- 1Department of Day Ward, Tianjin Medical University Cancer Institute and Hospital, Tianjin, China
- 2Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Tianjin, China
- 3Tianjin’s Clinical Research Center for Cancer, Tianjin, China
- 4Key Laboratory of Breast Cancer Prevention and Therapy, Tianjin Medical University, Ministry of Education, Tianjin, China
- 5Key Laboratory of Cancer Prevention and Therapy, Tianjin, China
- 6Department of Hematology, Tianjin Medical University Cancer Institute and Hospital, Tianjin, China
Objectives: To investigate the characteristics, diagnosis, survival and prognosis of second primary breast carcinoma (SPBC).
Materials and methods: Records of 123 patients with SPBC in Tianjin Medical University Cancer Institute & Hospital between December 2002 and December 2020 were retrospectively reviewed. Clinical characteristics, imaging features and survival were analyzed and comparisons between SPBC and breast metastases (BM) were made.
Results: Of 67156 newly diagnosed breast cancer patients, 123 patients (0.18%) suffered previous extramammary primary malignancies. Of the 123 patients with SPBC, approximately 98.37%(121/123)were female. The median age was 55 years old (27-87). The average diameter of breast mass was 2.7 cm (0.5-10.7). Approximately 77.24% (95/123) of the patients presented with symptoms. The most common types of extramammary primary malignancies were thyroid, gynecological cancers, lung, and colorectal. Patients with the first primary malignant tumor of lung cancer were more likely to develop synchronous SPBC, and those with the first primary malignant tumor of ovarian cancer were more likely to develop metachronous SPBC. When comparing with BM, patients with SPBC were more often older (≥45 years old), at earlier stages (I/II), more microcalcification and less multiple breast masses in imaging. More than half (55.88%) of patients in the metachronous group developed primary breast cancer within 5 years after diagnosis of extramammary primary cancer. The median overall survival time was 71 months. Within 90 months, the prognosis of patients with synchronous SPBC was worse than that of patients with metachronous SPBC (p=0.014). Patients with BM had the worst outcome compared with patients with synchronous SPBC and metachronous SPBC (p<0.001).ER/PR-negative status, an interval of less than 6 months between the onset of two tumors, a late stage of first primary malignancy, and an age of diagnosis of first primary malignancy greater than 60 years predicted a worse prognosis for patients with SPBC.
Conclusion: The possibility of SPBC should be considered during the follow-up of patients with primary extramammary malignancy, especially within 5 years of the onset of the first tumor. The stage of first primary malignancy and the age at diagnosis of first primary malignancy have an impact on the prognosis of patients with SPBC.
Introduction
Multiple primary malignant tumors (MPMTs) are defined as malignant tumors with two or more different histological features in the same individual at the same or different times, excluding metastasis or recurrence (1). The development of MPMTs is associated with genetic predisposition, potential immune deficiencies, history of chemoradiotherapy, and exposure to carcinogens (2, 3).The development of diagnostic and improved treatments for cancer contributes to an increase in the number of cancer survivors and makes it possible for cancer patients to live long enough to develop a second primary tumor (4–7).The second primary carcinoma is an important prognostic factor among patients who survive a prior cancer, and is estimated to be the sixth most common malignant tumor worldwide (8).Cancer survivors account for 3.5% of the total population in the United States, and approximately 10% of newly diagnosed malignant tumors develop in cancer survivors (9).
Previous studies have shown that different treatments for breast malignancy could play a crucial role in the development and progression of primary extramammary cancer as delayed effects and consequences of treatment (10–12).However, only a few studies have described the types and incidence of extramammary tumors that predate the diagnosis of breast cancer. Although breast cancer has been mentioned in some studies of MPMTs, the focus is not on second primary breast cancer (3, 13, 14).In our study, we identified possible risk factors for the development of second primary breast cancer in patients with previous extramammary malignancies and assessed the survival and clinical characteristics of patients with second primary breast cancer. The differential diagnosis of primary and metastatic tumors is also important, resulting in better clinical outcomes in cases of primary cancers. We also compared SPBC and BM.
