- 1Department of Orthodontics, University of Münster, Münster, Germany
- 2Department of Prosthodontics and Biomaterials, University of Münster, Münster, Germany
- 3Department of Pediatrics, University of Münster, Münster, Germany
Background: The fetal alcohol spectrum disorder is a group of developmental disorders caused by maternal alcohol consumption. Patients with fetal alcohol syndrome show abnormal orofacial features. This review presents an overview over the facial, oral, dental or orthodontic findings and diagnostic tools concerning these features.
Methods: For this systematic review Cochrane, Medline and Embase databases were considered and the review was performed according to the PRISMA checklist. Two independent reviewers evaluated all studies and recorded results in a summary of findings table. Risk of bias was analyzed via Quadas-2 checklist.
Results: 61 studies were eligible for inclusion. All included studies were clinical studies. Methods and results of the studies were not comparable, guidelines or methods for the detection of FASD varied across studies. Facial features most often measured or found as distinguishing parameter were: palpebral fissure length, interpupillary or innercanthal distance, philtrum, upper lip, midfacial hypoplasia or head circumference.
Conclusions: This review shows that to date a multitude of heterogeneous guidelines exists for the diagnosis of FASD. Uniform, objective diagnostic criteria and parameters for the orofacial region in FASD diagnosis are needed. A bio database with values and parameters for different ethnicities and age groups should be made available for diagnosis.
1. Introduction
1.1. What is FASD?
FASD is a group of developmental disorders, which result from maternal alcohol exposure. Symptoms comprise growth deficiencies, aberrant facial features and damage or dysfunction of the central nervous system (1–3). Individuals with FASD suffer from lifetime consequences, even more if the underlying disorder remains undiagnosed.
Different severity grades of the disorder are summarized by the term fetal alcohol spectrum disorder (FASD). The most severe form of FASD is the fetal alcohol syndrome (FAS), less severe forms are categorized as partial fetal alcohol syndrome (pFAS), alcohol-related birth defects (ARBD) or alcohol-related neurodevelopmental disorder (ARND) (4–6).
1.2. Prevalence of FASD, FAS, pFAS, ARND, ARBD
Unfortunately, the estimated worldwide prevalence of the fetal alcohol spectrum disorder is still very high (0,77%, Lange et al., 2017), although it should be common knowledge that any amount of alcohol consumption during pregnancy is harmful to the embryo. FASD shows regional differences ranging from up to 5% in first grade school children in the United States (7), 2%–3% in Canadian school children (8), 1.98% in Europe to 0.01% in the eastern Mediterranean region (9). According to a Canadian study by Popova et al. the estimated prevalence for school children aged 7–9 years is 1.2 per 1,000 for FAS, 2.0 per 1,000 for pFAS and 15 per 1,000 for ARND (8). There is no recent prevalence for ARBD in literature, a study from 1988 by Warren et al. mentions a high prevalence of 417 per 1,000 (10).
1.3. Diagnosis of FASD
FASD is a developmental disorder, which is, because of its complexity and variable severity, challenging to detect. Since Lemoine first described the distinctive features and established the disorder FAS, a great number of diagnostic systems have been developed (11).
The current variability of existing diagnostic methods concerning FASD impedes comparability and reproducibility (12, 13).
There seem to be four guidelines across the world, which are most commonly used but which contain differences in certain diagnostic parameters:
- The four-digit code by Astley et al.—not for the terms ARND/ARBD (14)
- The revised IOM by Hoyme (5)
- The Canadian guideline by Chudley (6)
- The CDC code—mainly for FAS, not for partial FAS/ARND/ARBD (15)
Most diagnostic guidelines for FASD include four parameters: growth deficiency, facial phenotype, central nervous system (CNS) damage or dysfunction as well as gestational exposure to alcohol (5, 16). The most uncertain parameter is in many cases the exposure to alcohol since many children with FASD live in foster care or mothers might not tell the truth about alcohol consumption during pregnancy (17).
Growth retardation is measured according to percentile curves and for the guidelines mentioned above, measurements should be below the 10th percentile to be considered relevant for FAS diagnosis. In the case of partial FAS this measurement is only required for the IOM guideline and not necessary for diagnosis in the other guidelines. For ARBD and ARND growth retardation is not part of the diagnosis (5, 6, 14, 15).
CNS involvement is measured differently in the mentioned guidelines. For FAS the four-digit code requires a head circumference, which is smaller by more than two standard deviations below the 2.5th percentile, whereas the revised IOM and the CDC require only measurements below the 10th percentile for FAS diagnosis. The Canadian guideline does not measure according to percentile curve but requires more than three impairments in the CNS domains, for example hard and soft neurologic signs, brain structure, cognition, academic achievement, memory. For partial FAS CNS involvement is the same as for FAS in the four-digit code and the Canadian guideline, not applicable for the CDC guideline and for the revised IOM head circumference should be below the 10th percentile or behavioral and cognitive abnormalities should be present. ARND requires neurobehavioral impairment for the IOM and for the Canadian guideline evidence of CNS neurodevelopmental abnormalities and/or evidence of a complex pattern of behavior or cognitive abnormalities. ARBD requires one or more specific cardiac, skeletal, renal, eye or ear malformation for both the IOM and the Canadian guideline (5, 6, 14, 15).
In all four diagnostic guidelines alcohol exposure must not necessarily be confirmed for FAS. For partial FAS the four-digit code and the Canadian guideline require confirmed alcohol exposure, whereas the revised IOM does not require confirmed alcohol exposure for diagnosis of partial FAS. For ARND and ARBD confirmed alcohol exposure is required in the revised IOM and Canadian Guideline (5, 6, 14, 15).
For the parameter facial phenotype a commonly used tool is the lip-philtrum guide by Astley and Clarren (18). Facial characteristics used in the diagnostic guidelines comprise short palpebral fissures, thin vermillion borders and a smooth philtrum. Short palpebral fissure lengths are measured using a ruler or are measured via computer using the FAS facial photographic analysis software by Astley et al. The smooth philtrum and thin vermillion border are diagnosed comparing the patient's facial features to pictures on the lip-philtrum guide by Astley and Clarren and then ranking them to a certain grade of the feature (14, 18, 19).
All currently available diagnostic guidelines for the detection of facial FASD-parameters are in parts based on subjective evaluation. Therefore, this requires a professional dysmorphologist with experience in this field and is otherwise another factor for possible bias in the FASD diagnosis.
1.4. Aim of the review
Newer methods for the evaluation of facial parameters have investigated shape analysis, morphometrics, 3D-facial scanning and stereophotogrammetry with promising results for objectifying facial analysis in the future (20–35).
To improve the diagnostic process in patients with FASD it seems important to find facial characteristics for reproducible and objective measurements.
The aim of this review was to analyze, which facial, oral, orthodontic and dental parameters for the detection of FASD have been found so far and what kind of different diagnostic methods have been described for the diagnosis of FASD.
2. Materials and methods
2.1. Protocol and eligibility criteria
This review was performed according to the PRISMA guidelines. It was confined to articles in English, French or German that were published until January 2022.
Studies were considered eligible for inclusion if facial or oral structures in patients with FASD were investigated or if diagnostic methods for abnormal facial or oral structures in patients with FASD were described or tested.
Exclusion criteria were: Animal studies, scientific papers in which exposure of the study subjects to alcohol during pregnancy was not clear and studies concerning exclusively brain structures or ophthalmologic findings other than external ophthalmologic findings such as palpebral fissure length, innercanthal or interpupillary distance.
