- Department of Hematology, Renji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, China
Introduction: Allogeneic hematopoietic cell transplantation (alloHCT) possessed direct cytotoxicity and graft-versus-multiple myeloma effect (GvMM). Growing trials have shown survival benefits of performing alloHCT in both newly diagnosed and relapsed MM.
Methods: We aimed to provide a comprehensive analysis in the recent 10 years to verify the efficacy and survival outcome of alloHCT in MM patients. A total of 61 studies which provide data between 14/04/2013 and 14/04/2023 and a total of 15,294 data from MM patients who had undergone alloSCT were included in our study. The best response rates (CR, VGPR, PR) and survival outcomes (1-, 2-, 3-,5-, and 10-year OS, PFS, NRM) were assessed. We further conducted meta-analysis in the NDMM/frontline setting and RRMM/salvage setting independently.
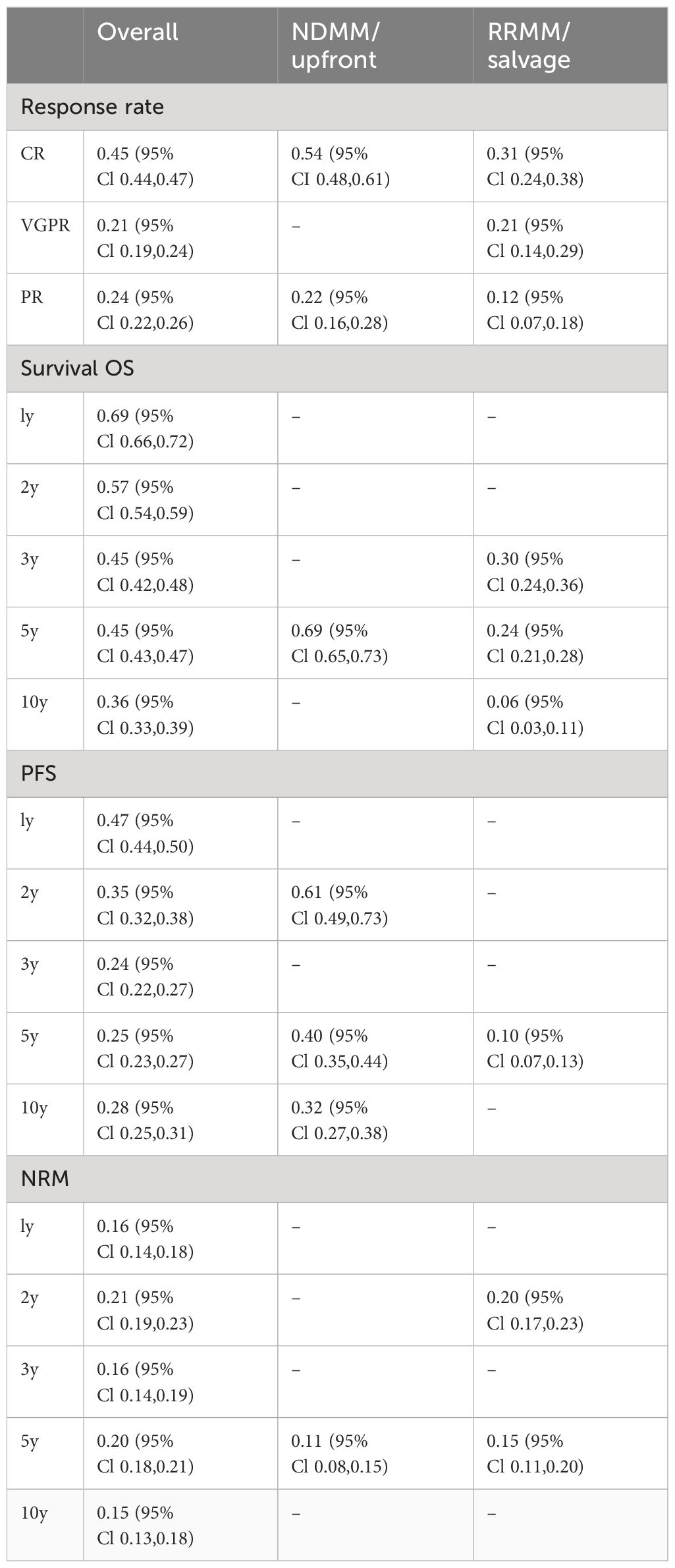
Results: The pooled estimate CR, VGPR, and PR rates were 0.45, 0.21, and 0.24, respectively. The pooled estimates of 1-, 2-, 3-, 5-, and 10-year OS were 0.69, 0.57, 0.45, 0.45, and 0.36, respectively; the pooled estimates of 1-, 2-, 3-, 5-, and 10-year PFS were 0.47, 0.35, 0.24, 0.25, and 0.28, respectively; and the pooled estimates of 1-, 2-, 3-, 5-, and 10-year NRM were 0.16, 0.21, 0.16, 0.20, and 0.15, respectively. In the NDMM/upfront setting, the pooled estimate CR rate was 0.54, and those for 5-year OS, PFS, and NRM were 0.69, 0.40, and 0.11, respectively. In a relapsed setting, the pooled estimate CR rate was 0.31, and those for 5-year OS, PFS, and NRM were 0.24, 0.10, and 0.15, respectively.
Discussion: Our results showed constant OS, PFS, and NRM from the third year onwards till the 10th year, suggesting that alloSCT has sustained survival benefits. Good response rate and promising survival outcome were observed in the NDMM/ frontline setting.
Conclusion: Although comparing with other treatments, alloSCT had a lower response rate and poorer short-term survival outcome, long-term follow-up could reveal survival benefits of alloSCT in MM patients.
Introduction
Multiple myeloma (MM), the second most common hematological malignancy, is a monoclonal tumor characterized by the expansion of malignant plasma cells in the bone marrow (BM) (1). Uncontrolled expansion interferes with osteogenesis in BM, leading to lytic bone disease (2). Moreover, progression of the disease may lead to acute kidney injury, anemia, and hypercalcemia (3).
Drug resistance arises due to the intratumor high heterogeneity nature of MM (2). Recently, the standard treatment for transplant eligible MM patients is a combination of induction therapy (injectable proteasome inhibitor, oral immunomodulatory agent, and dexamethasone) and autologous hematopoietic stem cell transplantation (autoSCT) followed by lenalidomide maintenance therapy (3).
Allogeneic hematopoietic cell transplantation (alloHCT) possesses direct cytotoxicity and graft-versus-multiple myeloma effect (GvMM) (4). It remains controversial due to the high occurrence of graft-versus-host disease (GVHD), treatment-related mortality (TRM), and relapse rate. Recent data showed the crucial role of GVHD in the GVMM effect, specifically chronic GVHD (5). In research comparing survival between auto-auto and auto-allo after induction therapy, results showed higher overall survival (OS) and progression-free survival (PFS) in the auto-allo group. Moreover, there was a higher non-relapsed mortality (NRM) and lower risk of disease progression in the auto-allo group, verifying the continuous GVMM effect on disease control (6).
In a phase 3 trial comparing auto-auto versus auto-alloSCT in newly diagnosed MM (NDMM) patients with high-risk cytogenetic abnormalities (del13q, del17p), patients undergoing auto-alloSCT had a much higher median PFS and OS, showing the effectiveness of prolonged GVMM effect in improving survival in high-risk NDMM patients (7). The BMT CTN 0102 trial with long-term follow-up over 10 years showed a reduction in relapse, better PFS, and similar OS among high-risk NDMM patients treated with auto-allo. This trial further revealed the potential of alloSCT in overcoming the deleterious effect brought by high-risk cytogenetic chromosomal abnormalities in NDMM patients (8). Furthermore, there are evidence showing the effectiveness of alloSCT in overcoming translocation t(4;14) (9), del(17p13) (10), and del(13) abnormalities (11).
In addition, multivariate analysis of several research showed age as an influencing factor for survival outcome in alloSCT. In a study from the Japanese Society of Myeloma, age ≥50 years would adversely affect PFS (12). Another research showed both reduction in PFS and OS in patients >55 years (13).
All in all, there are growing evidence verifying the positive effect of alloSCT in newly diagnosed and young MM patients. Our meta-analysis aimed to provide a more comprehensive and reliable analysis to verify the efficacy and survival outcome of alloHCT in MM patients, patients in NDMM/frontline, and patients in an RRMM/salvage setting.
Methods
Search strategy
We conducted our search on 14/04/2023 in PubMed, Embase, Cochrane Library, and Web of Science with “(Allogenic) AND (myeloma)” as our searching term. We aimed to analyze research published in the past 10 years; thus, the publication date was limited to 14/04/2013 in all databases. All results were then downloaded to EndNote 20, and duplicated studies were removed. Studies were further filtered by their title and abstract. Full text of the remaining studies was downloaded for the final screen. In addition, studies that do not provide sufficient data were identified and removed after data extraction.
Search criteria
The following are the inclusion criteria: (1) studies concerning patients diagnosed as multiple myeloma and have no other reported hematological disease; (2) studies that have a reported response rate and survival after allogenic transplantation (studies that did not regard allo-transplantation as their main intervention but have reported that the above data were also included in our analysis); (3) studies reported in English; (4) studies with full text.
The following are the exclusion criteria: (1) cord blood as the stem cell source; (2) insufficient reported data; (3) studies concerning pediatric cases; (4) sample size <5.
We further conducted meta-analysis in an NDMM/frontline setting and an RRMM/salvage setting independently. The study design of each study was carefully read, and studies which did not clarify research population or treatment setting were excluded.
