
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pediatr. , 18 October 2023
Sec. Pediatric Neurology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1149646
This article is part of the Research Topic Neuroimaging of Pediatric Brain Tumors View all 5 articles
The fifth edition of the World Health Organization Classification of Tumors of the Central Nervous System (WHO CNS5) has identified a new classification system for tumors of the brain and spinal cord, highlighting the pivotal role of molecular diagnosis in accurately categorizing neoplasms. In addition to previous classifications, one of the key distinctions lies in categorizing pediatric-type diffuse low-grade gliomas (pDLGGs) and pediatric-type diffuse high-grade gliomas (pDHGGs) as distinct tumor types. Although similar in histology and morphology, pediatric diffuse gliomas are completely different from the adult type with respect to the molecular genetic characteristics, prognosis, and treatment strategies. pDLGG includes four tumor types, namely, diffuse astrocytoma, MYB- or MYBL1-altered; angiocentric glioma; polymorphous low-grade neuroepithelial tumor of the young (PLNTY); and diffuse low-grade glioma, MAPK pathway-altered, three types of which are newly recognized tumor types. Herein, we review the clinical characteristics, histopathological and molecular genetic characteristics, neuroimaging features, and prognosis of pDLGG and summarize the neuroimaging key points in diagnosing different tumor types. This review aims to evaluate and update the relevant pDLGG features and advances in neuroimaging that may assist in differential diagnosis, surgery planning, and prognostic determination of these tumor types and provide accurate diagnostic information for clinical colleagues.
The 2016 World Health Organization (WHO) central nervous system (CNS) classification integrated molecular and histologic/phenotypic information for the first time (1). However, owing to advances in molecular diagnostics, the 2016 WHO CNS classification has some limitations in describing pediatric gliomas. The 2021 WHO CNS tumor classification includes molecular features for CNS tumors, adding new categories (2). The WHO CNS5 has classified diffuse gliomas into adult and pediatric types. Although pediatric diffuse glioma is similar to the adult type in histological morphology, its genetic characteristics and prognosis are molecularly distinct (3). Pediatric-type diffuse low-grade gliomas (pDLGGs) differ from adult forms with respect to molecular characteristics, biological behavior, clinical course, and prognosis. In the WHO CNS5, pDLGG is classified into four distinct tumor types, namely, (1) diffuse astrocytoma, MYB- or MYBL1-altered; (2) angiocentric glioma (AG); (3) polymorphous low-grade neuroepithelial tumor of the young (PLNTY); and (4) diffuse low-grade glioma, MAPK pathway-altered (Table 1). All four pDLGG tumor types are characterized by diffuse growth in the brain with overlapping histological features and poor specificity between them. Molecular analysis can be helpful in the characterization of lesions.
Low-grade gliomas are the most commonly encountered pediatric brain tumors. Although low-grade gliomas are a common type of CNS tumor in the pediatric age group, diffuse low-grade gliomas with infiltrative margins are relatively rare, with an incidence rate of only 8% (4). PDLGGs have a high incidence of BRAF p.V600E mutation, FGFR alteration, and/or MYB or MYBL1 rearrangement (5, 6).
According to the previous WHO classification criteria of CNS tumors, the prognosis of these tumors varies greatly. Here, we review the imaging and pathological features, clinical manifestations, treatment, and prognosis of pDLGG, aiming to enhance our comprehension of the disease and provide an initial interpretation from the perspective of clinical diagnosis and treatment. Table 2 shows the neuroimaging features of pDLGG (7–12).
Diffuse astrocytoma with alterations of MYB/MYBL1 is a diffuse, infiltrating tumor composed of astrocytic cells. Its histological appearance is not distinguishable from that of astrocytic tumors. The tumor belongs to the family of MYB/MYBL1-altered gliomas and is an IDH-wild type. It exhibits low-grade histopathologic features and represents a distinct group of tumors with indolent behavior and a favorable prognosis (9). The tumor cell proliferation index is low and usually classified as WHO grade 1 (13).
MYB is a proto-oncogene that plays an essential role in controlling the proliferation and differentiation of hematopoietic and other progenitor cells and acts as a proto-oncogene in leukemia and other solid tumors (14). MYBL1 is closely associated with MYB and has a similar function (15). Alterations of these genes have been associated with various hematologic diseases and solid malignancies. The alterations include MYB or MYBL1 rearrangements and MYB-QKI fusion (5). The MYB/MYBL1 alterations are associated with diffuse astrocytoma, while the MYB-QKI fusion is highly relevant to AG. Diffuse astrocytoma, MYB- or MYBL1-altered, is a diffuse infiltrative neoplasm composed of astrocytoid cells that exhibit histological morphology indistinguishable from conventional astrocytomas. The tumor exhibits invasiveness and primarily affects the cerebral hemispheres (16).
Patients were primarily young children (median age: 5 years; range 0–26 years), and no gender predilection was observed (17). The tumors most commonly involved the cerebral cortex, followed by cerebral white matter and/or deep gray nuclei (18). Most patients with diffuse MYB/MYBL1-altered astrocytoma have a history of epileptic seizures since childhood (13). Furthermore, symptoms such as movement disorders and behavioral changes occur in both encephalopathy and glioneuronal tumors (19).
The prognosis of diffuse astrocytoma with alterations of MYB/MYBL1 is generally excellent. Chiang et al. (17) found that the 10-year progression-free survival and overall survival rates were 89.6% and 95.2%, respectively. The conservative approach is recommended for inert IDH-wild-type diffuse astrocytoma owing to its more favorable prognosis and progression-free survival (9). Yang et al. (20) found that children with MYB amplification have a better prognosis and longer progression-free survival than those without MYB amplification.
