
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 06 December 2023
Sec. Cancer Epidemiology and Prevention
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1288197
This article is part of the Research TopicParasites effect on the gut microbiomeView all articles
Background: Only a few studies have focused on the association between Schistosoma japonicum and human malignancy. The aim of this study was to update the prevalence rate, mortality, and 5-year overall survival of S. japonicum patients with human malignancy.
Methods: From January 20, 2018, to January 31, 2021, 5,866 inpatients were included in the study. A total of 656 S. japonicum patients with malignancy were identified. Cases were stratified by gender and age groups. The cancer sites, prevalence rate, mortality, and 5-year overall survival of the patients were reported. The S. japonicum patients with malignancy were further divided into a non-digestive system tumor group (n = 309) and a digestive system tumor group (n = 347), including those with cancer in the esophagus, stomach, colon, rectum, liver, gallbladder, bile duct, or pancreas. Chi-squared test and odds ratio with confidence intervals were performed between these two groups.
Results: Lung cancer was found the most common malignancy, accounting for 18.6% of all malignancies, followed by colorectal, stomach, liver, and gallbladder cancers. These five leading malignancies accounted for approximately 61.8% of all cases. Colorectal cancer was the leading cause of malignancy death, followed by lung, stomach, gallbladder, and liver cancers. These five leading causes of death accounted for approximately 55.6% of all death cases. Statistical significance was found in the prevalence rate between S. japonicum and non-S. japonicum patients with/without digestive system tumor (p < 0.001). The odds ratio of S. japonicum patients with digestive system tumors was 1.6 (95%CI: 1.4–1.9).
Conclusion: S. japonicum contributes to a significant prevalence and mortality in digestive system tumors, including colorectal, stomach, liver, and gallbladder cancers.
Human malignancy remains a major health problem, which has become one of the leading causes of death worldwide (1). It is estimated that over 20% of human malignancies are attributable to infections (2). Schistosomiasis is one of the most common parasitic infectious diseases worldwide. At least 254.1 million schistosomiasis-infected patients required preventive treatment in 2021 (https://www.who.int/news-room/fact-sheets/detail/schistosomiasis). A previous study reported that schistosomiasis infection had a potential relation to the occurrence of human malignancy (3).
There are three major Schistosoma species that infect humans: Schistosoma mansoni, Schistosoma haematobium, and Schistosoma japonicum (4). Among these, S. haematobium is reported as a definite carcinogen to humans (human carcinogens Group 1). S. japonicum is reported as a probable carcinogen to humans (human carcinogens Group 2). There is insufficient evidence for determining the carcinogenicity of S. mansoni in humans (human carcinogens Group 3) (3).
S. japonicum is endemic in Southeast Asia (primarily in mainland China). The cercariae of Schistosoma penetrate the skin of humans after the human host comes into contact with contaminated water. The schistosomula live within the veins of the human hosts and need approximately 5–7 weeks before becoming adults and producing eggs. The eggs remain in the body and would die within 1–2 weeks after being released by the female worm. However, the dead eggs can induce inflammation in the human host continually, which leads to inflammatory granulomatous and malignancies (4). Several lines of evidence indicate that S. japonicum is a causative agent in the development of liver cancer and colorectal cancer (5, 6). However, only a few studies have focused on the association between S. japonicum and human malignancy. Furthermore, the data linking S. japonicum to other malignancy sites except the liver and colon and rectum are insufficient. To the best of our knowledge, no study has investigated the prevalence rate, mortality, and 5-year overall survival of S. japonicum patients with human malignancy.
The aim of this study was to investigate the concomitance of S. japonicum and human malignancy. The prevalence rate, mortality, and 5-year overall survival of S. japonicum patients with malignancy were reported.
This retrospective study was approved by the Ethics Committee of Jinshan Hospital, Fudan University (No. JIEC 2021-S55), and informed consent was waived. Patients’ confidentiality was protected. All the data were addressed anonymously. The personal information was appropriately de-identified.
From January 20, 2018, to January 31, 2021, 5,866 initial visit inpatients with schistosomiasis in our hospital were included in the study. All the patients were from an area (Jinshan District, Shanghai, China) that was highly endemic for S. japonicum. The diagnostic criteria were as follows: 1) patient with a contact history of S. japonicum; 2) anti-S. japonicum antibody detected by colloidal dye strip assay (DDIA), Schistosoma ovale test (COPT), enzyme-linked immunosorbent assay (ELISA), indirect red blood cell agglutination assay (IHA) or dot gold immunofiltration assay (DIGFA); and 3) patient received treatment of S. japonicum previously. The exclusion criteria were as follows: patients with positive results of rectal biopsy or stool examination were considered actively infected (n = 3). Finally, 5,863 patients were retained. The electronic medical records of all the patients were reviewed. Among these patients, malignancy cases diagnosed pathologically were identified. The malignancy sites were coded according to the International Statistical Classification of Diseases, 10th Revision (ICD-10). During the same time period, initial visit non-S. japonicum inpatients with pathologically diagnosed malignancy in our hospital were also enrolled. These patients served as the controls. According to the population structures of the S. japonicum patients, they were stratified into five groups by age (group 1, <50 years; group 2, 50–59 years; group 3, 60–69 years; group 4, 70–79 years; and group 5, ≥80 years). The workflow of this study is shown in Figure 1.
The following were recorded: indicators reflecting liver function including serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), total bilirubin (TTB), and prothrombin time (PT); indicators reflecting hematology including red blood cell (RBC), white blood cell (WBC), C-reactive protein (CRP), platelet count (PLT), and blood glucose (GLC); indicators reflecting renal function including urea (URE), creatinine (CRE), and uric acid (UAD); and indicators reflecting tumor markers including alpha-fetoprotein (AFP), carcino-embryonic antigen (CEA), cancer antigen 125 (CA125), and cancer antigen 199 (CA199).
For S. japonicum patients with malignancy, the prevalence rate (The number of cases of a certain tumor/The number of total tumor cases) and mortality (The number of death cases caused by a certain tumor/The number of total tumor cases) were calculated. Their gender- and age-specific prevalence rates and mortality were further calculated.
For non-S. japonicum patients with malignancy, the prevalence rate (The number of cases of a certain tumor/The number of total tumor cases) was calculated. Their gender- and age-specific prevalence rates were further calculated. The prevalence rates were compared between the S. japonicum and non-S. japonicum patients.
For S. japonicum patients, the survival curves of each malignancy site were plotted. Their gender- and age-specific 5-year overall survival were also shown.
Statistical analysis was performed using R software (version 4.2.0; http://www.Rproject.org). The “epitools” package was used for the chi-squared test to compare the differences between categorical variables and to calculate the odds ratio with confidence intervals by unconditional maximum likelihood estimation and bootstrap method. The “survival” and “survminer” packages were used for survival analysis, which was assessed using the Kaplan–Meier curves via log-rank tests. p-Value less than 0.05 was considered statistically significant.
