
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 10 March 2021
Sec. Neonatology
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.648979
This article is part of the Research Topic Intestinal Microbiota in the Pathogenesis and Management of Necrotizing Enterocolitis in Preterm Infants View all 11 articles
 Ying Li1,2,3†
Ying Li1,2,3† Chunhong Jia2,3†
Chunhong Jia2,3† Xiaojun Lin2†
Xiaojun Lin2† Lili Lin2
Lili Lin2 Lizhen Li2
Lizhen Li2 Xi Fan2
Xi Fan2 Xiaoxia Huang2
Xiaoxia Huang2 Zhanyuan Xu2
Zhanyuan Xu2 Huixin Wang2
Huixin Wang2 Fan Wu2,3*
Fan Wu2,3* Guosheng Liu1*
Guosheng Liu1*Background: Feeding intolerance (FI) is a common condition in premature infants that results in growth retardation and even necrotizing enterocolitis. The gut microbiome is linked to FI occurrence; however, the outcome after FI recovery is unclear.
Methods: Fecal samples were collected from 11 pairs of premature twins/triplets for 16S rRNA gene sequencing. Initial fecal samples were collected shortly after admission, and then every other week until 7 weeks or discharge.
Results: After FI recovery, there was no significant difference in the β-diversity of the intestinal flora between the FI group and the feeding tolerance (FT) group. By contrast, there was a significant difference in the β-diversity. Proteobacteria was the predominant phylum in the microbiome of the FI group, whereas Firmicutes was the predominant phylum in the microbiome of the FT group. The predominant bacteria with LDA >4 between the two groups at 13–15 days after birth, 19–28 days after birth, and at discharge were different, with the proportions of Bacillus, Clostridium butyricum, and Clostridium being highest in the FT group and Firmicutes, unidentified_Clostridiales, and Proteobacteria being highest in the FI group. Similarly, there were significant differences in the relative abundances of KEGG pathways, such as fatty acid metabolism, DNA repair and recombination proteins, energy metabolism, and amino acid metabolism, between the two groups (P < 0.01).
Conclusions: There was a significant difference in diversity of the intestinal flora after feeding intolerance recovery. Feeding intolerance may disturb the succession of the intestinal bacterial community.
Feeding intolerance (FI), a common condition in premature infants, is caused by an underdeveloped gastrointestinal tract or a disruption in intestinal function. It leads to the slow progression to enteral feeding and prolongs the length of hospitalization. More importantly, FI can lead to neonatal necrotizing enterocolitis (NEC), which threats the life of preterm infants (1).
The human gut is the natural habitat for a large and dynamic bacterial community, and the importance of resident bacteria on a host's physiology and pathology is gradually being elucidated. Recent studies have reported that the status of the intestinal microbiome is closely associated with several diseases, including NEC, inflammatory bowel disease, obesity, cardiovascular disease, and cancer (2–4).
To date, few studies have examined the composition of the gut microbial community in premature infants with FI. It has been reported that the intestinal microbiome changes drastically in cases of FI. Specifically, the proportion of Proteobacteria increases, while that of Firmicutes decreases (5, 6). However, no causative agent has been identified for FI. Furthermore, the composition of the intestinal microbiome after FI recovery remains unknown. The main reason for this lies in the complexity of the gut microbiome, especially in preterm infants. At the perinatal stage, the composition of the gut microbial community can be altered by various factors such as the delivery mode, antibiotic exposure, gestational age at birth, route of feeding, and gender (7, 8). In addition, the microbial composition varies greatly in preterm infants (9, 10). Therefore, the search for the causative agent is complicated by differences in the gut microbiome across different infants (11).
A previous study has demonstrated that the development of the gut microbiome is similar between twins, even within the complex environment of neonatal intensive care (12). Studying twins may provide unique insights into the differences in the gut microbial community. In this study, premature twins/triplets were enrolled to explore the diversity of the gut microbiome in the event of FI. To eliminate birth canal contamination, we only included cesarean section cases. Our results indicate that the effects of FI on the intestinal flora in premature infants continues long after recovery.
This study was conducted at the Third Affiliated Hospital of Guangzhou Medical University in Guangzhou, China. Fecal samples were collected from 11 pairs of premature twins/triplets for 16S rRNA gene sequencing from January 2020 to June 2020. Among these premature twins/triplets, eight pairs were discordant, that is, one infant exhibited clinical signs of FI, while the other infant did not. Initial fecal samples were collected in the first 2 days, and then every week until 7 weeks after birth or discharge. Good clinical practice (GCP) guidelines and regulations were followed for premature infants. Parents were well-informed about this study.
Premature infants diagnosed with FI presented with a gastric residual volume of more than 50% of the previous feeding volume, abdominal distension, gastric regurgitation and/or emesis. The disappearance of the aforementioned symptoms was defined as FI recovery (1, 13).
The inclusion criteria were as follows: (1) premature twins with a gestational age <34 weeks and a birth weight <2,000 g; (2) delivery in our hospital by cesarean section only; (3) premature infants were assigned to the FI group if they were diagnosed with FI and their condition improved to achieve full enteral feeding after treatment; and (4) premature infants were assigned to the FT group if they were not diagnosed with FI and achieved full enteral feeding during hospitalization.
