
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 31 March 2022
Sec. Neonatology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.817331
This article is part of the Research Topic Nutrition of the Premature Neonate: Physiology, Pathology and Management of the Immature and Developing Gut View all 9 articles
 Tanith Alexander1,2*
Tanith Alexander1,2* Michael Meyer2
Michael Meyer2 Jane E. Harding1
Jane E. Harding1 Jane M. Alsweiler3,4
Jane M. Alsweiler3,4 Yannan Jiang5
Yannan Jiang5 Clare Wall6
Clare Wall6 Mariana Muelbert1
Mariana Muelbert1 Frank H. Bloomfield1
Frank H. Bloomfield1 the DIAMOND Study Group
the DIAMOND Study GroupBackground: Exclusive breastmilk is the desired enteral nutrition for babies born moderate- and late-preterm between 32+0 and 36+6 weeks' gestation; however, this goal is often difficult to achieve.
Methods: A prospective cohort of babies 32+0 −35+6 weeks' gestation enrolled in the DIAMOND trial were randomized to a condition specifying that babies should receive mother's own milk (MOM) as the only enteral feed. Factors associated with the successful transition to MOM, defined as MOM being the sole enteral feeding at the time of the first cessation of intravenous (IV) fluids, were investigated by logistic regression. Time to commencement of a milk other than MOM was analyzed by Kaplan–Meier survival curves.
Results: A total of 151 eligible babies (60% boys) were included, 93 (63%) of whom successfully transitioned from IV fluids onto MOM only. Alternative sources of milk, mostly formula, were used to transition from IV fluids onto enteral feeds more often in multiples and Māori, and was commenced earlier in Māori than other ethnicities (p = 0.007) and in late-preterm compared with moderate-preterm babies (p=0.01). Receiving exclusively breastmilk at discharge was more likely for babies who successfully transitioned from IV fluids onto MOM only [OR (95% confidence intervals) 4.9 (2.3–10.6)] and who received only MOM in the first week after birth [4.8 (2.2–10.4)], both p < 0.0001. Receiving breastmilk exclusively at discharge was less likely for Māori than Caucasian babies [0.2 (0.1–0.6), p < 0.0006]. There was no difference in the use of alternative sources of milk in babies who received parenteral nutrition or dextrose or between small-for-gestational-age and appropriate-for-gestational-age babies.
Conclusions: Despite an intention to provide only MOM, significant numbers of moderate- and late-preterm babies received formula to transition from IV fluids, and this differed by ethnicity. The drivers underlying these decisions require further investigation. These data highlight an urgent need for quality initiatives to support and encourage mothers of moderate- and late-preterm babies in their lactation.
Worldwide, babies born moderate (MP)- to late (LP)-preterm (32+0 −36+6) account for >80% of all preterm babies born each year (1). It is becoming increasingly apparent that, compared with term babies, MP and LP babies are at higher risk of several complications following birth, including hypoglycaemia, jaundice, temperature instability, sepsis, and hospital readmission (2–9). They also are at an increased risk of worse long-term outcomes compared with term-born babies, including developmental delay (10), neurodevelopmental disability (11), need for special education (12) and behavioral problems (13). In babies born <32 weeks' gestation, better growth is associated with better neurodevelopmental outcomes (14), and growth is associated with nutrition (15). However, whether the same is true for MP and LP babies remains unknown. Given that the fetal brain continues to grow very rapidly during the final trimester, with brain volume at 32–34 weeks' gestation only 55–65% of the brain volume at term (16, 17), it is plausible that early nutrition and growth could also be important for optimizing neurodevelopmental outcomes of MP and LP babies.
One of the most significant issues facing clinicians following the birth of a MP or LP baby is how best to provide early nutritional support for optimal short- and long-term health. Breastmilk is the preferred nutrition for these babies but usually is not available in the first days after birth in sufficient quantities to meet the nutritional requirements, such as maintaining hydration and avoiding hypoglycaemia (18). Therefore, these requirements need to be met in other ways until sufficient breastmilk is available to meet the baby's physiological needs. The lack of robust evidence on which to base clinical practice is reflected in the great variation in the nutritional management of MP and LP babies (19–22). Common approaches include the provision of infant formula via a gastric feeding tube or provision of intravenous (IV) fluids (20) that may consist of dextrose alone or an amino acid solution with or without lipid emulsion. Provision of IV fluids means admission to a neonatal nursery, usually with separation of the mother–baby dyad, and the need for IV access. If IV fluids are commenced, how long one should wait for breastmilk supply to meet the baby's needs, thereby ensuring the baby only receives breastmilk rather than providing an alternative form of nutrition that may facilitate discharge and avoid the need for intravenous access, is a common dilemma (23).
The DIAMOND trial (DIfferent Approaches to MOderate & late preterm Nutrition: Determinants of feed tolerance, body composition, and development) is a randomized, controlled, factorial-design trial that aims to investigate the effects of different nutritional strategies in MP and LP babies on their body composition, time to full enteral feeds, and neurodevelopmental outcomes (24).
