Abstract
The patent ductus arteriosus frequently poses a significant morbidity in preterm infants, subjecting their immature pulmonary vascular bed to substantial volume overload. This, in turn, results in concurrent hypoperfusion to post-ductal organs, and subsequently alters cerebral blood flow. In addition, treatment has not demonstrated definitive improvements in patient outcomes. Currently, the optimal approach remains a subject of considerable debate with ongoing research controversy regarding the best approach. This article provides a comprehensive review of existing literature.
Introduction
Patent ductus arteriosus (PDA) and its approach remain a topic of major controversy in the field of neonatology. This article reviews the best available literature around the topic with its limitations. We searched the PubMed database using controlled vocabulary and key words representing the concept “PDA” and “neonate”. Main articles were selected to be included by all authors.
Why should we worry about patent ductus arteriosus in preterm infants?
The pathological entity of the PDA in preterm infants continues (1). PDA is linked to the most common preterm morbidities, including bronchopulmonary dysplasia (BPD) (2, 3), necrotizing enterocolitis (NEC) (4), etc. (Figure 1). Over the past decade, a shift of pendulum towards more conservative management, as opposed to pharmacological or surgical treatments has emerged (5). This trend is likely to be a response to potential side effects associated with pharmacological approach, as well as lack of marked inferiority in neonatal outcomes from conservative treatment (6–8) As an example, in the PDA-TOLERATE trial, preterm infants born <28 weeks’ gestation, were randomized to either early treatment, or to an observatory approach (9); there were no differences in primary outcomes (ligation or presence of a PDA at discharge), nor in secondary outcomes (NEC, BPD, BPD/death, weekly need for respiratory support) (9).
Figure 1

Pathophysiology of hsPDA.
Early on in life, PDA plays a critical role during transitional circulation, and potentially contributes to dysregulated transitional hemodynamics such as intraventricular hemorrhage (10), and pulmonary hemorrhage (11).
PDA is associated with significant pulmonary morbidities and BPD. Animal studies (lambs with PDA) confirm considerable engorgement and increase in lymphatic conspicuity due to dilated architecture, leading to pulmonary edema and heart failure (12). Primate studies suggest that surgical closure of PDA may improve ventilation scores (13).
In humans, preterm infants born before 28 weeks’ gestation, when exposed to prolonged ductal shunt, this will contribute to the remodelling of pulmonary vasculature and subsequently, chronic pulmonary hypertension with BPD, and an increase in the BPD baseline rate in preterm infants exposed to PDA (2). In fact, this continues to be an issue in preterm infants who are discharged home with persistent PDA (14). Interestingly, it only takes 7–13 days of exposure to a moderate-to-large duct, for a significant increase in the incidence of BPD/death to become evident (15). PDA also plays a significant role in pulmonary hemorrhage pathophysiology; it appears that early treatment or prophylaxis, significantly reduce the incidence of pulmonary hemorrhage (11). In a recent Canadian study, infants who underwent PDA ligation, exhibited higher respiratory morbidities as early as the first few days of life (16). In this study, PDA ligation did not improve outcomes of death or BPD (16).
PDA also contributes to extra-pulmonary morbidities. There is a change in the shape and size of the myocardium, which peaks at 4 weeks of volume overload, potentially contributing to an increased risk of new-onset heart failure in adulthood (17). These findings correlate with an increased cumulative incidence of heart failure in preterm babies shown in a large Swedish population-based study (18), and the newly defined “preterm cardiomyopathy” (19–21). When hemodynamically significant, the PDA also affects the coronary arteries, by compromising coronary perfusion pressure and oxygen delivery to the myocardium in preterm infants (22).
The impact of PDA on preterm bowel, is evident, by impaired tissue oxygenation as observed in near-infrared spectroscopy (NIRS) studies (23), physiological post prandial superior mesenteric artery (SMA) flow (24), increase in mortality associated with NEC (4), and a five-fold increase in NEC (25). In addition, the incidence of renal injury increases with hemodynamic significant PDA (hsPDA), and renal saturation levels by NIRS less than 66% seem to be sensitive and specific indicators of hsPDA (26). Sellmer et al. showed that a large PDA, as early as day 3 of life, is associated with a two-fold increase in mortality, and a six-fold increase in the intraventricular haemorrhage (IVH) (25).
For long term outcomes associated with PDA, there is a lack of literature, and it is an area for future research. It appears that the long-term respiratory outcomes are related to BPD and its association with PDA (27). However, theoretically, the PDA would impact the developing brain in preterm infants, hence potentially contributing to worsening long term outcomes (28). There are few studies evaluated the long-term neurodevelopmental outcomes of PDA. In a multicenter cohort, Collins et al., did not find the PDA in premature infants to affect their neurodevelopmental outcomes at 3–18 years (29). Oncel et al., found no neurodevelopmental effects observed in preterm infants when evaluated with Bayley Scales of Infant Development II (Bayley-II), at the corrected age of 18–24 months (30). Similarly, Elbayiyev at al. found no association between hsPDA and poor neurodevelopmental outcomes, in a retrospective case control observational cohort (31). On the other hand, in a retrospective cohort of preterm infants born <29 weeks’ gestation, Janz-Robinson et al. suggested unfavorable neurodevelopmental course at 2–3 years of age, possibly related to PDA (32). Overall, this is an area which potentially needs to be further investigated.
What is the definition of a hsPDA?
