- 1Department of Biology and Chemistry, School of Health Sciences, Liberty University, Lynchburg, VA, United States
- 2Department of Biology, School of Sciences and Agriculture, Ferrum College, Ferrum, VA, United States
Schistosomiasis is a group of both acute and chronic parasitic trematode infections of the genus Schistosoma. Research into schistosomiasis has been minimal, leading to its classification as a neglected tropical disease, yet more than 140 million people are infected with schistosomes globally. There are no treatments available for early-stage infections, schistosomal dermatitis, or Katayama syndrome, other than symptomatic control with steroids and antihistamines, as the maturing organisms seem to be mostly resistant to typical antiparasitics. However, praziquantel (PZQ) has been the drug of choice for schistosomiasis for decades in the latter stages of the disease. Though it is effective against all three clinically relevant species, heavy reliance on PZQ has led to concerns of schistosome resistance, especially in areas that have implemented this drug in mass drug administration (MDA) programs. This article summarizes the available literature concerning the available evidence for and against a warranted concern for PZQ resistance, genomic studies in schistosomes, proposed mechanisms of resistance, and future research in alternative methods of schistosomiasis treatment.
1 Introduction
Drug resistance is a well-known and key phenomenon that has impacted the effectiveness of medications such as some antibiotics and antihelminthics for treating poultry, livestock, and humans (Mohamed et al., 2022; Malik et al., 2022; Qamar and Alkheraije, 2023; Li et al., 2023). Resistance is defined as a significant increase in the frequency and unresponsiveness of individuals in a susceptible population to a compound (Prichard et al., 1980; Coles and Kinoti, 1997; Greenberg, 2013). Unlike tolerance, resistance is heritable and due to a population’s previous drug exposure (Prichard et al., 1980; Greenberg, 2013).
Emerging drug resistance against the broad-spectrum antihelminthic drug praziquantel (PZQ) (Figure 1) has been a growing public health concern (Berger et al., 2021; Cotton and Doyle, 2022). There has been much discussion as to whether PZQ resistance is imminent or widespread (Danso-Appiah and De Vlas, 2002; Botros et al., 2005; Melman et al., 2009; Crellen et al., 2016; Fukushige et al., 2021). Following its discovery in the 1970s by the pharmaceutical companies Merck and Bayer, PZQ has become the drug of choice to treat schistosomiasis, the second most debilitating tropical disease after malaria (Abdel Aziz et al., 2022). The causative schistosomes, or blood flukes, are also responsible for approximately 200,000 annual deaths and affect over 250 million people globally, making them the most important helminthic infectious agents (World Health Organization, 2021).
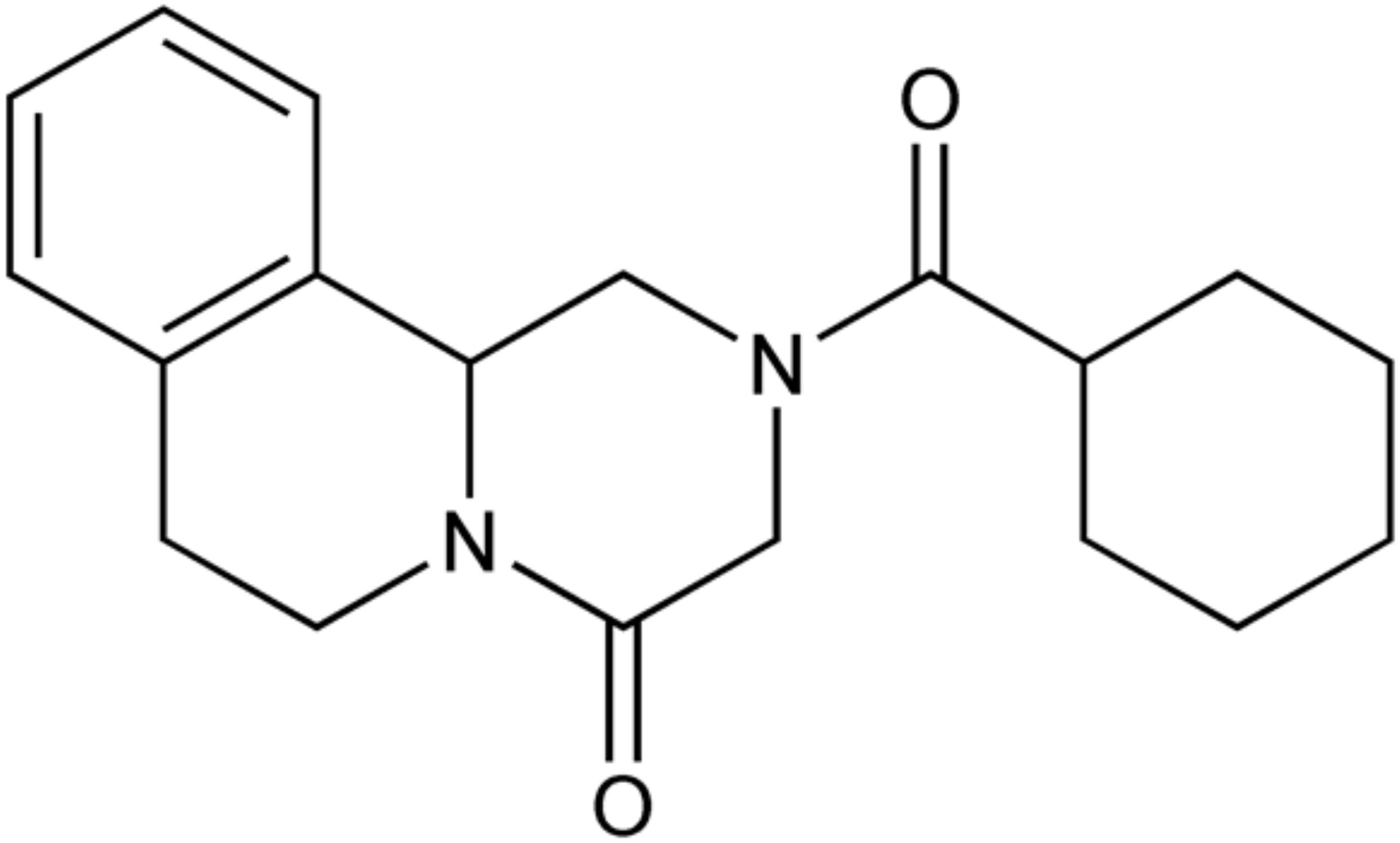
Figure 1. Chemical structure of praziquantel (Royal Society of Chemistry, 2024).