The objective of this study is to describe the actual nutritional management of a cohort of MP and LP babies who were randomized, as part of the DIAMOND trial, to nutritional management that stipulated MOM as the only enteral feed, with the provision of only IV fluids support until breastmilk supply met requirements. Clinicians were asked explicitly not to provide an alternative to MOM unless they believed that this was clinically indicated or the parents requested an alternative. We hypothesized that clinicians would consider that the smallest babies would benefit most from receiving only breastmilk, which is associated with a reduced risk of feed intolerance and necrotising enterocolitis (NEC) (25), although both are uncommon in MP and LP babies. We also hypothesized that parenteral nutrition (PN) would be considered by clinicians to provide more balanced nutrition than dextrose alone due to the administration of, at the least, additional protein. We therefore hypothesized that the smallest babies and those receiving PN would be more likely to transition successfully from IV fluids onto MOM only, and would be less likely to receive alternative forms of nutrition.
In 2017, the DIAMOND trial began recruiting babies born between 32+0 and 35+6 weeks' gestation across 4 neonatal units in Auckland, New Zealand, all of which are accredited through the baby-friendly hospital initiative (26). Babies with IV line placed for clinical reasons (type of line access was according to local practice) and whose mothers intended to breastfeed were eligible. Consent was required within 24 h of birth and babies were randomized to three interventions in a factorial design, leading to eight possible conditions (24). The three interventions (factors to which babies were randomized) were: [factor 1] either dextrose or PN (at a minimum amino acid solution, with lipid emulsion at the medical team's discretion); [factor 2] MOM as the only enteral feed or, if breastmilk was insufficient to meet prescribed fluid requirements, a milk supplement (peer-to-peer donor expressed breastmilk or infant formula according to local practice) while waiting for MOM to reach desired volumes; and [factor 3] exposure to smell and taste of milk before tube feeds or not. The goal for all babies was to achieve full feeds with MOM. Pasteurized donor human milk was not available at the recruiting sites during the study period. Each of the recruiting hospitals had dedicated lactation consultants and the provision of lactation support and advice was as per local practice; no extra support was provided for mothers within the DIAMOND trial protocol, details of which can be found in the published protocol (24).
This study cohort comprises babies recruited between March 2017 and 2020 who, within factor 2, were randomized to receive MOM as the only enteral feed. The protocol specified that babies should remain on IV fluids until milk feeds of MOM met the prescribed fluid requirements without the need for further IV fluids. Babies could receive an alternative enteral nutrition if the clinical team felt they no longer could wait for sufficient MOM or at parental request. Babies were categorized according to whether they received MOM only or received alternative enteral nutrition to transition from IV fluids onto enteral feeds. Successful transition from IV fluids onto MOM only was defined as MOM being the sole enteral feeding at the time of first cessation of intravenous fluids.
Babies' birth characteristics and nutrition status up to discharge are summarized as median and range or as frequencies and percentages as appropriate. Simple Chi-square (or Fisher's exact test with small counts) and logistic regression were used to compare the differences in proportions between subgroups. Time to transition from IV fluids onto enteral feeds was compared between groups using analysis of variance. Time to receive alternative nutrition was evaluated using Kaplan–Meier survival curves and log-rank test for gestational age, birth characteristics, and IV fluid type commenced at birth. Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). All statistical tests were two-sided at a 5% level of significance.
The New Zealand Health and Disability Ethics Committee has given ethical approval for the DIAMOND study (16/NTA/90), and each participating site has institutional approval through local institutional review processes. The DIAMOND trial is endorsed by the IMPACT clinical trials network (https://impact.psanz.com.au) and prospectively registered (ACTRN12616001199404).
The cohort comprises 151 babies, 77 (51%) MP (32+0 −33+6 weeks' gestation) and 74 (49%) LP (34+0 −35+6 weeks' gestation) (Table 1). Of these, 65 (43%) received IV dextrose and 86 (53%) received PN, with 70% of the latter receiving IV lipid emulsion in addition to an amino acid solution. A total of 93 (63%) babies successfully transitioned from IV fluids onto MOM only. For the babies who received alternative enteral nutrition to transition from IV fluids, infant formula was given to all but 3; these babies received unpasteurised (peer-to-peer) donor-expressed breastmilk.
Late preterm babies transitioned off IV fluids onto only enteral feeds more quickly than MP babies, but there was a significant interaction with sex, with LP boys taking the least time to transition off IV fluids and MP boys taking the most time (Table 2). Māori babies also transitioned off IV fluids more quickly than other ethnicities. There were no differences in time to transition off IV fluids between babies receiving different types of IV fluids, cared for in different hospitals, or with different modes of birth.
Multiples were less likely to achieve a successful transition to MOM only than singletons (46 vs. 67%, p = 0.02), and this was particularly the case for LP babies (33 vs. 68%, p = 0.007) (Table 3). Māori babies were less likely to achieve a successful transition to MOM only (43%) than Pacific (74%, p = 0.02) and Caucasian (70%, p = 0.03) babies. In LP babies only, the smallest (<1,500 g) and largest (>2,500 g) were more likely to achieve a successful transition to MOM only than babies with a birth weight between 1,500 and 2,500 g (p = 0.04). LP babies born by Cesarean section were less likely to achieve a successful transition to MOM only than LP babies born vaginally (49 vs. 74%, p = 0.03).
