- 1Allergy Unit, Department of Pediatrics, Meyer Children's University Hospital, Florence, Italy
- 2Pediatric Department, Latisana-Palmanova Hospital, Azienda Sanitaria Universitaria Friuli Centrale, Udine, Italy
- 3Department of Pediatrics, Salesi Children's Hospital, AOU Ospedali Riuniti Ancona, Ancona, Italy
- 4Pediatric Clinic, Department of Pediatrics, Fondazione IRCCS Policlinico San Matteo, University of Pavia, Pavia, Italy
- 5Translational Research in Pediatric Specialties Area, Division of Allergy, Bambino Gesù Children's Hospital (IRCCS), Rome, Italy
- 6Pediatric Unit and Emergency, University Hospital Consortium Corporation Polyclinic of Bari, Pediatric Hospital Giovanni XXIII, Bari, Italy
- 7Pediatric Unit, Department of Surgical Sciences, Dentistry, Gynecology and Pediatrics, University of Verona, Verona, Italy
- 8Department of Human Pathology in Adult and Development Age “Gaetano Barresi”, Allergy Unit, Department of Pediatrics, AOU Policlinico Gaetano Martino, Messina, Italy
- 9Sorbonne Universités, Service de Dermatologie et d'Allergologie, Hôpital Tenon, Paris HUEP, APHP, Paris, France
Linear Immunoglobulin A Bullous Disease (LABD) is a rare dermatosis whose pathomechanisms are not yet completely understood. LABD has different features characterizing adults and children in terms of potential triggers, clinical manifestations, and prognosis. The aim of the present study is to review all neonatal and pediatric cases of LABD and summarize the major characteristics. Childhood LABD is mainly idiopathic with a benign prognosis. Neonatal cases are difficult to differentiate from infectious diseases and usually have a poor prognosis. Drugs are one of the possible triggers that can activate autoimmune responses through antigen mimicry and epitope spreading as well as different stimuli (e.g., infections, inflammatory diseases, trauma). The gold standard for the diagnosis is based on direct immunofluorescence. Prognosis is generally favorable but often depends on the prompt dermatological diagnosis, treatment and follow-up guaranteed by a multidisciplinary team, including pediatricians for this group of age.
Introduction
Linear Immunoglobulin A Bullous Disease (LABD) is a rare cutaneous disease characterized by subepidermal linear deposition of immunoglobulin A (IgA) along the basement membrane zone. LABD was first described in the late 1970s as Chronic Bullous Dermatosis of Childhood (1).
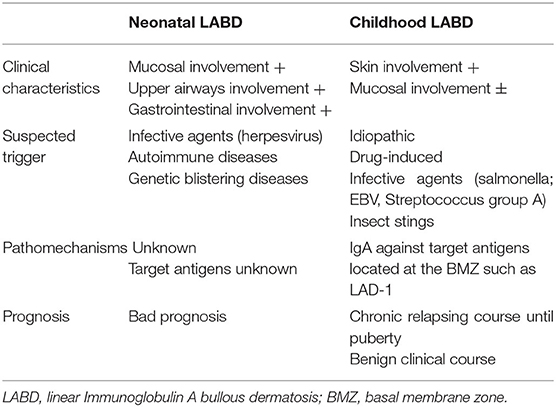
The two forms of the disease, one found in adults and one in children, have different clinical features but share the same immune-pathogenesis and microscopic findings (2). In children, it is more frequently idiopathic, but some drug-induced cases have been reported (3–8). Concomitant infections or malignancy are more often reported in adults, but their role has not yet been clearly defined. The pediatric form has a peak onset age of 4.5 years old (2), although it may also affect newborns, with a worse prognosis (9–13).
Epidemiology
Incidence is estimated to be 0.2–2.3 cases per million/year (14), and it seems to be higher in South Africa, North Africa, and Asia (15–18). So far, no gender or ethnicity prevalence has been observed. A PubMed research has been performed using the specific research strings, retrieving a total of 145 items for “linear IgA bullous dermatosis, humans, English, child: birth−18 years, 1976–2022” and 169 items for “linear IgA bullous disease, humans, English, child: birth−18 years, 1976–2022.” Most of these are case reports or small case series. Despite this lack of knowledge, LABD is the most frequent chronic autoimmune blistering disease in children (10, 19–22).
Pediatric Case Series
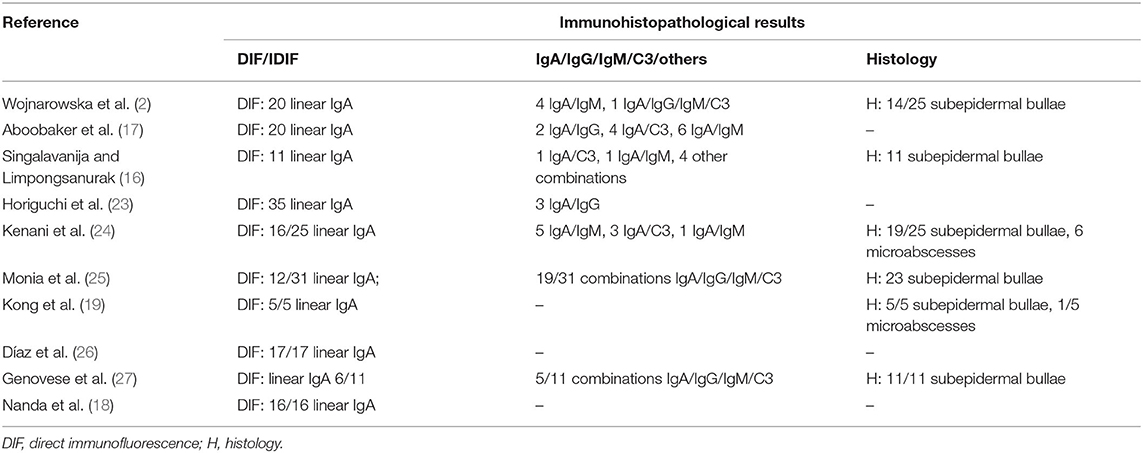
Spontaneous or drug-induced pediatric case series with complete clinical data are summarized in Tables 1, 2. Among a case series of 54 patients with the autoimmune bullous disease, there were 25 children (with a mean age of 4.5 years) who had a chronic bullous disease during childhood (2). Wojnarowska et al. (2) emphasized that 38% of children in this study had a previous infection (in the upper respiratory tract), and 31% of all patients were exposed to a drug (these were mainly anti-inflammatory drugs; these data were not reported for the pediatric subgroup). Some other case reports have been published reporting toddlers or young children with LABD mainly induced by antibiotics (3–8). Since vancomycin is not frequently used among children, it is more frequently reported as a causative drug in adults.