Schistosomiasis is caused by three main species: Schistosoma mansoni is the most widespread and is the only species known to exist in the Western Hemisphere, S. haematobium is found in Africa and the Middle East, and S. japonicum is found in regions of Southeast Asia (World Health Organization, 2023a). Schistosomes have a complex life cycle that involves two hosts—a mammalian definitive host and a freshwater snail from the genus Biomphalaria as an intermediate host. Schistosoma eggs are released from either the feces or urine of an infected individual, depending on the species of schistosome (Centers for Disease Control and Prevention, 2024). The larvae, called miracidia, infect the suitable snail host for two generations to produce free-swimming larval cercariae. These cercariae then shed their tails, penetrate the skin of the mammalian host, and become schistosomulae (Centers for Disease Control and Prevention, 2024). Following infection, the schistosomulae mature and undergo sexual reproduction. The adult females lay 300 – 3,000 eggs daily until the end of the worm’s lifespan—about three to five years (Cahill, 2011). Some of the eggs build up in the host’s tissue, causing inflammation and a host immune response that results in the disease’s morbidities (Burke et al., 2009; Colley et al., 2014). Other eggs are passed through the intestinal or bladder mucosa and are expelled in the feces or urine, completing the cycle (Cahill, 2011).
In endemic areas, MDA of PZQ is essential for schistosomiasis control as a form of preventative chemotherapy due to the drug’s affordability, availability, minimal side effects, and effectiveness against human infections of trematodes and cestodes (Cioli et al., 2014; Crellen et al., 2016; Nogueira et al., 2022; Villamizar-Monsalve et al., 2024). This extensive use has raised concerns about emerging drug resistance, which may develop following prolonged and repeated application, such as in MDA (Coles and Kinoti, 1997; Geerts et al., 1997).
Laboratory-induced resistance to PZQ have been successful, and there have been multiple reports of reduced PZQ efficacy in the field following continuous drug exposure (Ismail et al., 1994; Fallon, 1998; Ismail et al., 1999; Melman et al., 2009; Couto et al., 2011; Li et al., 2011; Lotfy et al., 2015; Crellen et al., 2016). However, it has also been observed that reduced PZQ sensitivity characteristics have dissipated after several generations, even in the presence of drug pressure (Botros et al., 2005; Melman et al., 2009). In addition, multiple areas have endured several years of PZQ treatment, and the efficacy rates remain high (Seto et al., 2011; Mduluza et al., 2020; Tetteh-Quarcoo et al., 2020).
This review draws together previous and recent literature about PZQ efficacy across the three main schistosome species in the context of emerging PZQ resistance. Brief updates on the PZQ mechanism of action, proposed mechanism of resistance, impacts of MDA on schistosome genetic diversity, PZQ alternatives, and schistosomiasis vaccine development are also covered.
2 Praziquantel efficacy
PZQ treatment efficacy is commonly measured by the egg reduction rate (ERR), which compares the pre-treatment and post-treatment number of eggs shed in the urine or feces. Another prevalent method is the cure rate (CR), which compares the number of infected individuals who become negative for schistosomiasis post-treatment (Fukushige et al., 2021).
Due to its effectiveness as an antiparasitic, PZQ is on the WHO’s list of essential medicines, and in 2017, approximately 100 million individuals received PZQ treatment for schistosomiasis (Park and Marchant, 2020). However, PZQ is less effective against juvenile worms or schistosomulae (Pica-Mattoccia and Cioli, 2004; Villamizar-Monsalve et al., 2024). It is thought that ATP-binding cassette (ABC) transporters, which can export toxins, play a role in this protection, as juvenile worms have about two and a half times the number of ABC transporters as the adult form (Kasinathan et al., 2010). In addition, PZQ must be administered in higher than recommended doses to efficiently kill schistosome eggs (Richards et al., 1989). Therefore, a follow-up dose 4 to 6 weeks after the initial dose may be necessary to prevent reinfection after any juvenile worms have matured (Gryseels et al., 2006).
2.1 Praziquantel mechanism of action and mechanism of resistance
Despite being the drug of choice against helminth infections for decades, the exact mechanism of action for PZQ is unclear. In trematodes and cestodes, PZQ may activate the transient receptor potential ion channel in the worm (Sm.TRPMPZQ) by engaging with a hydrophobic ligand-binding pocket, which opens the voltage-gated Ca2+ channels and pumps (Park and Marchant, 2020). This causes membrane depolarization, which is followed by rapid, involuntary tetanic muscle contractions and paralysis of the worm (Nogueira et al., 2022). PZQ may also change or destroy the worm’s integument, exposing its previously hidden parasitic antigens and leaving it vulnerable to host immune defenses (Eyoh et al., 2019).