2.2. Search strategy
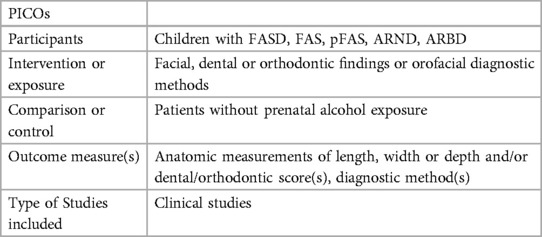
PICO Question was defined (Table 1).
Cochrane, Medline and Embase databases were considered. The search strategy was: [fetal alcohol syndrome OR “fetal alcohol spectrum disorders” (MESH)] AND (“Face” [MESH] OR “Mouth” [MESH] OR “Oral Health” [MESH] OR “orthodontics” [MESH] OR “dental” OR “Head” [MESH] OR “palpebral fissure length” OR “diagnostic” OR “diagnose”).
2.3. Study selection
All studies were screened for eligibility criteria.
2.4. Data extraction
All studies were screened by two independent reviewers MBL, AH in a blinded manner and classified into “eligible” or “not eligible” for this review. All “eligible” studies were read in full-text by the two reviewers and evaluated according to the Quadas-2 checklist. Data concerning facial features in patients with FASD and FASD diagnostic methods for the orofacial region were extracted independently by the two reviewers and recorded in a summary of findings table.
After reading all eligible studies full-text the two reviewers discussed their results and re-read those studies for which results differed in order to find a consensus.
2.5. Data items
The following outcomes were extracted:
• FASD or subgroup (FAS, pFAS, ARND, ARBD) diagnosis
• Control group
• Ethnicity
• Age
• Results, metric facial measurements or diagnostic scores of
• Head circumference (OFC = occipital frontal circumference)
• Palpebral fissure length (PFL), distance of inner and other canthi of the eye
• Innercanthal distance (ICD), distance between the inner canthi of right and left eye
• Interpupillary distance (IPD), distance between the centers of the pupils or right and left eye
• Philtrum anomalies in sagittal, vertical and transversal dimension
• decayed missing filled teeth index for deciduous teeth (dmft) and permanent teeth (DMFT), the sum of the number of decayed, missing due to caries and filled teeth
• DDE index (classification of developmental defects of enamel),
• facial or oral features associated with FASD
• applied/(new, for example 3D or computer aided) diagnostic methods for the orofacial region
• aim of the study
2.6. Synthesis
Synthesis of data was not possible due to inconsistent measuring methods and inhomogeneity across the studies.
2.7. Effect measures
Effect measures were not possible due to inhomogeneity across the studies.
2.8. Bias/quality assessment
To minimize bias, two reviewers independently selected eligible studies for this review according to afore defined criteria and did independent full-text reading and data extraction of all included studies.
The quality of the studies was assessed using the Quadas-2 checklist. The Quadas-2 checklist is supposed to evaluate the quality of diagnostic accuracy studies.
Possible bias for the synthesis of data was recorded if:
• the diagnostic guideline for FASD was unclear or not stated.
• the diagnosis FAS, FASD, pFAS, ARND or ARBD was not clearly defined.
• ethnicity was not described or unclear.
• the method was not fully described or unclear.
• the participants' ages did not match or were not clearly stated.
3. Results
3.1. Study selection (flow of studies)
The search strategy resulted in a total of 1,470 studies (Figure 1).
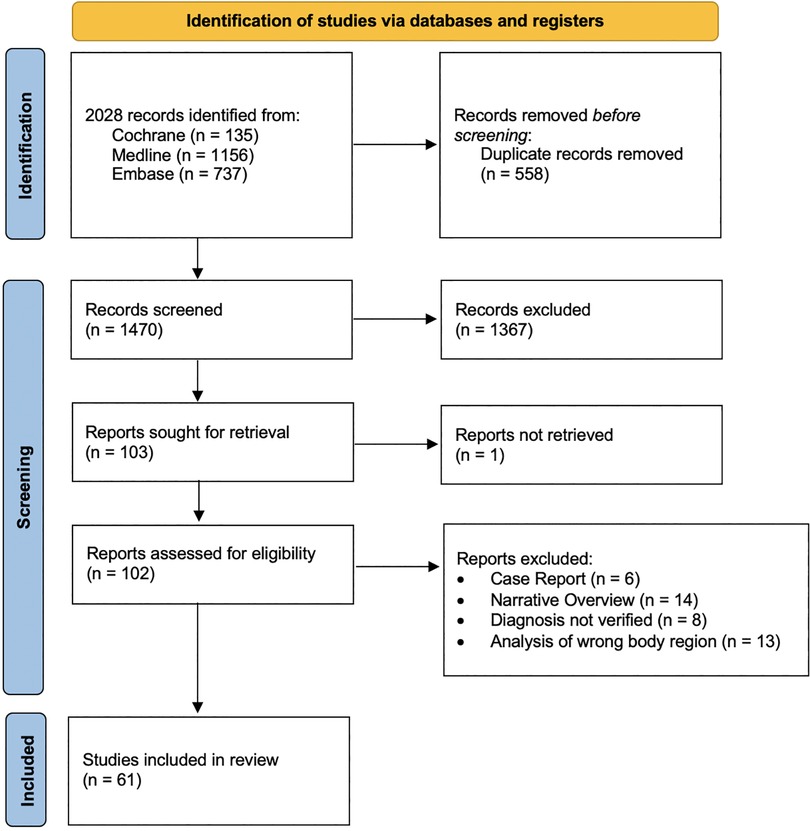
Figure 1. PRISMA flowchart. The search strategy resulted in 1,470 studies. A total of 1,409 studies were excluded. 61 studies were included in the review (84).
3.2. Study selection (excluded studies)
1,409 studies were excluded (Figure 1).
3.3. Study characteristics
All 61 included studies were clinical studies: 33 studies investigated diagnostic methods (Figure 2A) and 28 studies investigated the FASD phenotype (Figure 3A).