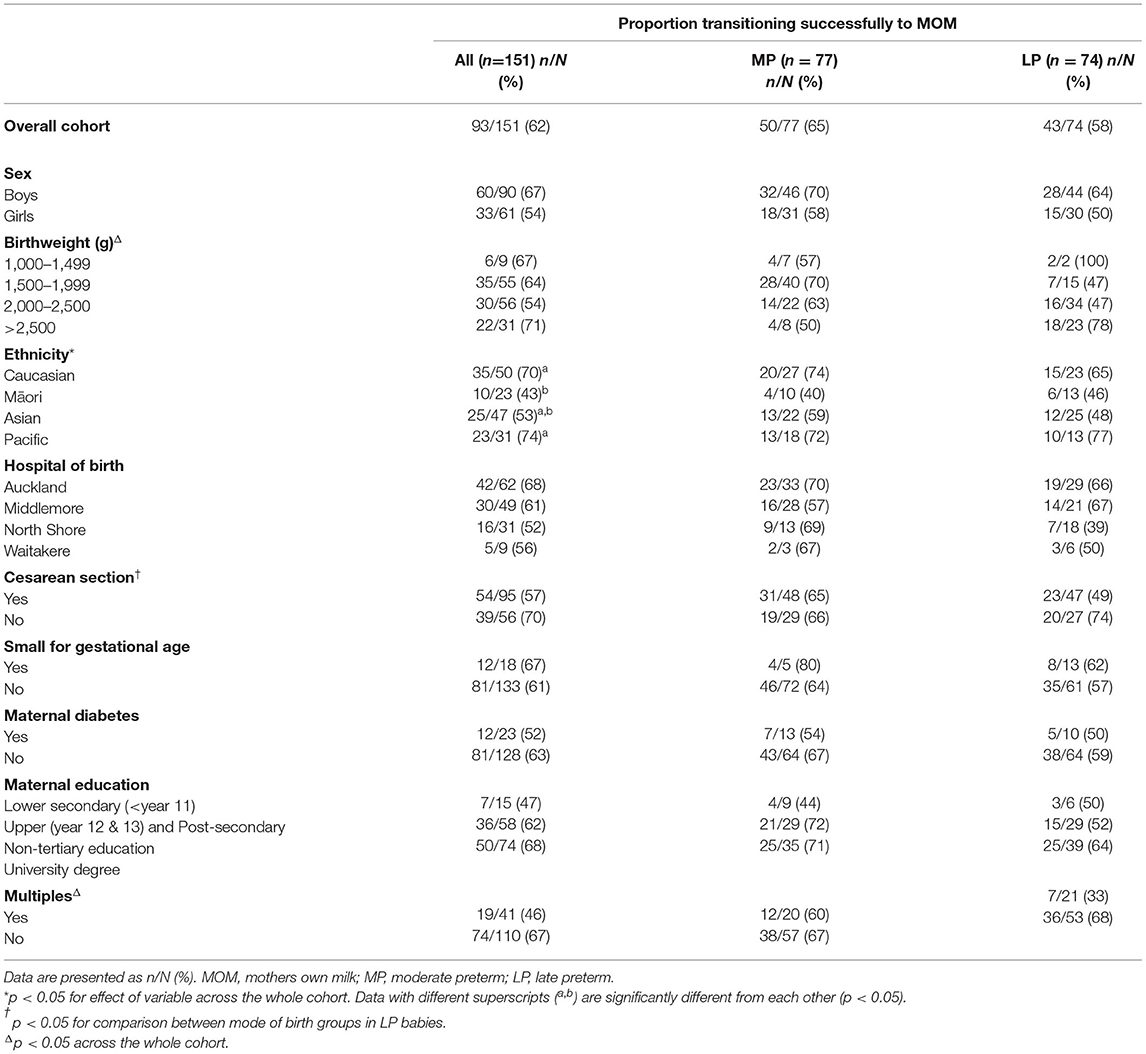
Table 3. Maternal and birth characteristics of babies who successfully transitioned onto only mothers own milk at the first cessation of IV fluids.
In babies who did not successfully transition to MOM only, LP babies received alternative nutrition earlier than MP babies (Figure 1A, p = 0.01), with 47% of LP babies receiving alternative nutrition by day 4 compared with only 24% of MP babies. This effect was seen across the gestational age spectrum with 19, 30, 43, and 54% of babies born at 32, 33, 34, and 35 completed weeks', respectively, transitioning from IV to alternative nutrition by day 4 after birth (Figure 1B, p = 0.048). Māori babies received alternative nutrition earlier than babies of other ethnicities (Figure 1C, p = 0.007); by day 3, 55% of Māori, 22% of Pacific, 20% of Caucasian, and 28% of Asian babies were receiving alternative nutrition that was not MOM. Whether babies received PN or dextrose was not related to the time to receiving alternative nutrition (Figure 1D, p = 0.84), either overall or in MP and LP babies separately (Figures 1E,F).