Materials and methods
A total of 67156 breast cancer patients were newly diagnosed in Tianjin Medical University Cancer Institute & Hospital between December 2002 and December 2020.The diagnosis of all patients was confirmed by histopathology. Of these patients, 123 cases had survived a previous primary extramammary malignancy. According to the diagnosis time from the primary extramammary malignancy to the primary breast malignancy, these 123 patients were classified into the synchronous SPBC group (interval within 6 months) or the metachronous SPBC group (interval longer than 6 months) (15). Seventeen patients with pathologically confirmed BM were also included in the study, as BM group, and compared with SPBC. We retrospectively collected data on clinical characteristics, imaging features, prognosis, and pathology of previous primary malignancies and the interval time between the occurrence of two types of tumor. Family history of cancers refers to the diagnosis of cancer in a first-degree relative of patient. Studies involving human participants were approved and reviewed by the Medical Ethics Committee of Tianjin Medical University Cancer Institute & Hospital.
A total of 109 patients with SPBC and 13 with BM were followed up. We recorded and analyzed metastasis, recurrence, and survival status. Overall survival (OS) was defined as the date from the diagnosis of SPBC to the date of death from any cause or the cut-off date of follow-up (September 30, 2022).
Data comparisons for categorical variables were analyzed using two-sided Fisher’s exact test or the chi-square test. The OS of the patients was calculated using the Kaplan–Meier method. The influence of different factors on OS was analyzed using log-rank test and landmark analysis. A Cox regression model was used for multivariate survival analysis. Differences were considered statistically significant at p < 0.05. Statistical analyses were performed using SPSS 27.0, Graphpad prism 9.5 and R software (version 4.2.2).
Results
Patients’ characteristics
