- 1Department of Child Health, Dalian Municipal Women and Children’s Medical Center (Group), Dalian, Liaoning, China
- 2Dalian Medical University, Dalian, Liaoning, China
Over the past few decades, the incidence of childhood allergic diseases has increased globally, and their impact on the affected child extends beyond the allergy itself. There is evidence of an association between childhood allergic diseases and the development of neurological disorders. Several studies have shown a correlation between allergic diseases and tic disorders (TD), and allergic diseases may be an important risk factor for TD. Possible factors influencing the development of these disorders include neurotransmitter imbalance, maternal anxiety or depression, gut microbial disorders, sleep disturbances, maternal allergic status, exposure to tobacco, and environmental factors. Moreover, gut microbial disturbances, altered immunological profiles, and DNA methylation in patients with allergic diseases may be potential mechanisms contributing to the development of TD. An in-depth investigation of the relationship between allergic diseases and TD in children will be important for preventing and treating TD.
1 Introduction
Allergic diseases in children include food allergy (FA), atopic dermatitis (AD), allergic rhinitis (AR), allergic conjunctivitis (AC) and allergic asthma (AA), which are chronic inflammatory disease with symptoms such as hives, swelling, vomiting, diarrhea, itchy rashes on the skin, watery or itchy eyes, sneezing, congestion, tightness of chest coughing and reversible airflow limitation (1, 2). Allergic diseases are systemic disorders caused by an impaired immune system, and their pathogenesis is complex and involves many factors, including genetics, epigenetics, environmental factors, and the body's immune status (3). IgE is a key factor in the pathogenesis of allergic diseases. Allergens and IgE sequentially activate dendritic cells, and T and B cells to initiate allergic immune responses (4). Simultaneously, they activate mast cells, basophils, and eosinophils to release inflammatory mediators, resulting in allergic reactions (5). Not only do allergic diseases lead to a rise in school absenteeism and a decrease in children's involvement in outdoor activities (6), but studies have also suggested a possible link between these conditions and the development of neurological disorders (7), particularly Tic disorder (TD) (8).
TD is a group of chronic neurological disorders that begin in childhood and adolescence, usually occurring for the first time at the age of 5–6 years and is characterized by sudden, rapid, repetitive, stereotypical, non-rhythmic, non-rhythmic single- or multi-located muscular and/or vocal tics of an involuntary nature (9). TD can be categorized into Provisional tic disorders (PTD), Chronic motor or vocal tic disorders (CTD), and Tourette's syndrome (TS), depending on their characteristics and the progression of the disease (10). TD is often accompanied by other neurodevelopmental or emotional behavior disorders, with the most common co-morbidities encompassing attention deficit hyperactivity disorder (ADHD), autism spectrum disorder (ASD) and obsessive-compulsive disorder (OCD) (11, 12). Children with TS or other persistent TD can experience co-occurring disorders, functional impairments, discrimination, and bullying victimization or perpetration (13, 14). However, the etiology and pathogenesis of TD are currently unknown.
An increasing number of studies indicate that allergic diseases could significantly impact TD. The presence of elevated serum IgE levels and a positive skin prick test in children with TD implies that the symptoms experienced by TD may bear resemblance to allergies or be connected to allergic diseases (15, 16). Furthermore, the primary indications of TD in children are blinking of the eyes, shrugging of the nose, clearing of the throat, coughing, and may experience discomfort in their eyes and nasal passages (17). Consequently, TD can be erroneously identified as AR, AC, or AA. Studies have indicated that certain children with TD may experience an amelioration of their symptoms following antiallergic therapy (18, 19). This suggests that allergic diseases are closely related to TD in terms of pathophysiology and immunity.
This review collected studies about the correlation between allergic diseases and TD, analyzed the common factors affecting the occurrence of both, and discussed the potential mechanisms through which allergic diseases may impact TD.
2 Methods
In order to discuss the relationship between allergic diseases and tic disorders in children, we undertook a systematized literature search that included clinical studies and experimental studies. Searchers were conducted using Pubmed, Google Scholar, EBSCO, Scopus and Medline with the following key terms: allergy OR allergic rhinitis OR allergic conjunctivitis OR asthma OR atopic dermatitis OR food allergy AND tic disorders AND children, allergy OR tic disorders AND immunology OR neurotransmitter agents OR genetics OR gut microbiota OR sleep OR environment, allergy OR tic disorders AND mechanism OR immunological OR DNA methylation. Studies from all years were included. Some review articles and their reference lists were also searched to identify related articles.
3 Epidemiology
Over the past 20 years, the prevalence of childhood allergic diseases has been increasing globally (20, 21), already affecting about 25% of children (22). In 2010, the occurrence of FA among children in the US stood at 8.0% (23), while in 2019, the prevalence of FA among children aged 2 years and below in Wenzhou, China, escalated to 11.1% (24). Approximately 13% of children in the US and 15%–38% of children under the age of 5 globally are affected by AD (25). In 2013, the occurrence of AR among 12–15 years old in Europe ranged from 15.1% to 37.8% (26). From 2015 to 2017, the prevalence of AR among children in inland areas of China was 26.6%–28.5% (27, 28). A population-based study indicated that AC alone has been estimated in 6%–30% of the general population and up to 30% in children alone or association with AR (29). The number of asthma cases in the US rose from 7.3% in 2001 to 7.9% in 2017 (30), and the total number of asthma cases among Chinese children under 14 years old rose from 1.97% in 2000 to 3.02% in 2010 (31, 32). Allergic diseases recurrence rate is high, which brings great pain to and imposes a severe financial burden on patients.
At the same time, the prevalence of TD has been on the rise in the last few years (33). According to a systematic review and meta-analysis of 13 studies of children, the prevalence of TS is estimated to be 0.77%, while the prevalence of PTD is estimated to be 2.99% (34), and TD is more common in boys than girls, with a ratio between 2 and 1 and 3.5 to 1 (35). A CDC study using parent-reported data found that 1 out of every 333 (0.3%) children 3–17 years of age in the US have received a diagnosis of TS (36). The combined prevalence of TS and other TD is estimated to be over 10 cases per 1,000 (1%, 1:100), suggesting that over ½ million children have a TD in the US. And the overall rate of TD in Chinese kids varies from 1.04% to 2.98% (37, 38). This variability in rates across different regions and populations could indicate a complex interplay of genetic, environmental, and possibly cultural factors influencing the manifestation of these disorders.
4 Correlation between allergic and TD
In 1984, in an overview of the clinical experience of 300 TS patients, Bruun noted that “although there is no evidence of allergy as a cause of TS, my clinical experience suggests that exacerbation of TS symptoms is usually associated with seasonal allergic reactions or the ingestion of allergens in food” (39). In 1985, Finegold first reported elevated serum IgE and positive skin prick test in four patients with TS who presented to an allergy specialist. He noted that the symptoms of patients with TS can resemble allergies or appear in combination with allergic diseases (34). Mandell's letter in response to Finegold's report, said his survey of 26 TS patients showed that 80% had allergies. Unfortunately, Mandell's report does not mention any details of the materials and methods used, or the laboratory tests performed on TS patients (40). In 1987, Comings et al. after comparing 247 patients with TS and 47 with the control group, concluded that there was no statistical difference between the two groups in terms of comorbid allergic disease (41). In 1997, Kim suggested that some foods may be involved in TS because they promote the production of certain neurotransmitters (42). In 1999, a study published by Ho showed that 41 of 72 TS patients (56.9%) had clinical allergies and tested positive for allergens, which was much higher than the 44.3% allergy rate in the local population. Therefore, this study concluded that the incidence of allergy in TS patients was significantly higher than that in the general population (43). Despite the conflicting results of the aforementioned studies, there is an increasing focus on the potential link between allergic conditions and the emergence of TD.
In 2011, Chang included 845 TS patients aged 2–18 years and 3,378 controls matched to the case group in terms of age, sex, and urbanization levels based on the Taiwan Health Database, to assess the correlation between allergic diseases and TS. The results showed a significant correlation between allergic diseases and TS, with subjects with AR having a doubled risk of TS (corrected OR = 2.18), and the corrected ORs for AA, AD, and AC were 1.82, 1.61, and 1.33, respectively, and the risk of TS rises with the number of comorbid allergic conditions (44). In 2013, Chen found a significantly increased risk of TD in patients with ADHD combined with allergies, following a further expansion of the study population, according to Taiwan Health Database (45). In 2014, a preliminary study in Turkey showed a higher rate of AR diagnosis in TD patients than in controls (40.6% vs. 17.1%, p = 0.033), suggesting a significant correlation between allergic diseases and TD (46). In 2019, Chen reported the association between AC and TD through a case-control study and found that children with TD had a significantly higher incidence of AC (74.3% vs. 17.1%, p < 0.001), higher skin prick test positivity than controls (80.0% vs. 20.0%), and a history of AR was significantly associated with TD (47). It can be seen that as research progresses, more and more studies show that children with TD are more likely to be diagnosed with allergies than healthy children.
In 2021, Liu showed that allergic diseases increased the severity of TD childhood (48). In 2022, a meta-analysis provided strong evidence for a relationship between allergic diseases and TD: TD is positively associated with AA, AR, and AC (21). In 2022, Zhang et al. showed some concordance with TD in the grading and distribution of IgE and sIgE levels in children with allergic diseases (49). In the same year, Chang et al. used a case-control study found that allergic diseases were associated with the development of TD in children, with AC having the highest correlation with TD (OR = 4.95), followed by eczema (OR = 2.64), AR (OR = 2.64), and FA (OR = 2.50), and this study also found that more than 80% of the children's allergic diseases preceded the development of TD (50).
The above studies have shown that not only is there a correlation between allergic diseases and TD, but that allergic diseases may be an important risk factor for the occurrence and severity of TD. Hence, it is crucial to analyze the factors that may contribute to the simultaneous occurrence of both and delve deeper into the mechanisms through which allergic diseases impact TD, to effectively prevent and treat TD.
5 Common risk factors for allergy and TD
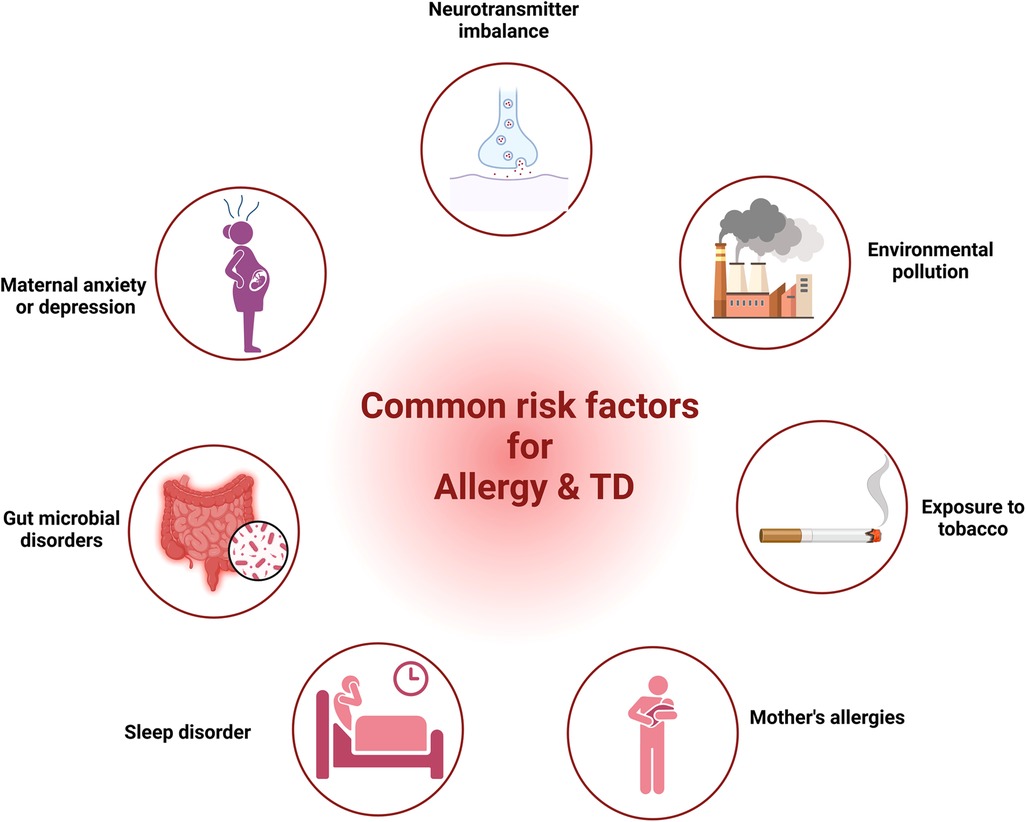
Research from various studies had highlighted common factors such as neurotransmitter imbalance, maternal anxiety or depression, gut microbial disorders, sleep disturbances, maternal allergic status, exposure to tobacco and environmental factors in both allergic diseases and TD. In the following segment, we provide proof regarding the role of these elements in allergic diseases and TD.
5.1 Neurotransmitter imbalance
Allergy and TD, both are governed by neurotransmitters—allergies by histamine and TD by serotonin and dopamine. Allergies can affect the nervous system, leading to symptoms such as red itchy eyes, sneezing, and altered gastrointestinal motility, which are mediated by the release of mediators that interact with sensory nerves and alter transmission in autonomic nerves (51).