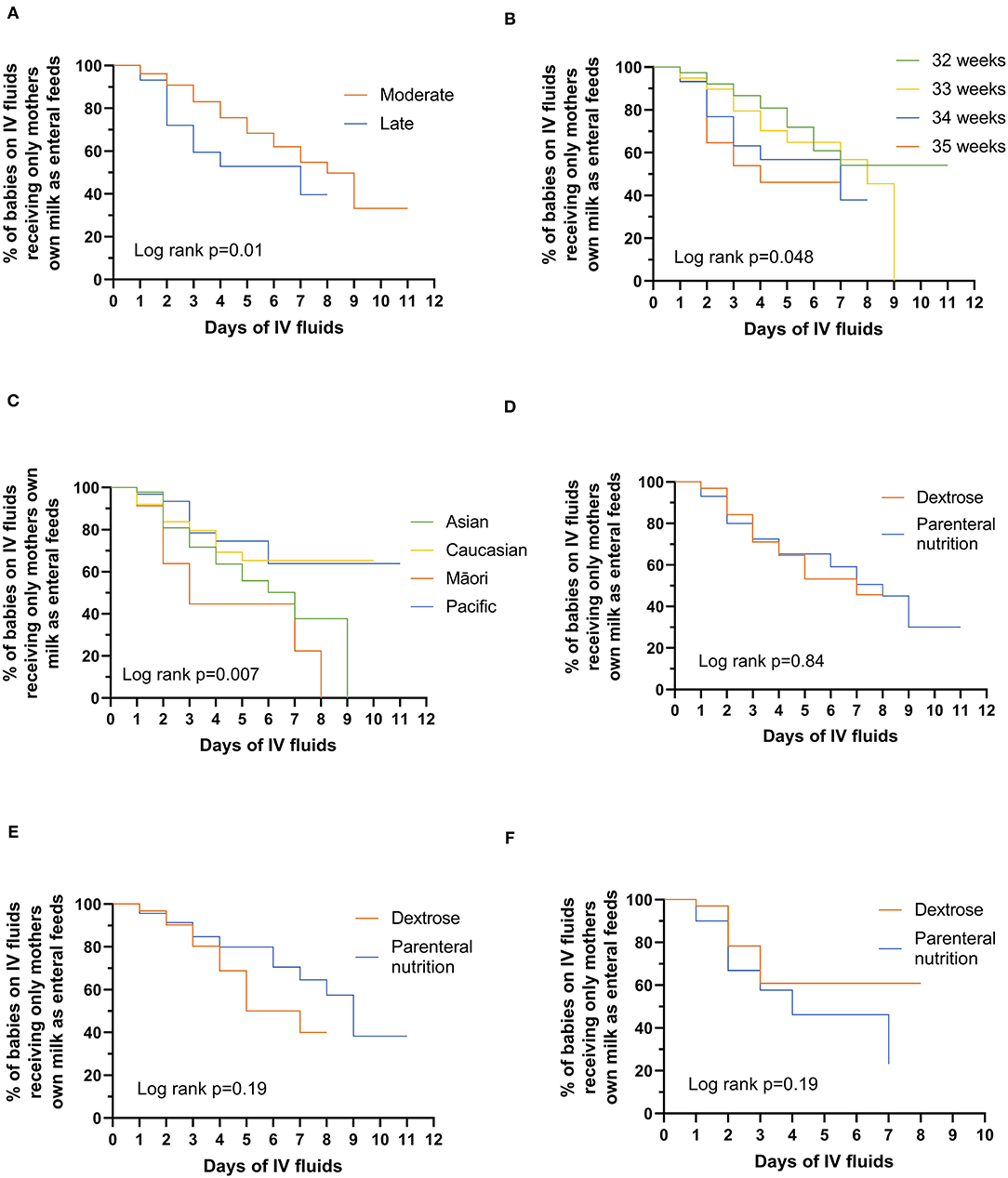
Figure 1. Days to alternative nutrition while on intravenous fluids by: (A) moderate vs. late preterm babies; (B) gestational age in weeks; (C) ethnicity; (D) whether babies are receiving dextrose or parenteral nutrition; (E) moderate-preterm babies receiving dextrose or parenteral nutrition; (F) late-preterm babies receiving dextrose or parenteral nutrition. Significant differences were tested using the log-rank test (two-sided at 5% level of significance).
Successful transition from IV fluids to MOM only was significantly associated with the type of feeding at discharge from the hospital. Of those babies discharged receiving exclusively breastmilk, 60% were exclusively breastfed, 38% breast and bottle-fed, and 2% were exclusively bottle-fed. Of babies who successfully transitioned to MOM only, 85% (n/N = 79/93) were discharged receiving exclusively breastmilk compared with 53% (n/N = 31/58) of those who did not [OR (95% confidence intervals) 4.9 (2.3–10.6), p < 0.0001]. Similarly, 86% (n/N = 73/85) of babies who received only MOM in the first week after birth were discharged from hospital receiving exclusively breastmilk compared with only 56% (n/N = 37/66) of those who received alternative nutrition within the first week [OR = 4.8 (2.2–10.4), p < 0.0001]. The type of feeding at discharge was significantly different based on ethnicity, where 84% of Caucasian (n/N = 42/50), 75% of Pacific (n/N = 23/31), 70% of the Asian (n/N = 33/47), and 52% of Māori (n/N = 12/23) babies were receiving exclusively breastmilk at discharge (p = 0.04). Māori babies were significantly less likely to receive exclusively breastmilk at discharge compared with Caucasian babies [OR = 0.2 (0.1–0.6), p < 0.01].