The imaging findings of this tumor type are similar to those of adult diffuse low-grade glioma, with invasive growth mainly involving the cerebral hemispheres. The brain stem and diencephalon are less commonly involved (18). The tumors are most commonly found in the temporal lobe, followed by the frontal and occipital lobes. They commonly involve the cortical and subcortical regions of the cerebral cortex (21). The tumors displayed a mass effect and mild peritumoral edema. The lesion showed an iso- to hypointense signal on T1-weighted imaging (WI) and mixed-signal or hyperintense signal on T2WI/fluid-attenuated inversion recovery (FLAIR) (Figure 1) (9, 17), without evidence of restricted diffusion. Contrast enhancement was never observed. Magnetic resonance spectroscopy (MRS) shows increased choline and decreased N-acetyl-aspartate (18, 22).
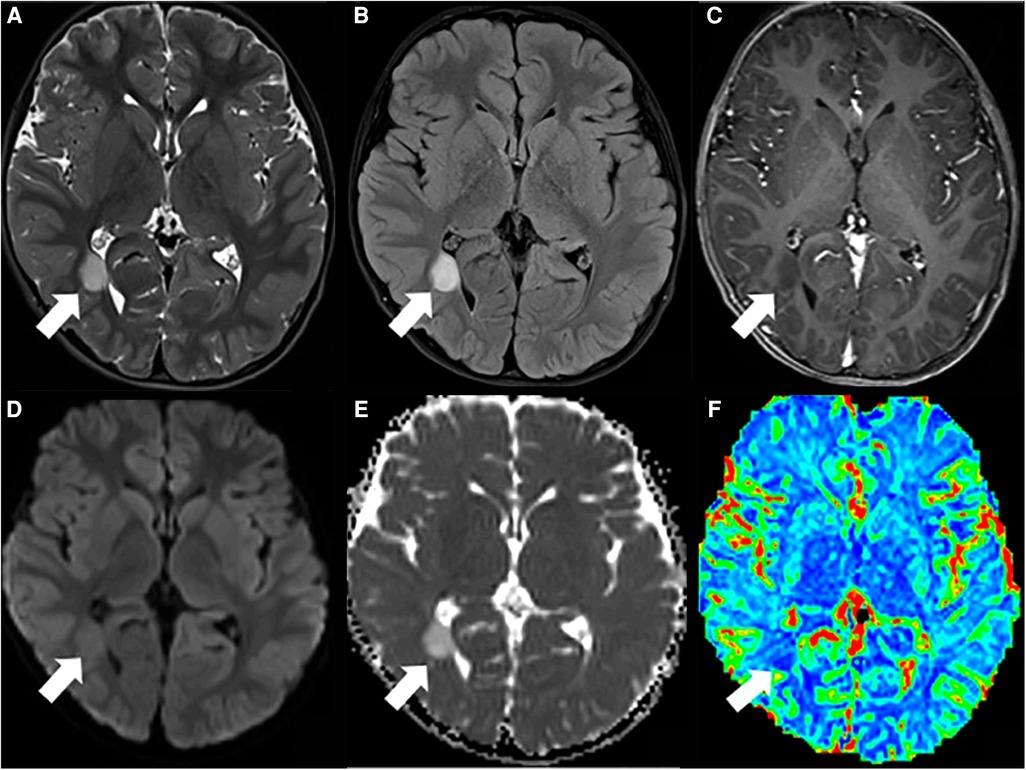
Figure 1. Pediatric-type diffuse low-grade glioma. (A) T2WI and (B) FLAIR hyperintense lesion in the right temporo-occipital (arrow). (C) There is no enhancement on the T1WI postcontrast image (arrow). (D) Facilitated diffusion seen on diffusion-weighted imaging (DWI) (arrow) and (E) ADC map (arrow). (F) Hypoperfusion is seen on the rCBV map (arrow). This figure was originally posted by Kalelioglu et al. in the Neuroradiology Journal in September 2022 (9).
Diffuse astrocytoma should be distinguished from oligodendroglioma and diffuse hemispheric glioma. Oligodendroglioma is mostly located in the frontal lobe and typically shows gyriform calcification (23). Diffuse hemispheric glioma belongs to pediatric-type diffuse high-grade gliomas (PDHGGs), with poor prognoses. The tumors most commonly involve the frontal lobe and parietal lobe, particularly the profound nuclei. The signal intensity on MRI images is intricately modulated by the coexistence of cystic, hemorrhagic, and necrotic constituents. They exhibit invasive growth patterns with indistinct margins and enhancement, with restricted diffusion (24, 25).
AG is a rare cortical/juxtacortical epilepsy-related low-grade glioma (WHO grade 1) that occurs in children and young adults (7). It is defined by characteristic MYB-QKI gene fusion and shows angiocentric patterns upon histopathological examination (14).
MYB is a proto-oncogene, and MYB proteins are transcription factors characterized by a highly conserved DNA-binding base sequence. MYB-QKI fusion is relevant to AG (8). MYB-QKI fusion is found in 87% of AGs and 41% of pDLGGs (18). The functional consequence of this fusion is the loss of the tumor suppressor function of QKI combined with the activation of MYB (21, 26). The histopathology of AG is characterized by “monomorphous bipolar fusiform cells” and “an angiocentric growth pattern,” which means that the tumor consists of monomorphic cells that align longitudinally around blood vessels, and the neurons form pseudo-rosettes (27). The tumor demonstrates indistinct boundaries and closely associates with the cerebral tissue. The tumor cells exhibited an elongated and bipolar morphology under microscopic examination, arranged in a radial pattern around the affected blood vessels and oriented vertically or parallel to the vascular endothelial cells (8).
Patients <20 years of age are the most affected, with no sex differences (8). The vast majority of patients have a long history of intractable seizures. The disease course is often accompanied by seizures owing to AGs that tend to be located superficially. Other clinical symptoms include facial weakness, double vision, irregular gait, and headache with vomiting (7).