For S. japonicum patients, 5,863 initially admitted S. japonicum patients were identified, with 2,552 women and 3,311 men. In these patients, 656 newly diagnosed malignancy cases were identified, with 223 women and 433 men. For non-S. japonicum patients, 5,415 newly diagnosed malignancy cases were identified, with 2,062 women and 3,353 men (Figure 2A). For S. japonicum patients, a total of 661 death cases were identified, with 276 women and 385 men. Of these patients, 241 cases died from malignancy, with 85 women and 156 men (Figure 2B).
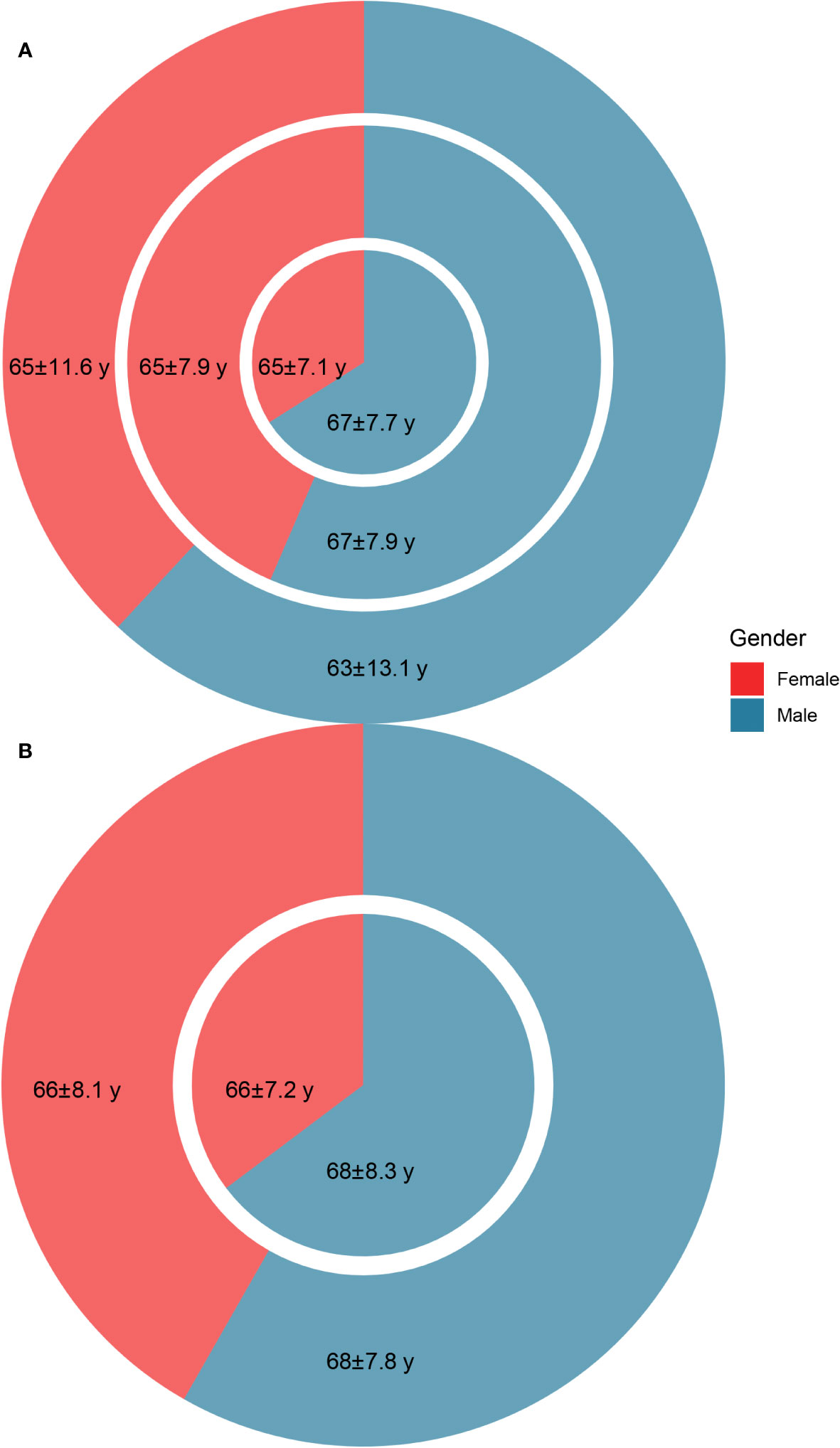
Figure 2 The age (mean ± standard deviation) and gender distribution of the patients. (A) The age and gender distribution of non-Schistosoma japonicum patients with malignancy (outer circle), S. japonicum patients without malignancy (middle circle), and S. japonicum patients with malignancy (inner pie). (B) The age and gender distribution of the total death cases of S. japonicum patients (outer circle) and the death cases of S. japonicum patients with malignancy (inner pie).
The S. japonicum patients with malignancy were further divided into a non-digestive system tumor group (n = 309) and a digestive system tumor group (n = 347), including those with cancer in the esophagus, stomach, colon, rectum, liver, gallbladder, bile duct, or pancreas. The clinical characteristics of the two groups are shown in Table 1. Statistical significance was found in the prevalence rate between S. japonicum and non-S. japonicum patients with/without digestive system tumor (p < 0.001). The odds ratio of S. japonicum patients with digestive system tumors was 1.6 (95%CI: 1.4–1.9). The co-occurrence network of digestive system tumors and clinical laboratory tests is shown in Figure 3. The prevalence rate and mortality of Schistosoma japonicum and non-S. japonicum patients with malignancy are shown in Table 2.