The exclusion criteria were as follows: (1) infants who suffered from systemic inflammatory reaction, Crohn's disease, inflammatory bowel disease, congenital gastrointestinal anomalies, or necrotizing enterocolitis; (2) infants who had severe asphyxia, sepsis, coagulation disorders, or those who underwent abdominal surgery during hospitalization; (3) infants whose mother had chorioamnionitis or premature rupture of membranes, as well as perinatal medication and illness were unknown; (4) infants whose symptoms reappeared after FI recovery; and (5) infants who had not achieved full enteral feeding during hospitalization.
Fecal samples were collected with disposable sterile fecal collection tubes from soiled patient diapers and delivered to the laboratory in an ice box. Fecal samples were collected from the first defecation until 7 weeks after birth or before discharge. All samples were stored at −80°C.
Total genomic DNA was extracted using the CTAB/SDS method. The concentration and purity of DNA were assessed on 1% agarose gels. 16S rRNA v4 genes were amplified using a specific primer pair (16S v4 515F-806R) with a barcode. All PCR reactions consisted of 15 μL of Phusion High-Fidelity PCR Master Mix (New England Biolabs), 0.2 μM each of forward and reverse primers, and 10 ng of template DNA. The thermal cycling conditions were pre-denaturation at 98°C for 1 min, followed by 30 cycles of denaturation at 98°C for 10 s, annealing at 50°C for 30s, and elongation at 72°C for 5 min. PCR products was purified with the Qiagen Gel Extraction Kit (Qiagen, Germany). Sequencing libraries were generated using the TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina, USA) following the manufacturer's protocol. The library was assessed using the Qubit® 2.0 Fluorometer (Thermo Scientific) and the Agilent Bioanalyzer 2100. The library was sequenced using the Illumina NovaSeq platform and 250 bp paired-end reads were generated according to standard protocols by Novogene Co., Ltd. (Beijing, China).
Paired-end reads derived from the original DNA fragments were merged using FLASH, which merged paired-end reads when there were overlaps between reads1 and reads2. Paired-end reads were assigned to each sample according to unique barcodes. Sequences were analyzed using QIIME software (Quantitative Insights Into Microbial Ecology), and in-house Perl scripts were used to analyze α- (within samples) and β- (among samples) diversity. First, reads were filtered by QIIME quality filters. Thereafter, we used pick_de_novo_otus.py to identify the operational taxonomic units (OTUs) by generating an OTU table. Sequences with ≥97% similarity were assigned to the same OTU. We selected a representative sequence for each OTU and used the RDP classifier to annotate taxonomic information for each representative sequence. To compute the α-diversity, we rarified the OTU table and calculated three metrics: Chao1, observed species, and Shannon index. Rarefaction curves were generated based on these three metrics. QIIME calculated both weighted and unweighted unifrac, which are phylogenetic measures of β-diversity. We used unweighted unifrac for principal coordinate analysis (PCoA) and unweighted pair group method with arithmetic mean (UPGMA) clustering. PCoA generated principal coordinates and visualized them from complex, multidimensional data. To mine deeper data of the differences in microbial diversity between the samples, significance tests were conducted using the t-test, MetaStat, LEfSe, Anosim, and MRPP.
All clinical data of the preterm infants were analyzed using SPSS 21.0 software. Continuous variables were reported as medians (interquartile interval, IQR), and categorical data were presented as ratios or percentages. A t-test and chi-square test were used to examine the significance of the differences in pairwise comparisons of the samples. Differences with P < 0.05 were considered statistically significant.
The 11 pairs of premature twins/triplets, including five pairs of monozygotic twins, three pairs of heterozygous twins, and three pairs of triplets, met the inclusion criteria. Among these premature twins/triplets, eight pairs were discordant, that is, one infant exhibited clinical signs of FI, while the other infant did not. The other three pairs exhibited no clinical signs of FI. Among the FI group the fecal flora from 5A to 9C failed to amplification. In summary, there were 14 cases in the FT group and nine cases in the FI group. All premature infants were fed with preterm formula after birth due to lack of breast milk. Only two pairs received antenatal antibiotics, and none of them received probiotics during hospitalization. Latamoxef was the most commonly prescribed antibiotic, and piperacillin was used occasionally, that is, if clinical conditions supported its use. The antibiotics were rarely used for more than 14 days. The demographic characteristics and the basic information of the twins and triplets is shown in Tables 1, 2, respectively.
A total of 131 fecal samples, including 70 in the FT group and 61 in the FI group, were collected. Among them, 33 samples could not be amplified. A total of 82 fecal samples were analyzed, including 46 fecal samples from the FT group and 36 fecal samples from the FI group. The information of the fecal samples is shown in Table 3 and Supplementary Table 1. The flowchart of our experiment is shown in Figure 1.
The OTUs, rarefaction curve, and rank abundance curve are shown in Supplementary Figures 1, 2. At the phylum level, the main OTUs in the FT group were Firmicutes (93.63%), Actinobacteria (5.41%), and Proteobacteria (0.81%), whereas those in the FI group were Firmicutes (79.40%), Proteobacteria (18.19%), and Actinobacteria (2.24%), as shown in Figure 2A. At the genus level, the top six OTUs in the FT group were unidentified_ Clostridiales (40.32%), Enterococcus (24.88%), Streptococcus (10.18%), Clostridioides (6.65%), Staphylococcus (5.87%), and Bifidobacterium (4.82%), whereas those in the FI group were unidentified_Clostridiales (32.18%), Streptococcus (9.50%), Staphylococcus (9.44%), Enterococcus (8.90%), Paenibacillus (7.14%), and Kluyvera (4.74%), as shown in Figure 2B. The observed species was similar between two groups as shown in Figure 2C. Additionally, there was no difference in the alpha-diversity presented with Shannon index, Simpson's index and ACE index, respectively, as shown in Figures 2D–F.