Defining hsPDA is challenging due to the lack of a standardized consensus in literature (33, 34). Clinical assessment has been found to be neither sensitive nor specific, in predicting PDA shunt volume, particularly in the early days of life (35). Echocardiographic assessment scores have been developed (34, 36–38), most of them rely on similar parameters, such as size of PDA and evidence of left heart pressure and volume overloading (Figure 2).
Figure 2
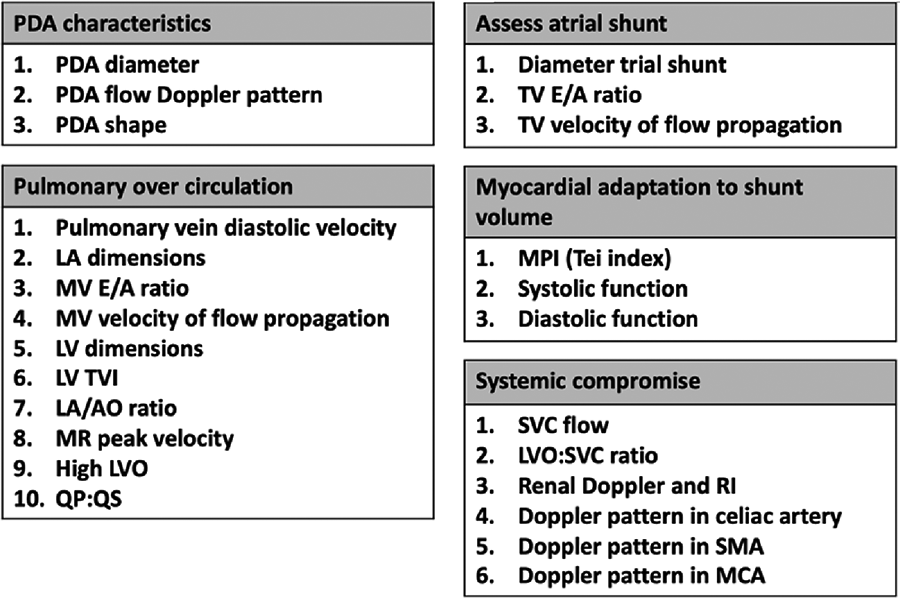
Targeted neonatal parameters needed to establish the hemodynamic significance of PDA.
Over the past two decades, the increasing application of targeted neonatal echocardiography (TnECHO), has provided a systematic approach to study the hemodynamic impact of PDA on circulation. This is through comprehensive assessment, which incorporates several domains (Figure 2), such as ductal size, flow Doppler pattern, and PDA shape (39). PDA is characterized by its length, width, tortuosity, and resistance to pharmacological closure (40). As a matter of fact, the PDA 3D structure is variable. While five types of ducts (labelled A-E) have been described, the increasing number of preterm infants referred for catheter closure, has led the identification of a additional type, known as the F type or fetal type ductus. This type is found mainly in prematurely born infants.
Evaluation of pulmonary circulation is achieved through analyzing multiple parameters obtained by TnECHO assessment (Figure 2). The preterm myocardium exhibits poor compliance due to its intrinsic characteristics, including a reduced number of calcium pumps, dependency on the L-type calcium channels, an underdeveloped sarcoplasmic reticulum and t-tubule system, disorganized mitochondria, and a higher proportion of non-contractile collagen and water in the myocardial interstitium (41). This makes it challenging for the myocardium to adapt to a high-volume ductal shunt.
Concurrently, the atrial shunt requires assessment, as it further enhance the pulmonary overcirculation, and subsequently, development of BPD (42–46). In addition, any evidence of a systemic compromise needs to be elaborated.
Recently, several cardiac biomarkers have been proposed for ductal assessment, particularly in resource-limited settings. B-type Natriuretic Peptide (BNP) and N-terminal-Pro-BNP (NTpBNP), traditionally used in adults to assess myocardial function and volume loading for prognostic identification post cardiac surgery, have gained increasing recognition in preterm infants. NTpBNP has shown promise as a potential screening tool for PDA, a marker for myocardial performance (47–51). Table 1 summarizes the most common suggested scoring tool used during TNE assessment (10). The application of a PDA score, defines hsPDA and guides management, and has demonstrated notable impacts on neonatal outcomes (10, 36). Also, such scores have been found to be reproducible (52).
Table 1
| Measurement | 0 | 1 | 2 |
|---|---|---|---|
| Pulmonary vein d wave velocity (cm/s) | <30 | 30–50 | >50 |
| Mitral valve E wave velocity (cm/s) | <45 | 45–80 | >80 |
| LV IVRT (ms) | >50 | 30–50 | <30 |
| LA:AO ratio | <1.3 | 1.3–2.2 | >2.2 |
| LVO:RVO | <1.5 | 1.5–2.0 | >2 |
| Aortic/Peripheral Doppler flow reversal | Forward/Absent | Reversed | |
| Ductus diameter indexed to weight (mm/kg) | <1.5 | 1.5–3.0 | >3 |
Suggested PDA scoring tool.
Why is there a lack of correlation between PDA treatment and improved neonatal outcomes?
Existing literature about PDA management in preterm infants, did not discernibly show improved neonatal outcomes. One example is the recent BeNeDuctus Trial (53, 54), showing that expectant management of PDA in preterm infants, was not inferior to early ibuprofen treatment with respect to neonatal outcomes (53, 54). In this trial, a total of 273 infants were randomized to receive either expectant management or early treatment with ibuprofen. Authors found that the expectant management is not inferior to the treatment when assessing the composite primary outcome of necrotizing enterocolitis, moderate to severe BPD, or death at 36 weeks’ postmenstrual age [46.3% vs. 63.5%, absolute risk difference, −17.2 percentage points; upper boundary of the one-sided 95% confidence interval (CI), −7.4; P < 0.001 for noninferiority] (53, 54).