Mediators linked to allergies can engage with sensory nerves, modify the central nervous system's (CNS) processing, and alter transmission in autonomic and enteric nerves, leading to neuronally-based symptoms of allergy. Synaptic interactions between presynaptic and postsynaptic components within the sympathetic, parasympathetic, and enteric ganglia are crucial for the autonomic and enteric nervous systems. Presynaptic neurons originating from the CNS are responsible for regulating efferent neurons in the majority of organs. Stimulation of the preganglionic nerve occurs in the CNS, where action potentials travel along the preganglionic axon, eventually establishing synapses with autonomic ganglia neurons. Rather than mere relay stations, these ganglia serve as locations for filtering and assimilating CNS inputs. In allergies, this could be significant as mast cells are frequently linked with ganglia that are sympathetic, parasympathetic, and enteric (51). Specifically within the intestinal tract, there exists a self-governing efferent regulation that operates independently from the CNS's neural processing. Here, a sensory nerve, upon sensing a local environmental stimulus, can relay this data straight to adjacent efferent enteric neurons via local afferent-efferent synapses. Known as a local “peripheral reflex”, these enteric ganglion neurons engage in communication via intraganglionic pathways. Other internal organs, like the airways and gall bladder, may also exhibit peripheral reflexes, though not as extensively as those in the gastrointestinal system (52).
Histamine is pivotal in the development of various allergic conditions like AD, AR, and AA, through differential regulation of T helper lymphocytes. Histamine is responsible for boosting the release of Th2 cytokines like IL-5, IL-4, IL-10, and IL-13, and suppressing the production of Th1 cytokines including IFN-γ, IL-12, and IL-2. Consequently, histamine plays a role in maintaining the equilibrium between Th1 and Th2 cells, aiding in the transition towards Th2 cells (53). Primarily found in mast cells and basophils, histamine plays a key role in allergic conditions, facilitating asthma's initial two primary symptoms (bronchospasm and edema) via its H1 receptor and mucus secretion through its H2 receptor (54). Therefore, histamine plays a crucial role in the pathophysiology of allergic diseases. Studies have revealed a critical role of the Gamma-Aminobutyric Acid (GABA) signaling pathway in the airway epithelium of AA through its ability to stimulate mucus production (55). Additionally, GABA can inhibit allergic reactions in the airways of guinea pigs by stimulating GABA receptors (56). GABA can also suppress the immune system, thereby easing the allergic responses (55). At the same time, there is evidence to suggest a relationship between serotonin, also known as 5-hydroxytryptamine (5-HT), and allergies. Allergic sensitization can modify the pulmonary expression of 5-HT receptors in guinea pigs (57). Supplementation with 5-hydroxytryptophan (5-HTP), a precursor to 5-HT, has been found to inhibit allergic lung inflammation and airway responsiveness induced by allergens (58). It can be seen that changes in various neurotransmitters are closely related to the development of allergies.
Although the exact cellular and molecular base of TS is as yet elusive, research in neuroanatomy and neurophysiology suggests the participation of cortical-striatal-thalamocortical pathways, connecting distinct areas of the frontal cortex with subcortical formations (59). Within these circuits, the transmission of messages is regulated through various neurotransmitters, including dopamine, histamine, GABA, and 5-HT (60).
While dopamine is the most regularly detected neurotransmitter alteration in TD, there's a strong probability that patients exhibit dysfunction in various neurotransmitter systems. Changes in the brain's histamine modulation system are recognized as a factor in the emergence of tics and TS, with genetic studies showing that histamine dysregulation can lead to TD. Histamine H3 receptor activation in the dorsal striatum has been found to trigger stereotypies in a mouse model of TD (61). Mice with a knockout of the histidine decarboxylase gene, which is involved in histamine production, demonstrate recurrent behavioral disorders, imbalanced dopamine levels, and modified indicators of cell function and internal signaling within the striatum (61). A study also found that individuals with TS exhibit increased GABA in brain areas linked to the planning and selection of movements (62). Another study found that GABA is involved in the pathophysiology of TD, and increased GABA levels may contribute to enhanced control over motor excitability in TS (63). In addition, 5-HT and 5-hydroxyindoleacetic acid levels may play a role in the genesis of TD, but these findings have no significant correlations with the severity of TD (64). In summary, neurotransmitter imbalance has been implicated in the development of TD, but the exact mechanisms involved are still not fully understood.
The above analyses showed that alterations in neurotransmitters, particularly histamine, GABA and 5-HT, all have an impact on allergic disease and TD.
5.2 Maternal anxiety or depression
Epidemiological research indicates that the health of a mother, both before conception and throughout pregnancy and after birth, play a significant influence in her child's health. The correlation between allergies and TD may manifest before birth. There is growing evidence that the presence of stress, depression, or anxiety in mothers before, during, and following pregnancy can significantly impact their offspring, rendering them more vulnerable to allergy (65–67) and/or TD (68).
A longitudinal study in Mexico, examining 601 pairs of mothers and infants, in order to comprehend the correlation between maternal depressive symptoms and childhood asthma. The study found that maternal postpartum depression or recurrent depression was highly associated with asthma in children at 48 months (66). A large data survey in Korea found that maternal depression was significantly associated with childhood asthma (OR = 2.03) and AD (OR = 1.76) (69). Schoolchildren in China were found to have a higher likelihood of developing rhinitis if their mothers had symptoms of depression during and after pregnancy (70). A cohort study from Singapore showed that elevated scores of maternal depression before conception and during pregnancy were associated with a greater chance of the offspring developing wheezing within the initial 18 months of life (71). A meta-analysis also showed a positive association between maternal depression or anxiety and asthma in offspring (72). In yet another study—a meta-analysis comprising 30 research investigations and a separate cross-sectional study involving 3,758 pairs of Italian mothers and children—it was found that prenatal maternal distress was linked to a higher likelihood of the offspring developing eczema, rhinitis and asthma (73, 74). Maternal depression or anxiety can impact the immune system of the offspring by influencing the hypothalamic-pituitary-adrenal (HPA) axis. This axis is crucial in regulating the body's immune responses to stressors. When mothers experience depression or anxiety, it can trigger increased production and release of cortisol. This reduces the expression of 11β-hydroxysteroid dehydrogenase 2 in the placenta, leading to higher levels of cortisol exposure in the fetus (75–77). An increase in cortisol levels in infants can lead to an imbalance in the HPA axis, which can activate a T-helper 2 (Th2) immune response by blocking interleukin-12, a Th1 cytokine (78), and raising Th2 levels (79, 80), thus exacerbating inflammation and causing IgE-mediated allergic reactions (67).
A British prospective cohort study investigated 14,541 pregnant women and their children and found that increased odds of TS/CTD in children at age 13 were significantly associated with maternal chronic anxiety (OR = 2.17) and antenatal depression (OR = 1.86) (81). In a separate study, it was found that a maternal background of non-specific psychiatric disorders—encompassing anxiety disorders and depressive disorders—was associated with higher odds of children experiencing TS/CTD during their childhood and adolescence (82). In addition, the severity of TS is also significantly related to maternal stress during pregnancy (83). Reduced placental monoamine oxidase A levels in maternal with depression can lead to increased 5-HT levels, which detrimental to fetal brain development (84). In addition, some studies have confirmed that regional connections in fetal brain function related to arousal and consciousness increase as maternal anxiety levels increase (85), and this pattern may be closely related to the occurrence of TD.
5.3 Gut microbial disorders
Gut microbial disturbance is another common factor between allergies and TD. In the past decade, many studies have been conducted to determine that microbial disturbance has an important impact on the occurrence of allergic diseases, and in TD patients, the role of microbial ecology has also received increasing attention.
A cross-sectional study of 1,440 children showed that altered gut microbial diversity is associated with reduced AD risk (86). A Japanese study shows that children with a higher abundance of Bacteroidetes in their gut microbiota during infancy are more likely to develop allergic diseases after the age of 2 years (87). A cohort study in Ecuador showed that increased fecal abundance of Streptococcus and Bacteroidetes and decreased abundance of Bifidobacterium and Ruminococcus in children as young as 3 months old was associated with allergy and wheezing at age 5 years (88). Research by Zhang et al. showed that the diversity of gut microbial in children with AR was reduced and the abundance of Bacteroidetes was significantly increased, and changes in the gut microbial were correlated with clinical symptoms (89). The gut microbiota plays a crucial role in the immune system by influencing the development of either responsive or tolerant reactions to various antigens. It achieves this by maintaining a balance between the activities of Th1 and Th2 cells while also regulating Th17 and T regulatory (Treg) cells specifically within the lamina propria (90). Alterations in gut microbial levels or diversity (dysbiosis) can disrupt mucosal immunological tolerance, leading to allergic diseases (91, 92).
The gut microbiota of children with untreated TD has higher abundance of Bacteroides plebeius and Ruminococcus lactaris, and lower abundance of Prevotella stercorea and Streptococcus lutetiensis (93). Chang et al. collected the feces of 15 TD children and 10 healthy children for 16S rRNA high-throughput sequencing. They observed that compared with healthy children, the relative abundance of Firmicutes, Agathobacter, Subdoligranulum, Ruminococcus_1 and Roseburia in TD children was decreased, while the relative abundance of Bacteroidetes and Erysipelatoclostridium increased (88). After 1 and 2 courses of acupuncture, the symptom of TD children was lower than those before treatment and the relative abundance of Firmicutes was decreased, while Bacteroidetes and Erysipelatoclostridium was increased. The above two studies showed that the gut microbial of TD children may be disordered, and the improvement of gut microbial is related to the relief of tic symptoms.
The common feature of children with allergies and TD is increased levels of Bacteroidetes. Increased levels of Bacteroidetes can lead to changes in short-chain fatty acid levels. Changes in SCFAs levels may be an important factor in the co-occurrence of the two.
5.4 Sleep disorders
Getting proper sleep is incredibly important for growth, development, and overall well-being. Healthy sleep involves getting the right amount of sleep suitable for one's age, ensuring high sleep quality, uninterrupted sleep, and the absence of sleep disorders. Sufficient sleep significantly contributes to brain development, learning, memory consolidation, emotional regulation, executive function, and various other critical functions (94). Sleep disorders encompass a broad spectrum of sleep-related issues. These can range from not getting enough sleep, experiencing trouble falling asleep, waking up too early, having low-quality sleep, facing disruptions in circadian rhythms, dealing with insomnia, to disorders related to breathing during sleep (95). Sleep disturbances can significantly affect the body's immune system, emotional state, and cognitive abilities (96).
Increasingly, there is mounting evidence indicating a correlation between sleep disorders and outcomes related to allergies. Research suggests that individuals who had insufficient sleep (≤6 h per night) had a 1.27 times higher probability of developing allergic sensitization compared to those who had adequate sleep (7–8 h per night) (97). The correlation between sleep and allergic skin conditions is particularly noteworthy in a clinical setting, as sleep disturbances can contribute to the development of allergic skin diseases (98, 99) and is positively correlated with the severity of AD (100). According to a recent study involving over 5,000 patients from 10 European countries, inadequate sleep duration (<6 h) was linked to respiratory and nasal symptoms (101). Moreover, sleep problems have been found to mediate the association between asthma and allergic rhinitis with psychological distress in children (102). A higher proportion of eosinophils in peripheral blood is associated with a more serious sleep disorder (103). Explain that allergies can also affect sleep, leading to issues such as insomnia, trouble falling asleep, trouble staying asleep, increased snoring, increased risk for sleep apnea, poor sleep efficiency, and short sleep duration (104).
In addition, sleep disorders are not uncommon in TD patients. Approximately 65% of children with TD have exhibited various forms of sleep disturbances. These issues can encompass challenges in initiating sleep and increased disruptions during sleep, leading to decreased sleep efficiency (105). A large US population-based survey showed that poor sleep quality is related to the severity of tics (106), and that proper sleep management can help reduce the intensity and effect of TD on life (107). Sleep disturbances in children with TS include increased night waking, parasomnias, and sleep onset delayed (108). The severity of TS symptoms experienced during the day has shown a significant positive correlation with the frequency of awakenings and changes in sleep stages during the night. Conversely, it has exhibited a negative correlation with sleep efficiency, indicating that more severe TS symptoms during waking hours often relate to increased disruptions in sleep and reduced overall sleep quality (109). The behavioral and cognitive impairments present in some TD patients may be related to sleep disturbances (110). Additionally, it has been reported that motor tics, associated with TS, can persist even during sleep (105). Research has indicated that TD patients combined with AR are more likely to develop sleep disorders (111). Therefore, sleep disorders may be an important factor in the co-occurrence of allergic diseases and TD.
5.5 Mother's allergies
Family history of allergic diseases is directly related to the occurrence and severity of allergies in offspring, especially in mothers. If the mother has allergies, the risk of the child developing allergies is approximately 45% (112). Maternal total IgE levels correlated with elevated infant IgE levels and infant eczema (113). A cohort study showed that the offspring of mothers with a history of AA and AR during pregnancy were at higher risk of developing AD and AR (20). Studies have shown that the levels of IL-10, TGF-β and Helios-induced Tregs in the cord blood of mothers with allergies all decrease, which means that the immune tolerance of such babies at the systemic level is weakened after birth, which may be caused by maternal allergies, an important mechanism for increased allergy risk in children (114).
Similarly, maternal allergies may also affect the occurrence and development of TD in children. A nationwide prospective cohort study conducted in Denmark revealed that 110 out of 2,442 children with TS had mothers with AD prior to pregnancy, indicating a correlation between maternal AD and an elevated likelihood of TS in the offspring (115). Although there is currently few studies related to maternal allergies and childhood TD, population-based cohort studies have shown that maternal asthma is significantly associated with the development of ASD and ADHD in offspring (116–118). ASD and ADHD are the most common comorbidities of TD in children, especially ADHD, which has a similar etiology and pathogenesis to TD (119).
5.6 Exposure to tobacco
Exposure to tobacco is a prevalent environmental factor. Extensive evidence indicates that tobacco exposure during early life doesn't just impact prenatal development but can also cause structural and functional changes. These alterations heighten the risks of immune, cardiovascular, and neuroendocrine disorders in offspring (120).