SPBC accounted for 0.18% (123/67156) of all breast cancers between December 2002 and December 2020.As shown in Table 1, the clinical characteristics of the SPBC and BM groups were compared. The median patient age was 55 (range 27-87 years of age), and 121 patients(98.37%)were female. The average diameter of breast mass was 2.7 cm (0.5-10.7). Of the patients, 76.42% were ER/PR positive and 30.08% were HER-2 positive. A family history of malignancies and smoking history were found in 42.28% and 6.50%of patients, respectively. There were 58 (47.15%) patients who had received chemoradiotherapy for their first primary malignancy. Approximately 77.24% (95/123) of the patients presented with symptoms, of which lumps(s) were the most common, accounting for 69.92% (86/95). A total of 122 patients (99.19%) had no distant metastases at the time of SPBC diagnosis. Regarding the first primary malignancies, the majority of patients (73.17%) were elderly at the time of diagnosis, and most (71.54%) were in the early stages (I/II).
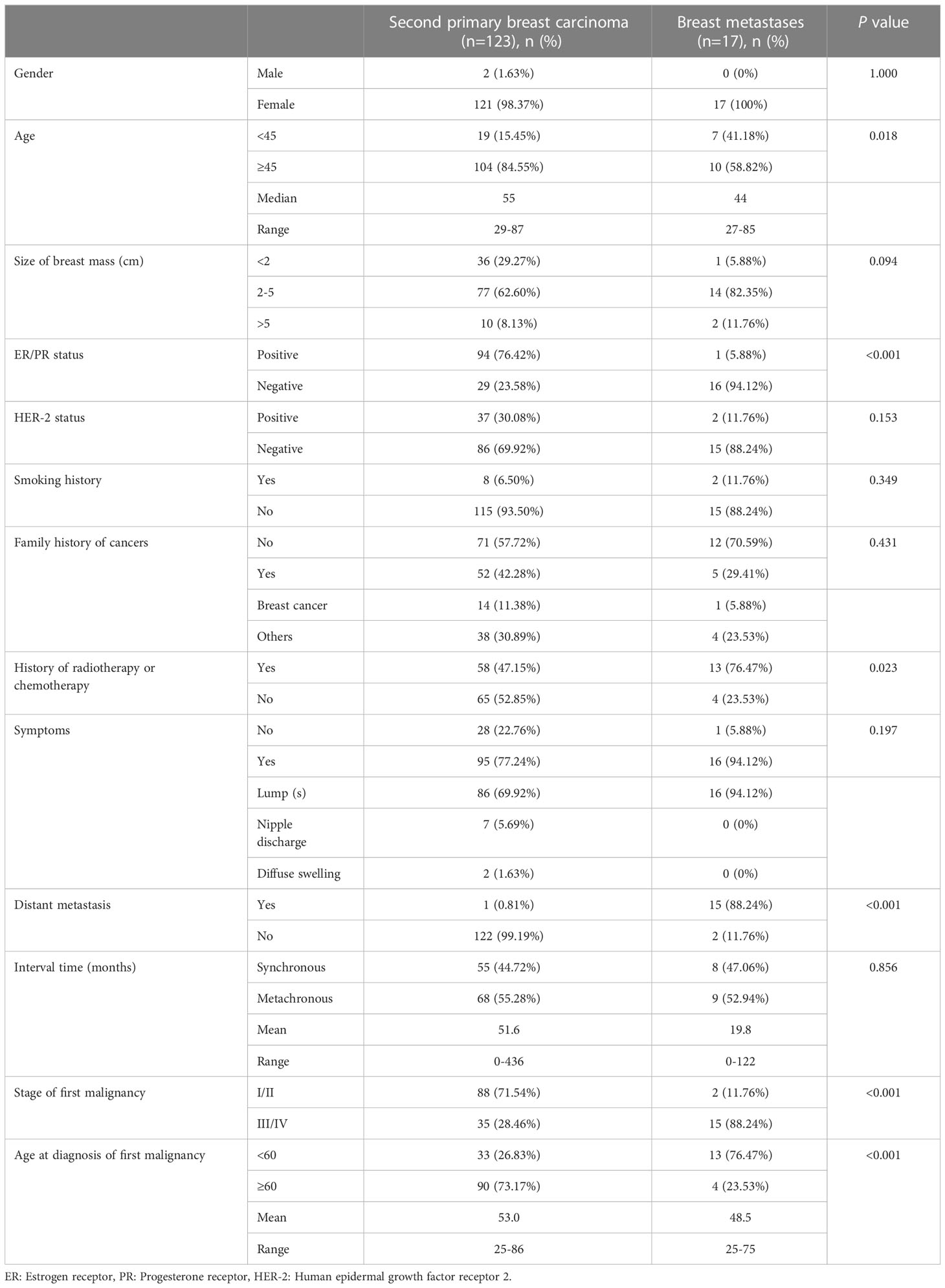
Table 1 Clinical characteristics of patients with second primary breast carcinoma and with those of breast metastases.
Treatment and pathology of SPBC
Of the 123 patients with SPBC, 112 underwent surgery, of which 13 received neoadjuvant chemotherapy before surgery and 58 received radiotherapy or chemotherapy after surgery. Nine patients were treated with radiotherapy or chemotherapy alone, and two refused treatment. In addition, patients with positive ER/PR and HER-2 status received endocrine therapy and targeted therapy, respectively. With regard to the treatment of the first primary malignant tumor, 101 patients received comprehensive treatment based on surgery, 18 patients received chemotherapy-based treatment, and four patients received radiotherapy alone.
The pathology of patients with SPBC included invasive ductal carcinoma (n=93, 75.61%), invasive lobular carcinoma (n=24, 19.51%), medullary carcinoma (n=2), mucinous carcinoma (n=2), mixed mucinous carcinoma (n=1), and invasive micropapillary carcinoma (n=1).