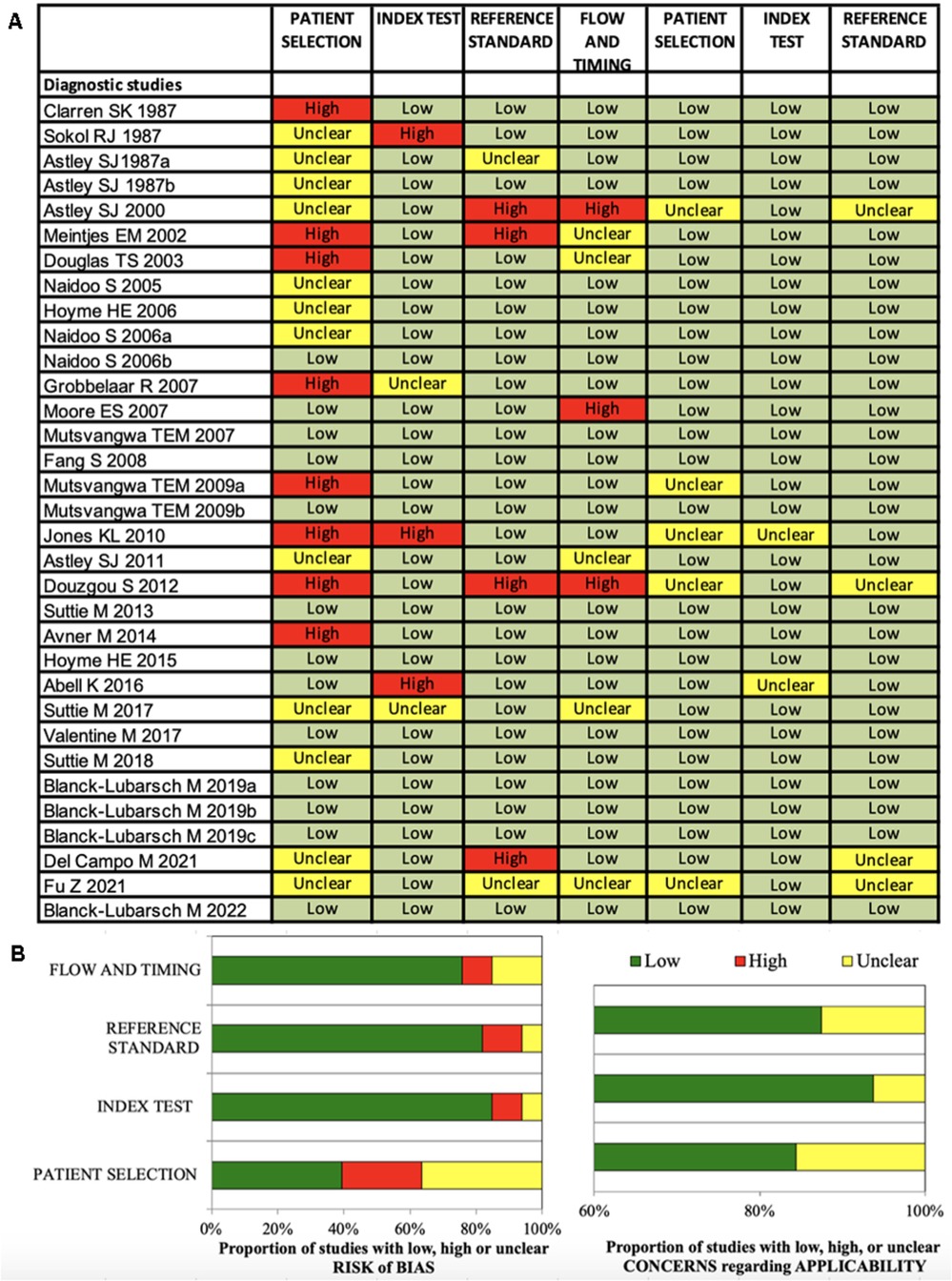
Figure 2. (A,B) Quadas-2 checklist for diagnostic studies. The evaluation showed low risk of bias concerning flow and timing, reference standard and index test. Risk of bias in patient selection was low for about 39% of the studies (85).
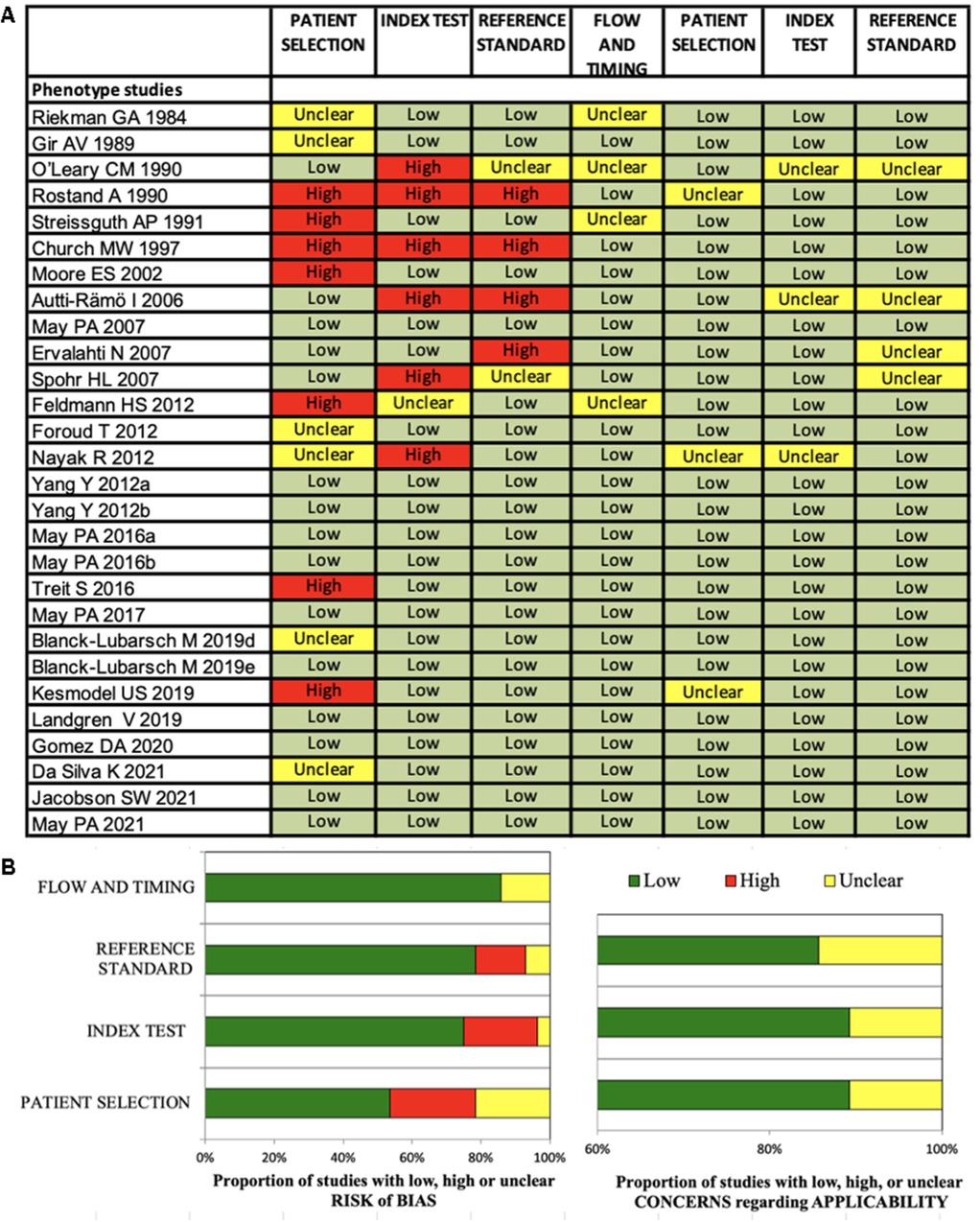
Figure 3. (A,B) Quadas-2 checklist for phenotype studies. The evaluation showed low risk of bias concerning flow and timing, reference standard and index test. Risk of bias in patient selection was low for about 54% of the studies (85).
3.4. Risk of bias in studies
The Quadas-2 evaluation showed low risk of bias concerning flow and timing (diagnostic studies 75%, phenotype studies 86%), reference standard (diagnostic studies 81%, phenotype studies 79%) and index test (diagnostic studies 84%, phenotype studies 75%) (Figures 2B, 3B). Risk of bias in patient selection was low for 39% of the diagnostic studies and for 54% of the phenotype studies. This was for example due to unclear or missing clarification of ethnicities or unclear verification of the FASD diagnosis.
None of the studies was rated with high concerns regarding applicability.
3.5. Results of individual studies
The results of the individual studies are displayed in Table 2.
3.6. Guidelines used for FASD diagnosis
The guidelines for FASD diagnosis used within the studies were the IOM (5 studies), the revised IOM (14 studies), the 4-digit diagnostic guide (4 studies), the German guideline (5 studies) and the Canadian guideline (1 study) (5, 6, 18, 36, 37).
Some studies only consulted expert dysmorphologists/pediatricians for FASD diagnosis (14 studies) or did not state which guideline was used (10 studies). For diagnosis, eight studies only considered the amount of alcohol consumed by pregnant women during pregnancy. 4 studies mentioned Hoyme as reference, two studies performed the Gestalt method. One study used the Chambers checklist, one study mentioned May et al. as reference and 1 study mentioned Clarren et al. as reference.