Such lack of correlation in literature, between treating PDA and improved outcomes, is often subject to criticism, and it could be attributed to various factors (
Table 2):
1- Absence of a standardized methodology for defining hsPDA across the literature. Roughly, 40% of the trials omitted any echocardiography data assessment (55). In most instances, hsPDA was primarily defined based on its dimeter alone, which has weak correlation with echocardiographic markers of shunt volume (56). Another example is Early PARacetamol Trial (EPAR) (57), preterm infants born at <29 weeks’ gestation, were randomized to receive early treatment with acetaminophen or placebo, based on ductal diameter >0.9 mm at 6 h of life (57). In addition, it is noteworthy that PDA diameter has significant inter-observer variability in 2D and color Doppler in preterm infants (58), and the PDA image on 2D view, does not accurately represent the PDA as a 3D structure, and it could potentially over- or underestimate ductal diameter (40). This highlights the importance of comprehensive echocardiographic evaluation, to provide a better understanding of the hemodynamic consequences of PDA.
2- Notable heterogeneity in the inclusion criteria, as well as the analyzed of outcomes among created difficulties for direct comparison. Variable outcomes were analyzed, and BPD is often an outcome in PDA-related literature (59, 60); few studies analyzed neurodevelopmental outcomes (29–32, 61), and others assessed composite outcomes of NEC, BPD, or death (53, 54). A standardized contemporary framework in PDA care that \supports the practice of evidence-based medicine is necessary (62).
3- Ductal shunt was not completely eliminated in the intervention arm in most studies, which leads to ongoing exposure to ductal shunt. Generally, the rate of ductal closure remains around 60%–70%, attributable to the partial effectiveness of pharmacotherapy as compared to surgical closure (63–65).
4- Lack of equipoise: In the control arm of many studies, almost two-thirds of infants received a rescue treatment. For example, in the DETECT trial, preterm infants born <29 weeks’ gestation were screened for a large PDA and randomized to receive either indomethacin or placebo before age 12 h of life (11). In the placebo arm, 40% of infants received an open-label treatment (11). This emphasizes the necessity of upholding equipoise in well-designed randomized controlled trials, a sentiment echoed by the Committee on Fetus and Newborn by American Academy of Pediatrics (66).
Table 2
|
|
|
|
Issues and deficits in available literature.
Ductal shunt limitation vs. elimination
Current practice when managing hsPDA, includes several pharmacological, non-pharmacological, and surgical interventions (67). The approach of limiting the ductal shunt, often referred to as conservative management, focuses on modulating the factors that dictate the shunt volume. Typically, pharmacological interventions, employing non-steroidal anti-inflammatory drugs (NSAIDs) or acetaminophen, induce ductal constriction (38, 68–71). Nonetheless, this does not assure complete ductal closure or shunt elimination, even when combined, with a success rate hovering around 60%–70% in most scenarios (38, 69–72). It is also worth mentioning the recent systematic review and meta-analysis regarding the high-dose ibuprofen, which seems to be more effecting compared to standard-dose ibuprofen, but still did not significantly decrease the failure rate of PDA closure in preterm infants after the first course (Relative risk (RR) 0.74, 95% confidence interval (CI) 0.53 −1.03, 6 studies, N = 369) (73).
A common clinical practice is regulating the systemic-pulmonary pressure gradient by increasing the pulmonary vascular resistance (by maintaining mean airway pressure) could be considered by optimizing the mean airway pressure and allowing for permissive hypercapnia (5, 74). When it comes to fluid restriction, clinicians should exercise caution with this practice (75–77), given most of trials are non-contemporary, and conducted in moderately preterm infants, which may not be applicable to extremely preterm infants currently (67).
Another strategy entails enhancing hemoglobin by packed red blood cell transfusion to limit the ductal shunt (78). In theory, blood transfusions can elevate blood viscosity, which may help in reduction of ductal shunt volume (79).
For definitive ductal shunt elimination, the only two strategies are surgical ligation, and percutaneous catheter closure. While PDA ligation ensures immediate shunt elimination, it is associated with unfavorable morbidities, and potentially long-term side effects. This encompasses post-ligation cardiac syndrome and respiratory failure, an increased risk of BPD with early ligation, vocal cord paresis retinopathy of prematurity, and neurodevelopmental impairment (80–84).
Recently, Food and Drug Administration (FDA) approved the use of the “The Amplatzer Piccolo” device for PDA closure in preterm infants. This was based of its proven efficacy and safety in this vulnerable population (85). This approach seems to be gaining popularity, as it is feasible, effective, and relatively safe (86, 87). It provides a definitive and complete ductal shunt elimination with improvement of respiratory status following the procedure (88). In a recent meta-analysis by Bischoff et al., this approach was feasible in infants ≤1.5 kg with only few major adverse events with high rate of success (89).
In fact, it can be utilized in preterm infants as small as 700 g, and as early as 3–4 weeks (85, 86). The left pulmonary stenosis and migration of the device are potential complications to this procedure (85, 86). Anecdotal data showed that the incidence of cardiorespiratory instability, might be less common with device closure as compared to ligation (40, 90, 91). The comparatively favorable side effects profile of device-closure versus ligation likely explains the decline in the rates of surgical ligation (86).