Statistical method
The following data were extracted: number of participants, age range, median follow-up, best response to allo-transplantation (complete remission (CR), very good partial remission (VGPR), and partial remission (PR)), and survival (1-, 2-, 3-, 5-, and 10-year OS, PFS, NRM). Our main outcome was best response rate and survival after allotransplantation in MM patients. Our secondary outcome was best response rate and survival after allotransplantation in the NDMM/frontline setting and RRMM/salvage setting.
Data were first collected in Excel, and all analyses were performed using STATA 15.1. This is a single-arm research; a random-effect model was adopted, and the results were presented in forest plot. The P value of Cochrane’s Q test <0.1 indicated the existence of heterogeneity. I2 statistic was used in the assessment of heterogeneity. Sensitivity analysis was then conducted. High heterogeneity (I2 statistic >50) was commonly observed in a single-armed study; thus, the change in I2 statistic after removal of a certain study was used to verify the extent of interference cause by the particular study. Sensitivity analysis could further confirm the stability of our study. Publication bias was assessed by funnel plots and was further confirmed by Begg’s and Egger’s tests. The P value in Begg’s and Egger’s tests >0.05 could confirm the absence of publication bias in our study.
Results
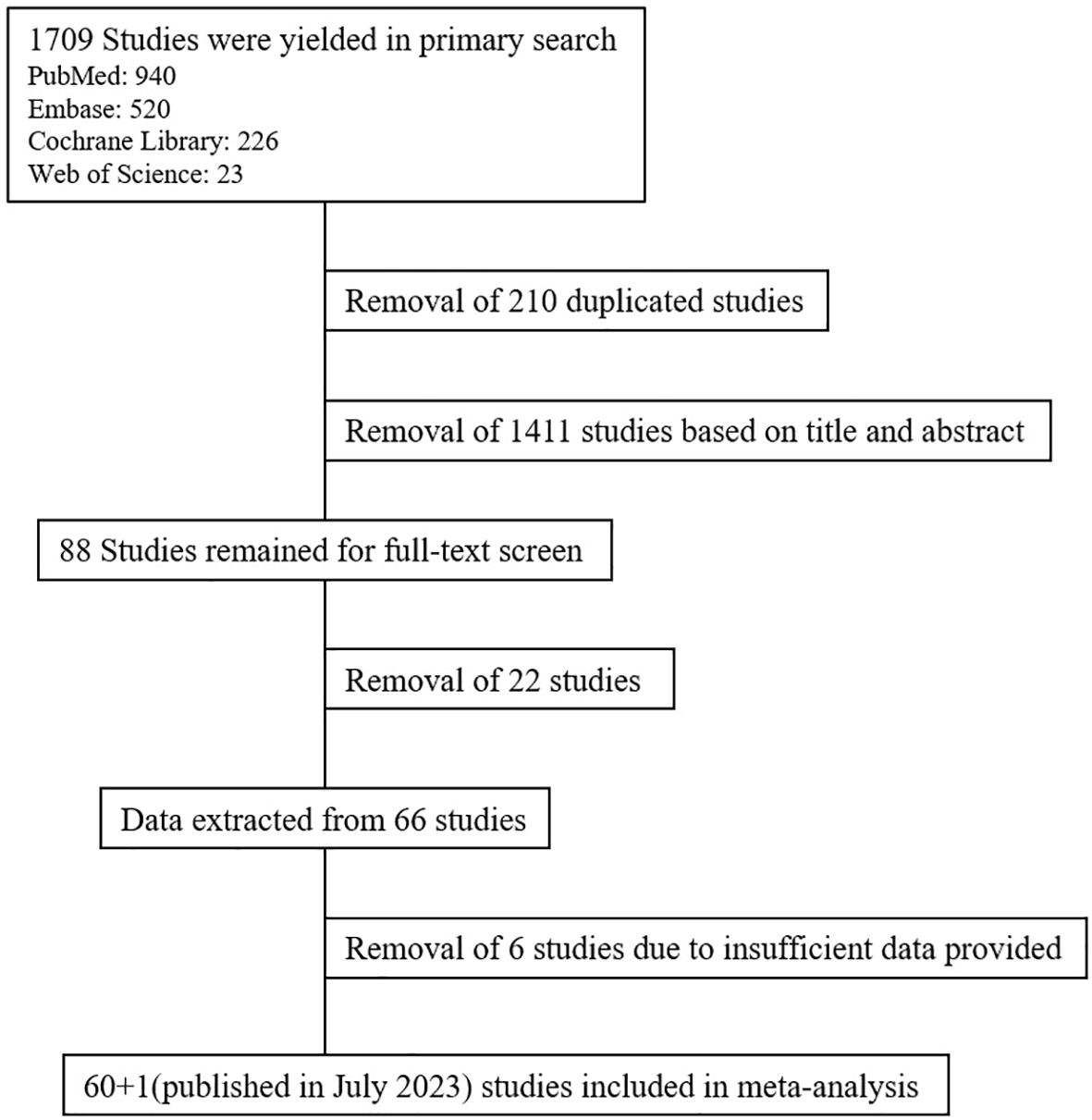
A total of 1,709 studies were yielded from the primary search, and 210 duplicated studies were removed. Based on title and abstract, 88 studies were selected for full-text screening. After full-text screening, 66 studies remained. After data extraction, six studies were further removed as there were insufficient data provided. Additionally, we have included a study which analyzed RRMM patients who underwent alloSCT between 2013 and 2022 published in July 2023 as to enhance the veracity of the analysis. Finally, 61 studies were included in our meta-analysis (7 (11, 14–19) in 2013, 5 (5, 20–23) in 2014, 3 (24–26) in 2015, 3 (27–29) in 2016, 6 (30–35) in 2017, 4 (12, 36–38) in 2018, 7 (7, 39–44) in 2019, 12 (8, 45–55) in 2020, 7 (56–62) in 2021, 3 (63–65) in 2022, 4 (66–69) in 2023) (Figure 1). The overall result is summarized in Table 1.
Response rate
Among the studies included, 31 studies (5, 7, 11, 12, 14–21, 23, 25, 26, 29, 34, 38–41, 44, 47, 48, 54, 56, 59, 60, 64, 68, 69) have reported the CR rate, 19 studies (5, 12, 14–16, 19, 21, 23, 25, 29, 34, 39, 40, 56, 59, 60, 64, 68, 69) have reported the VGPR rate, and 23 studies (5, 7, 11, 14, 16, 18–21, 23, 25, 26, 29, 34, 39, 40, 47, 54, 59, 60, 64, 68, 69) have reported the PR rate. Based on the random-effect model, the pooled estimate CR, VGPR, and PR rates were 0.45 (95% Cl 0.44, 0.47), 0.21 (95% Cl 0.19, 0.24), and 0.24 (95% Cl 0.22, 0.26), respectively (Figure 2).
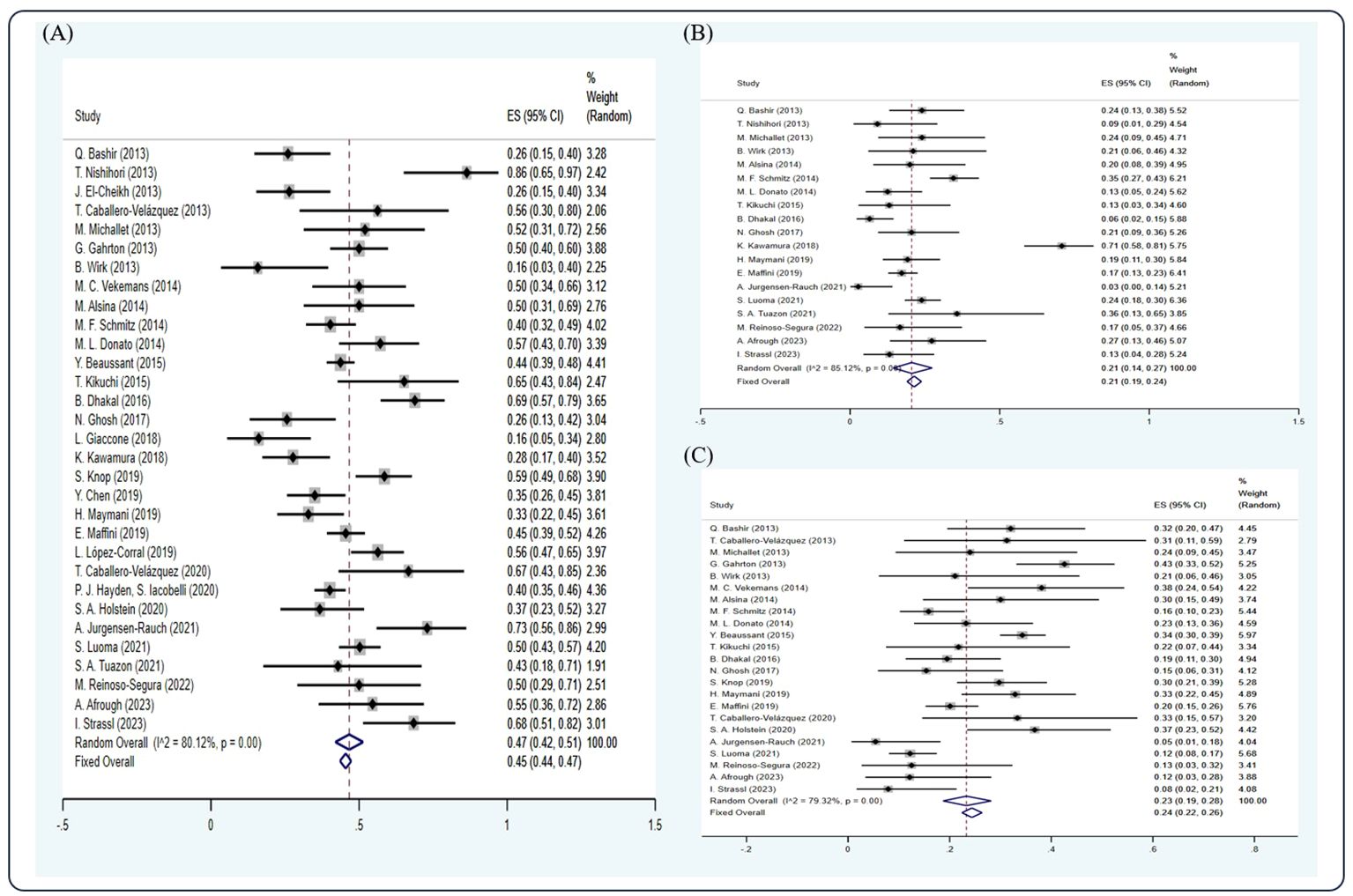
Figure 2 Forest plot of pooled weighted (A) CR, (B) VGPR, and (C) PR based on the random-effect model.