3.7. Age groups
The majority of studies only included infants or school children. Only three studies included adult patients. Three studies did not mention the patients' ages. 27 studies included children aged between 6 and 10 years, 10 studies included children 5 years or younger, 7 studies included children 10–14 years old. Only two studies included patients aged above 18 years. Some studies had a large age range (each 1 study: 0–27 years, 6–17 years, 1–17 years, 4–18 years, 12–40 years, 3–26 years, 8–16 years).
3.8. Group sizes
For FASD patients 7 studies included 20 subjects or less, 11 studies included 35 patients or less, 6 studies included 50 subjects or less, 16 studies included 100 patients or less and 19 studies included more than 100 participants.
For patients with FAS 10 studies included 20 patients or less, 14 studies included 35 patients or less, 4 studies included 50 subjects or less, 13 studies included 100 participants or less and 5 studies included more than 100 participants.
3.9. Ethnicities
Ten studies did not mention which ethnic group was analyzed. Many studies analyzed different ethnic populations. Some of these studies separately measured and analyzed these groups while other studies did not differentiate between different ethnicities.
3.10. Methods
Methods included direct facial measurements [7 studies (38–44)], 2D-photos [13 studies (20, 35, 45–52, 53–55)], 3D photographs [6 studies (27, 29, 33, 34, 56, 57)] and 3D-scans/stereophotogrammetry [9 studies (21–25, 28, 31, 32, 58)], landmark measurements, cephalometric measurements [2 studies (59, 60)], oral status [4 studies (61–64)], demographic and growth parameters [1 study (65)], heat maps [1 study (33)], telemedicine [1 study (66)], machine learning [1 study (21)] or automated diagnosis [7 studies (27, 31, 35, 50, 56, 58, 67)]. For decision trees the landmarks with highest scores for FAS detection in one study were midfacial length, palpebral fissure length and nose breadth at sulcus nasi (21).
3.11. Orofacial parameters
Small palpebral fissure length (PFL), smooth philtrum, upper lip circularity, thin vermillion border, midfacial hypoplasia, small head circumference and high dmft/DMFT (decayed, missing, filled teeth) score were the parameters, which were frequently found in the orofacial region.
3.11.1. Facial characteristics
Eyes
Reduced palpebral fissure length was found in 19 studies (20, 21, 23, 27, 29, 31, 38, 45, 46–48, 55, 57, 63, 65, 71). One study mentioned that higher alcohol exposure was not correlated with smaller PFLs and one study found that reduced PFLs were less frequently found in adult patients with FASD (42, 71). 4 studies found reduced IPD or ICD (43, 44, 63, 70). Epicanthal folds and ptosis were described by one study (70).
Lips and philtrum
12 studies (20, 24, 30, 38, 41, 42, 47, 49, 51, 55, 69, 70) found a smooth philtrum in patients with FASD and 12 studies (18, 30, 32, 38, 41, 47, 55, 68) reported a thin vermillion border or reduced upper lip thickness. Philtrum depth, when measured metrically on 3D-facial scans, significantly differed between patients with FAS and healthy controls (24).
Nose
One study found that nose breadth at the transition point of the sulcus alaris to the philtrum could be an indicator for FAS (23). Another study describes a flat nasal bridge (70).
Facial proportions
A hypoplastic or flat midface was mentioned in four studies (32, 43, 45, 70). Midfacial length was found to be significantly shorter when using profile analysis (22). Another study found overdevelopment of the upper and lower thirds in patients with FAS (60).
Impact of age on facial parameters
One study describes a decreased proportion of patients exhibiting small palpebral fissures in adulthood (73). Another study also describes fading of short palpebral fissures, whereas a thin vermillion was still apparent and the flat philtrum was variable. This study also describes that the physical phenotype of FAS was most apparent during early childhood and least apparent during puberty (71). Another study describes fading of facial parameters with age with the exception of elongated philtrum and thinner lips (72).
Impact of alcohol on facial findings
One study found that the offspring of alcoholics and heavy drinkers in the first trimester had significantly more craniofacial characteristics than those of moderate and light drinkers (40). Another study describes that higher prenatal alcohol exposure in any pattern was significantly associated with the incidence of a smooth philtrum but not with short palpebral fissures (42). In one study, children exposed to 1–4 drinks/week were 8.5-fold more likely to present with FAS phenotypes and the risk was 2.5-fold increased in children with a single binge exposure in gestational week 3–4 (52).
3.11.2. Findings of the dentition
Three studies found significantly higher dmft/DMFT scores in patients with FASD (1 of these studies could only find higher dmft and not DMFT scores) (61, 63, 64). One study found that the DDE-index measuring structural tooth anomalies was significantly higher in patients with FAS (63). Another study describes misaligned teeth (55).
3.11.3. Findings of the upper and lower jaw bones
Differences in mandibular and maxillary arcs were found in two studies (39, 43), abnormal vertical facial measurements, such as vertically and horizontally underdeveloped maxilla, large gonial angle and a short ramus, tendency to anterior open bite were found in three studies (22, 61, 74). One study found that the mandible was of normal overall size whereas the corpus of the mandible was significantly longer in patients with FAS (60). Another study described prognathism (43).
3.11.4. General oral health
Two studies found increased odds for gingivitis or periodontal diseases (60, 62). The odds ratio for children with FAS of having any dental admission by 5 years of age was 2.58 (62). Patients with FASD were 4.71 times more likely to be referred for treatment under general anaesthesia in one study (64). Additionally, one study found significantly higher rates of mouth breathing and habits in patients with FASD (63).
4. Discussion
This review shows that various parameters in the orofacial region for the diagnosis for FASD have been found so far. However different diagnostic methods or differing landmarks (for example manual/automated vs. 2D/3D measurements) for the same characteristic were used, which leads to difficulties in matching study results into uniform criteria.
In parts this is due to a multitude of heterogeneous diagnostic guidelines, which exist for the diagnosis of FASD. These diagnostic guidelines have in parts similarities but are not comparable or universal (12, 13). Riley et al. compared four different guidelines and could show that they are comparable in the sense that they all use facial characteristics, growth retardation, CNS involvement and alcohol exposure as the cardinal features (13). However, different cut-off values for diagnosis are used, for example the four-digit code requires a head circumference which is smaller by more than two standard deviations below the 2.5th percentile, whereas the revised IOM and the CDC require only measurements below the 10th percentile for FAS diagnosis.
The studies analyzed in this review most often used the IOM or revised IOM criteria, as well as the 4-digit diagnostic code or the German diagnostic guideline (5, 6, 14, 36, 37). Many studies however, only stated the verification of FASD via diagnosis by an expert dysmorphologist or reported alcohol consumption of the mother during pregnancy.
This lack in comparability and diagnostic standard concerning verification of a FASD diagnosis shows the importance of further clarification and standardization of this process. Another difficulty is that most studies included different ethnicities and therefore are not comparable. In addition, values or parameters for adults are not available. Only three studies investigated facial features in adult patients and Streissguth et al. concluded that characteristic facial features became less distinctive with increasing age (55, 72, 73).
Much progress seems to have been made concerning the investigation of new, objective and innovative diagnostic methods such as 3D-facial scans, stereophotogrammetry and machine learning approaches, which could facilitate and improve the diagnostic process in the future (20–35).
Many studies used 2D photographs in combination with the lip-philtrum guide as well as ruler measurements for the palpebral fissure length. Even if these are well-established methods in FASD detection, it seems that 3D measurements may be more accurate. Many studies used 3D-facial scans or 3D-photographs for further landmark analysis (21–25, 27, 29, 30, 32–34, 56–58).