Future directions and ideal study design
There is no controversial topic in the neonatal field like the PDA approach and its management. This continues to be a the most contentious topics in the care of preterm infants (92). Currently, there is no consensus about the ideal treatment. Catheter closure ensures a complete ductal shunt elimination (as opposed to limiting or reducing it), aligning more closely with the ideal goal of treatment; however, more research is needed to delineate safety profile. Future trials should consider randomizing infants with hsPDA to a complete shunt elimination vs. other approaches. Percutaneous Intervention Versus Observational Trial of Arterial Ductus in Low weight Infants (PIVOTAL) is an ongoing trial, where a complete shunt elimination would be compared to observational approach (https://www.pivotalstudy.org). in addition, emphasis should be put on standardized definitions of hsPDA with validation of the echocardiographic markers. Precise definition of outcomes in these trials is equally important.
Limitation of this review
This article is a general overview of the available literature pertaining the topic of PDA in preterm infants. There is a significant degree heterogeneity in the literature making a structured methodological search difficult. Since, the review is written by 3 authors who received similar structured training in TnECHO, and currently practicing in similar tertiary care neonatal settings in Canada, there might be an element of potential bias.
Statements
Author contributions
ASu: Conceptualization, Writing – original draft. ASi: Conceptualization, Writing – original draft. JT: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Powell ML . Patent ductus arteriosus in premature infants. Med J Aust. (1963) 2:58–60. 10.5694/j.1326-5377.1963.tb24612.x
2.
Reese J Shelton EL Slaughter JC McNamara PJ . Prophylactic indomethacin revisited. J Pediatr. (2017) 186:11–14.e1. 10.1016/j.jpeds.2017.03.036
3.
Gentle SJ Travers CP Clark M Carlo WA Ambalavanan N . Patent ductus arteriosus and development of bronchopulmonary dysplasia-associated pulmonary hypertension. Am J Respir Crit Care Med. (2023) 207(7):921–28. 10.1542/peds.2016-4258
4.
Palder SB Schwartz MZ Tyson KRT Marr CC . Association of closure of patent ductus arteriosus and development of necrotizing enterocolitis. J Pediatr Surg. (1988) 23(5):422–3. 10.1016/S0022-3468(88)80439-1
5.
Vanhaesebrouck S Zonnenberg I Vandervoort P Bruneel E van Hoestenberghe MR Theyskens C . Conservative treatment for patent ductus arteriosus in the preterm. Arch Dis Child Fetal Neonatal Ed. (2007) 92(4):244–7. 10.1136/adc.2006.104596
6.
Semberova J Sirc J Miletin J Kucera J Berka I Sebkova S et al Spontaneous closure of patent ductus arteriosus in infants ≤1500g. Pediatrics. (2017) 140(2):20–3. 10.1542/peds.2016-4258
7.
Alderliesten T Lemmers PMA Smarius JJM Van De Vosse RE Baerts W Van Bel F . Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular hemorrhage. J Pediatr. (2013) 162:698–704. 10.1016/j.jpeds.2012.09.038
8.
de Waal K Kluckow M . Functional echocardiography; from physiology to treatment. Early Hum Dev. (2010) 86(3):149–54. 10.1016/j.earlhumdev.2010.01.030
9.
Clyman RI Liebowitz M Kaempf J Erdeve O Bulbul A Håkansson S et al PDA-TOLERATE Trial: an exploratory randomized controlled trial of treatment of moderate-to-large patent ductus arteriosus at 1 week of age. J Pediatr. (2019) 205:41–8.e4. 10.1016/j.jpeds.2018.09.012
10.
Giesinger RE Hobson AA Bischoff AR Klein JM McNamara PJ . Impact of early screening echocardiography and targeted PDA treatment on neonatal outcomes in ‘22–23’ week and ‘24–26’ infants. Semin Perinatol. (2023) 47(2):151721. 10.1016/j.semperi.2023.151721
11.
Kluckow M Jeffery M Gill A Evans N . A randomised placebo-controlled trial of early treatment of the patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed. (2014) 99(2):F99–104. 10.1136/archdischild-2013-304695
12.
Datar SA Johnson EG Oishi PE Johengen M Tang E Aramburo A et al Altered lymphatics in an ovine model of congenital heart disease with increased pulmonary blood flow. Am J Physiol Lung Cell Mol Physiol. (2012) 302(6):530–40. 10.1152/ajplung.00324.2011
13.
Chang LY McCurnin D Yoder B Shaul PW Clyman RI . Ductus arteriosus ligation and alveolar growth in preterm baboons with a patent ductus arteriosus. Pediatr Res. (2008) 63(3):299–302. 10.1203/PDR.0b013e318163a8e4
14.
Bischoff AR Cavallaro Moronta S McNamara PJ . Going home with a patent ductus arteriosus: is it benign?J Pediatr. (2022) 240:10–13. 10.1016/j.jpeds.2021.09.009
15.
Clyman RI Liebowitz M Johng S Clyman RI Hills NK . Relationship between duration of infant exposure to a moderate-to-large patent ductus arteriosus shunt and the risk of developing bronchopulmonary dysplasia or death before 36 weeks. Am J Perinatol. (2020) 37(2):216–23. 10.1055/s-0039-1697672
16.