Survival
OS
Based on the random-effect model, the pooled estimates of 1-, 2-, 3-, 5-, and 10-year OS were 0.69 (10 studies (12, 22, 28, 29, 35, 42, 44, 55, 58, 60), 95% Cl 0.66, 0.72), 0.57 (12 studies (15, 19, 26, 32, 33, 51, 52, 54, 55, 58, 61, 64), 95% Cl 0.54, 0.59), 0.45 (11 studies (12, 18, 22, 28–31, 39, 44, 55, 69), 95% Cl 0.42, 0.48), 0.45 (21 studies (5, 11, 17, 20, 22, 25, 26, 34, 40, 43, 48, 50, 56, 57, 59–63, 66, 68), 95% Cl 0.43, 0.47), and 0.36 (11 studies (8, 17, 20, 34, 40, 43, 46, 59, 60, 63, 68), 95% Cl 0.33, 0.39), respectively (Figure 3).

Figure 3 Forest plot of pooled weighted (A) 1-year, (B) 2-year, (C) 3-year, (D) 5-year, (E) 10-year OS based on the random-effect model.
PFS
Based on the random-effect model, the pooled estimates of 1-, 2-, 3-, 5-, 10-year PFS were 0.47 (10 studies (12, 22, 28, 29, 35, 42, 44, 55, 58, 60), 95% Cl 0.44, 0.50), 0.35 (13 studies (7, 15, 19, 26, 32, 33, 41, 51, 52, 55, 58, 61, 65), 95% Cl 0.32, 0.38), 0.24 (9 studies (12, 22, 28–30, 39, 44, 55, 69), 95% Cl 0.22, 0.27), 0.25 (19 studies (5, 11, 17, 20, 25, 26, 40, 41, 43, 50, 54, 56, 57, 59–62, 66, 68), 95% Cl 0.23, 0.27), and 0.28 (9 studies (8, 17, 20, 40, 43, 46, 59, 60, 68), 95% Cl 0.25, 0.31), respectively (Figure 4).
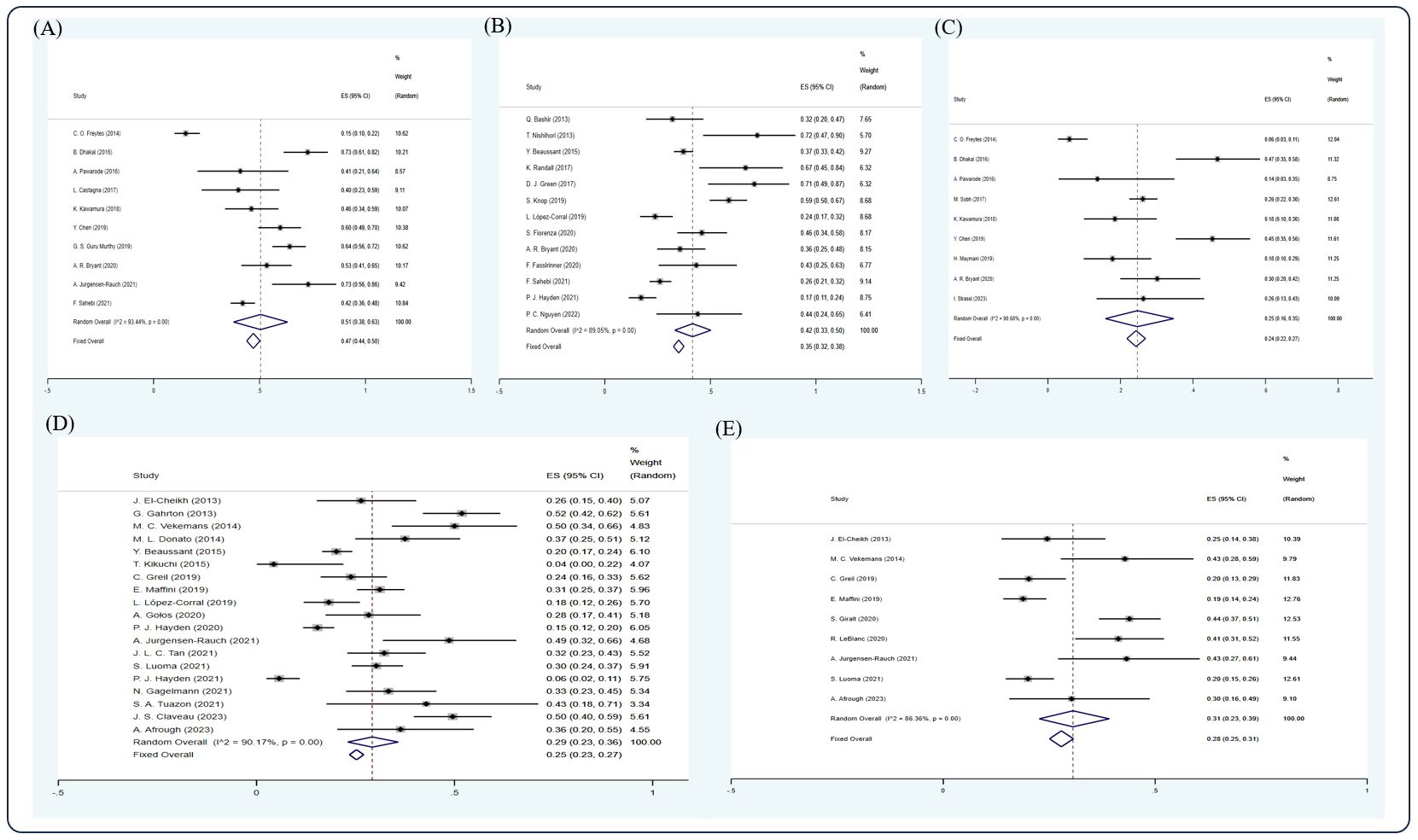
Figure 4 Forest plot of pooled weighted (A) 1-year, (B) 2-year, (C) 3-year, (D) 5-year, (E) 10-year PFS based on the random-effect model.
NRM
Based on the random-effect model, the pooled estimates of 1-, 2-, 3-, 5-, 10-year NRM were 0.16 (15 studies (5, 12, 20, 22, 29, 35, 39, 42–44, 48, 53–55, 58), 95% Cl 0.14, 0.18), 0.21 (11 studies (7, 15, 20, 26, 30, 32, 52, 55, 58, 61, 64), 95% Cl 0.19, 0.23), 0.16 (10 studies (11, 12, 14, 18, 22, 39, 44, 53, 55, 66), 95% Cl 0.14, 0.19), 0.20 (14 studies (20, 22, 26, 36, 38, 40, 43, 45, 53, 59, 61, 63, 66, 68), 95% Cl 0.18, 0.21), and 0.15 (7 studies (8, 40, 43, 46, 59, 63, 68), 95% Cl 0.13, 0.18), respectively (Figure 5).