Allergic disease in children has been linked to exposure to environmental tobacco during pregnancy and early life (121). A comprehensive analysis of 43 studies encompassing 29 distinct birth cohorts revealed that smoking during pregnancy heightened the likelihood of wheezing in children under the age of 6 by 36% (OR, 1.36; 95% CI: 1.19–1.55), while also elevating the risk of asthma in children aged 6 and above by 22% (OR, 1.22; 95% CI: 1.03–1.44) (122). The meta-analysis revealed that maternal smoking during pregnancy may elevate the likelihood of recurring wheezing in infancy (123). Consequently, it can be inferred that exposure to environmental tobacco smoke elevates the risk of wheezing or asthma in children by a minimum of 20% (124). Maternal smoking during pregnancy could potentially lead to a 13% increase in the risk of AR in offspring, particularly if the mother is a passive smoker (125). Smoking while pregnant and exposure to tobacco smoke during childhood might represent non-allergic factors linked to higher risk and increased occurrence of persistent asthma or rhinitis (126). In Ferrini's study, findings indicated that prenatal exposure to tobacco makes offspring more prone to heightened allergic airway inflammation. This condition was linked to a decrease in the functioning of pulmonary NK cells (127). Exposure to tobacco can trigger an increase in the expression of Toll-like receptors (TLRs) and alter the responses mediated by lipopolysaccharides. This exposure influences the activation of nuclear factor-kB, prompts the release of IL-8 by innate lymphoid cell-2 through epithelial cytokine production, and affects chemotactic activity toward neutrophils (128). Maternal smoking during pregnancy leads to epigenetic changes, including the increased expression of miRNA-233 and a reduction in the number of Treg cells in the offspring's cord blood at birth. These changes can contribute to an inclination towards atopic conditions and an increased risk that persists during early infancy (121). Exposure to tobacco during pregnancy is considered a potential factor contributing to the development of childhood allergic diseases and this connection is believed to occur through epigenetic mechanisms (125).
A study found that maternal smoking during pregnancy and a heightened risk for TS and CTD in offspring (129). A prospective study about the Danish National Birth Cohort, which analyzed 73,073 singleton pregnancies, concluded that heavy maternal prenatal smoking was associated with an increased risk and the severity of TS/CTD in offspring (130). Additionally, a meta-analysis shows that maternal smoking during pregnancy may be associated with a 35% increase in the risk of TS and CTD among offspring (131). These findings indicate a potential association between maternal smoking during pregnancy and the development of TS and CTD in offspring. Certainly, prenatal exposure to nicotine impacts various facets of fetal brain development, influencing processes such as neuronal migration, proliferation, and differentiation. These effects can have lasting implications for the maturation and functioning of the developing brain (132). Exposure of the fetal brain to nicotine disrupts the development of various neurotransmitter systems, notably affecting dopamine, which is consistently found to be altered in studies related to TD (133, 134). Indeed, prenatal exposure to smoking can induce subtle structural changes in the brain, specifically affecting regions like the striatum, thalamus, thalamocortical fibers, and cortex (135). Abnormalities within these brain circuits have been strongly associated with the underlying mechanisms of TD, as well as their frequently occurring comorbidities such as OCD, ADHD, and ASD. Dysfunction within these circuits often correlates with the manifestation of these conditions and their interconnected nature (130). Indeed, immune dysregulation has been associated with the risk of TS/CT. Maternal smoking, known to influence immune responses, might impact fetal brain development through this mechanism, potentially contributing to the risk of these neurodevelopmental conditions.
5.7 Environmental pollution
Particulate matter with an aerodynamic diameter of less than 2.5 μm (PM2.5) represents a criteria pollutant known for its ability to harm the alveoli within the respiratory system. These tiny particles can penetrate blood vessels after affecting the respiratory system (136). Epidemiological studies have revealed that exposure to PM2.5 can lead to various adverse health effects in humans. These effects include increased risks of allergies, congenital heart defects, and the induction of negative impacts on neurodevelopment (137). Infants and young children are particularly vulnerable to the detrimental effects of air pollution. This susceptibility is due to the relatively immature state of their immune and respiratory systems, making them more prone to experiencing severe health impacts from exposure to pollutants like PM2.5 (138). Furthermore, children tend to spend more time outdoors and breathe in about 50% more air per kilogram of body weight compared to adults. This higher respiration rate and outdoor activity expose them to relatively higher doses of ambient pollutants, further increasing their vulnerability to the adverse effects of air pollution (139).
PM2.5 has been found to have a strong correlation with allergies and allergic respiratory diseases (140). Exposure to PM2.5 can trigger allergic reactions and worsen existing allergic conditions (141). Among them, short- and long-term exposure to ambient PM2.5 could increase the risk of allergic nasal and eye symptoms, worsening dyspnea caused by allergens, and an increase in overall allergic symptoms (142). Elevated concentrations of PM2.5 can disrupt the balance of T helper cells. High levels of PM2.5 tend to increase the expression of TNF-α and cytokines associated with Th2 responses, such as IL-4 and IL-10. Conversely, they decrease the expression of the Th1-associated cytokine IFN-γ. This imbalance alters the Th1/Th2 ratio, which plays a critical role in immune responses (143). In a study conducted on rats with AR, the researchers noted that the expression levels of IFN-γ, IL-4, IL-5, IL-33, intercellular adhesion molecule 1 (ICAM1), and vascular cell adhesion molecule 1 (VCAM1) increased in correlation with the concentration of PM2.5. This suggested higher concentrations of PM2.5 led to increased expression levels of these markers associated with AR (144). Following exposure to PM2.5 can trigger a range of pathological alterations in the lungs, especially notable in asthmatic mice. These changes often include inflammatory cell infiltration, thickening of bronchial smooth muscles, and injury to the bronchial mucosa. These effects illustrate the impact of PM2.5 on exacerbating asthma-related symptoms and lung pathology (145). The above studies have shown that PM2.5 plays a role in promoting the occurrence and development of allergic diseases.
Prenatal and postnatal exposure to PM2.5 is associated with an increased risk of TD in infants delivered at term. A study conducted in central Taiwan found that exposure to PM2.5 during pregnancy and infancy was positively associated with the risk of TD, with a vulnerable time window for infants at 6–52 weeks after birth. The hazard ratio (HR) of TD was positively associated with a 10 μg/m3 increase in PM2.5 during pregnancy (HR 1.09, 95% CI 1.04, 1.15) and infancy (HR 1.12, 95% CI 1.06, 1.18). Indeed, the study found a non-linear relationship between exposure to PM2.5 and the risk of TD. Specifically, exposure levels between 16 and 64 μg/m3 of PM2.5 were associated with an increased risk of TD, particularly notable within the TS group. This non-linear association suggests a specific range of PM2.5 exposure that might significantly impact the risk of developing TD (146).
Our discussion covered shared elements leading to allergies and TD, encompassing neurotransmitter imbalance, maternal anxiety or depression, gut microbial disorders, sleep disturbances, maternal allergic status, exposure to tobacco, and environmental factors (Figure 1). Nonetheless, direct proof is absent to establish a link between these elements and both allergy and TD. Consequently, extended longitudinal research is essential to investigate the link between these factors and both conditions within the same group, as well as to explore how these factors mediate the relationship between allergies and TD.
6 Possible mechanisms linking allergy and TD
Emerging evidence pointing to potential links between allergies and TD underscores the importance to understanding the underlying mechanisms. Exploring mechanisms like alterations in gut microbial, changes in immunological and DNA methylation can provide valuable insights. These insights might lead to the identification of new treatment avenues and therapeutic targets for addressing both allergy-related TD.
6.1 Gut microbial
The ecosystem of the human gastrointestinal tract starts from the oral cavity, passes through the esophagus, stomach, small intestine, colon, and finally reaches the rectum. Its huge surface area of 150–200 m2 provides sufficient colonization space for microorganisms. The number of bacteria per milliliter of intestinal content is between 100,000 and 100 billion (147). Genome sequencing found that the gut microbial contains approximately 22.2 million genes, which is more than 700 times the length of the human genome (148). The gut microbial of a healthy human body is mainly composed of four bacterial phyla, namely Bacteroidetes, Firmicutes, Proteobacteria and Actinobacteria, accounting for more than 98% of all human gut microecologies (149). Gut microbial endows the host with multiple functions, including producing vitamins, absorbing calcium ions, resisting pathogens, enhancing immune function, etc. (150).
6.1.1 Gut-brain axis
The gut-brain axis, which refers to the bidirectional communication between the gastrointestinal tract and the CNS, has become one of the hotspots of research in the medical field. Research has shown that not only can the brain regulate gastrointestinal function and homeostasis, but the gut can also affect people's mood, sleep, and even neurological development and repair in a variety of ways, and this two-way communication between the gut and brain is known as the gut-brain axis (151). Gut-brain axis communication is based on neural, immune, endocrine and metabolic pathways. Gut microbial can produce neurotransmitters directly or indirectly through host biosynthetic pathways, and dysbiosis can cause neurotransmitter disorders and neurodevelopmental disorders. Beneficial bacteria such as Lactobacillus, Bifidobacterium, and Bacillus have been found to produce a variety of neurotransmitters, including dopamine, norepinephrine, 5-HT, GABA, acetylcholine and histamine, etc. (152). The above-mentioned neurotransmitters enter the CNS through the enteric nervous system (ENS) and affect the physiological functions of the brain. Available studies have shown that Lactobacillus and Bifidobacterium affect the synthesis of acetylcholine and GABA, while the synthesis of 5-HT, dopamine and norepinephrine is affected by Streptococcus, Enterococcus and Escherichia coli (153). Meanwhile, the effects of gut microbial on the nervous system are partly mediated by bacterial metabolites, the best-known of which are SCFAs, which can affect the development of the nervous system, immune signaling, and the integrity of the blood-brain barrier (154).
The two major barriers in the signal transduction process of the gut-brain axis are the gut barrier and the blood-brain barrier, of which the gut barrier is the first barrier between the human body and the intestinal lumen, and its normal functioning affects the exchange of substances between the body and the intestinal lumen and the invasion of pathogens (155). Gut microbial increase intestinal permeability by degrading biofilms on mucosal surfaces, which translocates lipopolysaccharide-containing Gram-negative bacteria, causing overactivation of the immune system and increased levels of pro-inflammatory cytokines. Excessive release of such pro-inflammatory factors is more destructive to CNS (156). The blood-brain barrier effectively regulates the exchange between the cerebrospinal fluid and the circulatory system, keeping the internal environment of the CNS relatively stable (157). Gut microbial reduce blood-brain barrier permeability by upregulating tight junction protein expression (158). At the same time, the use of many antipsychotics, such as fluoxetine and aripiprazole, can also alter the intestinal barrier (159), leading to alterations in signaling between the brain and the gut.
6.1.2 Allergy and SCFAs
The impact of allergic diseases on TD may be mediated by SCFAs, which mainly include acetic, propionic and butyric. SCFAs can influence the immune response in remote parts of the body and reduce airway inflammation caused by ovalbumin and house dust mites (160, 161). Furthermore, oral administration of SCFAs during pregnancy and weaning reduces the severity of allergic airway inflammation in offspring (162). Bacteroidetes and Firmicutes are the most common microorganisms in the intestine (163). Bacteroidetes are usually associated with the production of more acetic acid and propionic acid, while Firmicutes are associated with the production of more butyric acid (164). Butyrate is the most important presence in maintaining colon health. It can directly serve as an energy source for colon epithelial cells, maintain intestinal barrier integrity by regulating tight junction expression, and has systemic anti-inflammatory properties (165). Children with higher fecal propionic and butyric acid levels at 1 year of age are less likely to develop AA at 3–6 years of age (162). SCFAs are not only related to the integrity of the intestinal barrier, but also affect the ENS, promote the normal development of microglia, and affect the immune function and signaling of the CNS (166).
6.1.3 SCFAs and gut-brain axis
The ENS is also called the “Second Brain”. It is a neuron network composed of sensory neurons, motor neurons, interneurons and supporting cells embedded in the entire gastrointestinal wall. It is connected to the CNS through the vagus nerve, forming a gut-brain signal axis (167). Studies have shown that antibiotics induce a reduction in the ENS neuron network in mice (168). Gut function and enteric neurons restored after administration of SCFAs to germ-free mice (169). This shows that SCFAs deficiency can lead to the loss of intestinal neurons, thereby weakening ENS function. Damage to the ENS can produce pro-inflammatory factors that damage tight junction proteins and change the integrity of the blood-brain barrier, thereby affecting the maturation of microglia (170, 171). Microglia are resident macrophages in the brain's innate immune system and account for approximately 10% of nervous system cells. They are critical for the clearance of pathogens, senescent cells, and synaptic remodeling during development (172). Studies have shown that germ-free mice and antibiotic-treated mice have defects in microglial maturation, activation and differentiation, and morphological changes (173). Excessive activation of microglia function can trigger a cascade of neuroinflammation, including increased levels of IL-6, IFN-γ, TNF-α, and reactive oxygen species and reactive nitrogen species, leading to damage to the blood-brain barrier and neuronal cell death and brain damage (174). The addition of SCFAs (containing butyric acid, acetic acid, and propionic acid) to germ-free mouse feed improves microglia morphology and maturation defects (173). Sodium butyrate mediates neuroprotection by downregulating pro-inflammatory mediators TNF-α and NOS2 and upregulating IL-10 expression in microglia (175). Butyrate can also exert anti-inflammatory effects by limiting the formation of TGF-β1 and IL-6, enhancing Tregs cell activity and host immunity (176). SCFAs increase the expression of the transcription factor FOXP3 by inhibiting histone deacetylation, thereby expanding Tregs (177). At the same time, SCFAs can regulate genes encoding cAMP response element-binding proteins, regulate the synthesis of catecholamine neurotransmitters (such as dopamine), and affect gut-brain axis signal transmission (178).
