosuppressive activity in Swiss Albino mice when it was tested by performing hemagglutination antibody (HA) titer, delayed-type hypersensitivity (DTH) tests and flow cytometric techniques for the estimation of B lymphocytes (CD3 and CD19) and Th2 cytokines (IL-2, IFN-γ and IL-4). This plant significantly reduced primary and secondary antibody response and DTH response in a dose-related manner. At 200 mg/kg body weight, the maximal reductions occurred in the production of CD3, CD19, IL-2, IFN-γ and IL-4 at the level of 31.59, 32.12, 29.51, 32.45 and 33.53%, respectively (Ahirwal et al., 2015). However, it was also perceived that Gymnema sylvestre enhances the level of myeloid and lymphoid components of the immune system. Methanolic extract of this plant significantly increased (p < 0.05) the stimulation of Nitric oxide (NO) and Reactive Oxygen Species (ROS) by stimulation of macrophage activity and, also, significantly (p < 0.05) reduced nitroblue tetrazolium (Singh et al., 2015). Aqueous extract of Gymnema sylvestre also stimulated the phagocytic function of human neutrophils suggesting an immunostimulatory activity (Jitender et al., 2009). Ethanol extract of this plant was observed to improve immunosuppressed condition induced by cyclophosphamide in Albino Rats. In this study, the plant extract significantly improved haemagglutination titer, phagocytic activity and decreased paw edema (p < 0.01, p < 0.05 and p < 0.05 respectively), when compared with cyclophosphamide treated control (Kar et al., 2019). In another study, potent immunostimulatory potential of the aqueous extract of this plant was observed (Gupta et al., 2009).
Methanoic extract of Gymnema sylvestre showed anti-inflammatory activity in Wistar rats where carrageenan-induced inflammation was introduced in the rats. Methanolic extract of this plant reduced carrageenan-induced rat paw edema significantly (p < 0.05) (Kumar et al., 2012). In another study, aqueous extract of this plant displayed inhibitory potential against carrageenan-induced rat paw edema and peritoneal ascites in mice (Diwan et al., 1995). Furthermore, ethanolic extract of this plant was reported to show inhibitory effects against TPA-induced inflammation, with a 50% inhibitory dose of 50–555 nmol/ear where In vivo two-stage carcinogenesis was introduced in mice using 7,12-dimethylbenz[a]anthracene as an initiator and 12-O-tetradecanoyl phorbol-13-acetate (TPA) as a promoter (Yasukawa et al., 2014).
Methanolic extract of Gymnema sylvestre showed anti-ulcer activity in pylorus ligated Wister rat, forced swim stress-induced ulcer model as well as in rats where ulcer was induced by Indomethacin. It reduced the ulcer index significantly (p < 0.01) and also reduced free acidity, total acidity and gastric volume, and increased the pH of gastric juice. It was suggested that anti-ulcer activity was due to the presence of phytochemical constituents such as saponins, flavanoids, tannins, sterols, glycosides, alkaloids, resins, carbohydrates, proteins, triterpenoids (Swetha et al., 2012). A herbomineral formulation containing Gymnema sylvestre was found to improve impaired gastric emptying and intestinal transit associated with diabetes. In this study, Gymnema sylvestre containing formulation was found to significantly restore gastric emptying time and intestinal transit (p < 0.001 and p < 0.001 respectively), (Somani et al., 2013). However, Gymnema sylvestre was also shown to inhibit Glucose-Stimulated Gastric Inhibitory Peptide Secretion in Wister Rats significantly (p < 0.05) (Fushiki et al., 1992). Ethanolic extract of the leaves was described to improve mucosal injury induced by ethanol in Wister Albino rats. In this study it was observed that treatment of rats with this plant extract resulted in a significant depletion of stomach-wall mucus (p < 0.001), total proteins (p < 0.01), nucleic acids (p < 0.001), and non-protein sulfhydryl groups (p < 0.001) (Al-Rejaie et al., 2012). In a study, where gastric ulcer was induced in Swiss Albino male mice, aqueous extract of this plant was reported to show anti-ulcerative properties where it was observed that treatment with the plant extract exhibited significant (p < 0.05) protective activity against aspirin induced ulcer in rat models (Arun et al., 2014).
In an in-vitro investigation, hydro-alcoholic extract of Gymnema sylvestre was observed to render anti-hepatotoxic function in a dose-dependent manner in isolated rat hepatocytes where hepatotoxicity was induced using D – galactosamine. A significant increase in the level of ASAT, ALAT, ALP, total bilirubin and direct bilirubin (p < 0.001) was observed (Srividya et al., 2010). It was reported to lower urea and creatinine levels after acute and chronic administration of methanolic extract of this plant in Wister rats (Dholi and Raparla, 2014). In a study where methanolic poly herbal preparation containing this plant was used, it was observed that the preparation can reverse hepatotoxicity in Albino rats induced by paraffin and carbon tetrachloride (Yogi and Mishra, 2016).
Ethanol extract of this plant was found to reduce body weight in Wister rats (Shigematsu et al., 2001a). Similarly, when Streptozotocin-induced diabetic Albino rats were treated with ethanolic extract of Gymnema syvestre, a significant (p < 0.001) weight reduction was also observed (Fatani et al., 2015). However, in a different study, ethanol extract of this plant was reported to cause increase in the weight of the whole body, liver, pancreas in alloxan induced diabetic Wistar rats (Ahmed et al., 2010).
The methanol extract of Gymnema sylvestre disclosed antimicrobial activity against Streptococcus mutans which is responsible for the formation of dental caries (Devi and Ramasubramaniaraja, 2010). Gymnema Acid from this plant can reduce glucan as well as plaque formation by Streptococcus mutants (Porchezhian and Dobriyal, 2003).
Apart from various investigations on animal models, different extracts of this plant were tested on humans to inspect its therapeutic potential on the human body. Clinical investigations on Gymnema sylvestre revealed its potential to reduce body weight and glucose levels, triglyceride, LDL-c, total cholesterol and elevate the amount of insulin and C-peptide available in blood. A study conducted on 58 patients with type 2 diabetes mellitus for 90 days resulted in the reduction of fasting (p < 0.005) and post prandial blood glucose levels (p < 0.001) along with a reduction of triglyceride (p < 0.05) (Table 4) (Kumar et al., 2010). In another study, where 64 individuals with type 1 diabetes were treated with Gymnema leaf extract for 6 to 30 months resulted with the reduction of plasma glucose level, reduced external insulin dose and significant reduction in HbA1c (p < 0.001) (Shanmugasundaram et al., 1990b). Significant (p < 0.05) reduction in blood glucose level was observed in another study where 32 human subjects with type-2 diabetes were administered with Gymnema sylvestre leaf powder in hard gelatin capsule for 30 days. Reduction in triglyceride, cholesterol and LDL level was also observed in this study (Li et al., 2015) (Table 4). The therapeutic potential of Gymnema sylvestre (GS) observed from clinical studies so far conducted has been summarized in Table 4.