Neonatal Case Series
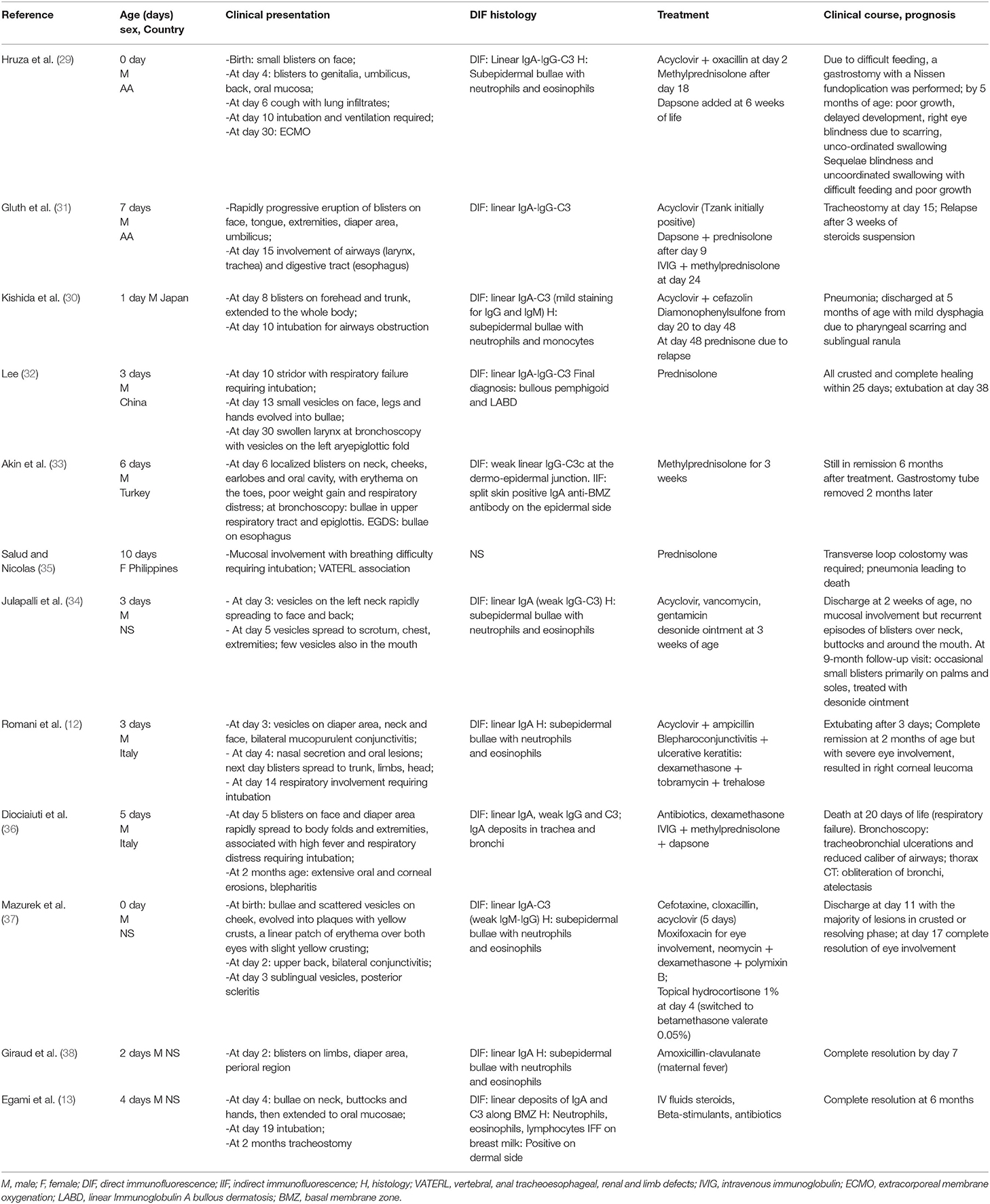
Some special considerations must be given for the neonatal age (Table 3). As reported above, newborns could be affected during the first days of life; unlike other bullous dermatoses, mothers usually have no clinical manifestations at all (28). The first neonatal case report dates back to 1993 (29), and some other have been published since then (12, 13, 30–38). Some case reports in newborns showed a worse outcome, even a deadly one (36), compared to older children, primarily due to respiratory (13, 36), eye (12), gastrointestinal involvement (31).
Pathology
The typical hallmark of LABD is the deposition along the basement membrane zone (BMZ) of the serum immunoglobulin (Ig) A, which binds to specific antigens located at the dermo-epidermal junction, eventually causing subepidermal blistering (39). It has been demonstrated that in most patients with LABD the main IgA belongs to the subclass A1 (40), although in some patients, IgA2 antibodies have been detected against LABD97 with heterogeneous clinical characteristics in terms of disease onset, duration, gastrointestinal signs and symptoms, or malignancies (40).
The major target antigens are the 120-kDa (LAD-1) and 97-kDa (LABD97) proteins, both parts of ectodomains of BP180 (collagen XVII), and there are cases with autoantibodies against collagen VII, BP230, α6β4 integrin, laminin, and other proteins (39, 41, 42). Target antigens in neonatal LABD are very poorly known. No target antigen has been identified in two patients who underwent immunoblotting studies (12, 33). According to immunoelectron microscopy, LABD shows three patterns of IgA deposition: sub-lamina dense deposition, lamina lucida deposition, and deposition in both layers (43–46). The antigens subset indicates a relationship between Bullous Pemphigoid (BP) and LABD that could be explained by “the epitope spreading phenomenon” in which the primary disease, such as ordinary BP, exposes the BMZ to the immune system, which induces autoantibodies against BMZ proteins without pathogenicity. For that reason, BP and LABD may overlap in infantile patients with mucosal involvement (47). LABD histologic and therapeutic findings are similar to those associated with BP and dermatitis herpetiformis (DH), even if LABD and DH are two different diseases in terms of patho-mechanisms and clinical features. LABD can be distinguished from BP and DH by direct immunofluorescence (IF), a demonstration of linear IgA deposits along the basement membrane zone (BMZ). On the contrary, BP has a linear IgG BMZ deposition. Sometimes in LABD, complement, IgG and IgM deposits are described in the BMZ. Indirect IF is frequently negative.
Pathogenesis
Genetic
As in DH, a strong association with HLA B8, CW7, and DR3 was reported, without association with gluten-sensitive enteropathy (2, 15, 48, 49). DH is more frequently associated with gluten-sensitive enteropathy and is characterized by granular IgA deposits in the dermal papillae, in direct IF, and the mucosa is not involved. Jejunal atrophy has been described in only one child with LABD (25). In addition, LABD is more likely to happen if these genes are present in a homozygote state (5). HLA B8 and HLADR3 haplotypes were positive in 80% of Tunisian children (24), and in a few studies the positivity of HLA B8 haplotype was associated with a good prognosis (2, 15, 50). Moreover, HLA-DR3-Q2 is considered high risk for type 1 diabetes mellitus (DM) (51), and this genetic pre-disposition, combined with environmental triggers, could probably explain the onset of DM in a child with LABD (52). At last, the role of the TNF2 gene has been described in the duration of the disease, and it was associated with a worse prognosis (53).
Infections
Most cases of childhood LABD are idiopathic, but infections, medications, skin traumas, and malignancies are potential inducers of LABD. The hypothesis of an infective agent triggering LABD as a result of an immunologic reaction involving IgA has been greatly discussed in the literature. So far, different infectious agents such as Salmonella enteritis (54), non-specific gastrointestinal infection, and Epstein Barr virus infections (55) have been related to LABD in childhood. It is not known if the development of IgA is due to the infection, treatment or both these triggers. Two cases have been reported with an upper respiratory tract infection preceding LABD. In one case, Streptococcus group A was detected in the skin lesions (56). LABD has also been described as following viral hepatitis, post-streptococcal glomerulonephritis, and beta-hemolytic streptococcal throat infections in children (18).