Primary extramammary malignancy in patients with SPBC
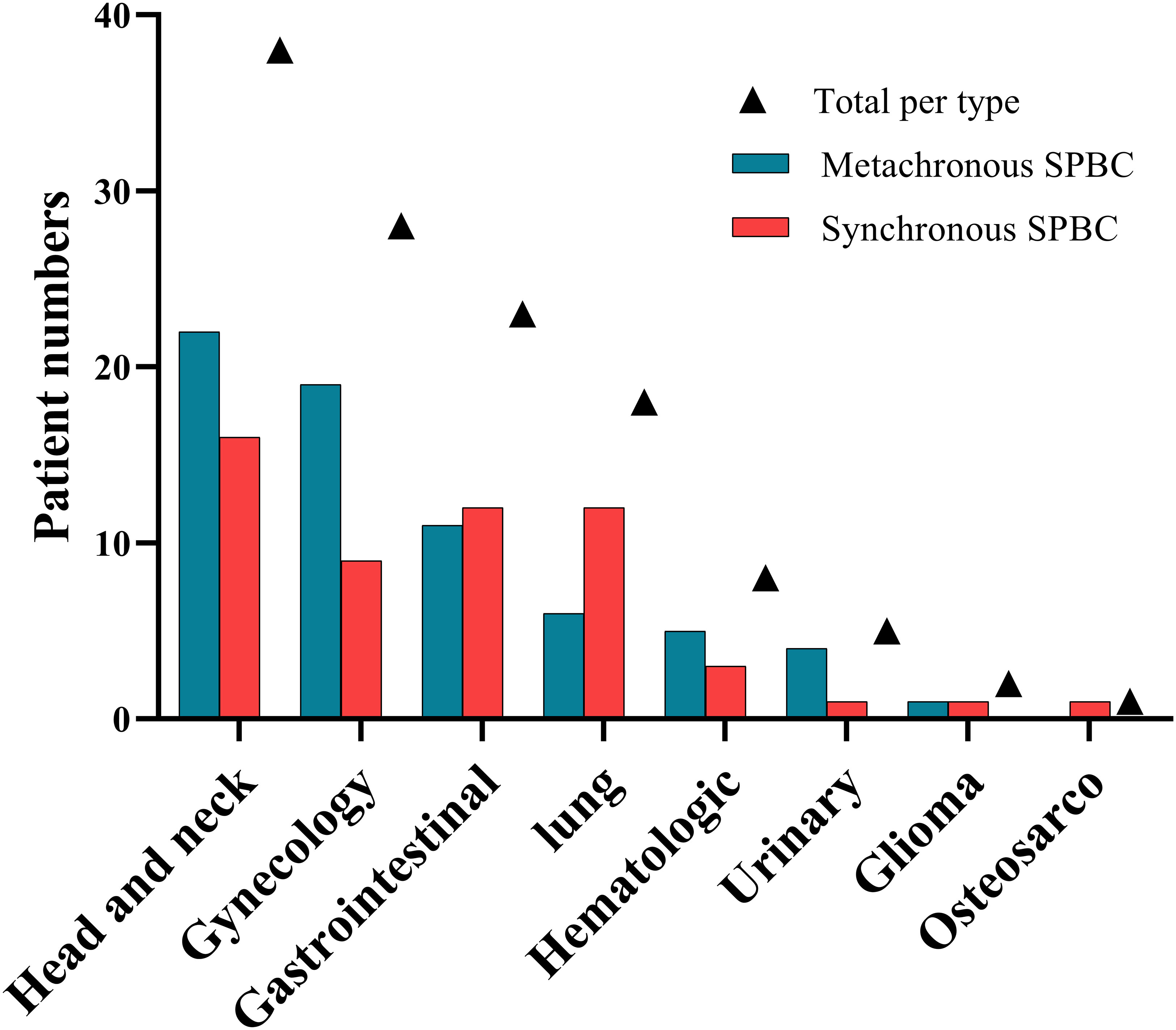
Of the 123 patients with SPBC, 68 (55.28%) had metachronous malignancies and 55 (44.72%) had synchronous malignancies. Most of the patients had double primary malignant tumors, except eight patients who developed additional malignancies after the diagnosis of SPBC, including three triple primary cancers in the metachronous group and four triple primary cancers in the synchronous group. We observed a rare case of quadruple primary cancer in the synchronous group. The types and number of primary non-breast malignancies in patients with SPBC are shown in Table 2 and Figure 1. The most common types of extramammary primary malignancies were thyroid (30.1%), gynecological (22.8%), and lung (14.6%), followed by colorectal (8.9%), hematologic (6.5%), gastric (4.1%), kidney (4.1%), and liver (3.3%) malignancies, with less common types including gallbladder (1.6%), esophageal (0.8%), oral (0.8%), glioma (1.6%), and osteosarcoma (0.8%). In the metachronous group, the most common types of first primary malignant tumors were head and neck, gynecology, and gastrointestinal, while in synchronous group, head and neck, gastrointestinal, and lung were the most common types. We further compared the distribution of the first primary malignant tumor type between the two groups and found that lung cancer was more common in the synchronous group than in the metachronous group (p =0.043), whereas ovarian cancer was more common in the metachronous group than in the synchronous group (p =0.040). This result suggests that, for women with lung cancer, targeted follow-up is needed within 6 months of diagnosis to alert them to the development of primary breast cancer. However, for patients with ovarian cancer, focused follow-up should be performed 6 months after diagnosis.