Although a mechanism of PZQ resistance has also not been well characterized, it has been suggested that resistant worms are simply better able to metabolize the drug compared to non-resistant worms (Zdesenko and Mutapi, 2020). According to a recent study using whole-genome sequencing, it is also possible that genetic variation at or near the Sm.TRPMPZQ channel could be involved (Le Clec’h et al., 2021). However, further research on wild-type schistosomes and their Sm.TRPMPZQ ion channels across a variety of regions is needed for more conclusive answers.
3 Praziquantel resistance
Several laboratory studies have successfully induced PZQ resistance in schistosomes, particularly S. mansoni. In 1994, an in vitro study subjected a population of S. mansoni-infected mice to increasing PZQ drug pressure. By the seventh generation, 93% of the resistant schistosomes survived three PZQ doses of 300 mg/kg, which killed 89% of the control group (Fallon, 1998; Vale et al., 2017). Another study showed that resistance to the therapeutic dose of PZQ can be induced in following generations of S. mansoni in mice through successive subcurative doses (Ismail et al., 1994). A simpler and less expensive technique was later developed to induce PZQ resistance in S. mansoni through successively treating infected Biomphalaria glabrata snails with 100 mg/kg of PZQ (Couto et al., 2011). More recently, a study in 2015 induced resistance in an Egyptian strain of S. mansoni through treating multiple subcurative doses of Biomphalaria alexandrina snails (Lotfy et al., 2015). Regarding S. japonicum, an unsuccessful attempt was made in 1990 to experimentally induce resistance using drug pressure through infected mice (Yue et al., 1990). Induction of resistance in S. japonicum in the three life stages—adult, cercaria, and miracidia— was later achieved (Li et al., 2011). These studies demonstrate that schistosomes are capable of developing resistance under PZQ drug pressure of subcurative doses. There is currently no knowledge of laboratory-induced resistance to PZQ in S. haematobium.
The first significant instance of reported PZQ resistance on the field occurred in 1994 during an S. mansoni infection outbreak in Senegal (Stelma et al., 1995). The standard single-dose treatment of 40 mg/kg resulted in alarmingly low cure rates of 18-36% rather than the usual 60-90% (Doenhoff et al., 2008; Stelma et al., 1995). However, a 20 mg/kg dose of oxamniquine, an alternative anthelminthic drug, showed a typical cure rate (Stelma et al., 1997). It has been suggested that the low cure rate of PZQ was due to an intense initial infection, as the average egg counts were notably high in patients (Stelma et al., 1995; Utzinger et al., 2000; Danso-Appiah and De Vlas, 2002). PZQ is less effective against juvenile schistosomes and eggs, which may have survived the initial treatment and matured into adults after treatment (Richards et al., 1989; Danso-Appiah and De Vlas, 2002; Pica-Mattoccia and Cioli, 2004).
Another early report of apparent S. mansoni field resistance to PZQ was in villages in the Nile Delta region of Egypt (Ismail et al., 1996). All patients were treated with the standard dose of 40 mg/kg followed by a second 40 mg/kg dose or a third 60 mg/kg dose if necessary (Ismail et al., 1996). Although the PZQ cure rate was 98.4%, eggs isolated from the uncured patients generated PZQ infections in mice that were 3-5 times less sensitive to PZQ, raising concerns about resistance to PZQ in the parent worms (Ismail et al., 1996, 1999). When treated with PZQ in vitro, the isolates from the uncured patients showed decreased muscle contraction, decreased tegumental disruption, and decreased calcium influx, all of which are well-characterized PZQ actions on schistosomes (Ismail et al., 1999; William et al., 2001; William and Botros, 2004; Eyoh et al., 2019; Nogueira et al., 2022). Although these results were concerning, a study conducted in the same villages using the same dosing regimen ten years after the initial studies revealed no resistance to PZQ despite a decade of continued and broad use of the drug. It is worth noting that the infections initially present for the follow-up study were generally light. In addition, detecting a change from the previous 98.4% cure rate was not possible due to the sample size (Botros et al., 2005).
Reduced sensitivity to PZQ was later reported in Kenya among isolates of S. mansoni gathered from patients who had been previously treated with PZQ but whose occupations continuously exposed them to infection. The study also analyzed an isolate from a patient who had been treated with PZQ 18 times and never fully cured (KCW). This isolate was significantly less susceptible to PZQ both in vivo and in vitro. However, one KCW sub-isolate retained its resistant characteristics through 6 generations without any PZQ treatment. Meanwhile, another KCW sub-isolate returned to PZQ sensitivity after retesting for 8 generations. Such an occurrence may inform the results of the Nile Delta villages studies, suggesting that reduced PZQ susceptibility is not a stable trait in schistosomes and may require a biological cost (Botros et al., 2005; Melman et al., 2009; Greenberg, 2013).
A repeated cross-sectional study in Uganda found statistically reduced PZQ efficacy against S. mansoni among children from schools that had received 8 or 9 rounds of mass drug administration (MDA) than children from schools that had received 5 rounds or 1 round. Interestingly, a whole-genome sequencing study of the miracidia collected revealed that genomic diversity remained varied and unstructured despite long-term PZQ use. Therefore, the previously reported low PZQ efficacy may have been due to factors other than resistance (Crellen et al., 2016; Berger et al., 2021).
