- 1Department of Translational and Precision Medicine, Policlinico Umberto I, La Sapienza University of Rome, Rome, Italy
- 2Hematology Unit, Businco Hospital, Azienda di Rilievo Nazionale ed Alta Specializzazione (ARNAS) G. Brotzu, Cagliari, Italy
We describe a case of second primary malignancy in a 65-year-old patient affected by polycythemia vera treated with the JAK 1/2 inhibitor ruxolitinib. The latter is recognized as a risk factor for the onset of non-melanoma skin cancers in many retrospective and perspective studies, but the concomitant use of ruxolitinib with new immunotherapies is very rarely reported, and the safety of this association is still not clear. In our case, ruxolitinib combined with the anti-PD-L1 avelumab demonstrated both safety and efficacy for hematological disease control and underlying carcinoma remission.
Introduction
Second primary malignancies (SPM) are not uncommon in myeloproliferative neoplasms (MPNs), as reported in retrospective studies (1). Ruxolitinib (RUX) is the first in the class JAK1/2 inhibitor approved for symptomatic myelofibrosis (MF) and in second-line therapy in patients with polycythemia vera (PV) resistant or intolerant to hydroxyurea (HU). An association between RUX treatment and an increased risk of SPM has been recently recognized, mainly non-melanoma skin cancers (NMSCs). Controversial data are reported regarding potential synergy and safety of ruxolitinib combination with immunotherapy (like programmed death and death-ligand, PD1/PD-L1 inhibitors) for the underlying skin cancer. We report a case of refractory PV successfully treated with ruxolitinib with a subsequent metastatic Merkel cell carcinoma (MCC). The patient received combined treatment with the anti-PD-L1 avelumab and ruxolitinib, resulting in both MPN and MCC control without safety concerns.
Case presentation
A 65-year-old man was diagnosed in 2014 as having a JAK2 V617F-mutated PV. According to international guidelines, he started prophylaxis with low-dose aspirin and treatment with HU. In March 2018, he developed thrombocytosis (>2.000 × 109/L) and poor hematocrit control, requiring additional phlebotomies. Considering that the patient was resistant, according to European LeukemiaNet (ELN) criteria (2), he started second-line ruxolitinib at the recommended dose of 10 mg BID. After 2 months, he had complete response with hematocrit control, without requiring further phlebotomies. Soon after, ruxolitinib was reduced to 15 mg daily for grade 2 liver toxicity. In February 2019, he underwent surgery and radiotherapy for laterocervical cutaneous melanoma. In November 2021, after approximately 2 years of RUX treatment, he developed slight bilateral laterocervical swelling. In May 2022, given the evidence of neck adenopathy, left lateral cervical lymph node excision was performed, and the biopsy result was positive for metastatic high-grade neuroendocrine carcinoma. Tumor cells expressed CK20 and neuroendocrine markers and lacked TTF-1, consistent with MCC. No cutaneous MCC was identified. The staging PET/CT scan documented a Ga68-avid 20 mm left retroperitoneal lesion, confirming the diagnosis of metastatic Merkel cell carcinoma with unknown primary lesion (MCC-UP). At the time of MCC diagnosis, the patient was in complete hematological response, with normal blood count and without any PV-related symptoms. He started therapy with the anti-programmed cell death-ligand 1 (PD-L1) antibody avelumab, administered intravenously according to the recommended schedule of 800 mg every 2 weeks until disease progression or unacceptable toxicity. PV was still in complete response, and ruxolitinib was not withdrawn. Avelumab was started on December 2022 with a close monitoring of complete blood count and blood chemistry. In January 2023, he developed mild severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and was treated with tixagevimab–cilgavimab without stopping ruxolitinib. Imaging performed after 3 months (six administrations of avelumab) to evaluate MCC revealed complete response. No hematological or extra-hematological toxicities were reported with ruxolitinib–avelumab combination in the first 5 months of follow-up (Figure 1). At the last evaluation, PV was in complete response according to ELN criteria (2).
