- 1Mushroom Research Centre, University of Malaya, Kuala Lumpur, Malaysia
- 2Department of Pharmacy, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia
- 3Department of Biochemistry, Karpagam Academy of Higher Education, Coimbatore, India
- 4Faculty of Science, Institute of Biological Sciences, University of Malaya, Kuala Lumpur, Malaysia
Edible and medicinal mushrooms are regularly used in natural medicines and home remedies since antiquity for ailments like fever, inflammation, and respiratory disorders. Lignosus rhinocerotis (Cooke) Ryvarden is a polypore found in Malaysia and other regions in South East Asia. It can be located on a spot where a tigress drips milk while feeding, hence the name “tiger's milk mushroom.” The sclerotium of L. rhinocerotis is highly sought after by the native communities in Malaysia to stave off hunger, relieve cough and asthma, and provide stamina. The genomic features of L. rhinocerotis have been described. The pharmacological and toxicity effects, if any, of L. rhinocerotis sclerotium have been scientifically verified in recent years. In this review, the validated investigations including the cognitive function, neuroprotection, immune modulation, anti-asthmatic, anti-coagulation, anti-inflammatory, anti-microbial/ anti-viral, anti-obesity, anti-cancer/ anti-tumor, and antioxidant properties are highlighted. These findings suggest that L. rhinocerotis can be considered as an alternative and natural medicine in the management of non-communicable diseases. However, there is a paucity of validation studies including human clinical trials of the mycochemicals of L. rhinocerotis.
Introduction
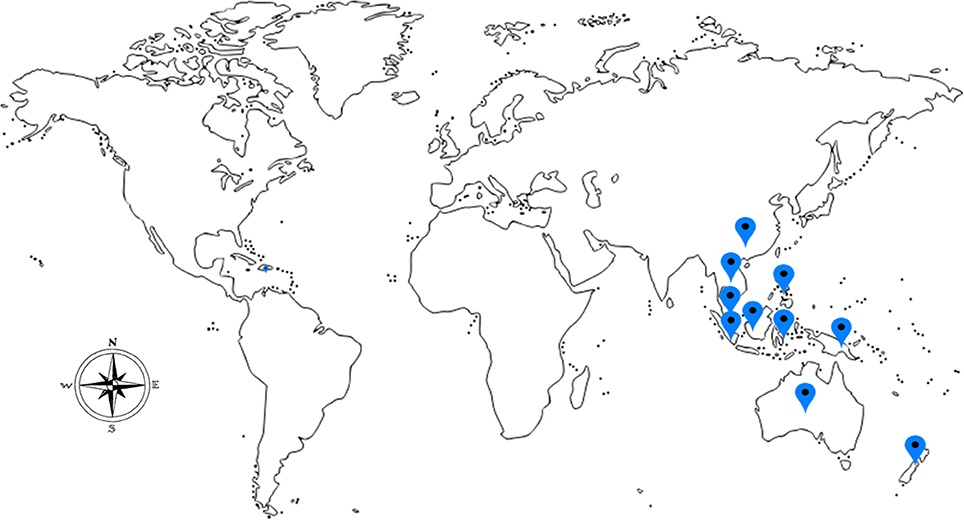
Medicinal mushrooms have been valued and used since ancient times by the Chinese, Korean, Japanese, Egyptians, and European communities. They are valued not only for the culinary purposes but also for their nutritional and medicinal values (Manzi et al., 1999). The greatest attribute of mushrooms, besides their taste, is their peculiar healing properties. Recently, ethnomycological knowledge of medicinal mushrooms for their curative properties is being tapped. Lignosus rhinocerotis (Cooke) Ryvarden, belonging to the Polyporaceae family is regarded as a rare and valuable traditional medicine and it can only be located in a certain geographic regions encompassing South China, Thailand, Malaysia, Indonesia, Philippines, Papua New Guinea, New Zealand, and Australia (Lai et al., 2011; Figure 1).
Lignosus rhinocerotis, however, was only collected from the wild. In the wild, the tiger milk mushroom grows solitary and makes the collection process time and energy consuming (Abdullah et al., 2013). Since the 2000s, large-scale cultivation of L. rhinocerotis in a controlled environment was made successful in Malaysia, overcoming the cost and supply problem (Lau et al., 2011, 2013a, 2015). Commercialization of this mushroom was then made possible and this opened the opportunity to investigate the potential pharmacological and nutraceutical properties of this mushroom for functional food or dietary supplements.
Description of L. rhinocerotis
This mushroom consists of the pileus (cap), stipe (stem), and sclerotium (tuber) (Figure 2). Its morphology is unusual for a polypore as the fruiting body (cap and stem) raises from the tuber under the ground, rather than from woody substrate. The cap and stem are woody while the sclerotium is a compacted mass of fungal mycelium containing food reserves. The sclerotium is white and gives a milk-like solution; and it even tastes like milk (Tan et al., 2010). As the irregular shaped sclerotium remains underground, the collection of the mushroom is challenging. Thus, due to lack of samples, limited study is done on this national treasure.
In Malaysia and Indonesia, this mushroom is known as “cendawan susu rimau” which literally means “tiger milk mushroom” (Burkill, 1930). Early documentation of this mushroom were given by Ridley and Corner (Ridley, 1900; Corner, 1989). In the early twentieth century, the taxonomy of L. rhinocerotis depended primarily on morphological observations. However, in recent years, specimens of “susu rimau” collected in these regions were confirmed as L. rhinocerotis on the basis of both micro- and macro-morphological characteristics, as well as molecular approaches. To date, L. rhinocerotis is noted to be the “most commonly occurring member of Lignosus in Malaysia” (Lai et al., 2009; Tan et al., 2010; Choong et al., 2014). Figure 2 shows the taxonomic classification of L. rhinocerotis.
Lignosus rhinocerotis was originally categorized as Polyporus rhinoceros (Cooke, 1879). This mushroom was taxonomically narrated under several genera, including Fomes, Scindalma, Polystictus, and Microporus by different authors before they were correctly re-named as Lignosus (Cooke, 1879). Some of the synonyms are “Polyporus rhinocerus Cooke (1879), Fomes rhinocerotis Cooke (1879), Fomes rhinocerus Cooke (1879), Scindalma rhinocerus (Cooke) Kuntze (1898), Scindalma rhinocerotis (Cooke) Kuntze (1898), Polyporus sacer var. rhinocerotis (Cooke) Llyod (1921), Polystictus rhinocerus (Cooke) Boedjin (1940), Polystictus rhinocerotis (Cooke) Boedjin (1940), Microporus rhinocerus (Cooke) Imazeki (1952), Microporus rhinocerotis (Cooke) Imazeki (1952), and Lignosus rhinocerus (Cooke) Ryvarden (1972)”. These synonyms were taken from MycoBank (http://www.mycobank.org).
Lignosus rhinocerotis was often mistaken for Pleurotus tuber-regium or Lentinus tuber-regium. Thus, the use of molecular markers in identifying L. rhinocerotis has proven to overcome the drawback of classic taxonomy methods (Sotome et al., 2008; Cui et al., 2011). There are even genetic markers and DNA barcode markers being developed to identify the Lignosus spp.
Recently, Tan et al. (2015) reported successful cultivation of the mushroom, which would overcome the supply problem and make possibilities for more investigation to be done on L. rhinocerotis. Other researchers also cultivated this mushroom using mycelium in submerged culture techniques. Further, the optimization of substrate formulation was done for the cultivation of L. rhinocerotis in conditions that mimic their natural environment. Based on the optimized formulation, the pilot cultivation was conducted. Both sclerotia and sporophores were successfully produced (Abdullah et al., 2013).
Ethnomycological Aspects of L. rhinocerotis
According to folklore, it is believed that the mushroom emerges on the spot where the milk of a tigress had accidentally dribbled during lactation. The sclerotium of the mushroom resembles the “congealed white mass of milk” (Corner, 1989; Chang, 2015). Different tribal communities in Malaysia have different referral names, such as “betes kismas” by the Semai (Chang and Lee, 2010), “tish am ong” by the Kensiu (Mohammad et al., 2012), and “Pěti' Aa” by the Besisi (Skeat, 1896). The Batak people from Indonesia denoted L. rhinocerotis as “Ndurabi” (Karo) (Hilton and Dhitaphichit, 1978). Lignosus rhinocerotis found in China were called “how gui kou or hurulingzhi” (in Chinese), which means “tiger milk Ganoderma” (Huang, 1999; Yokota, 2011). In Japan, it is known as “hijiritake” (Lee and Chang, 2007).
Besides the traditional beliefs that L. rhinocerotis was derived from the tiger's milk, there are many other folklore beliefs about this mushroom. The Semai (indigenous people of Malaysia) believes that L. rhinocerotis could reinstate the spirit of a crop and guarantee a lavish harvest. The sclerotia are habitually used during paddy farming and prayer ritual for a bountiful crop yields (Haji Taha, 2006). Alternatively, some crops, for instance, paddy is positioned in a flower-filled container, and suspended over the mushroom (Skeat and Ottoblagoen, 1906).