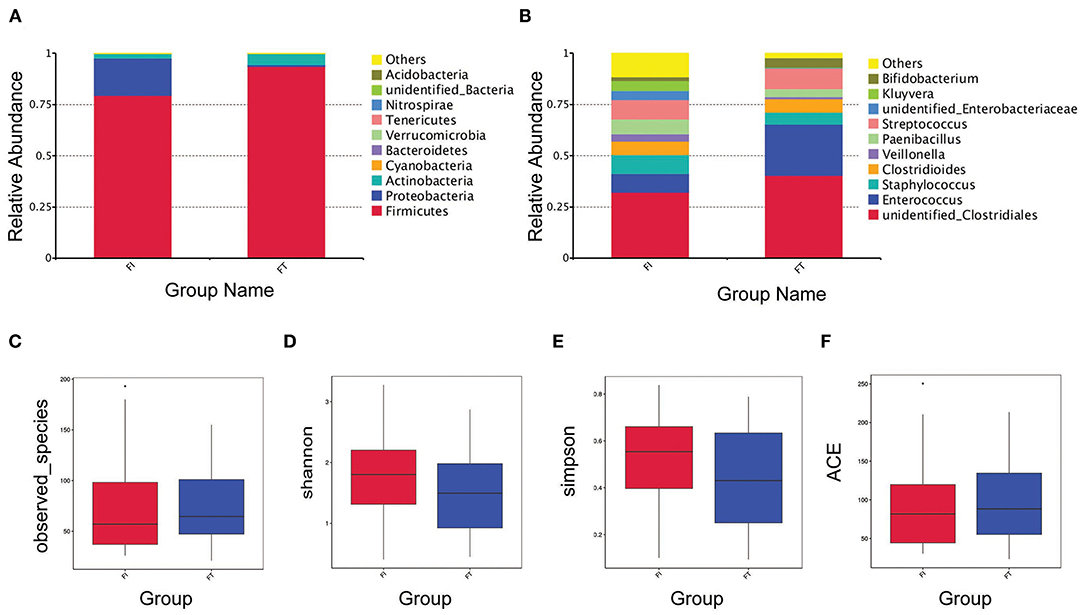
Figure 2. Overview of the preterm infant gut microbiome and the α-Diversity. Relative abundance of bacteria at phylum level (A) and at genus level (B) between FT and FI groups. α-diversity analysis in the sample groups including observed-species (C), Shannon index (D), Simpson index (E) and ACE index (F).
The box diagram based on unweighted and weighted UniFrac distance showed that there was a statistically significant difference in the β-diversity of intestinal flora between the FT group and the FI group after FI recovery (P < 0.05). PCoA analysis showed that similarities in the microbial community in the FT group were higher than those in the FI group (Figure 3). In particular, when the LDA score was set to >4, LEfSe analysis revealed that the predominant bacteria in the FI group were Proteobacteria, whereas those in the FT group were Firmicutes after FI recovery (Figure 4 and Supplementary Figure 3). As mentioned above, not all the twins or