AG is a slow-growing, stable tumor with a good prognosis after surgical resection, and the incidence of tumor recurrence after complete resection was low (7). Seizures/epilepsies improve when complete resection is performed (28).
AG is typically localized in the supratentorial cortex and subcortical white matter, predominantly affecting a single lobe, with the temporal lobe being the most commonly involved, followed by the frontal lobe, parietal lobe, brain stem, and thalamus, mostly involving the hippocampus (29, 30). The neuroimaging features of AG vary, showing low density, high density, or mixed density on computed tomography (CT), and calcification is generally rare. In some instances, a hyperdense lesion was observed on a CT scan, potentially indicating the presence of hemorrhage and deposition of hemosiderin (29). AG has a characteristic MRI appearance comprising an intratumoral T1WI high-intensity area, stalk-like sign, and atrophy of the adjacent brain parenchyma (Figure 2) (7). The stalk-like sign indicates that the tumor is located along the vessels extending from near the brain surface toward the ventricle. Solid ingredients showed isointense, hypointense, or hyperintense signals on T1WI and T2WI/FLAIR. Cystoid components are common and showed a hypointense signal on T1WI and a hyperintense signal on T2WI/FLAIR. Contrast enhancement was observed in more than 25% of patients despite the general consensus that AGs are considered to have no contrast enhancement. Typically, diffusion restriction in AGs is also atypical. On MRS, elevated levels of creatine and choline and decreased levels of NAA have been described, and lactate peaks were observed (8, 18, 31, 32). It is a well-circumscribed tumor in that peripheral edema and mass effect are usually absent (33). In addition, some studies have reported the coexisting nature of adjacent focal cortical dysplasia (FCD) (7).
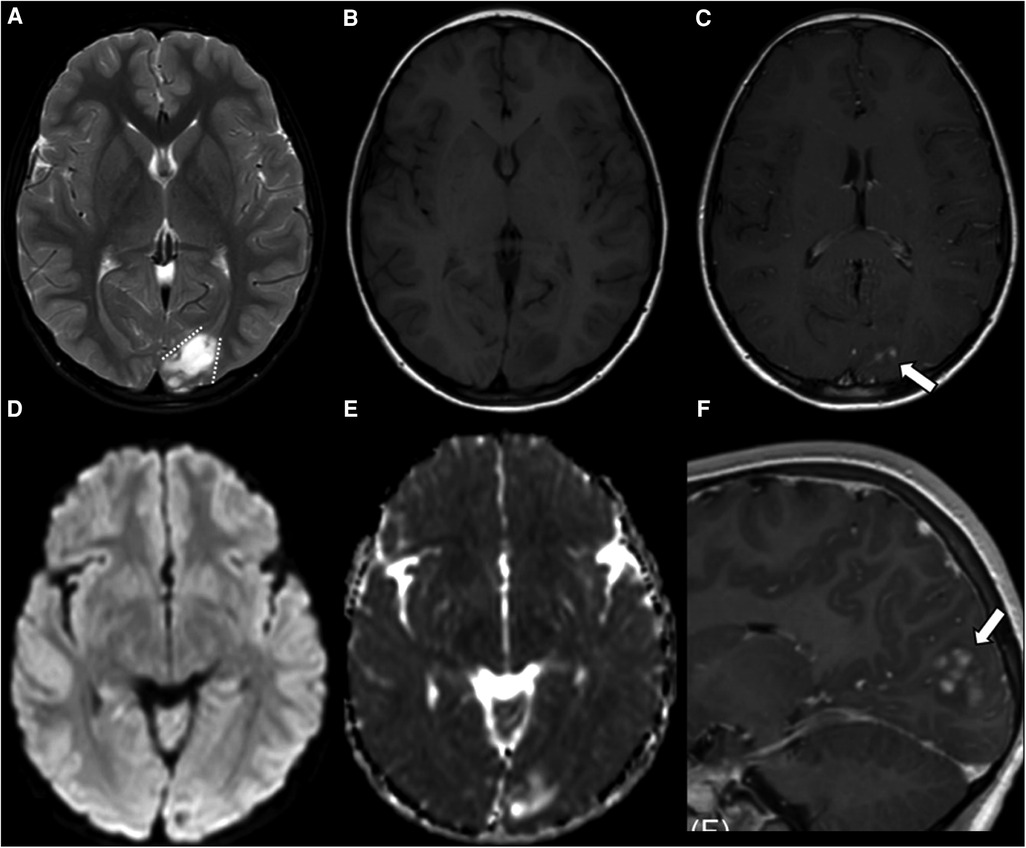
Figure 2. Supratentorial angiocentric glioma in a 10-year-old boy. The tumor shows high intensity on T2WI (A) and low intensity on T1WI (B). The stalk-like sign is observed without evidence of atrophy in the surrounding brain parenchyma (A, dotted lines). Diffusion restriction is not observed (D,E). Nodular enhancement is observed in the postcontrast sagittal T1WI (C,F, thick arrows). This figure was originally posted by Kurokawa et al. in the Journal of Neuroimaging in 2022 (7).
The differential diagnosis includes ganglioglioma, pleomorphic xanthoastrocytoma (PXA), and dysembryoplastic neuroepithelial tumor (DNET) (32). Gangliogliomas typically consist of cystic components and calcification, with approximately 50% demonstrating contrast enhancement. However, AGs generally lack such enhancement (19). On a CT scan, PXA shows hemorrhage more commonly, but calcification is rare (34). There is typically adjacent cortical thickening and fewer calcifications in DNET (10).
PLNTY is an epileptogenic tumor first described by Huse et al. in 2017, described as an epileptogenic histopathological tumor type with specific histological, immunohistochemical, and molecular profile combining an oligodendroglioma-like component, diffuse CD34 expression, and genetic alterations in the MAP kinase pathway (35).