Toxicology study on Albino mice treated with Gymnema sylvestre showed LD50 level at 3990 mg/kg and the safety ratio for normal and diabetic mice was found to be 11.08 and 16.03 respectively. In this study, no behavioral, neurologic and autonomic adverse effects were observed (Chattopadhyay, 1999). Another study reported LD50 of ethanol and water extract of Gymnema sylvestre to be 375mg/kg where mice were treated by intraperitoneal route with the plant extract (Bhakuni and Dhar, 1971). One case of drug-induced liver injury (DILI) was informed in the case of a patient who was treated with Gymnema sylvestre for diabetes mellitus (Shiyovich et al., 2010). It was stated that this plant can cause hypoglycemia in both diabetic and non-diabetic patient (Khare et al., 1983) and in the case of the diabetic animal model, one study reported persistent hypoglycemic effect even after the treatment with Gymnema sylvestre was stopped (Srivastava et al., 1986). However, no toxic effects were observed in a study where male and female Wistar rats were treated with Gymnema sylvestre for 52 weeks (Ogawa et al., 2004).
Concerning the use of extract of Gymnema sylvestre as a dietary supplement in Europe, the European Food Safety Agency recognizes the property of this plant which maintains normal sugar levels in organisms and in their conditions of use it must contain 400- 800 mg of gymnema extract, equal to 100- 200 mg kgymnemic acid (EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA), 2010).
Presently, there are considerable knowledge gaps for the risk assessment of G. sylvestre preparations and open questions for whether results obtained with one preparation can be extrapolated to another Gymnema preparation. Also, based on the lack of systematic data on dose and effect relationships, the available information was regarded as not being sufficient for the derivation of health-based guidance values for Gymnema or Gymnema preparations. Considering the uncertainties for the composition of different Gymnema preparations, potential herb–drug interactions and the concerns about glucose lowering or hypoglycaemic effects, the use of Gymnema-based food supplements in combination with (or as a substitute for) authorized antidiabetic drugs may be associated with risks when used without medical supervision (Marakis et al., 2018).
Phytochemicals account for numerous pharmacological properties. They are observed to have anti-metastatic, anti-diabetic, hypoglycemic, anti-oxidant, hepatoprotective, anti-inflammation, anti-bacterial, anti-fungal, anti-viral etc. activities. Plants contain compounds such as flavonoids, alkaloids, and tannins that render these life-saving therapeutic activities. It has been reported that about 80% of people from developing countries rely on natural medicines for the treatment of diseases and their primary health concerns (Hamilton, 2004). However, despite having great demand and therapeutic uses only 10% of the plants have been investigated for their therapeutic potential (Kumar V.H, et al., 2015). Furthermore, some of these plants which could be a great source of biologically important novel phytoconstituents are on the verge of extinction due to unsustainable use, destruction of forests, and habitat destruction (Brower, 2008). One of these therapeutically important plants that contain significant biologically important phytochemicals is Gymnema sylvestre. It constitutes saponins, flavonol, glycosides, gymnemanol, gurmarin etc. These phytochemicals isolated from Gymnema sylvestre can provide pharmacological activities such as anti-diabetic, anti-oxidative, anti-metastatic, anti-inflammatory, lipid-lowering and several other properties.
However, this plant is also subject to unsustainable use. It is disappearing very fast due to overexploitation and extensive collection to meet the demand (Choudhury, 1988). Many unauthorized preparations of this plant are found in the local market. People are using this plant as a cheap substitution for their anti-diabetic medicine without any knowledge of what part of the plant to be used which results in unnecessary destruction the whole plant. Thus, this plant is being wasted without being used up to their maximum potential. In order to prevent the waste of this plant, legal production of medicinal preparation from the plant should be ensured and sustainable use of this plant should be closely monitored. In addition to these, people should be also made aware of the proper use of the plant so that they can get maximum benefit from this plant.
MMRS conceived the concept. FK and MMRS wrote the initial draft and revised the manuscript. LCM, INM, CZ and MAR critically revised the manuscript. BYS and HFT significantly contributed to review the manuscript in reply to reviewers; FK, MMRS, LCM, INM, CZ, BYS, HFT and MAR finalized the manuscript. All authors read and approved the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Agrawal, R. C., Soni, S., Jain, N., Rajpoot, J., Maheshwari, S. K. (2016). Chemopreventive effect of Gymnema sylvestre in Swiss albino mice. Int. J. Sci. Res. Publ. 6 (1), 78–83.
Ahirwal, L., Singh, S., Kumar, M. D., Bharti, V., Mehta, A., Shukla, S. (2015). In vivo immunomodulatory effects of the methanolic leaf extract of Gymnema sylvestre in Swiss albino mice. Arch. Biol. Sci. 67 (2), 561–570. doi: 10.2298/ABS141027018A
Ahmed, A. B., Rao, A., Rao, M. (2010). In vitro callus and in vivo leaf extract of Gymnema sylvestre stimulate β-cells regeneration and anti-diabetic activity in Wistar rats. Phytomedicine 17 (13), 1033–1039. doi: 10.1016/j.phymed.2010.03.019
Ahmed, S. K., Cheekavolu, C., Alasyam, N., Sunil, M. (2017). Evaluation of hypoglycemic property of gurmar (Gymnema sylvestre) leaves methanolic extract (GSLME) in streptozocin (STZ) induced Diabetic Albino Rats. J. Med. Sci. Clin. Res. 5(12), 31753–31760. doi: 10.18535/jmscr/v5i12.78
Aleisa, A. M., Al-Rejaie, S. S., Abuohashish, H. M., Ola, M. S., Parmar, M. Y., Ahmed, M. M. (2014). Pretreatment of Gymnema sylvestre revealed the protection against acetic acid- induced ulcerative colitis in rats. BMC Complement Altern. Med. 14 (49). doi: 10.1186/1472-6882-14-49
Al-Rejaie, S. S., Abuohashish, H. M., Ahmed, M. M., Aleisa, A. M., Alkhamees, O. (2012). Possible biochemical effects following inhibition of ethanol-induced gastric mucosa damage by Gymnema sylvestre in male Wistar albino rats. Pharm. Biol. 50 (12), 1542–1550. doi: 10.3109/13880209.2012.694894
Al-Romaiyan, A., Liu, B., Asare-Anane, H., Maity, C. R., Chatterjee, S. K., Koley, N., et al. (2010). A novel Gymnema sylvestre extract stimulates insulin secretion from human islets In vivo and In vitro. Phytother. Res. 24 (9), 1370–1376. doi: 10.1002/ptr.3125
Anis, M., Sharma, M. P., Iqbal, M. (2000). Herbal ethnomedicine of the Gwalior forest division in Madhya Pradesh, India. Pharm. Biol. 38 (4), 241–253. doi: 10.1076/1388-0209(200009)3841-AFT241
Aralelimath, V. R., Bhise, S. B. (2012). Anti-diabetic effects of Gymnema sylvestre extract on streptozotocin induced diabetic rats and possible b-cell protective and regenerative evaluations. Dig. J. Nanomater. Bios. 7 (1), 135–142.