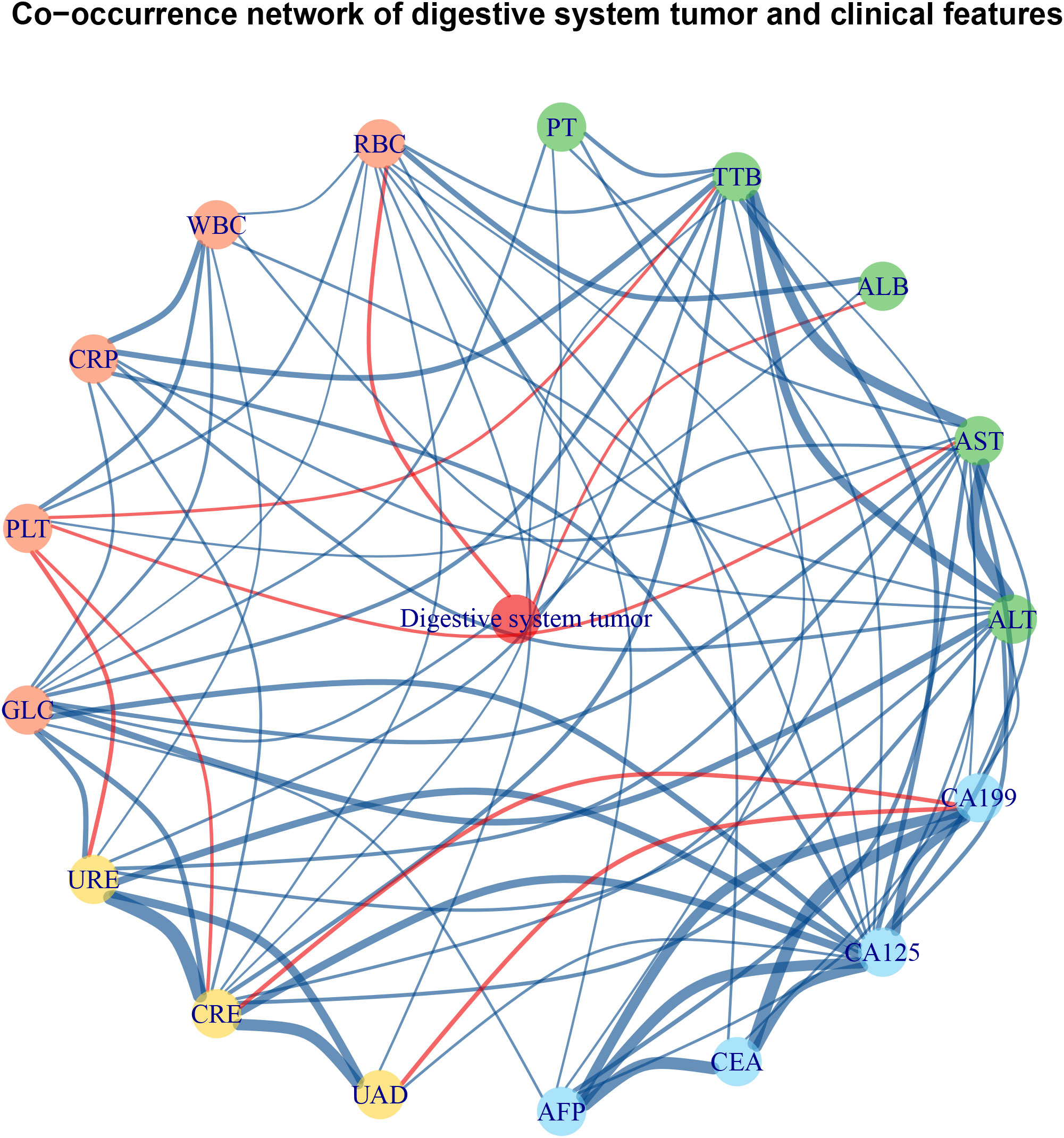
Figure 3 Co-occurrence network of digestive system tumor and clinical laboratory tests of Schistosoma japonicum patients with malignancy. The red edge indicates a negative correlation between two nodes, and the blue edge indicates positive correlation between two nodes. The green nodes are laboratory tests reflecting liver function. The red nodes are laboratory tests reflecting hematology. The yellow nodes are laboratory tests reflecting renal function. The blue nodes are laboratory tests reflecting tumor markers. ALB, albumin; ALT, alanine aminotransferase; AFP, alpha-fetoprotein; AST, aspartate aminotransferase; CA125, cancer antigen 125; CA199, cancer antigen 199; CEA, carcino-embryonic antigen; CRE, creatinine; CRP, C-reactive protein; GLC, blood glucose; PLT, platelet count; PT, prothrombin time; RBC, red blood cell; TTB, total bilirubin; UAD, uric acid; URE, urea; WBC, white blood cell.
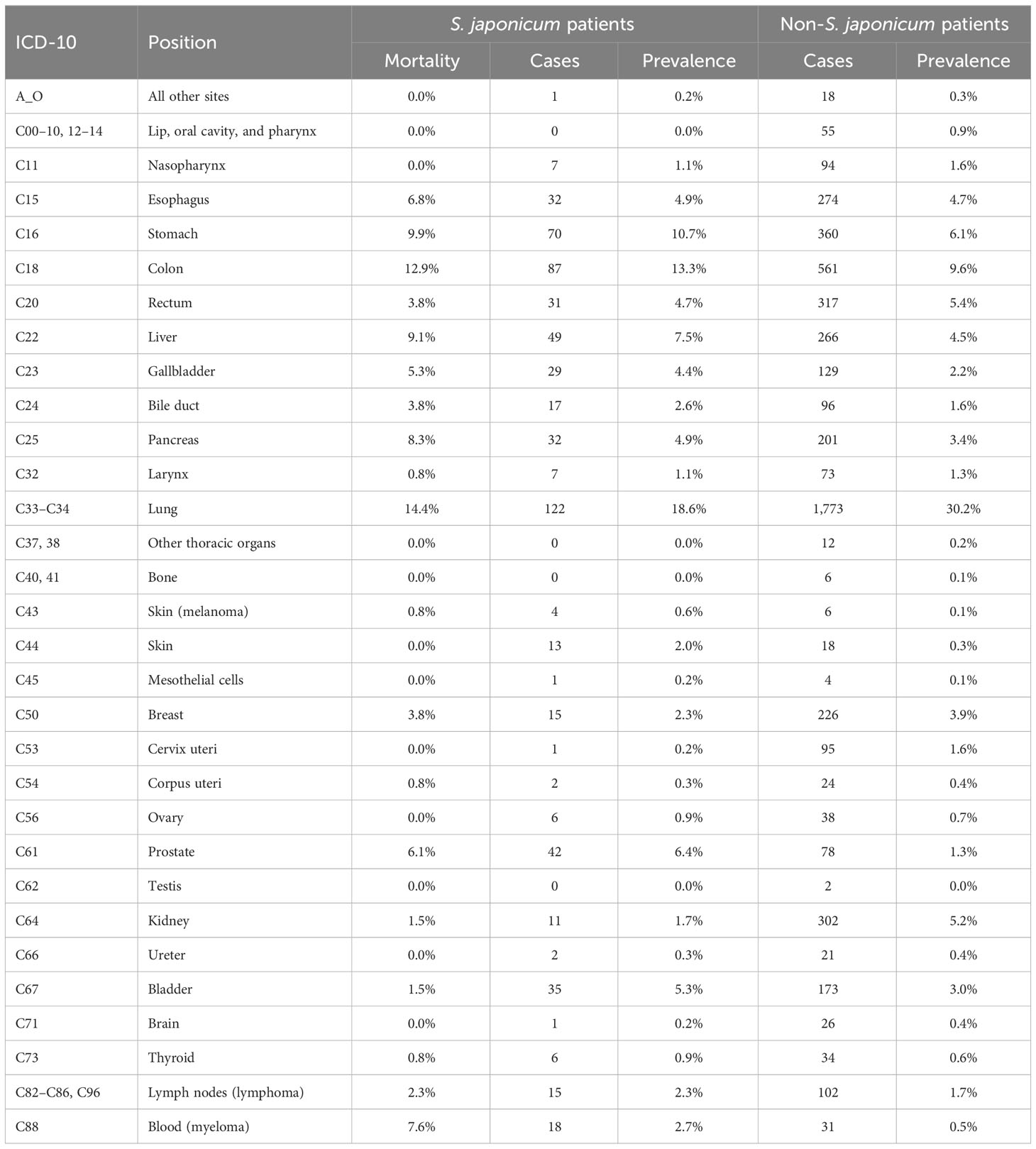
Table 2 The prevalence rate and mortality of Schistosoma japonicum and non-S. japonicum patients with malignancy.