An immunohistochemical examination reveals obvious differentiation of glial cells and intense positivity for CD34 and commonly shows abnormal MAPK pathway alterations. The expression of CD34 may be strong and diffuse or show focal distribution. Strong positivity for GFAP and OLIG2 could also be commonly seen. It is generally negative for IDH1, R132H, EMA, NeuN, and neuroendocrine markers, and the MIB-1 tag index is often low (<1%–2%). Molecular profiling of PLNTY shows that it carries a distinct DNA methylation signature, including a potential epigenetic subgroup defined by FGFR2 fusions. Molecular analysis of PLNTY includes genetic abnormalities involving either BRAF V600E mutation (≈40%) or FGFR 2/3 fusion (≈50%); common fusion genes include FGFR2-KIAA1598, FGFR2-CTNNA3, and FGFR-TACC3 (15).
Macroscopical examination reveals a generally well-circumscribed solid–cystic tumor with calcified and cystic components at the periphery that are unencapsulated. Histological examination reveals that characteristic manifestations of PLNTY are oligodendrocyte tumor-like components associated with other components, including fibrillary, fusiform, spindled, or pleomorphic astrocytic cells. Vague perivascular pseudorosettes are occasionally described, and the adjacent cortex may show evidence of FCD (36). Variable degrees of calcification are frequently observed in the tumor, which usually appears coarse and confluent.
PLNTY often occurs in children and young adults and shows slight female predominance (37). The median age of presentation is approximately 16 years (range: 4–57 years). The most prevalent clinical manifestation is epilepsy, also referred to as long-term epilepsy-associated brain tumors, which includes pilocytic astrocytoma, ganglioglioma, PXA, DNET, AG, pediatric oligodendroglioma, and other related conditions (23). Other clinical manifestations include headache, dizziness, and visual impairment mainly related to the tumor location.
Because of the very low proliferation rate, the WHO criteria currently classified PLNTY as grade 1. It often tends to exhibit a benign biological behavior and clinical course, most of which can be well controlled by gross total resection (36). However, a case report of malignant transformation of PLNTYs demonstrated FGFR3 (exon 18) and TACC3 (exon 11) fusion associated with additional somatic alterations in TP53, ATRX, PTEN, TEK, and RB1 consistent with more aggressive biology, typically seen in high-grade glioma (38). Therefore, although most cases are benign, long-term follow-up of PLNTY is warranted. Tateishi et al. (39) revealed that the activation of MAPK signaling and subsequent c-Myc induction, driven by the BRAF V600E mutation, elicit specific metabolic alterations in PLNTY cells. The superior potential of targeted drugs in unresectable regions is suggested.
The majority of PLNTY arises in the cerebral hemispheres, with a predilection for the temporal lobe, followed by the occipital lobe, frontal lobe, and parietal lobe. These regions of the brain are commonly located in the cortical and subcortical areas with clearly circumscribed margins. Approximately 80% of tumors are found in the temporal lobe, predominantly on the right side, but rarely in the ventricles (40). PLNTY has typical imaging features (Figures 3, 4) (41). On the CT scan, granular calcification in the tumor is one of the key radiological features. On magnetic resonance imaging (MRI), PLNTY shows the most commonly heterogeneous iso- to hypointense signal on T1WI and hyperintense signal on T2WI/FLAIR sequences with local slight or no enhancement after contrast enhancement (23). A cystic component can be present in the majority of PLNTY. No restricted diffusion is noted on the diffusion-weighted imaging sequence. Perfusion-weighted imaging shows increased focal cerebral blood volume. The “salt and pepper sign” on T2WI may be a distinctive manifestation of PLNTY, potentially attributed to calcification within the grit (42). In addition, cortical dysplasia is frequently observed in conjunction with low-grade tumors (43).
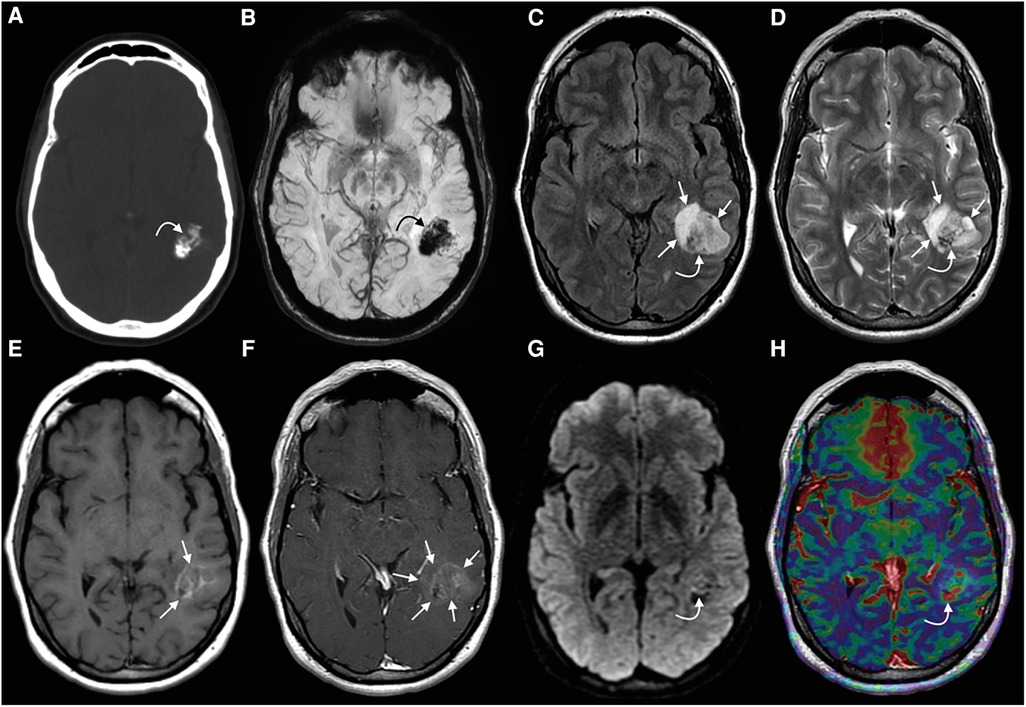
Figure 3. PLNTY. CT (A) image showing a mass within the left temporal lobe with dense intralesional calcifications, with a blooming signal on the corresponding SWI (B) (curved arrows in A and B). A heterogeneous signal is noted on both FLAIR (C) and T2WI (D) (curved arrows on C and D). A faint T1-hyperintense signal is noted in the central components of the tumor (straight arrows, E), while a greater extent of the mass demonstrates mild enhancement (straight arrows, F). A few tiny foci of mildly restricts diffusion were observed centrally (curved arrow, G). Some of the solid-appearing components demonstrates elevated relative CBV (curved arrow, H). This figure was originally posted by Benson et al. in AJNR (American Journal of Neuroradiology) in April 2020 (41).