Arora, D. S., Sood, H. (2017). In vitro antimicrobial potential of extracts and phytoconstituents from Gymnema sylvestre R.Br. leaves and their biosafety evaluation. AMB Express 7 (1), 115. doi: 10.1186/s13568-017-0416-z
Arun, L. B., Arunachalam, A. M., Arunachalam, K. D., Annamalai, S. K., Kumar, K. A. (2014). In vivo anti-ulcer, anti-stress, anti-allergic, and functional properties of Gymnemic Acid Isolated from Gymnema sylvestre R Br. BMC Complement Altern. Med. 14 (70). doi: 10.1186/1472-6882-14-70
Baskaran, K., Ahamath, B. K., Shanmugasundaram, K. R., Shanmugasundaram, E. R. B. (1990). Antidiabetic effect of a leaf extract from Gymnema sylvestre in non-insulin-dependent diabetes mellitus patients. J. Ethnopharmacol. 30 (3), 295–305. doi: 10.1016/0378-8741(90)90108-6
Bhakuni, D. S., Dhar, M. L. (1971). Screening of Indian plants for biological activity: Part III. Indian J. Exp. Biol. 9, 91–102.
Bishayee, A., Chatterjee, M. (1994). Hypolipidaemic and antiatherosclerotic effects of oral Gymnema sylvestre R. Br. Leaf extract in albino rats fed on a high fat diet. Phytother. Res. 8 (2), 118–120. doi: 10.1002/ptr.2650080216
Brower, V. (2008). Back to Nature: Extinction of Medicinal Plants Threatens Drug Discovery. J. Natl. Cancer. Inst. 100 (12), 838–839. doi: 10.1093/jnci/djn199
Chakraborty, D., Ghosh, S., Bishayee, K., Mukherjee, A., Sikdar, S., Khuda-Bukhsh, A. R. (2013). Antihyperglycemic Drug Gymnema sylvestre Also Shows Anticancer Potentials in Human Melanoma A375 Cells via Reactive Oxygen Species Generation and Mitochondria-Dependent Caspase Pathway. Integr. Cancer. Ther. 12 (5), 433–441. doi: 10.1177/1534735413485419
Chakrapani, L. N., Periandavan, K. (2018). Protective role of gymnemic acid in curbing high fat diet and high fructose induced pancreatic oxidative stress mediated type-2 diabetes in wistar rats. Int. J. Pharm. Sci. Res. 9 (5), 2130–2139. doi: 10.13040/IJPSR.0975-8232.9(5).2130-39
Chattopadhyay, R. (1999). A comparative evaluation of some blood sugar lowering agents of plant origin. J. Ethnopharmacol. 67 (3), 367–372. doi: 10.1016/S0378-8741(99)00095-1
Chen, G., Guo, M. (2017). Rapid Screening for α-Glucosidase Inhibitors from Gymnema sylvestre by Affinity Ultrafiltration–HPLC-MS. Front. Pharmacol. 8, 228. doi: 10.3389/fphar.2017.00228
Chen, Y., Liu, Y., Sarker, M. M. R., Yan, X., Yang, C., Zhao, L., et al. (2018). Structural characterization and antidiabetic potential of a novel heteropolysaccharide from Grifola frondosa via IRS1/PI3K-JNK signaling pathways. Carbohyd. Polym. 198, 452–461. doi: 10.1016/j.carbpol.2018.06.077
Chodisetti, B., Rao, K., Giri, A. (2013). Phytochemical analysis of Gymnema sylvestre and evaluation of its antimicrobial activity. Nat. Prod. Res. 27 (6), 583–587. doi: 10.1080/14786419.2012.676548
Chopra, R. N., Nayar, S. L., Chopra, I. C. (2002). Glossary of Indian medicinal plants. National Institute of Science Communication and Information Resources, New Delhi, India.
Choudhury, B. P. (1988). “Assessment and conservation of medicinal plants of Bhubaneswar and its neighbourhood,” in Proc. Indigenous medicinal plants Symp (New Delhi: Today & Tomorrow’s Printers & Publishers), 211–219.
Christopoulos, M. V., Rouskas, D., Tsantili, E., Bebeli, P. J. (2010). Germplasm diversity and genetic relationships among walnut (Juglans regia L.) cultivars and Greek local selections revealed by Inter-Simple Sequence Repeat (ISSR) markers. Sci. Hort. 125 (4), 584–592. doi: 10.1016/j.scienta.2010.05.006
Daisy, P., Eliza, J., Farook, K. A. (2009). A novel dihydroxy gymnemic triacetate isolated from Gymnema sylvestre possessing normoglycemic and hypolipidemic activity on STZ-induced diabetic rats. J. Ethnopharmacol. 126 (2), 339–344. doi: 10.1016/j.jep.2009.08.018
David, B. C., Sudarsanam, G. (2013). Antimicrobial activity of Gymnema sylvestre (Asclepiadaceae). J. Acute. Dis. 2 (3), 222–225. doi: 10.1016/S2221-6189(13)60131-6
Devi, B. P., Ramasubramaniaraja, R. (2010). Pharmacognostical and antimicrobial screening of Gymnema sylvestre R.Br, and evaluation of Gurmar herbal tooth paste and powder, composed of Gymnema sylvestre R.Br, extracts in dental caries. Int. J. Pharma. Bio. Sci. 1, 1–16.
Dholi, S. K., Raparla, R. K. (2014). In vivo anti-diabetic evaluation of gymnemic acid in streptozotocin induced rats. J. Pharm. Innov. 3 (7), 82–86.
Diwan, P. V., Margaret, I., Ramakrishna, S. (1995). Influence of Gymnema sylvestre on inflammation. Inflammopharmacol. 3 (3), 271–277. doi: 10.1007/BF02659124
EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). (2010). Scientific Opinion on the substantiation of health claims related to various food(s)/food constituent(s) claiming maintenance of normal blood glucose concentrations. EFSA Journal 8 (2), 149. doi: 10.2903/j.efsa.2010.1490
Elumalai, K., Dhanasekaran, S., Krishnappa, K. (2013). Larvicidal activity of Saponin isolated from Gymnema sylvestre R. Br. (Asclepiadaceae) against Japanese Encephalitis vector, Culex tritaeniorhynchus Giles (Diptera: Culicidae). Eur. Rev. Med. Pharmacol. Sci. 17 (10), 1404–1410.
Fatani, A. J., Al-Rejaie, S. S., Abuohashish, H. M., Al-Assaf, A., Parmar, M. Y., Ola, M. S., et al. (2015). Neuroprotective effects of Gymnema sylvestre on streptozotocin-induced diabetic neuropathy in rats. Exp. Ther. Med. 9 (5), 1670–1678. doi: 10.3892/etm.2015.2305
Fushiki, T., Kojima, A., Imoto, T., Inoue, K., Sugimoto, E. (1992). An Extract of Gymnema sylvestre Leaves and Purified Gymnemic Acid Inhibits Glucose-Stimulated Gastric Inhibitory Peptide Secretion in Rats. J. Nutr.122 (12), 2367–2373. doi: 10.1093/jn/122.12.2367
Galletto, R., Siqueira, V. L., Ferreira, E. B., Oliveira, A. J., Bazotte, R. B. (2004). Absence of antidiabetic and hypolipidemic effect of Gymnema sylvestre in non-diabetic and alloxan-diabetic rats. Braz. Arch. Biol. Technol. 47 (4), 545–551. doi: 10.1590/S1516-89132004000400007
Garai, S. (2014). Triterpenoid saponins. Nat. Prod. Chem. Res. 2 (6), 1000148. doi: 10.4172/2329-6836.1000148
Goto, T., Sarker, M. M. R., Zhong, M., Tanaka, S., Gohda, E. (2010). Enhancement of immunoglobulin M production in B cells by the extract of red bell pepper. J. Health Sci. 56 (3), 304–309. doi: 10.1248/jhs.56.304
Gunasekaran, V., Srinivasan, S., Rani, S. S. (2019). Potential antioxidant and antimicrobial activity of Gymnema sylvestre related to diabetes. J. Med. Plants. 7 (2), 05–11.