The Besisi (or MahMeri) declares that the mushroom is easier to be spotted after the full moon (Hartland, 1909). Lignosus rhinocerotis were also associated with childbirth customs. The Semang (or Negrito) people believe that L. rhinocerotis hold “the soul of an unborn tiger cub” and that “the soul is conveyed when the tiger eats it” (Cumming, 1903; Robert, 1986).
Lignosus rhinocerotis is mentioned in various stories from different cultural background, and in most cases, are linked to their medicinal properties. For example, in the story of “Indra Bangsawan”, “tigress' milk” is the remedy for a princess who became infected with an eye disease and lost her vision (Beveridge, 1909). A Mughal emperor, Jahangir (1568–1616) also wrote that the “milk of a tigress was of great use for brightening eyes” (Green, 2006; Lee et al., 2009).
Based on ethnobotanical uses, various tribes of the Orang Asli in Malaysia (the Semai, Temuan, and Jakun) use L. rhinocerotis to relief asthma, cough, food poisoning, swollen breasts, joint pain, liver illness, swollen body parts, and as a general tonic (Lee and Chang, 2007; Ismail, 2010). It is also used to nurse women after childbirth (during postpartum period) and also to stave off hunger. The L. rhinocerotis is not only popular among the indigenous people but also the urban population in Malaysia (Hattori et al., 2007). The sclerotia of L. rhinocerotis are occasionally sold in Traditional Chinese Medicine (TCM) store in Malaysia. They are used by the TCM practitioner to revitalize the body of the patients. According to Sabaratnam et al. (2013), infusions of L. rhinocerotis are said to improve the overall wellness of the individual by enhancing the vitality, energy, and alertness.
There are many ways this mushroom is prepared and consumed to treat illness. In earlier years, the sclerotium was pounded and the juice was infused with water and drunk as a tonic. The mushrooms are grounded or sliced, then boiled with water for drinking or soaked into Chinese wine for external applications (Chang and Lee, 2004). The sclerotium is also eaten raw and with betel leaves to relieve a cough and sore throat. The preparation methods of decoction and/or topical medicine vary among tribes.
Genomic and Proteomic Studies of L. rhinocerotis
Genome sequencing of L. rhinocerotis was carried out (Yap et al., 2014). A comparative genomics and phylogenetic analysis were performed. The L. rhinocerotis genome is widely composed of sesquiterpenoid biosynthesis genes. Moreover, the genome of L. rhinocerotis appears to code for 1,3-β- and 1,6-β- glucans, as well as lectin, laccase, and other fungal immune-modulatory proteins (FIPs).
Subsequently, proteomic profiling of L. rhinocerotis sclerotial proteins were carried out using various spectrometers. Table 1 shows the genomic and proteomic studies of L. rhinocerotis. While some of the proteins were unknown, a majority of the proteins were detected as lectin (39.13%) which were speculated for defense mechanisms in L. rhinocerotis. The other proteins identified from the study were of pharmacological interest, for example, FIPs (2.52%), antioxidant proteins i.e., manganese superoxide dismutase (Mn-SOD, 0.91%) and glutathione S-transferase (GST, 0.54%). There is a paucity of studies of the myconutrients in this mushroom. Furthermore, it is also necessary to study the molecular and genetic basis of the identified components, and the medicinal and/or nutraceutical properties of L. rhinocerotis.
Nutritional Composition of L. rhinocerotis
Analysis of the chemical compositions of L. rhinocerotis was carried out by Yap et al. (2013). The wild strain (WT Rhino) and the commercial strain (TM02) sclerotial powder were analyzed based on the Association of Official Analytical Chemists (AOAC) procedures. In general, the nutrient composition of the sclerotium of cultivated strain was higher when compared to the wild strain.
The major constituents of L. rhinocerotis sclerotia were carbohydrates (monosaccharides and disaccharides) while the fat content was <1%. As reported by Lau et al. (2013a), the beta (β)-glucans represented the dominant glucans in the aqueous extracts of L. rhinocerotis, which was 82–93% of total glucan (w/w). The protein content of the sclerotium of TM02 was 3.6-times higher than that of the wild-type. The essential amino acid content (g/kg dry weight) of the commercial strain was 4-times higher than that in the wild strain. The minerals (calcium, potassium, sodium, and magnesium) were also higher in the cultivated strain. Table 2 shows the chemical compositions of L. rhinocerotis.
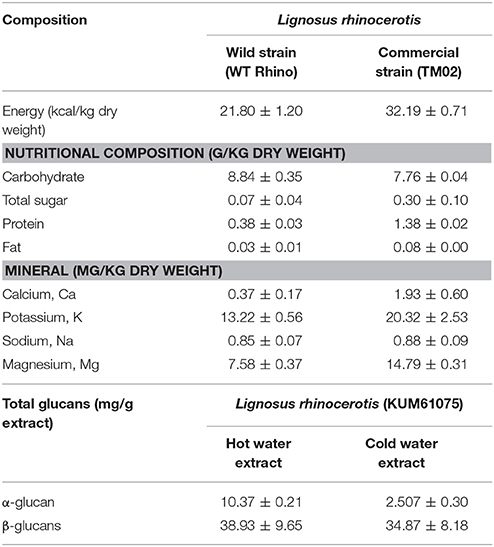
Table 2. The chemical and nutritional compositions of L. rhinocerotis (Lau et al., 2013a; Yap et al., 2013).
Therapeutic Values of L. rhinocerotis Anti-Asthmatic Activity
The efficacy of L. rhinocerotis in treating asthmatic symptoms has been validated in several in vitro and in vivo studies. The effects of L. rhinocerotis extracts on ovalbumin-induced allergic asthma in Sprague-Dawley rats were investigated (Malagobadan et al., 2014). The treatment with L. rhinocerotis extracts significantly reduced asthmatic parameters, for instance, the total immunoglobulin E (IgE) in serum, and T-helper type 2 (Th2) cytokine levels (IL-4, IL-5, and IL-13) in bronchoalveolar lavage fluid (BALF). It also inhibited the number of eosinophil in BAFL and diminished the infiltration of eosinophil in the lungs.
A study on airway inflammation in an asthmatic model was reported (Johnathan et al., 2016). The hot aqueous extract of L. rhinocerotis (500 mg/kg) was effective in reducing asthma-related parameters. The extract significantly ameliorated the increase of the total IgE (14.9 ng/ml) in serum, as well as IL-4 (16.2 pg/ml), IL-5 (38.8 ng/ml), and IL-13 (80.5 pg/ml) levels in BALF. It also effectively decreased eosinophils numbers in BALF while attenuating eosinophil infiltrations in the lungs. Sequential extraction using five solvents, i.e., petroleum ether, diethyl ether, hexane, ethyl acetate, and methanol, was conducted prior to gas chromatography-mass spectrometry (GC-MS) analysis (Johnathan et al., 2016). GC-MS examination uncovered five major groups (alkane, fatty acids, benzene, phenol, and dicarboxylic acid) with a total of 18 constituents in L. rhinocerotis. Linoleic acid, octadecane, and 2,3-dihydroxypropyl elaidate were present in abundance in the L. rhinocerotis extract (Johnathan et al., 2016).
Anti-coagulant and Fibrinolytic Activities
Cardiovascular diseases can be caused by thrombosis due to fibrin aggregation in the blood (Lee et al., 2005). Natural anti-coagulant and fibrinolytic agents have been used to treat thrombolytic conditions, especially edible mushrooms. Recently, the edible mushroom “wood ear fungus,” Auricularia polytricha (Mont.) Sacc was found to be able to produce protease-like fibrinolytic enzymes (Mohamed Ali et al., 2014).
The anti-coagulant activity of the crude aqueous extract of L. rhinocerotis was also reported. Kho (2014) demonstrated that the crude extracts of L. rhinocerotis caused a 1.2 cm-lytic zone in a fibrin plate assay. Subsequently, aqueous two phase system (ATPS) was employed to partition, purify, and concentrate the protein fraction of the mushroom extract. As a result, a fibrinolytic enzyme with a specific activity of 151.61 U/mg was isolated and the molecular size was estimated to be between 55 kDa and 60 kDa. The anti-platelet activity of the aqueous extract of L. rhinocerotis was also reported using fresh human blood (Teo, 2014). The crude extract, after subjected to ATPS, yielded a partially purified protease-like enzyme with size ranging from 50 to 55 kDa.
Sidek Ahmad et al. (2014) tested six different wild sclerotia (LR1 to LR6) of L. rhinocerotis obtained from Perak, Malaysia, for their fibrinolytic activities. Out of the six sclerotia used, the LR1 sclerotium exhibited proteolytic activity with a clear zone of 1.31 cm diameter in skim milk agar plates while giving a clear zone of 0.97 cm when tested with fibrin plates. It is presumed that the fibrinolytic activity of L. rhinocerotis is attributed to the presence of a protease.