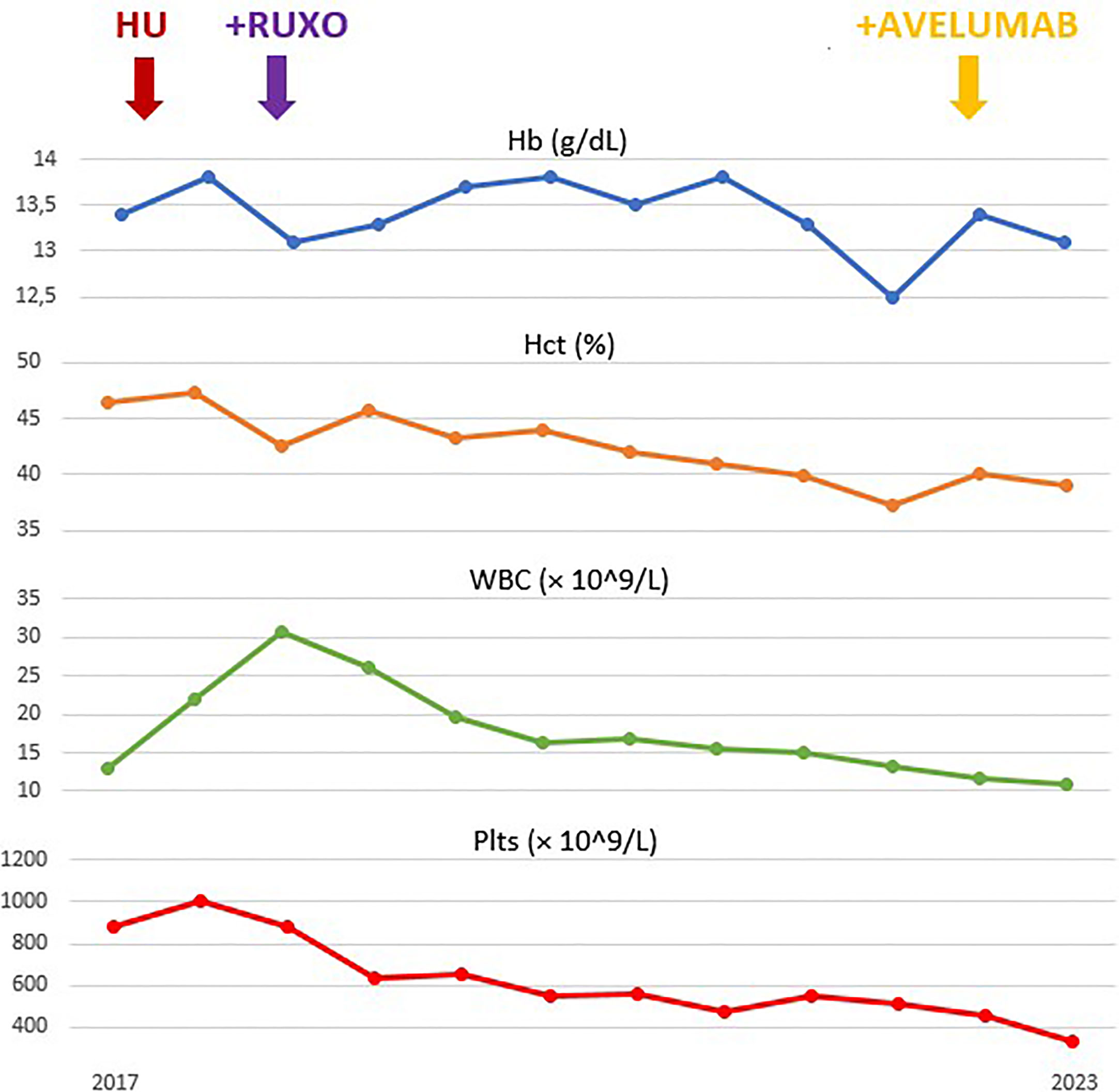
Figure 1 Evolution of hemoglobin (Hb), hematocrit (Hct), leukocyte (white blood cells, WBC) and platelets (Plts) count during treatment.