Concern for PZQ resistance in wild-type S. japonicum had received discussion due to its heavy use in endemic areas of China (Yu et al., 2001; Wu et al., 2011; Wang et al., 2012). Field studies have tested the efficacy of PZQ to S. japonicum in areas of varying endemicities throughout China using a single oral dose of 40 mg/kg (Liang et al., 2001; Wang et al., 2012). The results suggest that despite thirty years of heavy and expanded chemotherapy, sensitivity to PZQ in S. japonicum has not significantly decreased in China (Liang et al., 2001; Wang et al., 2012). In another study, the efficacy of PZQ against S. japonicum was compared between an area with repeated PZQ chemotherapy and a newly identified endemic area. The results indicated that the efficacy between the two areas were not significantly different (Yu et al., 2001). A cross-sectional study across 33 villages in Sichuan Province was organized to evaluate PZQ efficacy against S. japonicum (Seto et al., 2011). Out of 185 cases, only one remained uncured after receiving two doses of 40 mg/kg of PZQ, indicating that PZQ remains an effective treatment for S. japnonicum in China (Seto et al., 2011).
Regarding S. haebatobium, a recent study in Ghana detected no sign of its resistance to PZQ and attributed the more persistent schistosomiasis cases to reinfection (Tetteh-Quarcoo et al., 2020). Occasionally, there have been isolated reports of failed standard treatment of S. haematobium in travelers returning from endemic areas (Herwaldt et al., 1995; da Silva et al., 2005; Alonso et al., 2006). Various possible explanations exist for these instances, including the presence of a concurrent infection and the therapeutic failure of a single 40 mg/kg dose of PZQ (Herwaldt et al., 1995; da Silva et al., 2005). Since PZQ acts in synergy with the host immune system, it has been hypothesized that some individuals originating from non-endemic areas may lack the necessary immunological factor to overcome the infection (Wu et al., 2011; Vale et al., 2017).
A meta-analysis and systematic review article in 2023 have reported that PZQ efficacy has remained high, and there is no consistent evidence for the emergence of PZQ resistance in schistosomes (Fukushige et al., 2021; Aboagye and Addison, 2023). However, care should be taken to attempt to prevent schistosome resistance on the field, such as avoiding treatment with subcurative doses of PZQ, as this has been shown to experimentally induce resistance in S. mansoni and S. japonicum (Fallon, 1998; Li et al., 2011; Wang et al., 2012). Focus should also be placed on alternative methods of schistosomiasis control, such as snail control, clean tap water, health education, and building latrines (Wang et al., 2012; Villamizar-Monsalve et al., 2024). In addition, drug quality should continue to be monitored to ensure the effectiveness of praziquantel and detect further cases of suspected resistance (Wang et al., 2012; World Health Organization, 2022).
3.1 Continued use of praziquantel
Despite its ineffectiveness against juvenile schistosomes, inability to prevent reinfection, and heavy discussion of schistosome resistance, PZQ will remain the drug of choice for schistosomiasis for the foreseeable future. After decades of constant use, efficacy rates remain high and incidences of resistance are rare (Fukushige et al., 2021; World Health Organization, 2022). In 2022, the WHO published guidelines on the control and elimination of schistosomiasis in humans which recommended continued and expanded access to PZQ (World Health Organization, 2022).
3.2 Genetic diversity of schistosomes
The genetic consequences of MDA and drug pressure have been subject to recent investigation, especially in light of the decreased cost of genotyping technologies and increased research about schistosome molecular markers associated with PZQ resistance (Norton et al., 2010; Mendes et al., 2018; Gower et al., 2017; Doyle and Cotton, 2019; Berger et al., 2021; Summers et al., 2022). There have also been concerns that MDA would create a genetic bottleneck that selects for PZQ-resistant schistosomes (Norton et al., 2010; Rey et al., 2021). Genomic studies have reported genetic ramifications in S. mansoni worms following MDA and laboratory-induced resistance (Norton et al., 2010; Mendes et al., 2018; Gower et al., 2017). However, these studies are mainly aimed at investigating a limited number of molecular markers, and the vast number of unknown variables of genetic diversity makes the data difficult to attribute to the development of drug resistance (Coghlan et al., 2019; Doyle and Cotton, 2019; Berger et al., 2021). As a whole, genomic studies investigating PZQ resistance have found no long-term decrease in the genetic diversity of S. mansoni worms, even in ones that survived MDA (Gower et al., 2017; Faust et al., 2019).
3.3 Future research and vaccine
The WHO states the need for developing new drugs to co-administer with PZQ in case of resistance (World Health Organization, 2020). Several potential new compounds are PZQ derivatives, including sulphonamides, organometallics, and another agent with a minor structural variation to PZQ (Hess et al., 2015; Angeli et al., 2022; Xu et al., 2023). However, further testing and optimization is needed before such drugs become commercially available.
Because MDA alone is insufficient to eliminate schistosomiasis, the WHO also calls for the development of a schistosomiasis vaccine (World Health Organization, 2020). Recent advances in vaccine development increase the possibility of this goal being obtained. There are currently several schistosomiasis vaccines undergoing clinical testing (Molehin, 2020; Hotez and Bottazzi, 2023). Most are based on recombinant proteins and target S. mansoni (Zhang et al., 2020; Santini-Oliveira et al., 2022; Diemert et al., 2023). However, the task remains difficult due to the complex life cycle, host-evasion mechanisms, and hybridization between schistosome species (Fonseca et al., 2015). In addition, sustainable financing, uncertain manufacturer investment, and distribution issues remain considerable challenges. However, a schistosomiasis vaccine introduction in conjunction with MDA is a necessary factor in eliminating the disease, especially before any major sign of emerging schistosome resistance is detected (World Health Organization, 2023b).
