- 1Department of Gynecological Oncology, Cancer Center, Affiliated Hospital of Guangdong Medical University, Zhanjiang, China
- 2Department of Pathology, Affiliated Hospital of Guangdong Medical University, Zhanjiang, China
- 3Department of Imaging, Affiliated Hospital of Guangdong Medical University, Zhanjiang, China
Primary osteosarcoma of the uterus is an extremely rare pure heterologous sarcoma of the uterus. The relevant available information is limited to case reports. To date, only 31 cases of this type of cancer have been reported. Here, we report the first clinical experience with the administration of an immunotherapy-based combination regimen for multiple metastatic primary osteosarcomas of the uterus. The patient had undergone multiple treatments prior to this regimen, but her condition continued to progress. However, after 3 cycles of immunotherapy combined with targeted therapy and chemotherapy, a review showed that the disease was stable and even in partial remission. The patient has a good quality of life, and long-term survival is expected.
Introduction
Uterine sarcomas are unusual, accounting for less than 1% of all gynecologic tumors and 3-7% of all uterine tumors. Uterine sarcomas can be classified as homologous (composed of intrinsic uterine components) or heterologous (composed of foreign components such as cartilage and bone). Most uterine sarcomas are homologous, and heterologous uterine osteosarcomas are extremely rare (1). The majority of heterologous uterine tumors are classified as malignant mixed mullerian tumors (MMMTs). Heterologous MMMTs include carcinomas, heterologous sarcomas, and usually homologous mesenchymal components, such as those in leiomyosarcomas or stromal sarcomas. Most uterine sarcomas have been identified as rhabdomyosarcomas or chondrosarcomas, and heterogenous sarcomas have rarely been reported to produce bone and osteophytes (2). In general, the clinical course of osteosarcoma of the uterus is short, probably because it is difficult to detect a pelvic mass until symptoms appear. In addition, osteosarcoma of the uterus has an unusually malignant nature compared to osteosarcomas of other sites (3). The average age of the previously reported patients was 64 years. The majority of patients were perimenopausal or postmenopausal at the time of diagnosis (4). Due to the rarity of primary uterine osteosarcoma, there are currently no effective treatment options for this disease. In recent years, with the rise of immunotherapy and targeted therapy, we have tried to use immunotherapy combined with targeted therapy and chemotherapy to treat this disease. Herein, we report a case of primary uterine osteosarcoma and review the available literature.
Case report
A 60-year-old female patient presented to a local hospital in February 2021 with irregular vaginal bleeding. At the first visit, vaginal ultrasound showed a heterogeneous hypoechoic mass in the pelvic cavity, with a size of 9.0×7.4 cm. The results of other tests were unknown. An extensive total hysterectomy was subsequently performed.
The postoperative pathological diagnosis was a malignant mesenchymal tumor of the uterus, and the morphology was consistent with a malignant giant cell tumor, accompanied by a large amount of bleeding and necrosis. There was no tumor cell involvement in the bilateral adnexa. No metastasis was found in the lymph nodes examined.
Initial staining showed that the tumor was positive for EMA, CylinD1, Vim, SMA, and CD10, while it was negative for PAX8, ER, PR and S100. Keratin staining did not support the diagnosis of carcinosarcoma, which exhibits neither cartilage formation nor cancerous components. Ultimately, the pathological diagnosis was consistent with high-grade uterine sarcoma with heterologous differentiation (osteosarcoma).
According to the National Comprehensive Cancer Network (NCCN) guidelines for uterine sarcoma, the patient was treated with epirubicin combined with ifosfamide. After 2 cycles of chemotherapy, abdominal magnetic resonance imaging (MRI) showed that the pelvic lesions were smaller than before. Subsequently, chemotherapy was continued for 4 cycles according to the original regimen. A total dosage of 6000 rads of volumetric intensity-modulated radiation therapy was delivered to the pelvic region. Two months later, the patient found a palpable mass of approximately 6.0×6.0 cm in the surgical scar in the lower abdominal wall. Positron emission tomography/computed tomography (PET/CT) showed multiple metastases in the anterior abdominal wall incision, omentum, pelvic mesentery and lungs. After tumor progression, the patient was treated with gemcitabine and docetaxel combined with bevacizumab. After 2 cycles of treatment, abdominal MRI showed that the original mass was significantly larger than before, at approximately 9.7×9.0 cm in size. At that time, the patient was advised to undergo palliative tumor reduction surgery. One month later, preoperative examination was performed, and abdominal CT showed liver metastasis. Considering the risk of surgery and complications, the patient refused to undergo surgical treatment.