6.1.4 SCFAs and TD
The number of microglia in the striatum of TD patients is increased, accompanied by enriched expression of inflammatory genes (179). Using PET imaging found microglia activation in the bilateral caudate nuclei of TD children and suggested that activated microglia mediate neuroinflammation (180). In animal models, it was also found that microglial activation in the striatum of TD mice was enhanced, accompanied by increased levels of pro-inflammatory cytokines IL-1β and TNF-α. The above studies all point to the activation of microglia in TD (181). Compared with healthy controls, the levels of pro-inflammatory mediators such as TNF-α, IL-6, IL-1β, and IL-12 in the plasma of TD children were significantly higher (182). Other studies have shown that excessive activation of immune responses in children with TD may be caused by decreased levels of peripheral Tregs (183). A case-control study showed that the concentration of tetrahydroisoquinoline in the urine of TD children was significantly increased. Tetrahydroisoquinoline can regulate dopaminergic neurotransmission and metabolism in the CNS, indicating that dopaminergic overactivity exists in TD children (184). Changes in SCFAs in patients with allergic diseases, especially the decrease in butyrate levels, may damage the ENS, activate microglia, and trigger a neuroinflammation cascade, resulting in decreased Tregs levels and dopaminergic overactivity, thereby inducing the occurrence of TD. The specific mechanism is shown in Figure 2.
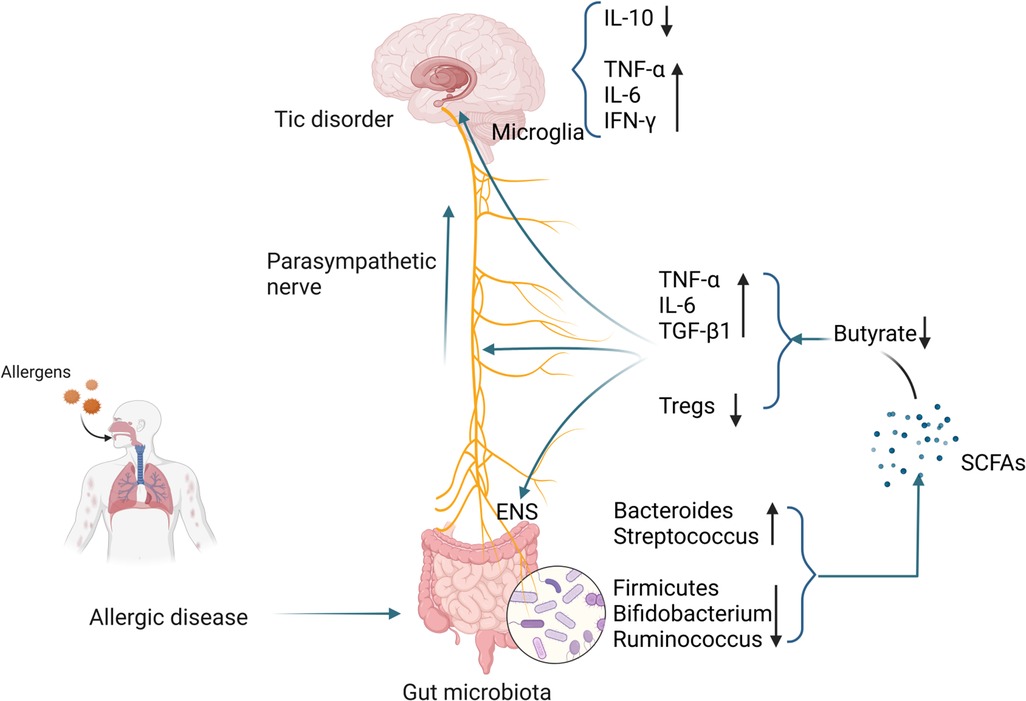
Figure 2 Possible mechanisms by which gut microbiota alterations occurring in patients with allergic diseases influence the development of TD. (Pictured by Biorender).
6.2 Changes in immunological
6.2.1 Allergy and immunity
The occurrence of allergic diseases is related to immune imbalance mediated by T lymphocytes. When a child is exposed to an allergen for the first time, dendritic cells (DC) process it and present it to CD4+ T cells. CD4+ T cells are activated and differentiate into Th2-type cells, producing IL-4 and IL-5. and IL-13 (185). IL-4 and IL-13 induce the activation of B cells and antibody class switching to produce sIgE (186). sIgE reaches various parts of the body through the circulation system and interacts with mast cells and basophils. The IgE high-affinity receptor (FcεRⅠ) on the cell surface binds, putting the body in a sensitized state (187). When the child is exposed to the same allergen again, the allergen binds to sIgE on the surface of mast cells and basophils, sIgE cross-links with FcεRⅠ, and mast cells and basophils degranulate, leading to histamine, etc. (188–190). IL-5 promotes the production and maturation of eosinophils, and the major basic protein released by IL-13 stimulates mast cells to release histamine and leukotrienes to trigger allergic symptoms (191).
Recent studies have also shown that epithelial cells are also involved in the development of allergies. Epithelial cells are the first line of defense against environmental damage and can produce a variety of cytokines after exposure to allergens, such as IL-25, IL-33, and thymic stromal lymphopoietin. These cytokines promote Th2 differentiation through DC and type 2 innate lymphocytes (192). In addition, reduced levels of Tregs were also observed in the peripheral blood of patients with allergic diseases (193). Animal studies have shown that infusion of Tregs from normal mice into ovalbumin-induced allergic mice significantly inhibited their airway hyperresponsiveness, while reducing eosinophil infiltration and lowering IL-5 in bronchoalveolar lavage fluid and IL-13 levels (194). Insufficient differentiation and functional defects of Tregs are key factors in the enhancement of Th2 responses and the occurrence of allergies, which are mainly manifested in the fact that TGF-β and IL-10 secreted by Tregs can significantly inhibit airway inflammation and hyperresponsiveness while blocking TGF-β or IL-10 aggravates airway inflammation and hyperresponsiveness (195). Studies have shown that the levels of TNF-α, IL-6 and other cytokines are increased in allergic diseases (196, 197). Although TNF-α is thought to synergize with IL-17 to promote neutrophil accumulation, TNF-α can also promote the production of Th2 cytokines, enhance airway smooth muscle contraction, and contribute to the occurrence of airway hyperresponsiveness (191). The above-mentioned studies have shown that patients with allergic diseases have significant immune system imbalances.
6.2.2 Immunity and TD
Increasing evidence shows that imbalances in the immune system also play an important role in the development of neurological diseases (198). Pro-inflammatory cytokines are released by the activation of immune cells when the host responds to pathogen invasion, tissue damage, and psychosocial stress. During an immune attack, pro-inflammatory cytokines are generally released for a short period and are regulated by anti-inflammatory mechanisms. Therefore, the immune signal released by the CNS in response to inflammation is an adaptive, temporary, and controllable response. However, when the immune attack becomes chronic and/or unregulated, the behavioral effects of cytokines and the resulting inflammatory response may promote the occurrence of TD (199, 200). Some studies have shown that the occurrence or worsening of tic symptoms in TD patients is related to abnormal immune activation caused by infection, and some TD patients have increased serum anti-streptolysin levels (201). Mycoplasma and enterovirus infections are also associated with tic severity (202). Therefore, when children with allergic diseases are repeatedly exposed to allergens, the immune imbalance caused by them and the continuous attack of pro-inflammatory cytokines may be important mechanisms in inducing TD.
6.2.3 Immune relationship between allergy and TD
Allergic diseases will aggravate the imbalance of T lymphocyte subsets and impairment of cellular immune function in TD children, mainly manifested by the decrease in the expression levels of CD3+ T cells, CD4+ T cells, and CD4+/CD8+ (48). Leckman found that the levels of pro-inflammatory cytokines such as TNF-α and IL-12 were increased in the peripheral blood of children with TD, and during the worsening of symptoms, the levels of these two cytokines further increased, suggesting that the occurrence of TD is related to innate immunity. Related to T cell immune imbalance (203). Another study showed that levels of pro-inflammatory cytokines such as IL-17A, IL-6, IL-12, and TNF-α were elevated in TD children without obsessive-compulsive disorder (202). Compared with healthy controls, TD children who did not receive drug treatment had higher TNF-α levels in the peripheral blood (204). Animal studies have shown that IL-6 increases 5-HT and dopamine activity in the hippocampus and frontal cortex of mice, showing more frequent digging, rearing, and grooming (205). Injection of IL-6 into mice during mid-pregnancy can lead to a lack of prepulse inhibition in offspring, and TD patients often have abnormal performance in this sensorimotor process (206). In addition, a study showed that the number of Tregs cells decreased in patients with moderate to severe TD, and further decreased during the symptom exacerbation period (183). This phenomenon may be related to the decrease in Tregs cells caused by the inflammatory response caused by allergies and the reactive pathogenic phenotype of Tregs (202). Histamine is an important mediator in the pathogenesis of allergic diseases, and its receptor-mediated signaling pathways are significantly enriched in TD patients, further indicating that the occurrence of allergic diseases may be an important risk factor for TD. In addition, some TD patients do not respond well to antipsychotic drugs, but they do respond well to adrenocorticotropic hormone or plasma exchange (37, 38). The above results all suggest that immune imbalance in allergic diseases and the resulting changes in related cytokine levels may be important factors in inducing TD. The specific mechanism is shown in Figure 3.

Figure 3 Possible mechanisms by which immunological changes occurring in patients with allergic diseases influence the development of TD. (Pictured by Biorender).
6.3 DNA methylation
The increasing prevalence of allergic diseases in children indicates that environmental exposures such as microbial imbalance, air pollution, and pet ownership have an important impact (207). Environmental factors can have persistent effects on gene expression by regulating epigenetic DNA methylation (208). DNA methylation refers to the transfer of methyl groups to cytosine-guanine (CpG) dinucleotides using S-adenosylmethionine as the methyl donor under the action of DNA methyltransferase. On the fifth carbon atom of cytosine. DNA methylation plays a pivotal role in modulating gene expression by changes in chromatin structure, DNA conformation, DNA stability, and the interactions between DNA and proteins. These modifications serve as a mechanism for controlling which genes are expressed or silenced within a cell (209).
6.3.1 Allergy and DNA methylation
At present, many studies have shown that DNA methylation testing in patients with allergic diseases may be helpful in the differential diagnosis of the disease (20). A large-scale cross-sectional study collected whole blood from 392 asthmatic children aged 4–8 years old and 1,156 control children for a whole-epigenome association study. This study discovered 14 differentially methylated CpG sites, which were subsequently verified through a meta-analysis of six additional European cohort studies (4–16 years old, 247 children with asthma and 2,949 controls). This study showed that all 14 differentially methylated CpG sites were significantly associated with asthma. These CpG sites are associated with whole blood transcriptional profiles, which are associated with increased activation of eosinophils, CD8+ T cells, NK cells, and reduced numbers of naive T cells (210). Another study showed that the DNA methylation of CpG sites in blood monocytes and airway epithelial cells of children with allergic diseases changed (211). After sublingual immunotherapy in patients with respiratory allergies, the CpG methylation level of the FOXP3 site in Tregs cells was reduced (95). In addition, studies have shown that DNA hypermethylation can cause a decrease in IFN-γ levels in AR patients, and this change may help distinguish allergic patients from healthy people (212). At the same time, DNA methylation in allergic diseases may also affect neurodevelopment, especially inducing the occurrence and development of TD.
6.3.2 TD and DNA methylation
A German study showed that compared with healthy people, the methylation level of the dopamine D2 receptor gene in TD patients was significantly increased and was positively correlated with the severity of tics. The dopamine transporter methylation level is negatively correlated with tic severity (213). Another epigenome-wide association study on TD investigated differences in DNA methylation in Dutch twins with TD. Among the top-ranked probes, an enrichment of differentially methylated neural genes previously associated with neurological diseases was detected (214).
6.3.3 DNA methylation link between allergy and TD
Studies have shown that compared with controls, patients with allergic diseases have differentially methylated CpG sites on Cadherin-26 (CDH26) (215). As an important cell adhesion molecule, CDH26 mediates cell-cell, cell-extracellular matrix interactions (216–218), and can regulate leukocyte migration, adhesion and activation, especially in the context of allergic inflammation (219). Tsetsos et al. also found in a genome-wide association study that CDH26 is related to TD and contains four single nucleotide polymorphisms (SNPs) (220). SNPs are the most common form of human genetic variation and represent changes in a single base pair in an individual's DNA sequence. Many SNPs are known to be associated with various human diseases, among which the genetic variations associated with TD are mainly concentrated in dopamine receptors (221). Dopamine receptors also play an important role in the occurrence of allergic diseases in children. Their combination with dopamine promotes Th2 cell differentiation and enhances Th2 inflammation in mice (222). At present, there are few epigenetic related studies on TD. Based on the analysis of existing research data, DNA methylation in allergic diseases, especially the differentially methylated CpG sites in CDH26, may be related to the occurrence of TD, but the relationship between DNA methylation between the two still needs to be further explored.
7 Conclusion
In summary, this study believes that there is a clear relationship between allergic diseases and TD, and allergic diseases may be an important risk factor for the occurrence of TD. Therefore, early and active treatment of allergic diseases may effectively prevent the occurrence of TD. There are currently few studies on the relationship between allergic diseases and TD, especially the lack of large-scale prospective cohort studies to further verify the causal relationship between the two. At the same time, it is of great significance to conduct in-depth research on the related mechanisms of allergic diseases affecting TD, aiming to provide new ideas and new directions for the prevention and treatment of TD.