Author contributions
GE: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. DF: Writing – review & editing, Writing – original draft, Validation, Methodology, Formal analysis. MB: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology. AG: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Conceptualization. WM: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Methodology, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank the Liberty University Office of Sponsored Programs and Research for funding the APC associated with this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel Aziz N., Musaigwa F., Mosala P., Berkiks I., Brombacher F. (2022). Type 2 immunity: a two-edged sword in schistosomiasis immunopathology. Trends. Immunol. 43, 657–673. doi: 10.1016/j.it.2022.06.005
Aboagye I. F., Addison Y. A. A. (2023). Praziquantel efficacy, urinary and intestinal schistosomiasis reinfection - a systematic review. Pathog. Glob. Health 117, 623–630. doi: 10.1080/20477724.2022.2145070
Alonso D., Munoz J., Gascon J., Valls M. E., Corachan M. (2006). Failure of standard treatment with praziquantel in two returned travelers with Schistosoma haematobium infection. Am. J. Trop. Med. Hyg 74, 342–344. doi: 10.4269/ajtmh.2006.74.342
Angeli A., Ferraroni M., Carta F., Häberli C., Keiser J., Costantino G., et al. (2022). Development of Praziquantel sulphonamide derivatives as antischistosomal drugs. J. Enzyme. Inhib. Med. Chem. 37, 1479–1494. doi: 10.1080/14756366.2022.2078970
Berger D. J., Crellen T., Lamberton P. H. L., Allan F., Tracey A., Noonan J. D., et al. (2021). Whole-genome sequencing of Schistosoma mansoni reveals extensive diversity with limited selection despite mass drug administration. Nat. Commun. 12, 4776. doi: 10.1038/s41467-021-24958-0
Botros S., Sayed H., Amer N., El-Ghannam M., Bennett J. L., Day T. A. (2005). Current status of sensitivity to praziquantel in a focus of potential drug resistance in Egypt. Int. J. Parasitol. 35, 787–791. doi: 10.1016/j.ijpara.2005.02.005
Burke M. L., Jones M. K., Gobert G. N., Li Y. S., Ellis M. K., Mcmanus D. P. (2009). Immunopathogenesis of human schistosomiasis. Parasite. Immunol. 31, 163–176. doi: 10.1111/j.1365-3024.2009.01098.x
Cahill K. M. (2011). Tropical medicine: A clinical text. 8th edn (New York: Fordham University Press).
Centers for Disease Control and Prevention. (2024). Schistosomiasis. Available online at: https://www.cdc.gov/dpdx/schistosomiasis/index.html (Accessed July 24, 2024).
Cioli D., Pica-Mattoccia L., Basso A., Guidi A. (2014). Schistosomiasis control: praziquantel forever? Mol. Biochem. Parasitol. 195, 23–29. doi: 10.1016/j.molbiopara.2014.06.002
Coghlan A., Tyagi R., Cotton J. A., Holroyd N., Rosa B. A., Tsai I. J., et al. (2019). Comparative genomics of the major parasitic worms. Nat. Genet. 51, 163–174. doi: 10.1038/s41588-018-0262-1
Coles G. C., Kinoti G. K. (1997). Defining resistance in schistosoma. Parasitol. Today 13, 157–158. doi: 10.1016/s0169-4758(97)89815-8
Colley D. G., Bustinduy A. L., Secor W. E., King C. H. (2014). Human schistosomiasis. Lancet 383, 2253–2264. doi: 10.1016/S0140-6736(13)61949-2
Cotton J. A., Doyle S. R. (2022). A genetic TRP down the channel to praziquantel resistance. Trends. Parasitol. 38, 351–352. doi: 10.1016/j.pt.2022.02.006
Couto F. F., Coelho P. M. Z., Araújo N., Kusel J. R., Katz N., Jannotti-Passos L. K., et al. (2011). Schistosoma mansoni: a method for inducing resistance to praziquantel using infected Biomphalaria glabrata snails. Mem. Inst. Oswaldo Cruz 106, 153–157. doi: 10.1590/S0074-02762011000200006
Crellen T., Walker M., Cotton J. A., Lamberton P., Webster J. P., Tukahebwa E. M., et al. (2016). Reduced efficacy of praziquantel against Schistosoma mansoni is associated with multiple rounds of mass drug administration. Clin. Infect. Dis. 63, 1151–1159. doi: 10.1093/cid/ciw506
Danso-Appiah A., De Vlas S. J. (2002). Interpreting low praziquantel cure rates of Schistosoma mansoni infections in Senegal. Trends. Parasitol. 18, 125–129. doi: 10.1016/S1471-4922(01)02209-7
da Silva I. M., Thiengo R., Conceição M. J., Rey L., Lenzi H. L., Pereira Filho E., et al. (2005). Therapeutic failure of praziquantel in the treatment of Schistosoma haematobium infection in Brazilians returning from Africa. Mem. Inst. Oswaldo Cruz 100, 445–449. doi: 10.1590/s0074-02762005000400018
Diemert D. J., Correa-Oliveira R., Fraga C. G., Talles F., Silva M. R., Patel S. M., et al. (2023). A randomized, controlled Phase 1b trial of the Sm-TSP-2 Vaccine for intestinal schistosomiasis in healthy Brazilian adults living in an endemic area. PLoS. Negl. Trop. Dis. 17, e0011236. doi: 10.1371/journal.pntd.0011236
Doenhoff M. J., Cioli D., Utzinger J. (2008). Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr. Opin. Infect. Dis. 21, 659–667. doi: 10.1097/qco.0b013e328318978f
Doyle S. R., Cotton J. A. (2019). Genome-wide approaches to investigate anthelmintic resistance. Trends. Parasitol. 35, 289–301. doi: 10.1016/j.pt.2019.01.004