The World Health Organization recommends exclusive breastfeeding for all babies, and this is even more important for moderate- to late-preterm babies who are at an increased risk of adverse health outcomes, both in the short- and long-term. The focus of this cohort study was the factors that were associated with the choice of nutrition received at the first cessation of IV fluids. Our findings also confirmed that these choices about early nutrition are associated with feeding at discharge and therefore are likely to be reflected in feeding type at home.
The nutritional plan for all babies in this cohort was for them to receive only MOM: mothers had stated that they intended to breastfeed; babies already had intravenous access secured, and they were randomized to a condition that stipulated nutritional support with IV fluids until MOM supply met baby's needs or unless the attending clinician or parents no longer felt able to do so. Despite this, almost 40% of babies received a breastmilk alternative, almost exclusively infant formula. A limitation is that the trial protocol did not collect the reason for the introduction of an alternative form of nutrition or whether this was a medical or parental decision.
We hypothesized that the smallest preterm babies would be more likely to transition onto MOM only to avoid exposing these babies to infant formula. However, this was not the case, as babies of lower birth weight or who were small-for-gestational age (SGA) were not less likely to receive formula. This may indicate that clinicians are comfortable using infant formula in smaller babies given that the risk of necrotising enterocolitis is low in MP compared with extremely preterm babies (28). The high level of formula use is consistent with our survey of nutritional management in MP and LP babies (22). The provision of infant formula to transition off IV fluids was much more likely in multiples than singletons, consistent with the published literature (29).
We also hypothesized that babies randomized to PN would be more likely than babies randomized to IV dextrose to transition successfully to MOM only, as dextrose provides only carbohydrates, leading inevitably to an accumulating nitrogen deficit (30). It has been proposed that this nitrogen deficit followed by restoration of adequate nutrition may contribute to altered body composition of MP and LP babies (31), resulting in a greater fat accumulation, inadequate accretion of lean body mass, and a higher fat mass percent at term-equivalent age (31, 32). However, PN provides protein (and fat when lipid emulsion also is provided, as was the case for 70% of babies receiving PN) in addition to carbohydrate, and we expected that this might encourage clinicians to delay the introduction of other protein sources. However, our findings did not support this hypothesis.
Although there was no statistical difference in the proportion of MP and LP babies transitioning successfully onto MOM, MP babies who received alternative forms of nutrition did so later than LP babies. This may reflect a number of factors including a greater willingness to commence infant formula in the more mature babies, a desire for less medicalisation in well LP babies, the pressure to limit the separation of the mother–infant dyad, pressure on bed status, and perhaps the acknowledgment that, as MP babies are likely to require at least 1–2 weeks in the newborn nursery, there is more time to wait for successful transition onto MOM. However, it is unclear why such factors did not lead to an overall difference in the proportion of MP and LP transitioning successfully to MOM.
Lactogenesis II, the secretory activation phase, typically occurs 48–72 h after birth and, following a normal term birth, breastmilk begins to be produced in significant quantities from day 4 post-birth (33). Delayed lactogenesis, defined as occurring after 72 h (34), is well described with stress and anxiety (35, 36), Caesarean section (37), diabetes and obesity (38), and following preterm birth (39). Therefore, if the goal is for babies to receive only MOM and avoid formula whenever possible, clinicians must be willing to wait for >4 days before commencing an alternative form of nutrition; this occurred in ~70% of MP but only 50% of LP babies.
The lack of donor breastmilk use in this study reflects the lack of a donor breastmilk bank accessible by the recruiting sites. However, donor unpasteurised breastmilk is sometimes provided through a mother screening and sharing system within the individual units and is most often used in the more preterm infants at a higher risk of NEC (40). With the high use of infant formula and clinician preference for enteral fluids rather than IV fluids, this does highlight the need for robust evidence on whether donor breastmilk in MP and LP babies has health benefits and is cost-effective.
Receiving milk other than MOM and, indeed, doing so only in the first week after birth, was negatively associated with breastmilk feeding at discharge. Rates of breastfeeding, the ability to exclusively breastfeed, and breastmilk production are well documented to be challenging to mothers of MP and LP babies (41–43). However, supportive care programs to encourage and support breastfeeding have been shown to improve breastmilk provision during admission, decrease the use of formula, and increase the rates of exclusive breastfeeding in moderate to late preterm infants (44–46). Therefore, there may be an opportunity for quality initiatives to increase breastmilk provision, particularly in the first week after birth in this high-risk group.