Gupta, P., Singh, P. (2014). Antimicrobial activity of Gymnemic acid on pathogens- Gymnema sylvestre R.Br. Int. J. Curr. Microbiol. App. Sc. 3 (5), 40–45.
Gupta, S. P., Pramanik, S., Tiwari, O., Thacker, N., Pande, M., Upmanyu, N. (2009). Immunomodulatory Activity of Gymnema sylvestre Leaves. Internet J. Pharmacol. 8 (2). Available online: http://ispub.com/IJPHARM/8/2/8383. doi: 10.5580/14ed
Gurnani, N., Mehta, D., Gupta, M., Mehta, B. K. (2014). Natural products: Source of potential drugs. Afr. J. Basic. Appl. Sci. 6, 171–186. doi: 10.5829/idosi.ajbas.2014.6.6.21983
Hamilton, A. C. (2004). Medicinal plants, conservation and livelihoods. Biodivers. Conserv. 13 (8), 1477–1517. doi: 10.1023/B:BIOC.0000021333.23413.42
Imai, Y., Tsukahara, S., Asada, S., Sugimoto, Y. (2004). Phytoestrogens/flavonoids reverse breast cancer resistance protein/ABCG2-mediated multidrug resistance. Cancer Res. 64 (12), 4346–4352. doi: 10.1158/0008-5472.CAN-04-0078
Imam, H., Mahbub, N. U., Khan, M. F., Hana, H. K., Sarker, M. M. R. (2013). Alpha Amylase Enzyme Inhibitory and Anti-inflammatory Effect of Lawsonia inermis. Pak. J. Biol. Sci. 16 (23), 1796–1800. doi: 10.3923/pjbs.2013.1796.1800
Imoto, T., Miyasaka, A., Ishima, R., Akasaka, K. (1991). A novel peptide isolated from the leaves of Gymnema sylvestre—I. Characterization and its suppressive effect on the neural responses to sweet taste stimuli in the rat. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 100 (2), 309–314. doi: 10.1016/0300-9629(91)90475-R
J-GLOBAL-Japan Science and Technology Agency. (n.d.a). Gymnemasin A, J-GLOBAL ID: 200907028621377193,Nikkaji number: J708.815I. Retrieved from https://jglobal.jst.go.jp/en/detail?JGLOBAL_ID=200907028621377193&q=GymnemasinA&t=0 (accessed 3rd August 2019).
J-GLOBAL-Japan Science and Technology Agency. (n.d.b). Gymnemasin B, J-GLOBAL ID: 200907070830782735,Nikkaji number:J708.816G. Retrieved from https://jglobal.jst.go.jp/en/detail?JGLOBAL_ID=200907070830782735&rel=1#%7B%22category%22%3A%220%22%2C%22keyword%22%3A%22Gymnemasin%20%22%7D (accessed 3rd August 2019).
J-GLOBAL-Japan Science and Technology Agency. (n.d.c). Gymnemasin C, J-GLOBAL ID: 200907030904250402,Nikkaji number: J708.817E. Retrieved from https://jglobal.jst.go.jp/en/detail?JGLOBAL_ID=200907030904250402&rel=1#%7B%22category%22%3A%220%22%2C%22keyword%22%3A%22Gymnemasin%20%22%7D (accessed 3rd August 2019).
J-GLOBAL-Japan Science and Technology Agency. (n.d.d). Gymnemasin D, -GLOBAL ID: 200907058480629513,Nikkaji number: J708.818C. Retrieved from https://jglobal.jst.go.jp/en/detail?JGLOBAL_ID=200907058480629513&rel=1#%7B%22category%22%3A%220%22%2C%22keyword%22%3A%22Gymnemasin%20%22%7D (accessed 3rd August 2019).
Jitender, K. M., Manvi, F. V., Nanjwade, B. K., Alagawadi, K. R., Sanjiv, S. (2009). Immuno-modulatory activity of Gymnema sylvestre leaves extract on In vitro human neutrophils. J. Pharmacy Res. 2 (8), 1284–1286.
Kanetkar, P., Singhal, R., Kamat, M. (2007). Gymnema sylvestre: A Memoir. J. Clin. Biochem. Nutr. 41 (2), 77–81. doi: 10.3164/jcbn.2007010
Kang, J. S., Koh, H. C., Suh, T. K. (1990). Effect of gymnemic acid on the elevation of blood glucose concentration induced with oral sucrose in streptozotocin-diabetic rats. Hanyang Uidae Haksulchi 10 (1), 587–601.
Kang, M., Lee, M. S., Choi, M., Min, K., Shibamoto, T. (2012). Hypoglycemic Activity of Gymnema sylvestre extracts on oxidative stress and antioxidant status in diabetic rats. J. Agric. Food. Chem. 60 (10), 2517–2524. doi: 10.1021/jf205086b
Kapoor, L. D. (2017). CRC Handbook of Ayurvedic Medicinal Plants. S.l. (Boca Raton, Florida, USA: CRC Press). doi: 10.1201/9780203719473
Kar, P. P., Rath, B., Ramani, Y. R., Maharana, C. S. (2019). Amelioration of Cyclophosphamide induced immunosupression by the hydro-alcoholic extract of Gymnema sylvestre leaves in albino rats. Biomed. Pharmacol. J. 11 (1), 251–258. doi: 10.13005/bpj/1635
Karthikeyan, M., Ahamed, A. J., Karthikeyan, C., Kumar, P. V. (2019). Enhancement of antibacterial and anticancer properties of pure and REM doped ZnO nanoparticles synthesized using Gymnema sylvestre leaves extract. SN Appl. Sci. 1 (4), 355. doi: 10.1007/s42452-019-0375-x
Kashima, H., Eguchi, K., Miyamoto, K., Fujimoto, M., Endo, M. Y., Aso-Someya, N., et al. (2017). Suppression of Oral Sweet Taste Sensation with Gymnema sylvestre Affects Postprandial Gastrointestinal Blood Flow and Gastric Emptying in Humans. Chem. Senses 42 (4), 295–302. doi: 10.1093/chemse/bjw126
Kaur, N., Chaudhary, J., Jain, A., Kishore, L. (2011). Stigmasterol: a comprehensive review. Int. J. Pharm. Sci. Res. 2 (9), 2259–2265.
Kazemipoor, M., Cordell, G. A., Sarker, M. M. R., Radzi, C. W. J. B. W. M., Hajifaraji, M., En Kiat, P. (2015). Alternative treatments for weight loss: Safety/risks and effectiveness of anti-obesity medicinal plants. Int. J. Food Prop. 18 (9), 1942–1963. doi: 10.1080/10942912.2014.933350
Khanna, G. (2010). Non-proliferative activity of saponins isolated from the leaves of Gymnema sylvestre and Eclipta prostrata on HepG2 cells-In vitro study. Int. J. Pharm. Sci. Res. 1 (8), 38–42. doi: 10.13040/IJPSR.0975-8232
Khare, A. K., Tondon, R. N., Tewari, J. P. (1983). Hypoglycaemic activity of an indigenous drug (Gymnema sylvestre, “Gurmar”) in normal and diabetic persons. Indian J. Physiol. Pharm. 27, 257–258.
