
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pediatr., 27 September 2024
Sec. Neonatology
Volume 12 - 2024 | https://doi.org/10.3389/fped.2024.1432808
This article is part of the Research TopicWhat is new on the Horizon in Neonatology? Recent Advances in Monitoring, Diagnostics, and Therapeutics in Neonatal CareView all 21 articles
Necrotizing enterocolitis (NEC) remains a devastating disease in preterm and term neonates. Despite significant progress made in understanding NEC pathogenesis over the last 50 years, the inability of current definitions to discriminate the various pathophysiological processes underlying NEC has led to an umbrella term that limits clinical and research progress. In this mini review, we provide a historical perspective on how NEC definitions and pathogenesis have evolved to our current understanding of NEC endotypes. We also discuss how artificial intelligence-based approaches are influencing our knowledge of risk-factors, classification and prognosis of NEC and other neonatal intestinal injury phenotypes.
Necrotizing enterocolitis (NEC) is a devastating disease in premature infants with an incidence of 5%–12% in very low birthweight infants (1–4). While less common than in preterm infants, recent studies have also identified risk-factors that predispose full-term neonates to NEC (5–8). Despite significant progress made in understanding NEC pathogenesis over the last 50 years, the inability of current definitions to discriminate the various pathophysiological processes underlying NEC has led to an umbrella term that limits clinical and research progress (9, 10). Further, the lack of precise clinical, biochemical and radiological tools to define NEC has hindered progress in delineating it from different conditions such as septic ileus (9). We propose that identifying endotypes of NEC based on pathophysiology, epidemiology and diagnostic tools will pave the way for precision approaches in preventing and treating NEC (11, 12). In this review, we provide a historical perspective on how NEC definitions and pathogenesis have evolved to our current understanding of NEC endotypes (Figure 1). We also discuss how artificial intelligence (AI)-based approaches are influencing our knowledge of risk-factors, classification and prognosis of NEC endotypes and other neonatal intestinal injury phenotypes.
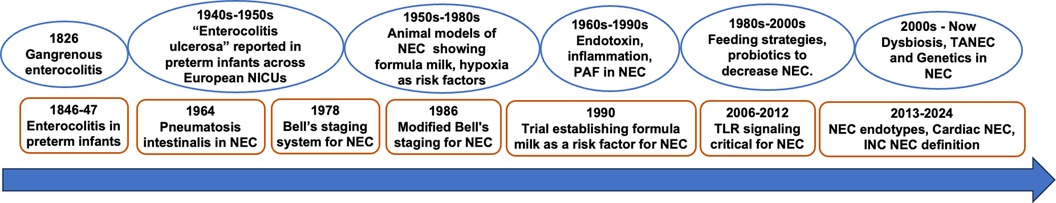
Figure 1. NEC – a brief history in time. INC, International Neonatal Consortium; PAF, platelet activating factor; TANEC, transfusion-associated NEC; TLR, Toll-Like Receptor.
The earliest report of an endotype resembling NEC could be Charles Billard's description 200 years ago from the Hôpital des Enfants Trouvés (13, 14). In his textbook, he describes “gangrenous enterocolitis” in neonates, characterized by abdominal distension, bloody stools, septicemia and death. Autopsy reports showed the ileum was particularly affected with erythema, swelling, ecchymosis, and friability (14). A systematic report of preterm NEC is found in Bednar's description of “entero-colitis” in 25 infants admitted to the Vienna hospital for foundlings between 1846 and 1847, 7 of whom were premature, with majority developing disease between 3 and 30 days of life. In 20 infants who died, autopsy showed evidence of necrosis, gangrene and hemorrhage, very similar to current descriptions of severe advanced NEC (15). In several neonatal intensive care units established across Europe to care for preterm infants between 1910 and 1940s, a NEC-like disease with pathological features including intestinal perforation in lethal cases is poignantly described (16, 17). Between 1948 and 1950, Schmidt and Kaiser et al. described “enterocolitis ulcerosa” in 85 mostly breast-fed preterm infants in Graz, Germany (18, 19). They accurately documented the ileocecal involvement, peritonitis, and perforation and speculated the role of a specific pathogen. Apart from the lack of corroborative radiological, cytological and bacteriology evidence, these physicians established the pathology of NEC and identified the at-risk preterm infant population. These studies did not delve into the pathophysiology of NEC, but hinted at infectious etiology, including viruses.
While pneumatosis intestinalis had been reported in NEC before, it was Berdon in 1964, who described the entire spectrum of what has become the signe qua non of NEC diagnosis (20–22). Bell et al. in 1978 proposed the first systematic classification of NEC, grading it from stage I to III based on clinical signs, biochemical markers, radiological signs and disease severity (23). This significant advance enabled consistency among clinicians and researchers to classify NEC more accurately than before, and also provided severity-based treatment guidelines. Around the time Bell et al. classified NEC, several investigators using animal and human studies suggested that hypoxia, formula feeding, speed of feed advancement and infection were risk factors for NEC in the late preterm population (24–26). Walsh and Kliegman in 1986 modified this classification to 6 categories with two subcategories for stage I, II and III (10). In their classical paper, they also summarized the existing thoughts on pathogenesis of NEC indicating the potential roles for direct intestinal infections, bacterial overgrowth, formula feeding, milk intolerance, ischemia, hypertonic enteral supplements in causing mucosal injury and inflammation in an immature gut (10). Interestingly, their summary hinted at multi-factorial causation and a broad spectrum of mechanisms underlying NEC evolution. While several other definitions including the Vermont Oxford Network, Centers of Disease Control and Prevention and the UK Neonatal Collaborative NEC Study group among others have defined NEC, Bell's staging and modified Bell's staging are still the most commonly accepted definitions for NEC (12). From mid-1980s to the current era, several investigators have unraveled the role of dysregulated Toll Like receptor 4 (TLR4) signaling, gut microbiome and dysbiosis, inflammatory mediators and genetic predisposition in NEC (1, 4, 27–35).
Recognizing the limitation of the Bell's criteria in not recognizing spontaneous ileal perforation (SIP), differentiating NEC in term infants vs. preterm infants, and standardizing definitions for research purposes, the International Neonatal Consortium NEC definition groups proposed to classify NEC based on gestational-age, timing of onset of disease, one of the two clinical signs (hematochezia and abdominal distension), and radiological evidence (9). These criteria demarcate NEC that develops in preterm infants from NEC in term infants, and also distinguishes SIP and septic ileus from NEC in preterm infants. While these criteria do address some of the short comings of the previous NEC definitions, it is agnostic with respect to the different pathogenic mechanisms and the resulting endotypes. In the subsequent paragraphs, we will briefly review current understanding of NEC and NEC-like intestinal injury endotypes in preterm and term infants focusing on differences in the pathogenic mechanisms, timing of onset, distinguishing features, and prognosis. A summary of different NEC endotypes is presented in Table 1.