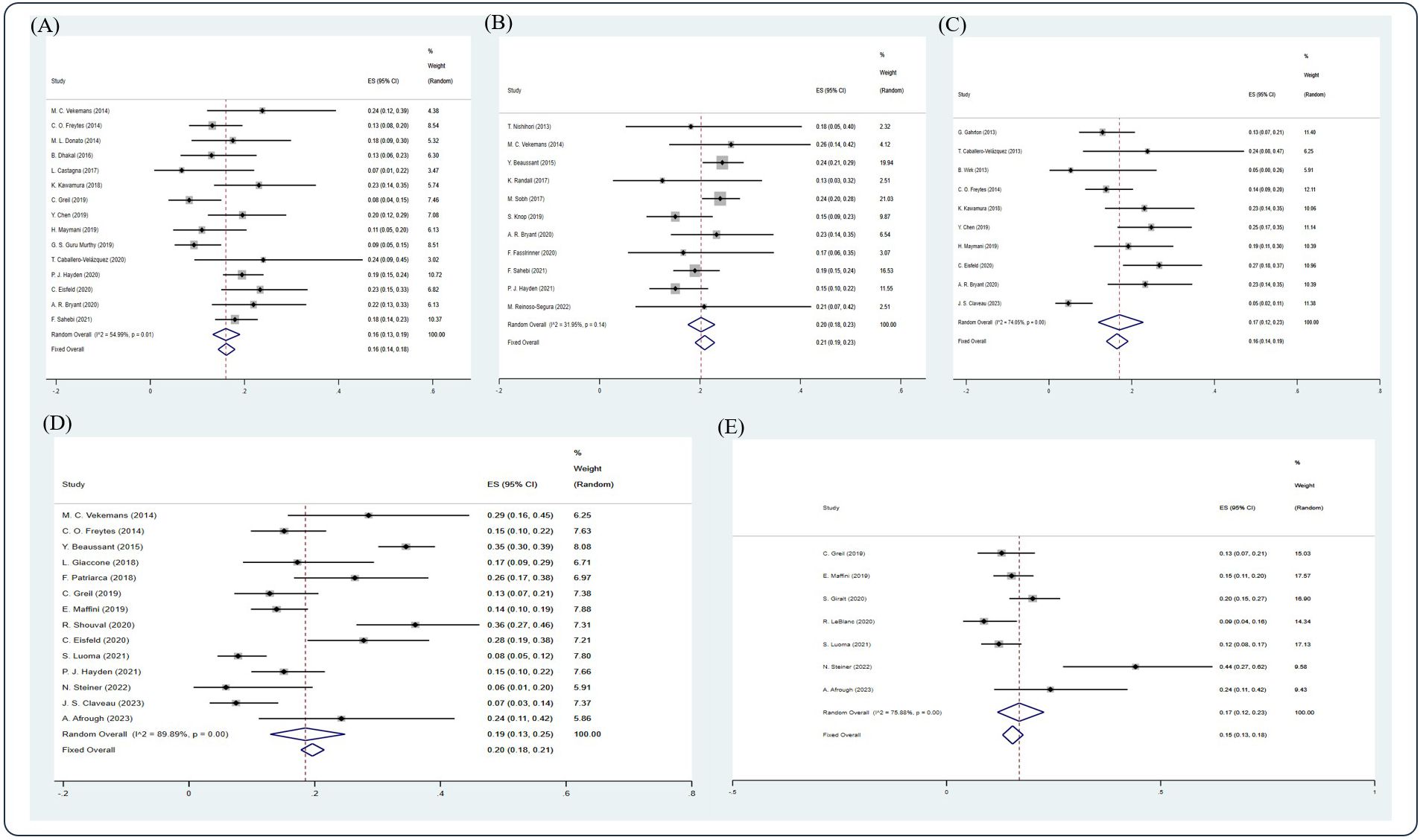
Figure 5. Forest plot of pooled weighted (A) 1-year, (B) 2-year, (C) 3-year, (D) 5-year, and (E) 10-year NRM based on the random-effect model.
Response rate and survival outcome in an NDMM/upfront setting
After screening, 13 studies (15, 17, 27, 33, 38, 43, 46, 57, 59, 62, 65, 66, 68) were included in the analysis. Due to insufficient data available, only CR, VGPR rate, 5-year OS, 2-, 5-, and 10-year PFS, and 5-year NRM could be analyzed in this section. In an NDMM/upfront setting, the pooled estimate CR rate and VGPR rate were 0.54 (4 studies (15, 38, 59, 68), 95% Cl 0.48, 0.61) and 0.22 (3 studies (15, 59, 68), 95% Cl 0.16, 0.28), respectively, whereas the pooled estimate of 5-year OS was 0.69 (7 studies (17, 43, 57, 59, 62, 66, 68), 95% Cl 0.65, 0.73). The pooled estimates of 2-, 5-, and 10-year PFS were 0.61 (3 studies (15, 33, 65), 95% Cl 0.49, 0.73), 0.40 (7 studies (17, 43, 57, 59, 62, 66, 68), 95% Cl 0.35, 0.44), and 0.32 (3 studies (43, 46, 59), 95% Cl 0.27, 0.38), respectively. The pooled estimate of 5-year NRM was 0.11 (4 studies (38, 59, 66, 68), 95% Cl 0.08,0.15). (Figure 6).
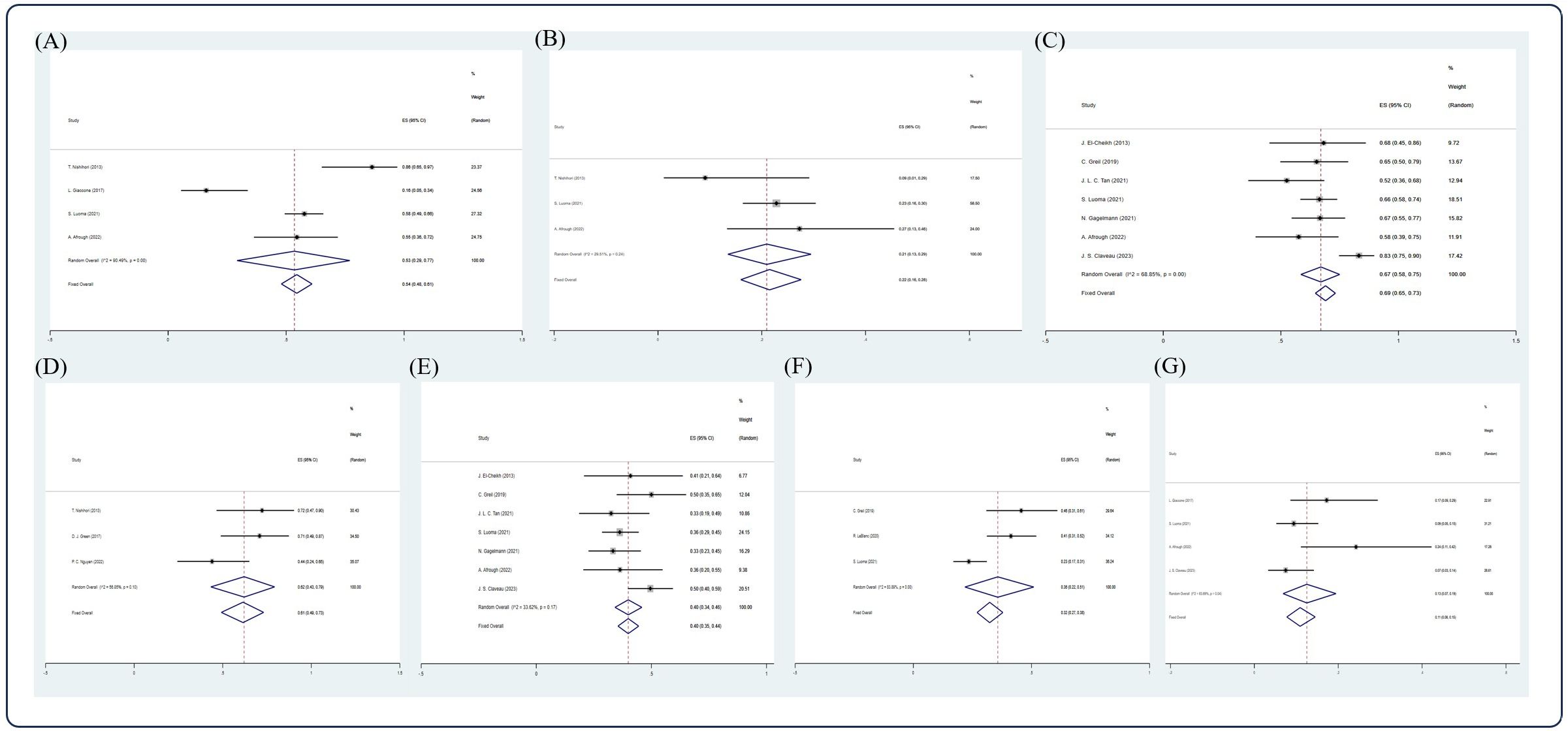
Figure 6. Forest plot of pooled weighted (A) CR, (B) PR, (C) 5-year OS, (D) 2-year PFS, (E) 5-year PFS, (F) 10-year PFS, (G) 5-year NRM in NDMM/frontline setting based on random effect model.
Response rate and survival outcome in an RRMM/salvage setting
After screening, 12 studies (14, 17, 22, 30–32, 43, 57, 59, 61, 63, 69) were included in the analysis. Due to insufficient data available, only CR, VGPR, PR rate, 5- and 10-year OS, 5-year PFS, and 2- and 5-year NRM could be analyzed in this section. In the RRMM/salvage setting, the pooled estimate CR, VGPR, and PR rates were 0.31 (5 studies (14, 31, 32, 59, 69), 95% Cl 0.24, 0.38), 0.21 (3 studies (14, 59, 69), 95% Cl 0.14, 0.29), and 0.12 (4 studies (14, 31, 59, 69), 95% Cl 0.07, 0.18), respectively, whereas the pooled estimates of 3-, 5-, and 10-year OS were 0.30 (3 studies (22, 31, 69), 95% Cl 0.24, 0.36), 0.24 (7 studies (17, 22, 43, 57, 59, 61, 63), 95% Cl 0.21, 0.28), and 0.06 (3 studies (43, 59, 63), 95 CI% 0.03, 0.11), respectively. The pooled estimate of 5-year PFS was 0.10 (5 studies (17, 22, 43, 57, 59, 61), 95% Cl 0.07, 0.13), whereas the pooled estimates of 2- and 5-year NRM were 0.20 (4 studies (30, 32, 43, 61), 95% Cl 0.17, 0.2) and 0.15 (3 studies (59, 61, 63), 95% Cl 0.11, 0.20), respectively (Figures 7, 8).