Khramov, V. A., Spasov, A. A., Samokhina, M. P. (2008). Chemical composition of dry extracts of Gymnema sylvestre leaves. Pharm. Chem. J. 42 (1), 30–32. doi: 10.1007/s11094-008-0051-8
Kirtikar, K. R., Basu, B. D. (1975). “Indian medicinal plants, vol. III,” in Periodical Experts D-42 (Vivek Vihar Delhi).
Kirtikar, K. R., Basu, B. D. (1987). Indian Medicinal Plants. Lalit Mohan Basu, Allahabad. Jayyd Press, New Delhi, India, 2, 146.
Kishore, L., Singh, R. (2015). Protective effect of Gymnema sylvestre L. against advanced glycation end-product, sorbitol accumulation and aldose reductase activity in Homoeopathic Formulation. Indian J. Res. Homoeopathy 9 (4), 240–248. doi: 10.4103/0974-7168.172866
Kosta, S., Tiwari, A. (2009). Screening and assessment of anti-diabetic and reactive oxygen scavenging (ros), effects of herbs in streptozotacin induced mice. Pharmacol. online 3, 695–704.
Kothe, A., Uppal, R. (1997). Antidiabetic effects of Gymnema sylvestre in NIDDM—a short study. Indian J. Homeopath. Med. 32, 61–62.
Kumar, A. R., Rathinam, K. S., Kumar, C. A. (2012). Evaluation of Anti-inflammatory Activity of some selected species of Asclepiadaceae Family. Int. J. Chem. Sci. 10 (1), 548–556.
Kumar, P., Rani, S., Arunjyothi, B., Chakrapani, P., Rojarani, A. (2017). Evaluation of Antidiabetic Activity of Gymnema sylvestre and Andrographis paniculata in Streptozotocin Induced Diabetic Rats. Int. J. Pharmacogn. Phytoch. Res. 9 (1), 22–25. doi: 10.25258/ijpapr.v9i1.8034
Kumar, P., Venkataranganna, M., Manjunath, K., Viswanatha, G., Ashok, G. (2016). Methanolic leaf extract of Gymnema sylvestre augments glucose uptake and ameliorates insulin resistance by upregulating GLUT-4, PPAR- and adiponectin and leptin levels In vitro. J. Intercult. Ethnopharmacol. 5 (2), 146–152. doi: 10.5455/jice.20160224051727
Kumar, S. N., Mani, U. V., Mani, I. (2010). An Open Label Study on the Supplementation of Gymnema sylvestre in Type 2 Diabetics. J. Die. Suppl. 7 (3), 273–282. doi: 10.3109/19390211.2010.505901
Kumar, S., Paul, S., Walia, Y., Kumar, A., Singhal, P. (2015). Therapeutic Potential of Medicinal Plants: A Review. J. Biol. Chem. Chron. 1, 46–54.
Kumar, V. H., Nayak, I. N., Huilgol, S. V., Yendigeri, S. M., Narendar, K. (2015). Antidiabetic and hypolipidemic activity of Gymnema sylvestre in dexamethasone induced insulin resistance in Albino rats. Int. J. Med. Res. Health Sci. 4 (3), 639–645. doi: 10.5958/2319-5886.2015.00122.8
Kumar, V., Bhandari, U., Tripathi, C., Khanna, G. (2013). Anti-obesity Effect of Gymnema sylvestre Extract on High Fat Diet-induced Obesity in Wistar Rats. Drug Res. 63 (12), 625–632. doi: 10.1055/s-0033-1349852
Li, Y., Zheng, M., Zhai, X., Huang, Y., Khalid, A., Malik, A., et al. (2015). Effect of Gymnema sylvestre, Citrullus colocynthis and Artemisia absinthium on blood glucose and lipid profile in diabetic human. Acta Pol. Pharm. 72, 981–985.
Liu, B., Asare-Anane, H., Al-Romaiyan, A., Huang, G., Amiel, S. A., Jones, P. M., et al. (2009). Characterisation of the Insulinotropic Activity of an Aqueous Extract of Gymnema sylvestre in Mouse β-Cells and Human Islets of Langerhans. Cell. Physiol. Biochem. 23 (1–3), 125–132. doi: 10.1159/000204101
Liu, H., Kiuchi, F., Tsuda, Y. (1992). Isolation and Structure Elucidation of Gymnemic Acids, Antisweet Principles of Gymnema sylvestre. Chem. Pharm. Bull. 40 (6), 1366–1375. doi: 10.1248/cpb.40.1366
Liu, X., Ye, W., Yu, B., Zhao, S., Wu, H., Che, C. (2004). Two new flavonol glycosides from Gymnema sylvestre and Euphorbia ebracteolata. Carbohydr. Res. 339 (4), 891–895. doi: 10.1016/j.carres.2003.12.017
Liu, Y., Xu, T.-H., Zhang, M.-Q., Li, X., Xu, Y.-J., Jiang, H.-Y., et al. (2014). Chemical constituents from the stems of Gymnema sylvestre. Chin. J. Nat. Med. 12 (4), 300–304. doi: 10.1016/S1875-5364(14)60059-5
Luo, H., Kashiwagi, A., Shibahara, T., Yamada, K. (2006). Decreased bodyweight without rebound and regulated lipoprotein metabolism by gymnemate in genetic multifactor syndrome animal. Mol. Cell Biochem. 299 (1–2), 93–98. doi: 10.1007/s11010-005-9049-7
Malik, J. K., Manvi, F. V., Nanjware, B. R., Dwivedi, D. K., Purohit, P., Chouhan, S. (2010). Anti-arthritic activity of leaves of Gymnema sylvestre R.Br. leaves in rats. Pharm. Lett. 2, 336–341.
Mall, G. K., Mishra, P. K., Prakash, V. (2009). Antidiabetic and hypolipidemic activity of Gymnema sylvestre in alloxan induced diabetic rats. Glob. J. Biotech. Biochem. 4 (1), 37–42.
Mao, Q. (2005). Role of the breast cancer resistance protein (ABCG2) in drug transport. AAPS J. 7 (1), E118–E133. doi: 10.1208/aapsj070112
Marakis, G., Ziegenhagen, R., Lampen, A., Hirsch-Ernst, K. I. (2018). Risk assessment of substances used in food supplements: the example of the botanical Gymnema sylvestre. EFSA Journal 16 (51), e16083. doi: 10.2903/j.efsa.2018.e16083
Mathew, M. (2004). Aromatic and Medicinal Plants Research Station, Odakkali-A Centre for Promoting Medicinal and Aromatic Plants. Indian Coconut J. Cochin 34 (10), 10–15.