Perhaps the most striking finding of this study was that Māori babies, the indigenous peoples of New Zealand, transitioned onto formula significantly earlier than babies of other ethnicities. Māori babies also were least likely to achieve breastmilk feeding on discharge, a concerning finding given the risk of poor health outcomes associated with low breastfeeding rates and the protective effects of breastfeeding on sudden infant death syndrome (47), childhood obesity (48), and respiratory infections (49), all of which are over-represented in Māori children. We are unable to determine from this study the reasons underlying these findings, which require further investigation. Possibilities include social factors impacting the mother's ability to visit and express regularly, less access to resources and equipment that support expressing breastmilk, and the knowledge that it might be difficult to continue completely breastfeeding after discharge. However, there may also be larger issues involved including a healthcare system that may not be aligned with the Māori perspective on healthcare, and an unconscious bias or systemic racism within the healthcare system.
Even when the intention is to provide only MOM within the context of a randomized control trial, this goal is not achieved for many MP and LP babies. Formula is often provided even before lactogenesis II is likely to be established, suggesting that mothers are not given the opportunity to establish a milk supply before breastmilk alternatives are provided. Māori babies were significantly more likely to receive formula, to be given formula at an earlier age, and were least likely to be breastfed at discharge. Further research should address the reasons behind these findings and find measures to address them.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the New Zealand Health and Disability Ethics Committee (16/NTA/90). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
TA conceptualized and designed the study and protocol, drafted the initial manuscript, obtained funding for the study, contributed to the acquisition and interpretation of the data, reviewed, and revised the manuscript. FB conceptualized and designed the study and protocol, obtained funding for the study, contributed to the interpretation of the data, reviewed and revised the manuscript. YJ contributed to the study design, protocol development, and analysis of the data. MM, JH, JA, and CW contributed to protocol development and have commented on all drafts of manuscript. MM was involved in the acquisition of the data and commented on all drafts of manuscript. All authors have approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
The DIAMOND trial is funded by the Health Research Council of New Zealand (16/605) and Counties Manukau Health (number 269).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank all those in the DIAMOND Study Group: Tanith Alexander1, 2, Jane M. Alsweiler3, 4, Sharin Asadi1, Friederike Beker5, 6 Frank H. Bloomfield1, 3, David Cameron-Smith1 Clara Y. L. Chong1, Caroline A. Crowther1, Laura Galante1, Jane E. Harding1, Yannan Jiang9, Michael P. Meyer2, 4, Amber Milan1, 7, Mariana Muelbert1, Justin M. O'Sullivan1, Clare R. Wall10.
1Liggins Institute, University of Auckland, Auckland, New Zealand, 2Neonatal Unit, Kidz First, Middlemore Hospital, Auckland, New Zealand, 3Newborn Services, Auckland City Hospital, Auckland, New Zealand, 4Department of Pediatrics: Child and Youth Health, 5Department of Newborn Services, Mater Mothers' Hospital, Brisbane, QLD, Australia, 6Mater Research Institute, The University of Queensland, Brisbane, QLD, Australia, 7Food and Bio-based Products, AgResearch Grasslands, Palmerston North, New Zealand, 9Department of Statistics, Faculty of Science, University of Auckland, Auckland, New Zealand, 10Department of Nutrition, Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand.
1. Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller A-B, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. (2013) 10:1–14. doi: 10.1186/1742-4755-10-S1-S2
2. Isayama T, Lewis-Mikhael AM, O'Reilly D, Beyene J, McDonald SD. Health services use by late preterm and term infants from infancy to adulthood: a meta-analysis. Pediatrics. (2017) 140:e20170266. doi: 10.1542/peds.2017-0266
3. Wang M, Dorer DJ, Fleming MP, Catlin EA. Clinical outcomes of near-term infants. Pediatrics. (2004) 114:372–6. doi: 10.1542/peds.114.2.372
4. Escobar GJ, Greene JD. Rehospitalisation after birth hospitalisation: patterns among infants of all gestations. Arch Dis Child. (2005) 90:125–31. doi: 10.1136/adc.2003.039974
5. Hibbard JU, Wilkins I, Sun L, Gregory K, Haberman S, Hoffman M, et al. Respiratory morbidity in late preterm births. J Am Med Assoc. (2010) 304:419–25. doi: 10.1001/jama.2010.1015