triplets were discordant, which means one of the pair suffered FI and the other did not. In 11 pairs of premature twins/triplets, eight pairs of them were discordant. We further analyzed the only eight discordant pairs, as it was shown in the Supplementary Figures 4, 5. There was still significant differences in the β-diversity between two groups based on the eight discordant pairs.
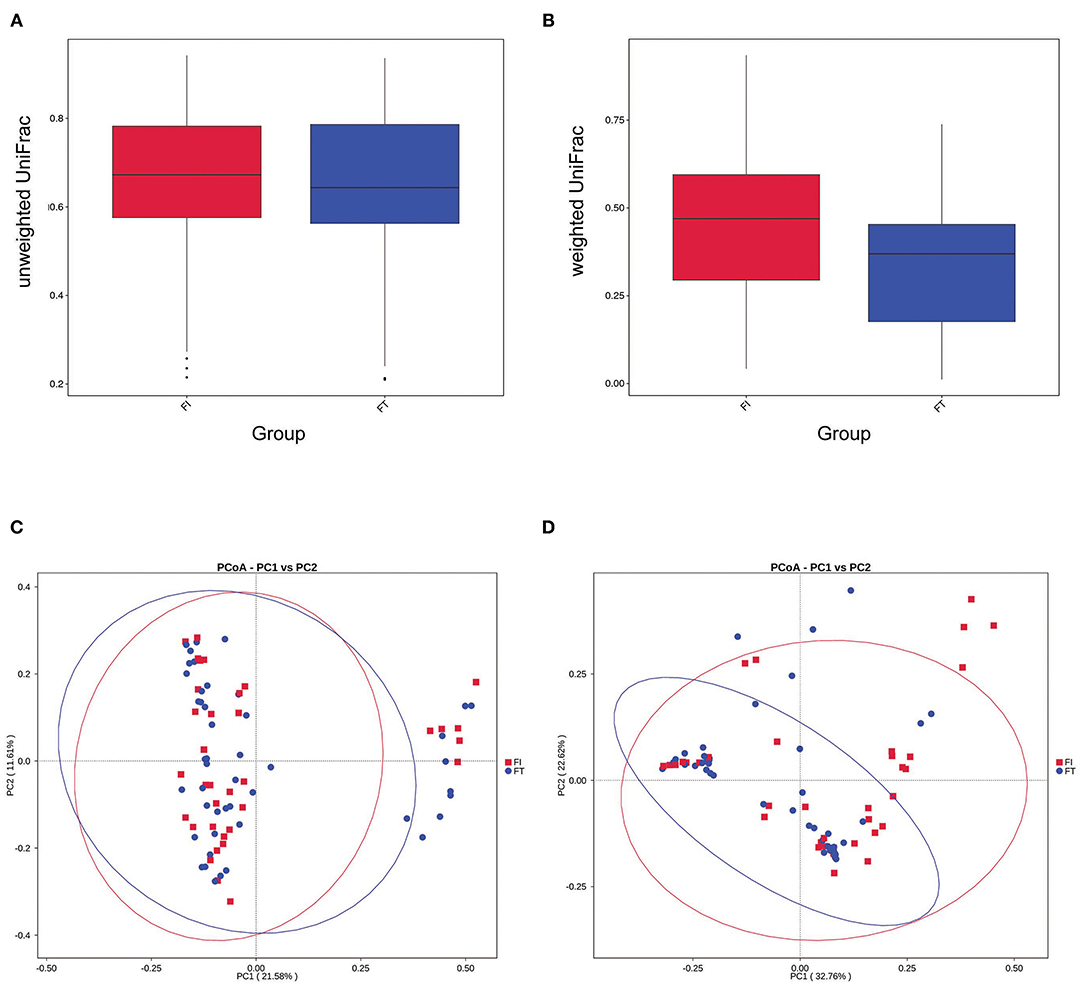
Figure 3. β- diversity analysis. (A) Box diagram based on unweighted UniFrac beta diversity P = 0.055 (t-test) and P = 0.040 (two-wilcox). (B) Box diagram based on weighted UniFrac beta diversity P < 0.001. (C) PCoA analysis based on unweighted UniFrac distance. (D) PCoA analysis based on weighted UniFrac distance.
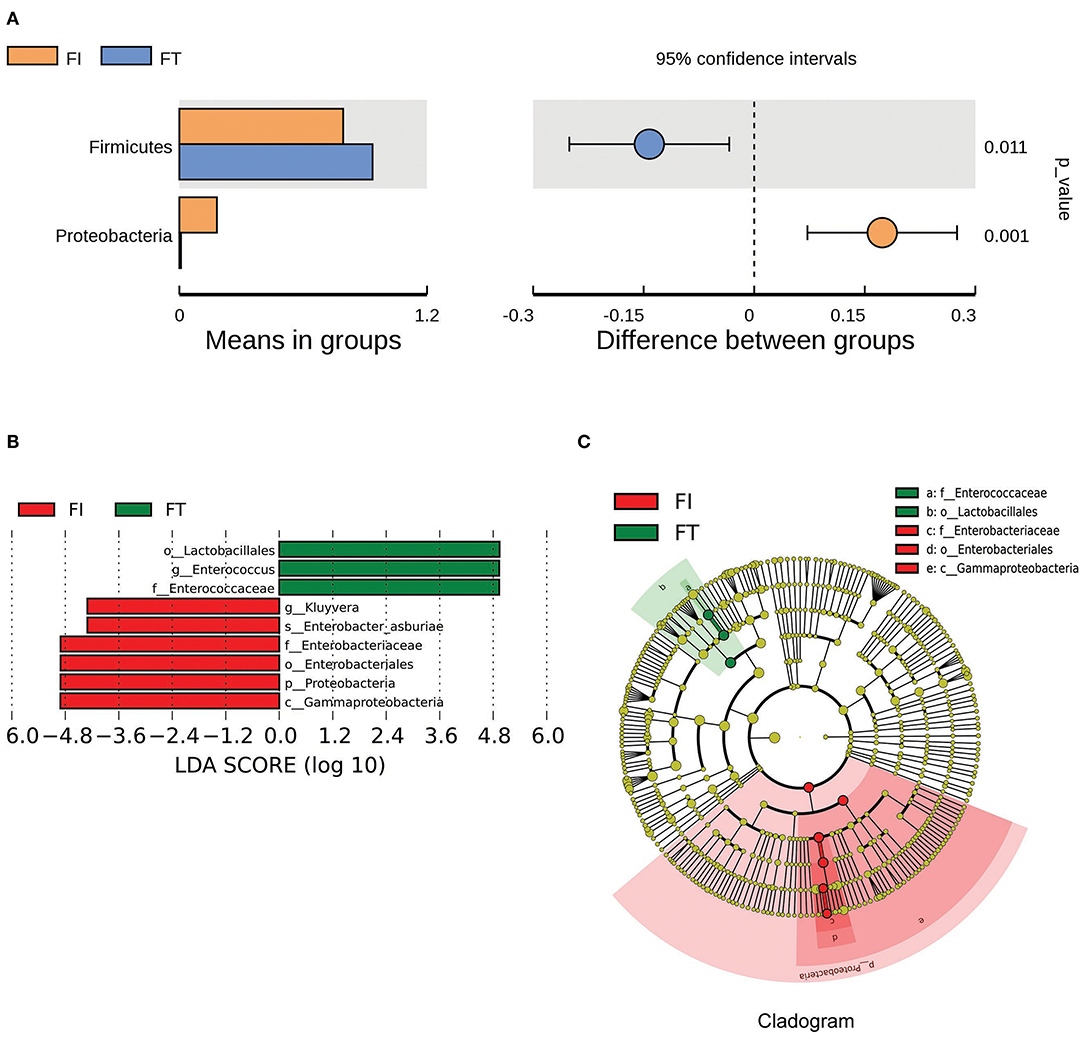
Figure 4. The differential microbes between FI and FT groups. (A) Analysis of difference in microbes between two groups using t-test. (B) LDA value distribution. (C) Evolutionary cladistics (phylogenetic distribution).