Eyoh E., McCallum P., Killick J., Amanfo S., Mutapi F., Astier A. L. (2019). The anthelmintic drug praziquantel promotes human Tr1 differentiation. Immunol. Cell. Biol. 97, 512–518. doi: 10.1111/imcb.12229
Fallon P. G. (1998). Schistosome resistance to praziquantel. Drug Resist. Update 1, 236–241. doi: 10.1016/S1368-7646(98)80004-6
Faust C. L., Crotti M., Moses A., Oguttu D., Wamboko A., Adriko M., et al. (2019). Two-year longitudinal survey reveals high genetic diversity of Schistosoma mansoni with adult worms surviving praziquantel treatment at the start of mass drug administration in Uganda. Parasitol. Vectors 12, 607. doi: 10.1186/s13071-019-3860-6
Fonseca C. T., Oliveira S. C., Alves C. C. (2015). Eliminating schistosomes through vaccination: what are the best immune weapons? Front. Immunol. 6. doi: 10.3389/fimmu.2015.00095
Fukushige M., Chase-Topping M., Woolhouse M. E. J., Mutapi F. (2021). Efficacy of praziquantel has been maintained over four decades (from 1977 to 2018): A systematic review and meta-analysis of factors influence its efficacy. PLoS. Negl. Trop. Dis. 15, e0009189. doi: 10.1371/journal.pntd.0009189
Geerts S., Coles G. C., Gryseels B. (1997). Anthelmintic resistance in human helminths: Learning from the problems with worm control in livestock. Parasitol. Today 13, 149–151. doi: 10.1016/s0169-4758(97)01024-7
Gower C. M., Gehre F., Marques S. R., Lamberton P. H. L., Lwambo N. J., Webster J. P. (2017). Phenotypic and genotypic monitoring of Schistosoma mansoni in Tanzanian schoolchildren five years into a preventative chemotherapy national control programme. Parasitol. Vectors 10, 593. doi: 10.1186/s13071-017-2533-6
Greenberg R. M. (2013). New approaches for understanding mechanisms of drug resistance in schistosomes. Parasitology 140, 1534–1546. doi: 10.1017/S0031182013000231
Gryseels B., Polman K., Clerinx J., Kestens L. (2006). Human schistosomiasis. Lancet 368, 1106–1118. doi: 10.1016/S0140-6736(06)69440-3
Herwaldt B. L., Tao L., van Pelt W., Tsang V. C. W., Bruce J. I. (1995). Persistence of schistosoma haematobium infection despite multiple courses of therapy with praziquantel. Clin. Infect. Dis. 20, 309–315. doi: 10.1093/clinids/20.2.309
Hess J., Keiser J., Gasser G. (2015). Toward organometallic antischistosomal drug candidates. Future. Med. Chem. 7, 821–830. doi: 10.4155/fmc.15.22
Hotez P. J., Bottazzi M. E. (2023). Human schistosomiasis vaccines as next generation control tools. Trop. Med. Infect. Dis. 8, 170. doi: 10.3390/tropicalmed8030170
Ismail M., Botros S., Metwally A., William S., Farghally A., Tao L. F., et al. (1999). Resistance to praziquantel: Direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am. J. Trop. Med. Hyg 60, 932–935. doi: 10.4269/ajtmh.1999.60.932
Ismail M., Metwally A., Farghaly A., Bruce J., Tao L., Bennett J. L. (1996). Characterization of isolates of schistosoma mansoni from Egyptian villagers that tolerate high doses of praziquantel. Am. J. Trop. Med. Hyg 55, 214–218. doi: 10.4269/ajtmh.1996.55.214
Ismail M. M., Taha S. A., Farghaly A. M., el-Azony A. S. (1994). Laboratory induced resistance to praziquantel in experimental schistosomiasis. J. Egypt. Soc Parasitol. 24, 685–695.
Kasinathan R. S., Goronga T., Messerli. S. M., Webb T. R., Greenberg R. M. (2010). Modulation of a Schistosoma mansoni multidrug transporter by the antischistosomal drug praziquantel. FASEB. J. 24, 128–135. doi: 10.1096/fj.09-137091
Le Clec’h W., Chevalier F. D., Mattos A. C. A., Strickland A., Diaz R., McDew-White M., et al. (2021). Genetic analysis of praziquantel response in schistosome parasites implicates a transient receptor potential channel. Sci. Transl. Med. 13, eabj9114. doi: 10.1126/scitranslmed.abj9114
Li H. J., Liang Y. J., Dai J. R., Wang W., Qu G. L., Li Y. Z., et al. (2011). Studies on resistance of Schistosoma to praziquantel XIV. Experimental comparison of susceptibility to praziquantel between praziquantel-resistant isolates and praziquantel-susceptible isolates of Schistosoma japonicum in stages of adult worms, miracidia, and cercariae. Chin. J. Schistosomiasis. Control. 23, 611–619. doi: 10.29261/pakvetj/2023.062
Li X., Zhu X., Xue Y. (2023). Drug resistance and genetic relatedness of Escherichia coli from mink in Northeast China. Pak. Vet. J. 43, 824–827.
Liang Y., Dai J., Ning A., Yu D., Xu X., Zhu Y., et al. (2001). Susceptibility of Schistosoma japonicum to praziquantel in China. Trop. Med. Int. Health 6, 707–714. doi: 10.1046/j.1365-3156.2001.00772.x
Lotfy W. M., Hishmat M. G., El Nashar A. S., Abu El Einin H. M. (2015). Evaluation of a method for induction of praziquantel resistance in Schistosoma mansoni. Pharm. Biol. 53, 1214–1219. doi: 10.3109/13880209.2014.970289
Malik F., Nawaz M., Anjum A. A., Firyal S., Shahid M. A., Irfan S., et al. (2022). Molecular characterization of antibiotic resistance in poultry gut origin enterococci and horizontal gene transfer of antibiotic resistance to Staphylococcus aureus. Pak. Vet. J. 42, 383–389. Available online at: https://web.p.ebscohost.com/ehost/pdfviewer/pdfviewer?vid=0&sid=df38bb88-a72a-49d1-bbfd-c4817e8a33b0%40redis.