Drugs
Drug-induced LABD may result from stimulation of the immune system to produce IgA in a susceptible individual. The drugs may disrupt the lamina lucida's antigenicity and act as a hapten, complexing with derma/epidermal proteins and eliciting an autoimmune response. IgA-stimulated neutrophils chemotaxis then results in the formation of neutrophilic microabscesses at the dermo-epidermal junction (14, 57). The interval between drug intake and LABD development ranges from 2 to 28 days and is usually characterized by rapid recovery after stopping the drug. Compared to adults, drug-induced LABD is less frequent in children, with two reports of amoxicillin-clavulanic acid leading to the development of the disease and one case in which remission occurred after cessation of the medication (3, 8). Such reactions to amoxicillin, minocycline, and vibramycin have also been reported in children aged 3, 15, and 17, respectively (58). A retrospective study identified 25 Tunisian children with LABD, and a 6-month-old infant developed LABD 10 days after beginning treatment with vancomycin for bronchopulmonary infection (24). In a report including childhood LABD, childhood cicatricial pemphigoid, and adult LABD, 31% of the patients had taken non-steroidal anti-inflammatory drugs or antibiotics, but no details were provided on which group had received these medications before the eruption. In the same paper, 38% of the children and 26% of the adults had a preceding infection (2). A penicillin-induced LABD has been described in a neonatal case, although recurrence of blistering after drug discontinuation made the diagnosis doubtful (34). Recovery of drug-induced LABD in adults has been reported to take up to several months after discontinuation of an associated agent. Also, a few pediatric cases of drug-induced LABD had a longer duration, such as the one triggered by amoxicillin-clavulanic acid in a 2.5-year-old girl described by Mori et al. (3) in which the recovery took 6 months. Recently, a pediatric case of cephalosporin-induced LABD has been described in a 4-year-old girl after 7 days of antibiotic treatment for a urinary tract infection (5). However, in this case, the concomitant role of the drug and infection in triggering the disease cannot be excluded. In adults, drug-induced LABD cases were described as more severe, resembling toxic epidermal necrolysis (59). In adult case studies of drug-induced LABD, it was found that a minority of patients (20%) have circulating antibodies (60). This contrasts with idiopathic LABD, in which indirect immunofluorescence (IIF) is positive in up to 80% of patients (61). This difference may imply a milder severity as well as a better prognosis for drug-induced LABD. The same conclusion has also been proposed for childhood LABD.
Underlying Diseases
Association between LABD and underlying diseases has been frequently reported in adults. However, underlying disease has been associated in pediatric patients: ulcerative colitis (62), autoimmune lymphoproliferative syndrome (63), and Crohn's disease (18). Immune activation secondary to the exposition of multiple intestinal epithelial antigens, including BP180, in patients with coexistence of LABD and autoinflammatory bowel disease, has been hypothesized as a possible patho-mechanism eliciting blister formation (64). A case of LABD has been described in a child with pancreatic lipase deficiency (65) and in a child with Gilbert's syndrome (18). Confounding underlying conditions, possible triggering therapies have been reported in pediatric cases of LABD, such as two cases with lymphoblastic leukemia in remission (66) or idiopathic congenital thrombocytopenia and cytomegalovirus infection in children treated with trimethoprim-sulfamethoxazole (7). In one case, the diagnosis of drug-induced LABD was confirmed by a positive cotrimoxazole provocation test (7). To our knowledge, only a case of LABD induced by insect bite in a 22-month-old Chinese girl has been reported in pediatric population (67).
Vaccinations
Concerning vaccinations as a LABD trigger in children, two cases have been documented after flu/varicella vaccination (26) and two cases after quadrivalent human papillomavirus vaccine (qHPV), respectively in a 14 years old Italian girl (68) and in a 16-year-old Japanese girl (69). It was assumed that, as in rhesus macaques, the qHPV vaccine induces a Th2-skewed immune response with high titers of IgA and IgG1 antibodies, and a high level of IgA could also occur in humans after qHPV vaccine (69). Two cases after SARS-CoV2 vaccination have been reported in adults, one after second dose of AstraZeneca vaccine (70) and one after Pfizer vaccine (71).
Special Consideration for Neonatal Cases
Today, few cases of neonatal LABD have been described, and the pathomechanism of neonatal LABD is unknown mainly because no data are available for the target antigens. Circulating IgA antibodies against BMZ can be detected, but the origin of IgA deposits in the skin of neonates with LABD remains speculative, as the low level of IgA detected in neonatal cord blood may be produced by the mother or the child (72). Moreover, in all neonatal cases, maternal disease or symptoms were absent during pregnancy, and this fact is striking compared with the mother's frequent involvement in other bullous diseases. It has been postulated that the lower transfer of IgA across the placenta may explain this fact (28). Recently it has been published a case-report of a newborn who developed LABD due to transfer of maternal IgA via breast milk. Authors have been able to demonstrate the presence of IgA reactive against BMZ in breast milk, and symptoms disappeared after suspension of breastfeeding (13).
Clinical Manifestations
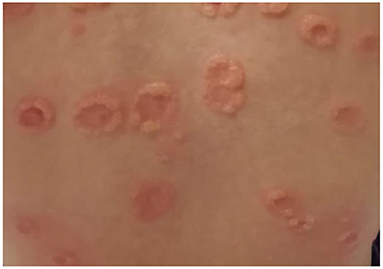
The childhood variant involves a sudden eruption of clear or serum-hematic vesicles and blisters on normal or erythematous skin with a typical onset at the abdominal and perioral areas (14, 73, 74). The face (eyes, mouth), hands, and feet may also be affected (75). Skin lesions may be accompanied by pruritus of variable intensity. Commonly there are excoriations and crusted papules, especially in cases of intense itching (76). New blisters develop at the periphery of resolving lesions, resulting in an annular or “rosette-like” pattern (“crown of jewels” or “string of pearls”) (Figures 1, 2, with the permissions of a parents' patient evaluated at Meyer Pediatric Hospital, Florence, Italy).
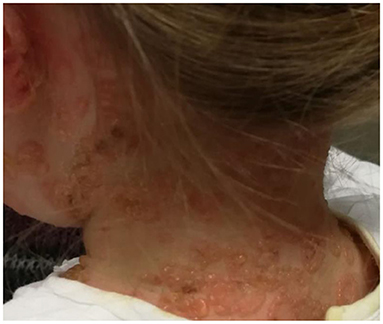
Figure 1. Typical lesions of LABD known as “string of pearls” in a girl evaluated at Meyer's Children Hospital (Florence).
Lesions may be symmetrically or asymmetrically distributed (77). Data about mucosal involvement are controversial; it has been reported from 8.3% (15) of cases up to 74% (50) of cases. Mucosal involvement, typically of the oral mucosa and conjunctiva, was reported in 64% of the childhood variant cases in Egan et al. study (78) but was rarer (12.9%) in other studies in children (23–25). Mucosal involvement is believed to be less frequent in children than in adults (25, 53), but in a recent study, mucosal involvement was more frequent in children than in adults (27). Even so far, only a few neonatal cases of LABD have been reported in the literature, and mucosal involvement seems to be characteristic of these cases (12, 29–34, 37). Typically, the onset of neonatal LABD is within 10–16 days of life, and in all cases, the skin is affected first. In only one case, lesions were documented at delivery (37). However, skin lesions seem to resolve quickly in neonates. On the other hand, the mucous membrane is involved later but persists longer than skin blisters. Oral lesions consist in erosions and painful ulcerations, desquamative gingivitis, and cicatricial lesions (14). Chronic conjunctivitis can lead to symblepharon, trichiasis, shrinkage of fornices, and even leucoma or blindness (12). Organ involvement, particularly upper airway involvement and less frequent involvement of the gastrointestinal tract, is reported in neonatal cases of LABD except for one (34). Eight patients ended with intubation, and in two cases, tracheostomy was necessary (12, 13, 29–33, 35, 36). Death due to respiratory distress was reported in one patient who also was affected by vertebral, anal, tracheoesophageal, renal and limb defects (VATERL) syndrome, and hypoplastic nasal sinuses (35). Neonatal cases of LABD can show feeding difficulties due to esophageal involvement requiring gastrostomy and ending with permanent scars in a few cases (30, 31).