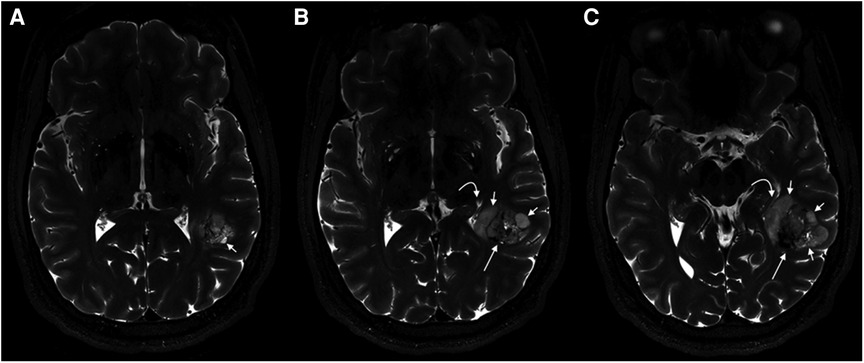
Figure 4. PLNTY. 7T MR imaging demonstrates internal characteristics of the mass on T2WI. From left to right (A–C), heterogeneous solid material is seen centrally (long straight arrows), while cystic components are located peripherally (short straight arrows). A mild associated mass effect is seen. This figure was originally posted by Benson et al. in AJNR (American Journal of Neuroradiology) in April 2020 (41).
PLNTY must be distinguished from ganglioblastoma, oligodendroglioma, and DNET, all of which are common in the temporal lobe (37). Ganglioblastoma is another epilepsy-relevant tumor, and it always appears as an isointense signal to the gray matter on T1WI (19, 44). Calcifications and cystic components are more common in PLNTYs than ganglioblastoma. There are usually more intratumoral septations and fewer calcifications in DNET (10). The characteristic gyriform calcification helps distinguish oligodendroglioma from PLNTY (23).
Diffuse low-grade glioma, MAPK pathway-altered is also a newly defined tumor type in the 2021 WHO CNS5, which requires a combination of histological evaluation and molecular characterization to diagnose. However, different from the other three types of pDLGG defined as WHO grade 1, the specific WHO grade classification of the diffuse low-grade glioma, MAPK pathway-altered is not assigned, which may be related to the small number of cases and lack of sufficient clinical course data.
Histological examination reveals minimal and atypical glial proliferation and diffuse astrocytic or oligodendroglial morphology, similar to other WHO grade 2 diffuse gliomas. In immunohistochemical examination, compared with angiocentric gliomas, diffuse low-grade glioma, MAPK pathway-altered lacks the characteristic growth pattern around the blood duct center, is EMA spot-positive, and shows MYB variation. In contrast to PLNTY, diffuse low-grade glioma, MAPK pathway-altered does not have a strong diffuse positive expression of CD34 (45). BRAF V600E mutation and FGFR1 alteration are commonly found upon molecular analysis. Common molecular alterations of diffuse low-grade glioma, MAPK pathway-altered include TKD duplication, FGFR1 mutation, FGFR1 fusion, BRAF V600E mutation, BRAF fusion, or BRAF insertion mutation (5).
MAPK pathway-altered is a rare tumor with a diffuse and invasive growth pattern, which mainly occurs in children, is commonly associated with epilepsy, and occasionally affects adults (26). Yang et al. (20) classified diffuse low-grade glioma, MAPK pathway-altered with BRAF V600E mutation into the intermediate risk group.
The imaging features of diffuse low-grade glioma, MAPK pathway-altered are related to the site and histological examinations. For tumors located in the cerebral cortical region, calcification is more common; hypointensity on the T1WI sequence and hyperintensity on the T2WI sequence with obvious and heterogeneous enhancement after contrast enhancement are typically seen (12). In some cases, both cystic and solid components can be observed instead of calcification. Atypically, no obvious enhancement was observed (Figure 5). The tumors in the diencephalon predominantly exhibit solid and lobular characteristics, demonstrating obvious and homogeneous enhancement without any signs of necrosis, edema, and space-occupying effects (46).