The most common endotype of NEC – coined “classical NEC” – occurs in preterm infants. The onset of presentation of classical NEC has an inverse relationship with gestational age (36), often occuring in the 28–32 week postmenstrual age with sudden onset of feeding intolerance, abdominal distention, and bloody stools that can rapidly progress towards intestinal perforation, peritonitis, and multi-organ dysfunction (4). The presence of pneumatosis intestinalis and/or portal venous gas on imaging is diagnostic of the disease, while free air heralds intestinal perforation requiring surgery (37). A gasless abdomen, or fixed, dilated loops – defined by persistent location and configuration for more than 24 h – are also a concerning imaging finding for NEC (38, 39).
While the pathogenesis of classical NEC is multifactorial, prematurity remains its single most important risk factor, with NEC incidence rising as gestational age and birth weight decrease. The immature preterm gut is structurally and functionally underdeveloped, with decreased mucosal integrity, reduced motility, and impaired barrier function. Preterm infants also possess an immature immune system that predisposes them to aberrant inflammatory responses. Experimental studies reveal excessive Toll-like receptor (TLR) activation as a key pathway that drives intestinal inflammation in NEC (40). The other major player in classical NEC pathogenesis is the gut microbiota. Dysbiosis – driven by formula-feeding, antibiotic exposure, and perinatal stress – can induce aberrant inflammation in the preterm gut causing mucosal injury, translocation of bacteria into the circulation and subsequent multi-organ dysfunction (1, 29). Conversely, factors that promote a healthy gut microbiome – such as breastmilk, avoidance of prolonged antibiotics, and probiotics – decreases the risk of NEC (41–44).
Despite these advances in our understanding, classical NEC remains a complex disease with significant morbidity and mortality risks. For instance, NEC continues to occur despite avoidance of formula-feeding, exclusive use of human breastmilk, probiotics, and judicious antibiotic stewardship. It remains a leading cause of mortality, especially in infants with extensive intestinal necrosis requiring surgery. Survivors of NEC are also at increased risk for complications including strictures, short gut syndrome, growth failure, and neurodevelopmental impairments.
Another endotype of NEC in preterm infants is TANEC or transfusion related acute gut injury. TANEC often develops much later than classical NEC – after the 4th or 5th week of life – in otherwise stable preterm infants who have been established on enteral feeds for several weeks (45). Infants who develop TANEC have chronic anemia for several weeks, and symptoms of NEC often are evident within 48 h after packed red blood cell transfusion (45).
The exact pathogenesis of TANEC remains unknown. One proposed mechanism is that chronic anemia could mimic a state of ischemia-hypoxia in the mesenteric bed, and transfusion could trigger a reperfusion injury of previously ischemic intestinal tissue (46, 47). The generation of reactive oxygen species with reperfusion injury, combined with physiological demads of feeding, could be sufficient to cause mucosal damage and compromise the intestinal barrier, leading to TANEC. Based on this pathophysiology, withholding feeds around transfusion has been adopted by some to prevent TANEC (48), although good-quality evidence supporting this practice remains lacking (49). Another proposed mechanism is that intestinal injury arises from hemolytic factors in the transfused blood. In an experimental model of TANEC, free hemoglobin and heme in packed red blood cells were shown to activate monocytes and macrophages in the intestine, triggering excessive TLR inflammation and NEC (50). Interestingly, the Transfusion of Prematurity trial did not show differences in NEC rates among extremely preterm infants randomized to high vs. low transfusion thresholds, and a secondary analyses showed no temporal relationship between red blood cell transfusion and NEC (51, 52).
TANEC poses significant morbidity and mortality risks for preterm infants. In one meta-analysis, TANEC had higher odds of mortality compared to classical NEC (53). In contrast, a study using the Canadian Neonatal Network database found no significant differences in mortality and morbidities between TANEC and classical NEC (54).
Gastroenteritis caused by viral pathogens can mimic NEC (55, 56). Rotavirus (57, 58), cytomegalovirus (59), norovirus (60), astrovirus (61, 62), and enterovirus have been implicated in neonatal gastroenteritis (63). As viral infections are typically not considered in the differential diagnosis of NEC, a high index of suspicion is required. A viral cause is suspected when NEC occurs in clusters, coinciding with peak seasons of viral transmission (55, 64, 65). While clinical presentation can vary, most cases present similarly as classical NEC with gastrointestinal symptoms such as abdominal distention, feeding intolerance, bilious emesis, and bloody stools. Some infants present with systemic signs such as fever, apnea, lethargy, or irritability (e.g., norovirus); while others present with extra-intestinal manifestations such as respiratory symptoms (e.g., enterovirus) or hepatic dysfunction (e.g., cytomegalovirus). Early recognition of viral NEC could limit the use of antibiotics and direct appropriate anti-viral treatment, such as ganciclovir for cytomegalovirus.
The pathogenesis of viral NEC includes direct invasion of virus into intestinal epithelial cells, leading to cellular injury, disruption of tight junctions, and loss of barrier function. Viral infection also triggers an inflammatory response which can mimic classical NEC. Nevertheless, viral NEC tends to have a more insidious onset and a less fulminant clinical course compared to classical NEC, which often presents with rapid progression.
Term infants can also develop NEC, although the incidence is much less than in preterm infants. NEC in term infants typically presents earlier than preterm NEC, with average age of onset in the 1st week of life (5, 66). Term NEC is also typically secondary to other underlying disease processes, most notably congenital heart disease (67).
The incidence of NEC in term infants with congenital heart disease is about 2%–6%. The highest risk seems to occurr in conditions with ductal-dependent systemic blood flow such as hypoplastic left heart disease (68–70), although other lesions such as truncus arteriosus and common ventricle have been reported (71). Some studies suggest that the colon is significantly more involved in cardiac NEC (6), but others indicate the small intestine remains the primary location (72).
The pathophysiology of cardiac NEC is thought to be from mesenteric ischemia caused by anatomic lesions that disrupt systemic perfusion during diastole (73). Reperfusion injury following ischemia could also play a major role, particularly in infants who remain at increased risk for NEC even after surgical correction. Other mechanisms include hemodynamic changes while on cardiopulmonary bypass during surgery, or from medications such as vasopressin and opiates post-surgery (74–76). Because feeding can alter gastrointestinal perfusion and hemodynamics, there is often hesitancy to feed infants with cardiac disease, despite the absence of high-quality evidence (77).