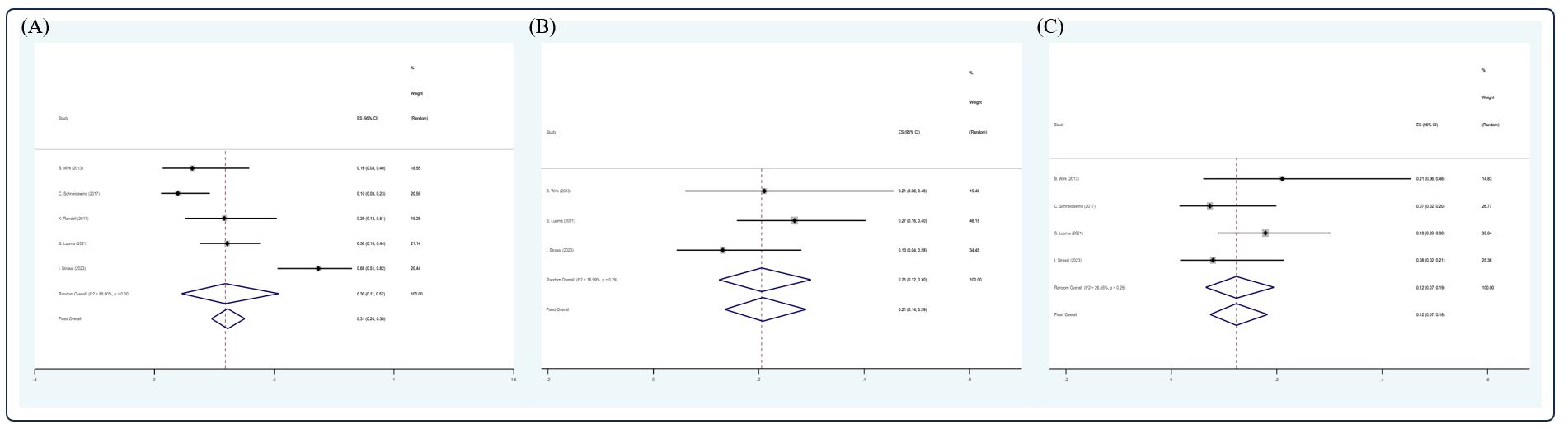
Figure 7 Forest plot of pooled weighted (A) CR, (B) VGPR, and (C) PR in RRMM/salvage setting based on the random-effect model.
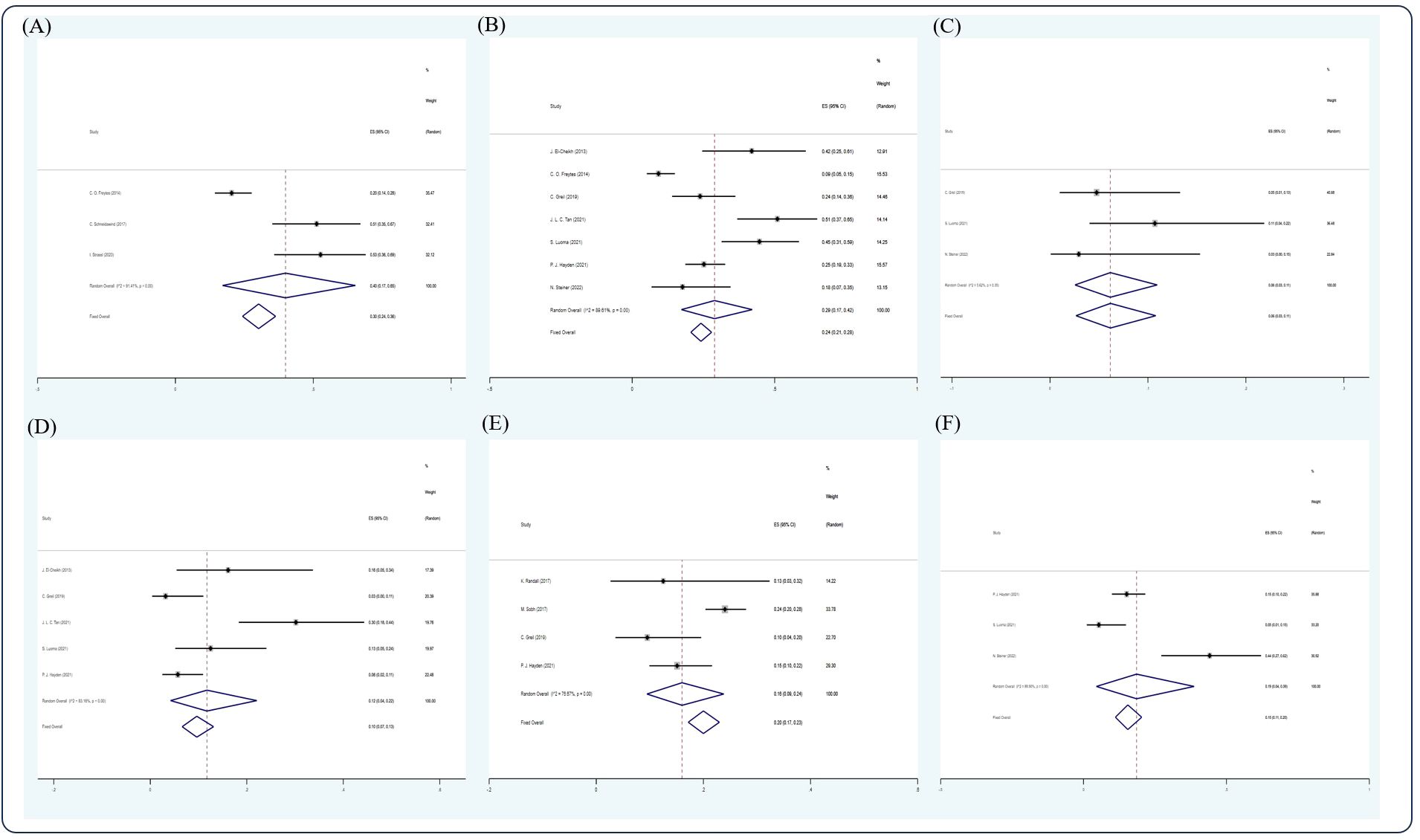
Figure 8 Forest plot of pooled weighted (A) 3-year OS, (B) 5-year OS, (C) 10-year OS, (D) 5-year PFS, (E) 2-year NRM, and (F) 5-year NRM in an RRMM/salvage setting based on the random-effect model.
Heterogeneity analyses and sensitivity analyses
I2 statistic was >50% in all analyses, which indicates large heterogeneity between data sources. We failed to identify the source of heterogeneity using sensitivity analysis. Sensitivity analysis showed good stability without obvious interference by particular study.
Publication bias
All funnel plots were visually symmetric. The P value in Begg’s and Egger’s tests of all analysis was >0.05, further confirming the symmetry of all funnel plots. No publication bias was found in our study.
Discussion
Having a history of 66 years since the first alloSCT held by Donnall Tomas in 1957 (70), there has been an accumulation of a certain number of studies with long-term follow-up. In the past 10 years, a simple search on engines could yield 1,709 studies related to alloSCT in MM patients. The IMWG group made consensus that allogeneic SCT or tandem auto-allo-SCT should be limited to clinical trials (71), alloSCT patient data were precious and scarce. Our meta was held to optimize the use of these limited data.
In our results, OS and PFS of alloSCT, both had a marked fall from first to second and second to third years and then remained constant till the 10th year. NRM increased from the first to the second year and then remained relatively stable. The response rate of alloSCT was comparatively low. The survival benefits from alloSCT sustained. Long-term follow-up could reflect a relatively better survival outcome in alloSCT. Due to insufficient data available, the analysis of relapse rate could not be performed. The gap between OS and PFS in both settings give a clue on the survival status of patients. Furthermore, good response rate and promising survival outcome were observed in the NDMM/frontline setting.
AlloSCT could be adapted in MM patients under different conditions, as mentioned; studies suggested that it was most effective in high-risk NDMM patients. Meanwhile, there is evidence showing the beneficial effect of using alloSCT in a relapse setting, especially in young relapse patients (72). A long-term follow-up trial comparing survival outcome of relapse patients using salvage treatment with bortezomib and/or immunomodulatory agents and salvage alloSCT showed a higher 7-year OS and PFS in the alloSCT group (36). However, there is contradicting research evidence. In research aiming to compare the outcome of alloSCT in NDMM or RRMM, results showed better patient outcome in the first-line setting, with a good CR rate of 48.3%, a median PFS of 30.2 months, and a 10-year OS of 51%. The median PFS was only 8 months in the relapse setting (73). The effectiveness of alloSCT in the relapse setting is inconsistent, and age might be a more important determinant influencing the outcome.
In our meta-analysis, the CR rate and survival outcome in the RRMM/relapsed setting was below the average of the overall result, with a low CR rate of 0.31, 5-year OS of 0.24, and 10-year OS of 0.06. Due to insufficient data on survival status before alloSCT, the improvement of survival outcome could not be assessed. Despite prognostic factors such as age and cytogenetic risk, conditioning therapy and consolidation regimen could cause great influence to the result.
Since the introduction of less ablative conditioning regimens reduced-intensity conditioning (RIC) and nonmyeloablative (NMA) in 1998, NRM and systemic toxicity greatly decreased while keeping promising GvMM effects (4). Traditional myeloablative conditioning regimens such as total body irradiation, cyclophosphamide, and busulfan were less used afterwards. The working party of the EBMT had held a retrospective study comparing outcomes of MM patients undergoing alloSCT after treosulfan-conditioning (Treo), non-Treo RIC or non-Treo MAC, higher 5-year OS (Treo: 62%, RIC: 57%, MAC: 47%), and lower NRM (Treo: 10%, RIC: 17%, MAC: 19%) were observed in the group using Treo-based conditioning (49).