Astley et al. compared accuracy of measurements of fissure length with a ruler with using the FAS facial photographic analysis software and found that the software was more accurate in comparison with a handheld ruler, which showed high interrater variability (75).
Meintjes et al. found high repeatability of PFL, ICD and IPD for 3D measurements in comparison with measurements with a handheld ruler (29).
A study by Mutsvangwa et al. could show that stereophotogrammetry precision results were better than those of manual measurements (31). Fang et al. found high precision for correct classification of FAS faces via automated techniques (59).
Suttie et al. found that facial curvature method assists the recognition of the effects of prenatal alcohol exposure (67). Another study by Suttie et al. could show that dense surface modelling achieved good agreement for FAS and pFAS and that heat map comparison of faces to matched controls revealed facial dysmorphisms with were otherwise overlooked (33). Valentine et al. also found an increased diagnostic accuracy for ARND via computer aided methods (35).
Machine learning methods such as decision trees, support vector machines and k-nearest neighbors proved to be accurate methods in detecting certain facial features in a study by Blanck-Lubarsch et al. (21). The above study results support that research is needed towards using 3D measurements as well as computer aided analysis methods or machine learning techniques since these methods seem to be accurate for FASD diagnosis.
Concerning the FASD phenotype, there were some parameters, which were repeatedly measured and mentioned as diagnostic features in FASD. These parameters comprise reduced palpebral fissure length, smooth philtrum, thin vermillion border and upper circularity, abnormal interpupillary or innercanthal distance and hypoplastic or flat midface (22, 23, 46, 48, 49, 70).
Unfortunately, the measuring or detection methods for most parameters were not metrically comparable between different studies because of different methodology (for 2D vs. 3D measurements) so that it was not possible to extract definite values for certain FASD parameters. For future research standardized measuring would improve comparability and enable the documentation of norm values for FASD diagnosis.
To date palpebral fissure length, smooth philtrum and thin vermillion border are cardinal parameters for the diagnostics of facial parameters in FASD diagnosis of most FASD guidelines (5, 6, 14, 15).
However, studies found that not all parameters are detectable in adulthood and another difficulty is that comparing facial parameters of patients to photographs in the lip-philtrum guide needs an expert dysmorphologist in the field of FASD (71–73).
Study results in various studies showed that multiple facial characteristics such as interpupillary or innercanthal distances, vertical measurements in x-rays or 3D-photographs, as well as nasal, maxillary or mandibular measurements could be used for FASD diagnosis (5, 6, 14, 15, 18, 21, 67, 74).
In addition, patients with FASD seem to have more dental and orthodontic treatment needs in comparison with healthy controls. Studies found higher dmft/DMFT values and more anomalies of the enamel structure (DDE-index) for FASD patients. Orthodontic findings included anterior open bite, horizontally and vertically underdeveloped maxilla, as well as prognathism. Mouth breathing and higher rates of habits were also found and can contribute to orthodontic problems such as open-bites, crossbites or increased sagittal distances between upper and lower front teeth (76). One study found a higher prevalence of crossbites and dental crowding in patients with FASD. These orthodontic problems need early treatment since dental crowding tends to aggravate with age and crossbites lead to asymmetric growth of the mandible or growth restriction of the upper jaw (77–83). Therefore, these dental and orthodontic parameters could not only contribute to the detection of FASD but also need to be kept in mind as these findings show the necessity for interdisciplinary treatment and early referral of the FASD patients to the dentist and/or orthodontist for prevention (43, 55, 61, 63, 64, 83). The included studies did not investigate dental findings in the deciduous dentition apart from dmft scores. In addition, it might be interesting to investigate, whether dental findings concerning crossbites or dental crowding are still characteristic parameters in the permanent dentition or whether these factors might be less frequent in adults because of orthodontic treatment in adolescence. Dental crowding is a malocclusion, which becomes worse with age even in healthy subjects (78), which in turn could result in dental crowding not being a distinguishing characteristic for FASD in adults. Therefore, these orofacial findings should be investigated for different age groups.
Further research should aim at finding values for abnormal parameters in FASD and defining universal, favorably digital measuring methods. The latter would help to store the data more easily and provide it to bio databanks, that should be accessible worldwide.
Once homogeneous and easy to apply measuring methods for the detection of FASD are defined, a routine screening of small patients could be applied by pediatricians within routine check-ups, or to for example kindergarten or primary school children, thus enabling early developmental care for patients with FASD. Early detection of FASD seems even more important since studies found a possible fading of facial parameters with age and in adult patients thus making diagnosis at older ages more difficult (55, 71, 73). If the applied methods were universal across different countries, this would allow for comparability and improvement of the diagnostic process. In addition, routine screening procedures in early childhood could reduce the number of undetected FASD patients which is suspected to be high across the world.
Furthermore, finding additional facial parameters could improve the development of machine learning methods in FASD screening.
Further studies investigating facial features in adults could find helpful parameters for the diagnosis of patients with FASD, which were not diagnosed in childhood or adolescence.
The results of this review stress the need for uniform diagnostic criteria and measuring methods. Comparable values are necessary in order to create bio databases with values for different degrees, ethnicities and ages.
5. Conclusion
This review could show that uniform diagnostic criteria and parameters for the orofacial region in FASD diagnosis are needed. Many facial parameters in the orofacial region for the detection of FASD have been found so far but only few of them are part of the diagnostic processes or current guidelines.
Further research should aim:
- to find among the existing characteristics the most comparable parameters
- to find parameters which are easy to measure for routine pediatric screenings as it is important to detect patients at young ages, even more since there seems to be a fading in facial parameters with age
- to examine larger groups of different ethnicities to find objective and uniform criteria
- to create a bio database with values and parameters for different ethnicities, degrees and age groups
- to address machine learning and 3D-facial measurement approaches as these seem to be more accurate and might facilitate the diagnostic process
- to find a consensus for one globally valid guideline with special consideration of different ethnicities and age groups
In addition, several orofacial findings hint at a higher need for dental and orthodontic treatment. This stresses the need for early referral to a dentist or orthodontist.
Author contributions
Conceptualization, MB and AH; methodology, MB, DD; software, DD; validation, MB, RF, AH and DD; formal analysis, MB, DD; investigation, MB, AH; resources, AH; data curation, MB, DD; writing—original draft preparation, MB; writing—review and editing, MB, RF, DD, AH; visualization, MB; supervision, AH; project administration, MB, AH. All authors have read and agreed to the published version of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We acknowledge Open Access support from the University of Muenster.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ARBD, Alcohol Related Birth Defects; ARND, Alcohol Related Neurodevelopmental Disorder; BMI, Body Mass Index; CNS, Central Nervous Systems; DDE, Developmental Defects of Enamel; dmft, decayed, missing, filled, teeth (deciduous dentition); DMFT, Decayed, Missing, Filled, Teeth (permanent dentition); DSM, Dense Surface Model; FAE, Fetal Alcohol Effects; FAS, Feta Alcohol Syndrome; FASD, Fetal Alcohol Spectrum Disorder; HE, Heavily Exposed; ICD, Innercanthal Distance; IOM, Institute Of Medicine; IPD, Interpupillary Distance; IQ, Intelligence Quotient; OCD, Outer Canthal Distance; OFC, Occipital Frontal Circumference; pFAE, partial Fetal Alcohol Effects; pFAS, partial Fetal Alcohol Syndrome; PFL, Palpebral Fissure Length.