Infants with cardiac NEC can have poor outcomes despite its occurrence predominantly in term infants. In one study, mortality rates were higher in infants with cardiac NEC compared to non-cardiac NEC (38% vs. 27%) (78). Prolonged hospital stay, mechanical ventilation, and parenteral nutrition were also noted (70, 71, 74). Although less common, NEC associated with severe congenital heart disease has also been reported in preterm infants, showing higher mortality compared to NEC in preterm infants without congenital heart disease (79).
Term and late-preterm infants with gastrointestinal anomalies – such as intestinal atresia, malrotation with volvulus, Hirschsprung's disease, gastroschisis, and omphalocele – are also at increased risk of NEC (80–82). In particular, NEC has been reported to occur in up to 20% of infants with gastroschisis (81, 82). The pathogenesis of NEC in these conditions is related to structural and functional anomalies of the intestine including intestinal obstruction, dysmotility, and vascular compromise. The anatomical complexity of gastrointestinal anomalies can increase morbidity and mortality of these infants who also develop NEC, as the presence of gastrointestinal anomalies would typically require surgical repair of the underlying anatomical defect.
Other conditions that could impair mesenteric blood flow or result in mesenteric ischemia – such as perinatal asphyxia, polycythemia, and septic shock – have been reported in term infants with NEC (67). In a review of term NEC cases at Intermountain Health, all cases of term NEC occurred in infants with gavage feeding, overfeeding, and/or feeding with formula (83). Moreover, overfeeding has been shown to be sufficient to elicit NEC injury in a mouse model (84). Thus, a possible unifying hypothesis regarding the pathogenesis of term NEC is the combination of underlying conditions that impair mesenteric blood flow with feeding.
While SIP also develops in extremely preterm infants it is a distinct entity from NEC. SIP tends to occur earlier, even before feeds have been initiated, and is associated with exposure to postnatal indomethacin and hydrocortisone (85, 86). While both can present with abdominal distention, SIP often presents with a bluish discoloration of the abdomen (87). Gross examination of the intestine in SIP reveals the perforation localized to the antimesenteric border of the small intestine with healthy tissue surrounding it. In NEC with perforation, the surrounding bowel is not healthy (88). The absence of bowel injury allows for SIP to have simpler surgical treatment with either peritoneal drainage, direct repair, or resection and primary anastomosis (87). In contrast, surgical NEC often requires resection of injured tissue with creation of stomas. Mortality from SIP is also lower compared to surgical NEC (89), but surprisingly morbidity and neurodevelopmental outcomes were not better (90, 91).
CMPA, also known as Food Protein-Induced Enterocolitis or FPIES, can present in both term and preterm infants with bloody stools and is thus an important differential for NEC (92). CMPA tends to occur much later, after several weeks of established feeds with formula or breastmilk that contains cow's milk protein (8). Severe cases can present with pneumatosis intestinalis, but otherwise do not progress towards systemic and multi-organ dysfunction (93). It can be difficult to distinguish CMPA from NEC. Typically, CMPA is suspected when reintroduction of feeds that contain cow-milk protein leads to “reoccurrence” of NEC-like symptoms (94). The pathogenesis of CMPA is non-IgE mediated allergic reaction to cow milk protein resulting in infiltration of intestinal mucosa with eosinophils, lymphocytes, and mast cells (95). In contrast to NEC, CMPA is a benign condition with low morbidity and typically improves with elimination of cow's milk protein from the infant's or mothers diet.
While clinicians have strived to define NEC more precisely and differentiate it from conditions that mimic NEC, these classifications still rely on prior conditioned learning (11). The potential for artificial intelligence (AI)-based approaches to classify in an unbiased fashion disease endotypes has resulted in several studies applying machine learning (ML) to classify NEC. AI is particularly useful in analyzing large datasets to detect intricate patterns not discoverable to human intelligence, and holds promise to improve diagnosis, classification, and management of acquired intestinal injury (96). When AI is utilized to analyze data within frameworks such as specific diagnosis/labels, known risk factors, or pre-determined outcomes it is commonly known as supervised ML. When AI is used to analyze data without labels or pre-determined framework/outcome, it is considered unsupervised analysis. In Table 2, a collection of ML models is depicted, along with a concise explanation and their contextual applications in NEC research.Through these models, patterns of disease in NEC that were not readily appreciated can be discovered (97).
Apart from prematurity, formula milk feeding, African American race (either as a social determinant of health or genetic risk), and potentially genetic factors, risk factors that are consistently associated with NEC remain unclear (1, 98, 99). Recently, Mueller et al. (100) used artificial neural networks to identify small for gestational age and use of artificial ventilation as additional risk factors for NEC. The utilization of continuous vital signs data has proven to be highly beneficial in diagnosing life-threatening diseases and improving outcomes for sepsis and other conditions in preterm infants (101). Vital signs data can also be leveraged using ML techniques to accurately predict NEC. Doheny et al. (102) analyzed the high frequency component of heart rate variability, an indicator of baseline vagal tone, in 70 preterm infants. The authors found that decreased vagal tone was a highly accurate predictor of NEC. ML can be used to identify white blood cell patterns to diagnose and prognosticate NEC. In a retrospective cohort of 246 infants, Pantalone et al. (103) reported that the onset of NEC in more mature infants (born after 33 weeks) was associated with lower neutrophil counts at diagnosis compared to controls. In less mature infants, a sharp decrease in monocytes and lymphocytes, as well as an elevation in bands at the time of diagnosis, predicted surgical intervention. The type of ML algorithm employed can also affect the findings as shown by Cho et al. (104) who compared the ability of several ML models to predict NEC. The study dataset consisted of over 10,000 very low birthweight infants and 74 variables, including environmental factors. Logistic regression and random forest (RF) exhibited superior performance achieving accuracy rates of 0.93, compared to artificial neural network, decision tree, naïve Bayes, and support vector machine methods. Birth weight, maternal age, gestational age, sepsis, male sex, and environmental factors such as ambient temperature were highlighted as key predictors, among others.
ML models have been used to predict intestinal perforations in NEC patients (NEC-IP). Using a Back-propagation neural network model, Irles et al. (105) identified that platelet counts, neutrophil counts, intubation, birth weight and arterial blood gas parameters can accurately predict NEC-IP. Recent ML studies have also demonstrated its utility to differentiate SIP from NEC-IP. Models such as random forest, ridge logistic regression model (106), and artificial neural network (107) have shown accuracy rates higher than 90% in differentiating these two conditions even before surgery, which can help guide optimal management strategies. Recent studies have also used ML models to predict need for surgical intervention in NEC (108, 109).