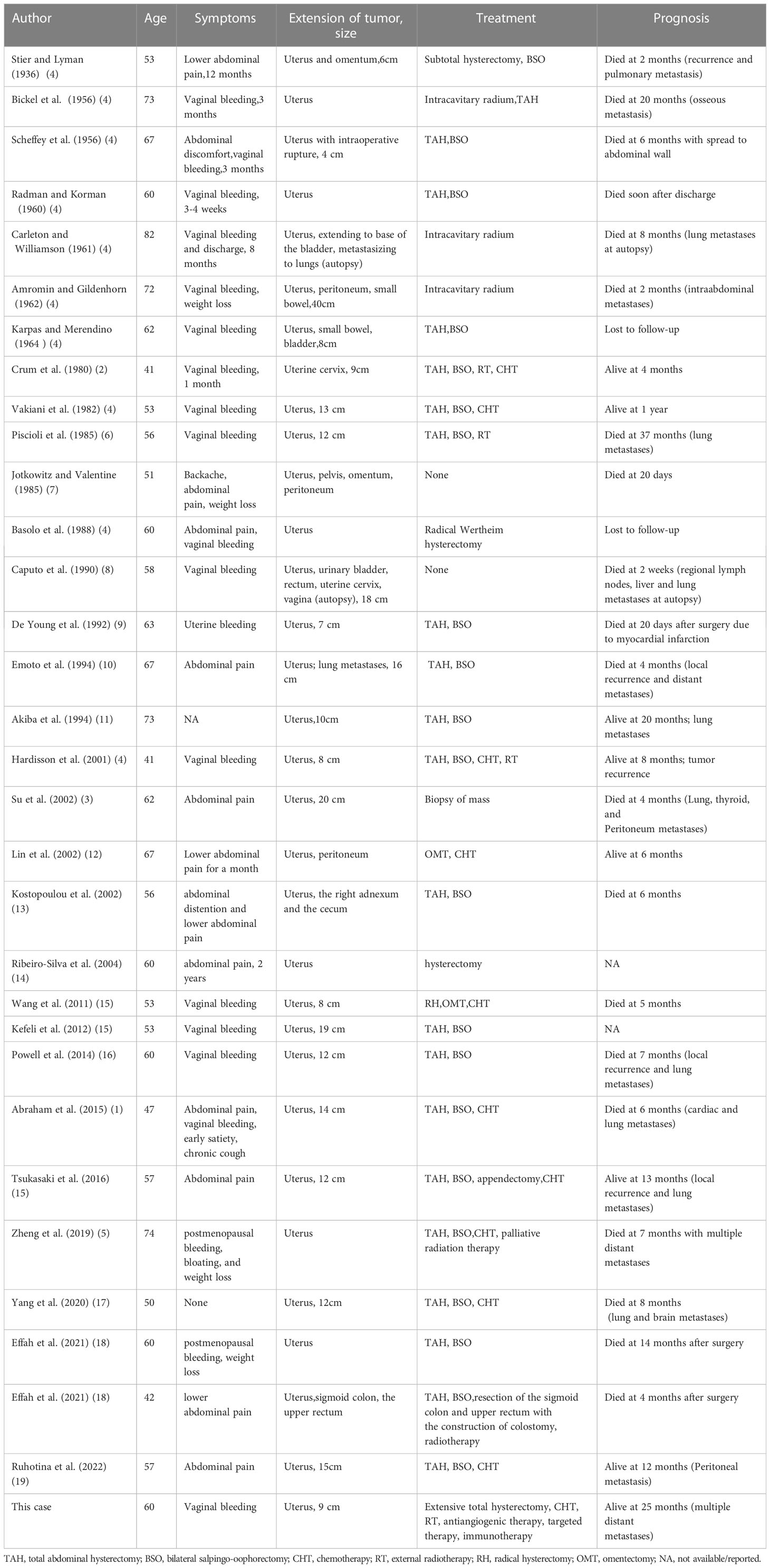
For further treatment, the patient visited our hospital. Abdominal CT showed large metastases in the anterior abdominal wall and multiple metastases in the liver (Figures 1A, I). Chest CT showed multiple metastatic tumors in both lungs (Figure 1E). Treatment with tislelizumab and anlotinib was then considered. After 3 cycles of treatment, chest CT showed that the metastatic tumors in both lungs had increased in size, and disease progression was considered. Therefore, paclitaxel injection was added to the original treatment regimen. After 2 cycles of treatment, chest CT and abdominal MRI showed that the metastatic tumors in both lungs, anterior abdominal wall mass and liver metastases were larger than before (Figures 1B, F, J). As the disease progressed, the patient gradually developed abdominal pain and bloating.