Diagnosis
The diagnosis of LABD is established on clinical, histopathological, and immunological data.
Although direct immunofluorescence (DIF) remains the gold standard for diagnosis, the clinician should complete a diagnosis profiling with serum laboratory tests (such a circulating antibodies, complement fractions), serological tests for infective agents, specific exams for autoimmune diseases, complete blood counts, inflammatory and infection biomarkers (such as RCP, ERS).
Histopathological findings are not specific for LABD. Skin biopsy sample usually reveals subepidermal bullae with a predominantly neutrophilic inflammatory infiltrate and sparse eosinophils and lymphocytes. In drug-induced LABD, focal necrotic keratinocytes are more frequent than in idiopathic cases, but the difference is not statistically significant (59). Microabscesses in the dermal papillae can occasionally be seen (25). Laboratory tests are usually normal, although hypereosinophilia is evidenced in some patients (24).
Immunofluorescence studies are suitable, as LABD has many overlapping features with other bullous dermatoses (79). DIF on perilesional skin, shows isolated or predominant continuous IgA deposits at the dermo-epidermal junction (80). Complement fraction 3 (C3c), IgG and/or IgM deposits along the basement membrane zone (BMZ) can also be present at the same time as IgA antibodies (27, 81, 82). There are no specific immunofluorescence patterns that are peculiar for idiopathic rather than drug-induced LABD (59), although, in the latter, linear deposition of C3 at the BMZ is evidenced in almost 1/3 of the cases (83, 84).
A heterogeneous humoral response characterizes LABD. Indirect immunofluorescence (IIF) with salt-split skin was performed to detect circulating autoantibodies anti-BMZ, and its sensitivity ranges between 30% and 50% (26). However, as reported by Antiga et al. (39), circulating IgA are not an exclusive finding in LABD since they could be detected in other subepidermal blistering diseases too (85, 86). Moreover, in LABD, circulating IgG anti-BMZ could be found too (87, 88).
Many cases report associations between LABD and drugs, but there is inadequate evidence of proven causality of them (89). In order to obtain a correct diagnosis, the use of algorithms (e.g., Naranjo score) (59) to evaluate the probability of ADR could be helpful. In particular, a cause–effect relationship is considered when there is definitive evidence that the adverse event occurred after the medication was started, as well as the adverse event diminishes or disappears at any time after stopping the medication.
Provocation tests could be helpful for diagnosis, but they are not usually performed (59) because a severe recurrence (i.e., shorter latency and longer disease course) can occur in rechallenged patients (83).
The primary differential diagnoses in LABD are DH, bullous impetigo, herpes simplex, scabies, arthropod bites, parasitic infections, drug eruptions, dermatitis herpetiformis, and erythema multiforme. Frequently in neonatal cases, the first suspected diagnosis is a bacterial or viral infection (i.e., herpes virus) and most of them received as first treatment intravenous acyclovir plus antibiotics. The differential diagnosis of neonatal blistering includes infectious, autoimmune, and genetic blistering conditions (Table 4).
Treatment
The primary treatment of LABD is initially dapsone 0.5 mg/kg/ day, which is gradually increased until signs resolve and symptoms are controlled (usually up to 2 mg/kg/day) (9, 10), with a quick response in most of the cases (18, 24, 25, 90). It is effective as monotherapy or in combination with corticosteroids, antibiotics, or colchicine (26). Treatment with dapsone can have adverse effects such as hemolysis in glucose-6-phosphate dehydrogenase (G6PD) deficient patients, methemoglobinemia, bone marrow suppression, and peripheral neuropathy. Therefore, screening for G6PD deficiency must be performed before treatment initiation and levels of methemoglobin, and the blood count with reticulocytes must be monitored regularly (10).
Corticosteroids
For better disease control, some patients require a low dose of prednisone (0.5 mg/kg/day) in order to suppress blister formation or when there is severe mucosal involvement; on the other hand, the use of corticosteroids should generally be avoided in children because of its long-term side-effects (24).
Sulfapyridine has also been used (91) in patients with G6PD deficiency, or in cases of intolerance to dapsone or sulfapyridine, colchicine (usually 0.6 mg twice daily) can be considered as a possible alternative (9, 92).
Antibiotics
Antibiotics, including erythromycin, oxacillin, flucloxacillin, sulfamethoxypyridazine, and cotrimoxazole, have also been used with variable results (9, 10, 93) even though tetracyclines are not suitable in children <8 years due to the risk of permanent tooth discoloration. Kenani et al. (24) reported favorable outcomes in pediatric patients treated with oxacillin and erythromycin. As these drugs have been proven to be safer than dapsone or steroids, they can be considered a good alternative therapy in LABD. Alajlan et al. (94) described seven pediatric patients with LABD treated with flucloxacillin. They underlined that the precocious beginning of treatment (within 1 month) might be relevant to induce early and long-lasting remission. However, this drug can also determine side effects, such as cholestatic hepatitis, hemolytic anemia, bone marrow suppression, and acute interstitial nephritis (95). Even though these side effects are rare, their monitoring is strongly recommended. According to Mervic et al. (96) miocamycin and a topical corticosteroid (betamethasone dipropionate 0.05% cream, twice daily) can be an effective and safe alternative treatment for LABD patients. It is unclear how these antibiotics work for LABD, although probably through their anti-inflammatory properties.
Other Drugs
Another drug that has been proven effective in treating LABD is nicotinamide, which has been tested alone (67) or in combination with dapsone (97). The therapeutic function of nicotinamide is to inhibit local factors causing blister formation.
Several adult patients (98, 99) and one pediatric patient (100) were treated with mycophenolate mofetil. Furthermore, patients with LABD have been successfully treated with intravenous immunoglobulins (101).
In some patients with severe skin involvement, in association with systemic therapy, wounded areas can be treated with topic eosin 2%-gentamycin and covered with emollient sterile gauze to prevent bacterial superinfections (6).
Only a few cases of neonatal LABD have been reported in the literature, some of them with significant mucosal involvement (12), ocular lesions, and upper airway and upper aerodigestive tract involvement. In these cases, the use of corticosteroid aerosol therapy and topical ocular corticosteroids were useful.
As LABD is often a benign disease, which in many cases leads to a spontaneous resolution, many authors suggest carefully considering the potential adverse effects of additional treatments by weighing the potential harms and benefits of all drugs. According to a recent study, drug-induced forms of LABD show a more severe clinical evolution than idiopathic LABD (58). Long-term immunosuppression may not be necessary for drug-induced LABD (89) in contrast to idiopathic LABD. In the literature, many studies report a favorable outcome for LABD in childhood. Nanda et al. (18) observed complete remission in 71% of patients after an average treatment period of 1 year.