Anti-inflammatory Activity
The anti-inflammatory activity of the sclerotium of L. rhinocerotis was previously reported with its hot aqueous, cold aqueous, and methanol extracts (Lee et al., 2012b, 2013b). Lee et al. (2014) reported that the three extracts of L. rhinocerotis exhibited anti-inflammatory properties as shown by the carrageenan-induced paw edema test using Sprague-Dawley rats. The cold aqueous extract, the most potent extract, was subjected to separation by Sephadex G50 gel filtration chromatography. The resulting high-molecular-weight protein fraction was further assessed for anti-inflammatory activity in lipopolysaccharide (LPS)-induced RAW 264.7 macrophage cells. The protein fraction was shown to inhibit tumor necrosis factor alpha (TNF-α) production.
The anti-inflammatory effect of L. rhinocerotis hot aqueous and ethanol extracts on RAW 264.7 cells was further tested (Baskaran et al., 2012; Baskaran, 2015). The ethanol extract showed significant decrease (48.3–88.5%) of nitric oxide (NO) production from 0.01 to 100 μg/mL dose-dependently but the aqueous extract did not show a significant reduction. The ethanol extract was able to activate signal transducer and activator of transcription 3 (STAT3) pathway by reducing inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) expressions while increasing the interleukin 10 (IL-10) expression.
Nallathamby et al. (2016) analyzed the ethyl acetate fraction from the ethanol extract of L. rhinocerotis. The fraction significantly reduced the NO production in microglial (BV2) cells by 12 to 70% at 10 and 100 μg/mL; respectively. The major compounds of the ethyl acetate fraction were revealed as linoleic acid, oleic acid, and ethyl linoleate. The identified compounds were further tested individually for their anti-inflammatory activities. Treatment with linoleic acid significantly suppressed iNOS and COX2 expression by 1.2-fold as compared to the control. In another study, LPS-induced BV2 cells pretreated with hot aqueous extract (500 μg/mL), n-butanol fraction of hot aqueous extract (250 μg/mL), and ethyl acetate fraction of hot aqueous extract (250 μg/mL), showed maximum inhibition of NO production by 88.95, 86.50, and 85.93%, respectively (Seow et al., 2017). These studies represent the first evidence of anti-inflammatory properties of L. rhinocerotis using brain microglial BV2 cells.
Anti-microbial Activity
Four extracts of the wild L. rhinocerotis sclerotium, i.e., petroleum ether, chloroform, methanol, and aqueous extracts, were screened for their anti-microbial properties (Mohanarji et al., 2012). The four extracts were tested against 15 pathogenic bacteria, including Staphylococcus, Corynebacterium, Bacillus, Streptococcus, Klebsiella, Salmonella, Pseudomonas, Escherichia, and Micrococcus spp.; as well as four fungi species including Candida spp and Mucor sp. Antifungal and antibacterial activities of the extracts were evaluated by measuring the inhibition zone using disc diffusion assay. The methanol and aqueous extracts (30 mg/mL) showed significant inhibition against the tested microbes except for Streptococcus pyogenes and Serratia marcescens. A qualitative phytochemical analysis showed the presence of alkaloids, protein, gums and mucilage, and flavonoids in the extracts of L. rhinocerotis (Mohanarji et al., 2012).
Anti-obesity and Hepatoprotective Activities
A study by Hoe (2014) provided evidence that the aqueous extract of L. rhinocerotis mitigated non-alcoholic fatty liver disease in high-fat-diet induced obese hamsters. Further, the extract inhibited weight gain in high-fat-diet fed hamsters. The L. rhinocerotis extract did not show any side effects in the experimented animals with regards to the weight of organs i.e., brain, heart, thymus, liver, kidney, adrenal glands, spleen, testicles, and epididymis.
The L. rhinocerotis at low dose demonstrated the highest effect in reducing the weights of liver and adipose tissues with no histological abnormalities found in the organs and tissues. The serum biochemical, as well as the liver and kidney function parameters, were also measured. The results showed that the extract reversed the serum levels of several biochemical parameters in the hamsters. The L. rhinocerotis aqueous extract was also shown to inhibit reactive oxygen species (ROS) production and reduced cluster of differentiation 68 (CD68) and COX2 expressions in the liver. The extract also downregulated mRNA expressions of different genes, namely TNF-α, IL-1β, IL-6, transforming growth factor beta (TGF-β), and collagen-type 1 (Colla1)-related inflammation and collagen-type 1 (Colla1)-related inflammation and fibrosis caused by obesity.
Antioxidant Properties
The cold aqueous, hot aqueous, and methanol extracts of wild and cultivated sclerotium of L. rhinocerotis were assessed for their antioxidant capacities (Yap et al., 2013). The total phenolic content (TPC) of the extracts were calculated in terms of gallic acid equivalents (mg GAE/g). The TPC of the extracts ranged from 19.3 to 29.4 mg GAE/g extract. The ability of the extracts to reduce ferric ions and scavenge free radicals was also measured by using Ferric reducing antioxidant power (FRAP) assay and DPPH• (1,1-diphenyl-2-picrylhydrazyl) radical scavenging assay; respectively. The FRAP value was between 0.006 to 0.016 mmol/min g extract. The DPPH radical scavenging values were 0.05–0.2 mmol Trolox equivalents (TE)/g. The extracts from wild sclerotium of L. rhinocerotis had higher antioxidant capacity compared to the cultivated sclerotium. The methanol extract displayed higher activity compared to the aqueous extracts. It is noteworthy to mention that both strains exhibited significantly higher superoxide anion radical scavenging activity when compared to rutin.
Similar analyses were done using aqueous extract of L. rhinocerotis (Hoe, 2014). The extract showed half maximal effective concentration (EC50) values of DPPH scavenging and ferrous ion chelating activity at 135.16 ± 2.47 mg/mL and 503.34 ± 10.44 mg/mL; respectively. The TPC and flavonoid contents were 366.23 ± 5.06 mg GAE and 28.67 ± 2.5 mg rutin equivalent/g; respectively. In a study by Suziana Zaila (2013), the ferric reducing capacity of pressurized methanol extract was higher than the aqueous extract.
The free radical scavenging abilities, reducing properties, metal chelating activities, and inhibitory effects of lipid peroxidation by L. rhinocerotis extracts of mycelium from submerged cultivated and the culture broth were reported by Lau et al. (2014). The aqueous methanol extracts from the mycelium and culture broths showed comparable antioxidant effects with the aqueous methanol extracts of sclerotium. The results suggested that the mycelia of L. rhinocerotis obtained in submerged cultivation can also be a valuable source of antioxidant compounds.
The antioxidant activity of hexane and ethyl acetate fractions obtained from ethanol sclerotial extracts of L. rhinocerotis were determined (Nallathamby et al., 2014, 2016). The ethanol extract at 5 mg/mL had the highest antioxidant activity among the three extracts with ferric reducing ability of 122.6 mmol ferrous sulfate equivalent (FSE)/g extract. The 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS•+) and DPPH radical scavenging activity were 86.5 ± 4 mg TE /g and 29.4 ± 1.7%; respectively. The hexane and ethyl acetate fractions had moderate antioxidant activities compared to methanol /ethanol extract.
Anti-tumor/Anti-cancer Activities
The traditional claims that L. rhinocerotis had anti-tumor/anti-cancer properties were investigated in several cancer and solid tumor cell lines. The first study on the anti-tumor properties of L. rhinocerotis sclerotium specified that the water-soluble polysaccharide–protein complex (PR-HW) and alkali-soluble β-glucan (PR-CA) possessed notable host-mediated anti-tumor activity on the Sarcoma 180 implanted male BALB/c mice (Lai, 2005). The PR-HW exhibited apoptotic effect on human acute promyelocytic leukemia cells (HL-60), human chronic myelogenous leukemia cells (K562), and human acute monocytic leukemia cells (THP-1) with IC50 of 100 mg/ml, 400 mg/ml, and >400 mg/ml; respectively after 72 h of incubation (Lai, 2005; Lai et al., 2008). Besides, flow cytometric analysis revealed that the onset of apoptosis could be linked to the cell cycle arrest at Gap1 (G1) phase.
The cold-water extract (LR-CW) prepared from the sclerotium of L. rhinocerotis exhibited antiproliferative activity in human breast carcinoma cells (MCF-7) and human lung carcinoma cells (A549), with IC50 of 96.7 and 466.7 μg/mL, respectively. In contrast, the LR-CW did not show significant cytotoxicity toward the two corresponding normal human cells, i.e., human breast cells (184B5) and human lung cells (NL 20). From the results of DNA fragmentation assay, the cell death was attributed to apoptosis. The high-molecular-weight fraction of the cold water extract exhibited cytotoxicity toward MCF7 and A549 cancer cells, with IC50 of 70.0 and 76.7 μg/mL, respectively (Lee et al., 2012a).