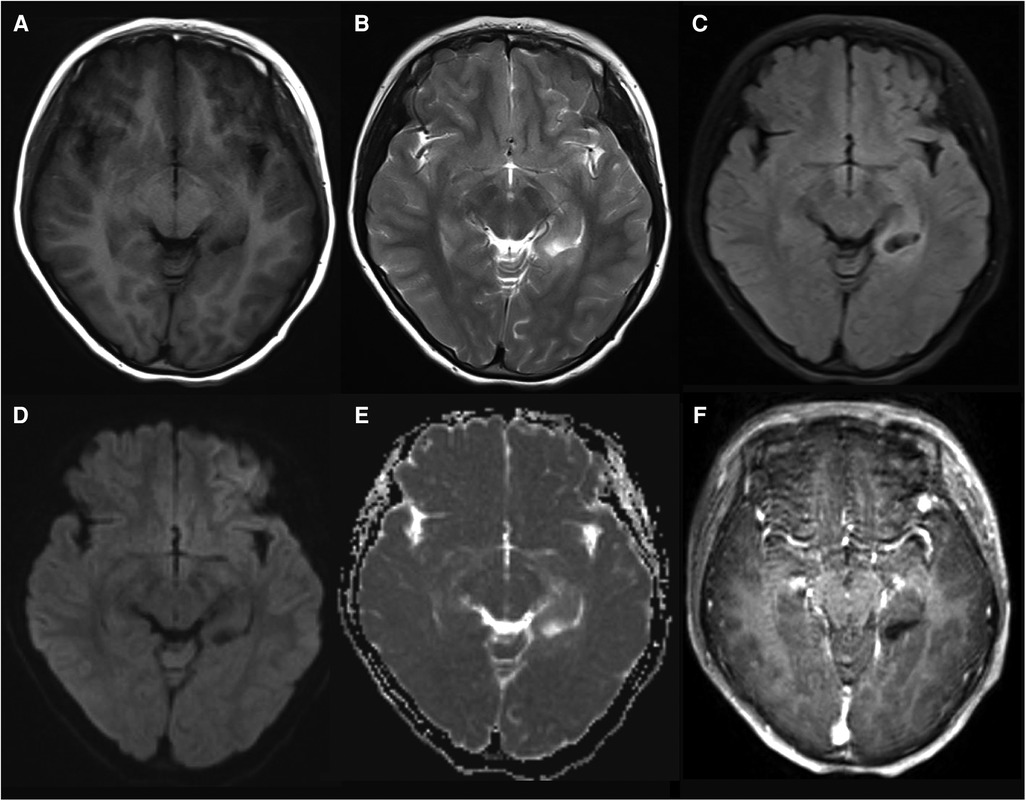
Figure 5. Pediatric-type diffuse low-grade glioma, MAPK pathway-altered in a 10-year-old girl. The tumor is located in the left hippocampus and shows hypointense on T1WI (A) and hyperintense on T2WI (B) and FLAIR (C). Diffusion is not restricted (D,E). No enhancement is observed (F).
The primary differential diagnosis includes ganglioblastoma, DNET, and PLNTY. Both ganglioblastoma and DNET have been previously mentioned. Granular calcification and the “salt and pepper sign” on T2WI are characteristic features of PLNTY. Furthermore, PLNTY exhibits minimal or negligible enhancement after contrast enhancement, while diffuse low-grade glioma, MAPK pathway-altered demonstrates conspicuous enhancement with contrast.
The revised tumor classification reflects the comprehensive understanding of CNS tumors by domain experts in light of current scientific knowledge. With the widespread implementation of novel detection techniques, an increasing number of novel molecular variants associated with tumors will be identified. Simultaneously, as the relevant clinical trials progress, our comprehension of the classification system for CNS tumors will be further enhanced.
MRI is the first choice and optimal imaging modality for evaluating pediatric CNS tumors. It accurately depicts various imaging features of the tumor, including size, margin, morphology, location, signal characteristics, mass effect, peritumoral edema, and enhancement features. CT is particularly sensitive in detecting subtle calcifications. In summary, we have highlighted the distinctive features of pDLGG to enhance the understanding of these tumor types and provide additional diagnostic information.
JC and XQ performed the conception and design; CW and CC provided the administrative support; JZ and TH performed the provision of patient materials; MZ performed the collection and assembly of data. All authors contributed to the article and approved the submitted version.
This work was supported by the Public Health and Technology Project of Tianjin (grant number TJWJ2021ZD007) and the Program of Tianjin Science and Technology Plan (grant number 21JCZDJC00390).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016World health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. (2016) 131(6):803–20. doi: 10.1007/s00401-016-1545-1
2. WHO Classifcation of Tumours Editorial Board. World health organization classifcation of tumours of the central nervous system. 5th ed. Lyon: International Agency for Research on Cancer (2021).
3. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. (2021) 23(8):1231–51. doi: 10.1093/neuonc/noab106
4. Lassaletta A, Zapotocky M, Bouffet E, Hawkins C, Tabori U. An integrative molecular and genomic analysis of pediatric hemispheric low-grade gliomas: an update. Childs Nerv Syst. (2016) 32(10):1789–97. doi: 10.1007/s00381-016-3163-6
5. Ellison DW, Hawkins C, Jones DTW, Onar-Thomas A, Pfister SM, Reifenberger G, et al. cIMPACT-NOW update 4: diffuse gliomas characterized by MYB, MYBL1, or FGFR1 alterations or BRAF(V600E) mutation. Acta Neuropathol (2019) 137(4):683–7. doi: 10.1007/s00401-019-01987-0
6. Suh YY, Lee K, Shim YM, Phi JH, Park CK, Kim SK, et al. MYB/MYBL1:QKI fusion-positive diffuse glioma. J Neuropathol Exp Neurol. (2023) 82(3):250–60. doi: 10.1093/jnen/nlac123
7. Kurokawa R, Baba A, Emile P, Kurokawa M, Ota Y, Kim J, et al. Neuroimaging features of angiocentric glioma: a case series and systematic review. J Neuroimaging. (2022) 32(3):389–99. doi: 10.1111/jon.12983
8. Wang H, Zhu J, Zhu P, Luo C. Angiocentric glioma: a case report and review of the literature. J Clin Neurosci. (2021) 94:179–85. doi: 10.1016/j.jocn.2021.10.016
9. Kalelioglu T, Rama B, Cho BB, Lopes BM, Patel SH. Pediatric-type diffuse low-grade glioma with MYB/MYBL1 alteration: report of 2 cases. Neuroradiol J. (2022) 36(2):232–5. doi: 10.1177/19714009221126015
10. Kurokawa M, Kurokawa R, Capizzano AA, Baba A, Ota Y, Pinarbasi E, et al. Neuroradiological features of the polymorphous low-grade neuroepithelial tumor of the young: five new cases with a systematic review of the literature. Neuroradiology. (2022) 64(6):1255–64. doi: 10.1007/s00234-021-02879-5
11. Danyeli AE, Altinoz MA, Sari R, Elmaci I. Polymorphous low-grade neuroepithelial tumor of the young: a detailed pathomolecular analysis and discussion of a case. Clin Neuropathol. (2021) 40(5):271–8. doi: 10.5414/NP301370