For S. japonicum patients, lung cancer was the most common malignancy, accounting for 18.6% of all cases, followed by colorectal cancer (18.0%), stomach cancer (10.7%), liver cancer (7.5%), and gallbladder cancer (7.0%). These five leading malignancies accounted for approximately 61.8% of all newly diagnosed malignancy cases. Lung cancer was found to be the most common malignancy in men, accounting for 13.6% of all cases, followed by colorectal cancer (9.9%), stomach cancer (8.4%), prostate cancer (6.4%), and liver cancer (5.2%). These five leading cancers accounted for approximately 43.5% of all newly diagnosed malignancy cases in men. For women, colorectal cancer was the most common malignancy, accounting for 8.1% of all cases, followed by lung cancer (5.0%), gallbladder cancer (4.3%), stomach cancer (2.3%), and liver cancer (2.3%). These five leading malignancies accounted for approximately 22.0% of all cases in women (Figure 4).
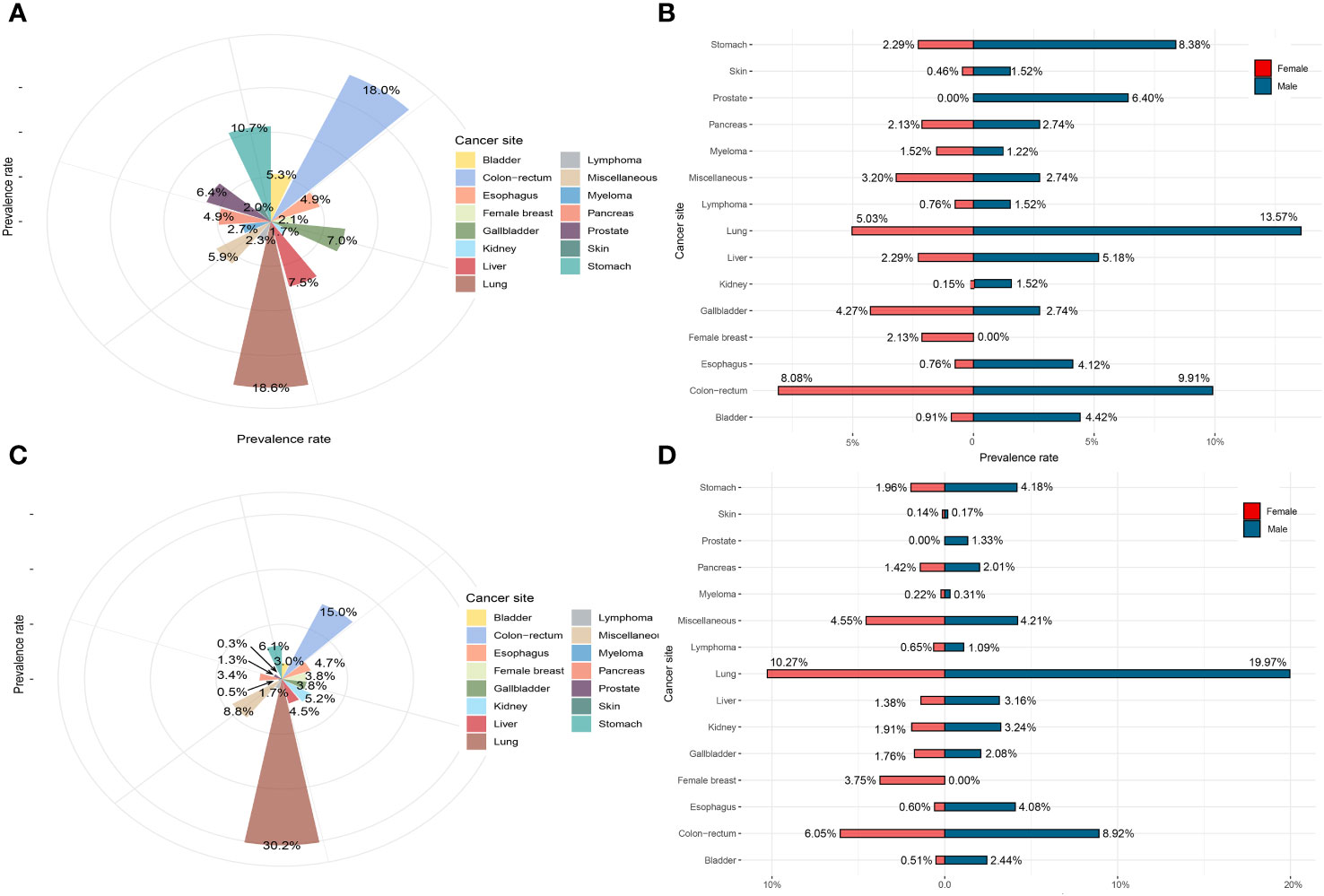
Figure 4 The prevalence rate of Schistosoma japonicum and non-S. japonicum patients with malignancy. (A) The prevalence rate of S. japonicum patients with malignancy (all the cases). (B) The prevalence rate of S. japonicum patients with malignancy (stratified by gender). (C) The prevalence rate of non-S. japonicum patients with malignancy (all the cases). (D) The prevalence rate of non-S. japonicum patients with malignancy (stratified by gender).
For non-S. japonicum patients, lung cancer was the most common malignancy, accounting for 30.2% of all cases, followed by colorectal cancer (15.0%), stomach cancer (6.1%), kidney cancer (5.2%), and esophageal cancer (4.7%). These five leading malignancies accounted for approximately 61.2% of all newly diagnosed malignancy cases. Lung cancer was found the most common malignancy in men, accounting for 20.0% of all cases, followed by colorectal cancer (8.9%), stomach cancer (4.2%), esophageal cancer (4.1%), and kidney cancer (3.2%). These five leading malignancies accounted for approximately 61.2% of all new cases in men. For women, lung cancer was the most common malignancy, accounting for 27% of all cancers, followed by colorectal cancer (6.1%), female breast cancer (3.8%), stomach cancer (2.0%), and kidney cancer (1.9%). These five leading malignancies accounted for approximately 40.8% of all cases in women (Figure 4).