Subsequently, the cytotoxicity of hot aqueous and cold aqueous extract of L. rhinocerotis sclerotium were screened using 11 human cell lines, namely HL-60 (human acute promyelocytic leukemia cells), MCF7, MDA-MB-231 (human breast adenocarcinoma cells), HCT116 (human colorectal carcinoma cells), PC-3 (human prostate adenocarcinoma cells), A549 (human lung carcinoma cells), MRC-5 (human lung fibroblast cells), HepG2 (human hepatocellular carcinoma cells), WRL68 (human embryonic liver cells), HSC2 (human squamous carcinoma cells), and HK1 (human nasopharyngeal carcinoma cells) (Lau et al., 2013a). The cold aqueous extract was cytotoxic toward solid tumor cells with IC50 of 37–120 mg/mL, whereas the hot aqueous extract was inactive toward the solid tumor cells.
The pressurized methanol extract exerted a higher cytotoxic activity (IC50: 600 μg/mL) when compared to the pressurized aqueous extract (IC50: 1200 μg/mL) in HCT 116 (human colorectal carcinoma) cells as determined by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Suziana Zaila, 2013). Both extracts did not exert cytotoxic activity on CCD-18co (human normal colon fibroblast cells) and V79-4 (Chinese hamster lung fibroblast cells) (IC50> 2000 μg/mL). The mode of cell death induced by methanol and aqueous extracts was primarily apoptosis as assessed by Annexin V-fluorescein isothiocyanate/propidium iodide (FITC/PI) dual staining. Both extracts arrested HCT 116 cells at G0/G1 phases with the corresponding decrease of S-phase population.
Lai et al. (2014) indicated that the polysaccharide at concentrations ranging from 4 to 8 μg/mL of L. rhinocerotis (collected from the forest in Kuala Lipis, Pahang, Malaysia) inhibited 45% growth of human lung carcinoma cells (A549). This is 100-fold lower than the results reported by Lee et al. (2012a). A preliminary study found that the antiproliferative ingredient in the polysaccharide extract was the β-glucan.
The mycelial extract of L. rhinocerotis cultured in a stirred tank reactor was also reported to exhibit cytotoxic effect on cervical cancer cells (Ca Ski) (Abdullah et al., 2010). The ammonium sulfate precipitate obtained from the mycelial protein fraction showed a growth inhibition of 72–82% against Ca Ski cells. The aqueous methanol extract of mycelial and culture broth of L. rhinocerotis from liquid fermentation did not exert cytotoxicity toward the normal cell lines (Lau et al., 2014).
In conclusion, there is a consistency among the reported studies, that the alcoholic extracts were not cytotoxic toward various cancer cell lines (Yap et al., 2013; Lau et al., 2014). However, the selectivity of the extracts on non-tumorigenic cells lines is not consistent (Lau et al., 2013a; Lee et al., 2014). The cultivation, processing, and preparation of extract may affect the cytotoxicity toward different cell lines. The chemical compositions of the extract may also influence the degree of cytotoxic level toward the cell lines. According to the reported literature, the primary identified bioactive components are more to high-molecular-weight hydrophilic components (Lai et al., 2008; Lau et al., 2014), protein-carbohydrates complex (Lee et al., 2014), and protein/peptides (Lau et al., 2013b).
Anti-viral Activity
The mycelial protein fractions of L. rhinocerotis were previously reported to inhibit human papilloma virus (HPV) activity (Abdullah et al., 2010). The anti-dengue activity tested by plaque reduction assay against dengue virus type-2 (DENV-2) strain was detected in hot aqueous extract of L. rhinocerotis at IC50 of 520 μg/mL (Ellan et al., 2013). Hot aqueous extract of L. rhinocerotis also inhibited dengue viral RNA synthesis by 99.68%. The extract showed anti-dengue activity in viral penetration assay but did not have a significant effect on DENV-2 virucidal activity and viral attachment. The anti-dengue activity was correlated with the carbohydrate content in the hot aqueous extract of L. rhinocerotis (2.0 mg/mL) (Ellan et al., 2014). This suggested that the L. rhinocerotis has anti-dengue activity attributed to the polysaccharide content in the sclerotium.
Immunomodulatory Actions
Immune modulatory activity can lead to anti-inflammation and anti-tumor effects. The immune responses are mediated by various immune cells and their secondary secretory components (Wong and Cheung, 2009). Guo et al. (2011) investigated the effect of aqueous extract of L. rhinocerotis sclerotium on RAW 264.7 and primary macrophages isolated from BALB/c mice. The mushroom treatment promoted a significant up-regulation of pinocytosis, leading to an increase in the production of ROS, NO, and TNF-α production. The iNOS expression in both RAW 264.7 cells and primary macrophages was also increased. The expression of Dectin-1+ cells on the cell surface was shown to decrease. On the other hand, the complement receptor (CR3+) and toll-like receptor (TLR2+) were increased in response to aqueous extract-treated primary macrophage. The aqueous extract also increased the phosphorylation of I-kappa-B alpha (IKBα), which then triggered the nuclear factor kappa B (NF-κB) signaling pathway. Therefore, the immunomodulatory effect of L. rhinocerotis could be intervened by macrophage activation via the NF-κB signal pathway.
The in vitro results from the previous studies were further tested in in vivo studies using healthy BALB/c mice and athymic nude mice. Their serum cytokine profile, splenocytes, and peritoneal exudate cells (PECs) were analyzed (Wong et al., 2011). The hot water extract and sonication-assisted cold alkali-soluble polysaccharides (PRS) of L. rhinocerotis increased the spleen weight of both healthy BALB/c mice and athymic nude mice. The hot water extract activated the neutrophil production whereas the PRS stimulated other innate immune cells.
The combined effect of L. rhinocerotis supplementation and resistance training on immune functions/parameters such as CD3+ and CD4+T lymphocytes; and CD4+ B lymphocytes were measured in young males between 19 to 25 years (Chen et al., 2016). The results indicated that the combination of extracts and resistance training did not affect the immune functions of the subjects.
In another study, a water-soluble polysaccharide-protein complex (PRW1) was isolated from the sclerotium of Polyporus rhinocerus Cooke (synonym of L. rhinocerotis) and further purified by membrane ultrafiltration (Liu et al., 2016). It showed a significant increase of NO production and enhanced the release of a variety of cytokines, for example, granulocyte colony stimulating factor (GCSF) and granulocyte-macrophage colony-stimulating factor (GMCSF). Accompanying the release of stimulating factors were the IL-6, IL12p40/70, monocyte chemoattractant protein-1 (MCP-1), MCP-5, macrophage inflammatory protein (MIP-1-α), MIP-2, regulated on activation normal T cell expressed and secreted (RANTES), soluble tumor necrosis factor receptor I (sTNFRI), and TNF-α. L. rhinocerotis also triggered extracellular signal-regulated kinase (ERK) phosphorylation and increased the iNOS expression. It was the first report that provided the molecular mechanism of the immunostimulatory properties of Polyporus-derived protein complex on RAW 264.7cells. Polyporus rhinocerus sclerotium may have a potential application for cancer immunotherapy.
It is noteworthy to clarify that while the myconutrients of L. rhinocerotis can improve immune system by activating several immune effector cells (pro-inflammatory), they too, can suppress the immune system by inhibiting certain inflammation markers (anti-inflammation). This “immunomodulating properties” is very common among fungal metabolites, especially the mushroom polysaccharides.
Neuritogenic Properties
The sclerotium of L. rhinocerotis was investigated for neurite outgrowth in rat pheochromocytoma adherent (PC12 adh) cells (Eik et al., 2011). The aqueous extract increased the percentage of neurite bearing cells by 9.8 to 23.6% in PC12 cells. The aqueous and ethanol extracts stimulated maximal neurite outgrowth of 23.6% and 18.5%; respectively at 20 μg/mL. It is also shown that the L. rhinocerotis aqueous extract showed better neurite outgrowth compared to other medicinal mushrooms tested. Furthermore, the combination of 20 μg/mL aqueous extract and 30 ng/mL of nerve growth factor (NGF) enhanced the neurite outgrowth by 42.1% compared to either aqueous extract (24.4%) or NGF (24.6%) alone (Eik et al., 2012).
The aqueous extract of the L. rhinocerotis mycelium also had high neurite outgrowth activity of 21.1% at 20 μg/mL in PC12 cells (John et al., 2011). The synergistic effects of L. rhinocerotis mycelium extract combined with other natural products were further tested on PC12 cells. The treatments with the combination of aqueous extract (20 μg/mL) and Gingko biloba (30 μg/mL) resulted in 39.89% of neurite outgrowth activity when compared to G. biloba alone (31.15%) (John et al., 2012). The increase of neurite outgrowth activity was significant although the synergistic effects are not remarkably high. The effects of L. rhinocerotis mycelium extract (20 μg/mL) bio-augmented with 1 μg/mL of curcumin were subsequently tested (John et al., 2013). The combination has enhanced neurite outgrowth by 27.2% when compared to curcumin alone.