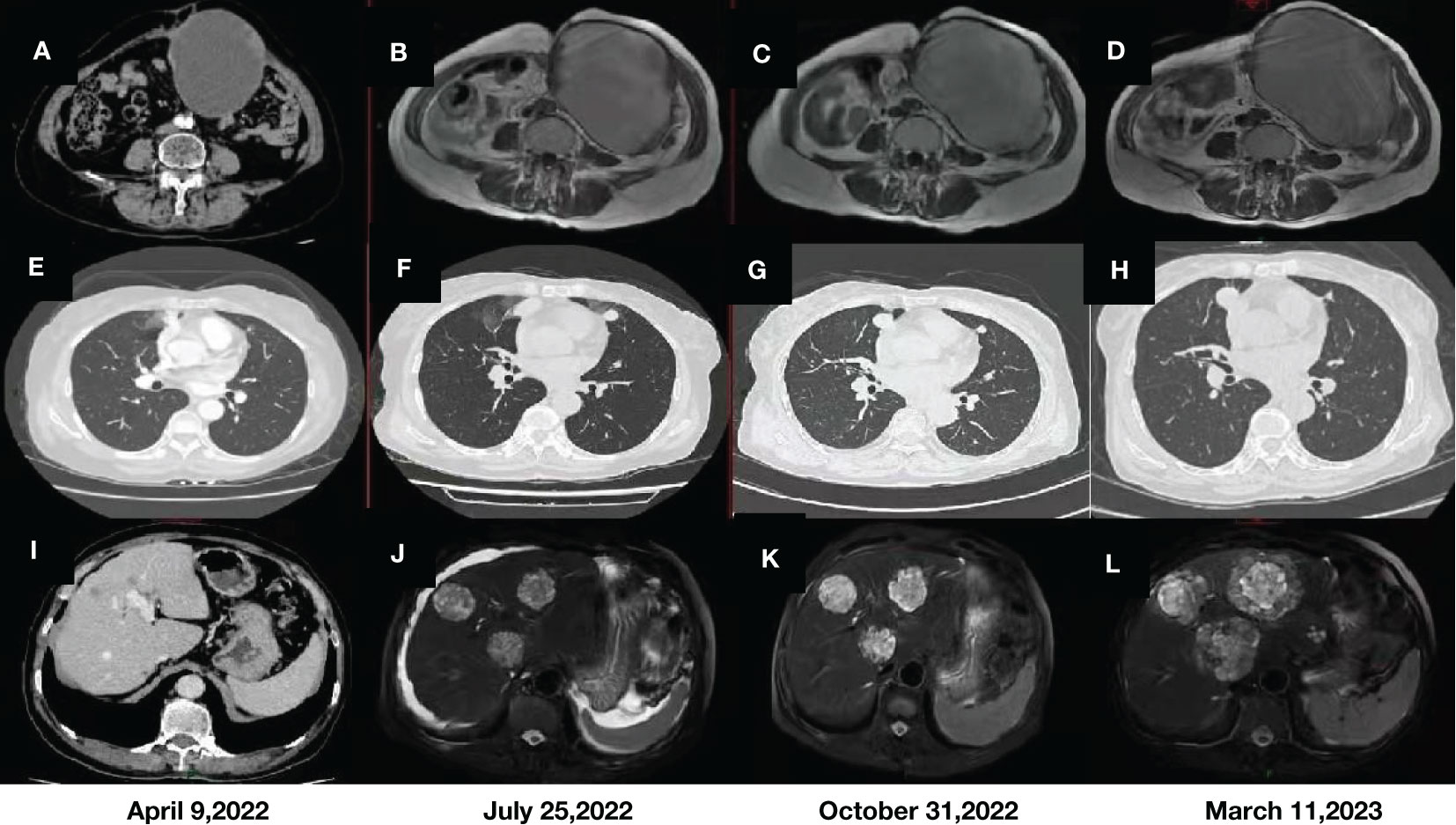
Figure 1 CT and MRI images of the anterior abdominal wall mass (A, B) and liver metastases (I, J) before immunotherapy. MRI images of the anterior abdominal wall mass (C, D) and liver metastases (K, L) after immunotherapy. Comparison of CT images of metastatic tumors in both lungs before (E, F) and after immunotherapy (G, H).