Weisz DE Mirea L Rosenberg E Jang M Ly L Church PT et al Association of patent ductus arteriosus ligation with death or neurodevelopmental impairment among extremely preterm infants. JAMA Pediatr. (2017) 171(5):443–9. 10.1001/jamapediatrics.2016.5143
17.
de Waal K Phad N Collins N Boyle A . Cardiac remodeling in preterm infants with prolonged exposure to a patent ductus arteriosus. Congenit Heart Dis. (2017) 12(3):364–72. 10.1111/chd.12454
18.
Crump C Groves A Sundquist J Sundquist K . Association of preterm birth with long-term risk of heart failure into adulthood. JAMA Pediatr. (2021) 175:689–97. 10.1001/jamapediatrics.2021.0131
19.
Lewandowski AJ . The preterm heart: a unique cardiomyopathy?Pediatr Res. (2019) 85:738–39. 10.1038/s41390-019-0301-3
20.
Greer C Troughton RW Adamson PD Harris SL . Preterm birth and cardiac function in adulthood. Heart. (2022) 108:172–77. 10.1136/heartjnl-2020-318241
21.
Carr H Cnattingius S Granath F Ludvigsson JF Edstedt Bonamy AK . Preterm birth and risk of heart failure up to early adulthood. J Am Coll Cardiol. (2017) 69(21):2634–42. 10.1016/j.jacc.2017.03.572
22.
Vaisbourd Y Sharif D Riskin A Yaniv L Dinur G Amen K et al The effect of patent ductus arteriosus on coronary artery blood flow in premature infants: a prospective observational pilot study. J Perinatol. (2020) 40(9):1366–74. 10.1038/s41372-020-0622-4
23.
Lemmers PMA Toet MC Van Bel F . Impact of patent ductus arteriosus and subsequent therapy with indomethacin on cerebral oxygenation in preterm infants. Pediatrics. (2008) 121:142–7. 10.1542/peds.2007-0925
24.
McCurnin D Clyman RI . Effects of a patent ductus arteriosus on postprandial mesenteric perfusion in premature baboons. Pediatrics. (2008) 122(6):e1262–7. 10.1542/peds.2008-2045
25.
Sellmer A Bjerre JV Schmidt MR McNamara PJ Hjortdal VE Høst B et al Morbidity and mortality in preterm neonates with patent ductus arteriosus on day 3. Arch Dis Child Fetal Neonatal Ed. (2013) 98(6):505–10. 10.1136/archdischild-2013-303816
26.
Chock VY Rose LA Mante J Punn R . Near-infrared spectroscopy for detection of a significant patent ductus arteriosus. Pediatr Res. (2016) 80(5):675–80. 10.1038/pr.2016.148
27.
Bancalari E . Patent ductus arteriosus and short- and long-term respiratory outcomes. Am J Perinatol. (2016) 33:1055–7. 10.1055/s-0036-1586112
28.
Osborn DA Evans N Kluckow M . Effect of early targeted indomethacin on the ductus arteriosus and blood flow to the upper body and brain in the preterm infant. Arch Dis Child Fetal Neonatal Ed. (2003) 88(6):F477–82. 10.1136/fn.88.6.F477
29.
Collins RT Lyle RE Rettiganti M Gossett JM Robbins JM Casey PH . Long-term neurodevelopment of low-birthweight, preterm infants with patent ductus arteriosus. J Pediatr. (2018) 203:170–76.e1. 10.1016/j.jpeds.2018.08.004
30.
Oncel MY Eras Z Uras N Canpolat FE Erdeve O Oguz SS . Neurodevelopmental outcomes of preterm infants treated with oral paracetamol versus ibuprofen for patent ductus arteriosus. Am J Perinatol. (2017) 34(12):1185–89. 10.1055/s-0037-1601564
31.
Elbayiyev S Canpolat FE Kadıoğlu Şimşek G Işık S Büyüktiryaki M Kanmaz Kutman HG . Long-term neurodevelopmental outcomes in very low birth weight infants with and without patent ductus arteriosus: a retrospective case control observational study. Child Care Health Dev. (2022) 48(5):862–8. 10.1111/cch.12997
32.
Janz-Robinson EM Badawi N Walker K Bajuk B Abdel-Latif ME Bowen J et al Neurodevelopmental outcomes of premature infants treated for patent ductus arteriosus: a population-based cohort study. J Pediatr. (2015) 167(5):1025–32.e3. 10.1016/j.jpeds.2015.06.054
33.
Shepherd JL Noori S . What is a hemodynamically significant PDA in preterm infants?Congenit Heart Dis. (2019) 14(1):21–6. 10.1111/chd.12727
34.
El-Khuffash A James AT Corcoran JD Dicker P Franklin O Elsayed YN et al A patent ductus arteriosus severity score predicts chronic lung disease or death before discharge. J Pediatr. (2015) 167(6):1354–1361.e2. 10.1016/j.jpeds.2015.09.028
35.
Skelton R Evans N Smythe J . A blinded comparison of clinical and echocardiographic evaluation of the preterm infant for patent ductus arteriosus. J Paediatr Child Health. (1994) 30:406–11. 10.1111/j.1440-1754.1994.tb00689.x
36.
Bussmann N Smith A Breatnach CR McCallion N Cleary B Franklin O et al Patent ductus arteriosus shunt elimination results in a reduction in adverse outcomes: a post hoc analysis of the PDA RCT cohort. J Perinatol. (2021) 41:1134–41. 10.1038/s41372-021-01002-z
37.
O’Rourke DJ El-Khuffash A Moody C Walsh K Molloy EJ . Patent ductus arteriosus evaluation by serial echocardiography in preterm infants. Acta Paediatr Int J Paediatr. (2008) 97(5):574–8. 10.1111/j.1651-2227.2008.00745.x
38.