To examine the developmental trajectory of the fecal microbiota after FI recovery, all samples were analyzed according to the collection time. The samples collected at 7–8 days after birth were grouped as time 1, 13–15 days after birth were grouped as time 2, 19–28 days after birth were grouped as time 3, and the remaining samples were grouped as time 4. LEfSe analysis revealed that the predominant bacteria with linear discriminant analysis (LDA) >4 in the FT group were Bacillus, Clostridium_Butyricum, and Clostridiales at time 2, time 3, and time 4, respectively. However, the predominant bacteria with LDA >4 in the FI group were Firmicutes, unidentified_Clostridiales, and γ-Proteobacteria at time 2, time 3, and time 4, respectively, as shown in Figure 5. Taking into account the differences in body weight and gestational age of subjects, stratification analysis was further conducted. According to weight or gestational age, it was found that there was still a statistically significant differences in the flora between FI and FT groups, as it was shown in the Supplementary Figures 6, 7. Because the samples are limited, the conclusion may be not sufficient.
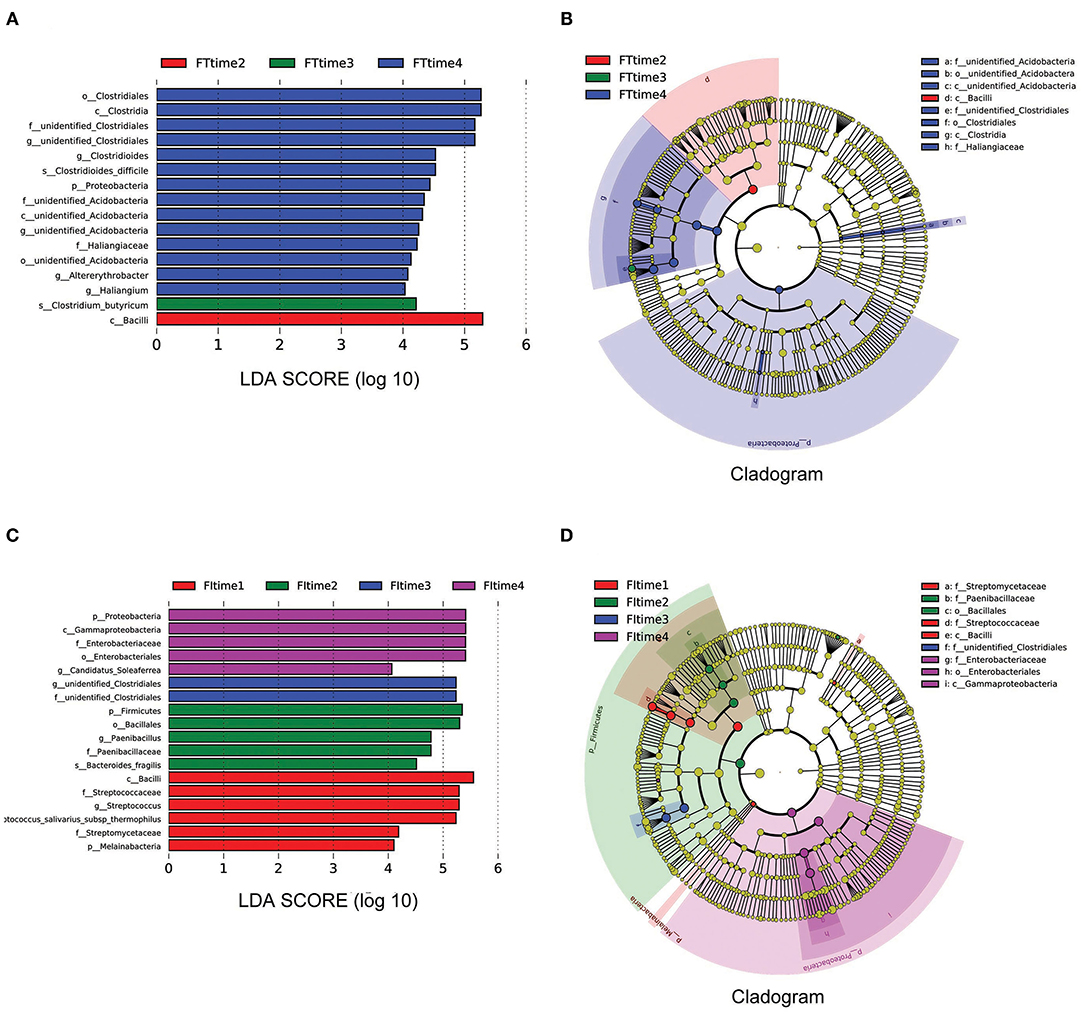
Figure 5. LEfSe analysis based on the different time. LEfSe analysis (A) and Cladogram (B) in the FT groups over time. LEfSe analysis (C) and Cladogram (D) in the FT groups over time. Time 1: 7–8 days after birth, Time 2: 13–15 days after birth, Time 3: 19–28 days after birth, Time 4: until the time before discharge.