Given the advantages of current immunotherapy, we tested the patient for programmed cell death-1 (PD-1) and programmed cell death-ligand 1 (PD-L1), but the results were negative (tumor cell (TC)<1%). Eventually, however, we changed the treatment to tislelizumab, liposomal doxorubicin and lenvatinib. After three cycles of treatment, reexamination by abdominal MRI showed no significant changes in the anterior abdominal wall mass and liver metastases (Figures 1C, K), but chest CT showed that multiple metastases in both lungs were smaller than before (Figure 1G). In addition, the patient’s abdominal pain and bloating symptoms were significantly less than before. Reexamination after 3 cycles of treatment indicated that some of the lung lesions were smaller and some were larger than before (Figure 1H), and the anterior abdominal wall masses were the same as before (Figure 1D), but the liver metastases were significantly larger (Figure 1L). On March 20, 2023, transhepatic arterial chemoembolization and puncture biopsy of the liver mass were performed. The pathological findings after puncture biopsy showed necrotic tissue in some areas, a few heterotrophic cells in some tissues, and pathological mitosis, which were considered to indicate a malignant tumor. Immunohistochemical and genetic analyses were not available because of the small amount of tissue collected. Although the patient’s condition progressed with the previous treatment regimen, her disease was moderately controlled through immunotherapy combined with chemotherapy and targeted therapy, and her survival was expected to be prolonged.
Discussion
Primary osteosarcoma of the uterus is distinctly rare, and the detailed mechanism of its carcinogenic process remains unclear (5). The available literature contains little information on its relative incidence, clinical behavior, and treatment outcomes (4). A review of the available literature (Table 1) showed that the previously reported patients ranged in age from 41 to 82 years, and all but 3 patients were perimenopausal or menopausal at the time of diagnosis. The clinical manifestations of most patients were vaginal bleeding and abdominal pain (1–19). In our case, the patient presented with vaginal bleeding in the early stage and mainly abdominal distension in the later stage.
Microscopically, uterine malignancy was associated with extensive necrosis, neoplastic osteogenesis in most areas, diffuse distribution of giant cells, local spindle-shaped tumor cells, rich red cytoplasm, severe nuclear atypia, and clear mitosis (Figure 2). According to the description published by Piscioli et al., primary uterine osteosarcoma must meet the following three criteria: exclusion of a primary source of bone, presence of neoplastic osteoids, and absence of epithelial components and other specific homologous or heterologous components after confirmation of the presence of sufficient tissue samples (1, 6). Accordingly, the histological findings of this patient fully met the above criteria.
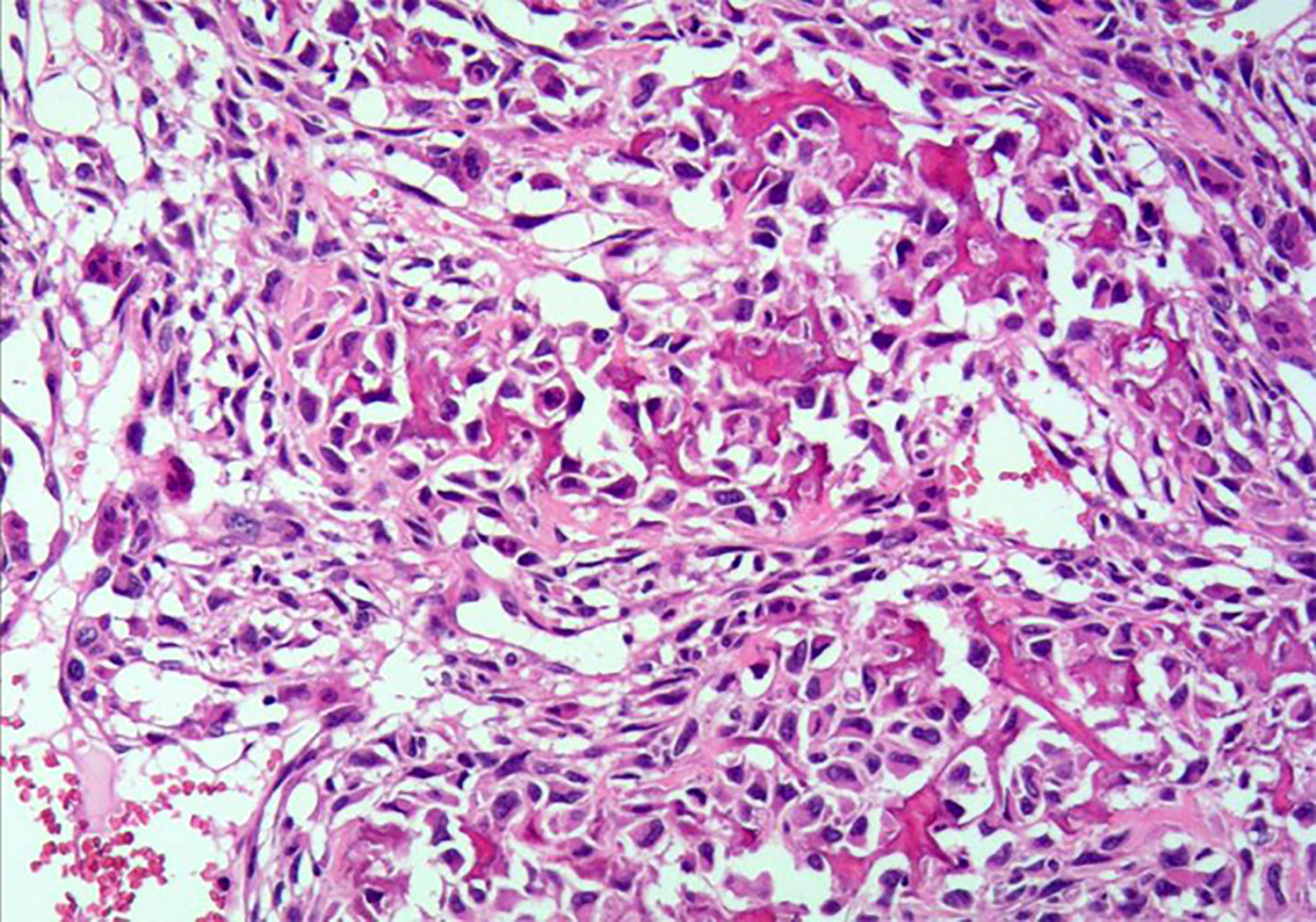
Figure 2 Hematoxylin and eosin staining of primary uterine osteosarcoma tissue sample (under 200× magnification).