12. Fukuoka K, Mamatjan Y, Ryall S, Komosa M, Bennett J, Zapotocky M, et al. BRAF V600e mutant oligodendroglioma-like tumors with chromosomal instability in adolescents and young adults. Brain Pathol. (2020) 30(3):515–23. doi: 10.1111/bpa.12799
13. Wefers AK, Stichel D, Schrimpf D, Coras R, Pages M, Tauziede-Espariat A, et al. Isomorphic diffuse glioma is a morphologically and molecularly distinct tumour entity with recurrent gene fusions of MYBL1 or MYB and a benign disease course. Acta Neuropathol. (2020) 139(1):193–209. doi: 10.1007/s00401-019-02078-w
14. Ryall S, Tabori U, Hawkins C. Pediatric low-grade glioma in the era of molecular diagnostics. Acta Neuropathol Commun. (2020) 8(1):30. doi: 10.1186/s40478-020-00902-z
15. Purkait S, Mahajan S, Sharma MC, Sarkar C, Suri V. Pediatric-type diffuse low grade gliomas: histomolecular profile and practical approach to their integrated diagnosis according to the WHO CNS5 classification. Indian J Pathol Microbiol. (2022) 65(Supplement):S42–9. doi: 10.4103/ijpm.ijpm_1043_21
16. Nikolaus M, Koch A, Stenzel W, Elezkurtaj S, Sahm F, Tietze A, et al. Atypical NMDA receptor expression in a diffuse astrocytoma, MYB- or MYBL1-altered as a trigger for autoimmune encephalitis. Acta Neuropathol. (2022) 144(2):385–9. doi: 10.1007/s00401-022-02447-y
17. Chiang J, Harreld JH, Tinkle CL, Moreira DC, Li X, Acharya S, et al. A single-center study of the clinicopathologic correlates of gliomas with a MYB or MYBL1 alteration. Acta Neuropathol. (2019) 138(6):1091–2. doi: 10.1007/s00401-019-02081-1
18. Bag AK, Chiang J, Patay Z. Radiohistogenomics of pediatric low-grade neuroepithelial tumors. Neuroradiology. (2021) 63(8):1185–213. doi: 10.1007/s00234-021-02691-1
19. Quiroz Tejada AR, Miranda-Lloret P, Llavador Ros M, Plaza Ramirez E, Pancucci G, Roca Barber A, et al. Gangliogliomas in the pediatric population. Childs Nerv Syst. (2021) 37(3):831–7. doi: 10.1007/s00381-020-04900-3
20. Yang RR, Aibaidula A, Wang WW, Chan AK, Shi ZF, Zhang ZY, et al. Pediatric low-grade gliomas can be molecularly stratified for risk. Acta Neuropathol. (2018) 136(4):641–55. doi: 10.1007/s00401-018-1874-3
21. Thomas DL. 2021 Updates to the world health organization classification of adult-type and pediatric-type diffuse gliomas: a clinical practice review. Chin Clin Oncol. (2023) 12(1):7. doi: 10.21037/cco-22-120
22. AlRayahi J, Alwalid O, Mubarak W, Maaz AUR, Mifsud W. Pediatric brain tumors in the molecular era: updates for the radiologist. Semin Roentgenol. (2023) 58(1):47–66. doi: 10.1053/j.ro.2022.09.004
23. Chen Y, Tian T, Guo X, Zhang F, Fan M, Jin H, et al. Polymorphous low-grade neuroepithelial tumor of the young: case report and review focus on the radiological features and genetic alterations. BMC Neurol. (2020) 20(1):123. doi: 10.1186/s12883-020-01679-3
24. Kurokawa R, Baba A, Kurokawa M, Pinarbasi ES, Makise N, Ota Y, et al. Neuroimaging features of diffuse hemispheric glioma, H3 G34-mutant: a case series and systematic review. J Neuroimaging. (2021) 32(1):17–27. doi: 10.1111/jon.12939
25. Marlow C, Cuoco JA, Hoggarth AR, Stump MS, Apfel LS, Rogers CM. Pediatric diffuse hemispheric glioma H3 G34-mutant with gains of the BRAF locus: an illustrative case. Rare Tumors. (2023) 15:203636132311687. doi: 10.1177/20363613231168704
26. Bale TA, Rosenblum MK. The 2021 WHO classification of tumors of the central nervous system: an update on pediatric low-grade gliomas and glioneuronal tumors. Brain Pathol. (2022) 32(4):e13060. doi: 10.1111/bpa.13060
27. Wang Q, Xiong Y, Chen J, Shao Q. Cystic angiocentric glioma: a case report and literature review. Childs Nerv Syst. (2021) 37(8):2701–5. doi: 10.1007/s00381-020-04882-2
28. Ampie L, Choy W, DiDomenico JD, Lamano JB, Williams CK, Kesavabhotla K, et al. Clinical attributes and surgical outcomes of angiocentric gliomas. J Clin Neurosci. (2016) 28:117–22. doi: 10.1016/j.jocn.2015.11.015
29. Park YW, Vollmuth P, Foltyn-Dumitru M, Sahm F, Ahn SS, Chang JH, et al. The 2021 WHO classification for gliomas and implications on imaging diagnosis: part 2-summary of imaging findings on pediatric-type diffuse high-grade gliomas, pediatric-type diffuse low-grade gliomas, and circumscribed astrocytic gliomas. J Magn Reson Imaging. (2023) 58(3):690–708. doi: 10.1002/jmri.28740