Discussion
Ruxolitinib is the only JAK1/2 inhibitor approved for HU-resistant/intolerant PV, since the pivotal trials “RESPONSE 1” and “RESPONSE 2” showed an increased rate of hematological response, reduction in splenic volume, and improvement in PV-related symptoms in comparison with best available therapy (BAT) (3, 4). However, several studies conducted in MF, such as the COMFORT-II trial, showed an increased risk of SPMs, mainly lymphoma and non-melanoma skin cancers (NMSCs) (5). Among NMSCs, Merkel cell carcinoma, a rare neuroendocrine carcinoma, is the second most common cause of skin-cancer-related death. It has been described that immune system plays an active role in the control, prevention, and progression of MCC. Approximately 10% of patients affected by MCC are on immunesuppression (6). JAK1/2 inhibitors suppress the activity of different proinflammatory cytokines and impair T lymphocytes function (7). Immune checkpoint inhibitors (ICIs) have recently demonstrated promising efficacy in metastatic MCC by enhancing the cytotoxic activity of T cells (8). There are concerns that JAK inhibitors may compromise the effectiveness of ICI in NMSC, since acquired inactivating mutations in JAK1 and JAK2 genes resulted in a reduction in PD-L1 expression in melanoma patients, leading to acquired resistance to ICI treatment (9). Conversely, JAK2 V617F mutation was also found to increase PD-L1 expression by phosphorylating STAT3/STAT5 on the surfaces of platelets, megakaryocytes, and myeloid-derived suppressor cells (MDSCs) (10). Additionally, the 9p24.1 amplification, identified in various malignancies as leading to increased PD-L1 expression, has been discovered to frequently contain focal amplification of the JAK2 gene (11). Thus, it is plausible that the PD-1/PD-L1 and JAK2 pathway could represent complementary therapeutic targets, indicating that ruxolitinib and ICI may be combined to achieve synergy. This mechanism could account for the sustained complete response in NMSCs reported in our patient and in other case reports with the combination of ICI and RUX (12, 13). In similar, limited case series, controversial data have been reported about the toxicity of combination therapy: hematological toxicity due to ruxolitinib–avelumab combination led to discontinuation of both therapies by the fourth cycle of immunotherapy in two patients (14), while a case of combination of ruxolitinib and cemiplimab without hematological concerns was also reported (15). In our patient, combined treatment was associated with the absence of hematological toxicity. As been reported previously, our case highlights the possible occurrence of solid skin cancer in patients with MPNs treated with ruxolitinib, suggesting routine dermatological screening and follow-up during continuous treatment. Considering the increasing use of JAK inhibitors, more and more patients may be diagnosed with secondary malignancies and may potentially require combination therapy. This case report offers crucial details to physicians seeking advice on how to combine these medications in the future. Combined treatment of JAK2 inhibitors with novel anti-cancer drugs is possible, after discussion of each case in multidisciplinary settings, in our case without increased toxicity and good clinical efficacy. However, further investigations are necessary to conclusively evaluate the association of the two drugs.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patient was not required to participate in this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CM and MP participated in the care of the patient, conceived the idea for publication, and wrote the manuscript; AC, CI, MC, IC and ES participated in the clinical care of the patient and contributed to the manuscript. MM and MB guided the direction of the report, and contributed to the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Polverelli N, Elli EM, Abruzzese E, Palumbo GA, Benevolo G, Tiribelli M, et al. Second primary malignancy in myelofibrosis patients treated with ruxolitinib. Br J Haematol (2021) 193(2):356–68. doi: 10.1111/bjh.17192