This tumor is highly aggressive with a poor prognosis, and the mean survival time is 8.1 months (15). As shown in the Table 1, hysterectomy was performed in 24 patients, and bilateral salpingo-oophorectomy was also performed in 21 of these patients. Seven patients were treated with radiation therapy, including luminal radium therapy and external radiation therapy. Eleven patients received chemotherapy. The table shows that even after a combination of surgery, radiotherapy and chemotherapy, the treatment outcome is still unsatisfactory. Survival ranged from 2 weeks to 20 months, except for one patient who had survived for 37 months. In comparison, our patient has now survived for 25 months, and the survival time is expected to be extended. The results of tracking indicate that this cancer has a high probability of recurrence and distant metastasis, as well as a mortality rate. Regardless of treatment, most patients develop local or pulmonary metastases early after surgery and die within one year of starting treatment (15). Due to the rarity of uterine osteosarcoma, there is currently no standard for treatment. In this patient, previous first- and second-line treatments for uterine sarcoma failed. Her third-line therapy was challenging, and we referred to the NCCN guidelines for soft tissue sarcoma to recommend the application of pembrolizumab in third-line therapy (20). Due to the patient’s financial situation, we chose domestic tislelizumab as an alternative. Moreover, because our hospital only has SP263 antibody to detect the expression of PD-L1, although the result showed TC < 1%, this result may not necessarily reflect the real immune microenvironment of the patient. Sarcomas are cold tumors, and the effect of immunotherapy alone is poor. Existing studies have shown that the immune microenvironment can be changed by using targeted drugs to turn cold tumors into hot tumors (21, 22), and anlotinib is recommended in the domestic guidelines for soft tissue sarcoma (23); thus, we chose tislelizumab in combination with anlotinib. However, after treatment with this regimen, the patient’s disease progressed. We reviewed the NCCN guidelines for endometrial cancer and found that lenvatinib in combination with pembrolizumab has been approved for advanced endometrial cancer with pMMR (24). Therefore, we replaced anlotinib with lenvatinib. Considering the poor effect of tislelizumab in combination with anlotinib in the treatment of this patient, we referred to the NCCN guidelines for uterine sarcoma and added liposomal doxorubicin (25). As a combined result of the above analysis, we chose a regimen of lenvatinib in combination with tislelizumab and liposomal doxorubicin to treat this patient. As a result, the patient’s condition remained stable, with no obvious adverse reactions, and the symptoms of abdominal pain and abdominal distension were relieved. The patient has survived for nearly 25 months since treatment began and is in good condition (Figure 3). This is the first clinical experience with using a combination regimen based on immunotherapy to treat this disease. This treatment regimen may serve as a new option for controlling this tumor.