References
1. Popova S, Lange S, Probst C, Gmel G, Rehm J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: a systematic review and meta-analysis. Lancet Glob Health. (2017) 5(3):e290–e9. doi: 10.1016/S2214-109X(17)30021-9
2. Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. (1973) 302(7836):999–1001. doi: 10.1016/S0140-6736(73)91092-1
3. Jones KL, Smith DW. The fetal alcohol syndrome. Teratology. (1975) 12(1):1–10. doi: 10.1002/tera.1420120102
4. May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, et al. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics. (2014) 134(5):855–66. doi: 10.1542/peds.2013-3319
5. Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais AS, et al. Updated clinical guidelines for diagnosing fetal alcohol Spectrum disorders. Pediatrics. (2016) 138(2). doi: 10.1542/peds.2015-4256
6. Chudley AE, Conry J, Cook JL, Loock C, Rosales T, LeBlanc N, et al. Fetal alcohol spectrum disorder: canadian guidelines for diagnosis. Can Med Assoc J. (2005) 172(5 Suppl):S1–S21. doi: 10.1503/cmaj.1040302
7. May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, et al. Prevalence of fetal alcohol Spectrum disorders in 4 US communities. JAMA. (2018) 319(5):474–82. doi: 10.1001/jama.2017.21896
8. Popova S, Lange S, Chudley AE, Reynolds JN, Rehm J. World Health Organization International Study on the Prevalence of Fetal Alcohol Spectrum Disorder (FASD) (2018).
9. Lange S, Probst C, Gmel G, Rehm J, Burd L, Popova S. Global prevalence of fetal alcohol Spectrum disorder among children and youth: a systematic review and meta-analysis. JAMA Pediatr. (2017) 171(10):948–56. doi: 10.1001/jamapediatrics.2017.1919
10. Warren KR, Bast RJ. Alcohol-related birth defects: an update. Public Health Rep. (1988) 103(6):638–42.3141958
11. Lemoine P, Harousseau H, Borteyru JP, Menuet JC. Children of alcoholic parents—anomalies in 127 cases. Arch Fr Pediatr. (1968) 25(7):830.
12. Coles CD, Gailey AR, Mulle JG, Kable JA, Lynch ME, Jones KL. A comparison among 5 methods for the clinical diagnosis of fetal alcohol Spectrum disorders. Alcohol Clin Exp Res. (2016) 40(5):1000–9. doi: 10.1111/acer.13032
13. Riley EP, Infante MA, Warren KR. Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev. (2011) 21(2):73–80. doi: 10.1007/s11065-011-9166-x
14. Astley SJ. Diagnostic guide for fetal alcohol Spectrum disorders: The 4- digit diagnostic code. Seattle WA: University of Washington Publication Services (2004).
15. Bertrand JFR, Weber MK, O’Connor M, Riley EP, Johnson KA, Cohen DE, et al. Fetal Alcohol Syndrome: Guidelines for Referral and Diagnosis. (2004). Available at: https://www.cdc.gov/ncbddd/fasd/documents/FAS_guidelines_accessible-P.pdf
16. Clarren SK, Chudley AE, Wong L, Friesen J, Brant R. Normal distribution of palpebral fissure lengths in Canadian school age children. Can J Clin Pharmacol. (2010) 17(1):e67–78.20147771
17. Greenbaum R, Koren G. Fetal alcohol spectrum disorder—new diagnostic initiatives. Paediatr Child Health. (2002) 7(3):139–41. doi: 10.1093/pch/7.3.139
18. Astley SJ, Clarren SK. Diagnosing the full spectrum of fetal alcohol-exposed individuals: introducing the 4-digit diagnostic code. Alcohol Alcohol. (2000) 35(4):400–10. doi: 10.1093/alcalc/35.4.400
19. Astley SJ, Stachowiak J, Clarren SK, Clausen C. Application of the fetal alcohol syndrome facial photographic screening tool in a foster care population. J Pediatr. (2002) 141(5):712–7. doi: 10.1067/mpd.2002.129030
20. Avner M, Henning P, Koren G, Nulman I. Validation of the facial photographic in fetal alcohol spectrum disorder screening and diagnosis. J Popul Ther Clin Pharmacol. (2014) 21(1):e106–13.24615428
21. Blanck-Lubarsch M, Dirksen D, Feldmann R, Bormann E, Hohoff A. Simplifying diagnosis of fetal alcohol syndrome using machine learning methods. Front Pediatr. (2022) 9. doi: 10.3389/fped.2021.707566
22. Blanck-Lubarsch M, Dirksen D, Feldmann R, Sauerland C, Hohoff A. Children with fetal alcohol syndrome (FAS): 3D-analysis of palatal depth and 3D-metric facial length. Int J Environ Res Public Health. (2019) 17(1). doi: 10.3390/ijerph17010095
23. Blanck-Lubarsch M, Dirksen D, Feldmann R, Sauerland C, Hohoff A. 3D-Analysis of mouth, nose and eye parameters in children with fetal alcohol syndrome (FAS). Int J Environ Res Public Health. (2019) 16(14). doi: 10.3390/ijerph16142535
24. Blanck-Lubarsch M, Dirksen D, Feldmann R, Sauerland C, Kirschneck C, Hohoff A. 3D Analysis of philtrum depth in children with fetal alcohol syndrome. Alcohol Alcohol. (2019) 54(2):152–8. doi: 10.1093/alcalc/agy088
25. Blanck-Lubarsch M, Dirksen D, Feldmann R, Sauerland C, Kirschneck C, Hohoff A. Asymmetry-index and orthodontic facial analysis of children with foetal alcohol syndrome using 3D-facial scans. Pediatr Res. (2019) 88(2):243–9. doi: 10.1038/s41390-019-0559-5
26. Chik L, Sokol RJ, Martier SS, editors. Computer aided morphometry of the neonatal fetal alcohol syndrome face. Bellingham, WA, United States: Publ by Society of Photo-Optical Instrumentation Engineers (1993).