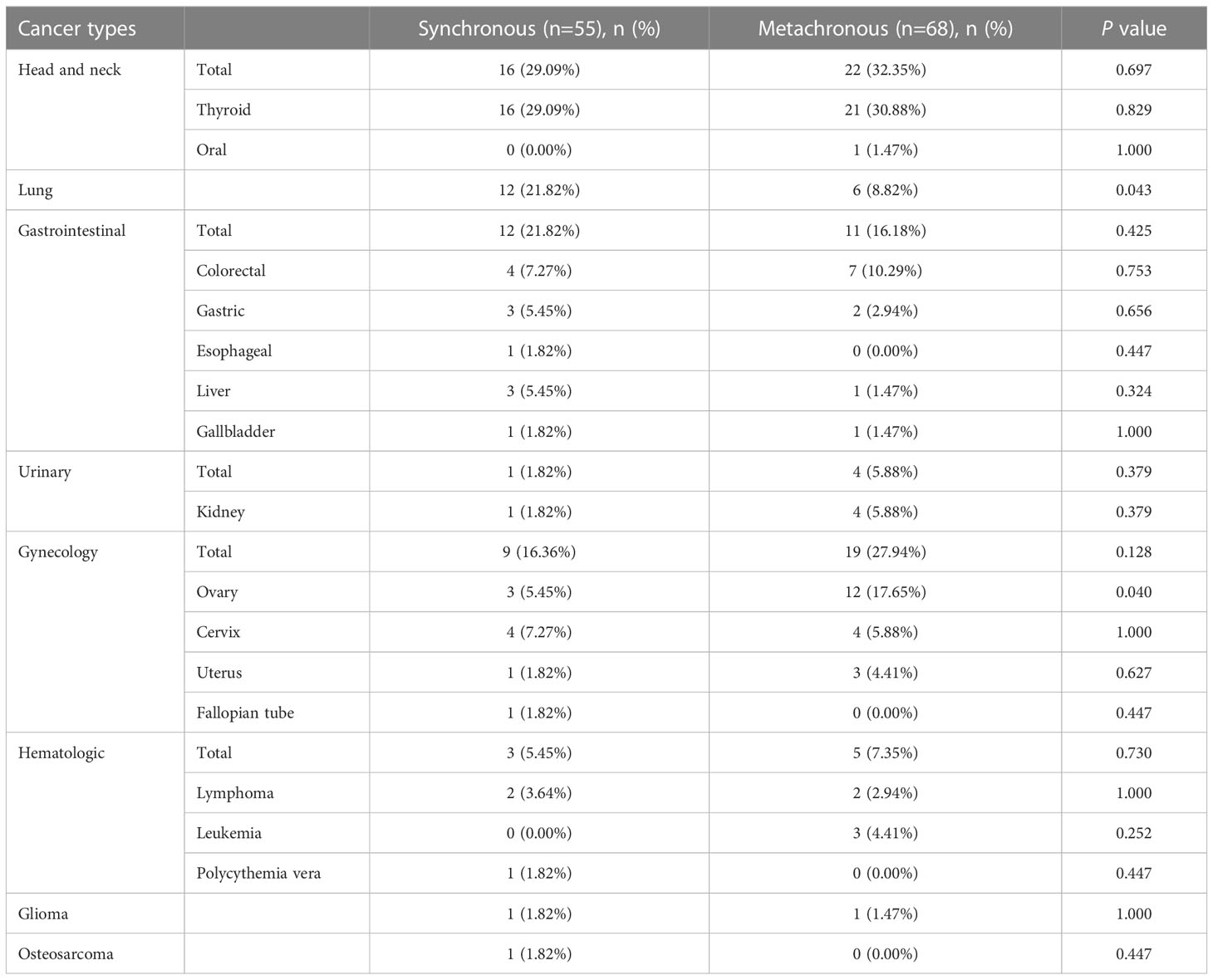
Table 2 Numbers and types of primary non-breast malignancy in patients with second primary breast carcinoma.