Researchers have also examined stool microbiome and metabolomic data using unsupervised ML algorithms to predict NEC (97). Notably, Lin et al. (110) used a multiple instance learning (MIL) architecture for predicting NEC based on stool microbiota data. Through incorporating past data with analysis of crucial bacterial taxa, this approach achieved timely and precise prediction of NEC risk, with an average lead time of 8.3 days. Their RF model emphasized the importance of Firmicutes, Proteobacteria and Enterobacteriaceae in NEC prediction with high level of sensitivity and specificity, thus emphasizing its potential in enabling personalized risk assessment and disease prevention (110). Other studies examined stool metabolomic (111) and urine peptides data (112) to find specific patterns to predict NEC. Recent endeavors have undertaken an unbiased assessment of distinct patterns of acquired intestinal injuries in preterm infants. By utilizing an unsupervised hierarchical clustering algorithm, Gipson et al. (113) successfully identified five distinct clusters of acquired neonatal intestinal injuries from a sample of 183 infants who experienced 210 episodes of such injuries. These clusters were classified as (1) low mortality, (2) immature with high mortality, (3) mature with inflammation, (4) late injury at full feeds, and (5) late injury with intestinal necrosis. These studies provide encouraging data for improving the prediction, accurate diagnosis and prognosis of NEC and other intestinal phenotypes that mimic NEC. One limitation of current AI studies includes limited data from single center design, smaller cohorts, and non-uniformity of variables used for analysis. As the accuracy and reliability of AI models rely solely on the quality of the input provided, reliability and generalizability of these models can be enhanced by using standardized datasets across multiple centers. Another important limitation is the complex and multi-factorial nature of NEC pathogenesis, which makes capturing all relevant variables and their interactions in an AI model challenging. AI models would also need to be continuously updated to incorporate new insights and research findings as our understanding of NEC evolves.
While our understanding of NEC has evolved over time from a clinical/pathological description to a better understanding of pathophysiology and NEC endotypes, only limited progress has been made in in differentiating classical NEC from endotypes that mimic it but have different etiologies and prognosis. We speculate that characterizing endotypes of NEC based on pathophysiology, clinical variables and radiological/biochemical tests using traditional clustering methods augmented by machine learning (ML) is important for precision approaches directed at disease prevention and management of NEC and acquired intestinal injury phenotypes in neonates.
AC: Investigation, Writing – original draft, Writing – review & editing. NK: Investigation, Writing – original draft, Writing – review & editing. VS: Investigation, Supervision, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Venkatash Sampath (5R01DK117296) and Alain Cuna (5K08DK125735) received support from the National Institute of Health.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hackam DJ, Sodhi CP. Bench to bedside—new insights into the pathogenesis of necrotizing enterocolitis. Nat Rev Gastroenterol Hepatol. (2022) 19:468–79. doi: 10.1038/s41575-022-00594-x
2. Bell EF, Hintz SR, Hansen NI, Bann CM, Wyckoff MH, DeMauro SB, et al. Mortality, in-hospital morbidity, care practices, and 2-year outcomes for extremely preterm infants in the US, 2013–2018. JAMA. (2022) 327(3):248–63. doi: 10.1001/jama.2021.23580
3. Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. (2015) 314(10):1039–51. doi: 10.1001/jama.2015.10244
4. Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. (2011) 364:255–64. doi: 10.1056/NEJMra1005408
5. Overman RE, Criss CN, Gadepalli SK. Necrotizing enterocolitis in term neonates: a different disease process? J Pediatr Surg. (2019) 54:1143–6. doi: 10.1016/j.jpedsurg.2019.02.046
6. Bubberman JM, van Zoonen A, Bruggink JLM, van der Heide M, Berger RMF, Bos AF, et al. Necrotizing enterocolitis associated with congenital heart disease: a different entity? J Pediatr Surg. (2019) 54(9):1755–60. doi: 10.1016/j.jpedsurg.2018.11.012
7. Kinstlinger N, Fink A, Gordon S, Levin TL, Friedmann P, Nafday S, et al. Is necrotizing enterocolitis the same disease in term and preterm infants? J Pediatr Surg. (2021) 56(8):1370–4. doi: 10.1016/j.jpedsurg.2021.01.007
8. Burris AD, Burris J, Järvinen KM. Cow’s milk protein allergy in term and preterm infants: clinical manifestations, immunologic pathophysiology, and management strategies. Neoreviews. (2020) 21:e795–808. doi: 10.1542/neo.21-12-e795
9. Caplan MS, Underwood MA, Modi N, Patel R, Gordon PV, Sylvester KG, et al. Necrotizing enterocolitis: using regulatory science and drug development to improve outcomes. J Pediatr. (2019) 212:208–15.e1. doi: 10.1016/j.jpeds.2019.05.032
10. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin N Am. (1986) 33:179–201. doi: 10.1016/S0031-3955(16)34975-6
11. Kim JH, Sampath V, Canvasser J. Challenges in diagnosing necrotizing enterocolitis. Pediatr Res. (2020) 88:16–20. doi: 10.1038/s41390-020-1090-4
12. Patel RM, Ferguson J, McElroy SJ, Khashu M, Caplan MS. Defining necrotizing enterocolitis: current difficulties and future opportunities. Pediatr Res. (2020) 88:10–5. doi: 10.1038/s41390-020-1074-4
13. Obladen M. Necrotizing enterocolitis–150 years of fruitless search for the cause. Neonatology. (2009) 96:203–10. doi: 10.1159/000215590
14. Billard CM, Ollivier CP. Traité Des Maladies Des Enfans Nouveau-Nés et à La Mamelle. Paris: Baillière (1928) Obs. 50.
15. Bednař A. Die Krankheiten der Neugeborenen und Säuglinge vom Clinischen Und Pathologisch-Anatomischen Standpunkte Bearbeitet. Wien: Gerold (1850). p. 101–3.
16. Ylppö A. Pathologie der frühgeborenen einschließlich der “debilen” und “lebensschwachen” kinder. In: Pfaundler MV, editor. Ergänzungswerk. Berlin, Heidelberg: Springer Berlin Heidelberg (1942). p. 96–108. doi: 10.1007/978-3-642-90967-2_4
17. von Willi H. Über eine bösartige enteritis bei säuglingen des ersten trimenons. Ann Pediatr. (1944) 162:87–112.