For S. japonicum patients, the mortality stratified by gender in all the cancer sites is shown in Figure 5. Colorectal cancer was the leading cause of malignancy death, which accounted for 18.7% of all death cases, followed by lung cancer (17.4%), stomach cancer (10.4%), liver cancer (9.1%), gallbladder cancer (7.1%), and pancreatic cancer (7.1%). These six leading malignancies accounted for approximately 55.6% of all death cases. Colorectal cancer was the leading cause of death cases in both genders. For men, colorectal cancer accounted for 12.0% of all death cases, followed by lung cancer (11.2%), stomach cancer (8.7%), liver cancer (6.2%), and prostate cancer (5.8%). The five leading malignancies accounted for approximately 43.9% of all death cases in men. For women, colorectal cancer accounted for 6.6% of all malignancy deaths, followed by lung cancer (6.2%), gallbladder cancer (4.6%), breast cancer (3.7%), and liver cancer (2.9%). These five leading malignancies accounted for approximately 24% of all death cases in women.
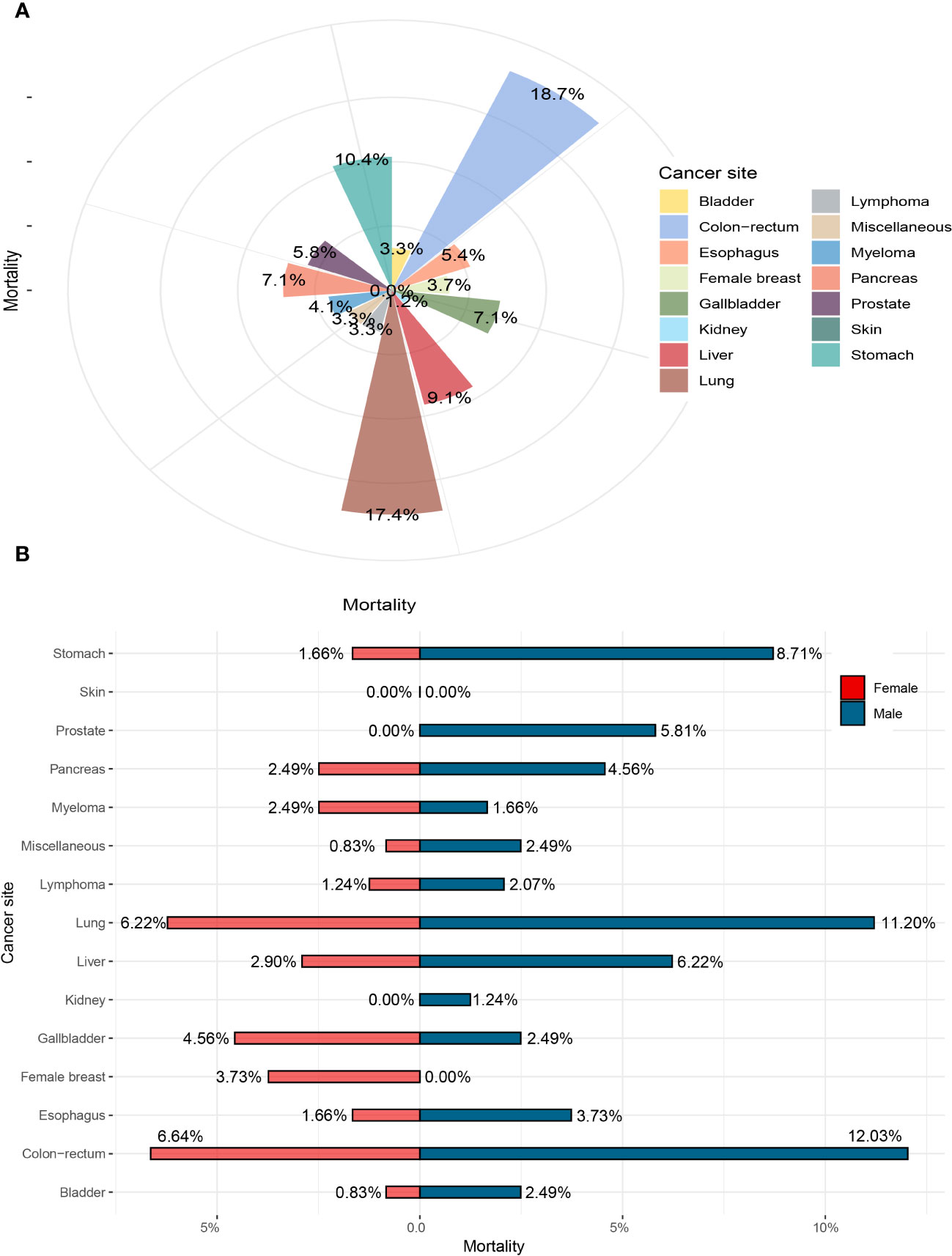
Figure 5 The mortality of Schistosoma japonicum patients with human malignancy. (A) The mortality of all the cases. (B) The mortality stratified by gender.
For both S. japonicum and non-S. japonicum patients with malignancy, their prevalence rates increased with age (Table 3). The prevalence rates reached a peak in group 3 (60–69 years). The rates in group 4 (70–79 years) and group 2 (50–59 years) were comparable with those in group 3.