Author contributions
PZ: Conceptualization, Investigation, Software, Writing – original draft, Writing – review & editing. ZZ: Data curation, Investigation, Writing – review & editing. HS: Data curation, Investigation, Writing – review & editing. TG: Formal Analysis, Supervision, Validation, Writing – review & editing. XX: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the Liaoning Provincial Doctoral Research Start-up Fund of China (2023010655-JH3/101).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hesse L, Oude Elberink JNG, van Oosterhout AJM, Nawijn MC. Allergen immunotherapy for allergic airway diseases: use lessons from the past to design a brighter future. Pharmacol Ther. (2022) 237:108115. doi: 10.1016/j.pharmthera.2022.108115
2. Chad Z. Allergies in children. Paediatr Child Health. (2001) 6(8):555–66. doi: 10.1093/pch/6.8.555
3. Wang J, Zhou Y, Zhang H, Hu L, Liu J, Wang L, et al. Pathogenesis of allergic diseases and implications for therapeutic interventions. Signal Transduct Targe Ther. (2023) 8(1):138. doi: 10.1038/s41392-023-01344-4
4. Maeda K, Caldez MJ, Akira S. Innate immunity in allergy. Allergy. (2019) 74(9):1660–74. doi: 10.1111/all.13788
5. Chinthrajah RS, Hernandez JD, Boyd SD, Galli SJ, Nadeau KC. Molecular and cellular mechanisms of food allergy and food tolerance. J Allergy Clin Immunol. (2016) 137(4):984–97. doi: 10.1016/j.jaci.2016.02.004
6. Devillier P, Bousquet J, Salvator H, Naline E, Grassin-Delyle S, de Beaumont O. In allergic rhinitis, work, classroom and activity impairments are weakly related to other outcome measures. Clin Exp Allergy. (2016) 46(11):1456–64. doi: 10.1111/cea.12801
7. Chua RXY, Tay MJY, Ooi DSQ, Siah KTH, Tham EH, Shek LP, et al. Understanding the link between allergy and neurodevelopmental disorders: a current review of factors and mechanisms. Front Neurol. (2020) 11:603571. doi: 10.3389/fneur.2020.603571
8. Huang J, Li R, Li L, Song Y, Jin L. The relationship between allergic diseases and tic disorders: a systematic review and meta-analysis. Neurosci Biobehav Rev. (2022) 132:362–77. doi: 10.1016/j.neubiorev.2021.12.004
9. Szejko N, Robinson S, Hartmann A, Ganos C, Debes NM, Skov L, et al. European clinical guidelines for Tourette syndrome and other tic disorders-version 2.0. Part I: assessment. Eur Child Adolesc Psychiatry. (2022) 31(3):383–402. doi: 10.1007/s00787-021-01842-2
10. Li F, Cui Y, Li Y, Guo L, Ke X, Liu J, et al. Prevalence of mental disorders in school children and adolescents in China: diagnostic data from detailed clinical assessments of 17,524 individuals. J Child Psychol Psychiatry. (2022) 63(1):34–46. doi: 10.1111/jcpp.13445
11. Kuhn J, Huys D. Tic-disorder - a disease between neurology and psychiatry. Fortschr Neurol Psychiatr. (2019) 87(10):538–9. doi: 10.1055/a-0963-1419
12. Deeb W, Malaty IA, Mathews CA. Tourette disorder and other tic disorders. Handb Clin Neurol. (2019) 165:123–53. doi: 10.1016/B978-0-444-64012-3.00008-3
13. Charania SN, Danielson ML, Claussen AH, Lebrun-Harris LA, Kaminski JW, Bitsko RH. Bullying victimization and perpetration among US children with and without tourette syndrome. J Dev Behav Pediatr. (2022) 43(1):23–31. doi: 10.1097/DBP.0000000000000975
14. Conelea CA, Woods DW, Zinner SH, Budman C, Murphy T, Scahill LD, et al. Exploring the Impact of Chronic Tic Disorders on Youth: Results from the Tourette Syndrome Impact Survey. Great Britain: Springer Science + Business Media (2011). 219–42.
16. Liu Y, Li Y, Ma X, Yu L, Liang Y, Li C. Comparative analysis of serum total IgE levels and specific IgE levels in children aged 6 to 9 years with tic disorder and normal children. BMC Pediatr. (2023) 23(1):399. doi: 10.1186/s12887-023-04233-5
17. Tagwerker Gloor F, Walitza S. Tic disorders and Tourette syndrome: current concepts of etiology and treatment in children and adolescents. Neuropediatrics. (2016) 47(2):84–96. doi: 10.1055/s-0035-1570492
18. He L, Ran M, Cui K, Li HY, Li T, et al. Analyze the multiple pathogenic factors of children’s abnormal blinking. Chin J Strab Pediatr Ophthalmol. (2017) 25(3):42–4. doi: 10.3969/J.ISSN.1005-328X.2017.03.014
19. Robertson NP. Advances in Tourette’s syndrome. J Neurol. (2023) 270(3):1808–10. doi: 10.1007/s00415-023-11588-3
20. Gezmu AM, Kung SJ, Shifa JZ, Nakstad B, Brooks M, Joel D, et al. Pediatric spectrum of allergic diseases and asthma in a tertiary level hospital in Botswana: an exploratory retrospective cross-sectional study. J Asthma Allergy. (2020) 13:213–23. doi: 10.2147/JAA.S253618
21. Zhang MZ, Chu SS, Xia YK, Wang DD, Wang X. Environmental exposure during pregnancy and the risk of childhood allergic diseases. World J Pediatr. (2021) 17(5):467–75. doi: 10.1007/s12519-021-00448-7
22. Ranasinghe JC, Karunarathne RR, Munasinghe TS, Vidanapathirana GU, Kudagammna ST. Childhood allergic diseases across geographical regions of Kandy and Anuradhapura districts of Sri Lanka; where do the rates stand among other regions: experience from global asthma network phase 1 study. Allergy Asthma Clin Immunol. (2022) 18(1):79. doi: 10.1186/s13223-022-00720-z
23. Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. (2011) 128(1):e9–17. doi: 10.1542/peds.2011-0204
24. Ma Z, Chen L, Xian R, Fang H, Wang J, Hu Y. Time trends of childhood food allergy in China: three cross-sectional surveys in 1999, 2009, and 2019. Pediatr Allergy Immunol. (2021) 32(5):1073–9. doi: 10.1111/pai.13490
25. Al-Naqeeb J, Danner S, Fagnan LJ, Ramsey K, Michaels L, Mitchell J, et al. The burden of childhood atopic dermatitis in the primary care setting: a report from the meta-LARC consortium. J Am Board Fam Med. (2019) 32(2):191–200. doi: 10.3122/jabfm.2019.02.180225
26. Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort study. BMC Pediatr. (2016) 16:133. doi: 10.1186/s12887-016-0673-z
27. Ma T, Zhuang Y, Shi H, Ning H, Gao M, He H, et al. Epidemiology of allergic rhinitis in children in grassland of Inner Mongolia. Chin J Otorhinolaryngol Head Neck Surg. (2019) 8:571–5. doi: 10.3760/ema.j.issn.1673-0860.2019.08.003
28. Hu S, Yao H, Peng Y, Wu X, Wei P, Kou W. Epidemiologic investigation and analysis of children allergic rhinitis in Chongqing city. Chongqing Med. (2017) 46(33):4700–14. doi: 10.3969/j.issn.1671-8348.2017.33.030
29. Leonardi A, Castegnaro A, Valerio AL, Lazzarini D. Epidemiology of allergic conjunctivitis: clinical appearance and treatment patterns in a population-based study. Curr Opin Allergy Clin Immunol. (2015) 15(5):482–8. doi: 10.1097/ACI.0000000000000204
30. Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. (2019) 53(5):1900164. doi: 10.1183/13993003.00164-2019
31. China Childhood asthma prevention and control collaborative group. A nationwide survey in China on prevalence of asthma in urban children. Chin J Pediatr. (2003) 41(02):123–7. doi: 10.3760/cma.j.issn.0578-1310.2003.02.116
32. Liu C, Hong J, Shang X, Sun J, Liu L, Duo L, et al. Third nationwide survey of childhood asthma in urban areas of China. Chin J Pediatr. (2013) 51(10):729–35. doi: 10.3760/cma.j.issn.0578-1310.2013.10.003
33. Sun LY, Li QP, Zhao LL, Ding YQ. Traditional Chinese medicine inheritance system analysis of professor Ding Yuanqing in treating tic disorder medication based on experience. China J Chin Mater Med. (2015) 40(16):3314–8. doi: 10.4268/cjcmm20151636
34. Knight T, Steeves T, Day L, Lowerison M, Jette N, Pringsheim T. Prevalence of tic disorders: a systematic review and meta-analysis. Pediatr Neurol. (2012) 47(2):77–90. doi: 10.1016/j.pediatrneurol.2012.05.002
35. Scahill L, Specht M, Page C. The prevalence of tic disorders and clinical characteristics in children. J Obsessive Compuls Relat Disord. (2014) 3(4):394–400. doi: 10.1016/j.jocrd.2014.06.002
36. Bitsko RH, Claussen AH, Lichstein J, Black LI, Jones SE, Danielson ML, et al. Mental health surveillance among children—United States, 2013–2019. MMWR Suppl. (2022) 71(2):1–42. doi: 10.15585/mmwr.su7102a1
37. Liu Y, Zheng Y, Han S, Cui Y. Tic disorders in children aged 6–16 years in Daxing district of Beijing: a cross-sectional study. Chin J Psychiatry. (2009) 42(4):231–4. doi: 10.3760/cma.j.issn.1006-7884.2009.04.016
38. Zheng R, Jin R, Xu H, Huang W, Chen H, Shao B, et al. Study on the prevalence of tic disorders in schoolchildren aged 7–16 years old in Wenzhou. Chin J Epidemiol. (2004) 25(9):745–7.
39. Bruun RD. Gilles de la Tourette’s syndrome. An overview of clinical experience. J Am Acad Child Psychiatry. (1984) 23(2):126–33. doi: 10.1097/00004583-198403000-00002
41. Comings D, Comings B. A controlled study of Tourette syndrome. VI. Early development, sleep problems, allergies, and handedness. Am J Hum Genet. (1987) 41(5):822–38.3479017
42. Kim H, Moote W, Mazza J. Tourette’s syndrome in patients referred for allergy evaluation. Ann Allergy Asthma Immunol. (1997) 79(4):347–9. doi: 10.1016/S1081-1206(10)63026-8
43. Ho CS, Shen EY, Shyur SD, Chiu NC. Association of allergy with Tourette’s syndrome. J Formos Med Assoc. (1999) 98(7):492–5.10462998
44. Chang YT, Li YF, Muo CH, Chen SC, Chin ZN, Kuo HT, et al. Correlation of Tourette syndrome and allergic disease: nationwide population-based case-control study. J Dev Behav Pediatr. (2011) 32(2):98–102. doi: 10.1097/DBP.0b013e318208f561
45. Chen MH, Su TP, Chen YS, Hsu JW, Huang KL, Chang WH, et al. Attention deficit hyperactivity disorder, tic disorder, and allergy: is there a link? A nationwide population-based study. J Child Psychol Psychiatry. (2013) 54(5):545–51. doi: 10.1111/jcpp.12018
46. Yuce M, Guner SN, Karabekiroglu K, Baykal S, Kilic M, Sancak R, et al. Association of Tourette syndrome and obsessive-compulsive disorder with allergic diseases in children and adolescents: a preliminary study. Eur Rev Med Pharmacol Sci. (2014) 18(3):303–10.24563428
47. Chen L, Chen X, Ke N, Pi L, Liu Q. Association between allergic conjunctivitis and provisional tic disorder in children. Int Ophthalmol. (2020) 40(1):247–53. doi: 10.1007/s10792-019-01174-w
48. Liu X, Wang X, Zhang X, Cao AH. Allergic diseases influence symptom severity and T lymphocyte subgroups of children with tic disorders. J Investig Med. (2021) 69(8):1453–7. doi: 10.1136/jim-2021-001788
49. Zhang Q, Xu B, Wang Q, Wang H, Chen T, Chen Z. Research on the relationship between tic disorder and allergic diseases in children. J Med Res. (2022) 51(7):72–5. doi: 10.11969/j.issn.1673-548X.2022.07.016
50. Chang Y, Li M, Li F, Qiu X, Qi Y, et al. A study on the correlation between tic disorders and allergic diseases in children. Chin J Clin. (2022) 50(5):610–3. doi: 10.3969/j.issn.2095-8552.2022.05.033
51. Undem BJ, Taylor-Clark T. Mechanisms underlying the neuronal-based symptoms of allergy. J Allergy Clin Immunol. (2014) 133(6):1521–34. doi: 10.1016/j.jaci.2013.11.027
52. Mawe GM. Tachykinins as mediators of slow EPSPs in Guinea-pig gall-bladder ganglia: involvement of neurokinin-3 receptors. J Physiol (Lond). (1995) 485(Pt 2):513–24. doi: 10.1113/jphysiol.1995.sp020747
53. Thangam EB, Jemima EA, Singh H, Baig MS, Khan M, Mathias CB, et al. The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: the hunt for new therapeutic targets. Front Immunol. (2018) 9:1873. doi: 10.3389/fimmu.2018.01873
54. White MV. The role of histamine in allergic diseases. J Allergy Clin Immunol. (1990) 86(4 Pt 2):599–605. doi: 10.1016/S0091-6749(05)80223-4
55. Lu WY, Inman MD. Gamma-aminobutyric acid nurtures allergic asthma. Clin Exp Allergy. (2009) 39(7):956–61. doi: 10.1111/j.1365-2222.2009.03265.x
56. Gentilini G, Franchi-Micheli S, Mugnai S, Bindi D, Zilletti L. GABA-mediated inhibition of the anaphylactic response in the Guinea-pig trachea. Br J Pharmacol. (1995) 115(3):389–94. doi: 10.1111/j.1476-5381.1995.tb16345.x