18. Schmidt K. Über eine besonders schwer verlaufende form von enteritis beim säugling, “enterocolitis ulcerosa necroticans”. I. Pathologisch-anatomische studien. Oesterr Z Kinderheilkd. (1952) 8:114–36.
19. Quaiser K. A specially severe form of enteritis in newborn, enterocolitis ulcerosa necroticans. II. Clinical studies. Osterr Z Kinderheilkd Kinderfuersorge. (1952) 8:136–52.13003088
20. Berdon WE, Grossman H, Baker DH, Mizrahi A, Barlow O, Blanc WA. Necrotizing enterocolitis in the premature infant. Radiology. (1964) 83:879–87. doi: 10.1148/83.5.879
21. Paris L. Pneumatosis cystoides intestinalis in infancy. J Pediatr. (1955) 46:1–17. doi: 10.1016/S0022-3476(55)80233-8
22. Stiennon OA. Pneumatosis Intestinalis in the newborn. AMA Am J Dis Child. (1951) 81:651–63. doi: 10.1001/archpedi.1951.02040030664004
23. Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. (1978) 187(1):1–7. doi: 10.1097/00000658-197801000-00001
24. Pitt J, Barlow B, Heird WC. Protection against experimental necrotizing enterocolitis by maternal milk. I. Role of milk leukocytes. Pediatr Res. (1977) 11:906–9. doi: 10.1203/00006450-197708000-00011
25. Barlow B, Santulli TV. Importance of multiple episodes of hypoxia or cold stress on the development of enterocolitis in an animal model. Surgery. (1975) 77:687–90.1173200
26. Brown EG, Sweet AY. Preventing necrotizing enterocolitis in neonates. JAMA. (1978) 240:2452–4. doi: 10.1001/jama.1978.03290220064019
27. Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol. (2006) 177:3273–82. doi: 10.4049/jimmunol.177.5.3273
28. Sodhi CP, Shi XH, Richardson WM, Grant ZS, Shapiro RA, Prindle T, et al. Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology. (2010) 138(1):185–96. doi: 10.1053/j.gastro.2009.09.045
29. Warner BB, Deych E, Zhou Y, Hall-Moore C, Weinstock GM, Sodergren E, et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet. (2016) 387(10031):1928–36. doi: 10.1016/S0140-6736(16)00081-7
30. Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome. (2017) 5:31. doi: 10.1186/s40168-017-0248-8
31. Cuna A, Morowitz MJ, Ahmed I, Umar S, Sampath V. Dynamics of the preterm gut microbiome in health and disease. Am J Physiol Gastrointest Liver Physiol. (2021) 320:G411–9. doi: 10.1152/ajpgi.00399.2020
32. Caplan MS, Simon D, Jilling T. The role of PAF, TLR, and the inflammatory response in neonatal necrotizing enterocolitis. Semin Pediatr Surg. (2005) 14:145–51. doi: 10.1053/j.sempedsurg.2005.05.002
33. Sampath V, Menden H, Helbling D, Li K, Gastonguay A, Ramchandran R, et al. SIGIRR genetic variants in premature infants with necrotizing enterocolitis. Pediatrics. (2015) 135(6):e1530–4. doi: 10.1542/peds.2014-3386
34. Sampath V, Martinez M, Caplan M, Underwood MA, Cuna A. Necrotizing enterocolitis in premature infants-A defect in the brakes? Evidence from clinical and animal studies. Mucosal Immunol. (2023) 16:208–20. doi: 10.1016/j.mucimm.2023.02.002
35. Bhandari V, Bizzarro MJ, Shetty A, Zhong X, Page GP, Zhang H, et al. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics. (2006) 117(6):1901–6. doi: 10.1542/peds.2005-1414
36. Neu J. Neonatal necrotizing enterocolitis: an update. Acta Paediatr Suppl. (2005) 94:100–5. doi: 10.1111/j.1651-2227.2005.tb02163.x
37. Epelman M, Daneman A, Navarro OM, Morag I, Moore AM, Kim JH, et al. Necrotizing enterocolitis: review of state-of-the-art imaging findings with pathologic correlation. Radiographics. (2007) 27(2):285–305. doi: 10.1148/rg.272055098
38. Morrison SC, Jacobson JM. The radiology of necrotizing enterocolitis. Clin Perinatol. (1994) 21:347–63. doi: 10.1016/S0095-5108(18)30350-6
39. Pierro A, Hall N. Surgical treatments of infants with necrotizing enterocolitis. Semin Neonatol. (2003) 8:223–32. doi: 10.1016/S1084-2756(03)00025-3
40. Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. (2007) 179(7):4808–20. doi: 10.4049/jimmunol.179.7.4808
41. Good M, Sodhi CP, Egan CE, Afrazi A, Jia H, Yamaguchi Y, et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. (2015) 8(5):1166–79. doi: 10.1038/mi.2015.30
42. Morgan RL, Preidis GA, Kashyap PC, Weizman AV, Sadeghirad B, , McMaster Probiotic, Prebiotic, and Synbiotic Work Group. Probiotics reduce mortality and morbidity in preterm, low-birth-weight infants: a systematic review and network meta-analysis of randomized trials. Gastroenterology. (2020) 159(2):467–80. doi: 10.1053/j.gastro.2020.05.096
43. Patel RM, Underwood MA. Probiotics and necrotizing enterocolitis. Semin Pediatr Surg. (2018) 27:39–46. doi: 10.1053/j.sempedsurg.2017.11.008
44. Cuna A, Morowitz MJ, Sampath V. Early antibiotics and risk for necrotizing enterocolitis in premature infants: a narrative review. Front Pediatr. (2023) 11:1112812. doi: 10.3389/fped.2023.1112812
45. Mally P, Golombek SG, Mishra R, Nigam S, Mohandas K, Depalhma H, et al. Association of necrotizing enterocolitis with elective packed red blood cell transfusions in stable, growing, premature neonates. Am J Perinatol. (2006) 23(8):451–8. doi: 10.1055/s-2006-951300
46. Krimmel GA, Baker R, Yanowitz TD. Blood transfusion alters the superior mesenteric artery blood flow velocity response to feeding in premature infants. Am J Perinatol. (2009) 26:99–105. doi: 10.1055/s-0028-1090595
47. La Gamma EF, Blau J. Transfusion-related acute gut injury: feeding, flora, flow, and barrier defense. Semin Perinatol. (2012) 36:294–305. doi: 10.1053/j.semperi.2012.04.011