6. Sharma D, Padmavathi V, Tabatabaii A, Farahbakhsh N, Vara Padmavathi I. Late preterm: a new high risk group in neonatology. J Matern Neonatal Med. (2019) 34:2717–30. doi: 10.1080/14767058.2019.1670796
7. Boyle EM, Poulsen G, Field DJ, Kurinczuk JJ, Wolke D, Alfirevic Z, et al. Effects of gestational age at birth on health outcomes at 3 and 5 years of age: population based cohort study. BMJ. (2012) 344:e896. doi: 10.1136/bmj.e896
8. Harris DL, Weston PJ, Harding JE. Incidence of neonatal hypoglycemia in babies identified as at risk. J Pediatr. (2012) 161:787–91. doi: 10.1016/j.jpeds.2012.05.022
9. Williams LZJ, Mcnamara D, Alsweiler JM. Intermittent hypoxemia in infants born late preterm: a prospective cohort observational study. J Pediatr. (2019) 204:89–95. doi: 10.1016/j.jpeds.2018.08.048
10. Schonhaut L, Armijo I, Perez M. Gestational age and developmental risk in moderately and late preterm and early term infants. Pediatrics. (2015) 135:e835–41. doi: 10.1542/peds.2014-1957
11. Johnson S, Evans TA, Draper ES, Field DJ, Manktelow BN, Marlow N, et al. Neurodevelopmental outcomes following late and moderate prematurity: a population-based cohort study. Arch Dis Childhood-Fetal Neonatal Ed. (2015) 100:F301–8. doi: 10.1136/archdischild-2014-307684
12. MacKay DF, Smith GC, Dobbie R, Pell JP. Gestational age at delivery and special educational need: retrospective cohort study of 407,503 schoolchildren. PLoS Med. (2010) 7:e1000289. doi: 10.1371/journal.pmed.1000289
13. Brumbaugh JE, Weaver AL, Myers SM, Voigt RG, Katusic SK. Gestational age, perinatal characteristics, and autism spectrum disorder: a birth cohort study. J Pediatr. (2020) 220:175–183.e8. doi: 10.1016/j.jpeds.2020.01.022
14. Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. (2006) 117:1253–61. doi: 10.1542/peds.2005-1368
15. Chan SHT, Johnson MJ, Leaf AA, Vollmer B, Chan SHT. Nutrition and neurodevelopmental outcomes in preterm infants: a systematic review. Acta Paediatr. (2016) 105:587–99. doi: 10.1111/apa.13344
16. Guihard-Costa A-M, Larroche J-C. Differential growth between the fetal brain and its infratentorial part. Early Hum Dev. (1990) 23:27–40. doi: 10.1016/0378-3782(90)90126-4
17. Kinney HC. The near-term (late preterm) human brain and risk for periventricular leukomalacia: a review. Semin Perinatol. (2006) 30:81–8. doi: 10.1053/j.semperi.2006.02.006
18. Lapillonne A, Bronsky J, Campoy C, Embleton N, Fewtrell M, Fidler Mis N, et al. Feeding the late and moderately preterm infant: a position paper of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on nutrition. J Pediatr Gastroenterol Nutr. (2019) 69:259–70. doi: 10.1097/MPG.0000000000002397
19. Eichenwald EC, Blackwell M, Lloyd JS, Tran T, Wilker RE, Richardson DK. Inter-neonatal intensive care unit variation in discharge timing: influence of apnea and feeding management. Pediatrics. (2001) 108:928–33. doi: 10.1542/peds.108.4.928
20. Blackwell MT, Eichenwald EC, McAlmon K, Petit K, Linton PT, McCormick MC, et al. Interneonatal intensive care unit variation in growth rates and feeding practices in healthy moderately premature infants. J Perinatol. (2005) 25:478–85. doi: 10.1038/sj.jp.7211302
21. McCormick MC, Escobar GJ, Zheng Z, Richardson DK. Place of birth and variations in management of late preterm (“near-term”) infants. Semin Perinatol. (2006) 30:44–7. doi: 10.1053/j.semperi.2006.01.012
22. Alexander T, Bloomfield FH. Nutritional management of moderate-late preterm infants: survey of current practice. J Paediatr Child Health. (2018) 5:338–42. doi: 10.1111/jpc.14201
23. Harding JE, Cormack BE, Alexander T, Alsweiler JM, Bloomfield FH. Advances in nutrition of the newborn infant. Lancet. (2017) 389:1660–8. doi: 10.1016/S0140-6736(17)30552-4
24. Bloomfield FH, Harding JE, Meyer MP, Alsweiler JM, Jiang Y, Wall CR, et al. The DIAMOND trial – DIfferent Approaches to MOderate & late preterm Nutrition: Determinants of feed tolerance, body composition and development: protocol of a randomised trial. BMC Pediatr. (2018) 18:1–6. doi: 10.1186/s12887-018-1195-7
25. de la Cruz D, Bazacliu C. Enteral feeding composition and necrotizing enterocolitis. Semin Fetal Neonatal Med. (2018) 23:406–10. doi: 10.1016/j.siny.2018.08.003
26. World Health Organization and United Nations Children's Fund (UNICEF). Baby-Friendly Hospital Initiative : Revised, Updated and Expanded for Integrated Care. World Health Organization (2009). Available online at: https://apps.who.int/iris/handle/10665/43593
27. Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. (2013) 13:59. doi: 10.1186/1471-2431-13-59
28. Walsh MC, Bell EF, Kandefer S, Saha S, Carlo WA, D'angio CT, et al. Neonatal outcomes of moderately preterm infants compared to extremely preterm infants. Pediatr Res. (2017) 82:297–304. doi: 10.1038/pr.2017.46