57. Córdoba-Rodríguez G, Vargas MH, Ruiz V, Carbajal V, Campos-Bedolla P, Mercadillo-Herrera P, et al. Allergic sensitization modifies the pulmonary expression of 5-hydroxytryptamine receptors in Guinea pigs. Respir Physiol Neurobiol. (2016) 223:9–15. doi: 10.1016/j.resp.2015.11.018
58. Abdala-Valencia H, Berdnikovs S, McCary CA, Urick D, Mahadevia R, Marchese ME, et al. Inhibition of allergic inflammation by supplementation with 5-hydroxytryptophan. Am J Physiol Lung Cell Mol Physiol. (2012) 303(8):L642–60. doi: 10.1152/ajplung.00406.2011
59. Wang Z, Maia TV, Marsh R, Colibazzi T, Gerber A, Peterson BS. The neural circuits that generate tics in Tourette’s syndrome. Am J Psychiatry. (2011) 168(12):1326–37. doi: 10.1176/appi.ajp.2011.09111692
60. Paschou P, Fernandez TV, Sharp F, Heiman GA, Hoekstra PJ. Genetic susceptibility and neurotransmitters in Tourette syndrome. Int Rev Neurobiol. (2013) 112:155–77. doi: 10.1016/B978-0-12-411546-0.00006-8
61. Rapanelli M, Frick L, Pogorelov V, Ohtsu H, Bito H, Pittenger C. Histamine H3R receptor activation in the dorsal striatum triggers stereotypies in a mouse model of tic disorders. Transl Psychiatry. (2017) 7(1):e1013. doi: 10.1038/tp.2016.290
62. Jackson GM, Draper A, Dyke K, Pépés SE, Jackson SR. Inhibition, disinhibition, and the control of action in Tourette syndrome. Trends Cogn Sci (Regul Ed). (2015) 19(11):655–65. doi: 10.1016/j.tics.2015.08.006
63. Draper A, Stephenson MC, Jackson GM, Pépés S, Morgan PS, Morris PG, et al. Increased GABA contributes to enhanced control over motor excitability in Tourette syndrome. Curr Biol. (2014) 24(19):2343–7. doi: 10.1016/j.cub.2014.08.038
64. Augustine F, Singer HS. Merging the pathophysiology and pharmacotherapy of tics. Tremor Other Hyperkinet Mov (N Y). (2019) 8:595. doi: 10.5334/tohm.442
65. Stepanikova I, Thon V, Mikes O, Klanova J. A model of perinatal stress and childhood wheezing: eLSPAC-CZ cohort. Pediatr Pulmonol. (2021) 56(6):1471–83. doi: 10.1002/ppul.25346
66. Alcala CS, Orozco Scott P, Tamayo-Ortiz M, Hernández Chávez MDC, Schnaas L, Carroll KN, et al. Longitudinal assessment of maternal depression and early childhood asthma and wheeze: effect modification by child sex. Pediatr Pulmonol. (2023) 58(1):98–106. doi: 10.1002/ppul.26164
67. Brew BK, Lundholm C, Viktorin A, Lichtenstein P, Larsson H, Almqvist C. Longitudinal depression or anxiety in mothers and offspring asthma: a Swedish population-based study. Int J Epidemiol. (2018) 47(1):166–74. doi: 10.1093/ije/dyx208
68. Han VX, Patel S, Jones HF, Nielsen TC, Mohammad SS, Hofer MJ, et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: a systematic review. Transl Psychiatry. (2021) 11(1):71. doi: 10.1038/s41398-021-01198-w
69. Kim CH, Kim SH, Lee JS. Association of maternal depression and allergic diseases in Korean children. Allergy Asthma Proc. (2017) 38(4):300–8. doi: 10.2500/aap.2017.38.4059
70. Li Y, Jiang Y, Li S, Shen X, Liu J, Jiang F. Pre- and postnatal risk factors in relation to allergic rhinitis in school-aged children in China. PLoS One. (2015) 10(2):e0114022. doi: 10.1371/journal.pone.0114022
71. Lau HX, Kee MZL, Yap QV, Tham EH, Chan YH, Goh AEN, et al. Associations between maternal distress during early life periods and offspring respiratory infections and allergic outcomes. Front Pediatr. (2022) 10:49323. doi: 10.3389/fped.2022.749323
72. Pierce M, Hope HF, Kolade A, Gellatly J, Osam CS, Perchard R, et al. Effects of parental mental illness on children’s physical health: systematic review and meta-analysis. Br J Psychiatry. (2020) 217(1):354–63. doi: 10.1192/bjp.2019.216
73. Flanigan C, Sheikh A, DunnGalvin A, Brew BK, Almqvist C, Nwaru BI. Prenatal maternal psychosocial stress and offspring’s asthma and allergic disease: a systematic review and meta-analysis. Clin Exp Allergy. (2018) 48(4):403–14. doi: 10.1111/cea.13091
74. de Marco R, Pesce G, Girardi P, Marchetti P, Rava M, Ricci P, et al. Foetal exposure to maternal stressful events increases the risk of having asthma and atopic diseases in childhood. Pediatr Allergy Immunol. (2012) 23(8):724–9. doi: 10.1111/j.1399-3038.2012.01346.x
75. Fan F, Zou Y, Zhang Y, Ma X, Zhang J, Liu C, et al. The relationship between maternal anxiety and cortisol during pregnancy and birth weight of Chinese neonates. BMC Pregnancy Childbirth. (2018) 18(1):265. doi: 10.1186/s12884-018-1798-x
76. Duthie L, Reynolds RM. Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: influences on maternal and fetal outcomes. Neuroendocrinology. (2013) 98(2):106–15. doi: 10.1159/000354702
77. O'Donnell KJ, Bugge Jensen A, Freeman L, Khalife N, O'Connor TG, Glover V. Maternal prenatal anxiety and downregulation of placental 11β-HSD2. Psychoneuroendocrinology. (2012) 37(6):818–26. doi: 10.1016/j.psyneuen.2011.09.014
78. Von Hertzen LC. Maternal stress and T-cell differentiation of the developing immune system: possible implications for the development of asthma and atopy. J Allergy Clin Immunol. (2002) 109(6):923–8. doi: 10.1067/mai.2002.124776
79. Mcgowan PO, Matthews SG. Prenatal stress, glucocorticoids, and developmental programming of the stress response. Endocrinology. (2018) 159(1):69–82. doi: 10.1210/en.2017-00896
80. Buske-Kirschbaum A, Geiben A, Höllig H, Morschhäuser E, Hellhammer D. Altered responsiveness of the hypothalamus-pituitary-adrenal axis and the sympathetic adrenomedullary system to stress in patients with atopic dermatitis. J Clin Endocrinol Metab. (2002) 87(9):4245–51. doi: 10.1210/jc.2001-010872
81. Ben-Shlomo Y, Scharf JM, Miller LL, Mathews CA. Parental mood during pregnancy and post-natally is associated with offspring risk of Tourette syndrome or chronic tics: prospective data from the avon longitudinal study of parents and children (ALSPAC). Eur Child Adolesc Psychiatry. (2016) 25(4):373–81. doi: 10.1007/s00787-015-0742-0
82. Leivonen S, Scharf JM, Mathews CA, Chudal R, Gyllenberg D, Sucksdorff D, et al. Parental psychopathology and Tourette syndrome/chronic tic disorder in offspring: a nationwide case-control study. J Am Acad Child Adolesc Psychiatry. (2017) 56(4):297–303.e4. doi: 10.1016/j.jaac.2017.01.009
83. Leckman JF, Dolnansky ES, Hardin MT, Clubb M, Walkup JT, Stevenson J, et al. Perinatal factors in the expression of Tourette’s syndrome: an exploratory study. J Am Acad Child Adolesc Psychiatry. (1990) 29(2):220–6. doi: 10.1097/00004583-199003000-00010
84. Blakeley PM, Capron LE, Jensen AB, O'Donnell KJ, Glover V. Maternal prenatal symptoms of depression and down regulation of placental monoamine oxidase A expression. J Psychosom Res. (2013) 75(4):341–5. doi: 10.1016/j.jpsychores.2013.07.002
85. De Asis-Cruz J, Krishnamurthy D, Zhao L, Kapse K, Vezina G, Andescavage N, et al. Association of prenatal maternal anxiety with fetal regional brain connectivity. JAMA Netw Open. (2020) 3(12):e2022349. doi: 10.1001/jamanetworkopen.2020.22349
86. Hu C, van Meel ER, Medina-Gomez C, Kraaij R, Barroso M, Kiefte-de Jong J, et al. A population-based study on associations of stool microbiota with atopic diseases in school-age children. J Allergy Clin Immunol. (2021) 148(2):612–20. doi: 10.1016/j.jaci.2021.04.001
87. Songjinda P, Nakayama J, Tateyama A, Tanaka S, Tsubouchi M, Kiyohara C, et al. Differences in developing intestinal microbiota between allergic and non-allergic infants: a pilot study in Japan. Biosci Biotechnol Biochem. (2007) 71(9):2338–42. doi: 10.1271/bbb.70154
88. Chang H, Tang Y, Wang Z, Jia M, Shi S, RE N, et al. Effect of acupuncture combined with infantile tuina on intestinal flora in children with tic disorders. Chin Acupunct Moxibustion. (2023) 43(5):509–16. doi: 10.13703/j.0255-2930.20220724-0002
89. Zhang P, Zhou X, Tan H, Jian F, Jing Z, Wu H, et al. Microbial signature of intestine in children with allergic rhinitis. Front Microbiol. (2023) 14:1208816. doi: 10.3389/fmicb.2023.1208816
90. Pascal M, Perez-Gordo M, Caballero T, Escribese MM, Lopez Longo MN, Luengo O, et al. Microbiome and allergic diseases. Front Immunol. (2018) 9:1584. doi: 10.3389/fimmu.2018.01584
91. Aitoro R, Paparo L, Amoroso A, Di Costanzo M, Cosenza L, Granata V, et al. Gut microbiota as a target for preventive and therapeutic intervention against food allergy. Nutrients. (2017) 9(7). doi: 10.3390/nu9070672
92. Bisgaard H, Li N, Bonnelykke K, Chawes BL, Skov T, Paludan-Müller G, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. (2011) 128(3):646–52.e1–5. doi: 10.1016/j.jaci.2011.04.060
93. Xi W, Gao X, Zhao H, Luo X, Li J, Tan X, et al. Depicting the composition of gut microbiota in children with tic disorders: an exploratory study. J Child Psychol Psychiatry. (2021) 62(10):1246–54. doi: 10.1111/jcpp.13409
94. Goldstein AN, Walker MP. The role of sleep in emotional brain function. Annu Rev Clin Psychol. (2014) 10:679–708. doi: 10.1146/annurev-clinpsy-032813-153716
95. Xie Z, Chen F, Li WA, Geng X, Li C, Meng X, et al. A review of sleep disorders and melatonin. Neurol Res. (2017) 39(6):559–65. doi: 10.1080/01616412.2017.1315864
96. Liu J, Zhang X, Zhao Y, Wang Y. The association between allergic rhinitis and sleep: a systematic review and meta-analysis of observational studies. PLoS One. (2020) 15(2):e0228533. doi: 10.1371/journal.pone.0228533
97. Xi Y, Deng YQ, Chen SM, Kong YG, Xu Y, Li F, et al. Allergy-related outcomes and sleep-related disorders in adults: a cross-sectional study based on NHANES 2005–2006. Allergy Asthma Clin Immunol. (2022) 18(1):27. doi: 10.1186/s13223-022-00669-z
98. Mann C, Dreher M, Weeß HG, Staubach P. Sleep disturbance in patients with urticaria and atopic dermatitis: an underestimated burden. Acta Derm Venereol. (2020) 100(6):adv00073. doi: 10.2340/00015555-3416
99. Ramirez FD, Chen S, Langan SM, Prather AA, McCulloch CE, Kidd SA, et al. Association of atopic dermatitis with sleep quality in children. JAMA Pediatr. (2019) 173(5):e190025. doi: 10.1001/jamapediatrics.2019.0025
100. Camfferman D, Kennedy JD, Gold M, Martin AJ, Winwood P, Lushington K. Eczema, sleep, and behavior in children. J Clin Sleep Med. (2010) 6(6):581–8. doi: 10.5664/jcsm.27992
101. Björnsdóttir E, Janson C, Lindberg E, Arnardottir ES, Benediktsdóttir B, Garcia-Aymerich J, et al. Respiratory symptoms are more common among short sleepers independent of obesity. BMJ Open Respir Res. (2017) 4(1):e000206. doi: 10.1136/bmjresp-2017-000206
102. Sherrey J, Biggs S, Dorrian J, Martin J, Gold M, Kennedy D, et al. Allergic disease, sleep problems, and psychological distress in children recruited from the general community. Ann Allergy Asthma Immunol. (2022) 129(3):366–72. doi: 10.1016/j.anai.2022.05.008
103. Chang HY, Seo JH, Kim HY, Kwon JW, Kim BJ, Kim HB, et al. Allergic diseases in preschoolers are associated with psychological and behavioural problems. Allergy Asthma Immunol Res. (2013) 5(5):315–21. doi: 10.4168/aair.2013.5.5.315
104. Koinis-Mitchell D, Craig T, Esteban CA, Klein RB. Sleep and allergic disease: a summary of the literature and future directions for research. J Allergy Clin Immunol. (2012) 130(6):1275–81. doi: 10.1016/j.jaci.2012.06.026
105. Ghosh D, Rajan PV, Das D, Datta P, Rothner AD, Erenberg G. Sleep disorders in children with Tourette syndrome. Pediatr Neurol. (2014) 51(1):31–5. doi: 10.1016/j.pediatrneurol.2014.03.017
106. Ricketts EJ, Rozenman M, Choy C, Goldberg HB, Kim JS, Colwell CS, et al. Sleep sufficiency in pediatric and adolescent Tourette’s disorder: national survey of children’s health. J Dev Behav Pediatr. (2018) 39(1):72–6. doi: 10.1097/DBP.0000000000000518
107. Singer HS. Tics and Tourette syndrome. Continuum (Minneapolis, Minn). (2019) 25(4):936–58. doi: 10.1212/CON.0000000000000752
108. Mi Y, Zhao R, Sun X, Yu P, Wang W, Li J, et al. Sleep disturbances and sleep patterns in children with tic disorder: a case-control study. Front Pediatr. (2022) 10:911343. doi: 10.3389/fped.2022.911343