Intervals between primary extramammary malignancy and SPBC
For the 123 patients included, the mean interval between the onset of primary non-breast malignancy and SPBC was 51.6 months (range, 0-436 months). Among the 68 patients in the metachronous group, the mean duration between the onset of the two cancers was 92.9 months. As shown in Table 3 and Figure 2, in the metachronous group, more than half (55.88%) of the patients developed primary breast cancer within 5 years after diagnosis of the first primary extramammary malignancy. Fortunately, the risk of developing SPBC decreased over time (Figure 2). The longest interval observed was nearly 37 years. This result suggests that patients should be closely followed for a second primary breast cancer within 5 years of the diagnosis of extramammary malignancy, and that the frequency of follow-up could be reduced, but not interrupted, 5 years after the diagnosis of the first extramammary malignancy.
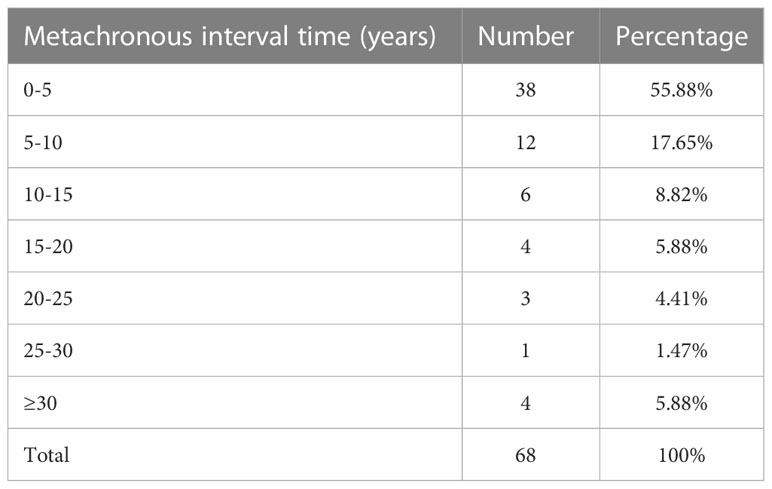
Table 3 Average interval time for different cancer types to develop second primary breast carcinoma.