Saker A Surak A Kimani S De La Hoz A Miller MR Lalitha R et al Combination therapy for patent ductus arteriosus in preterm infants: echocardiographic changes and clinical use. Prog Pediatr Cardiol. (2023) 68. 10.1016/j.ppedcard.2022.101611
39.
Resende MHF More K Nicholls D Ting J Jain A McNamara PJ . The impact of a dedicated patent ductus arteriosus ligation team on neonatal health-care outcomes. J Perinatol. (2016) 36(6):463–8. 10.1038/jp.2015.213
40.
Philip R Rush Waller B Agrawal V Wright D Arevalo A Zurakowski D et al Morphologic characterization of the patent ductus arteriosus in the premature infant and the choice of transcatheter occlusion device. Catheter Cardiovasc Interv. (2016) 87(2):310–7. 10.1002/ccd.26287
41.
Xu A Hawkins C Narayanan N . Ontogeny of sarcoplasmic reticulum protein phosphorylation by Ca2+-calmodulin-dependent protein kinase. J Mol Cell Cardiol. (1997) 29:405–18. 10.1006/jmcc.1996.0284
42.
Evans N Lyer P . Assessment of ductus arteriosus shunt in preterm infants supported by mechanical ventilation: effect of interatrial shunting. J Pediatr. (1994) 125:778–85. 10.1016/s0022-3476(94)70078-8
43.
Evans N Iyer P . Incompetence of the foramen ovale in preterm infants supported by mechanical ventilation. J Pediatr. (1994) 125:786–92. 10.1016/s0022-3476(94)70079-6
44.
Rios DR Martins F El-Khuffash A Weisz DE Giesinger RE McNamara PJ . Early role of the atrial-level communication in premature infants with patent ductus arteriosus. J Am Soc Echocardiogr. (2021) 34(4):423–32.e1. 10.1016/j.echo.2020.11.008
45.
Choi EK Jung YH Kim HS Shin SH Choi CW Kim EK et al The impact of atrial left-to-right shunt on pulmonary hypertension in preterm infants with moderate or severe bronchopulmonary dysplasia. Pediatr Neonatol. (2015) 56(5):317–23. 10.1016/j.pedneo.2014.12.006
46.
Kumar KR Clark DA Kim EM Perry JD Wright K Thomas SA et al Association of atrial septal defects and bronchopulmonary dysplasia in premature infants. J Pediatr. (2018) 202:56–62.e2. 10.1016/j.jpeds.2018.07.024
47.
Elsayed Y Seshia M Soni R Buffo I Baier RJ McNamara PJ et al Pre-symptomatic prediction of morbitidies in preterm infants with patent ductus arteriosus by targeted neonatal echocardiography and brain-type natriuretic peptide. J Pediatr Neonatal Individ Med. (2016) 5(2):e050210. 10.7363/050210
48.
EL-Khuffash A Molloy E . The use of N-terminal-pro-BNP in preterm infants. Int J Pediatr. (2009) 2009:1–7. 10.1155/2009/175216
49.
Sanjeev S Pettersen M Lua J Thomas R Shankaran S L’Ecuyer T . Role of plasma B-type natriuretic peptide in screening for hemodynamically significant patent ductus arteriosus in preterm neonates. J Perinatol. (2005) 25(11):709–13. 10.1038/sj.jp.7211383
50.
Byung MC Kee HL Baik LE Kee HY Young SH Chang SS et al Utility of rapid B-type natriuretic peptide assay for diagnosis of symptomatic patent ductus arteriosus in preterm infants. Pediatrics. (2005) 115(3):e255–61. 10.1542/peds.2004-1837
51.
Chen S Tacy T Clyman R . How useful are B-type natriuretic peptide measurements for monitoring changes in patent ductus arteriosus shunt magnitude. J Perinatol. (2010) 30(12):780–5. 10.1038/jp.2010.47
52.
Smith A Mullaly R Franklin O EL-Khuffash A . Reproducibility of the EL-khuffash PDA severity score and PDA diameter measurements in extremely preterm infants. Early Hum Dev. (2023) 184. 10.1016/j.earlhumdev.2023.105832
53.
Hundscheid T Onland W van Overmeire B Dijk P van Kaam AHLC Dijkman KP et al Early treatment versus expectative management of patent ductus arteriosus in preterm infants: a multicentre, randomised, non-inferiority trial in Europe (BeNeDuctus trial). BMC Pediatr. (2018) 18(1):1–14. 10.1186/s12887-018-1215-7
54.
Hundscheid T Onland W Kooi EMW Vijlbrief DC de Vries WB Dijkman KP et al Expectant management or early ibuprofen for patent ductus arteriosus. N Engl J Med. (2023) 388(11):980–90. 10.1056/NEJMoa2207418
55.
Zonnenberg I de Waal K . The definition of a haemodynamic significant duct in randomized controlled trials: a systematic literature review. Acta Paediatr Int J Paediatr. (2012) 101(3):247–51. 10.1111/j.1651-2227.2011.02468.x
56.
de Freitas Martins F Ibarra Rios D Maura MH Javed H Weisz D Jain A et al Relationship of patent ductus arteriosus size to echocardiographic markers of shunt volume. J Pediatr. (2018) 202:50–55.e3. 10.1016/j.jpeds.2018.06.045
57.