Tax 4Fun was used to predict bacterial functions in the fecal samples. After FI recovery, the relative abundances of the KEGG pathways of fatty acid metabolism, DNA repair and recombination proteins, mitochondrial biogenesis, and terpenoid backbone biosynthesis in the FT group were significantly higher than those in the FI group (P < 0.01), whereas energy metabolism, amino acid metabolism, bacteria motility proteins, biofilm formation in Escherichia coli, cationic antimicrobial peptide resistance, and biofilm formation in Vibrio cholerae were significantly higher in the FI group than those in the FT group (P < 0.01), as shown in Figure 6.
The initial acquisition and early development of the intestinal microbiome during infancy are important to human health (14). It is now recognized that intestinal microbes are key modulators of disease status. Feeding intolerance (FI) is a common condition in preterm neonates, and it disrupts the homeostasis of the intestinal tract (15). However, the search for its causative agent during early life is complicated due to the mode of birth, antibiotic administration, environment, nutrition, and other individual differences (16). A previous study has demonstrated that microbiome development in preterm twins is similar (12). Thus, in this study, the gut microbiome of premature twins/triplets was examined, where one of the twins exhibited clinical signs of FI, while the other infant did not. To eliminate birth canal contamination, only cesarean section cases were included.
We found that the microbial β-diversity differed significantly between the FT group and the FI group after FI recovery. Enterobacteria from the Proteobacteria phylum were the predominant organisms in the FI group, whereas Enterococcus from the Firmicutes phylum were the predominant organisms in the FT group. As previously reported, there was an increase in the relative abundance of Proteobacteria before and during the diagnosis of NEC and feeding intolerance. Enterococcus faecalis plays an immunomodulatory role by decreasing TGF-β and increasing IL-8 levels, and it also regulates the inflammatory response through TLR3, TLR4, TLR9, and TRAF6, which has a protective effect on the body. Our results showed that after FI recovery, the gut environment remained different from preterm infants without FI for at least 7 weeks after birth or discharge, which was also reflected in the different KEEG pathways between the two groups of premature infants. Our results were inconsistent with those of Yuan et al. who reported that Firmicutes was the predominant intestinal flora after FI recovery (6). We speculate the reason for this difference is as follows. Firstly, FI occurred in less than a week in our study, while it occurred at 3–18 d after birth in their study. Secondly, they collected stool samples 1 week after FI recovery, but we collected stool samples from the day of FI recovery to several weeks after recovery. As the development of the gut microbial environment has recently been shown to evolve in a pattern associated with PMA (postmenstrual age, that is, gestational age at birth plus week of life) (17), the different collection times of stool samples, which correspond to different PMAs, may have affected our results.
Our study also found that after FI recovery, the bacterial succession in preterm infants of the FI group was different from that of the FT group in the several weeks after birth. The fecal microbiota of FI infants was dominated by Bacilli at early PMA, followed by Clostridia and γ-Proteobacteria, while that of FT infants was dominated by Bacilli and Clostridia, with the latter class remaining unchanged until 7 weeks after birth before discharge. Grier et al. reported (18) an association between a diagnosis of NEC and microbial reverse transitions from γ-Proteobacteria to Bacilli, Clostridia, and γ-Proteobacteria or a delayed transition from Bacilli to Clostridia. Furthermore, previous studies have revealed that FI and NEC have similarities in clinical manifestations and intestinal flora changes (6, 19). We found that FI may affect the evolution of the gut microbiome in a manner similar to that of NEC. Therefore, enteral feeding of preterm infants that have recovered from FI should be monitored for several weeks after birth.
The accumulated evidence indicates that microbiota development is shaped by host biology and related to the environment. Previous studies have shown that the composition of the intestinal flora of preterm infants was affected by many factors, such as maternal illness and nutritional status during pregnancy (20), delivery mode (21), feeding mode (22, 23), gestational age (24, 25), type and duration of antibiotic use (26, 27), intrauterine or nosocomial infection (28, 29), environment in NCU (30), use of probiotics and so on (31, 32). In our study, we choose those infants that were deliverd by cesarean, no breastmilk feeding, no NEC or sepsis, no probiotics and asphyxia, so we did not analyze those factors mentioned above. As previous studies have shown that the smaller the gestational age, the lower the diversity of intestinal flora, and the later the colonization of anaerobic bacteria such as bifidobacteria and lactic acid bacteria (24, 25). In this study, the range of the gestational age in eight pairs of infants discordant with FI were 28–32 weeks, and the range of the gestational age in three pairs of preterm infants without FI were 32–34 weeks, all of which are extremely premature infants. After preliminary stratified analysis according to gestational age, the main outcome is similar to the original results. Whether gestational age is an important factor that affects the composition of the intestinal flora after FI is cured is not clear, which need a larger sample to conclude. Previous studies have pointed out that the empirical use of antibiotics (including antenatal antibiotics and postnatal antibiotics) can lead to an decrease in Alpha diversity, delayed colonization of anaerobic bacteria, and the impact of antibiotics on the intestinal flora can last several months after the drug was stopped (33, 34). The pre-and post-natal antibiotics used in our study were Piperacillin/cephalosporin. Because there were only two pairs of infants (No 3 and No 5) had antenatal antibiotics, we did not analyze the effect of this factor on the intestinal microflora. In addition, although all preterm infants received either or both of these antibiotics mentioned above after birth, and there were differences in the type and duration of antibiotics usage among different pairs of infants, the type and duration of postnatal antibiotics used between the same pair of twins/triplets are relatively similar, so we did not analyze the intestinal flora of the two groups of preterm infants based on the duration and type of antibiotics under limited numbers.