Matsuda, H., Murakami, T., Ninomiya, K., Inadzuki, M., Yoshikawa, M. (1997). New hepatoprotective saponins, bupleurosides III, VI, IX, and XIII, from Chinese Bupleuri Radix: Structure-requirements for the cytoprotective activity in primary cultured rat hepatocytes. Bioorg. Med. Chem. Lett. 7 (17), 2193–2198. doi: 10.1016/S0960-894X(97)00418-6
Mcburney, D. H., Gent, J. F. (1978). Taste of methyl-α-D-mannopyranoside: Effects of cross adaptation and Gymnema sylvestre. Chem. Senses 3 (1), 45–50. doi: 10.1093/chemse/3.1.45
Nadkarni, K. M. editor. (1996). “Indian materia medica,” in Dr. KM Nadkarni’s Indian materia medica: with Ayurvedic, Unani-Tibbi, Siddha, allopathic, homeopathic, naturopathic & home remedies, appendices & indexes, vol. 1 (Popular Prakashan).
Nadkarni, K. M. (1986). “Gymnema sylvestre,” in Indian Materia Medica with Ayurvedic Unani, vol.I (Bombay, India: Popular Prakashan), 596–599.
Najafi, S., Deokule, S. S. (2011). Studies on Gymnema sylvestre-a medicinally important plant of the family Asclepiadaceae. Trakia. J. Sci. 9 (2), 26–32.
Nakamura, Y., Tsumura, Y., Tonogai, Y., Shibata, T. (1999). Fecal Steroid Excretion is Increased in Rats by Oral Administration of Gymnemic Acids Contained in Gymnema sylvestre Leaves. J. Nutr.129 (6), 1214–1222. doi: 10.1093/jn/129.6.1214
Ogawa, Y., Sekita, K., Umemura, T., Saito, M., Ono, A., Kawasaki, Y., et al. (2004). Gymnema sylvestre Leaf Extract: A 52-Week Dietary Toxicity Study in Wistar Rats. J. Food Hyg. Soc. JPN. 45 (1), 8–18. doi: 10.3358/shokueishi.45.8
Ohmori, R., Iwamoto, T., Tago, M., Takeo, T., Unno, T., Itakura, H., et al. (2005). Antioxidant activity of various teas against free radicals and LDL oxidation. Lipids 40 (8), 849–853. doi: 10.1007/s11745-005-1447-4
Pasha, C., Sayeed, S., Ali, M. S., Khan, M. Z. (2009). Antisalmonella Activity of Selected Medicinal Plants. Turkish J. Biol. 33, 59–64. doi: 10.3906/biy-0804-3
Patel, M. R. (2017). Pharmacognostic and Phytochemical Evaluation of Gymnema sylvestre Leaf. World J. Pharm. Pharm. Sci. 6 (7), 1532–1538. doi: 10.20959/wjpps20177-9574
Paul, J., Jayapriya, K. P. (2009). Screening of antibacterial effects of Gymnema sylvestre (L.) R.Br. - A medicinal plant. Pharmacol. online 3, 832–836.
Porchezhian, E., Dobriyal, R. M. (2003). An overview on the advances of Gymnema sylvestre: chemistry, pharmacology and patents. Pharmazie 58 (1), 5–12. doi: 10.1002/chin.200319223
Potawale, S. E., Shinde, V. M., Libi, A., Boradem, S., Dhalawatm, H., Deshmukh, R. S. (2008). Gymnema sylvestre: a comprehensive review. Pharmacol. online 2, 144–157.
Pramanick, D. D. (2016). “Anatomical studies on the leaf of Gymnema sylvestre (Retz.) R. Br. ex Schult. (Apocynaceae),” in A magical herbal medicine for diabetes. Int. J. Herb. Med. 4 (1), 70–72.
Preuss, H. G., Garis, R. I., Bramble, J. D., Bagchi, D., Bagchi, M., Rao, C. V., Satyanarayana, S. (2005). Efficacy of a novel calcium/potassium salt of (-)-hydroxycitric acid in weight control. Int. J. Clin. Pharmacol. Res. 25 (3), 133–144.
Rachh, P. R., Patel, S. R., Hirpara, H. V., Rupareliya, M. T., Rachh, M. R., Bhargava, A. S., et al. (2009). In-vitro evaluation of antioxidant activity of Gymnema sylvestre R.Br. leaf extract. Rom. J. Biol. Plant Biol. 54 (2), 141–148.
Rachh, P., Rachh, M., Ghadiya, N., Modi, D., Modi, K., Patel, N., et al. (2010). Antihyperlipidemic Activity of Gymenma sylvestre R. Br. Leaf Extract on Rats Fed with High Cholesterol Diet. Int. J. Pharmacol. 6 (2), 138–141. doi: 10.3923/ijp.2010.138.141
Rahman, M. M., Habib, M. R., Hasan, M. A., Saha, A., Mannan, A. (2014). Comparative assessment on In vitro antioxidant activities of ethanol extracts of Averrhoa bilimbi, Gymnema sylvestre and Capsicum frutescens. Pharmacog. Res. 6, 36–41. doi: 10.4103/0974-8490.122915
Ramalingam, R., Dhand, C., Leung, C. M., Ong, S. T., Annamalai, S. K., Kamruddin, M., et al. (2019). Antimicrobial properties and biocompatibility of electrospun poly-ε-caprolactone fibrous mats containing Gymnema sylvestre leaf extract. Mat. Sci. Eng. 98, 503–514. doi: 10.1016/j.msec.2018.12.135
Rouhi, S. Z. T., Sarker, M. M. R., Rahmat, A., Alkahtani, S. A., Othman, F. (2017). The effect of pomegranate fresh juice versus pomegranate seed powder on metabolic indices, lipid profile, inflammatory biomarkers, and the histopathology of pancreatic islets of Langerhans in streptozotocin-nicotinamide induced type 2 diabetic Sprague–Dawley rats. BMC Complement. Altern. Med. 17 (1), 156. doi: 10.1186/s12906-017-1724-1
Rupanar, S. V., Pingale, S. S., Dandge, C. N., Kshirsagar, D. (2012). Phytochemical screening and In vitro evaluation of antioxidant antimicrobial activity of Gymnema sylvestre. Int. J. curr. Res. 8 (12), 43480–43486. doi: 10.7897/2230-8407.080327
Sahu, N., Mahato, S. B., Sarkar, S. K., Poddar, G. (1996). Triterpenoid saponis from Gymnema sylvestre. Phytochem. 41, 1181–1185. doi: 10.1016/0031-9422(95)00782-2
Sarker, M. M. R. (2015). Antihyperglycemic, insulin-sensitivity and anti-hyperlipidemic potential of Ganoderma lucidum, a dietary mushroom, on zalloxan-and glucocorticoid-induced diabetic Long-Evans rats. Funct. Food Health Dis. 5 (12), 450–466. doi: 10.31989/ffhd.v5i12.220
Sarker, M. M. R., Mazumder, M. E. H., Rashid, M. H. O. (2011). In vitro enhancement of polyclonal IgM production by ethanolic extract of Nigella sativa L. seeds in whole spleen cells of female BALB/c mice. Bangladesh Pharm. J. 14 (1), 73–77.
Sarker, M. M. R., Nahar, S., Shahriar, M., Seraj, S., Choudhuri, M. S. K. (2012a). Preliminary study of the immunostimulating activity of an ayurvedic preparation, Kanakasava, on the splenic cells of BALB/c mice in vitro. Pharm. Biol. 50 (11), 1467–1472. doi: 10.3109/13880209.2012.681329
Sarker, M. M. R., Nimmi, I., Kawsar, M. H. (2012b). Preliminary screening of six popular fruits of Bangladesh for in vitro IgM production and proliferation of splenocytes. Bangladesh Pharm. J. 15 (1), 31–37.