109. Cohrs S, Rasch T, Altmeyer S, Kinkelbur J, Kostanecka T, Rothenberger A, et al. Decreased sleep quality and increased sleep related movements in patients with Tourette’s syndrome. J Neurol Neurosurg Psychiatr. (2001) 70(2):192–7. doi: 10.1136/jnnp.70.2.192
110. Martino D, Ganos C, Pringsheim TM. Tourette syndrome and chronic tic disorders: the clinical spectrum beyond tics. Int Rev Neurobiol. (2017) 134:1461–90. doi: 10.1016/bs.irn.2017.05.006
111. Lee WT, Huang HL, Wong LC, Weng WC, Vasylenko T, Jong YJ, et al. Tourette syndrome as an independent risk factor for subsequent sleep disorders in children: a nationwide population-based case-control study. Sleep. (2017) 40(3). doi: 10.1093/sleep/zsw072
112. Jiao L, Su CW, Cao T, Zheng S, Walker WA, Shi HN. Maternal influences and intervention strategies on the development of food allergy in offspring. Front Immunol. (2022) 13:817062. doi: 10.3389/fimmu.2022.817062
113. Liu CA, Wang CL, Chuang H, Ou CY, Hsu TY, Yang KD. Prenatal prediction of infant atopy by maternal but not paternal total IgE levels. J Allergy Clin Immunol. (2003) 112(5):899–904. doi: 10.1016/j.jaci.2003.08.030
114. Černý V, Hrdý J, Novotná O, Petrásková P, Boráková K, Kolářová L, et al. Distinct characteristics of tregs of newborns of healthy and allergic mothers. PLoS One. (2018) 13(11):e0207998. doi: 10.1371/journal.pone.0207998
115. Dalsgaard S, Waltoft BL, Leckman JF, Mortensen PB. Maternal history of autoimmune disease and later development of Tourette syndrome in offspring. J Am Acad Child Adolesc Psychiatry. (2015) 54(6):495–501.e1. doi: 10.1016/j.jaac.2015.03.008
116. Gong T, Lundholm C, Rejnö G, Bölte S, Larsson H, D'Onofrio BM, et al. Parental asthma and risk of autism spectrum disorder in offspring: a population and family-based case-control study. Clin Exp Allergy. (2019) 49(6):883–91. doi: 10.1111/cea.13353
117. Hisle-Gorman E, Susi A, Stokes T, Gorman G, Erdie-Lalena C, Nylund CM. Prenatal, perinatal, and neonatal risk factors of autism spectrum disorder. Pediatr Res. (2018) 84(2):190–8. doi: 10.1038/pr.2018.23
118. Liu X, Dalsgaard S, Munk-Olsen T, Li J, Wright RJ, Momen NC. Parental asthma occurrence, exacerbations and risk of attention-deficit/hyperactivity disorder. Brain Behav Immun. (2019) 82:302–8. doi: 10.1016/j.bbi.2019.08.198
119. Amiri S, Fakhari A, Golmirzaei J, Mohammadpoorasl A, Abdi S. Tourette’s syndrome, chronic tics, and comorbid attention deficit/hyperactivity disorder in elementary students. Arch Iran Med. (2012) 15(2):76–8.22292574
120. Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. (2008) 359(1):61–73. doi: 10.1056/NEJMra0708473
121. Yang HJ. Impact of perinatal environmental tobacco smoke on the development of childhood allergic diseases. Korean J Pediatr. (2016) 59(8):319–27. doi: 10.3345/kjp.2016.59.8.319
122. Silvestri M, Franchi S, Pistorio A, Petecchia L, Rusconi F. Smoke exposure, wheezing, and asthma development: a systematic review and meta-analysis in unselected birth cohorts. Pediatr Pulmonol. (2015) 50(4):353–62. doi: 10.1002/ppul.23037
123. Duan C, Wang M, Ma X, Ding M, Yu H, Han Y. Association between maternal smoking during pregnancy and recurrent wheezing in infancy: evidence from a meta-analysis. Int J Clin Exp Med. (2015) 8(5):6755–61.26221213
124. Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. (2012) 129(4):735–44. doi: 10.1542/peds.2011-2196
125. Zhou Y, Chen J, Dong Y, Shen J, Tian M, Yang Y, et al. Maternal tobacco exposure during pregnancy and allergic rhinitis in offspring: a systematic review and meta-analysis. Medicine (Baltimore). (2021) 100(34):e26986. doi: 10.1097/MD.0000000000026986
126. Arruda LK, Solé D, Baena-Cagnani CE, Naspitz CK. Risk factors for asthma and atopy. Curr Opin Allergy Clin Immunol. (2005) 5(2):153–9. doi: 10.1097/01.all.0000162308.89857.6c
127. Ferrini M, Carvalho S, Cho YH, Postma B, Miranda Marques L, Pinkerton K, et al. Prenatal tobacco smoke exposure predisposes offspring mice to exacerbated allergic airway inflammation associated with altered innate effector function. Part Fibre Toxicol. (2017) 14(1):30. doi: 10.1186/s12989-017-0212-6
128. Li X, Jing R, Feng S, Zhang H, Zhang J, Li J, et al. Association between prenatal or postpartum exposure to tobacco smoking and allergic rhinitis in the offspring: an updated meta-analysis of nine cohort studies. Tob Induc Dis. (2022) 20:37. doi: 10.18332/tid/146905
129. Chen D, Niu Q, Liu S, Shao W, Huang Y, Xu Y, et al. The correlation between prenatal maternal active smoking and neurodevelopmental disorders in children: a systematic review and meta-analysis. BMC Public Health. (2023) 23(1):611. doi: 10.1186/s12889-023-15496-z
130. Browne HA, Modabbernia A, Buxbaum JD, Hansen SN, Schendel DE, Parner ET, et al. Prenatal maternal smoking and increased risk for Tourette syndrome and chronic tic disorders. J Am Acad Child Adolesc Psychiatry. (2016) 55(9):784–91. doi: 10.1016/j.jaac.2016.06.010
131. Ayubi E, Mansori K, Doosti-Irani A. Effect of maternal smoking during pregnancy on Tourette syndrome and chronic tic disorders among offspring: a systematic review and meta-analysis. Obstet Gynecol Sci. (2021) 64(1):1–12. doi: 10.5468/ogs.20252
132. Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry. (2001) 40(6):630–41. doi: 10.1097/00004583-200106000-00007
133. Steeves TD, Ko JH, Kideckel DM, Rusjan P, Houle S, Sandor P, et al. Extrastriatal dopaminergic dysfunction in Tourette syndrome. Ann Neurol. (2010) 67(2):170–81. doi: 10.1002/ana.21809
134. Liu J, Lester BM, Neyzi N, Sheinkopf SJ, Gracia L, Kekatpure M, et al. Regional brain morphometry and impulsivity in adolescents following prenatal exposure to cocaine and tobacco. JAMA Pediatr. (2013) 167(4):348–54. doi: 10.1001/jamapediatrics.2013.550
135. Derauf C, Lester BM, Neyzi N, Kekatpure M, Gracia L, Davis J, et al. Subcortical and cortical structural central nervous system changes and attention processing deficits in preschool-aged children with prenatal methamphetamine and tobacco exposure. Dev Neurosci. (2012) 34(4):327–41. doi: 10.1159/000341119
136. Kim KH, Kabir E, Kabir S. A review on the human health impact of airborne particulate matter. Environ Int. (2015) 74:136–43. doi: 10.1016/j.envint.2014.10.005
137. Chang YC, Chen WT, Su SH, Jung CR, Hwang BF. PM(2.5) exposure and incident attention-deficit/hyperactivity disorder during the prenatal and postnatal periods: a birth cohort study. Environ Res. (2022) 214(Pt 1):113769. doi: 10.1016/j.envres.2022.113769
138. Wright RJ, Brunst KJ. Programming of respiratory health in childhood: influence of outdoor air pollution. Curr Opin Pediatr. (2013) 25(2):232–9. doi: 10.1097/MOP.0b013e32835e78cc
139. Friedrich MJ. Global impact of air pollution on children’s health. JAMA. (2018) 320(23):2412. doi: 10.1001/jama.2018.19559
140. Wu JZ, Ge DD, Zhou LF, et al. Effects of particulate matter on allergic respiratory diseases. Chronic Dis Transl Med. (2018) 4(2):95–102. doi: 10.1016/j.cdtm.2018.04.001
141. Bowatte G, Lodge C, Lowe AJ, Erbas B, Perret J, Abramson MJ, et al. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy. (2015) 70(3):245–56. doi: 10.1111/all.12561
142. Wei S, Liao J, Xue T, Yu K, Fu X, Wang R, et al. Ambient fine particulate matter and allergic symptoms in the middle-aged and elderly population: results from the PIFCOPD study. Respir Res. (2023) 24(1):139. doi: 10.1186/s12931-023-02433-2
143. Zhang X, Zhong W, Meng Q, Lin Q, Fang C, Huang X, et al. Ambient PM2.5 exposure exacerbates severity of allergic asthma in previously sensitized mice. J Asthma. (2015) 52(8):785–94. doi: 10.3109/02770903.2015.1036437
144. Wang YL, Gao W, Li Y, Wang YF. Concentration-dependent effects of PM2.5 mass on expressions of adhesion molecules and inflammatory cytokines in nasal mucosa of rats with allergic rhinitis. Eur Arch Otorhinolaryngol. (2017) 274(8):3221–9. doi: 10.1007/s00405-017-4606-8
145. Liu H, Fan X, Wang N, Zhang Y, Yu J. Exacerbating effects of PM2.5 in OVA-sensitized and challenged mice and the expression of TRPA1 and TRPV1 proteins in lungs. J Asthma. (2017) 54(8):807–17. doi: 10.1080/02770903.2016.1266495
146. Chang YT, Jung CR, Chang YC, Chuang BR, Chen ML, Hwang BF. Prenatal and postnatal exposure to PM(2) (.5) and the risk of tic disorders. Paediatr Perinat Epidemiol. (2023) 37(3):191–200. doi: 10.1111/ppe.12943
147. Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. (2016) 164(3):337–40. doi: 10.1016/j.cell.2016.01.013
148. Tierney BT, Yang Z, Luber JM, Beaudin M, Wibowo MC, Baek C, et al. The landscape of genetic content in the gut and oral human microbiome. Cell Host Microbe. (2019) 26(2):283–95.e8. doi: 10.1016/j.chom.2019.07.008
149. Barcik W, Boutin RCT, Sokolowska M, Finlay BB. The role of lung and gut Microbiota in the pathology of asthma. Immunity. (2020) 52(2):241–55. doi: 10.1016/j.immuni.2020.01.007
150. Hillman ET, Lu H, Yao T, Nakatsu CH. Microbial ecology along the gastrointestinal tract. Microbes Environ. (2017) 32(4):300–13. doi: 10.1264/jsme2.ME17017
151. Gershon MD, Margolis KG. The gut, its microbiome, and the brain: connections and communications. J Clin Invest. (2021) 131(18):e143768. doi: 10.1172/JCI143768
152. Yang J, Fu X, Liao X, Li Y. Effects of gut microbial-based treatments on gut microbiota, behavioral symptoms, and gastrointestinal symptoms in children with autism spectrum disorder: a systematic review. Psychiatry Res. (2020) 293:113471. doi: 10.1016/j.psychres.2020.113471
153. Saurman V, Margolis KG, Luna RA. Autism spectrum disorder as a brain-gut-microbiome axis disorder. Dig Dis Sci. (2020) 65(3):818–28. doi: 10.1007/s10620-020-06133-5
154. Ahmed H, Leyrolle Q, Koistinen V, Kärkkäinen O, Layé S, Delzenne N, et al. Microbiota-derived metabolites as drivers of gut-brain communication. Gut Microbes. (2022) 14(1):2102878. doi: 10.1080/19490976.2022.2102878
155. Takiishi T, Fenero CIM, Câmara NOS. Intestinal barrier and gut microbiota: shaping our immune responses throughout life. Tissue Barriers. (2017) 5(4):e1373208. doi: 10.1080/21688370.2017.1373208
156. Góralczyk-Bińkowska A, Szmajda-Krygier D, Kozłowska E. The microbiota-gut-brain axis in psychiatric disorders. Int J Mol Sci. (2022) 23(19):11245. doi: 10.3390/ijms231911245
157. Armstead WM. Cerebral blood flow autoregulation and dysautoregulation. Anesthesiol Clin. (2016) 34(3):465–77. doi: 10.1016/j.anclin.2016.04.002
158. Alaish SM, Smith AD, Timmons J, Greenspon J, Eyvazzadeh D, Murphy E, et al. Gut microbiota, tight junction protein expression, intestinal resistance, bacterial translocation and mortality following cholestasis depend on the genetic background of the host. Gut Microbes. (2013) 4(4):292–305. doi: 10.4161/gmic.24706
159. Cussotto S, Strain CR, Fouhy F, Strain RG, Peterson VL, Clarke G, et al. Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology. (2019) 236(5):1671–85. doi: 10.1007/s00213-018-5006-5
160. Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. (2014) 20(2):159–66. doi: 10.1038/nm.3444
161. Cait A, Hughes MR, Antignano F, Cait J, Dimitriu PA, Maas KR, et al. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunol. (2018) 11(3):785–95. doi: 10.1038/mi.2017.75
162. Roduit C, Frei R, Ferstl R, Loeliger S, Westermann P, Rhyner C, et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy. (2019) 74(4):799–809. doi: 10.1111/all.13660
164. Besten GD, Van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. (2013) 54(9):2325–40. doi: 10.1194/jlr.R036012
165. Rauf A, Khalil AA, Rahman UU, Khalid A, Naz S, Shariati MA, et al. Recent advances in the therapeutic application of short-chain fatty acids (SCFAs): an updated review. Crit Rev Food Sci Nutr. (2022) 62(22):6034–54. doi: 10.1080/10408398.2021.1895064
166. Romano S, Savva GM, Bedarf JR, Charles IG, Hildebrand F, Narbad A. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinson’s Dis. (2021) 7(1):27. doi: 10.1038/s41531-021-00156-z
167. Rao M, Gershon MD. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol. (2016) 13(9):517–28. doi: 10.1038/nrgastro.2016.107