There were several limitations in this study. Firstly, the sample size was relatively small. However, our study is the first longitudinal analysis of the development of the gut microbiome in premature twins/triplets, where one infant had FI and the other did not. Secondly, this was a single-center study, and it was difficult to eliminate inclusion bias. Thirdly, because most of our initially collected stool samples provided insufficient DNA for sequencing, we only analyzed the stool samples of infants that recovered from FI. Last but not least, we have not been able to compare the intestinal flora of different preterm infants according to the use of antibiotics, but we try to make up for this deficiency by selecting those premature twin/multiple fetuses with similar antibiotic usage. Regardless, the changes in the major bacterial phyla reported in this study are consistent with the current understanding of the effect of those microbes. In conclusion, we found that FI may disturb the diversity of intestinal flora even after FI recovery.
The authors acknowledge that the data presented in this study must be deposited and made publicly available in an acceptable repository, prior to publication. The data presented in the study are deposited in the (https://www.ncbi.nlm.nih.gov/sra/PRJNA698588, PRJNA698588) repository, accession number (SUB8976175).
The studies involving human participants were reviewed and approved by Institutional Review Board of the Third Affiliated Hospital of Guangzhou Medical University ([2020]009). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
FW and GL: conceptualization, project administration, formal analysis, and writing - review & editing. YL and CJ: writing -original draft, writing - review & editing, data curation, formal analysis, and methodology. XL, LLin, XF, and XH: methodology, investigation, validation, and formal analysis. HW and ZX: investigation, validation, and supervision. All authors contributed to the article and approved the submitted version.
This study was supported by National Natural Science Foundation of China (NSFC) (81741083 and 81801492), Natural Science Foundation of Guangdong Province (GDNSF) (2018A030310598), Medical Scientific Research Foundation of Guangdong Province (A2019102, A2019069), and General Guidance Project of Guangzhou Health Technology (20191A010064).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank all participating children and their parents. The International Science Editing Compuscript Ltd. provides language help.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2021.648979/full#supplementary-material
1. Fanaro S. Feeding intolerance in the preterm infant. Early Hum Dev. (2013) 89(Suppl. 2):S13–20. doi: 10.1016/j.earlhumdev.2013.07.013
2. Althani AA, Marei HE, Hamdi WS, Nasrallah GK, El Zowalaty ME, Al Khodor S, et al. Human microbiome and its association with health and diseases. J Cell Physiol. (2016) 231:1688–94. doi: 10.1002/jcp.25284
3. Rios-Covian D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilan CG, Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol. (2016) 7:185. doi: 10.3389/fmicb.2016.00185
4. Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. (2017) 15:55–63. doi: 10.1038/nrmicro.2016.142
5. Putignani L, Del Chierico F, Petrucca A, Vernocchi P, Dallapiccola B. The human gut microbiota: a dynamic interplay with the host from birth to senescence settled during childhood. Pediatr Res. (2014) 76:2–10. doi: 10.1038/pr.2014.49
6. Yuan Z, Yan J, Wen H, Deng X, Li X, Su S. Feeding intolerance alters the gut microbiota of preterm infants. PLoS ONE. (2019) 14:e0210609. doi: 10.1371/journal.pone.0210609
7. Lim ES, Zhou Y, Zhao G, Bauer IK, Droit L, Ndao IM, et al. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nat Med. (2015) 21:1228–34. doi: 10.1038/nm.3950
8. Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. (2016) 352:539–44. doi: 10.1126/science.aad9378
9. Dicksved J, Floistrup H, Bergstrom A, Rosenquist M, Pershagen G, Scheynius A, et al. Molecular fingerprinting of the fecal microbiota of children raised according to different lifestyles. Appl Environ Microbiol. (2007) 73:2284–9. doi: 10.1128/AEM.02223-06
10. Simioni J, Hutton EK, Gunn E, Holloway AC, Stearns JC, McDonald H, et al. A comparison of intestinal microbiota in a population of low-risk infants exposed and not exposed to intrapartum antibiotics: the Baby & Microbiota of the Intestine cohort study protocol. BMC Pediatr. (2016) 16:183. doi: 10.1186/s12887-016-0724-5
11. Stewart CJ, Marrs EC, Nelson A, Lanyon C, Perry JD, Embleton ND, et al. Development of the preterm gut microbiome in twins at risk of necrotising enterocolitis and sepsis. PLoS ONE. (2013) 8:e73465. doi: 10.1371/journal.pone.0073465
12. Moore TA, Wilson ME. Feeding intolerance: a concept analysis. Adv Neonatal Care. (2011) 11:149–54. doi: 10.1097/ANC.0b013e31821ba28e
13. Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. (2011) 27:2957–63. doi: 10.1093/bioinformatics/btr507