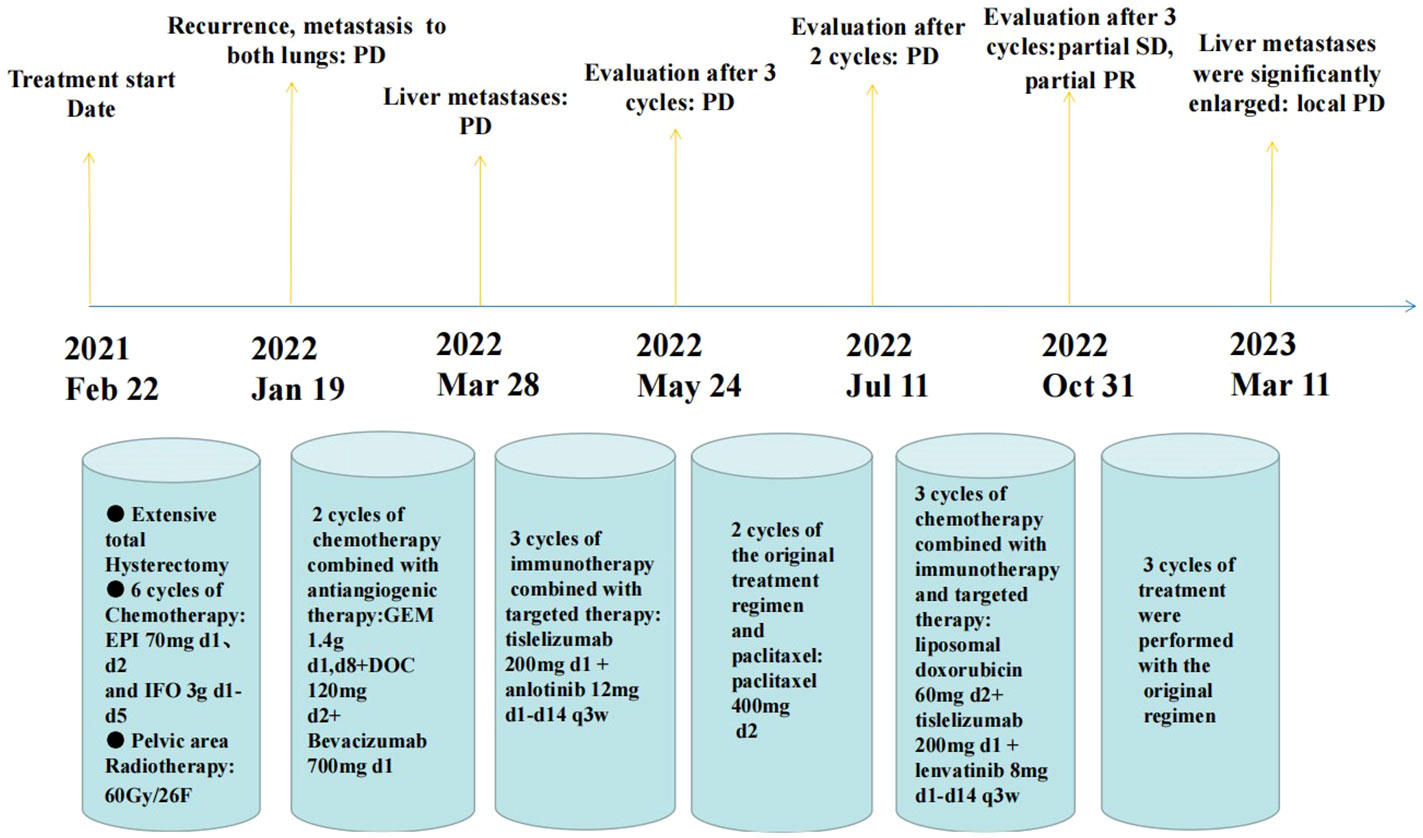
Figure 3 A nearly 25-month treatment history of the patient with primary uterine osteosarcoma. (PD, progressive disease; SD, stable disease; PR, partial remission).