Schindler T Smyth J Bolisetty S Michalowski J Mallitt KA Singla A et al Early PARacetamol (EPAR) trial: a randomized controlled trial of early paracetamol to promote closure of the ductus arteriosus in preterm infants. Neonatology. (2021) 118(3):274–81. 10.1159/000515415
58.
Babla K Duffy D Dumitru R Richards J Kulkarni A . Repeatability of PDA diameter measurements on echocardiography. Eur J Pediatr. (2022) 181(1):403–6. 10.1007/s00431-021-04178-w
59.
Mirza H Garcia J McKinley G Hubbard L Sensing W Schneider J et al Duration of significant patent ductus arteriosus and bronchopulmonary dysplasia in extremely preterm infants. J Perinatol. (2019) 39(12):1648–55. 10.1038/s41372-019-0496-5
60.
Willis KA Weems MF . Hemodynamically significant patent ductus arteriosus and the development of bronchopulmonary dysplasia. Congenit Heart Dis. (2019) 14(1):27–32. 10.1111/chd.12691
61.
Zhong B Tan K Razak A Sackett V Machipisa C Zhou L et al Early neurodevelopmental outcomes of extreme preterm infants exposed to paracetamol: a retrospective cohort study. Pediatr Res. (2023) 94(5):1714–9. 10.1038/s41390-023-02649-4
62.
Backes CH Hill KD Shelton EL Slaughter JL Lewis TR Weisz DE et al Patent ductus arteriosus: a contemporary perspective for the pediatric and adult cardiac care provider. J Am Heart Assoc. (2022) 11:e025784. 10.1161/JAHA.122.025784
63.
Olgun H Ceviz N Kartal İ Caner İ Karacan M Taştekin A et al Repeated courses of oral ibuprofen in premature infants with patent ductus arteriosus: efficacy and safety. Pediatr Neonatol. (2017) 58(1):29–35. 10.1016/j.pedneo.2015.04.017
64.
Bagnoli F Rossetti A Messina G Mori A Casucci M Tomasini B . Treatment of patent ductus arteriosus (PDA) using ibuprofen: renal side-effects in VLBW and ELBW newborns. J Matern Fetal Neonatal Med. (2013) 26(4):423–9. 10.3109/14767058.2012.733775
65.
Gersony WM Peckham GJ Ellison RC Miettinen OS Nadas AS . Effects of indomethacin in premature infants with patent ductus arteriosus: results of a national collaborative study. J Pediatr. (1983) 102(6):895–906. 10.1016/S0022-3476(83)80022-5
66.
Benitz WE . Patent ductus arteriosus in preterm infants. Pediatrics. (2016) 137(1). 10.1542/peds.2015-3730
67.
Mitra S de Boode WP Weisz DE Shah PS . Interventions for patent ductus arteriosus (PDA) in preterm infants: an overview of cochrane systematic reviews. Cochrane Database Syst Rev. (2023) 2023(4):CD013588. 10.1002/14651858.CD013588
68.
Starling MB Elliott RB . The Effects of Prostaglandins, Prostaglandin Inhibitors, and Oxygen on the Closure of the Ductus Arteriosus, Pulmonary Arteries and Umbilical Vessels in Vitro Department of Paediatrics School of Medicine University of Auckland New Zealand Acknowledgmen. 8(3).
69.
Hochwald O Mainzer G Borenstein-Levin L Jubran H Dinur G Zucker M et al Adding paracetamol to ibuprofen for the treatment of patent ductus arteriosus in preterm infants: a double-blind, randomized, placebo-controlled pilot study. Am J Perinatol. (2018) 35:1319–25. 10.1055/s-0038-1653946
70.
Yurttutan S Bozkaya A Hüdayioglu F Oncel MY . The effect of combined therapy for treatment of monotherapy-resistant PDA in preterm infants. J Matern Fetal Neonatal Med. (2019) 32:3662–5. 10.1080/14767058.2018.1481043
71.
Kimani S Surak A Miller M Bhattacharya S . Use of combination therapy with acetaminophen and ibuprofen for closure of the patent ductus arteriosus in preterm neonates. Paediatr Child Health. (2020) 26:e177–83. 10.1093/pch/pxaa057
72.
Jasani B Weisz DE Reese J Jain A . Combination pharmacotherapy for patent ductus arteriosus: rationale and evidence. Semin Perinatol. (2023) 47(2). 10.1016/j.semperi.2023.151720
73.
Yeung T Shahroor M Jain A Weisz D Jasani B . Efficacy and safety of high versus standard dose ibuprofen for patent ductus arteriosus treatment in preterm infants: a systematic review and meta-analysis. J Neonatal Perinatal Med. (2022) 15:501–10. 10.3233/NPM-210968
74.
Shekerdemian L . BohnD. Cardiovascular effects of Mechanical Ventilation. Arch Dis Child. (1999) 2:475–80. 10.1136/adc.80.5.475
75.
Lalitha R Surak A Bitar E Hyderi A Kumaran K . Fluid and electrolyte management in preterm infants with patent ductus arteriosus. J Neonatal Perinatal Med. (2022) 15:689–97. 10.3233/NPM-210943
76.
De Buyst J Rakza T Pennaforte T Johansson AB Storme L . Hemodynamic effects of fluid restriction in preterm infants with significant patent ductus arteriosus. J Pediatr. (2012) 161(3):404–8. 10.1016/j.jpeds.2012.03.012
77.
Bell EF Acarregui MJ . Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. (2014) 2014:CD000503. 10.1002/14651858.CD000503.pub3
78.