Sarker, M. M. R., Gohda, E. (2013). Promotion of anti-keyhole limpet hemocyanin IgM and IgG antibody productions in vitro by red bell pepper extract. J. Funct. Foods 5 (4), 1918–1926.
Sathya, S., Kokilavani, R., Gurusamy, K. (2008). Hypoglycemic effect of Gymnema sylvestre (retz.), R. Br leaf in normal and alloxan induced diabetic rats. Anc. Sci. Life 28 (2), 12–14.
Senthilkumar, M. (2015). Phytochemical Screening and Antibacterial Activity of Gymnema sylvestre R.Br. Ex Schult. Int. J. Pharm. Sci. Res. 6 (6), 2496–2503. doi: 10.13040/IJPSR.0975-8232.6(6).2496-03
Shafey, A. A., El-Ezabi, M. M., Seliem, M. M., Ouda, H. H., Ibrahim, D. S. (2013). Effect of Gymnema sylvestre R. Br. leaves extract on certain physiological parameters of diabetic rats. J. King Saud. Univ. Sci. 25 (2), 135–141. doi: 10.1016/j.jksus.2012.11.001
Shah, K. K., Shiradkar, M. R., Bindu, V. H. (2012). In vitro permeation of aceclofenac through the shed skin of two different species. Der. Pharmacia Sinic. 3, 11–19.
Shah, K. K., Shiradkar, M. R., Hima Bindu, V. (2011). Transdermal delivery of aceclofenac: Effect of Gymnema sylvestre and Caralluma adscendens with its mechanism of action. Res. J. Pharm. Biol. Chem. Sci. 2, 762–772.
Shah, M. A., Sarker, M. M. R., Gousuddin, M. (2016). Antidiabetic potential of Brassica Oleracea Var. Italica in type 2 diabetic sprague dawley (sd) rats. Int. J. Pharmacogn. Phytochem. Res. 8 (3), 462–469.
Shankar, K. R., Rao, B. G. (2008). Anti-arthritic activity of Gymnema sylvestre root extract. Biosci. Biotechnol. Res. Asia 5 (1), 469–471.
Shanmugasundaram, E. R. B., Venkatasubrahmanyam, M., Vijendran, N., Shanmugasundaram, K. R. (1988). Effect of an Isolate from Gymnema sylvestre, R. Br. in The Control of Diabetes Mellitus and The Associated Pathological Changes. Anc. Sci. Life 7 (3–4), 183–194.
Shanmugasundaram, E., Gopinath, K., Shanmugasundaram, K., Rajendran, V. (1990a). Possible regeneration of the islets of langerhans in streptozotocin-diabetic rats given Gymnema sylvestre leaf extracts. J. Ethnopharmacol. 30 (3), 265–279. doi: 10.1016/0378-8741(90)90106-4
Shanmugasundaram, E., Rajeswari, G., Baskaran, K., Kumar, B., Shanmugasundaram, K., Ahmath, B. (1990b). Use of Gymnema sylvestre leaf extract in the control of blood glucose in insulin-dependent diabetes mellitus. J. Ethnopharmacol. 30 (3), 281–294. doi: 10.1016/0378-8741(90)90107-5
Sheikh, B. Y., Sarker, M. M. R., Kamarudin, M. N. A., Ismail, A. (2017a). Prophetic medicine as potential functional food elements in the intervention of cancer: A review. Biomed. Pharmacother. 95, 614–648. doi: 10.1016/j.biopha.2017.08.043
Sheikh, B. Y., Sarker, M. M. R., Kamarudin, M. N. A., Mohan, G. (2017b). Antiproliferative and apoptosis inducing effects of citral via p53 and ROS-induced mitochondrial-mediated apoptosis in human colorectal HCT116 and HT29 cell lines. Biomed. Pharmacother. 96, 834–846. doi: 10.1016/j.biopha.2017.10.038
Shenoy, R. S., Prashanth, K. V., Manonmani, H. K. (2018). In vitro antidiabetic effects of isolated triterpene glycoside fraction from Gymnema sylvestre. J. Evid. Based Complement. Altern. Med. 2018, 1–12. doi: 10.1155/2018/7154702
Shigematsu, N., Asano, R., Shimosaka, M., Okazaki, M. (2001a). Effect of administration with the extract of Gymnema sylvestre R. Br leaves on lipid metabolism in rats. Biol. Pharm. Bull. 24 (6), 713–717. doi: 10.1248/bpb.24.713
Shigematsu, N., Asano, R., Shimosaka, M., Okazaki, M. (2001b). Effect of long term-administration with Gymnema sylvestre R. BR on Plasma and Liver Lipid in Rats. Biol. Pharm. Bull. 24 (6), 643–649. doi: 10.1248/bpb.24.643
Shimizu, K., Iino, A., Nakajima, J., Tanaka, K., Nakajyo, S., Urakawa, N., et al. (1997). Suppression of glucose absorption by some fractions extracted from Gymnema sylvestre Leaves. J. Vet. Med. Sci. 59 (4), 245–251. doi: 10.1292/jvms.59.245
Shivanna, Y., Raveesha, K. A. (2009). In01-vitro antibacterial effect of selected medicinal plant extracts. J. Nat. Prod. (India) 2, 64–69.
Shiyovich, A., Nesher, L., Sztarkier, I. (2010). Toxic hepatitis induced by Gymnema sylvestre, a natural remedy for type 2 diabetes mellitus. Am. J. Med. Sci. 340 (6), 514–517. doi: 10.1097/MAJ.0b013e3181f41168
Singh, D. K., Kumar, N., Sachan, A., Lakhani, P., Tutu, S., Nath, R., et al. (2017). Hypolipidaemic effects of Gymnema sylvestre on high fat diet induced dyslipidaemia in wistar rats. J. Clin. Diagn. Res. 11 (5), FF01–FF05. doi: 10.7860/JCDR/2017/27430.9859
Singh, V. K., Dwivedi, P., Chaudhary, B. R., Singh, R. (2015). Immunomodulatory effect of Gymnema sylvestre (R.Br.) Leaf Extract: an in vitro study in rat model. PLoS One 10 (10), 1–15. doi: 10.1371/journal.pone.0139631
Singh, V. K., Umar, S., Ansari, S. A., Iqbal, M. (2008). Gymnema sylvestre for diabetics. Journal of Herbs. J. Herbs Spices Med. Plants 14 (1–2), 88–106. doi: 10.1080/10496470802341508
Sinsheimer, J. E., Rao, G. S., Mcilhenny, H. M. (1970). Constituents from Gymnema sylvestre leaves V: isolation and preliminary characterization of the gymnemic acids. J. Pharm. Sci. 59 (5), 622–628. doi: 10.1002/jps.2600590510
Somani, R., Deshmukh, P., Shah, P., Soni, R., Jain, D., Khaserao, S. (2013). Prokinetic effect of hyponidd, a herbomineral formulation in STZ- induced diabetic rats. Pharmacologia 4 (1), 48–52. doi: 10.5567/pharmacologia.2013.48.52
Srikanth, A. V., Sayeeda, M., Lakshmi, N., Ravi, M., Kumar, P., Madhava, R. B. (2010). Anticancer activity of Gymnema sylvestre R.Br. Int. J. Pharm. Sci. Nanotech. 3, 897–899.