48. El-Dib M, Narang S, Lee E, Massaro AN, Aly H. Red blood cell transfusion, feeding and necrotizing enterocolitis in preterm infants. J Perinatol. (2011) 31:183–7. doi: 10.1038/jp.2010.157
49. Yeo KT, Kong JY, Sasi A, Tan K, Lai NM, Schindler T. Stopping enteral feeds for prevention of transfusion-associated necrotising enterocolitis in preterm infants. Cochrane Database Syst Rev. (2019) 2019(10):CD012888. doi: 10.1002/14651858.CD012888.pub2
50. MohanKumar K, Namachivayam K, Song T, Jake Cha B, Slate A, Hendrickson JE, et al. A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nat Commun. (2019) 10(1):3494. doi: 10.1038/s41467-019-11199-5
51. Kirpalani H, Bell EF, Hintz SR, Tan S, Schmidt B, Chaudhary AS, et al. Higher or lower hemoglobin transfusion thresholds for preterm infants. N Engl J Med. (2020) 383(27):2639–51. doi: 10.1056/NEJMoa2020248
52. Salas AA, Gunn E, Carlo WA, Bell EF, Das A, Josephson CD, et al. Timing of red blood cell transfusions and occurrence of necrotizing enterocolitis: a secondary analysis of a randomized clinical trial. JAMA Netw Open. (2024) 7(5):e249643. doi: 10.1001/jamanetworkopen.2024.9643
53. Mohamed A, Shah PS. Transfusion associated necrotizing enterocolitis: a meta-analysis of observational data. Pediatrics. (2012) 129:529–40. doi: 10.1542/peds.2011-2872
54. Stritzke AI, Smyth J, Synnes A, Lee SK, Shah PS. Transfusion-associated necrotising enterocolitis in neonates. Arch Dis Child Fetal Neonatal Ed. (2013) 98:F10–4. doi: 10.1136/fetalneonatal-2011-301282
55. Coggins SA, Wynn JL, Weitkamp J-H. Infectious causes of necrotizing enterocolitis. Clin Perinatol. (2015) 42:133–54, ix. doi: 10.1016/j.clp.2014.10.012
56. Mani S, Hazra S, Hagan J, Sisson A, Nair J, Pammi M. Viral infections and neonatal necrotizing enterocolitis: a meta-analysis. Pediatrics. (2023) 152(1):e2022060876. doi: 10.1542/peds.2022-060876
57. Rotbart HA, Nelson WL, Glode MP, Triffon TC, Kogut SJ, Yolken RH, et al. Neonatal rotavirus-associated necrotizing enterocolitis: case control study and prospective surveillance during an outbreak. J Pediatr. (1988) 112(1):87–93. doi: 10.1016/S0022-3476(88)80128-8
58. Rotbart HA, Levin MJ, Yolken RH, Manchester DK, Jantzen J. An outbreak of rotavirus-associated neonatal necrotizing enterocolitis. J Pediatr. (1983) 103:454–9. doi: 10.1016/S0022-3476(83)80427-2
59. Omarsdottir S, Agnarsdottir M, Casper C, Orrego A, Vanpée M, Rahbar A, et al. High prevalence of cytomegalovirus infection in surgical intestinal specimens from infants with necrotizing enterocolitis and spontaneous intestinal perforation: a retrospective observational study. J Clin Virol. (2017) 93:57–64. doi: 10.1016/j.jcv.2017.05.022
60. Stuart RL, Tan K, Mahar JE, Kirkwood CD, Andrew Ramsden C, Andrianopoulos N, et al. An outbreak of necrotizing enterocolitis associated with norovirus genotype GII.3. Pediatr Infect Dis J. (2010) 29(7):644–7. doi: 10.1097/INF.0b013e3181d824e1
61. Bagci S, Eis-Hübinger AM, Franz AR, Bierbaum G, Heep A, Schildgen O, et al. Detection of astrovirus in premature infants with necrotizing enterocolitis. Pediatr Infect Dis J. (2008) 27(4):347–50. doi: 10.1097/INF.0b013e318162a17a
62. Chappé C, Minjolle S, Dabadie A, Morel L, Colimon R, Pladys P. Astrovirus and digestive disorders in neonatal units. Acta Paediatr. (2012) 101(5):e208–12. doi: 10.1111/j.1651-2227.2011.02569.x
63. Alidjinou EK, Lazrek M, Schuffenecker I, Pindi B, Lapeyre F, Kacet N, et al. Necrotizing enterocolitis cases associated with nosocomial enterovirus transmission in a neonatal unit. Pediatr Infect Dis J. (2018) 37(9):954–7. doi: 10.1097/INF.0000000000001928
64. Snyder CL, Hall M, Sharma V, St Peter SD. Seasonal variation in the incidence of necrotizing enterocolitis. Pediatr Surg Int. (2010) 26:895–8. doi: 10.1007/s00383-010-2675-5
65. Meinzen-Derr J, Morrow AL, Hornung RW, Donovan EF, Dietrich KN, Succop PA. Epidemiology of necrotizing enterocolitis temporal clustering in two neonatology practices. J Pediatr. (2009) 154(5):656–61. doi: 10.1016/j.jpeds.2008.11.002
66. Ostlie DJ, Spilde TL, St Peter SD, Sexton N, Miller KA, Sharp RJ, et al. Necrotizing enterocolitis in full-term infants. J Pediatr Surg. (2003) 38(7):1039–42. doi: 10.1016/S0022-3468(03)00187-8
67. Christensen RD, Lambert DK, Baer VL, Gordon PV. Necrotizing enterocolitis in term infants. Clin Perinatol. (2013) 40:69–78. doi: 10.1016/j.clp.2012.12.007
68. Kelleher ST, McMahon CJ, James A. Necrotizing enterocolitis in children with congenital heart disease: a literature review. Pediatr Cardiol. (2021) 42:1688–99. doi: 10.1007/s00246-021-02691-1
69. Burge KY, Gunasekaran A, Makoni MM, Mir AM, Burkhart HM, Chaaban H. Clinical characteristics and potential pathogenesis of cardiac necrotizing enterocolitis in neonates with congenital heart disease: a narrative review. J Clin Med. (2022) 11(14):3987. doi: 10.3390/jcm11143987
70. ElHassan NO, Tang X, Gossett J, Zakaria D, Ross A, Kona SK, et al. Necrotizing enterocolitis in infants with hypoplastic left heart syndrome following stage 1 palliation or heart transplant. Pediatr Cardiol. (2018) 39(4):774–85. doi: 10.1007/s00246-018-1820-0
71. Spinner JA, Morris SA, Nandi D, Costarino AT, Marino BS, Rossano JW, et al. Necrotizing enterocolitis and associated mortality in neonates with congenital heart disease: a multi-institutional study. Pediatr Crit Care Med. (2020) 21(3):228–34. doi: 10.1097/PCC.0000000000002133