Joye S McNamara PJ Giesinger RE Tolsa JF Sekarski N . Association of hemoglobin and spontaneous closure of the ductus arteriosus during the transitional period in very low birth weight infants. J Neonatal Perinatal Med. (2021) 14(4):493–502. 10.3233/NPM-200518
79.
Lister G Hellenbrand WE Kleinman CS Talner NS . Physiologic effects of increasing hemoglobin concentration in left-to-right shunting in infants with ventricular septal defects. N Engl J Med. (1982) 306(9):502–6. 10.1056/NEJM198203043060902
80.
Wickremasinghe AC Rogers EE Piecuch RE Johnson BC Golden S Moon-Grady AJ et al Neurodevelopmental outcomes following two different treatment approaches (early ligation and selective ligation) for patent ductus arteriosus. J Pediatr. (2012) 161(6):1065–72. 10.1016/j.jpeds.2012.05.062
81.
Kabra NS Schmidt B Roberts RS Doyle LW Papile L Fanaroff A . Neurosensory impairment after surgical closure of patent ductus arteriosus in extremely low birth weight infants: results from the trial of indomethacin prophylaxis in preterms. J Pediatr. (2007) 150(3):229–34.e1. 10.1016/j.jpeds.2006.11.039
82.
Chorne N Leonard C Piecuch R Clyman RI . Patent ductus arteriosus and its treatment as risk factors for neonatal and neurodevelopmental morbidity. Pediatrics. (2007) 119(6):1165–74. 10.1542/peds.2006-3124
83.
Teixeira LS Shivananda SP Stephens D van Arsdell G McNamara PJ . Postoperative cardiorespiratory instability following ligation of the preterm ductus arteriosus is related to early need for intervention. J Perinatol. (2008) 28(12):803–10. 10.1038/jp.2008.101
84.
Weisz DE More K McNamara PJ Shah PS . PDA liǵation and health outcomes: a meta-analysis. Pediatrics. (2014) 133:e1024–46. 10.1542/peds.2013-3431
85.
Sathanandam SK Gutfinger D O’Brien L Forbes TJ Gillespie MJ Berman DP et al Amplatzer piccolo occluder clinical trial for percutaneous closure of the patent ductus arteriosus in patients ≥700 grams. Catheter Cardiovasc Interv. (2020) 96(6):1266–76. 10.1002/ccd.28973
86.
Mitchell CC Rivera BK Cooper JN Smith C V Berman DP Slaughter JL et al Percutaneous closure of the patent ductus arteriosus: opportunities moving forward. Congenit Heart Dis. (2019) 14(1):95–9. 10.1111/chd.12704
87.
Philip R Waller BR Chilakala S Graham B Stecchi N Apalodimas L et al Hemodynamic and clinical consequences of early versus delayed closure of patent ductus arteriosus in extremely low birth weight infants. J Perinatol. (2021) 41(1):100–8. 10.1038/s41372-020-00772-2
88.
Wheeler CR Vogel ER Cusano MA Friedman KG Callahan R Porras D et al Definitive closure of the patent ductus arteriosus in preterm infants and subsequent short-term respiratory outcomes. Respir Care. (2022) 67(5):594–606. 10.4187/respcare.09489
89.
Bischoff AR Jasani B Sathanandam SK Backes C Weisz DE McNamara PJ . Percutaneous closure of patent ductus arteriosus in infants 1.5kg or less: a meta-analysis. J Pediatr. (2021) 230:e14. 10.1016/j.jpeds.2020.10.035
90.
Sathanandam S Balduf K Chilakala S Washington K Allen K Knott-Craig C et al Role of transcatheter patent ductus arteriosus closure in extremely low birth weight infants. Catheter Cardiovasc Interv. (2019) 93(1):89–96. 10.1002/ccd.27808
91.
Grabitz RG Neuss MB Coe JY Handt S Redel DA von Bernuth G . A small interventional device to occlude persistently patent ductus arteriosus in neonates: evaluation in piglets. J Am Coll Cardiol. (1996) 28(4):1024–30. 10.1016/S0735-1097(96)00242-2
92.
Mitra S Rønnestad A Holmstrøm H . Management of patent ductus arteriosus in preterm infants-where do we stand?Congenit Heart Dis. (2013) 8:500–12. 10.1111/chd.12143
Summary
Keywords
PDA, preterm, ligation, piccolo, shunt
Citation
Surak A, Sidhu A and Ting JY (2024) Should we “eliminate” PDA shunt in preterm infants? A narrative review. Front. Pediatr. 12:1257694. doi: 10.3389/fped.2024.1257694
Received
12 July 2023
Accepted
24 January 2024
Published
06 February 2024
Volume
12 - 2024
Edited by
Jeroen J. van Vonderen, Leiden University Medical Center (LUMC), Netherlands
Reviewed by
Daniel Vijlbrief, University Medical Center Utrecht, Netherlands
Funda Yavanoğlu Atay, University of Health Sciences, Türkiye
Updates
Copyright
© 2024 Surak, Sidhu and Ting.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Aimann Surak aimann@ualberta.ca
Abbreviations AAP, American academy of pediatrics: BPD, bronchopulmonary dysplasia; FDA, food and drug administration; hsPDA, hemodynamically significant patent ductus arteriosus; IVH, intraventricular haemorrhage; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; NIRS, near-infra red spectroscopy; NSAID, non-steroidal anti-inflammatory drugs; PDA, patent ductus arteriosus; SMA, superior mesenteric artery; TNE, targeted neonatal echocardiography.
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.