Phan et al. (2013) reported neurite outgrowth of aqueous extract of L. rhinocerotis sclerotium and mycelium in mouse neuroblastoma (N2a) cells. At 20 μg/mL, the aqueous extract of sclerotium resulted in 38.1% of neurite bearing cells, which was approximately twice the number of NGF-treated neurite bearing cells. However, the aqueous extract of the mycelium did not cause a significant increase in neurite outgrowth when compared to NGF treatment. In all the studies, neuronal differentiation in the cell lines treated by L. rhinocerotis extracts were further demonstrated by indirect immunofluorescence staining of neurofilament protein.
According to the studies by Seow et al. (2013a, 2015), the maximal neuritogenic activity in PC12 at 25 μg/mL of aqueous extract was 20.99% followed by ethanolic extract (17.4%) and crude polysaccharides (16.4%). The hot aqueous extract (25 μg/mL) stimulated neuritogenesis as equivalent to NGF (50 μg/mL). However, all the extracts promoted neuritogenesis without stimulating the release of NGF in PC12 cells. The tyrosine kinase (Trk) and ERK1/2 inhibitors (K252a, U0126, and PD98059) showed a decrease in the neurite bearing cells percentage by 82.2, 86.2, and 91.6% in NGF-treated cells; and 80.9, 86.7, and 84.6% in hot aqueous extract-treated cells. The NGF-mimicking potential of L. rhinocerotis is postulated to follow the phosphoinositide 3-kinase protein kinase B (P13K-Akt) and ERK1/2 pathways (Eik et al., 2012; Seow et al., 2013b).
On the other hand, the ethyl acetate and n-butanol fractions of L. rhinocerotis were found to mimic the neuritogenic activity of NGF by targeting the TrkA receptor and activated the mammalian target of rapamycin (mTOR) signaling pathway with the phosphorylation of the transcription factors, cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB), and leading to the increased expressions of neuritogenesis biomarker, i.e., the growth associated protein 43 (GAP43), tubulin alpha 4A (TUBA4A), and tubulin beta 1 (TUBB1) in PC12 cells (Seow, 2016).
In a different study, aqueous extract of L. rhinocerotis sclerotium was investigated for stimulation of neurite outgrowth in dissociated cells of the brain, spinal cord, and retina of chick embryo (Samberkar et al., 2015). After 48 h incubation, the aqueous extract at 50 μg/mL induced maximum neurite outgrowth of 20.8 and 24.7% in brain and spinal cord. On the other hand, 20.8% of neurite outgrowth was achieved in retinal cells at 25 μg/mL. The neuronal differentiation by L. rhinocerotis aqueous extract-treated cells was then confirmed by immunofluorescence staining.
Toxicology Evaluation
It is crucial that the toxicity assessment is done both in in vitro and in vivo assays in order to develop L. rhinocerotis as health supplements. The teratogenicity effects of L. rhinocerotis was carried out by Lee et al. (2013a). The freeze-dried sclerotium of L. rhinocerotis was orally administrated to both male and female mice for 28 days and then the animals were allowed to mate for 10 days. The female rats were continuously fed with the sclerotium powder until the pups were delivered (around 7–8 weeks after mating period). The pups were assessed for anti-fertility and teratogenic effects, if any. The 100 mg/kg sclerotium of L. rhinocerotis did not affect the fertility of the rats. Further, the dosage tested did not trigger teratogenic effects or aberrations in the litter.
The genotoxic effects of the sclerotium of the L. rhinocerotis or its possibility to cause gene mutations were evaluated in the plate incorporation and pre-incubation tests (Lee et al., 2013a). The bacteria strains were Salmonella typhimurium and Escherichia coli strains. There was no genotoxic effects or mutation in all the five strains (TA98, TA100, TA1535, TA1537, and WP2 uvrA) tested.
The genotoxic effects of L. rhinocerotis sclerotia were further evaluated by the bacterial reverse mutation or Ames test, the in-vitro chromosome aberration and in vivo mammalian erythrocyte micronucleus assays (Chen et al., 2013). At the concentration of the mycelium of L. rhinocerotis at 100 mg/mL (5 mg/plate) dose no mutagenic activity in the presence and absence of S9 metabolic activation system was observed in all the five Salmonella strains. The number of structural aberrations in Chinese hamster ovary (CHO-K1) cells was comparable to the negative control. The cells were unaltered and frequencies across the treatment had insignificant damage. The treatment with L. rhinocerotis displayed no adverse effect on the natural micronucleus frequency in both male and female mice. Further polychromatic erythrocytes (PCE) percentage and micronucleus frequency in 2,000 mg/kg L. rhinocerotis orally administrated animals had no significant difference when compared to the control animals.
The effect on cellular viability of L. rhinocerotis extracts of non-tumorigenic cell lines was investigated. The aqueous extract of L. rhinocerotis sclerotium was not cytotoxic to normal human breast (184B5) and normal lung (NL20) cells (Lee et al., 2012a). The methanol extract of L. rhinocerotis sclerotium also was not cytotoxic to normal human colon (CCD-18co), kidney (HEK-293), nasopharyngeal (NP69), oral (OKF6), rat kidney (NRK-52E), and Vero cell lines (Lau et al., 2013a; Suziana Zaila, 2013).
Eik et al. (2012) and Seow et al. (2015) reported that the aqueous and ethanol extracts of the L. rhinocerotis sclerotium were non-cytotoxic to PC12 cells after 48 h incubation. Phan et al. (2013) also reported that the aqueous extracts of the mycelium and sclerotium of L. rhinocerotis were not cytotoxic with IC50 values of 1.75–5.93 mg/mL to mouse embryonic fibroblasts (BALB/3T3) and N2a cells after 24 h incubation. Baskaran (2015) reported that the aqueous and ethanol extracts were non-cytotoxic to RAW264.7 macrophages cells. Nallathamby et al. (2013, 2016), too found that the ethanol, hexane, and ethyl acetate fractions obtained from the sclerotium was non-cytotoxic to BV2 microglial cells. The crude polysaccharides, hot aqueous and ethanol extracts of the sclerotium of L. rhinocerotis were shown to be non-cytotoxic to BV2 microglial cells. Further, the hexane, ethyl acetate, n-butanol and aqueous fractions from hot aqueous and ethanol extracts were not cytotoxic to BV2 microglial cells (Nallathamby et al., 2013, 2016).
In a sub-acute toxicity in vivo study using Sprague Dawley rats, the sclerotium powder of cultivated and wild L. rhinocerotis were orally administrated at 250, 500, and 1,000 mg/kg doses (Shien et al., 2011). Neither the cultivated nor the wild L. rhinocerotis sclerotium had adversative effects on the growth rate, blood, and clinical biochemical parameters. There were also no pathological changes in the vital organs like liver, kidneys, spleen, and lungs.
Findings obtained from the chronic toxicity study on L. rhinocerotis TM02 cultivar were also similar (Lee et al., 2013a). The oral administration of the sclerotium powder at the highest dose of 1,000 mg/kg did not cause adverse effects. Therefore, the no-observed-adverse-effect-level (NOAEL) dose of the sclerotium powder was higher than 1,000 mg/kg. Based on both in vitro and in vivo toxicity assessments reported, indicate that cultivated L. rhinocerotis, the mycelium or sclerotium, were safe for consumption.
Future Perspectives
Table 3 summarizes the medicinal properties of this mushroom. In order to expand the application of this mushroom, certain aspects of research should be further studied in detail.
First, since there is a high demand for this mushroom and the current lack of supply, more studies could be conducted on the domestication of L. rhinocerotis by optimizing the cultivation conditions to produce more consistent yield and bioactive components in extracts. Second, in most studies conducted so far, the medicinal properties of L. rhinocerotis were demonstrated using crude mushroom extracts or mixture containing different constituents. It is therefore necessary to identify the active bio-molecules for a better grasp of the medicinal properties and the mechanistic pathways of the novel compounds. Figure 3 provides an overview of the recent findings of the medicinal properties of L. rhinocerotis and the proposed mechanistic pathways of some of the activities.