72. Cozzi C, Aldrink J, Nicol K, Nicholson L, Cua C. Intestinal location of necrotizing enterocolitis among infants with congenital heart disease. J Perinatol. (2013) 33:783–5. doi: 10.1038/jp.2013.49
73. Carlo WF, Kimball TR, Michelfelder EC, Border WL. Persistent diastolic flow reversal in abdominal aortic Doppler-flow profiles is associated with an increased risk of necrotizing enterocolitis in term infants with congenital heart disease. Pediatrics. (2007) 119:330–5. doi: 10.1542/peds.2006-2640
74. McElhinney DB, Hedrick HL, Bush DM, Pereira GR, Stafford PW, Gaynor JW, et al. Necrotizing enterocolitis in neonates with congenital heart disease: risk factors and outcomes. Pediatrics. (2000) 106(5):1080–7. doi: 10.1542/peds.106.5.1080
75. Zhang W, Shibamoto T, Kuda Y, Shinomiya S, Kurata Y. The responses of the hepatic and splanchnic vascular beds to vasopressin in rats. Biomed. Res. (2012) 33:83–8. doi: 10.2220/biomedres.33.83
76. Menon G, Boyle EM, Bergqvist LL, McIntosh N, Barton BA, Anand KJS. Morphine analgesia and gastrointestinal morbidity in preterm infants: secondary results from the NEOPAIN trial. Arch Dis Child Fetal Neonatal Ed. (2008) 93(5):F362–7. doi: 10.1136/adc.2007.119297
77. Kataria-Hale J, Osborne SW, Hair A, Hagan J, Pammi M. Preoperative feeds in ductal-dependent cardiac disease: a systematic review and meta-analysis. Hosp Pediatr. (2019) 9:998–1006. doi: 10.1542/hpeds.2019-0111
78. Siano E, Lauriti G, Ceccanti S, Zani A. Cardiogenic necrotizing enterocolitis: a clinically distinct entity from classical necrotizing enterocolitis. Eur J Pediatr Surg. (2019) 29:14–22. doi: 10.1055/s-0038-1668144
79. Fisher JG, Bairdain S, Sparks EA, Khan FA, Archer JM, Kenny M, et al. Serious congenital heart disease and necrotizing enterocolitis in very low birth weight neonates. J Am Coll Surg. (2015) 220(6):1018–26.e14. doi: 10.1016/j.jamcollsurg.2014.11.026
80. Urushihara N, Kohno S, Hasegawa S. Pseudomembranous enterocolitis and hemorrhagic necrotizing enterocolitis in Hirschsprung’s disease. Surg Today. (1994) 24:221–4. doi: 10.1007/BF02032891
81. Jayanthi S, Seymour P, Puntis JW, Stringer MD. Necrotizing enterocolitis after gastroschisis repair: a preventable complication? J Pediatr Surg. (1998) 33:705–7. doi: 10.1016/S0022-3468(98)90191-9
82. Oldham KT, Coran AG, Drongowski RA, Baker PJ, Wesley JR, Polley TZ. The development of necrotizing enterocolitis following repair of gastroschisis: a surprisingly high incidence. J Pediatr Surg. (1998) 23(10):945–9. doi: 10.1016/S0022-3468(88)80392-0
83. Lambert DK, Christensen RD, Henry E, Besner GE, Baer VL, Wiedmeier SE, et al. Necrotizing enterocolitis in term neonates: data from a multihospital health-care system. J Perinatol. (2007) 27(7):437–43. doi: 10.1038/sj.jp.7211738
84. Okada K, Fujii T, Ohtsuka Y, Yamakawa Y, Izumi H, Yamashiro Y, et al. Overfeeding can cause NEC-like enterocolitis in premature rat pups. Neonatology. (2010) 97(3):218–24. doi: 10.1159/000253150
85. Berrington JE, Embleton ND. Time of onset of necrotizing enterocolitis and focal perforation in preterm infants: impact on clinical, surgical, and histological features. Front Pediatr. (2021) 9:724280. doi: 10.3389/fped.2021.724280
86. Thakkar PV, Sutton KF, Detwiler CAB, Henegar JG, Narayan JR, Perez-Romero M, et al. Risk factors and epidemiology of spontaneous intestinal perforation among infants born at 22–24 weeks’ gestational age. J Perinatol. (2024) 44(1):94–9. doi: 10.1038/s41372-023-01782-6
87. Rausch LA, Hanna DN, Patel A, Blakely ML. Review of necrotizing enterocolitis and spontaneous intestinal perforation clinical presentation, treatment, and outcomes. Clin Perinatol. (2022) 49:955–64. doi: 10.1016/j.clp.2022.07.005
88. Pumberger W, Mayr M, Kohlhauser C, Weninger M. Spontaneous localized intestinal perforation in very-low-birth-weight infants: a distinct clinical entity different from necrotizing enterocolitis. J Am Coll Surg. (2002) 195:796–803. doi: 10.1016/S1072-7515(02)01344-3
89. Fisher JG, Jones BA, Gutierrez IM, Hull MA, Kang KH, Kenny M, et al. Mortality associated with laparotomy-confirmed neonatal spontaneous intestinal perforation: a prospective 5-year multicenter analysis. J Pediatr Surg. (2014) 49(8):1215–9. doi: 10.1016/j.jpedsurg.2013.11.051
90. Culbreath K, Keefe G, Edwards EM, Morrow KA, Soll RF, Jaksic T, et al. Morbidity associated with laparotomy-confirmed spontaneous intestinal perforation: a prospective multicenter analysis. J Pediatr Surg. (2022) 57(6):981–5. doi: 10.1016/j.jpedsurg.2022.01.058
91. Chugh PV, Nes E, Culbreath K, Keefe G, Edwards EM, Morrow KA, et al. Comparing healthcare needs in extremely low birth weight infants with NEC and spontaneous intestinal perforation. J Pediatr Surg. (2024) 59(9):1759–64. doi: 10.1016/j.jpedsurg.2024.03.006
92. Srinivasan P, Brandler M, D’Souza A, Millman P, Moreau H. Allergic enterocolitis presenting as recurrent necrotizing enterocolitis in preterm neonates. J Perinatol. (2010) 30:431–3. doi: 10.1038/jp.2009.153
93. Liu H, Turner TWS. Allergic colitis with pneumatosis Intestinalis in an infant. Pediatr Emerg Care. (2018) 34:e14–5. doi: 10.1097/PEC.0000000000001369