27. Douglas TS, Martinez F, Meintjes EM, Vaughan CL, Viljoen DL. Eye feature extraction for diagnosing the facial phenotype associated with fetal alcohol syndrome. Med Biol Eng Comput. (2003) 41(1):101–6. doi: 10.1007/BF02343545
28. Grobbelaar R, Douglas TS. Stereo image matching for facial feature measurement to aid in fetal alcohol syndrome screening. Med Eng Phys. (2007) 29(4):459–64. doi: 10.1016/j.medengphy.2006.06.005
29. Meintjes EM, Douglas TS, Martinez F, Vaughan CL, Adams LP, Stekhoven A, et al. A stereo-photogrammetric method to measure the facial dysmorphology of children in the diagnosis of fetal alcohol syndrome. Med Eng Phys. (2002) 24(10):683–9. doi: 10.1016/S1350-4533(02)00114-5
30. Mutsvangwa T, Douglas TS. Morphometric analysis of facial landmark data to characterize the facial phenotype associated with fetal alcohol syndrome. J Anat. (2007) 210(2):209–20. doi: 10.1111/j.1469-7580.2006.00683.x
31. Mutsvangwa TE, Smit J, Hoyme HE, Kalberg W, Viljoen DL, Meintjes EM, et al. Design, construction, and testing of a stereo-photogrammetric tool for the diagnosis of fetal alcohol syndrome in infants. IEEE Trans Med Imaging. (2009) 28(9):1448–58. doi: 10.1109/TMI.2009.2017375
32. Mutsvangwa TEM, Meintjes EM, Viljoen DL, Douglas TS. Morphometric analysis and classification of the facial phenotype associated with fetal alcohol syndrome in 5- and 12-year-old children. Am J Med Genet A. (2010) 152(1):32–41. doi: 10.1002/ajmg.a.33137
33. Suttie M, Foroud T, Wetherill L, Jacobson JL, Molteno CD, Meintjes EM, et al. Facial dysmorphism across the fetal alcohol spectrum. Pediatrics. (2013) 131(3):e779–e88. doi: 10.1542/peds.2012-1371
34. Suttie M, Wozniak JR, Parnell SE, Wetherill L, Mattson SN, Sowell ER, et al. Combined face–brain morphology and associated neurocognitive correlates in fetal alcohol Spectrum disorders. Alcohol Clin Exp Res. (2018) 42(9):1769–82. doi: 10.1111/acer.13820
35. Valentine M, Bihm DCJ, Wolf L, Hoyme HE, May PA, Buckley D, et al. Computer-Aided recognition of facial attributes for fetal alcohol Spectrum disorders. Pediatrics. (2017) 140(6). doi: 10.1542/peds.2016-2028
36. Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, et al. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. (2005) 115(1):39–47. doi: 10.1542/peds.2004-0259
37. Landgraf MN, Nothacker M, Heinen F. Diagnosis of fetal alcohol syndrome (FAS): german guideline version 2013. Eur J Paediatr Neurol. (2013) 17(5):437–46. doi: 10.1016/j.ejpn.2013.03.008
38. Astley SJ, Clarren SK. A fetal alcohol syndrome screening tool. Alcohol Clin Exp Res. (1995) 19(6):1565–71. doi: 10.1111/j.1530-0277.1995.tb01025.x
39. Abell K, May W, May PA, Kalberg W, Hoyme HE, Robinson LK, et al. Fetal alcohol spectrum disorders and assessment of maxillary and mandibular arc measurements. Am J Med Genet A. (2016) 170(7):1763–71. doi: 10.1002/ajmg.a.37656
40. Rostand A, Kaminski M, Lelong N, Dehaene P, Delestret I, Klein-Bertrand C, et al. Alcohol use in pregnancy, craniofacial features, and fetal growth. J Epidemiol Community Health. (1990) 44(4):302–6. doi: 10.1136/jech.44.4.302
41. Autti-Rämö I, Fagerlund A, Ervalahti N, Loimu L, Korkman M, Hoyme HE. Fetal alcohol spectrum disorders in Finland: clinical delineation of 77 older children and adolescents. Am J Med Genet A. (2006) 140(2):137–43. doi: 10.1002/ajmg.a.31037
42. Feldman HS, Jones KL, Lindsay S, Slymen D, Klonoff-Cohen H, Kao K, et al. Prenatal alcohol exposure patterns and alcohol-related birth defects and growth deficiencies: a prospective study. Alcohol Clin Exp Res. (2012) 36(4):670–6. doi: 10.1111/j.1530-0277.2011.01664.x
43. May PA, De Vries MM, Marais AS, Kalberg WO, Buckley D, Adnams CM, et al. Replication of high fetal alcohol spectrum disorders prevalence rates, child characteristics, and maternal risk factors in a second sample of rural communities in South Africa. Int J Environ Res Public Health. (2017) 14(5). doi: 10.3390/ijerph14050522
44. Gomez DA, May PA, Tabachnick BG, Hasken JM, Lyden ER, Kalberg WO, et al. Ocular measurements in fetal alcohol spectrum disorders. Am J Med Genet A. (2020) 182(10):2243–52. doi: 10.1002/ajmg.a.61759
45. Clarren SK, Sampson PD, Larsen J, Donnell DJ, Barr HM, Bookstein FL, et al. Facial effects of fetal alcohol exposure: assessment by photographs and morphometric analysis. Am J Med Genet. (1987) 26(3):651–66. doi: 10.1002/ajmg.1320260321
46. Sokol RJ, Chik L, Martier SS, Salari V. Morphometry of the neonatal fetal alcohol syndrome face from “snapshots”. Alcohol Alcohol Suppl. (1991) 1:531–4.1845594
47. Astley SJ, Clarren SK. A case definition and photographic screening tool for the facial phenotype of fetal alcohol syndrome. J Pediatr. (1996) 129(1):33–41. doi: 10.1016/S0022-3476(96)70187-7
48. Astley SJ. Canadian Palpebral fissure length growth charts reflect a good fit for two school and fasd clinic-based U.S. Populations. J Clin Pharmacol. (2011) 18(2):e231–e41.
49. Hoyme HE, Hoyme DB, Elliott AJ, Blankenship J, Kalberg WO, Buckley D, et al. A South African mixed race lip/philtrum guide for diagnosis of fetal alcohol spectrum disorders. Am J Med Genet A. (2015) 167(4):752–5. doi: 10.1002/ajmg.a.37023
50. Fu Z, Jiao J, Suttie M, Noble JA. Facial anatomical landmark detection using regularized transfer learning with application to fetal alcohol syndrome recognition. IEEE J Biomed Health Inform. (2021) 26(4):1591–601. doi: 10.1109/JBHI.2021.3110680
51. Nayak R, Murthy P, Girimaji S, Navaneetham J. Fetal alcohol spectrum disorders–a case-control study from India. J Trop Pediatr. (2012) 58(1):19–24. doi: 10.1093/tropej/fmr015
52. Kesmodel US, Nygaard SS, Mortensen EL, Bertrand J, Denny CH, Glidewell A, et al. Are low-to-moderate average alcohol consumption and isolated episodes of binge drinking in early pregnancy associated with facial features related to fetal alcohol syndrome in 5-year-old children? Alcohol Clin Exp Res. (2019) 43(6):1199–212. doi: 10.1111/acer.14047
53. May PA, Marais AS, De Vries MM, Buckley D, Kalberg WO, Hasken JM, et al. The prevalence, child characteristics, and maternal risk factors for the continuum of fetal alcohol spectrum disorders: a sixth population-based study in the same South African community. Drug Alcohol Depend. (2021) 218:108408. doi: 10.1016/j.drugalcdep.2020.108408
54. Riekman GA. Oral findings of fetal alcohol syndrome patients. J Can Dent Assoc. (1984) 50(11):841–2.6391625
55. Streissguth AP, Aase JM, Clarren SK, Randels SP, Ladue RA, Smith DF. Fetal alcohol syndrome in adolescents and adults. J Am Med Assoc. (1991) 265(15):1961–7. doi: 10.1001/jama.1991.03460150065025
56. Moore ES, Ward RE, Wetherill LF, Rogers JL, Autti-Rämö I, Fagerlund A, et al. Unique facial features distinguish fetal alcohol syndrome patients and controls in diverse ethnic populations. Alcohol Clin Exp Res. (2007) 31(10):1707–13. doi: 10.1111/j.1530-0277.2007.00472.x