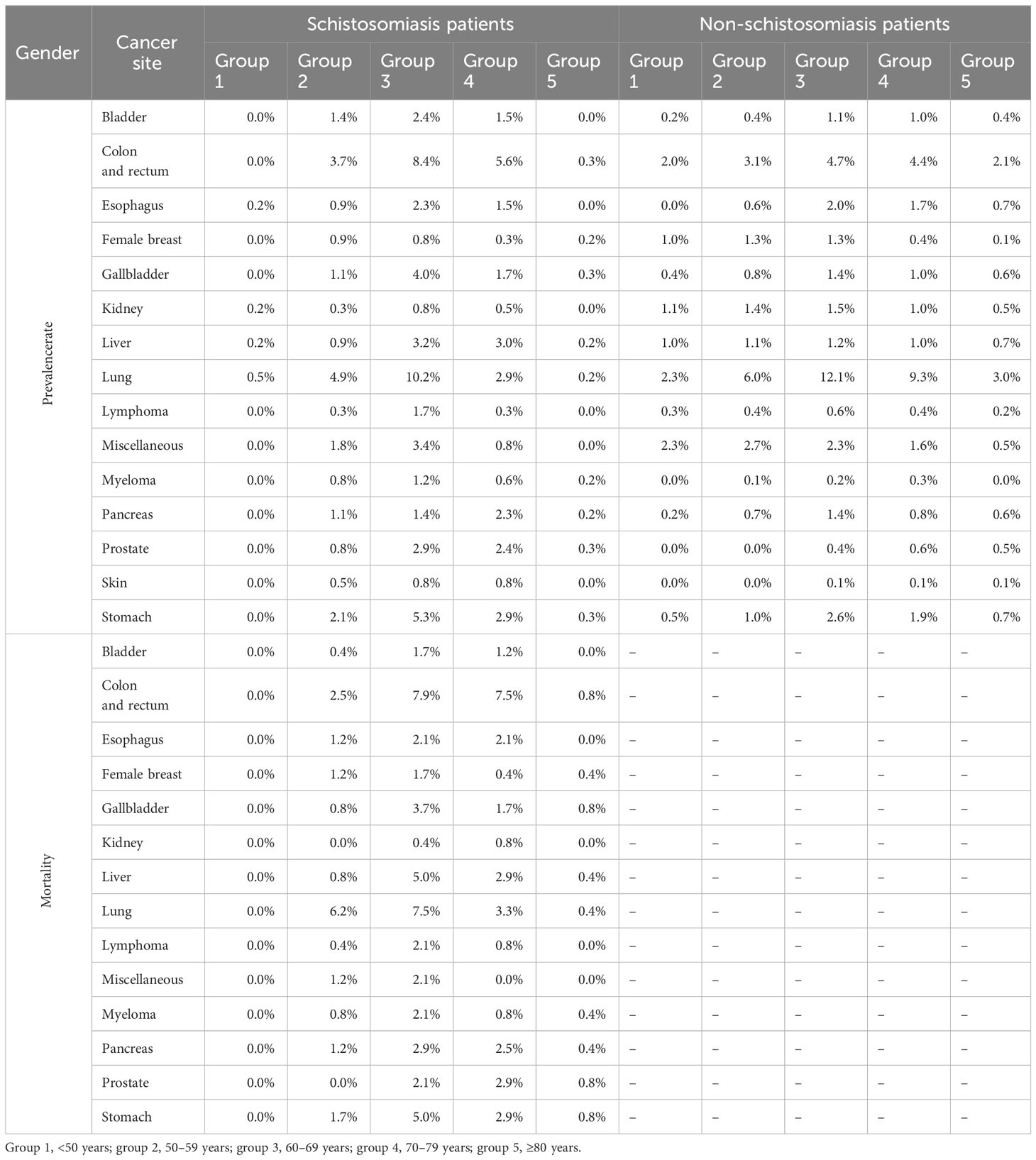
Table 3 The prevalence rate and mortality by age groups in Schistosoma japonicum and non-S. japonicum patients.
For S. japonicum patients, group 3 (60–69 years) had the most malignancy cases (lung cancer) in men. Group 4 (70–79 years) had the most malignancy cases (colorectal cancer) in women (Supplementary Table 1).
For non-S. japonicum patients, group 3 (60–69 years) had the most malignancy cases (lung cancer) in men and the most malignancy cases (lung cancer) in women (Supplementary Table 1).
For S. japonicum patients, lung cancer caused the highest number of death cases in group 2 (50–59 years) in men. Gallbladder cancer and colorectal cancer caused the highest number of death cases in group 2 (50–59 years) and group 3 (60–69 years) in women, respectively.
For S. japonicum patients, their 5-year overall survival of each cancer site is shown in Supplementary Figure 1. The age- and gender-specific 5-year overall survival of the patients with five leading malignancies are shown in Figure 6.
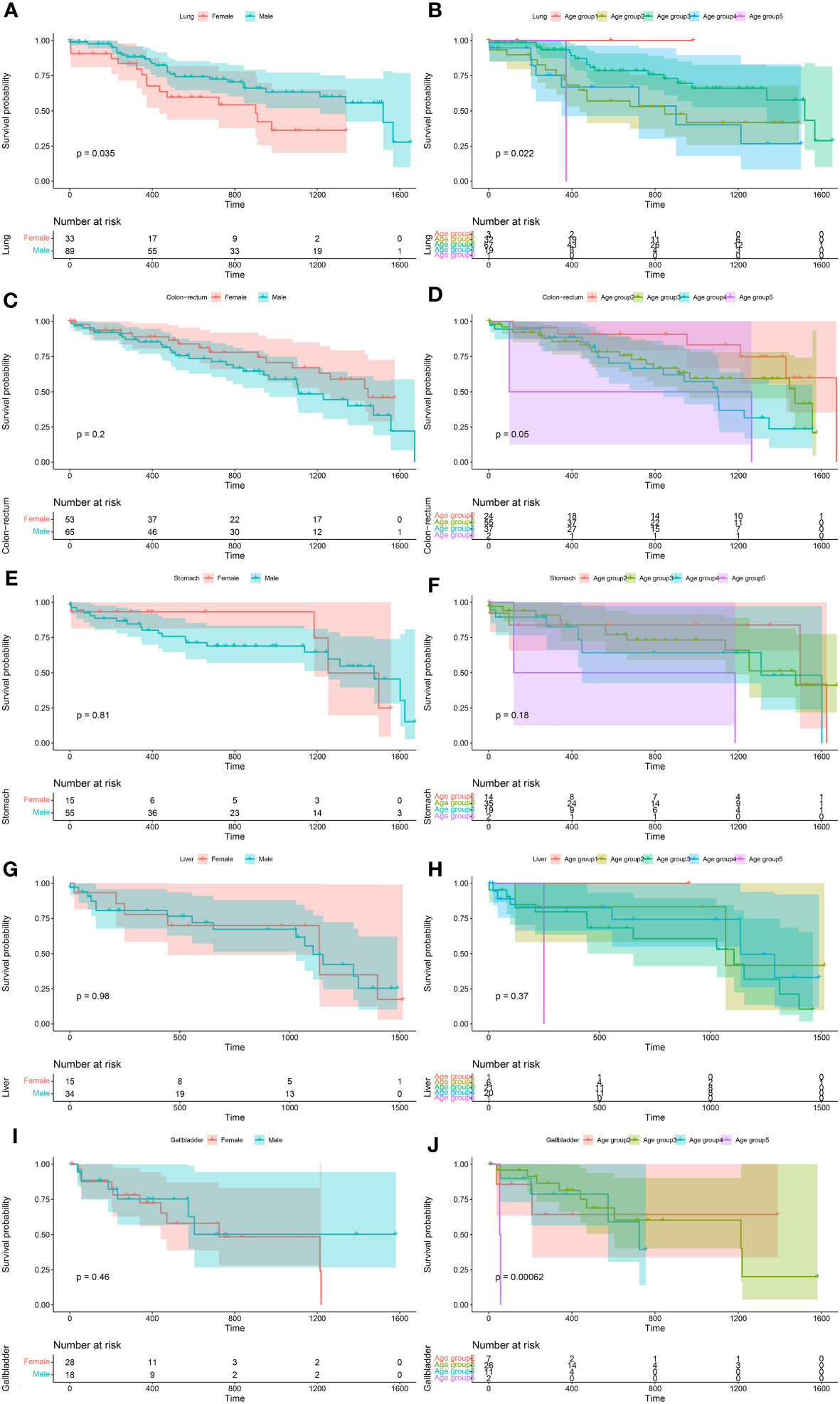
Figure 6 The age- and gender-specific 5-year overall survival of the five leading malignancies of Schistosoma japonicum patients. Lung cancer (A, B), colorectal cancer (C, D), stomach cancer (E, F), liver cancer (G, H), and gallbladder cancer (I, J).