Prognosis
Childhood LABD has a chronic relapsing course and resolves before puberty in most cases, often curing without sequelae (10, 14, 22). However, cases with severe morbidity and persisting in adulthood have been reported (8). The disease's mean duration is 14 months (25) and remission has been reported in 64% of children, usually within 2 years. Prompt diagnosis and the right treatment positively affect the prognosis (102). As the disease has a shorter duration and fewer relapses, the clinical course is more benign in children than in adults (73).
In neonatal LABD, respiratory involvement represents the major prognostic factor. The presence of IgA deposits can explain the pathogenesis of respiratory distress through the trachea and bronchi. Indeed, the cylindrical pseudostratified bronchial epithelium forms hemidesmosomes that express BP180, BP230, and α6β4 integrin, leading to mucosal epithelium disruption and fatal bronchial obstruction (36). Few case-reports in neonatal LABD have been described so far, nevertheless most of them showed the severity of such a disease in this age frame. Seven out of twelve (58%) required intubation and only one third showed a complete remission in the follow-up phase. Two patients died and three ended with severe sequelae involving eyes, gastrointestinal or respiratory tracts with scars. The remaining patients still show relapses of the disease.
Conclusion
Linear immunoglobulin A bullous disease is the most common bullous disease in the pediatric age. It is a disease with unknown exact pathogenesis, and it has been proposed that various stimuli (i.e., infections, drugs, inflammatory diseases, trauma) can activate autoimmune responses through antigen mimicry and epitope spreading. If spontaneous remission occurs, the prognosis is generally favorable. A prompt diagnosis to identify precipitating factors and to timely initiate therapy is crucial to assure a better prognosis.
Author Contributions
FM and EN conceived the study and supervised it. FM, FS, LL, and AB wrote the manuscript. All the authors performed the research and the selection of the sources, critically revised the manuscript, and accepted the final version of the manuscript.
Funding
The publication fee was financed by the Italian Society of Pediatric Allergy and Immunology. However, no significant funding source could have influenced the outcomes of this work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors want to thank the Italian Society of Pediatric Allergy and Immunology for its support in relation to this work.
References
1. Prystowsky S, Gilliam JN. Benign chronic bullous dermatosis of childhood. Linear IgA and C3 deposition on the basement membrane. Arch Dermatol. (1976) 112:837–8. doi: 10.1001/archderm.112.6.837
2. Wojnarowska F, Marsden RA, Bhogal B, Black MM. Chronic bullous disease of childhood, childhood cicatricial pemphigoid, and linear IgA disease of adults. J Am Acad Dermatol. (1988) 19:792–805. doi: 10.1016/S0190-9622(88)70236-4
3. Mori F, Filippeschi C, Parronchi P, Capone M, Liccioli G, Barni S, et al. Linear immunoglobulin A bullous disease (LABD) triggered by amoxicillin clavulanic acid in a child. J Allergy Clin Immunol Pract. (2020) 8:1398–9.e1. doi: 10.1016/j.jaip.2019.11.037
4. Stamenkovic HM, Lazarevic D, Stankovic T, Vojinovic J, Lekic B, Marinkovic A, et al. Linear IgA dermatosis of the childhood—report of an amoxicillin-induced case Dermatol Ther. (2020) 33:e13173. doi: 10.1111/dth.13173
5. Sarikaya Solak S, Ficicioglu S. Cephalosporin-induced linear IgA dermatosis in a child: case report and literature review. Dermatol Ther. (2019) 32:e12927. doi: 10.1111/dth.12927
6. Gatto A, Guerriero C, Moretta G, Valentini P, Giorgio V, Lazzareschi I, et al. Linear IgA bullous dermatosis in a 2-year-old child: possible association with aspirin. Eur J Dermatol. (2017) 27:417–8. doi: 10.1684/ejd.2017.3030
7. Nantel-Battista M, Al Dhaybi R, Hatami A, Marcoux D, DesRoches A, Kokta V. Childhood linear IgA bullous disease induced by trimethoprim-sulfamethoxazole. J Dermatol Case Rep. (2010) 4:33–5. doi: 10.3315/jdcr.2010.1053
8. Ho JCC, Ng PLP, Tan SH, Giam YC. Childhood linear IgA bullous disease triggered by amoxicillin-clavulanic acid. Pediatr Dermatol. (2007) 24:E40–3. doi: 10.1111/j.1525-1470.2007.00438.x
9. Schultz B, Hook K. Bullous diseases in children: a review of clinical features and treatment options. Pediatr Drugs. (2019) 21:345–56. doi: 10.1007/s40272-019-00349-3
10. Mintz EM, Morel KD. Clinical features, diagnosis, and pathogenesis of chronic bullous disease of childhood. Dermatol Clin. (2011) 29:459–62. doi: 10.1016/j.det.2011.03.022
11. Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. (2001) 19:719–27. doi: 10.1016/S0738-081X(00)00185-1
12. Romani L, Diociaiuti A, D'Argenio P, Hachem M, Gargiullo L, Boldrini R, et al. Case of neonatal linear IgA bullous dermatosis with severe eye involvement. Acta Derm Venereol. (2015) 95:1015–7. doi: 10.2340/00015555-2074
13. Egami S, Suzuki C, Kurihara Y, Yamagami Y, Kubo A, Funakoshi T, et al. Neonatal linear IgA bullous dermatosis mediated by breast milk–borne maternal IgA. JAMA Dermatol. (2021) 157:1107–11. doi: 10.1001/jamadermatol.2021.2392
14. Fortuna G, Marinkovich MP. Linear immunoglobulin A bullous dermatosis. Clin Dermatol. (2012) 30:38–50. doi: 10.1016/j.clindermatol.2011.03.008
15. Kanwar AJ, Sandhu K, Handa S. Chronic bullous dermatosis of childhood in north India. Pediatr Dermatol. (2004) 21:610–2. doi: 10.1111/j.0736-8046.2004.21521.x
16. Singalavanija S, Limpongsanurak W. Immunobullous diseases in Thai children: report of 24 cases. J Med Assoc Thai. (2003) 86 Suppl 3:S681–8.