14. Lindberg TP, Caimano MJ, Hagadorn JI, Bennett EM, Maas K, Brownell EA, et al. Preterm infant gut microbial patterns related to the development of necrotizing enterocolitis. J Matern Fetal Neonatal Med. (2020) 33:349–58. doi: 10.1080/14767058.2018.1490719
15. Chong CYL, Bloomfield FH, O'Sullivan JM. Factors affecting gastrointestinal microbiome development in neonates. Nutrients. (2018) 10:274. doi: 10.3390/nu10030274
16. Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome. (2017) 5:31. doi: 10.1186/s40168-017-0248-8
17. Grier A, Qiu X, Bandyopadhyay S, Holden-Wiltse J, Kessler HA, Gill AL, et al. Impact of prematurity and nutrition on the developing gut microbiome and preterm infant growth. Microbiome. (2017) 5:158. doi: 10.1186/s40168-017-0377-0
18. Elgin TG, Kern SL, McElroy SJ. Development of the neonatal intestinal microbiome and its association with necrotizing enterocolitis. Clin Ther. (2016) 38:706–15. doi: 10.1016/j.clinthera.2016.01.005
19. La Rosa PS, Warner BB, Zhou Y, Weinstock GM, Sodergren E, Hall-Moore CM, et al. Patterned progression of bacterial populations in the premature infant gut. Proc Natl Acad Sci USA. (2014) 111:12522–7. doi: 10.1073/pnas.1409497111
20. Chu DM, Meyer KM, Prince AL, Aagaard KM. Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microbes. (2016) 7:459–70. doi: 10.1080/19490976.2016.1241357
21. Akagawa S, Tsuji S, Onuma C, Akagawa Y, Yamaguchi T, Yamagishi M, et al. Effect of delivery mode and nutrition on gut microbiota in neonates. Ann Nutr Metab. (2019) 74:132–9. doi: 10.1159/000496427
22. Cong X, Judge M, Xu W, Diallo A, Janton S, Brownell EA, et al. Influence of feeding type on gut microbiome development in hospitalized preterm infants. Nur Res. (2017) 66:123–33. doi: 10.1097/Nnr.0000000000000208
23. Lyons KE, Ryan CA, Dempsey EM, Ross RP, Stanton C. Breast milk, a source of beneficial microbes and associated benefits for infant health. Nutrients. (2020) 12:1039. doi: 10.3390/nu12041039
24. Chernikova DA, Madan JC, Housman ML, Zain-ul-Abideen M, Lundgren SN, Morrison HG, et al. The premature infant gut microbiome during the first 6 weeks of life differs based on gestational maturity at birth. Pediatr Res. (2018) 84:71–9. doi: 10.1038/s41390-018-0022-z
25. Yee AL, Miller E, Dishaw LJ, Gordon JM, Ji M, Dutra S, et al. Longitudinal microbiome composition and stability correlate with increased weight and length of very-low-birth-weight infants. Msystems. (2019) 4:e00229–18. doi: 10.1128/mSystems.00229-18
26. Greenwood C, Morrow AL, Lagomarcino AJ, Altaye M, Taft DH, Yu ZT, et al. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of enterobacter. J Pediatr. (2014) 165:23–9. doi: 10.1016/j.jpeds.2014.01.010
27. Ruoss JL, Bazacliu C, Russell JT, Cruz D, Li N, Gurka MJ, et al. Routine early antibiotic use in symptomatic preterm neonates: a pilot randomized controlled trial. J Pediatr. (2021) 229:294–8 e293. doi: 10.1016/j.jpeds.2020.09.056
28. Mukhopadhyay S, Puopolo KM. Clinical and microbiologic characteristics of early-onset sepsis among very low birth weight infants: opportunities for antibiotic stewardship. Pediatr Infect Dis J. (2017) 36:477–81. doi: 10.1097/INF.0000000000001473
29. Raspini B, Porri D, De Giuseppe R, Chieppa M, Liso M, Cerbo RM, et al. Prenatal and postnatal determinants in shaping offspring's microbiome in the first 1000 days: study protocol and preliminary results at one month of life. Ital J Pediatr. (2020) 46:45. doi: 10.1186/s13052-020-0794-8
30. Brooks B, Olm MR, Firek BA, Baker R, Geller-McGrath D, Reimer SR, et al. The developing premature infant gut microbiome is a major factor shaping the microbiome of neonatal intensive care unit rooms. Microbiome. (2018) 6:112. doi: 10.1186/s40168-018-0493-5
31. Kaban RK, Wardhana BH, Rohsiswatmo R, Handryastuti S, Amelia N, Muktiarti D, et al. Lactobacillus reuteri DSM 17938 improves feeding intolerance in preterm infants. Pediatr Gastroenterol Hepatol Nutr. (2019) 22:545–53. doi: 10.5223/pghn.2019.22.6.545
32. Underwood MA. Probiotics and human milk oligosaccharides in premature infants. Neoreviews. (2019) 20:e1–11. doi: 10.1542/neo.20-1-e1
33. Zwittink RD, Renes IB, van Lingen RA, van Zoeren-Grobben D, Konstanti P, Norbruis OF. Association between duration of intravenous antibiotic administration and early-life microbiota development in late-preterm infants. Eur J Clin Microbiol Infect Dis. (2018) 37:475–83. doi: 10.1007/s10096-018-3193-y
Keywords: premature infants, twins, triplets, feeding intolerance, microbiome
Citation: Li Y, Jia C, Lin X, Lin L, Li L, Fan X, Huang X, Xu Z, Wang H, Wu F and Liu G (2021) The Diversity of the Intestinal Flora Disturbed After Feeding Intolerance Recovery in Preterm Twins. Front. Pediatr. 9:648979. doi: 10.3389/fped.2021.648979
Received: 03 January 2021; Accepted: 16 February 2021;
Published: 10 March 2021.
Edited by:
Yuan Shi, Children's Hospital of Chongqing Medical University, ChinaReviewed by:
Lu-Quan Li, Chongqing Medical University, ChinaCopyright © 2021 Li, Jia, Lin, Lin, Li, Fan, Huang, Xu, Wang, Wu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guosheng Liu, dGxnc0BqbnUuZWR1LmNu; Fan Wu, Z2R3dWZhbkAxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.