For S. japonicum patients, no significant differences in the survival rates were seen between male and female patients in the cancer sites of the bladder (p = 0.21), colon and rectum (p = 0.20), esophagus (p = 0.97), gallbladder (p = 0.46), kidney (p = 0.52), liver (p = 0.98), lymph nodes (lymphoma) (p = 0.98), blood (myeloma) (p = 0.92), pancreas (p = 0.15), skin (p = 1.00), stomach (p = 0.81), and miscellaneous (p = 0.17). However, higher 5-year overall survival were found in female patients with lung cancer (p = 0.04) (Supplementary Figure 2).
For S. japonicum patients, no significant differences in the survival rates were seen among the age groups in the cancer sites of the bladder (p = 0.54), esophagus (p = 0.98), kidney (p = 0.26), liver (p = 0.37), lymphoma (p = 0.73), pancreas (p = 0.34), skin (p = 1.00), stomach (p = 0.18), and miscellaneous (p = 0.57). Significant differences in the survival rates were seen among the age groups in the cancer sites of the colon and rectum (p = 0.05), female breast (p = 0.004), lung (p = 0.02), gallbladder (p < 0.001), myeloma (p = 0.04), and prostate (p < 0.001) (Supplementary Figure 3).
This study found that lung cancer was the most common malignancy in male S. japonicum patients, followed by colorectal cancer. On the contrary, colorectal cancer was found as the most common in female S. japonicum patients, followed by lung cancer. However, colorectal cancer was the leading cause of death cases in both genders of S. japonicum patients.
The association between S. japonicum infection and colorectal cancer has been reported in previous studies (6, 7). A high incidence of colorectal cancer was observed in endemic regions, which was attributed to the high prevalence rate of individuals infected by S. japonicum (8). The standardized mortality for colorectal cancer was reported to be significantly higher in female patients who lived in the endemic area for more than 50 years (9). The peak prevalence rate of sporadic colorectal cancer was reported to occur in the 60–80 years of life worldwide (10). However, due to early exposure to Schistosoma in childhood, colorectal cancer occurs 6–16 years earlier in patients with schistosomiasis infection than in patients with ordinary colorectal cancer (6, 11). Similar results were shown in this study. The peak prevalence rate of colorectal cancer occurred in the 60–69 years of life in S. japonicum patients, while the peak prevalence rate occurred in the 60–69 and 70–79 years of life in non-S. japonicum patients. Colorectal cancer was found to be the leading cause of malignancy death in both S. japonicum and non-S. japonicum patients. The peak mortality occurred in the 60–80 years of life. Previous studies have reported that schistosomiasis would lead to the onset of colon cancer at an age less than 40. The results of this study showed that the onset time of colon cancer for S. japonicum patients was approximately 50 years old. This disparity may be a result of the Shanghai colorectal cancer screening program, which was launched in 2012. Precancerous lesions such as colonic polyps could be detected early and treated before developing into colon cancer. However, this study did not include precancerous lesions. Thus, a decreased number of colon cancer cases was observed in younger patients who had been recently infected by S. japonicum.
A previous study demonstrated an association between S. mansoni and liver cancer, which was probably through the effect of co-infection of hepatitis virus (12). Patients infected by S. mansoni have higher rates of co-infection of the hepatitis B virus and hepatitis C virus than the controls (13). Another study analyzed the age distribution among S. mansoni patients with liver cancer. Results revealed that patients with co-infection of hepatitis C virus and S. mansoni were younger (50–59 years) in age at the time of diagnosis of liver cancer than the patients with hepatitis C virus alone (at the age of 60 years). The combination of S. mansoni and hepatitis C virus is frequently associated with more rapid progression to liver cancer (14). A previous study suggested that S. japonicum infection may contribute to the disease burden of the liver and finally cause liver cancer (5). In this study, we did not investigate hepatitis B infection among S. japonicum patients with liver cancer. However, the results showed that both the peak prevalence rate and peak mortality occurred in the sixth decade of life in S. japonicum patients with liver cancer, which was consistent with Shousha’s research (15).
A previous study reported that the clinicopathologic pattern of bladder cancer was different in schistosomiasis and non-schistosomiasis patients. In Western countries, bladder cancer was three times more common in men than in women, with the prevalence rate peaking in the 60–90 years of life. Over 90% of the tumors were transitional cell carcinomas (16). The ratio of bladder cancer incidence (men to women) in countries with Schistosoma infection was reported to be within the range of 4:1 to 5.9:1 (17). In this study, the result was consistent with the previous study. A ratio of 4.9 (men to women) was found in the prevalence rate of bladder cancer in S. japonicum patients.
Lung cancer was found the most common malignant tumor in both S. japonicum and non-S. japonicum patients. To the best of our knowledge, there are no clinical reports about the relationship between schistosomiasis and lung cancer. Results of this study showed that the proportion of lung cancer in malignant tumors was 18.6% for S. japonicum patients, which was lower than that (30.2%) of non-S. japonicum patients. This result indicated that lung cancer may not be related to parasite infection. However, controversy remains in basic studies on the potential relationship between S. japonicum and lung neoplasm. Chen et al. reported that S. japonicum infection may contribute to pulmonary granulomatous inflammation (18). Lin et al. reported that infection of S. japonicum was likely to enhance the proliferation and migration of human breast cancer cells rather than lung A549 cancer cells (19).
Previous studies have reported that hepatitis B, Schistosoma, and multiple pregnancies may affect the cancer process of the gallbladder (20). However, the mechanism was still unclear. One case report presented a case of co-occurrence of S. mansoni and stomach cancer. The pathological mechanism may lead to pseudo-tumor formation due to excessive hyper-plastic neoformation and anomalous response to Schistosoma (21). Other cancer co-occurrences with schistosomiasis such as prostate adenocarcinoma associated with S. haematobium infection were previously reported (22). However, the specific mechanism was not clear either.