Mduluza T., Jones C., Osakunor D. N. M., Lim R., Kuebel J. K., Phiri I., et al. (2020). Six rounds of annual praziquantel treatment during a national helminth control program significantly reduced schistosome infection and morbidity levels in a cohort of schoolchildren in Zimbabwe. PLoS. Negl. Trop. Dis. 14, e0008388. doi: 10.1371/journal.pntd.0008388
Melman S. D., Steinauer M. L., Cunningham C., Kubatko L. S., Mwangi I. N., Wynn N. B., et al. (2009). Reduced Susceptibility to Praziquantel among Naturally Occurring Kenyan Isolates of Schistosoma mansoni. PLoS. Negl. Trop. Dis. 3, e504. doi: 10.1371/journal.pntd.0000504
Mendes T., Calado M., Clemente I., Belo S., Afonso A. (2018). Genetic analysis of Schistosoma mansoni sensitive and resistant strains to praziquantel using RAPD-PCR. An. Inst. Hig. Med. Trop. Lisb. 12, 41–45. doi: 10.25761/anaisihmt.189
Mohamed E. S., Hamouda A. M., El Enbaawy M. I. (2022). Current status of multidrug resistance of Ornithobacterium rhinotracheale from avian host. Int. J. Vet. Sci. 11, 539–543. doi: 10.47278/journal.ijvs/2021.127
Molehin A. J. (2020). Schistosomiasis vaccine development: update on human clinical trials. J. BioMed. Sci. 27, 28. doi: 10.1186/s12929-020-0621-y
Nogueira R. A., Lira M. G. S., Licá I. C. L., Frazão G. C. C. G., dos Santos V. A. F., Filho A. C. C. M., et al. (2022). Praziquantel: An update on the mechanism of its action against schistosomiasis and new therapeutic perspectives. Mol. Biochem. Parasitol. 252, 111531. doi: 10.1016/j.molbiopara.2022.111531
Norton A. J., Gower C. M., Lamberton P. H. L., Webster B. L., Lwambo N. J. S., Blair L., et al. (2010). Genetic consequences of mass human chemotherapy for schistosoma mansoni: population structure pre- and post-praziquantel treatment in Tanzania. Am. J. Trop. Med. Hyg 83, 951–957. doi: 10.4269/ajtmh.2010.10-0283
Park S., Marchant J. S. (2020). The journey to discovering a flatworm target of praziquantel: A long TRP. Trends. Parasitol. 36, 182–194. doi: 10.1016/j.pt.2019.11.002
Pica-Mattoccia L., Cioli D. (2004). Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int. J. Parasitol. 34, 527–533. doi: 10.1016/j.ijpara.2003.12.003
Prichard R. K., Hall C. A., Kelly J. D., Martin I. C. A., Donald A. D. (1980). The problem of anthelmintic resistance in nematodes. Aust. Vet. J. 56, 239–250. doi: 10.1111/j.1751-0813.1980.tb15983.x
Qamar W., Alkheraije K. A. (2023). Anthelmintic Resistance in Haemonchus contortus of sheep and goats from Asia–a review of in vitro and in vivo studies. Pak. Vet. J. 43, 376–387. Available online at: http://www.pvj.com.pk/pdf-files/23-292.pdf.
Rey O., Webster B. L., Huyse T., Rollinson D., Van den Broeck F., Kincaid-Smith J., et al. (2021). Population genetics of African Schistosoma species. Infect. Genet. Evol. 89, 104727. doi: 10.1016/j.meegid.2021.104727
Richards F., Sullivan J., Ruiz-Tiben E., Eberhard M., Bishop H. (1989). Effect of praziquantel on the eggs of Schistosoma mansoni, with a note on the implications for managing central nervous system schistosomiasis. Ann. Trop. Med. Parasitol. 83, 465–472. doi: 10.1080/00034983.1989.11812373
Royal Society of Chemistry. (2024). Praziquantel. Available online at: https://merckindex.rsc.org/monographs/m9107 (Accessed July 14, 2024).