The use of novel agents such as PI, IMID, and ADC as post-transplant consolidation regimens have shown promising results with acceptable toxicity. The use of DLI or in combination with PI and IMID in patients relapsing after alloSCT showed sustained GvMM effects in early studies. However, GVHD was commonly observed in patients treated with DLI after SCT (74). CD19 CAR “DLI” was developed and studied in patients relapsing after allo-HCT in B-cell malignancies. Studies have shown comparatively low incidence of GVHD with promising effects (75). Besides, a case report has shown relief of myelosuppression due to rapid proliferation of BCMA CAR-T cells using auto-SCT in MM patients (76). Small sample research has shown acceptable efficacy and safety of using CAR-T in post-alloSCT RRMM patients (77, 78). In addition, Liana Nikolaenko conducted a retrospective study to evaluate GVHD of using monoclonal antibody daratumumab in a post-alloSCT setting; 41% of the 34 RRMM patients included achieved PR or better, five developed acute GVHD, and none developed chronic GVHD (79). CAR-T, antibody, or immunoconjugate may have potential synergistic immunomodulatory effect with SCT; further studies are required to verify the effectiveness and safety of treatment.
There were only a few meta-analyses with regards to alloSCT. The data were hard to organize as the fundamental state of each patient varied and there were numerous preconditioning and maintenance regimens. Our meta could only provide a comprehensive but rough overview of the relative effectiveness and trend of survival of alloSCT over the years.
Conclusion
Our research gathered alloSCT MM data over the recent 10 years and provided a comprehensive analysis to verify the response rate and survival outcome of alloSCT in MM patients. AlloSCT has sustained OS, PFS, and NRM rates from the third year on. Long-term follow-up could reveal survival benefits of alloSCT in MM patients. Moreover, results have shown a promising effect of AlloSCT in an NDMM/upfront setting, whereas its effect in an RRMM/salvage setting is below average in our meta-analysis. Novel treatments such as CAR-T, antibody, or immunoconjugate may have potential synergistic immunomodulatory effects with SCT, whereas further studies were required to verify the effectiveness and safety of treatment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft. KL: Formal analysis, Methodology, Software, Validation, Writing – review & editing. XZ: Conceptualization, Data curation, Validation, Writing – review & editing. JH: Project administration, Supervision, Validation, Writing – review & editing. TL: Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hemminki K, Försti A, Houlston R, Sud A. Epidemiology, genetics and treatment of multiple myeloma and precursor diseases. Int J Cancer. (2021) 149:1980–96. doi: 10.1002/ijc.33762
2. Kuehl WM, Bergsagel PL. Molecular pathogenesis of multiple myeloma and its premalignant precursor. J Clin Invest. (2012) 122:3456–63. doi: 10.1172/JCI61188
3. Cowan AJ, Green DJ, Kwok M, Lee S, Coffey DG, Holmberg LA, et al. Diagnosis and management of multiple myeloma: A review. JAMA. (2022) 327:464–77. doi: 10.1001/jama.2022.0003
4. Claveau JS, Buadi FK, Kumar S. Current role of allogeneic stem cell transplantation in multiple myeloma. Oncol Ther. (2022) 10:105–22. doi: 10.1007/s40487-022-00195-3
5. Donato ML, Siegel DS, Vesole DH, McKiernan P, Nyirenda T, Pecora AL, et al. The graft-versus-myeloma effect: chronic graft-versus-host disease but not acute graft-versus-host disease prolongs survival in patients with multiple myeloma receiving allogeneic transplantation. Biol Blood Marrow Transplant. (2014) 20:1211–6. doi: 10.1016/j.bbmt.2014.04.027
6. Costa LJ, Iacobelli S, Pasquini MC, Modi R, Giaccone L, Blade J, et al. Long-term survival of 1338 MM patients treated with tandem autologous vs. autologous-allogeneic transplantation. Bone Marrow Transplant. (2020) 55:1810–6. doi: 10.1038/s41409-020-0887-4
7. Knop S, Engelhardt M, Liebisch P, Meisner C, Holler E, Metzner B, et al. Allogeneic transplantation in multiple myeloma: long-term follow-up and cytogenetic subgroup analysis. Leukemia. (2019) 33:2710–9. doi: 10.1038/s41375-019-0537-2
8. Giralt S, Costa LJ, Maloney D, Krishnan A, Fei M, Antin JH, et al. Tandem autologous-autologous versus autologous-allogeneic hematopoietic stem cell transplant for patients with multiple myeloma: long-term follow-up results from the blood and marrow transplant clinical trials network 0102 trial. Biol Blood Marrow Transplant. (2020) 26:798–804. doi: 10.1016/j.bbmt.2019.11.018
9. Schilling G, Hansen T, Shimoni A, Zabelina T, Pérez-Simón JA, Gutierrez NC, et al. Impact of genetic abnormalities on survival after allogeneic hematopoietic stem cell transplantation in multiple myeloma. Leukemia. (2008) 22:1250–5. doi: 10.1038/leu.2008.88
10. Kröger N, Badbaran A, Zabelina T, Ayuk F, Wolschke C, Alchalby H, et al. Impact of high-risk cytogenetics and achievement of molecular remission on long-term freedom from disease after autologous-allogeneic tandem transplantation in patients with multiple myeloma. Biol Blood Marrow Transplant. (2013) 19:398–404. doi: 10.1016/j.bbmt.2012.10.008
11. Gahrton G, Iacobelli S, Björkstrand B, Hegenbart U, Gruber A, Greinix H, et al. Autologous/reduced-intensity allogeneic stem cell transplantation vs autologous transplantation in multiple myeloma: long-term results of the EBMT-NMAM2000 study. Blood. (2013) 121:5055–63. doi: 10.1182/blood-2012-11-469452
12. Kawamura K, Tsukada N, Kanda Y, Ikeda T, Yoshida A, Ueda Y, et al. The role of allogeneic transplantation for multiple myeloma in the era of novel agents: A study from the Japanese society of myeloma. Biol Blood Marrow Transplant. (2018) 24:1392–8. doi: 10.1016/j.bbmt.2018.03.012
13. Montefusco V, Mussetti A, Rezzonico F, Maura F, Pennisi M, de Philippis C, et al. Allogeneic stem cell transplantation and subsequent treatments as a comprehensive strategy for long-term survival of multiple myeloma patients. Bone Marrow Transplant. (2017) 52:1602–8. doi: 10.1038/bmt.2017.183
14. Wirk B, Byrne M, Dai Y, Moreb JS. Outcomes of salvage autologous versus allogeneic hematopoietic cell transplantation for relapsed multiple myeloma after initial autologous hematopoietic cell transplantation. J Clin Med Res. (2013) 5:174–84. doi: 10.4021/jocmr1274w
15. Nishihori T, Ochoa-Bayona JL, Kim J, Pidala J, Shain K, Baz R, et al. Allogeneic hematopoietic cell transplantation for consolidation of VGPR or CR for newly diagnosed multiple myeloma. Bone Marrow Transplant. (2013) 48:1179–84. doi: 10.1038/bmt.2013.37
16. Michallet M, Sobh M, El-Cheikh J, Morisset S, Sirvent A, Reman O, et al. Evolving strategies with immunomodulating drugs and tandem autologous/allogeneic hematopoietic stem cell transplantation in first line high risk multiple myeloma patients. Exp Hematol. (2013) 41:1008–15. doi: 10.1016/j.exphem.2013.08.003
17. El-Cheikh J, Crocchiolo R, Furst S, Stoppa AM, Ladaique P, Faucher C, et al. Long-term outcome after allogeneic stem-cell transplantation with reduced-intensity conditioning in patients with multiple myeloma. Am J Hematol. (2013) 88:370–4. doi: 10.1002/ajh.23412
18. Caballero-Velázquez T, López-Corral L, Encinas C, Castilla-Llorente C, Martino R, Rosiñol L, et al. Phase II clinical trial for the evaluation of bortezomib within the reduced intensity conditioning regimen (RIC) and post-allogeneic transplantation for high-risk myeloma patients. Br J Haematol. (2013) 162:474–82. doi: 10.1111/bjh.12410
19. Bashir Q, Khan H, Thall PF, Liu P, Shah N, Kebriaei P, et al. A randomized phase II trial of fludarabine/melphalan 100 versus fludarabine/melphalan 140 followed by allogeneic hematopoietic stem cell transplantation for patients with multiple myeloma. Biol Blood Marrow Transplant. (2013) 19:1453–8. doi: 10.1016/j.bbmt.2013.07.008
20. Vekemans MC, Michaux L, Van Den Neste E, Ferrant A. Long-term survival after allogeneic stem cell transplantation for advanced stage multiple myeloma. Br J Haematol. (2014) 166:616–8. doi: 10.1111/bjh.12881
21. Schmitz MF, Otten HG, Franssen LE, van Dorp S, Strooisma T, Lokhorst HM, et al. Secondary monoclonal gammopathy of undetermined significance after allogeneic stem cell transplantation in multiple myeloma. Haematologica. (2014) 99:1846–53. doi: 10.3324/haematol.2014.111104