Srivastava, Y., Venkatakrishna-Bhatt, H., Jhala, C. I., Nigam, S. K., Kumar, A., Verma, Y., et al. (1986). Oral Gymnema sylvestre R.Br. Leaf Extracts Inducing Protracted Longevity and Hypoglycemia in Alloxan Diabetic Rats: Review and Experimental Study. Int. J. Crude Drug Res. 24 (4), 171–176. doi: 10.3109/13880208609060895
Srividya, A. R., Varma, S., Dhanapal, S. P., Vadivelan, R., Vijayan, P. (2010). In vitro and in vivo evaluation of hepatoprotective activity of Gymnema sylvestre. Int. J. Pharm. Sci. Nanotechnol. 2, 768–773.
Sugihara, Y., Nojima, H., Matsuda, H., Murakami, T., Yoshikawa, M., Kimura, I. (2000). Antihyperglycemic effects of gymnemic acid IV, a compound derived from Gymnema sylvestre leaves in streptozotocin-diabetic mice. J. Asian Nat. Prod. Res. 2 (4), 321–327. doi: 10.1080/10286020008041372
Sujin, R. M. (2008). Anti-diabetic effect of Gymnema sylvestre (asclepiadaceae) powder in the stomach of rats. Ethnobot. Leaflets 12, 1158–1167.
Swami, J., Prabakaran (2012). Studies on antibacterial activity of Gymnema sylvestre against respiratory infection causing bacteria. Int. J. Curr. Adv. Res. 1 (1), 1–4.
Swetha, Y., Sunanda, Rajanikar, Reddy, A. K., Srivally, Masoom, M. (2012). Phytochemical and pharmacological studies of methanolic extract of Gymnema sylvestre leaves: an approach for in vivo antiulcer activity. Res. J. Pharmacol. Pharmacodyn. 4 (6), 368–372.
Tahir, M., Rasheed, M. A., Niaz, Q., Ashraf, M., Anjum, A. A., Ahmed, M. U. (2017). Evaluation of antibacterial effect of Gymnema sylvestre R.Br. species cultivated in Pakistan. Pak. Vet. J. 37 (3), 245–250.
Tamaki, H., Satoh, H., Hori, S., Ohtani, H., Sawada, Y. (2010). Inhibitory effects of herbal extracts on breast cancer resistance protein (BCRP) and structure-Inhibitory Potency Relationship of Isoflavonoids. Drug Metab. Pharmacok. 25 (2), 170–179. doi: 10.2133/dmpk.25.170
Thanwar, M., Dwivedi, D., Gharia, D., Chouhan, S. (2016). Antibacterial study of Gymnema sylvestre plant. Int. J. Chem. 4 (3), 80–83.
The Plant List. (2013). Version 1.1. Published on the Internet; http://www.theplantlist.org/(accessed 20th June 2019).
Tiwari, P., Mishra, B. N., Sangwan, N. S. (2014). Phytochemical and pharmacological properties of Gymnema sylvestre: An important medicinal plant. Biomed. Res. Int. 2014, 1–18. doi: 10.1155/2014/830285
Wu, X., Mao, G., Fan, Q., Zhao, T., Zhao, J., Li, F., et al. (2012). Isolation, purification, immunological and anti-tumor activities of polysaccharides from Gymnema sylvestre. Food. Res. Int. 48 (2), 935–939. doi: 10.1016/j.foodres.2012.02.006
Yasmin, H., Kaiser, M. A., Sarker, M. M. R., Rahman, M. S., Rashid, M. A. (2009). Preliminary anti-bacterial activity of some indigenous plants of Bangladesh. Dhaka Univ. J. Pharm. Sci. 8 (1), 61–65. doi: 10.3329/dujps.v8i1.5337
Yasukawa, K., Okuda, S., Nobushi, Y. (2014). Inhibitory effects of Gymnema (Gymnema sylvestre) leaves on tumour promotion in two-stage mouse skin carcinogenesis. J. Evid. Based Complement. Altern. Med. 2014, 1–5. doi: 10.1155/2014/328684
Ye, W., Zhang, Q., Liu, X., Che, C., Zhao, S. (2000). Oleanane saponins from Gymnema sylvestre. Phytochemistry 53 (8), 893–899. doi: 10.1016/S0031-9422(99)00483-5
Yogi, B., Mishra, A. (2016). Hepatoprotective effects of polyherbal formulation against carbon tetrachloride-induced hepatic injury in albino rats: a toxicity screening approach. Asian J. Pharm. Clin. Res. 10 (1), 192–198. doi: 10.22159/ajpcr.2017.v10i1.14757
Yoshikawa, K., Kondo, Y., Arihara, S., Matsuura, K. (1993). Antisweet natural products IX structures of gymnemic acids XV-XVIII from Gymnema sylvestre R. Br. Chem. Pharm. Bull. 41 (12), 1730–1732. doi: 10.1248/cpb.41.1730
Yoshikawa, M., Murakami, T., Matsuda, H. (1997). Medicinal foodstuffs. X. Structures of new triterpene glycosides, gymnemosides-c,-d,-e, and-f, from the leaves of Gymnema sylvestre R. Br.: influence of gymnema glycosides on glucose uptake in rat small intestinal fragments. Chem. Pharm. Bull. 45 (12), 2034–2038. doi: 10.1248/cpb.45.2034
Yoshikawa, M., Murakami, T., Kadoya, M., Li, Y., Murakami, N., Yamahara, J., et al. (1998). ChemInform abstract: medicinal foodstuffs. Part 9. The inhibitors of glucose absorption from the leaves of Gymnema sylvestre R. BR. (Asclepiadaceae): Structures of Gymnemosides a and b. Chem. Inform. 29 (12). doi: 10.1002/chin.199812156
Keywords: Gymnema sylvestre, phytomedicine, antidiabetic, herbal medicine, traditional medicine, complementary and alternative medicine, immunomodulating, lipid lowering
Citation: Khan F, Sarker MMR, Ming LC, Mohamed IN, Zhao C, Sheikh BY, Tsong HF and Rashid MA (2019) Comprehensive Review on Phytochemicals, Pharmacological and Clinical Potentials of Gymnema sylvestre. Front. Pharmacol. 10:1223. doi: 10.3389/fphar.2019.01223
Received: 24 May 2019; Accepted: 23 September 2019;
Published: 29 October 2019.
Edited by:
Monique S.J. Simmonds, Royal Botanic Gardens, Kew, United KingdomReviewed by:
Francisco Pérez García, University of Barcelona, SpainCopyright © 2019 Khan, Sarker, Ming, Mohamed, Zhao, Sheikh, Tsong and Rashid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Md. Moklesur Rahman Sarker, bW9rbGVzdXIyMDAyQHlhaG9vLmNvbQ==; ZHIubW9rbGVzdXIyMDE0QGdtYWlsLmNvbQ==; Isa Naina Mohamed, aXNhbmFpbmFAeWFob28uY28udWs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.