Santini-Oliveira M., MaChado Pinto P., Santos T. D., Vilar M. M., Grinsztejn B., Veloso V., et al. (2022). Development of the sm14/GLA-SE schistosomiasis vaccine candidate: an open, non-placebo-controlled, standardized-dose immunization phase ib clinical trial targeting healthy young women. Vaccines 10, 1724. doi: 10.3390/vaccines10101724
Seto E. Y. W., Wong B. K., Lu D., Zhong B. (2011). Human schistosomiasis resistance to praziquantel in China: Should we be worried? Am. J. Trop. Med. Hyg. 85 (1), 74–82. doi: 10.4269/ajtmh.2011.10-0542
Stelma F. F., Sall S., Daff B., Sow S., Niang M., Gryseels B. (1997). Oxamniquine cures schistosoma mansoni infection in a focus in which cure rates with praziquantel are unusually low. J. Infect. Dis. 176, 304–307. doi: 10.1086/517273
Stelma F. F., Talla I., Sow S., Kongs A., Niang M., Polman K., et al. (1995). Efficacy and side effects of praziquantel in an epidemic focus of Schistosoma mansoni. Am. J. Trop. Med. Hyg 53, 167–170. doi: 10.4269/ajtmh.1995.53.167
Summers S., Bhattacharyya T., Allan F., Stothard J. R., Edielu A., Webster B. L., et al. (2022). A review of the genetic determinants of praziquantel resistance in Schistosoma mansoni: Is praziquantel and intestinal schistosomiasis a perfect match? Front. Trop. Dis. 3. doi: 10.3389/fitd.2022.933097
Tetteh-Quarcoo P. B., Forson P. O., Amponsah S. K., Ahenkorah J., Opintan J. A., Ocloo J. E. Y., et al. (2020). Persistent urogenital schistosomiasis and its associated morbidity in endemic communities within southern Ghana: suspected praziquantel resistance or reinfection? Med. Sci. 8, 10. doi: 10.3390/medsci8010010
Utzinger J., N’goran E. K., N’dri A., Lengeler C., Tanner M. (2000). Efficacy of praziquantel against Schistosoma mansoni with particular consideration for intensity of infection. Trop. Med. Int. Health 5, 771–778. doi: 10.1046/j.1365-3156.2000.00646.x
Vale N., Gouveia M. J., Rinaldi G., Brindley P. J., Gärtner F., Correia da Costa J. M. (2017). Praziquantel for schistosomiasis: single-drug metabolism revisited, mode of action, and resistance. Antimicrob. Agents. Chemother. 61, e02582–e02516. doi: 10.1128/AAC.02582-16
Villamizar-Monsalve M. A., López-Abán J., Vicente B., Peláez R., Muro A. (2024). Current drug strategies for the treatment and control of schistosomiasis. Expert. Opin. Pharmacother. 25, 409–420. doi: 10.1080/14656566.2024.2333372
Wang W., Wang L., Liang Y. (2012). Susceptibility or resistance of praziquantel in human schistosomiasis: a review. Parasitol. Res. 111, 1871–1877. doi: 10.1007/s00436-012-3151-z
William S., Botros S. (2004). Validation of sensitivity to praziquantel using Schistosoma mansoni worm muscle tension and Ca 2+-uptake as possible in vitro correlates to in vivo ED 50 determination. Int. J. Parasitol. 34, 971–977. doi: 10.1016/j.ijpara.2004.04.005
William S., Botros S., Ismail M., Farghally A., Day T. A., Bennett J. L. (2001). Praziquantel-induced tegumental damage in vitro is diminished in schistosomes derived from praziquantel-resistant infections. Parasitology 122, 63–66. doi: 10.1017/S0031182000007137
World Health Organization. (2020). Ending the neglect to attain the sustainable development goals: a road map for neglected tropical diseases 2021–2030. (Geneva: World Health Organization).
World Health Organization. (2021). Schistosomiasis and soil-transmitted helminthiases: progress report. Available online at: https://www.who.int/publications/i/item/who-wer9748-621-632 (Accessed July 14, 2024).
World Health Organization. (2022). WHO guideline on control and elimination of schistosomiasis (Geneva: World Health Organization).
World Health Organization. (2023a). Schistosomiasis. https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (Accessed July 14, 2024).
World Health Organization. (2023b). 34th meeting of the international task force for disease eradication, 19-20 september 2022. (Geneva: World Health Organization).
Wu W., Wang W., Huang Y. (2011). New insight into praziquantel against various developmental stages of schistosomes. Parasitol. Res. 109, 1501–1507. doi: 10.1007/s00436-011-2670-3
Xu J., Dong L., Sun H., Huang P., Zhang R., Wang X., et al. (2023). Small change, big difference: A promising praziquantel derivative designated P96 with broad-spectrum antischistosomal activity for chemotherapy of schistosomiasis japonica. PLoS. Negl. Trop. Dis. 17, e0011215. doi: 10.1371/journal.pntd.0011215
Yu D. B., Li Y., Sleigh A. C., Yu X. L., Li Y. S., Wei W. Y., et al. (2001). Efficacy of praziquantel against Schistosoma japonicum: field evaluation in an area with repeated chemotherapy compared with a newly identified endemic focus in Hunan, China. Trans. R. Soc Trop. Med. Hyg 95, 537–541. doi: 10.1016/S0035-9203(01)90032-X
Yue W. J., Yu S. H., Xu X. J. (1990). Failure to induce resistance of Schistosoma japonicum to praziquantel. Southeast. Asian. J. Trop. Med. Public. Health 21, 85–89.
Zdesenko G., Mutapi F. (2020). Drug metabolism and pharmacokinetics of praziquantel: A review of variable drug exposure during schistosomiasis treatment in human hosts and experimental models. PLoS. Negl. Trop. Dis. 14, e0008649. doi: 10.1371/journal.pntd.0008649
Zhang W., Le L., Ahmad G., Molehin A. J., Siddiqui A. J., Torben W., et al. (2020). Fifteen Years of Sm-p80-Based Vaccine Trials in Nonhuman Primates: Antibodies From Vaccinated Baboons Confer Protection in vivo and in vitro From Schistosoma mansoni and Identification of Putative Correlative Markers of Protection. Front. Immunol. 11. doi: 10.3389/fimmu.2020.01246
Keywords: schistosomiasis, praziquantel, resistance, efficacy, mass drug administration
Citation: Eastham G, Fausnacht D, Becker MH, Gillen A and Moore W (2024) Praziquantel resistance in schistosomes: a brief report. Front. Parasitol. 3:1471451. doi: 10.3389/fpara.2024.1471451
Received: 27 July 2024; Accepted: 10 September 2024;
Published: 02 October 2024.
Edited by:
Zia ud Din Sindhu, University of Agriculture, Faisalabad, PakistanReviewed by:
Muhammad Imran, University of Agriculture, Faisalabad, PakistanLaura Oliveira, State University of Montes Claros, Brazil
Copyright © 2024 Eastham, Fausnacht, Becker, Gillen and Moore. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: William Moore, d3Rtb29yZUBsaWJlcnR5LmVkdQ==
 Gabriela Eastham
Gabriela Eastham Dane Fausnacht
Dane Fausnacht Matthew H. Becker
Matthew H. Becker Alan Gillen
Alan Gillen William Moore
William Moore