22. Freytes CO, Vesole DH, LeRademacher J, Zhong X, Gale RP, Kyle RA, et al. Second transplants for multiple myeloma relapsing after a previous autotransplant-reduced-intensity allogeneic vs autologous transplantation. Bone Marrow Transplant. (2014) 49:416–21. doi: 10.1038/bmt.2013.187
23. Alsina M, Becker PS, Zhong X, Adams A, Hari P, Rowley S, et al. Lenalidomide maintenance for high-risk multiple myeloma after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. (2014) 20:1183–9. doi: 10.1016/j.bbmt.2014.04.014
24. Mir MA, Kapoor P, Kumar S, Pandey S, Dispenzieri A, Lacy MQ, et al. Trends and outcomes in allogeneic hematopoietic stem cell transplant for multiple myeloma at Mayo Clinic. Clin Lymphoma Myeloma Leuk. (2015) 15:349–57.e2. doi: 10.1016/j.clml.2015.03.016
25. Kikuchi T, Mori T, Koda Y, Kohashi S, Kato J, Toyama T, et al. Outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for multiple myeloma. Int J Hematol. (2015) 102:670–7. doi: 10.1007/s12185-015-1873-2
26. Beaussant Y, Daguindau E, Pugin A, Mohty M, Avet-Loiseau H, Roos-Weil D, et al. Hematopoietic Stem Cell Transplantation in Multiple Myeloma: A Retrospective Study of the Société Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC). Biol Blood Marrow Transplant. (2015) 21:1452–9. doi: 10.1016/j.bbmt.2015.04.020
27. Sobh M, Michallet M, Gahrton G, Iacobelli S, van Biezen A, Schönland S, et al. Allogeneic hematopoietic cell transplantation for multiple myeloma in Europe: trends and outcomes over 25 years. A study by the EBMT Chronic Malignancies Working Party. Leukemia. (2016) 30:2047–54. doi: 10.1038/leu.2016.101
28. Pawarode A, Mineishi S, Reddy P, Braun TM, Khaled YA, Choi SW, et al. Reducing treatment-related mortality did not improve outcomes of allogeneic myeloablative hematopoietic cell transplantation for high-risk multiple myeloma: A university of Michigan prospective series. Biol Blood Marrow Transplant. (2016) 22:54–60. doi: 10.1016/j.bbmt.2015.07.021
29. Dhakal B, D'Souza A, Martens M, Kapke J, Harrington AM, Pasquini M, et al. Allogeneic hematopoietic cell transplantation in multiple myeloma: impact of disease risk and post allograft minimal residual disease on survival. Clin Lymphoma Myeloma Leuk. (2016) 16:379–86. doi: 10.1016/j.clml.2016.03.001
30. Sobh M, Michallet M, Dubois V, Iacobelli S, Koster L, Van Biezen A, et al. Salvage use of allogeneic hematopoietic stem cell transplantation after reduced intensity conditioning from unrelated donors in multiple myeloma. A study by the Plasma Cell Disorders subcommittee of the European Group for Blood and Marrow Transplant Chronic Malignancies Working Party. Haematologica. (2017) 102:e271–e4. doi: 10.3324/haematol.2017.165399
31. Schneidawind C, Duerr-Stoerzer S, Faul C, Kanz L, Weisel K, Bethge W, et al. Follow-up of patients with refractory or relapsed multiple myeloma after allogeneic hematopoietic cell transplantation. Clin Transplant. (2017) 31. doi: 10.1111/ctr.12994
32. Randall K, Kaparou M, Xenou E, Paneesha S, Kishore B, Kanellopoulos A, et al. Reduced-intensity conditioning allogeneic transplantation after salvage treatment with DT-PACE in myeloma patients relapsing early after autologous transplant. Eur J Haematol. (2017) 99:300–5. doi: 10.1111/ejh.12917
33. Green DJ, Maloney DG, Storer BE, Sandmaier BM, Holmberg LA, Becker PS, et al. Tandem autologous/allogeneic hematopoietic cell transplantation with bortezomib maintenance therapy for high-risk myeloma. Blood Adv. (2017) 1:2247–56. doi: 10.1182/bloodadvances.2017010686
34. Ghosh N, Ye X, Tsai HL, Bolaños-Meade J, Fuchs EJ, Luznik L, et al. Allogeneic blood or marrow transplantation with post-transplantation cyclophosphamide as graft-versus-host disease prophylaxis in multiple myeloma. Biol Blood Marrow Transplant. (2017) 23:1903–9. doi: 10.1016/j.bbmt.2017.07.003
35. Castagna L, Mussetti A, Devillier R, Dominietto A, Marcatti M, Milone G, et al. Haploidentical allogeneic hematopoietic cell transplantation for multiple myeloma using post-transplantation cyclophosphamide graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. (2017) 23:1549–54. doi: 10.1016/j.bbmt.2017.05.006
36. Patriarca F, Bruno B, Einsele H, Spina F, Giaccone L, Montefusco V, et al. Long-Term Follow-Up of a Donor versus No-Donor Comparison in Patients with Multiple Myeloma in First Relapse after Failing Autologous Transplantation. Biol Blood Marrow Transplant. (2018) 24:406–9. doi: 10.1016/j.bbmt.2017.10.014
37. Htut M, D'Souza A, Krishnan A, Bruno B, Zhang MJ, Fei M, et al. Autologous/allogeneic hematopoietic cell transplantation versus tandem autologous transplantation for multiple myeloma: comparison of long-term postrelapse survival. Biol Blood Marrow Transplant. (2018) 24:478–85. doi: 10.1016/j.bbmt.2017.10.024
38. Giaccone L, Evangelista A, Patriarca F, Sorasio R, Pini M, Carnevale-Schianca F, et al. Impact of new drugs on the long-term follow-up of upfront tandem autograft-allograft in multiple myeloma. Biol Blood Marrow Transplant. (2018) 24:189–93. doi: 10.1016/j.bbmt.2017.09.017
39. Maymani H, Lin P, Saliba RM, Popat U, Bashir Q, Shah N, et al. Comparison of outcomes of allogeneic hematopoietic cell transplantation for multiple myeloma using three different conditioning regimens. Biol Blood Marrow Transplant. (2019) 25:1039–44. doi: 10.1016/j.bbmt.2019.01.009
40. Maffini E, Storer BE, Sandmaier BM, Bruno B, Sahebi F, Shizuru JA, et al. Long-term follow up of tandem autologous-allogeneic hematopoietic cell transplantation for multiple myeloma. Haematologica. (2019) 104:380–91. doi: 10.3324/haematol.2018.200253
41. López-Corral L, Caballero-Velázquez T, López-Godino O, Rosiñol L, Pérez-Vicente S, Fernandez-Avilés F, et al. Response to Novel Drugs before and after Allogeneic Stem Cell Transplantation in Patients with Relapsed Multiple Myeloma. Biol Blood Marrow Transplant. (2019) 25:1703–12. doi: 10.1016/j.bbmt.2019.04.026
42. Guru Murthy GS, Hari PN, Szabo A, Pasquini M, Narra R, Khan M, et al. Outcomes of reduced-intensity conditioning allogeneic hematopoietic cell transplantation performed in the inpatient versus outpatient setting. Biol Blood Marrow Transplant. (2019) 25:827–33. doi: 10.1016/j.bbmt.2018.12.069
43. Greil C, Engelhardt M, Ihorst G, Schoeller K, Bertz H, Marks R, et al. Allogeneic transplantation of multiple myeloma patients may allow long-term survival in carefully selected patients with acceptable toxicity and preserved quality of life. Haematologica. (2019) 104:370–9. doi: 10.3324/haematol.2018.200881
44. Chen Y, Fu WJ, Xu LP, Ren HY, Lai YR, Liu DH, et al. Comparison of outcomes after human leukocyte antigen-matched and haploidentical hematopoietic stem-cell transplantation for multiple myeloma. Chin Med J (Engl). (2019) 132:1765–72. doi: 10.1097/CM9.0000000000000341
45. Shouval R, Teper O, Fein JA, Danylesko I, Shem Tov N, Yerushalmi R, et al. LDH and renal function are prognostic factors for long-term outcomes of multiple myeloma patients undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. (2020) 55:1736–43. doi: 10.1038/s41409-020-0829-1
46. LeBlanc R, Claveau JS, Ahmad I, Delisle JS, Bambace N, Bernard L, et al. Newly diagnosed multiple myeloma patients treated with tandem auto-allogeneic stem cell transplant have better overall survival with similar outcomes at time of relapse compared to patients who received autologous transplant only. Clin Transplant. (2020) 34:e14099. doi: 10.1111/ctr.14099
47. Holstein SA, Suman VJ, Owzar K, Santo K, Benson DM Jr., Shea TC, et al. Long-term follow-up of CALGB (Alliance) 100001: autologous followed by nonmyeloablative allogeneic transplant for multiple myeloma. Biol Blood Marrow Transplant. (2020) 26:1414–24. doi: 10.1016/j.bbmt.2020.03.028
48. Hayden PJ, Iacobelli S, Pérez-Simón JA, van Biezen A, Minnema M, Niittyvuopio R, et al. Conditioning-based outcomes after allogeneic transplantation for myeloma following a prior autologous transplant (1991-2012) on behalf of EBMT CMWP. Eur J Haematol. (2020) 104:181–9. doi: 10.1111/ejh.13352
49. Gran C, Wang J, Nahi H, Koster L, Gahrton G, Einsele H, et al. Treosulfan conditioning for allogeneic transplantation in multiple myeloma - improved overall survival in first line haematopoietic stem cell transplantation - a large retrospective study by the Chronic Malignancies Working Party of the EBMT. Br J Haematol. (2020) 189:e213–e7. doi: 10.1111/bjh.16642
50. Gołos A, Gil L, Puła B, Boguradzki P, Hałaburda K, Sawicki W, et al. Allogeneic hematopoietic cell transplantation for multiple myeloma: A retrospective analysis of the Polish Myeloma Group. Adv Med Sci. (2020) 65:429–36. doi: 10.1016/j.advms.2020.08.003