17. Aboobaker J, Wojnarowska FT, Bhogal B, Black MM. Chronic bullous dermatosis of childhood-clinical and immunological features seen in African patients. Clin Exp Dermatol. (1991) 16:160–4. doi: 10.1111/j.1365-2230.1991.tb00336.x
18. Nanda A, Lazarevic V, Rajy JM, Almasry IM, AlSabah H, AlLafi A. Spectrum of autoimmune bullous diseases among children in Kuwait. Pediatr Dermatol. (2021) 38:50–7. doi: 10.1111/pde.14368
19. Kong YL, Lim YL, Chandran NS. Retrospective study on autoimmune blistering disease in pediatric patients. Pediatr Dermatol. (2015) 32:845–52. doi: 10.1111/pde.12684
20. Sansaricq F, Stein SL, Petronic-Rosic V. Autoimmune bullous diseases in childhood. Clin Dermatol. (2012) 30:114–27. doi: 10.1016/j.clindermatol.2011.03.018
21. Venning VA. Linear IgA disease: clinical presentation, diagnosis, and pathogenesis. Dermatol Clin. (2011) 29:453–8. doi: 10.1016/j.det.2011.03.013
22. Welfringer-Morin A, Bekel L, Bellon N, Gantzer A, Boccara O, Hadj-Rabia S, et al. Long-term evolving profile of childhood autoimmune blistering diseases: retrospective study on 38 children. J Eur Acad Dermatol Venereol. (2019) 33:1158–63. doi: 10.1111/jdv.15456
23. Horiguchi Y, Ikoma A, Sakai R, Masatsugu A, Ohta M, Hashimoto T. Linear IgA dermatosis: report of an infantile case and analysis of 213 cases in Japan. J Dermatol. (2008) 35:737–43. doi: 10.1111/j.1346-8138.2008.00561.x
24. Kenani N, Mebazaa A, Denguezil M, Ghariani N, Sriha B, Belajouza C, et al. Childhood linear IgA bullous dermatosis in Tunisia. Pediatr Dermatol. (2009) 26:28–33. doi: 10.1111/j.1525-1470.2008.00817.x
25. Monia K, Aida K, Amel K, Ines Z, Becima F, Ridha KM. Linear IGA bullous dermatosis in Tunisian children : 31 cases. Indian J Dermatol. (2011) 56:145. doi: 10.4103/0019-5154.80406
26. Díaz MS, Morita L, Ferrari B, Sartori S, Greco MF, Sobrevias Bonells L, et al. Dermatosis ampollar IgA lineal: serie de 17 casos. Actas Dermosifiliogr. (2019) 110:673–80. doi: 10.1016/j.ad.2018.06.017
27. Genovese G, Venegoni L, Fanoni D, Muratori S, Berti E, Marzano AV. Linear IgA bullous dermatosis in adults and children: a clinical and immunopathological study of 38 patients. Orphanet J Rare Dis. (2019) 14:115. doi: 10.1186/s13023-019-1089-2
28. Zhao CY, Chiang YZ, Murrell DF. Neonatal autoimmune blistering disease: a systematic review. Pediatr Dermatol. (2016) 33:367–74. doi: 10.1111/pde.12859
29. Hruza L, Mallory SB, Fitzgibbons J, Mallory GB. Linear IgA bullous dermatosis in a neonate. Pediatr Dermatol. (1993) 10:171–6. doi: 10.1111/j.1525-1470.1993.tb00049.x
30. Kishida Y, Kameyama J, Nei M, Hashimoto T, Baba K. Linear IgA bullous dermatosis of neonatal onset: case report and review of the literature. Acta Paediatr. (2004) 93:850–2. doi: 10.1080/08035250310007899
31. Gluth MB, Witman PM, Thompson DM. Upper aerodigestive tract complications in a neonate with linear IgA bullous dermatosis. Int J Pediatr Otorhinolaryngol. (2004) 68:965–70. doi: 10.1016/j.ijporl.2004.02.014
32. Lee SYR. Linear IgA bullous dermatosis in a neonate. Arch Dis Child-Fetal Neonatal Ed. (2004) 89:F280. doi: 10.1136/adc.2003.037911
33. Akin MA, Gunes T, Akýn L, Ohyama B, Kontas O, Hashimoto T. A newborn with bullous pemphigoid associated with linear IgA bullous dermatosis. Acta dermatovenerologica Alpina, Pannonica, Adriat. (2009) 18:66–70.
34. Julapalli MR, Brandon KL, Rosales CM, Grover RK, Plunkett RW, Metry DW. Neonatal linear immunoglobulin A bullous dermatosis: a rare presentation. Pediatr Dermatol. (2012) 29:610–3. doi: 10.1111/j.1525-1470.2011.01507.x
35. Salud CMD, Nicolas MEO. Chronic bullous disease of childhood and pneumonia in a neonate with VATERL association and hypoplastic paranasal sinuses. J Am Acad Dermatol. (2010) 62:895–6. doi: 10.1016/j.jaad.2009.02.009
36. Diociaiuti A, Zambruno G, Diomedi Camassei F, Di Zenzo G, Capolupo I, Stoppa F, et al. IgA tracheobronchial deposits underlie respiratory compromise in neonatal linear IgA bullous dermatosis. J Eur Acad Dermatol Venereol. (2017) 31:e333–5. doi: 10.1111/jdv.14120
37. Mazurek MT, Banihani R, Wong J, Weinstein M, Alnutayfi A, Etoom Y. Uncomplicated neonatal linear IgA bullous dermatosis: a case report. J Cutan Med Surg. (2018) 22:431–4. doi: 10.1177/1203475418760458
38. Giraud L, Welfringer-Morin A, Boccara O, Frassati-Biaggi A, Leclerc-Mercier S, Grootenboer-Mignot S, et al. Neonatal and self-healing linear immunoglobulin A dermatosis. J Eur Acad Dermatol Venereol. (2020) 34:e86–e87. doi: 10.1111/jdv.15989
39. Antiga E, Caproni M, Fabbri P. Linear immunoglobulin a bullous dermatosis: need for an agreement on diagnostic criteria. Dermatology. (2013) 226:329–32. doi: 10.1159/000350818
40. Egan CA, Martineau MT, Taylor TB, Meyer LJ, Petersen MJ, Zone JJ. IgA antibodies recognizing LABD97 are predominantly IgA1 subclass. Acta Derm Venereol. (1999) 79:343–6. doi: 10.1080/000155599750010229
41. Zone JJ. Clinical spectrum, pathogenesis and treatment of linear IgA bullous dermatosis. J Dermatol. (2001) 28:651–3. doi: 10.1111/j.1346-8138.2001.tb00056.x
42. Zillikens D. BP180 as the common autoantigen in blistering diseases with different clinical phenotypes. Keio J Med. (2002) 51:21–8. doi: 10.2302/kjm.51.21
43. Stingl G, Hönigsmann H, Holubar K, Wolff K. Ultrastructural localization of immunoglobulins in skin of patients with dermatitis herpetiformis. J Invest Dermatol. (1976) 67:507–12. doi: 10.1111/1523-1747.ep12664537
44. Yaoita H, Katz SI. Circulating IgA anti-basement membrane zone antibodies in dermatitis herpetiformis. J Invest Dermatol. (1977) 69:558–60. doi: 10.1111/1523-1747.ep12688382
45. Bhogal B, Wojnarowska F, Marsden RA, Das A, Black MM, McKee PH. Linear IgA bullous dermatosis of adults and children: an immunoelectron microscopic study. Br J Dermatol. (1987) 117:289–96. doi: 10.1111/j.1365-2133.1987.tb04134.x
46. Prost C, De Leca AC, Combemale P, Labeille B, Martin N, Cosnes A, et al. Diagnosis of adult linear IgA dermatosis by immunoelectronmicroscopy in 16 patients with linear IgA deposits. J Invest Dermatol. (1989) 92:39–45. doi: 10.1111/1523-1747.ep13070851
47. Martinez-De Pablo MI, González-Enseñat MA, Vicente A, Gilaberte M, Mascaró JM. Childhood bullous pemphigoid. Arch Dermatol. (2007) 143:215–20. doi: 10.1001/archderm.143.2.215
48. Denguezli M, Ben Nejma B, Nouira R, Korbi S, Bardi R, Ayed K, et al. Iga linear bullous dermatosis in children. A series of 12 Tunisian patients. Ann Dermatol Venereol. (1994) 121:888–92.