Since this study is a case report, there are not sufficient data to support the efficacy of this treatment regimen. As the incidence of primary uterine osteosarcoma is extremely low, there are few relevant studies, all of which are case reports. There are not enough patients to conduct a large randomized controlled clinical trial, which is a limitation for the future treatment of this tumor. For this rare disease, a global multicenter collaboration is needed to conduct analyses of larger cohorts.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Data collection and article writing: JY, XC. Pathological picture analysis: XL. Image analysis: WL. Clinical data analysis: SL, YW. Subject design and article revision: YZ. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Medical Research Foundation of Guangdong Province (No. B2021114), Guangdong Medical College Doctoral Initiation Program (XB1228) and Natural Science Foundation of Guangdong province (No.2014A020212299).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Abraham C, O’Cearbhaill R, Soslow R. Primary osteosarcoma of the uterus with cardiac and pulmonary metastases. Gynecol Oncol Rep (2015) 11:4–6. doi: 10.1016/j.gore.2014.07.002
2. Crum CP, Rogers BH, Andersen W. Osteosarcoma of the uterus: case report and review of the literature. Gynecol Oncol (1980) 9:256–68. doi: 10.1016/0090-8258(80)90036-0
3. Su M, Tokairin T, Nishikawa Y, Yoshioka T, Takahashi O, Watanabe H, et al. Primary osteosarcoma of the uterine corpus: case report and review of the literature. Pathol Int (2002) 52:158–63. doi: 10.1046/j.1440-1827.2002.01325.x
4. Hardisson D, Simón RS, Burgos E. Primary osteosarcoma of the uterine corpus: report of a case with immunohistochemical and ultrastructural study. Gynecol Oncol (2001) 82:181–6. doi: 10.1006/gyno.2001.6262
5. Zheng G, Richmond A, Liu C, Berrebi A, Pallavajjala A, Haley L, et al. Clinicopathologic features and genetic alterations of a primary osteosarcoma of the uterine corpus. Int J Gynecol Pathol (2019) 38:414–9. doi: 10.1097/PGP.0000000000000511
6. Piscioli F, Govoni E, Polla E, Pusiol T, Dalri P, Antolini M. Primary osteosarcoma of the uterine corpus. report of a case and critical review of the literature. Int J Gynecol Obstet (1985) 23:377–85. doi: 10.1016/0020-7292(85)90146-8
7. Jotkowitz MW, Valentine R. A rare pelvic mass: osteosarcoma of the body of the uterus. Aust N Z J Obstet Gynaecol (1985) 25:132–6. doi: 10.1111/j.1479-828X.1985.tb00627.x
8. Caputo MG, Reuter KL, Reale F. Primary osteosarcoma of the uterus. Br J Radiol (1990) 63:578–80. doi: 10.1259/0007-1285-63-751-578
9. De Young B, Bitterman P, Lack EE. Primary osteosarcoma of the uterus: report of a case with immunohistochemical study. Mod Pathol Off J U S Can Acad Pathol Inc (1992) 5:212–5.