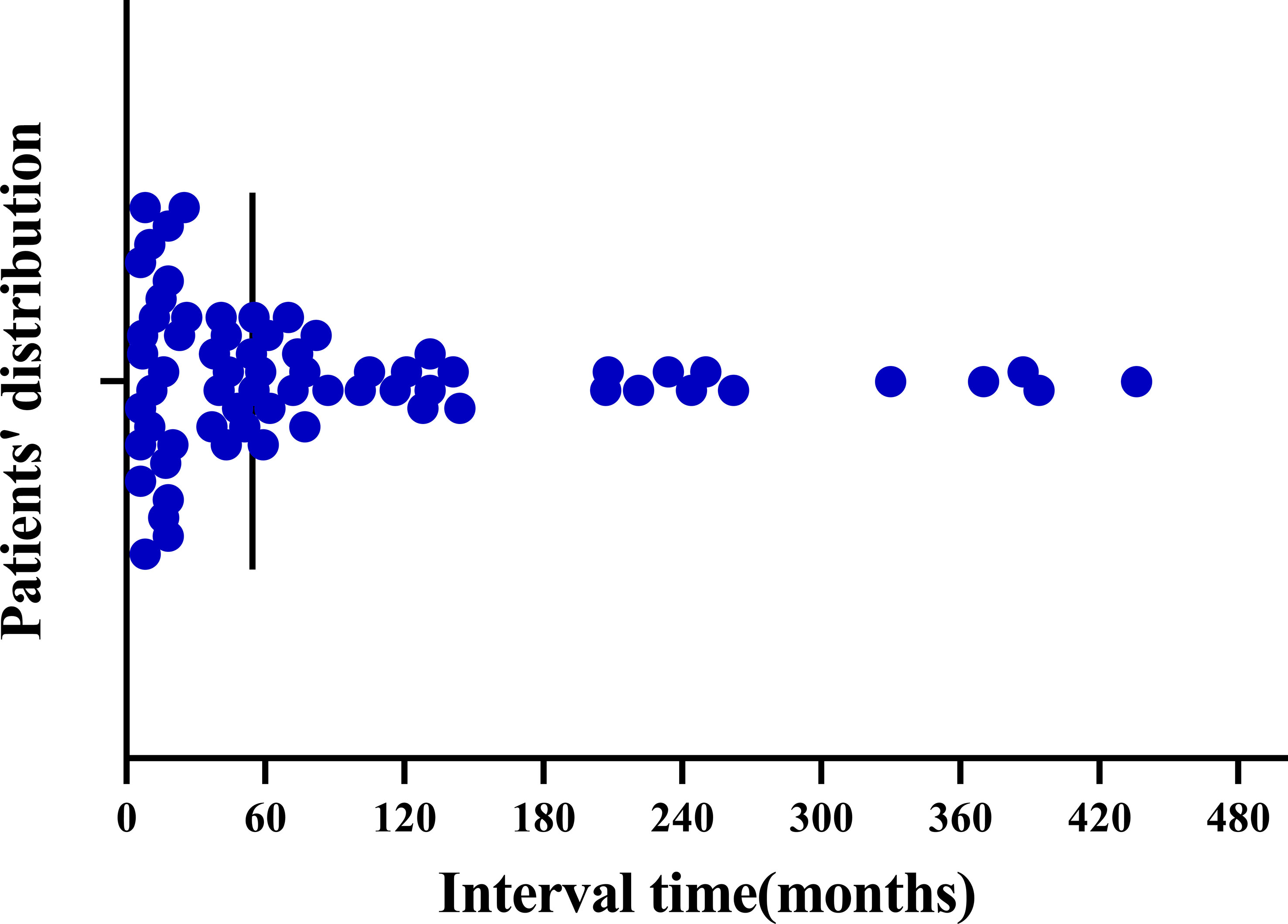
Figure 2 Distribution of time intervals between the development of metachronous second primary breast cancer in patients with primary non-breast malignancy.