168. Dicks LMT. Gut bacteria and neurotransmitters. Microorganisms. (2022) 10(9):1838. doi: 10.3390/microorganisms10091838
169. Vicentini FA, Keenan CM, Wallace LE, Woods C, Cavin JB, Flockton AR, et al. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome. (2021) 9(1):210. doi: 10.1186/s40168-021-01165-z
170. Claudino Dos Santos JC, Lima MPP, Brito GAC, Viana GSB. Role of enteric glia and microbiota-gut-brain axis in Parkinson disease pathogenesis. Ageing Res Rev. (2023) 84:101812. doi: 10.1016/j.arr.2022.101812
171. Sun MF, Shen YQ. Dysbiosis of gut microbiota and microbial metabolites in Parkinson’s disease. Ageing Res Rev. (2018) 45:53–61. doi: 10.1016/j.arr.2018.04.004
172. Pavek P, Stejskalova L, Krausova L, Bitman M, Vrzal R, Dvorak Z. Rifampicin does not significantly affect the expression of small heterodimer partner in primary human hepatocytes. Front Pharmacol. (2012) 3:1. doi: 10.3389/fphar.2012.00001
173. Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. (2015) 18(7):965–77. doi: 10.1038/nn.4030
174. Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. (2007) 8(1):57–69. doi: 10.1038/nrn2038
175. Patnala R, Arumugam TV, Gupta N, Dheen ST. HDAC inhibitor sodium butyrate-mediated epigenetic regulation enhances neuroprotective function of microglia during ischemic stroke. Mol Neurobiol. (2017) 54(8):6391–411. doi: 10.1007/s12035-016-0149-z
176. Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. (2007) 450(7169):566–9. doi: 10.1038/nature06306
177. Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. (2013) 504(7480):451–5. doi: 10.1038/nature12726
178. Nankova BB, Agarwal R, MacFabe DF, La Gamma EF. Enteric bacterial metabolites propionic and butyric acid modulate gene expression, including CREB-dependent catecholaminergic neurotransmission, in PC12 cells–possible relevance to autism spectrum disorders. PLoS One. (2014) 9(8):e103740. doi: 10.1371/journal.pone.0103740
179. Lennington JB, Coppola G, Kataoka-Sasaki Y, Fernandez TV, Palejev D, Li Y, et al. Transcriptome analysis of the human striatum in Tourette syndrome. Biol Psychiatry. (2016) 79(5):372–82. doi: 10.1016/j.biopsych.2014.07.018
180. Kumar A, Williams MT, Chugani HT. Evaluation of basal ganglia and thalamic inflammation in children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection and Tourette syndrome: a positron emission tomographic (PET) study using 11C-[R]-PK11195. J Child Neurol. (2015) 30(6):749–56. doi: 10.1177/0883073814543303
181. Frick L, Pittenger C. Microglial dysregulation in OCD, Tourette syndrome, and PANDAS. J Immunol Res. (2016) 2016:8606057. doi: 10.1155/2016/8606057
182. Cheng YH, Zheng Y, He F, Yang JH, Li WB, Wang ML, et al. Detection of autoantibodies and increased concentrations of interleukins in plasma from patients with Tourette’s syndrome. J Mol Neurosci. (2012) 48(1):219–24. doi: 10.1007/s12031-012-9811-8
183. Kawikova I, Leckman JF, Kronig H, Katsovich L, Bessen DE, Ghebremichael M, et al. Decreased numbers of regulatory T cells suggest impaired immune tolerance in children with Tourette syndrome: a preliminary study. Biol Psychiatry. (2007) 61(3):273–8. doi: 10.1016/j.biopsych.2006.06.012
184. Capetian P, Roessner V, Korte C, Walitza S, Riederer F, Taurines R, et al. Altered urinary tetrahydroisoquinoline derivatives in patients with Tourette syndrome: reflection of dopaminergic hyperactivity? J Neural Transm (Vienna). (2021) 128(1):115–20. doi: 10.1007/s00702-020-02289-6
185. Humbert M, Bousquet J, Bachert C, Palomares O, Pfister P, Kottakis I, et al. IgE-mediated multimorbidities in allergic asthma and the potential for omalizumab therapy. J Allergy Clin Immunol Pract. (2019) 7(5):1418–29. doi: 10.1016/j.jaip.2019.02.030
186. Iinuma T, Okamoto Y, Morimoto Y, Arai T, Sakurai T, Yonekura S, et al. Pathogenicity of memory Th2 cells is linked to stage of allergic rhinitis. Allergy. (2018) 73(2):479–89. doi: 10.1111/all.13295
187. Wang B, Rieger A, Kilgus O, Ochiai K, Maurer D, Födinger D, et al. Epidermal Langerhans cells from normal human skin bind monomeric IgE via Fc epsilon RI. J Exp Med. (1992) 175(5):1353–65. doi: 10.1084/jem.175.5.1353
188. Crosson T, Wang JC, Doyle B, Merrison H, Balood M, Parrin A, et al. FcεR1-expressing nociceptors trigger allergic airway inflammation. J Allergy Clin Immunol. (2021) 147(6):2330–42. doi: 10.1016/j.jaci.2020.12.644
189. Justiz Vaillant AA, Vashisht R, Zito PM. Immediate Hypersensitivity Reactions. Treasure Island (FL): StatPearls, StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC (2022).
190. Shamji MH, Valenta R, Jardetzky T, Verhasselt V, Durham SR, Würtzen PA, et al. The role of allergen-specific IgE, IgG and IgA in allergic disease. Allergy. (2021) 76(12):3627–41. doi: 10.1111/all.14908
191. Habib N, Pasha MA, Tang DD. Current understanding of asthma pathogenesis and biomarkers. Cells. (2022) 11(17):2764. doi: 10.3390/cells11172764
192. Morita H, Moro K, Koyasu S. Innate lymphoid cells in allergic and nonallergic inflammation. J Allergy Clin Immunol. (2016) 138(5):1253–64. doi: 10.1016/j.jaci.2016.09.011
193. Wang X, Li R, Ma X. Relationship between serum lncRNA MEG3 expression and Th17/treg balance in children with allergic asthma. China Med Herald. (2023) 20(20):113–6. doi: 10.20047/j.issn1673-7210.2023.20.24
194. Joetham A, Schedel M, O'Connor BP, Kim S, Takeda K, Abbott J, et al. Inducible and naturally occurring regulatory T cells enhance lung allergic responses through divergent transcriptional pathways. J Allergy Clin Immunol. (2017) 139(4):1331–42. doi: 10.1016/j.jaci.2016.06.051
195. Zhao ST, Wang CZ. Regulatory T cells and asthma. J Zhejiang Univ Sci B. (2018) 19(9):663–73. doi: 10.1631/jzus.B1700346
196. Lechner A, Henkel FDR, Hartung F, Bohnacker S, Alessandrini F, Gubernatorova EO, et al. Macrophages acquire a TNF-dependent inflammatory memory in allergic asthma. J Allergy Clin Immunol. (2022) 149(6):2078–90. doi: 10.1016/j.jaci.2021.11.026
197. Tashiro H, Shore SA. Obesity and severe asthma. Allergol Int. (2019) 68(2):135–42. doi: 10.1016/j.alit.2018.10.004
198. Ferencova N, Visnovcova Z, Ondrejka I, Hrtanek I, Bujnakova I, Kovacova V, et al. Peripheral inflammatory markers in autism spectrum disorder and attention deficit/hyperactivity disorder at adolescent age. Int J Mol Sci. (2023) 24(14):11710. doi: 10.3390/ijms241411710
199. Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. (2011) 130(2):226–38. doi: 10.1016/j.pharmthera.2011.01.014
200. Spinello C, Laviola G, Macrì S. Pediatric autoimmune disorders associated with streptococcal infections and Tourette’s syndrome in preclinical studies. Front Neurosci. (2016) 10:310. doi: 10.3389/fnins.2016.00310
201. Lamothe H, Tamouza R, Hartmann A, Mallet L. Immunity and Gilles de la Tourette syndrome: a systematic review and meta-analysis of evidence for immune implications in Tourette syndrome. Eur J Neurol. (2021) 28(9):3187–200. doi: 10.1111/ene.14983
202. Hsu CJ, Wong LC, Lee WT. Immunological dysfunction in Tourette syndrome and related disorders. Int J Mol Sci. (2021) 22(2):853. doi: 10.3390/ijms22020853
203. Leckman JF, Katsovich L, Kawikova I, Lin H, Zhang H, Krönig H, et al. Increased serum levels of interleukin-12 and tumor necrosis factor-alpha in Tourette’s syndrome. Biol Psychiatry. (2005) 57(6):667–73. doi: 10.1016/j.biopsych.2004.12.004
204. Yeon SM, Lee JH, Kang D, Bae H, Lee KY, Jin S, et al. A cytokine study of pediatric Tourette’s disorder without obsessive compulsive disorder. Psychiatry Res. (2017) 247:90–6. doi: 10.1016/j.psychres.2016.11.005
205. Zalcman S, Murray L, Dyck DG, Greenberg AH, Nance DM. Interleukin-2 and -6 induce behavioral-activating effects in mice. Brain Res. (1998) 811(1–2):111–21. doi: 10.1016/S0006-8993(98)00904-4
206. Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. (2007) 27(40):10695–702. doi: 10.1523/JNEUROSCI.2178-07.2007
207. Kabesch M, Tost J. Recent findings in the genetics and epigenetics of asthma and allergy. Semin Immunopathol. (2020) 42(1):43–60. doi: 10.1007/s00281-019-00777-w
208. Potaczek DP, Harb H, Michel S, Alhamwe BA, Renz H, Tost J. Epigenetics and allergy: from basic mechanisms to clinical applications. Epigenomics. (2017) 9(4):539–71. doi: 10.2217/epi-2016-0162
209. Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Science (New York, NY). (1975) 187(4173):226–32. doi: 10.1126/science.187.4173.226
210. Xu CJ, Söderhäll C, Bustamante M, Baïz N, Gruzieva O, Gehring U, et al. DNA methylation in childhood asthma: an epigenome-wide meta-analysis. Lancet Respir Med. (2018) 6(5):379–88. doi: 10.1016/S2213-2600(18)30052-3
211. Stefanowicz D, Hackett TL, Garmaroudi FS, Günther OP, Neumann S, Sutanto EN, et al. DNA methylation profiles of airway epithelial cells and PBMCs from healthy, atopic and asthmatic children. PLoS One. (2012) 7(9):e44213. doi: 10.1371/journal.pone.0044213
212. Bayrak Degirmenci P, Aksun S, Altin Z, Bilgir F, Arslan IB, Colak H, et al. Allergic rhinitis and its relationship with IL-10, IL-17, TGF-β, IFN-γ, IL 22, and IL-35. Dis Markers. (2018) 2018:9131432. doi: 10.1155/2018/9131432
213. Müller-Vahl KR, Loeber G, Kotsiari A, Müller-Engling L, Frieling H. Gilles de la Tourette syndrome is associated with hypermethylation of the dopamine D2 receptor gene. J Psychiatr Res. (2017) 86:1–8. doi: 10.1016/j.jpsychires.2016.11.004
214. Zilhão NR, Padmanabhuni SS, Pagliaroli L, Barta C, Smit DJ, Cath D, et al. Epigenome-wide association study of tic disorders. Twin Res Hum Genet. (2015) 18(6):699–709. doi: 10.1017/thg.2015.72
215. Forno E, Wang T, Qi C, Yan Q, Xu CJ, Boutaoui N, et al. DNA methylation in nasal epithelium, atopy, and atopic asthma in children: a genome-wide study. Lancet Respir Med. (2019) 7(4):336–46. doi: 10.1016/S2213-2600(18)30466-1
216. Stevens AJ, Harris AR, Gerdts J, Kim KH, Trentesaux C, Ramirez JT, et al. Programming multicellular assembly with synthetic cell adhesion molecules. Nature. (2023) 614(7946):144–52. doi: 10.1038/s41586-022-05622-z
217. Martino D, Church AJ, Defazio G, Dale RC, Quinn NP, Robertson MM, et al. Soluble adhesion molecules in Gilles de la Tourette’s syndrome. J Neurol Sci. (2005) 234(1–2):79–85. doi: 10.1016/j.jns.2005.03.032
218. Bochner BS. Adhesion molecules as therapeutic targets. Immunol Allergy Clin North Am. (2004) 24(4):615–30, vi. doi: 10.1016/j.iac.2004.06.003
219. Caldwell JM, Collins MH, Kemme KA, Sherrill JD, Wen T, Rochman M, et al. Cadherin 26 is an alpha integrin-binding epithelial receptor regulated during allergic inflammation. Mucosal Immunol. (2017) 10(5):1190–201. doi: 10.1038/mi.2016.120
220. Tsetsos F, Yu D, Sul JH, Huang AY, Illmann C, Osiecki L, et al. Synaptic processes and immune-related pathways implicated in Tourette syndrome. Transl Psychiatry. (2021) 11(1):56. doi: 10.1038/s41398-020-01082-z
221. Pagliaroli L, Vető B, Arányi T, Barta C. From genetics to epigenetics: new perspectives in Tourette syndrome research. Front Neurosci. (2016) 10:277. doi: 10.3389/fnins.2016.00277
Keywords: children, allergic diseases, tic disorders, common factors, mechanism
Citation: Zhang P, Zheng Z, Sun H, Gao T and Xiao X (2024) A review of common influencing factors and possible mechanisms associated with allergic diseases complicating tic disorders in children. Front. Pediatr. 12:1360420. doi: 10.3389/fped.2024.1360420
Received: 5 January 2024; Accepted: 31 May 2024;
Published: 18 June 2024.
Edited by:
Alenka Gagro, Children’s Hospital Zagreb, CroatiaReviewed by:
Iva Mihatov Štefanović, University Hospital Center, CroatiaZhifeng Huang, First Affiliated Hospital of Guangzhou Medical University, China
© 2024 Zhang, Zheng, Sun, Gao and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuwu Xiao, dlxiaoxw@163.com
 Panpan Zhang
Panpan Zhang Zhimin Zheng
Zhimin Zheng Hao Sun1,2
Hao Sun1,2