This study had some limitations. First, the present retrospective study included limited samples of single-center data. Further study should be carried out in prospective studies in multi-centers with larger sample sizes. Second, with the limited clinical information, the relationship between various malignancies other than digestive system tumors and S. japonicum could not be clarified. Third, personal lifestyle and habits were not investigated in the current study, which may be related to the occurrence of malignancies such as smoking or the presence of carcinogens in the work environment. Furthermore, co-infection with other pathogens may exist in S. japonicum patients, which may lead to potential bias. Thus, more comprehensive research needs to be further performed.
In conclusion, S. japonicum is attributed to a high prevalence rate and mortality in digestive system tumors, including colorectal cancer, stomach cancer, liver cancer, and gallbladder cancer.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of Jinshan Hospital, Fudan University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Informed consent was waived. Patients’ confidentiality was protected. All the data were addressed in anonymous. The personal information was appropriately de-identified.
X-FL: Data curation, Formal Analysis, Investigation, Writing – original draft. SJ: Data curation, Writing – review & editing. K-YW: Data curation, Writing – review & editing. YL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing. J-WQ: Funding acquisition, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The results reported herein correspond to specific aims of grant No. 2021-3-01 to investigator YL from Jinshan Science and Technology Committee. This work was also supported by grant No. ZK2019B01 to investigator J-WQ from Shanghai Municipal Health Commission.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1288197/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens–Part B: biological agents. Lancet Oncol (2009) 10:321–2. doi: 10.1016/S1470-2045(09)70096-8
3. Humans IWGOTEOCRT. Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum (2012) 100:1–441. Available at: https://www.ncbi.nlm.nih.gov/books/NBK304348/
4. Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet (2014) 383:2253–64. doi: 10.1016/S0140-6736(13)61949-2
5. Qiu DC, Hubbard AE, Zhong B, Zhang Y, Spear RC. A matched, case-control study of the association between Schistosoma japonicum and liver and colon cancers, in rural China. Ann Trop Med Parasitol (2005) 99:47–52. doi: 10.1179/136485905X19883
6. Hamid HKS. Schistosoma japonicum-associated colorectal cancer: A review. Am J Trop Med Hyg (2019) 100:501–5. doi: 10.4269/ajtmh.18-0807
7. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
8. Guo W, Zheng W, Li JY, Chen JS, Blot WJ. Correlations of colon cancer mortality with dietary factors, serum markers, and schistosomiasis in China. Nutr Cancer (1993) 20:13–20. doi: 10.1080/01635589309514266
9. Inaba Y. A cohort study on the causes of death in an endemic area of schistosomiasis japonica in Japan. Ann Acad Med Singap (1984) 13:142–8.
10. Silla IO, Rueda D, Rodriguez Y, Garcia JL, de la Cruz Vigo F, Perea J. Early-onset colorectal cancer: a separate subset of colorectal cancer. World J Gastroenterol (2014) 20:17288–96. doi: 10.3748/wjg.v20.i46.17288
11. Xu Z, Su DL. Schistosoma japonicum and colorectal cancer: an epidemiological study in the People's Republic of China. Int J Cancer (1984) 34:315–8. doi: 10.1002/ijc.2910340305
12. El-Tonsy MM, Hussein HM, Helal Tel S, Tawfik RA, Koriem KM, Hussein HM. Schistosoma mansoni infection: is it a risk factor for development of hepatocellular carcinoma? Acta Trop (2013) 128:542–7. doi: 10.1016/j.actatropica.2013.07.024
13. El-Tonsy MM, Hussein HM, Helal Tel S, Tawfik RA, Koriem KM, Hussein HM. Human Schistosomiasis mansoni associated with hepatocellular carcinoma in Egypt: current perspective. J Parasit Dis (2016) 40:976–80. doi: 10.1007/s12639-014-0618-0
14. Kamal S, Madwar M, Bianchi L, Tawil AE, Fawzy R, Peters T, et al. Clinical, virological and histopathological features: long-term follow-up in patients with chronic hepatitis C co-infected with S. mansoni Liver (2000) 20:281–9. doi: 10.1034/j.1600-0676.2000.020004281.x
15. Shousha HI, Abdelaziz AO, Nabeel MM, Omran DA, Abdelmaksoud AH, Elbaz TM, et al. Schistosoma mansoni infection and the occurrence, characteristics, and survival of patients with hepatocellular carcinoma: an observational study over a decade. Pathog Glob Health (2022) 116:119–27. doi: 10.1080/20477724.2021.1975081
16. Khaled H. Schistosomiasis and cancer in Egypt: review. J Adv Res (2013) 4:461–6. doi: 10.1016/j.jare.2013.06.007
17. Mostafa MH, Sheweita SA, O'connor PJ. Relationship between schistosomiasis and bladder cancer. Clin Microbiol Rev (1999) 12:97–111. doi: 10.1128/CMR.12.1.97
18. Chen D, Xie H, Luo X, Yu X, Fu X, Gu H, et al. Roles of Th17 cells in pulmonary granulomas induced by Schistosoma japonicum in C57BL/6 mice. Cell Immunol (2013) 285:149–57. doi: 10.1016/j.cellimm.2013.09.008
19. Lin YL, Ramanujum R, He S. Infection of Schistosomiasis Japanicum is likely to enhance proliferation and migration of human breast cancer cells: mechanism of action of differential expression of MMP2 and MMP9. Asian Pac J Trop Biomed (2011) 1:23–8. doi: 10.1016/S2221-1691(11)60063-4
20. Xu LN, Zou SQ. A clinicopathological analysis in unsuspected gallbladder carcinoma: a report of 23 cases. World J Gastroenterol (2007) 13:1857–60. doi: 10.3748/wjg.v13.i12.1857
21. Ramos MFKP, Duarte VC, Pereira MA, de Castria TB, Schmerling CK, Zilberstein B, et al. Schistosomiasis misleading gastric cancer treatment. J Gastrointest Cancer (2020) 51:643–6. doi: 10.1007/s12029-019-00334-6
Keywords: Schistosoma japonicum, cancer, prevalence, mortality, five-year overall survival
Citation: Liu X-F, Ju S, Wang K-Y, Li Y and Qiang J-W (2023) The prevalence rate, mortality, and 5-year overall survival of Schistosoma japonicum patients with human malignancy. Front. Oncol. 13:1288197. doi: 10.3389/fonc.2023.1288197
Received: 04 September 2023; Accepted: 20 November 2023;
Published: 06 December 2023.
Edited by:
José Manuel Correia Da Costa, National Health Institute Doutor Ricardo Jorge (INSA), PortugalReviewed by:
Lei Deng, Massachusetts General Hospital and Harvard Medical School, United StatesCopyright © 2023 Liu, Ju, Wang, Li and Qiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Li, ZHIueWluZ2xpQGZveG1haWwuY29t; Jin-Wei Qiang, ZHIuamlud2VpcWlhbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.