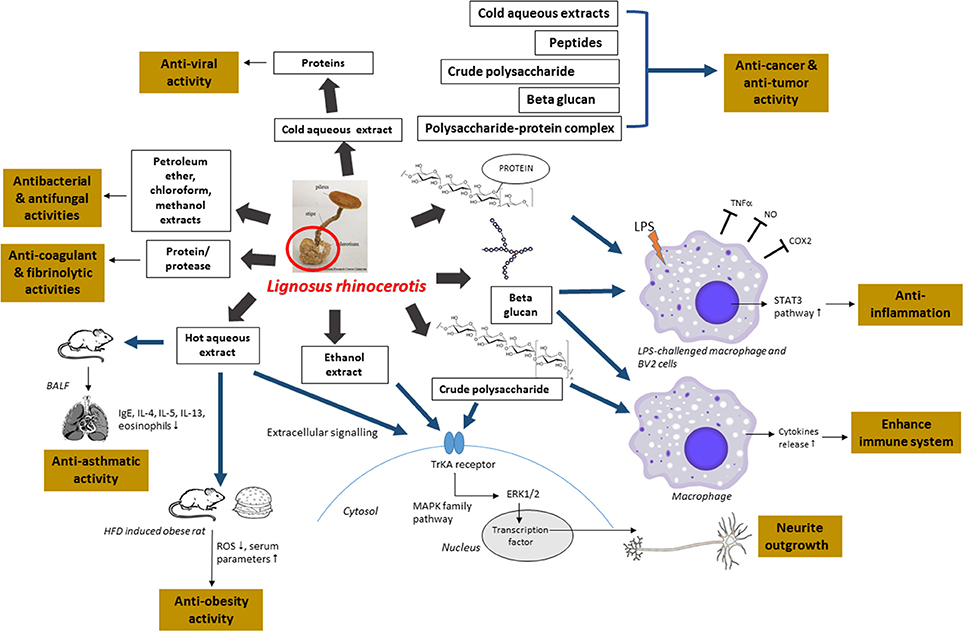
Figure 3. An overview of the recent findings of the medicinal properties of L. rhinocerotis and the proposed mechanistic pathways. Ig, immunoglobulin; BALF, bronchoalveolar lavage fluid; IL, interleukin; TNF-α, tumor necrosis factor alpha; LPS, lipopolysaccharide; NO, nitric oxide; COX2, cyclooxygenase-2; STAT3, signal transducer and activator of transcription 3; ROS, reactive oxygen species; HFD, high fat diet; ERK, extracellular signal-regulated kinase; Trk, tyrosine kinase; MAPK, mitogen activated protein kinase.
Third, many findings were based on in vitro results. Thus, these studies should be further investigated in in vivo and human/ clinical studies to validate the uses scientifically. Besides, more applications for L. rhinocerotis extracts/ bioactive compounds should be ventured such as nanotechnology and genomic identifications. Finally, more surveys should be conducted in cooperation with the indigenous people to discover more traditional medicine applications. This will enhance the understanding of the medicinal properties based on traditional knowledge and applications for evidential documentation.
Conclusion
The L. rhinocerotis sclerotium is well-known for its ethnomedicinal uses in curing many ailments. The efforts to research, compile, and validate the information scientifically is a continuous process in the development of medicinally relevant products. This review showed that the sclerotium of L. rhinocerotis possessed several potential therapeutic properties that would be useful to human's health. Further research is warranted to identify and isolate chemical components/bioactive components and their mode of actions.
Author Contributions
NN, C-WP, SL-SS, AB, and VS have written the first draft of the manuscript. HL and SNAM revised and improved the first draft. All authors have seen and agreed on the finally submitted version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank University of Malaya and the Ministry of Education Malaysia for the High Impact Research Grants UM-MOHE UM.C/625/1/HIR/MOHE/SC/02 and UM-MOHEUM.C/625/1/HIR/MOHE/ASG/01. We also acknowledge the Postgraduate Research Grant (PG110-2012B) from the Institute of Research Management & Monitoring (IPPP), University of Malaya, for the support of postgraduate students.
References
Abdullah, N., Haimi, M. Z. D., Lau, B. F., Annuar, M. S. M., Zuhayr, M., Haimi, D., et al. (2013). Domestication of a wild medicinal sclerotial mushroom, Lignosus rhinocerotis (Cooke) Ryvarden. Ind. Crops Prod. 47, 256–261. doi: 10.1016/j.indcrop.2013.03.012
Abdullah, N., Wahab, A. I. A., Lau, B. F., Abidin, N. Z., and Aminudin, N. (2010). “Anti-cervical cancer activity and SELDI-TOF-MS analysis of proteins from Lignosus rhinocerus (Tiger's Milk Mushroom) grown in stirred tank reactor,” in Proceedings of the Fifth International Peptide Symposium (Kyoto).
Baskaran, A. (2015). Suppression of Lipopolysaccharide and Hydrogen Peroxide-Induced Inflammatory Responses in Raw 264.7 Macrophage by Pleurotus giganteus and Lignosus rhinocerotis. University of Malaya.
Baskaran, A., Sabaratnam, V., and Kuppusamy, U. R. (2012). “Inhibition of lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophage by extracts of selected medicinal mushrooms,” in 17th Biological Science Graduate Congress (Bangkok).
Beveridge, H. (1909). Tuzuk-e Jahangiri or Memoirs of Jahangir. Trans. R. Alexander (London: Royal Asiatic Society).
Burkill, I. H. (1930). An Index to the Malay Vernacular Names with Comments. Straits Settlements: Gardens' Bull.
Chang, Y. S. (2015). Tiger's Milk is a Fungus with Medicinal Value. Kuala Lumpur: New Straits Times Press.
Chang, Y. S., and Lee, S. S. (2004). Utilisation of macrofungi species in Malaysia. Fungal Divers. 15, 15–22. Available online at: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.459.4963&rep=rep1&type=pdf
Chang, Y. S., and Lee, S. S. (2010). Traditional knowledge about mushroom utilization by the sub-tribe Semaiin Peninsular Malaysia. Moeszia 5–6, 84–88.
Chen, C., Hamdan, N., Ooi, F., and Wan Abd Hamid, W. (2016). Combined effects of Lignosus rhinocerotis supplementation and resistance training on isokinetic muscular strength and power, anaerobic and aerobic fitness level, and immune parameters in young males. Int. J. Prev. Med. 7, 107. doi: 10.4103/2008-7802.190604
Chen, T.-I., Zhuang, H.-W., Chiao, Y.-C., and Chen, C.-C. (2013). Mutagenicity and genotoxicity effects of Lignosus rhinocerotis mushroom mycelium. J. Ethnopharmacol. 149, 70–74. doi: 10.1016/j.jep.2013.06.001
Choong, Y., Xu, C., Lan, J., Chen, X., and Jamal, J. (2014). Identification of geographical origin of Lignosus samples using Fourier transform infrared and two-dimensional infrared correlation spectroscopy. J. Mol. Struct. 1069, 188–195. doi: 10.1016/j.molstruc.2014.04.001
Cooke, M. (1879). XV. Enumeration of Polyporus. Trans. Proc. Bot. Soc. Edinb. 13, 131–159. doi: 10.1080/03746607909468768
Cui, B.-K., Tang, L.-P., and Dai, Y.-C. (2011). Morphological and molecular evidences for a new species of Lignosus (Polyporales, Basidiomycota) from tropical China. Myco. Prog. 10, 267–271. doi: 10.1007/s11557-010-0697-y
Eik, L.-F., Naidu, M., David, P., Wong, K.-H., Tan, Y.-S., and Sabaratnam, V. (2012). Lignosus rhinocerus (Cooke) Ryvarden: A medicinal mushroom that stimulates neurite outgrowth in PC-12 cells. Evid. Based Complement. Alternat. Med. 2012:320308. doi: 10.1155/2012/320308
Eik, L. F., Naidu, M., David, R. P., Wong, K. H., and Sabaratnam, V. (2011). “Stimulation of neurite outgrowth in PC-12 cells by sclerotium of the national treasure mushroom: Lignosus rhinocerus (Cooke) Ryvarden,” in Proceedings of the International Congress of the Malaysian Society for Microbiology (Penang).
Ellan, K., Sabaratnam, V., and Thayan, R. (2013). “Antiviral activity and mode of action of mushroom extracts against dengue virus type-2,” in Proceedings of the 3rd International Conference Dengue Dengue Haemorrhagic Fever (Bangkok).
Ellan, K., Thayan, R., Hidari, K. I. P. J., Raman, J., and Sabaratnam, V. (2014). “Mode of anti dengue activity of selected culinary and medicinal mushroom,” in 32nd Symposium of the Malaysian society of Microbiology (Terengganu).
Guo, C., Wong, K.-H., and Cheung, P. C. K. (2011). Hot water extract of the sclerotium of Polyporus rhinocerus Cooke enhances the immune functions of murine macrophages. Int. J. Med. Mushrooms 13, 237–244. doi: 10.1615/IntJMedMushr.v13.i3.30
Hartland, E. S. (1909). Primitive Paternity: The Myth of Supernatural Birth in Relation to the History of the Family. London: D. Nutt.
Hattori, T., Noraswati, M., and Salmiah, U. (2007). “Basidiomycota: diversity of Malaysian polypores,” in Malaysian Fungal Diversity, eds S. Jones, E. B. G. Hyde, and K. D. Vikineswary (Kuala Lumpur: Mushroom Research Centre, University of Malaya and Ministry of Natural Resources and Environment), 55–68
Hilton, R. N., and Dhitaphichit, P. (1978). Procedures in Thai ethnomycology. Nat. Hist. Bull. Siam Soc. 41, 75–92.
Hoe, T. L. (2014). Lignosus rhinocerus Attenuated High Fat Diet Induced Non-Alcoholic Fatty Liver. National Chung Hsing University.
John, P. A., Sabaratnam, V., David, R. P., and Naidu, M. (2011). “A combination of curcumin and Lignosus rhinocerus mycelium aqueous extract for neurite outgrowth stimulation activity,” Proceedings of the International Congress of the Malaysian Society for Microbiology (Penang).