94. Cordova J, Sriram S, Patton T, Jericho H, Gokhale R, Weinstein D, et al. Manifestations of cow’s-milk protein intolerance in preterm infants. J Pediatr Gastroenterol Nutr. (2016) 62(1):140–4. doi: 10.1097/MPG.0000000000000933
95. Maloney J, Nowak-Wegrzyn A. Educational clinical case series for pediatric allergy and immunology: allergic proctocolitis, food protein-induced enterocolitis syndrome and allergic eosinophilic gastroenteritis with protein-losing gastroenteropathy as manifestations of non-IgE-mediated cow’s milk allergy. Pediatr Allergy Immunol. (2007) 18:360–7. doi: 10.1111/j.1399-3038.2007.00561.x
96. Alowais SA, Alghamdi SS, Alsuhebany N, Alqahtani T, Alshaya AI, Almohareb SN, et al. Revolutionizing healthcare: the role of artificial intelligence in clinical practice. BMC Med Educ. (2023) 23(1):689. doi: 10.1186/s12909-023-04698-z
97. McElroy SJ, Lueschow SR. State of the art review on machine learning and artificial intelligence in the study of neonatal necrotizing enterocolitis. Front Pediatr. (2023) 11:1182597. doi: 10.3389/fped.2023.1182597
98. Cuna A, George L, Sampath V. Genetic predisposition to necrotizing enterocolitis in premature infants: current knowledge, challenges, and future directions. Semin Fetal Neonatal Med. (2018) 23:387–93. doi: 10.1016/j.siny.2018.08.006
99. Cuna A, Sampath V, Khashu M. Racial disparities in necrotizing enterocolitis. Front Pediatr. (2021) 9:633088. doi: 10.3389/fped.2021.633088
100. Mueller M, Taylor SN, Wagner CL, Almeida JS. Using an artificial neural network to predict necrotizing enterocolitis in premature infants. 2009 International Joint Conference on Neural Networks 2172–2175 (IEEE, Atlanta, Ga, USA, 2009). doi: 10.1109/IJCNN.2009.5178635
101. Kumar N, Akangire G, Sullivan B, Fairchild K, Sampath V. Continuous vital sign analysis for predicting and preventing neonatal diseases in the twenty-first century: big data to the forefront. Pediatr Res. (2020) 87:210–20. doi: 10.1038/s41390-019-0527-0
102. Doheny KK, Palmer C, Browning KN, Jairath P, Liao D, He F, et al. Diminished vagal tone is a predictive biomarker of necrotizing enterocolitis-risk in preterm infants. Neurogastroenterol Motil. (2014) 26(6):832–40. doi: 10.1111/nmo.12337
103. Pantalone JM, Liu S, Olaloye OO, Prochaska EC, Yanowitz T, Riley MM, et al. Gestational age-specific complete blood count signatures in necrotizing enterocolitis. Front Pediatr. (2021) 9:604899. doi: 10.3389/fped.2021.604899
104. Cho H, Lee EH, Lee K-S, Heo JS. Machine learning-based risk factor analysis of necrotizing enterocolitis in very low birth weight infants. Sci Rep. (2022) 12:21407. doi: 10.1038/s41598-022-25746-6
105. Irles C, González-Pérez G, Carrera Muiños S, Michel Macias C, Sánchez Gómez C, Martínez-Zepeda A, et al. Estimation of neonatal intestinal perforation associated with necrotizing enterocolitis by machine learning reveals new key factors. Int J Environ Res Public Health. (2018) 15(11):2509. doi: 10.3390/ijerph15112509
106. Lure AC, Du X, Black EW, Irons R, Lemas DJ, Taylor JA, et al. Using machine learning analysis to assist in differentiating between necrotizing enterocolitis and spontaneous intestinal perforation: a novel predictive analytic tool. J Pediatr Surg. (2021) 56(10):1703–10. doi: 10.1016/j.jpedsurg.2020.11.008
107. Son J, Kim D, Na JY, Jung D, Ahn JH, Kim TH, et al. Development of artificial neural networks for early prediction of intestinal perforation in preterm infants. Sci Rep. (2022) 12(1):12112. doi: 10.1038/s41598-022-16273-5
108. Qi G, Huang S, Lai D, Li J, Zhao Y, Shen C, et al. An improved joint non-negative matrix factorization for identifying surgical treatment timing of neonatal necrotizing enterocolitis. Bosn J Basic Med Sci. (2022) 22(6):972–81. doi: 10.17305/bjbms.2022.7046
109. Gao W, Pei Y, Liang H, Lv J, Chen J, Zhong W. Multimodal AI system for the rapid diagnosis and surgical prediction of necrotizing enterocolitis. IEEE Access. (2021) 9:51050–64. doi: 10.1109/ACCESS.2021.3069191
110. Lin YC, Salleb-Aouissi A, Hooven TA. Interpretable prediction of necrotizing enterocolitis from machine learning analysis of premature infant stool microbiota. BMC Bioinformatics. (2022) 23:104. doi: 10.1186/s12859-022-04618-w
111. Rusconi B, Jiang X, Sidhu R, Ory DS, Warner BB, Tarr PI. Gut sphingolipid composition as a prelude to necrotizing enterocolitis. Sci Rep. (2018) 8(1):10984. doi: 10.1038/s41598-018-28862-4
112. Sylvester KG, Ling XB, Liu GY, Kastenberg ZJ, Ji J, Hu Z, et al. A novel urine peptide biomarker-based algorithm for the prognosis of necrotising enterocolitis in human infants. Gut. (2014) 63(8):1284–92. doi: 10.1136/gutjnl-2013-305130
Keywords: necrotizing enterocolitis, machine learning, phenotype, endotype, prematurity, neonate
Citation: Cuna A, Kumar N and Sampath V (2024) Understanding necrotizing enterocolitis endotypes and acquired intestinal injury phenotypes from a historical and artificial intelligence perspective. Front. Pediatr. 12:1432808. doi: 10.3389/fped.2024.1432808
Received: 14 May 2024; Accepted: 13 September 2024;
Published: 27 September 2024.
Edited by:
Minesh Khashu, University Hospitals Dorset NHS Foundation Trust, United KingdomReviewed by:
Rachana Singh, Tufts University, United StatesCopyright: © 2024 Cuna, Kumar and Sampath. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Venkatesh Sampath, dnNhbXBhdGhAY21oLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.