29. Porta R, Capdevila E, Botet F, Ginovart G, Moliner E, Nicolàs M, et al. Breastfeeding disparities between multiples and singletons by NICU discharge. Nutrients. (2019) 11:2191. doi: 10.3390/nu11092191
30. Embleton ND, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? Pediatrics. (2001) 107:270–3. doi: 10.1542/peds.107.2.270
31. Gianni ML, Roggero P, Liotto N, Amato O, Piemontese P, Morniroli D, et al. Postnatal catch-up fat after late preterm birth. Pediatr Res. (2012) 72:637–40. doi: 10.1038/pr.2012.128
32. Olhager E, Törnqvist C. Body composition in late preterm infants in the first 10 days of life and at full term. Acta Paediatr. (2014) 103:737–43. doi: 10.1111/apa.12632
33. Boss M, Gardner H, Hartmann P. Normal human lactation: closing the gap. F1000Research. (2018) 7:801. doi: 10.12688/f1000research.14452.1
34. Dewey KG, Nommsen-Rivers LA, Heinig MJ, Cohen RJ. Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics. (2003) 112:607–19. doi: 10.1542/peds.112.3.607
35. Lau C. Effect of stress on lactation. Pediatr Clin North Am. (2001) 48:221–34. doi: 10.1016/S0031-3955(05)70296-0
36. Zanardo V, Gambina I, Begley C, Litta P, Cosmi E, Giustardi A, et al. Psychological distress and early lactation performance in mothers of late preterm infants. Early Hum Dev. (2011) 87:321–3. doi: 10.1016/j.earlhumdev.2011.01.035
37. Hobbs AJ, Mannion CA, Mcdonald SW, Brockway M, Tough SC. The impact of caesarean section on breastfeeding initiation, duration and difficulties in the first four months postpartum. BMC Pregnancy Childbirth. (2016) 16:1–9. doi: 10.1186/s12884-016-0876-1
38. Matias SL, Dewey KG, Quesenberry CP, Gunderson EP. Maternal prepregnancy obesity and insulin treatment during pregnancy are independently associated with delayed lactogenesis in women with recent gestational diabetes mellitus. Am J Clin Nutr. (2014) 99:115–36. doi: 10.3945/ajcn.113.073049
39. Cregan MD, de Mello TR, Kershaw D, Mcdougall K, Hartmann PE. Initiation of lactation in women after preterm delivery. Acta Obstet Gynecol Scand. (2002) 81:870–7. doi: 10.1034/j.1600-0412.2002.810913.x
40. Battersby C, Longford N, Costeloe K, Modi N. Development of a gestational age-specific case definition for neonatal necrotizing enterocolitis. JAMA Pediatr. (2017) 171:256–63. doi: 10.1001/jamapediatrics.2016.3633
41. Brown K, Johnson MJ, Leaf AA. Suboptimal nutrition in moderately preterm infants. Acta Paediatr Int J Paediatr. (2014) 103:e510–2. doi: 10.1111/apa.12755
42. Hill PD, Aldag JC, Chatterton RT, Zinaman M. Comparison of milk output between mothers of preterm and term infants: the first 6 weeks after birth. J Hum Lact. (2005) 21:22–30. doi: 10.1177/0890334404272407
43. Radtke JV. The paradox of breastfeeding-associated morbidity among late preterm infants. J Obstet Gynecol Neonatal Nurs. (2011) 40:9–24. doi: 10.1111/j.1552-6909.2010.01211.x
44. Mitha A, Piedvache A, Khoshnood B, Fresson J, Glorieux I, Roué J, et al. The impact of neonatal unit policies on breast milk feeding at discharge of moderate preterm infants: the EPIPAGE-2 cohort study. Matern Child Nutr. (2019) 15:e12875. doi: 10.1111/mcn.12875
45. Moudi Z, Molashahi B, Imani M, Ansari H. Effects of a feasible supportive care program on breastfeeding behaviors and neonatal outcomes among the late preterm newborns in the south east of Iran. J Neonatal Nurs. (2017) 23:238–41. doi: 10.1016/j.jnn.2017.02.008
46. Jang GJ, Hong YR. Effects of a breastfeeding support program on the prevalence of exclusive breastfeeding and growth in late preterm infants. Child Heal Nurs Res. (2020) 26:90–7. doi: 10.4094/chnr.2020.26.1.90
47. Vennemann MM, Hauck FR, Thompson JMD, Tanabe KO, Moon RY. Breastfeeding and reduced risk of sudden infant death syndrome: a meta-analysis. Pediatrics. (2011) 128:103–10. doi: 10.1542/peds.2010-3000
48. Yan J, Liu L, Zhu Y, Huang G, Wang PP. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health. (2014) 14:1–11. doi: 10.1186/1471-2458-14-1267
Keywords: moderate preterm, late preterm, nutrition, breastmilk, intravenous fluid, Māori
Citation: Alexander T, Meyer M, Harding JE, Alsweiler JM, Jiang Y, Wall C, Muelbert M, Bloomfield FH and the DIAMOND Study Group (2022) Nutritional Management of Moderate- and Late-Preterm Infants Commenced on Intravenous Fluids Pending Mother's Own Milk: Cohort Analysis From the DIAMOND Trial. Front. Pediatr. 10:817331. doi: 10.3389/fped.2022.817331
Received: 17 November 2021; Accepted: 03 March 2022;
Published: 31 March 2022.
Edited by:
Letizia Capasso, Federico II University Hospital, ItalyReviewed by:
Arianna Aceti, University of Bologna, ItalyCopyright © 2022 Alexander, Meyer, Harding, Alsweiler, Jiang, Wall, Muelbert, Bloomfield and the DIAMOND Study Group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tanith Alexander, VGFuaXRoLmFsZXhhbmRlckBtaWRkbGVtb3JlLmNvLm56
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.