49. Tse Y, Lim HW. Chronic bullous dermatosis of childhood: differentiation from other autoimmune blisterig diseases in children. Int J Dermatol. (1994) 33:507–9. doi: 10.1111/j.1365-4362.1994.tb02868.x
50. Leigh G, Marsden RA, Wojnarowska F. Linear IgA dermatosis with severe arthralgia. Br J Dermatol. (1988) 119:789–92. doi: 10.1111/j.1365-2133.1988.tb03505.x
51. Achenbach P, Bonifacio E, Koczwara K, Ziegler A-G. Natural history of type 1 diabetes. Diabetes. (2005) 54:S25–31. doi: 10.2337/diabetes.54.suppl_2.S25
52. Moleiro S, Santos V, Calha M, Pessoa G. Atypical response to treatment in linear IgA bullous dermatosis of childhood: revision of literature. Dermatol Online J. (2011) 17:12–4. doi: 10.5070/D31379S6T6
53. Collier PM, Wojnarowska F, Welsh K, Mcguire W, Black MM. Adult linear IgA disease and chronic bullous disease of childhood: the association with human lymphocyte antigens Cw7, B8, DR3 and tumor necrosis factor influences disease expression. Br J Dermatol. (1999) 141:867–75. doi: 10.1046/j.1365-2133.1999.03110.x
54. Simon JC, Dietrich A, Kapp A, Schopf E. [Chronic bullous dermatosis in childhood. Association with salmonella enteritis]. Hautarzt. (1995) 46:485–9. doi: 10.1007/s001050050287
55. Baldari U, Raccagni AA, Celli B, Righini MG. Chronic bullous disease of childhood following Epstein-Barr virus seroconversion: a case report. Clin Exp Dermatol. (1996) 21:123–6. doi: 10.1111/j.1365-2230.1996.tb00034.x
56. Wojnarowska F, Venning VA, Burge SM. Immunobullous disease. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 7th ed. Blackwell Science Ltd (2004). doi: 10.1002/9780470750520.ch41
57. Durdu M, Baba M, Seçkin D. A case of bullous pemphigoid induced by aspirin. J Am Acad Dermatol. (2011) 65:443–4. doi: 10.1016/j.jaad.2010.02.032
58. Garel B, Ingen-Housz-Oro S, Afriat D, Prost-Squarcioni C, Tétart F, Bensaid B, et al. Drug-induced linear immunoglobulin A bullous dermatosis: a French retrospective pharmacovigilance study of 69 cases. Br J Clin Pharmacol. (2019) 85:570–9. doi: 10.1111/bcp.13827
59. Chanal J, Ingen-Housz-Oro S, Ortonne N, Duong T-A, Thomas M, Valeyrie-Allanore L, et al. Linear IgA bullous dermatosis: comparison between the drug-induced and spontaneous forms. Br J Dermatol. (2013) 169:1041–8. doi: 10.1111/bjd.12488
60. Plunkett RW, Chiarello SE, Beutner EH. Linear IgA bullous dermatosis in one of two piroxicam-induced eruptions: a distinct direct immunofluorescence trend revealed by the literature. J Am Acad Dermatol. (2001) 45:691–6. doi: 10.1067/mjd.2001.117390
61. Collier PM, Wojnarowska F. Drug-inducer linear immunoglobulin A disease. Clin Dermatol. (1993) 11:529–33. doi: 10.1016/0738-081X(93)90161-5
62. Handley J, Shields M, Dodge J, Walsh M, Bingham A. Chronic bullous disease of childhood and ulcerative colitis. Pediatr Dermatol. (1993) 10:256–8. doi: 10.1111/j.1525-1470.1993.tb00371.x
63. Wong CSM, Arkwright PD, Rieux-laucat F, Cant AJ, Stevens RF, Judge MR. Childhood linear IgA disease in association with autoimmune lymphoproliferative syndrome. Br J Dermatol. (2004) 150:578–80. doi: 10.1111/j.1365-2133.2004.05850.x
64. Shipman AR, Reddy H, Wojnarowska F. Association between the subepidermal autoimmune blistering diseases linear IgA disease and the pemphigoid group and inflammatory bowel disease: two case reports and literature review. Clin Exp Dermatol. (2012) 37:461–8. doi: 10.1111/j.1365-2230.2012.04383.x
65. Olivera Olmedo JE, Sanchez–Valverde Visus F, Espana Alonso A, Barriuso Lapresa L, Ruiz de Eranchun Lasa F. Deficit de lipasa pancreatica y enfermedad IgA lineal en la infancia. An Esp Pediatr. (1996) 44:73–5.
66. Polat M, Lenk N, Kürekçi E, Öztaş P, Artüz F, Alli N. Chronic bullous disease of childhood in a patient with acute lymphoblastic leukemia. Am J Clin Dermatol. (2007) 8:389–91. doi: 10.2165/00128071-200708060-00010
67. Cui YX, Yang BQ, Zhou GZ, Zhang FR. Childhood linear IgA bullous dermatosis successfully treated with oral nicotinamide. Clin Exp Dermatol. (2016) 41:816–8. doi: 10.1111/ced.12892
68. Corrà A, Bonciolini V, Quintarelli L, Verdelli A, Caproni M. Linear IgA bullous dermatosis potentially triggered by vaccination. Int J Immunopath Pharmacol. (2022) 36:1–5. doi: 10.1177/20587384211021218
69. Ikeya S, Urano S, Tokura Y. Linear IgA bullous dermatosis following human papillomavirus vaccination. Eur J Dermatol. (2012) 22:787–8. doi: 10.1684/ejd.2012.1851
70. Hali F, Kerouach A, Alatawna H, Chiheb S, Lakhdar H. Linear IgA bullous dermatosis following Oxford AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. (2022) 47:611–3. doi: 10.1111/ced.15007
71. Coto-Segura P, Fernández-Prada M, Mir-Bonafé M, García-García B, González-Iglesias I, Alonso-Penanes P, et al. Vesiculobullous skin reactions induced by COVID-19 mRNA vaccine: report of four cases and review of the literature. Clin Exp Dermatol. (2022) 47:141–3. doi: 10.1111/ced.14835
72. Borte S, Janzi M, Pan-Hammarström Q, von Döbeln U, Nordvall L, Winiarski J, et al. Placental transfer of maternally-derived IgA precludes the use of guthrie card eluates as a screening tool for primary immunodeficiency diseases. PLoS ONE. (2012) 7:e43419. doi: 10.1371/journal.pone.0043419
73. Vico-Alonso C, Palencia-Pérez S. Linear IgA bullous dermatosis. N Engl J Med. (2020) 382:2248. doi: 10.1056/NEJMicm1913412
74. Jabłońska S, Chorzelski TP, Rosinska D, Maciejowska E. Linear IgA bullous dermatosis of childhood (Chronic bullous dermatosis of childhood). Clin Dermatol. (1991) 9:393–401. doi: 10.1016/0738-081X(91)90031-F
75. Wojnarowska F, Frith P. “Linear IgA disease.” In: Bernauer W, Dart JKG, Elder MJ, editors. Cicatrising Conjunctivitis, Vol. 28. Basel: KARGER (1997). p. 64–72. doi: 10.1159/000060705
76. Patrício P, Ferreira C, Gomes MM, Filipe P. Autoimmune bullous dermatoses: a review. Ann N Y Acad Sci. (2009) 1173:203–10. doi: 10.1111/j.1749-6632.2009.04737.x
77. Brenner S, Mashiah J. Autoimmune blistering diseases in children: signposts in the process of evaluation. Clin Dermatol. (2000) 18:711–24. doi: 10.1016/S0738-081X(00)00154-1