57. Foroud T, Wetherill L, Vinci-Booher S, Moore ES, Ward RE, Hoyme HE, et al. Relation over time between facial measurements and cognitive outcomes in fetal alcohol-exposed children. Alcohol Clin Exp Res. (2012) 36(9):1634–46. doi: 10.1111/j.1530-0277.2012.01750.x
58. Fang S, McLaughlin J, Fang J, Huang J, Autti-Rämö I, Fagerlund A, et al. Automated diagnosis of fetal alcohol syndrome using 3D facial image analysis. Orthod Craniofacial Res. (2008) 11(3):162–71. doi: 10.1111/j.1601-6343.2008.00425.x
59. Naidoo S, Norval G, Swanevelder S, Lombard C. Foetal alcohol syndrome: a dental and skeletal age analysis of patients and controls. Eur J Orthod. (2006) 28(3):247–53. doi: 10.1093/ejo/cji109
60. Gir AV, Aksharanugraha K, Harris EF. A cephalometric assessment of children with fetal alcohol syndrome. Am J Orthod Dentofac. (1989) 95(4):319–26. doi: 10.1016/0889-5406(89)90165-0
61. Naidoo S, Chikte U, Laubscher R, Lombard C. Fetal alcohol syndrome: anthropometric and oral health status. J Contemp Dent Pract. (2005) 6(4):101–15. doi: 10.5005/jcdp-6-4-101
62. O'Leary CM, Slack-Smith LM. Dental hospital admissions in the children of mothers with an alcohol-related diagnosis: a population-based, data-linkage study. J Pediatr. (2013) 163(2):515–20.e1. doi: 10.1016/j.jpeds.2013.02.020
63. Blanck-Lubarsch M, Dirksen D, Feldmann R, Sauerland C, Hohoff A. Tooth malformations, dmft index, speech impairment and oral habits in patients with fetal alcohol syndrome. Int J Environ Res Public Health. (2019) 16(22). doi: 10.3390/ijerph16224401
64. Da Silva K, Wood D. The oral health status and treatment needs of children with fetal alcohol spectrum disorder. Clin Oral Investig. (2021) 25(6):3497–503. doi: 10.1007/s00784-020-03671-0
65. May PA, Gossage JP, Marais AS, Adnams CM, Hoyme HE, Jones KL, et al. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend. (2007) 88(2–3):259–71. doi: 10.1016/j.drugalcdep.2006.11.007
66. del Campo M, Beach D, Wells A, Jones KL. Use of telemedicine for the physical examination of children with fetal alcohol Spectrum disorders. Alcohol Clin Exp Res. (2021) 45(2):409–17. doi: 10.1111/acer.14533
67. Suttie M, Wetherill L, Jacobson SW, Jacobson JL, Hoyme HE, Sowell ER, et al. Facial curvature detects and explicates ethnic differences in effects of prenatal alcohol exposure. Alcohol Clin Exp Res. (2017) 41(8):1471–83. doi: 10.1111/acer.13429
68. Yang Y, Phillips OR, Kan E, Sulik KK, Mattson SN, Riley EP, et al. Callosal thickness reductions relate to facial dysmorphology in fetal alcohol Spectrum disorders. Alcohol Clin Exp Res. (2012) 36(5):798–806. doi: 10.1111/j.1530-0277.2011.01679.x
69. May PA, de Vries MM, Marais AS, Kalberg WO, Adnams CM, Hasken JM, et al. The continuum of fetal alcohol spectrum disorders in four rural communities in South Africa: prevalence and characteristics. Drug Alcohol Depend. (2016) 159:207–18. doi: 10.1016/j.drugalcdep.2015.12.023
70. May PA, Marais AS, de Vries MM, Kalberg WO, Buckley D, Hasken JM, et al. The continuum of fetal alcohol spectrum disorders in a community in South Africa: prevalence and characteristics in a fifth sample. Drug Alcohol Depend. (2016) 168:274–86. doi: 10.1016/j.drugalcdep.2016.09.025
71. Jacobson SW, Hoyme HE, Carter RC, Dodge NC, Molteno CD, Meintjes EM, et al. Evolution of the physical phenotype of fetal alcohol Spectrum disorders from childhood through adolescence. Alcohol Clin Exp Res. (2021) 45(2):395–408. doi: 10.1111/acer.14534
72. Spohr HL, Willms J, Steinhausen HC. Fetal alcohol spectrum disorders in young adulthood. J Pediatr. (2007) 150(2):175–9. 9 e1. doi: 10.1016/j.jpeds.2006.11.044
73. Landgren V, Svensson L, Gyllencreutz E, Aring E, Gronlund MA, Landgren M. Fetal alcohol spectrum disorders from childhood to adulthood: a Swedish population-based naturalistic cohort study of adoptees from Eastern Europe. BMJ Open. (2019) 9(10):e032407. doi: 10.1136/bmjopen-2019-032407
74. Naidoo S, Harris A, Swanevelder S, Lombard C. Foetal alcohol syndrome: a cephalometric analysis of patients and controls. Eur J Orthod. (2006) 28(3):254–61. doi: 10.1093/ejo/cji110
75. Astley SJ. Palpebral fissure length measurement: accuracy of the FAS facial photographic analysis software and inaccuracy of the ruler. J Popul Ther Clin Pharmacol. (2015) 22(1):e9–e26.25594840
76. Festa P, Mansi N, Varricchio AM, Savoia F, Cali C, Marraudino C, et al. Association between upper airway obstruction and malocclusion in mouth-breathing children. Acta Otorhinolaryngol Ital. (2021) 41(5):436–42. doi: 10.14639/0392-100X-N1225
77. Lippold C, Stamm T, Meyer U, Vegh A, Moiseenko T, Danesh G. Early treatment of posterior crossbite–a randomised clinical trial. Trials. (2013) 14:20. doi: 10.1186/1745-6215-14-20
78. Lombardo G, Vena F, Negri P, Pagano S, Barilotti C, Paglia L, et al. Worldwide prevalence of malocclusion in the different stages of dentition: a systematic review and meta-analysis. Eur J Paediatr Dent. (2020) 21(2):115–22. doi: 10.23804/ejpd.2020.21.02.05
79. McNamara JA. Maxillary transverse deficiency. Am J Orthod Dentofacial Orthop. (2000) 117(5):567–70. doi: 10.1016/s0889-5406(00)70202-2
80. McNamara JA Jr. Early intervention in the transverse dimension: is it worth the effort? Am J Orthod Dentofacial Orthop. (2002) 121(6):572–4. doi: 10.1067/mod.2002.124167
81. Melink S, Vagner MV, Hocevar-Boltezar I, Ovsenik M. Posterior crossbite in the deciduous dentition period, its relation with sucking habits, irregular orofacial functions, and otolaryngological findings. Am J Orthod Dentofacial Orthop. (2010) 138(1):32–40. doi: 10.1016/j.ajodo.2008.09.029
82. Pinto AS, Buschang PH, Throckmorton GS, Chen P. Morphological and positional asymmetries of young children with functional unilateral posterior crossbite. Am J Orthod Dentofacial Orthop. (2001) 120(5):513–20. doi: 10.1067/mod.2001.118627a
83. Blanck-Lubarsch M, Flieger S, Feldmann R, Kirschneck C, Sauerland C, Hohoff A. Malocclusion can give additional hints for diagnosis of fetal alcohol spectrum disorder. Alcohol Alcohol. (2019) 54(1):56–61. doi: 10.1093/alcalc/agy071
84. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ (OPEN ACCESS). (2021) 372:n160. doi: 10.1136/bmj.n160
Keywords: review, craniofacial anomalies, orofacial disorder, diagnostic methods, fetal alcohol syndrome (FAS)
Citation: Blanck-Lubarsch M, Dirksen D, Feldmann R and Hohoff A (2023) A systematic review: facial, dental and orthodontic findings and orofacial diagnostics in patients with FASD. Front. Pediatr. 11:1169570. doi: 10.3389/fped.2023.1169570
Received: 19 February 2023; Accepted: 23 May 2023;
Published: 8 June 2023.
Edited by:
Carlos Fernando Valenzuela, University of New Mexico, United StatesReviewed by:
Michael Suttie, University of Oxford, United KingdomDevi Sewvandini Atukorallaya, University of Manitoba, Canada
© 2023 Blanck-Lubarsch, Dirksen, Feldmann and Hohoff. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Moritz Blanck-Lubarsch YmxhbmNrbHViYXJzY2hAdW5pLW11ZW5zdGVyLmRl
 Moritz Blanck-Lubarsch
Moritz Blanck-Lubarsch Dieter Dirksen
Dieter Dirksen Reinhold Feldmann3
Reinhold Feldmann3