51. Fiorenza S, Routledge D, Collins J, Shipton M, Harrison S, Bajel A, et al. Time from autologous to allogeneic hematopoietic stem cell transplantation impacts post-transplant outcomes in multiple myeloma. Bone Marrow Transplant. (2020) 55:1172–4. doi: 10.1038/s41409-019-0642-x
52. Fasslrinner F, Stölzel F, Kramer M, Teipel R, Brogsitter C, Morgner A, et al. Radioimmunotherapy in combination with reduced-intensity conditioning for allogeneic hematopoietic cell transplantation in patients with advanced multiple myeloma. Biol Blood Marrow Transplant. (2020) 26:691–7. doi: 10.1016/j.bbmt.2019.11.007
53. Eisfeld C, Eßeling E, Wullenkord R, Khandanpour C, Reusch J, Mikesch JH, et al. Long-term survival and polyclonal immunoglobulin reconstitution after allogeneic stem cell transplantation in multiple myeloma. Ann Hematol. (2020) 99:1907–15. doi: 10.1007/s00277-020-04068-5
54. Caballero-Velázquez T, Calderón-Cabrera C, López-Corral L, Puig N, Marquez-Malaver F, Pérez-López E, et al. Efficacy of bortezomib to intensify the conditioning regimen and the graft-versus-host disease prophylaxis for high-risk myeloma patients undergoing transplantation. Bone Marrow Transplant. (2020) 55:419–30. doi: 10.1038/s41409-019-0670-6
55. Bryant AR, Hilden P, Giralt S, Chung DJ, Maloy M, Landau H, et al. Presalvage international staging system stage and other important outcome associations in CD34(+)-selected allogeneic hematopoietic stem cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. (2020) 26:58–65. doi: 10.1016/j.bbmt.2019.08.023
56. Tuazon SA, Sandmaier BM, Gooley TA, Fisher DR, Holmberg LA, Becker PS, et al. (90)Y-labeled anti-CD45 antibody allogeneic hematopoietic cell transplantation for high-risk multiple myeloma. Bone Marrow Transplant. (2021) 56:202–9. doi: 10.1038/s41409-020-01000-3
57. Tan JLC, Das T, Kliman D, Muirhead J, Gorniak M, Kalff A, et al. Evaluation of EuroFlow minimal residual disease measurement and donor chimerism monitoring following tandem auto-allogeneic transplantation for multiple myeloma. Bone Marrow Transplant. (2021) 56:1116–25. doi: 10.1038/s41409-020-01148-y
58. Sahebi F, Eikema DJ, Koster L, Kroger N, Meijer E, van Doesum JA, et al. Post-transplantation cyclophosphamide for graft-versus- host disease prophylaxis in multiple myeloma patients who underwent allogeneic hematopoietic cell transplantation: first comparison by donor type. A study from the chronic Malignancies working party of the European society for blood and marrow transplantation. Transplant Cell Ther. (2021) 27:999.e1–.e10. doi: 10.1016/j.jtct.2021.09.008
59. Luoma S, Silvennoinen R, Rauhala A, Niittyvuopio R, Martelin E, Lindström V, et al. Long-term outcome after allogeneic stem cell transplantation in multiple myeloma. Ann Hematol. (2021) 100:1553–67. doi: 10.1007/s00277-021-04514-y
60. Jurgensen-Rauch A, Gibbs S, Farrell M, Aries J, Grantham M, Eccersley L, et al. Reduced intensity allogeneic hematopoietic stem cell transplantation is a safe and effective treatment option in high-risk myeloma patients - a single centre experience. Br J Haematol. (2021) 193:420–3. doi: 10.1111/bjh.17379
61. Hayden PJ, Eikema DJ, de Wreede LC, Koster L, Kröger N, Einsele H, et al. Second allogeneic transplants for multiple myeloma: a report from the EBMT Chronic Malignancies Working Party. Bone Marrow Transplant. (2021) 56:2367–81. doi: 10.1038/s41409-021-01286-x
62. Gagelmann N, Eikema DJ, de Wreede LC, Rambaldi A, Iacobelli S, Koster L, et al. Upfront stem cell transplantation for newly diagnosed multiple myeloma with del(17p) and t(4;14): a study from the CMWP-EBMT. Bone Marrow Transplant. (2021) 56:210–7. doi: 10.1038/s41409-020-01007-w
63. Steiner N, Schober P, Willenbacher W, Kircher B, Gunsilius E, Wolf D, et al. Autologous and allogeneic stem cell transplantation as salvage treatment options for relapsed/refractory multiple myeloma: A single-center experience over 20 years. Anticancer Res. (2022) 42:5825–32. doi: 10.21873/anticanres.16090
64. Reinoso-Segura M, Caballero-Velázquez T, Herrera P, Patriarca F, Fanin R, Bruno B, et al. Phase II trial of allogeneic transplantation plus novel drugs in multiple myeloma: effect of intensifying reduced-intensity conditioning with bortezomib and adding maintenance treatment. Transplant Cell Ther. (2022) 28:258.e1–.e8. doi: 10.1016/j.jtct.2022.01.026
65. Nguyen PC, Muirhead J, Tan J, Kalff A, Bergin K, Walker P, et al. Upfront tandem autologous non-myeloablative allogeneic stem cell transplant in high-risk multiple myeloma: a long-term single-centre experience. Intern Med J. (2022) 52:1263–7. doi: 10.1111/imj.15842
66. Claveau JS, LeBlanc R, Ahmad I, Delisle JS, Cohen S, Kiss T, et al. Bortezomib maintenance after allogeneic transplantation in newly diagnosed myeloma patients results in decreased incidence and severity of chronic GVHD. Transplant Cell Ther. (2023) 29:44.e1–.e9. doi: 10.1016/j.jtct.2022.10.022
67. Kříž T, Jungová A, Lysák D, Karas M, Hrabětová M, Šrámek J, et al. Allogeneic stem cell transplantation in patients with multiple myeloma-single center experience. Neoplasma. (2023) 70:294–9. doi: 10.4149/neo_2023_220720N733
68. Afrough A, Alsfeld LC, Milton DR, Delgado R, Popat UR, Nieto Y, et al. Long-term outcomes of allogeneic hematopoietic cell transplantation in patients with newly diagnosed multiple myeloma. Transplant Cell Ther. (2023) 29:264.e1–.e9. doi: 10.1016/j.jtct.2022.05.023
69. Strassl I, Nikoloudis A, Machherndl-Spandl S, Buxhofer-Ausch V, Binder M, Wipplinger D, et al. Allogeneic stem cell transplantation in multiple myeloma: risk factors and outcomes in the era of new therapeutic options-A single-center experience. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15245738
70. Thomas ED, Lochte HL Jr., Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. (1957) 257:491–6. doi: 10.1056/NEJM195709122571102
71. Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. (2016) 127:2955–62. doi: 10.1182/blood-2016-01-631200
72. Greil C, Engelhardt M, Finke J, Wäsch R. Allogeneic stem cell transplantation in multiple myeloma. Cancers (Basel). (2021) 14. doi: 10.3390/cancers14010055
73. Franssen LE, Raymakers RA, Buijs A, Schmitz MF, van Dorp S, Mutis T, et al. Outcome of allogeneic transplantation in newly diagnosed and relapsed/refractory multiple myeloma: long-term follow-up in a single institution. Eur J Haematol. (2016) 97:479–88. doi: 10.1111/ejh.12758
74. Frey NV, Porter DL. Graft-versus-host disease after donor leukocyte infusions: presentation and management. Best Pract Res Clin Haematol. (2008) 21:205–22. doi: 10.1016/j.beha.2008.02.007
75. Smith M, Zakrzewski J, James S, Sadelain M. Posttransplant chimeric antigen receptor therapy. Blood. (2018) 131:1045–52. doi: 10.1182/blood-2017-08-752121
76. Huang R, Wang X, Zhang X. Unity brings strength: Combination of CAR-T cell therapy and HSCT. Cancer Lett. (2022) 549:215721. doi: 10.1016/j.canlet.2022.215721
77. Htut M, Dhakal B, Cohen AD, Martin T, Berdeja JG, Usmani SZ, et al. Ciltacabtagene autoleucel in patients with prior allogeneic stem cell transplant in the CARTITUDE-1 study. Clin Lymphoma Myeloma Leuk. (2023) 23:882–8. doi: 10.1016/j.clml.2023.08.012
78. John L, Sauer S, Hegenbart U, Dreger P, Hundemer M, Müller-Tidow C, et al. Idecabtagene vicleucel is well tolerated and effective in relapsed/refractory myeloma patients with prior allogeneic stem cell transplantation. Transplant Cell Ther. (2023) 29:609.e1–.e6. doi: 10.1016/j.jtct.2023.06.010
Keywords: multiple myeloma, allogeneic stem cell transplantation, response rate, survival outcome, OS, PFS
Citation: Lin SY, Lu KJ, Zheng XN, Hou J and Liu TT (2024) Efficacy and survival outcome of allogeneic stem-cell transplantation in multiple myeloma: meta-analysis in the recent 10 years. Front. Oncol. 14:1341631. doi: 10.3389/fonc.2024.1341631
Received: 20 November 2023; Accepted: 09 July 2024;
Published: 31 July 2024.
Edited by:
Jacopo Mariotti, Humanitas Research Hospital, ItalyReviewed by:
Luca Castagna, Azienda Ospedaliera Ospedali Riuniti Villa Sofia Cervello, ItalyJohannes Clausen, Ordensklinikum Linz, Austria
Copyright © 2024 Lin, Lu, Zheng, Hou and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Ting Liu, bGl1dGluZ3RpbmdfMDEzMUBzaW5hLmNvbQ==; Jian Hou, aG91amlhbkBtZWRtYWlsLmNvbS5jbg==
 Si Yu Lin
Si Yu Lin Ting Ting Liu
Ting Ting Liu