30. Almubarak AO, Alahmari A, Hindi HA, AlShail E. Angiocentric glioma of brainstem. Neurosciences. (2021) 25(5):416–20. doi: 10.17712/nsj.2020.5.20200026
31. Chiang J, Diaz AK, Makepeace L, Li X, Han Y, Li Y, et al. Clinical, imaging, and molecular analysis of pediatric pontine tumors lacking characteristic imaging features of DIPG. Acta Neuropathol Commun. (2020) 8(1):57. doi: 10.1186/s40478-020-00930-9
32. Han G, Zhang J, Ma Y, Gui Q, Yin S. Clinical characteristics, treatment and prognosis of angiocentric glioma. Oncol Lett. (2020) 20(2):1641–8. doi: 10.3892/ol.2020.11723
33. Zhang R, Xu X, Zhou H, Yao D, Wei R, Muhammad S. Pediatric angiocentric glioma with acute intracerebral hemorrhage: a case report with 36 months follow-up. Surg Neurol Int. (2021) 12:499. doi: 10.25259/SNI_791_2021
34. Takamine Y, Yamamuro S, Sumi K, Ohta T, Shijo K, Nakanishi Y, et al. A case of pleomorphic xanthoastrocytoma with intracranial hemorrhage in a child. NMC Case Rep J. (2019) 6(1):39–42. doi: 10.2176/nmccrj.cr.2018-0174
35. Huse JT, Snuderl M, Jones DT, Brathwaite CD, Altman N, Lavi E, et al. Polymorphous low-grade neuroepithelial tumor of the young (PLNTY): an epileptogenic neoplasm with oligodendroglioma-like components, aberrant CD34 expression, and genetic alterations involving the MAP kinase pathway. Acta Neuropathol. (2017) 133(3):417–29. doi: 10.1007/s00401-016-1639-9
36. Gupta VR, Giller C, Kolhe R, Forseen SE, Sharma S. Polymorphous low-grade neuroepithelial tumor of the young: a case report with genomic findings. World Neurosurg. (2019) 132:347–55. doi: 10.1016/j.wneu.2019.08.221
37. Lelotte J, Duprez T, Raftopoulos C, Michotte A. Polymorphous low-grade neuroepithelial tumor of the young: case report of a newly described histopathological entity. Acta Neurol Belg. (2020) 120(3):729–32. doi: 10.1007/s13760-019-01241-0
38. Bale TA, Sait SF, Benhamida J, Ptashkin R, Haque S, Villafania L, et al. Malignant transformation of a polymorphous low grade neuroepithelial tumor of the young (PLNTY). Acta Neuropathol. (2021) 141(1):123–5. doi: 10.1007/s00401-020-02245-4
39. Tateishi K, Ikegaya N, Udaka N, Sasame J, Hayashi T, Miyake Y, et al. BRAF V600e mutation mediates FDG-methionine uptake mismatch in polymorphous low-grade neuroepithelial tumor of the young. Acta Neuropathol Commun. (2020) 8(1):1–8. doi: 10.1186/s40478-020-01023-3
40. Bitar M, Danish SF, Rosenblum MK. A newly diagnosed case of polymorphous low-grade neuroepithelial tumor of the young. Clin Neuropathol. (2018) 37(07):178–81. doi: 10.5414/np301081
41. Benson JC, Summerfield D, Carr C, Cogswell P, Messina S, Gompel JV, et al. Polymorphous low-grade neuroepithelial tumor of the young as a partially calcified intra-axial mass in an adult. AJNR Am J Neuroradiol. (2020) 41(4):573–8. doi: 10.3174/ajnr.A6500
42. Johnson DR, Giannini C, Jenkins RB, Kim DK, Kaufmann TJ. Plenty of calcification: imaging characterization of polymorphous low-grade neuroepithelial tumor of the young. Neuroradiology. (2019) 61(11):1327–32. doi: 10.1007/s00234-019-02269-y
43. Prayson RA, Gales JM. Coexistent ganglioglioma, focal cortical dysplasia, and hippocampal sclerosis (triple pathology) in chronic epilepsy. Ann Diagn Pathol. (2015) 19(5):310–3. doi: 10.1016/j.anndiagpath.2015.07.003
44. Nafe R, Porto L, Samp PF, You SJ, Hattingen E. Adult-type and pediatric-type diffuse gliomas: what the neuroradiologist should know. Clin Neuroradiol. (2023) 33(3):611–24. doi: 10.1007/s00062-023-01277-z
45. Ryall S, Zapotocky M, Fukuoka K, Nobre L, Guerreiro Stucklin A, Bennett J, et al. Integrated molecular and clinical analysis of 1,000 pediatric low-grade gliomas. Cancer Cell. (2020) 37(4):569–83.e5. doi: 10.1016/j.ccell.2020.03.011
Keywords: brain tumor, gliomas, pediatric-type diffuse low-grade gliomas, 2021 WHO classification, neuroimaging
Citation: Chen J, Qi X, Zhang M, Zhang J, Han T, Wang C and Cai C (2023) Review on neuroimaging in pediatric-type diffuse low-grade gliomas. Front. Pediatr. 11:1149646. doi: 10.3389/fped.2023.1149646
Received: 22 January 2023; Accepted: 22 September 2023;
Published: 18 October 2023.
Edited by:
Fabrício Guimarães Gonçalves, Children’s Hospital of Philadelphia, United StatesReviewed by:
Flavio Giordano, University of Florence, Italy© 2023 Chen, Qi, Zhang, Zhang, Han, Wang and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunxiang Wang dGpjaGN0QDE2My5jb20= Chunquan Cai Y3FjbnM2QDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.