John, P. A., Wong, K. H., David, R. P., Naidu, M., and Sabaratnam, V. (2012). “Combination effects on Lignosus rhinocerus (Cooke) Ryvarden mycelium and Gingko biloba aqueous extracts on PC-12 cells neurite outgrowth stimulation activity,” in National Postgraduate Seminar (Kuala Lumpur).
John, P. A., Wong, K.-H., Naidu, M., Sabaratnam, V., and David, M. (2013). Combination effects of curcumin and aqueous extract of Lignosus rhinocerotis mycelium on neurite outgrowth stimulation activity in PC-12 cells. Nat. Prod. Commun. 8, 711–714.
Johnathan, M., Gan, S. H., Ezumi, M. F. W., Faezahtul, A. H., and Nurul, A. A. (2016). Phytochemical profiles and inhibitory effects of Tiger Milk mushroom (Lignosus rhinocerus) extract on ovalbumin-induced airway inflammation in a rodent model of asthma. BMC Complement. Alternat. Med. 16, 167. doi: 10.1186/s12906-016-1141-x
Kho, T. T. (2014). Fibrinolytic Activities of a Medicinal Mushroom: Lignosus rhinocerotis (Cooke) Ryvarden. University of Malaya.
Lai, C. K. M. (2005). Antitumor Effects of Polysaccharides Extracted from Mushroom Sclerotia: an in vitro and in vivo Study. The Chinese University of Hong Kong.
Lai, C., Wong, K. H., and Cheung, P. C. K. (2008). Antiproliferative effects of sclerotial polysaccharides from Polyporus rhinocerus Cooke (Aphyllophoromycetideae) on different kinds of leukemic cells. Int. J. Med. Mushrooms 10, 255–264. doi: 10.1615/IntJMedMushr.v10.i3.60
Lai, W. H., Siti Murni, M. J., Fauzi, D., Abas Mazni, O., and Saleh, N. M. (2011). Optimal culture conditions for mycelial growth of Lignosus rhinocerus. Mycobiol. 39, 92–95. doi: 10.4489/MYCO.2011.39.2.092
Lai, W. H., Zainal, Z., and Daud, F. (2014). Preliminary study on the potential of polysaccharide from indigenous Tiger's Milk mushroom (Lignosus rhinocerus) as anti-lung cancer agent. AIP Conf. Proc. 517, 517–519. doi: 10.1063/1.4895252
Lai, W., Tei, V., Loo, S., and Abas Mazni, O. (2009). “Molecular identification of Lignosus rhinocerus (tiger-milk mushroom) using 28SrRNA,” in Proceedings of the 8th Malaysia Congress on Genetics (Pahang).
Lau, B. F., Abdullah, N., Aminudin, N., Lee, H. B., and Tan, P. J. (2015). Ethnomedicinal uses, pharmacological activities, and cultivation of Lignosus spp. (tiger's milk mushrooms) in Malaysia- A review. J. Ethnopharmacol. 169, 441–458. doi: 10.1016/j.jep.2015.04.042
Lau, B. F., Abdullah, N., Aminudin, N., Lee, H. B., Yap, K. C., and Sabaratnam, V. (2014). The potential of mycelium and culture broth of Lignosus rhinocerotis as substitutes for the naturally occurring sclerotium with regard to antioxidant capacity, cytotoxic effect, and low-molecular-weight chemical constituents. PLoS ONE 9:e102509. doi: 10.1371/journal.pone.0102509
Lau, B. F., Aminudin, N., and Abdullah, N. (2011). Comparative SELDI-TOF-MS profiling of low-molecular-mass proteins from Lignosus rhinocerus (Cooke) Ryvarden grown under stirred and static conditions of liquid fermentation. J. Microbiol. Methods 87, 56–63. doi: 10.1016/j.mimet.2011.07.005
Lau, F., Abdullah, N., and Aminudin, N. (2013a). Chemical composition of the tiger's milk mushroom, Lignosus rhinocerotis (Cooke) Ryvarden, from different developmental stages. J. Agric. Food Chem. 61, 4890–4897. doi: 10.1021/jf4002507
Lau, F., Abdullah, N., Aminudin, N., and Boon, H. (2013b). Chemical composition and cellular toxicity of ethnobotanical-based hot and cold aqueous preparations of the tiger′s milk mushroom (Lignosus rhinocerotis). J. Ethnopharmacol. 150, 252–262. doi: 10.1016/j.jep.2013.08.034
Lee, M. L., Tan, N. H., Fung, S. Y., Tan, C. S., and Ng, S. T. (2012a). The antiproliferative activity of sclerotia of Lignosus rhinocerus (Tiger Milk Mushroom). Evid. Based Complem. Alternat. Med. 2012:697603. doi: 10.1155/2012/697603
Lee, S. S., and Chang, Y. S. (2007). “Ethnomycology,” in Malaysian Fungal Diversity, eds E. B. G. Jones, K. D. Hyde, and V. Sabaratnam (Kuala Lumpur: Mushroom Research Centre, University of Malaya and Ministry of Natural Resources and Environment).
Lee, S. S., Chang, Y. S., and Noraswati, M. N. R. (2009). Utilization of macrofungi by some indigenous communities for food and medicine in Peninsular Malaysia. Forest Ecol. Manag. 257, 2062–2065. doi: 10.1016/j.foreco.2008.09.044
Lee, S. S., Enchang, F. K., Tan, N. H., Fung, S. Y., and Pailoor, J. (2013a). Preclinical toxicological evaluations of the sclerotium of Lignosus rhinocerus (Cooke), the Tiger Milk mushroom. J. Ethnopharmacol. 147, 157–163. doi: 10.1016/j.jep.2013.02.027
Lee, S. S., Fung, S. Y., Sim, S. M., and Tan, N. H. (2013b). “Anti-inflammatory effect of the cultivated sclerotium of Lignosus rhinocerus (Cooke), the tiger milk mushroom,” in Proceedings of the 38th Annual Conference of the Malaysian Society for Biochemistry and Molecular Biology (Kuala Lumpur).
Lee, S. S., Tan, N. H., Fung, S. Y., Sim, S. M., Tan, C. S., and Ng, S. T. (2014). Anti-inflammatory effect of the sclerotium of Lignosus rhinocerotis (Cooke) Ryvarden, the Tiger Milk mushroom. BMC Complem. Alternat. Med. 14:359. doi: 10.1186/1472-6882-14-359
Lee, S. S., Tan, N. H., Fung, S. Y., Tan, C. S., Ng, S. T., and Sim, S. M. (2012b). “Anti-inflammatory activity of Lignosus rhinocerus (tiger milk mushroom) sclerotia,” in Abstracts of the 18th Congress of the International Society for Mushroom Science (Beijing), 150–151.
Lee, S.-Y., Kim, J.-S., Kim, J.-E., Sapkota, K., Shen, M.-H., Kim, S., et al. (2005). Purification and characterization of fibrinolytic enzyme from cultured mycelia of Armillaria mellea. Prot. Expr. Pur. 43, 10–17. doi: 10.1016/j.pep.2005.05.004
Liu, C., Chen, J., Chen, L., Huang, X., and Cheung, P. C. K. (2016). Immunomodulatory activity of polysaccharide-protein complex from the mushroom sclerotia of Polyporus rhinocerus in murine macrophages. J. Agric. Food Chem. 64, 3206–3214. doi: 10.1021/acs.jafc.6b00932
Malagobadan, J., Gan, S. H., Fuad, W. E. M., Wan Mohamad, W. M., Hussein, F. A., and Abdullah, N. A. (2014). “Inhibitory effects of Tiger Milk mushroom extract on airway inflammation in ovalbumin-induced allergic asthma,” in Proceeding of the Immunology Symposium (Serdang).
Manzi, P., Gambelli, L., Marconi, S., Vivanti, V., and Pizzoferrato, L. (1999). Nutrients in edible mushrooms: an inter-species comparative study. Food Chem. 65, 477–482. doi: 10.1016/S0308-8146(98)00212-X
Mohamed Ali, S., Ling, T. C., Muniandy, S., Tan, Y. S., Raman, J., and Sabaratnam, V. (2014). Recovery and partial purification of fibrinolytic enzymes of Auricularia polytricha (Mont.) Sacc by an aqueous two-phase system. Separ. Pur. Technol. 122, 359–366. doi: 10.1016/j.seppur.2013.11.016
Mohammad, N. S., Milow, P., and Ong, H. C. (2012). Traditional medicinal plants used by the Kensiu Tribe of Lubuk Ulu Legong, Kedah, Malaysia. Ethno Med. 6, 149–153. doi: 10.1080/09735070.2012.11886432
Mohanarji, S., Dharmalingam, S., Kalusalingam, A., and Science, A. (2012). Screening of Lignosus rhinocerus extracts as antimicrobial agents against selected human pathogens. J. Pharm. Biomed. Sci. 18, 1–4.
Nallathamby, N., Lee, G. S., Raman, J., Malek, S. N. A., Vidyadaran, S., Naidu, M., et al. (2016). Identification and in vitro evaluation of lipids from sclerotia of Lignosus rhinocerotis for antioxidant and anti-neuroinflammatory activities. Nat. Prod. Commun. 11, 1485–1490.