10. Emoto M, Iwasaki H, Kawarabayashi T, Egami D, Yoshitake H, Kikuchi M, et al. Primary osteosarcoma of the uterus: report of a case with immunohistochemical analysis. Gynecol Oncol (1994) 54:385–8. doi: 10.1006/gyno.1994.1229
11. Akiba T, Ujiie H, Takasaki N, Ohki T, Kurihara H, Endo Y, et al. Endobronchial metastasis from a primary uterine osteosarcoma in a patient with multiple myeloma: report of a case. Surg Today (1994) 24:179–82. doi: 10.1007/BF02473406
12. Lin J-W, Ko S-F, Ng S-H, Eng H-L, Changchien C-C. Primary osteosarcoma of the uterus with peritoneal osteosarcomatosis: CT features. Br J Radiol (2002) 75(897):772–4. doi: 10.1259/bjr.75.897.750772
13. Kostopoulou E, Dragoumis K, Zafrakas M, Myronidou Z, Agelidou S, Bontis I. Primary osteosarcoma of the uterus with immunohistochemical study: case report. Acta Obstet Gynecol Scand (2002) 81:678–80. doi: 10.1034/j.1600-0412.2002.810716.x
14. Ribeiro-Silva A, Ximenes L. Pathologic quiz case: a 60-Year-Old woman with diffuse uterine enlargement. Arch Pathol Lab Med (2004) 128:e172–4. doi: 10.5858/2004-128-e172-PQCAYW
15. Tsukasaki N, Mori T, Yasukawa S, Konishi E, Kokabu T, Kitawaki J. Primary osteosarcoma of the uterine corpus: a case report: osteosarcoma arising from the uterus. J Obstet Gynaecol Res (2016) 42:1604–8. doi: 10.1111/jog.13079
16. Powell G, Barth L, Todd R, Ganesan R. Primary uterine osteosarcoma presenting synchronously with bilateral breast carcinomas. Case Rep (2014) 2014:bcr2013201502–bcr2013201502. doi: 10.1136/bcr-2013-201502
17. Yang VS, Lim JQ, Tay TKY, Selvarajan S, Ng CC-Y, Farid M, et al. Clinicopathologic features and whole genome sequencing of a primary osteosarcoma of the uterus. J Immunother Precis Oncol (2020) 3:90–5. doi: 10.36401/JIPO-19-34
18. Effah K, Hiadzi E, Osabutey A, K. Boateng A, B. Akosa A, T. Anim J. Primary osteosarcoma of the uterus: a report of two cases. Ghana Med J (2021) 55:232–5. doi: 10.4314/gmj.v55i3.10
19. Ruhotina M, Kukla J, Wilcox A, Murphy C, Menderes G. Primary uterine osteosarcoma arising in a leiomyoma with rapid local recurrence: a case report. Gynecol Oncol Rep (2022) 44:101102. doi: 10.1016/j.gore.2022.101102
20. von Mehren M, Kane JM, Agulnik M, Bui MM, Carr-Ascher J, Choy E, et al. Soft tissue sarcoma, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw JNCCN (2022) 20:815–33. doi: 10.6004/jnccn.2022.0035
21. Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discovery (2019) 18:197–218. doi: 10.1038/s41573-018-0007-y
22. Zhang J, Huang D, Saw PE, Song E. Turning cold tumors hot: from molecular mechanisms to clinical applications. Trends Immunol (2022) 43:523–45. doi: 10.1016/j.it.2022.04.010
23. Chi Y, Fang Z, Hong X, Yao Y, Sun P, Wang G, et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft-tissue sarcoma. Clin Cancer Res Off J Am Assoc Cancer Res (2018) 24:5233–8. doi: 10.1158/1078-0432.CCR-17-3766
24. Makker V, Rasco D, Vogelzang NJ, Brose MS, Cohn AL, Mier J, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol (2019) 20:711–8. doi: 10.1016/S1470-2045(19)30020-8
25. Judson I, Radford JA, Harris M, Blay JY, van Hoesel Q, le Cesne A, et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC soft tissue and bone sarcoma group. Eur J Cancer Oxf Engl 1990 (2001) 37:870–7. doi: 10.1016/s0959-8049(01)00050-8
Keywords: sarcomas, osteosarcoma, immunotherapy, targeted therapy, chemotherapy
Citation: Yang J, Chen X, Li X, Liu W, Liao S, Wang Y and Zuo Y (2023) Case Report: Clinical application of immunotherapy-based combination regimen in primary osteosarcoma of the uterus. Front. Oncol. 13:1198765. doi: 10.3389/fonc.2023.1198765
Received: 06 April 2023; Accepted: 01 June 2023;
Published: 04 July 2023.
Edited by:
Chao Liu, Shandong Cancer Hospital, ChinaReviewed by:
Bo Tian, Shanghai University, ChinaShihao Hu, Janssen Pharmaceuticals, Inc., United States
Yuting Liu, Akoya Biosciences, Inc., United States
Copyright © 2023 Yang, Chen, Li, Liu, Liao, Wang and Zuo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yufang Zuo, eXVmYW5nenVvMDEwMkAxNjMuY29t; Yuzhou Wang, eXV6aG91d2FuZzE3QDEyNi5jb20=; Sihai Liao, bGlhb3NpaGFpMTYzQDE2My5jb20=
†These authors share first authorship
 Jing Yang
Jing Yang Xiaowen Chen1†
Xiaowen Chen1† Yufang Zuo
Yufang Zuo