2. Barosi G, Mesa R, Finazzi G, Harrison C, Kiladjian JJ, Lengfelder E, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood (2013) 121(23):4778–81. doi: 10.1182/blood-2013-01-478891
3. Vannucchi AM, Kiladjian JJ, Griesshammer M, Masszi T, Durrant S, Passamonti F, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med (2015) 372(5):426–35. doi: 10.1056/NEJMoa1409002
4. Passamonti F, Griesshammer M, Palandri F, Egyed M, Benevolo G, Devos T, et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. Lancet Oncol (2017) 18(1):88–99. doi: 10.1016/S1470-2045(16)30558-7
5. Harrison CN, Vannucchi AM, Kiladjian JJ, Al-Ali HK, Gisslinger H, Knoops L, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia (2016) 30(8):1701–7. doi: 10.1038/leu.2016.148
6. Lin JQ, Li SQ, Li S, Kiamanesh EF, Aasi SZ, Kwong BY, et al. A 10-year retrospective cohort study of ruxolitinib and association with nonmelanoma skin cancer in patients with polycythemia vera and myelofibrosis. J Am Acad Dermatol (2022) 86(2):339–44. doi: 10.1016/j.jaad.2021.10.004
7. Paulson KG, Iyer JG, Blom A, Warton EM, Sokil M, Yelistratova L, et al. Systemic immune suppression predicts diminished merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol (2013) 133(3):642–6. doi: 10.1038/jid.2012.388
8. Parampalli Yajnanarayana S, Stübig T, Cornez I, Alchalby H, Schönberg K, Rudolph J, et al. JAK1/2 inhibition impairs T cell function in vitro and in patients with myeloproliferative neoplasms. Br J Haematol (2015) 169(6):824–33. doi: 10.1111/bjh.13373
9. Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D'Angelo SP, et al. Avelumab in patients with chemotherapy-refractory metastatic merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol (2016) 17(10):1374–85. doi: 10.1016/S1470-2045(16)30364-3
10. Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med (2016) 375(9):819–29. doi: 10.1056/NEJMoa1604958
11. Prestipino A, Emhardt AJ, Aumann K, O'Sullivan D, Gorantla SP, Duquesne S, et al. Oncogenic JAK2V617F causes PD-L1 expression, mediating immune escape in myeloproliferative neoplasms. Sci Transl Med (2018) 10(429):eaam7729. doi: 10.1126/scitranslmed.aam7729
12. Haddad N, Delyon J, Trabelsi Messai S, Herms F, Leccia MT, Lebbe C, et al. Clinical response to immune checkpoint inhibition in patients with advanced skin cancers receiving concurrent ruxolitinib therapy for haematological malignancy. Br J Dermatol (2021) 184(3):564–6. doi: 10.1111/bjd.19604
13. Debureaux PE, Arrondeau J, Bouscary D, Goldwasser F. Nivolumab combined with ruxolitinib: antagonism or synergy? Ann Oncol (2018) 29(5):1334–5. doi: 10.1093/annonc/mdy077
14. Buonerba L, Di Trolio R, Grimaldi A, Tucci A, Leo E, Ingenito C, et al. Hematological toxicity during concomitant treatment with ruxolitinib and avelumab for merkel cell carcinoma. Front Oncol (2020) 10:579914. doi: 10.3389/fonc.2020.579914
Keywords: avelumab, Merkel cell carcinoma, ruxolitinib, polycytemia vera, second malignancies
Citation: Masucci C, Passucci M, Scalzulli E, Carmosino I, Capriata M, Costa A, Ielo C, Martelli M and Breccia M (2023) Case Report: Avelumab and ruxolitinib to manage polycythemia vera and secondary metastatic Merkel cell carcinoma: a possible successful combination. Front. Oncol. 13:1212638. doi: 10.3389/fonc.2023.1212638
Received: 26 April 2023; Accepted: 22 May 2023;
Published: 07 June 2023.
Edited by:
Mohamed A. Yassin, Hamad Medical Corporation, QatarReviewed by:
Marina Kremyanskaya, Icahn School of Medicine at Mount Sinai, United StatesMario Tiribelli, University of Udine, Italy
Copyright © 2023 Masucci, Passucci, Scalzulli, Carmosino, Capriata, Costa, Ielo, Martelli and Breccia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Massimo Breccia, YnJlY2NpYUBiY2UudW5pcm9tYTEuaXQ=
 Chiara Masucci
Chiara Masucci Mauro Passucci
Mauro Passucci Emilia Scalzulli
Emilia Scalzulli Ida Carmosino1
Ida Carmosino1 Maurizio Martelli
Maurizio Martelli Massimo Breccia
Massimo Breccia