Nallathamby, N., Malek, S., Kuppusamy, U., and Sabaratnam, V. (2014). “Antioxidant properties of ethanol extract and its fractions of Lignosus rhinocerotis (Cooke) Ryvarden,” in 18th Biological Sciences Graduate Congress (Kuala Lumpur).
Nallathamby, N., Malek, S. N. A., Naidu, M., Wong, K. H., David, P., and Sabaratnam, V. (2013). “Cytotoxicity effect of selected medicinal mushrooms on BV2 microglial cells,” in Proceedings of the 7th International Medicinal Mushroom Conference (Beijing).
Phan, C.-W., David, P., Naidu, M., Wong, K.-H., and Sabaratnam, V. (2013). Neurite outgrowth stimulatory effects of culinary-medicinal mushrooms and their toxicity assessment using differentiating Neuro-2a and embryonic fibroblast BALB/3T3. BMC Complem. Alternat. Med. 13:261. doi: 10.1186/1472-6882-13-261
Robert, W. (1986). The Soul of Ambiguity: The Tiger in Southeast Asia. Illinois: Northern Illinois University; Center for Southeast Asian Studies.
Sabaratnam, V., Wong, K.-H., Naidu, M., and David, P. R. (2013). Neuronal health – can culinary and medicinal mushrooms help? J. Tradit. Complement. Med. 3, 62–68. doi: 10.4103/2225-4110.106549
Samberkar, S., Gandhi, S., Naidu, M., Wong, K.-H., Raman, J., and Sabaratnam, V. (2015). Lion's Mane, Hericium erinaceus and Tiger Milk, Lignosus rhinocerotis (Higher Basidiomycetes) medicinal mushrooms stimulate neurite outgrowth in dissociated cells of brain, spinal cord, and retina: an in vitro study. Int. J. Med. Mushrooms 17, 1047–1054. doi: 10.1615/IntJMedMushrooms.v17.i11.40
Seow, S. L. S. (2016). Ethyl Acetate and n-Butanol Fractions of Lignosus rhinocerotis (Cooke) Ryvarden Induced Neuritogenesis in PC-12 Cells via MEK/ERK1/2/CREB and PI3K/AKT/mTOR/CREB Signaling Pathways. University of Malaya.
Seow, S. L.-S., Eik, L.-F., Naidu, M., David, P., Wong, K.-H., and Sabaratnam, V. (2015). Lignosus rhinocerotis (Cooke) Ryvarden mimics the neuritogenic activity of nerve growth factor via MEK/ERK1/2 signaling pathway in PC-12 cells. Sci. Rep. 5:16349. doi: 10.1038/srep16349
Seow, S. L. S., Naidu, M., David, P., Wong, K. H., and Sabaratnam, V. (2013b). “Tiger's milk mushrooms – Nature's hidden treasure that promotes neuro health,” in Proceedings of the International Functional Food Conference (Cyberjaya).
Seow, S. L.-S., Naidu, M., David, P., Wong, K., and Sabaratnam, V. (2013a). Potentiation of neuritogenic activity of medicinal mushrooms in rat pheochromocytoma cells. BMC Complem. Alternat. Med. 13:157. doi: 10.1186/1472-6882-13-157
Seow, S. L. S., Naidu, M., Sabaratnam, V., Vidyadaran, S., and Wong, K. H. (2017). Tiger's milk medicinal mushroom, Lignosus rhinocerotis (Cooke) Ryvarden sclerotium inhibits nitric oxide production in LPS-stimulated BV2 microglia. Int. J. Med. Mushrooms 19, 405–418. doi: 10.1615/IntJMedMushrooms.v19.i5.30
Shien, S., Hong, N., Yee, S., Pailoor, J., and Mui, S. (2011). Evaluation of the sub-acute toxicity of the sclerotium of Lignosus rhinocerus (Cooke), the Tiger Milk mushroom. J. Ethnopharmacol. 138, 192–200. doi: 10.1016/j.jep.2011.09.004
Sidek Ahmad, M., Noor, Z. M., and Ariffin, Z. Z. (2014). New thrombolytic agent from endophytic fungi and Lignosus rhinocerus. Open Conf. Proc. J. 4, 95–98. doi: 10.2174/2210289201304020095
Skeat, W. W. (1896). A vocabulary of the Besisi dialect. J. Straits Branch Royal Asiatic Soc. 29, 13–31.
Skeat, W. W., and Ottoblagoen, C. (1906). Pagan Races of the Malay Peninsular. New York, NY: MacMillan and Co, Limited.
Sotome, K., Hattori, T., Ota, Y., To-anun, C., Salleh, B., and Kakishima, M. (2008). Phylogenetic relationships of Polyporus and morphologically allied genera. Mycologia 100, 603–615. doi: 10.3852/07-191R
Suziana Zaila, C. F. (2013). Antiproliferative effect of Lignosus rhinocerotis, the Tiger Milk Mushroom on HCT 116 human colorectal cancer cells. Open Conf. Proc. J. 4, 65–70. doi: 10.2174/2210289201304020065
Tan, C. S., Ng, S. T., Vikineswary, S., Lo, F. P., and Tee, C. S. (2010). Genetic markers for identification of a Malaysian medicinal mushroom, Lignosus rhinocerus (cendawan susu rimau) Acta Hortic. 859, 161–167. doi: 10.17660/ActaHortic.2010.859.19
Tan, W.-C., Kuppusamy, U. R., Phan, C.-W., Tan, Y.-S., Raman, J., Anuar, A. M., et al. (2015). Ganoderma neo-japonicum Imazeki revisited: domestication study and antioxidant properties of its basidiocarps and mycelia. Sci. Rep. 5:12515. doi: 10.1038/srep12515
Teo, C. P. (2014). In-vitro Investigation of Anticoagulant Activities in Edible and Medicinal Mushrooms. Kuala Lumpur: University of Malaya
Wong, K.-H., and Cheung, P. C. K. (2009). “Sclerotia: emerging functional food derived from mushrooms,” in Mushrooms as Functional Foods, ed P. C. K. Cheung (Hoboken, NJ: John Wiley & Sons, Inc), 111–146.
Wong, K. H., Lai, C. K. M., and Cheung, P. C. K. (2011). Immunomodulatory activities of mushroom sclerotial polysaccharides. Food Hydrocol. 25, 150–158. doi: 10.1016/j.foodhyd.2010.04.008
Yap, H.-Y. Y., Chooi, Y.-H., Firdaus-Raih, M., Fung, S.-Y., Ng, S.-T., Tan, C.-S., et al. (2014). The genome of the Tiger Milk mushroom, Lignosus rhinocerotis, provides insights into the genetic basis of its medicinal properties. BMC Genom. 15:635. doi: 10.1186/1471-2164-15-635
Yap, H. Y. Y., Fung, S. Y., Ng, S. T., Tan, C. S., and Tan, N. H. (2015). Genome-based proteomic analysis of Lignosus rhinocerotis (Cooke) Ryvarden sclerotium. Int. J. Med. Sci. 12, 23-31. doi: 10.7150/ijms.10019
Yap, Y. H. Y., Tan, N., Fung, S., Aziz, A. A., Tan, C., and Ng, S. (2013). Nutrient composition, antioxidant properties, and anti-proliferative activity of Lignosus rhinocerus Cooke sclerotium. J. Sci. Food Agric. 93, 2945–2952. doi: 10.1002/jsfa.6121
Keywords: Lignosus rhinocerotis, medicinal mushroom, sclerotium, medicinal properties, neuroprotection, antioxidant, ethnomedicine, mycomedicine
Citation: Nallathamby N, Phan C-W, Seow SL-S, Baskaran A, Lakshmanan H, Abd Malek SN and Sabaratnam V (2018) A Status Review of the Bioactive Activities of Tiger Milk Mushroom Lignosus rhinocerotis (Cooke) Ryvarden. Front. Pharmacol. 8:998. doi: 10.3389/fphar.2017.00998
Received: 11 September 2017; Accepted: 26 December 2017;
Published: 15 January 2018.
Edited by:
Banasri Hazra, Jadavpur University, IndiaReviewed by:
Tauqeer Hussain Mallhi, University of Science, Malaysia, MalaysiaWilliam Chi-Shing Tai, Hong Kong Polytechnic University, Hong Kong
Copyright © 2018 Nallathamby, Phan, Seow, Baskaran, Lakshmanan, Abd Malek and Sabaratnam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vikineswary Sabaratnam, dmlraUB1bS5lZHUubXk=
 Neeranjini Nallathamby1
Neeranjini Nallathamby1 Chia-Wei Phan
Chia-Wei Phan Syntyche Ling-Sing Seow
Syntyche Ling-Sing Seow Hariprasath Lakshmanan
Hariprasath Lakshmanan Sri N. Abd Malek
Sri N. Abd Malek Vikineswary Sabaratnam
Vikineswary Sabaratnam