78. Egan CA, Zone JJ. Linear IgA bullous dermatosis. Int J Dermatol. (1999) 38:818–27. doi: 10.1046/j.1365-4362.1999.00813.x
79. Barnadas MA. Dermatosis ampollar IgA lineal. Piel. (2001) 16:324–30. doi: 10.1016/S0213-9251(01)72485-9
80. Ingen-Housz-Oro S. Dermatose à IgA linéaire : revue de la littérature. Ann Dermatol Venereol. (2011) 138:214–20. doi: 10.1016/j.annder.2011.01.010
81. Morrison LH. Direct immunofluorescence microscopy in the diagnosis of autoimmune bullous dermatoses. Clin Dermatol. (2001) 19:607–13. doi: 10.1016/S0738-081X(00)00179-6
82. Aoki V, Sousa Jr JX, Fukumori LMI, Périgo AM, Freitas EL, Oliveira ZNP. Imunofluorescência direta e indireta. An Bras Dermatol. (2010) 85:490–500. doi: 10.1590/S0365-05962010000400010
83. Lammer J, Hein R, Roenneberg S, Biedermann T, Volz T. Drug-induced Linear IgA bullous dermatosis: a case report and review of the literature. Acta Derm Venereol. (2019) 99:508–15. doi: 10.2340/00015555-3154
84. Neughebauer BI, Negron G, Magnussen R, Pelton S, Plunkett RW, Beutner EH. Bullous skin disease: an unusual allergic reaction to vancomycin. Am J Med Sci. (2002) 323:273–8. doi: 10.1097/00000441-200205000-00009
85. Horvath B, Niedermeier A, Podstawa E, Müller R, Hunzelmann N, Karpati S, et al. IgA autoantibodies in the pemphigoids and linear IgA bullous dermatosis. Exp Dermatol. (2010) 19:648–53. doi: 10.1111/j.1600-0625.2010.01080.x
86. Cozzani E, Drosera M, Parodi A, Carrozzo M, Gandolfo S, Rebora A. Frequency of IgA antibodies in pemphigus, bullous pemphigoid and mucous membrane pemphigoid. Acta Derm Venereol. (2004) 84:381–4. doi: 10.1080/00015550410031825
87. Georgi M, Scheckenbach C, Kromminga A, Partscht K, Messer G, Brcker EB, Zillikens D. Mapping of epitopes on the BP180 ectodomain targeted by IgA and IgG autoantibodies in patients with the lamina lucida-type of linear IgA disease. Arch Dermatol Res. (2001) 293:109–14. doi: 10.1007/s004030000205
88. Viglizzo G, Cozzani E, Nozza P, Occella C, Parodi A. A case of linear IgA disease in a child with IgA and IgG circulating antibodies directed to BPAg2. Int J Dermatol. (2007) 46:1302–4. doi: 10.1111/j.1365-4632.2007.03317.x
89. Fortuna G, Salas-Alanis JC, Guidetti E, Marinkovich MP. A critical reappraisal of the current data on drug-induced linear immunoglobulin A bullous dermatosis: a real and separate nosological entity? J Am Acad Dermatol. (2012) 66:988–94. doi: 10.1016/j.jaad.2011.09.018
90. Sandoval M, Farias MM, Gonzalez S. Linear IgA bullous dermatosis: report of five cases in Chile. Int J Dermatol. (2012) 51:1303–6. doi: 10.1111/j.1365-4632.2011.05324.x
91. Lings K, Bygum A. Linear IgA bullous dermatosis: a retrospective study of 23 patients in Denmark. Acta Derm Venereol. (2015) 95:466–71. doi: 10.2340/00015555-1990
92. Benbenisty KM, Bowman PH, Davis LS. Localized linear IgA disease responding to colchicine. Int J Dermatol. (2002) 41:56–8. doi: 10.1046/j.1365-4362.2002.01321.x
93. Navedo de las Heras M. Linear IgA bullous dermatosis of childhood: good response to antibiotic treatment. Clin Exp Dermatol. (2014) 39:395–7. doi: 10.1111/ced.12220
94. Alajlan A, Al-Khawajah M, Al-Sheikh O, Al-Saif F, Al-Rasheed S, Al-Hoqail I, et al. Treatment of linear IgA bullous dermatosis of childhood with flucloxacillin. J Am Acad Dermatol. (2006) 54:652–6. doi: 10.1016/j.jaad.2005.11.1102
95. Dobson JL, Angus PW, Jones R, Crowley P, Gow PJ. Flucloxacillin-induced aplastic anemia and liver failure. Transpl Int. (2005) 18:487–9. doi: 10.1111/j.1432-2277.2004.00014.x
96. Mervic L, Dragoš V, Pavlović MD. Linear IgA bullous dermatosis of childhood: successful treatment with miocamycin and topical corticosteroid. Clin Exp Dermatol. (2009) 34:e391–2. doi: 10.1111/j.1365-2230.2009.03366.x
97. Khanna N, Pandhi R, Gupta S, Singh M. Response of chronic bullous dermatosis of childhood to a combination of dapsone and nicotinamide. J Eur Acad Dermatol Venereol. (2001) 15:368. doi: 10.1046/j.0926-9959.2001.00269-9.x
98. Gläser R, Sticherling M. Successful treatment of linear IgA bullous dermatosis with mycophenolate mofetil. Acta Derm Venereol. (2002) 82:308–9. doi: 10.1080/000155502320323351
99. Talhari C, Mahnke N, Ruzicka T, Megahed M. Successful treatment of linear IgA disease with mycophenolate mofetil as a corticosteroid sparing agent. Clin Exp Dermatol. (2005) 30:297–8. doi: 10.1111/j.1365-2230.2005.01741.x
100. Farley-Li J. treatment of linear IgA bullous dermatosis of childhood with mycophenolate mofetil. Arch Dermatol. (2003) 139:1121. doi: 10.1001/archderm.139.9.1121
101. Segura S, Iranzo P, Pablo IM, Mascaró JM, Alsina M, Herrero J, et al. High-dose intravenous immunoglobulins for the treatment of autoimmune mucocutaneous blistering diseases: evaluation of its use in 19 cases. J Am Acad Dermatol. (2007) 56:960–7. doi: 10.1016/j.jaad.2006.06.029
Keywords: linear IgA bullous dermatosis (LABD), newborn, children, drug hypersensitivity, epidemiology, diagnosis, treatment
Citation: Mori F, Saretta F, Liotti L, Giovannini M, Castagnoli R, Arasi S, Barni S, Mastrorilli C, Pecoraro L, Caminiti L, Marseglia GL, Barbaud A and Novembre E (2022) Linear Immunoglobulin a Bullous Dermatosis in Children. Front. Pediatr. 10:937528. doi: 10.3389/fped.2022.937528
Received: 10 May 2022; Accepted: 20 June 2022;
Published: 08 July 2022.
Edited by:
Claudio Pignata, University of Naples Federico II, ItalyCopyright © 2022 Mori, Saretta, Liotti, Giovannini, Castagnoli, Arasi, Barni, Mastrorilli, Pecoraro, Caminiti, Marseglia, Barbaud and Novembre. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca Saretta, ZnJhbmNlc2Nhc2FyZXR0YUBnbWFpbC5jb20=
†These authors share last authorship
 Francesca Mori
Francesca Mori Francesca Saretta
Francesca Saretta Lucia Liotti
Lucia Liotti Mattia Giovannini
Mattia Giovannini Riccardo Castagnoli
Riccardo Castagnoli Stefania Arasi5
Stefania Arasi5 Simona Barni
Simona Barni Carla Mastrorilli
Carla Mastrorilli Gian Luigi Marseglia
Gian Luigi Marseglia Annick Barbaud
Annick Barbaud Elio Novembre
Elio Novembre