Survival and prognostic factors of patients with SPBC
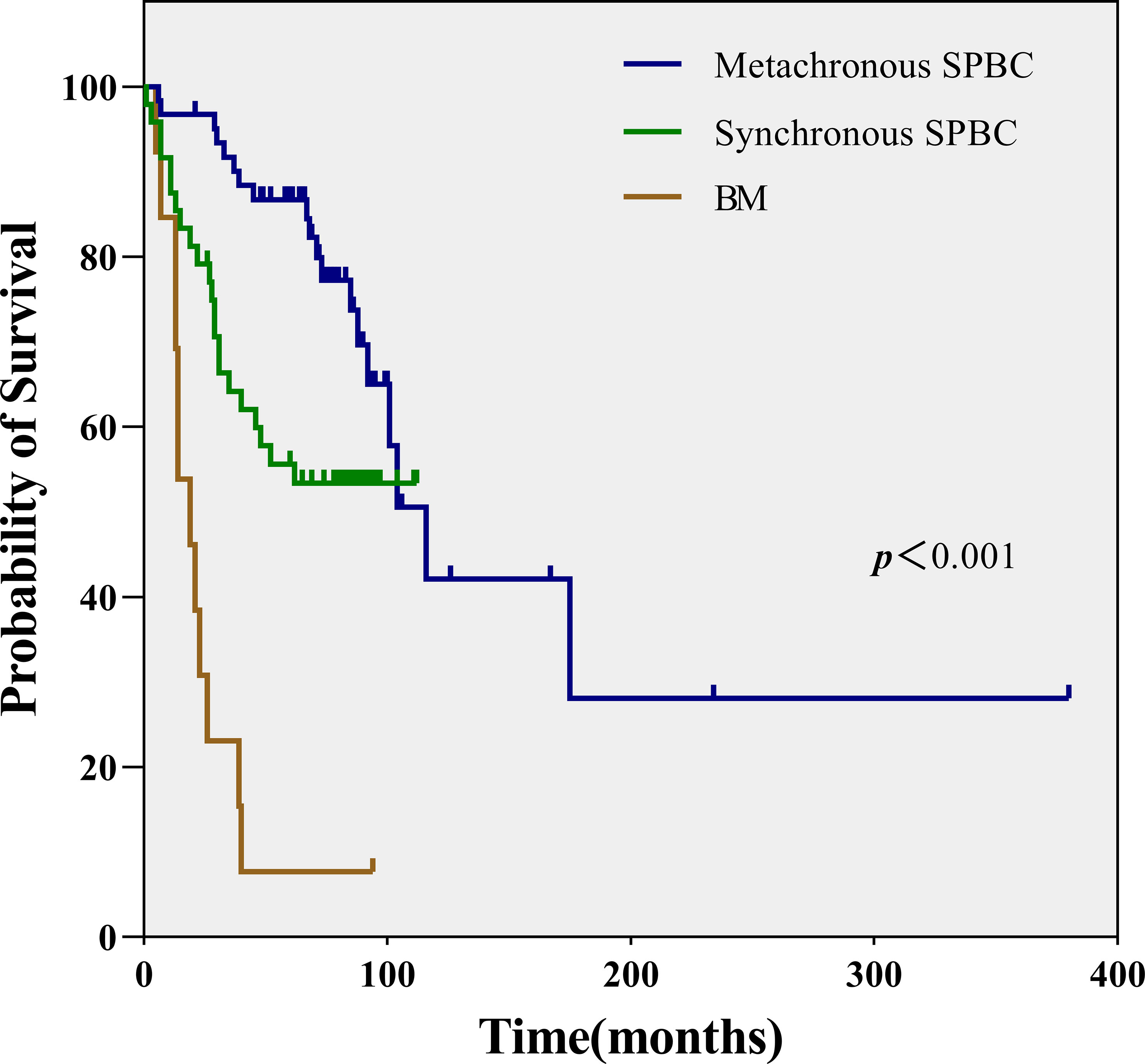
Of the 123 patients with SPBC, 109 were followed up, including 48 in the synchronous group and 61 in the metachronous group. The median overall survival time was 71 months (range, 1-380 months). We also followed up 13 patients with BM. Survival data of these patients were collected and analyzed. As shown in Figures 3, 3.1, patients with metachronous SPBC had the longest median survival time (MST) of 72 months, followed by patients with synchronous SPBC, with an MST of 63.5 months. Within 90 months, the prognosis of metachronous SPBC patients was better than that of synchronous SPBC patients (p=0.014); however, after 90 months, there was no significant difference in overall survival between the two groups (p=0.522).BM patients showed the worst prognosis, with a significantly shorter MST of 19 months (range, 5-94 months) than patients with synchronous SPBC and metachronous SPBC (MST: 19, 63.5, 72 months, p<0.001).
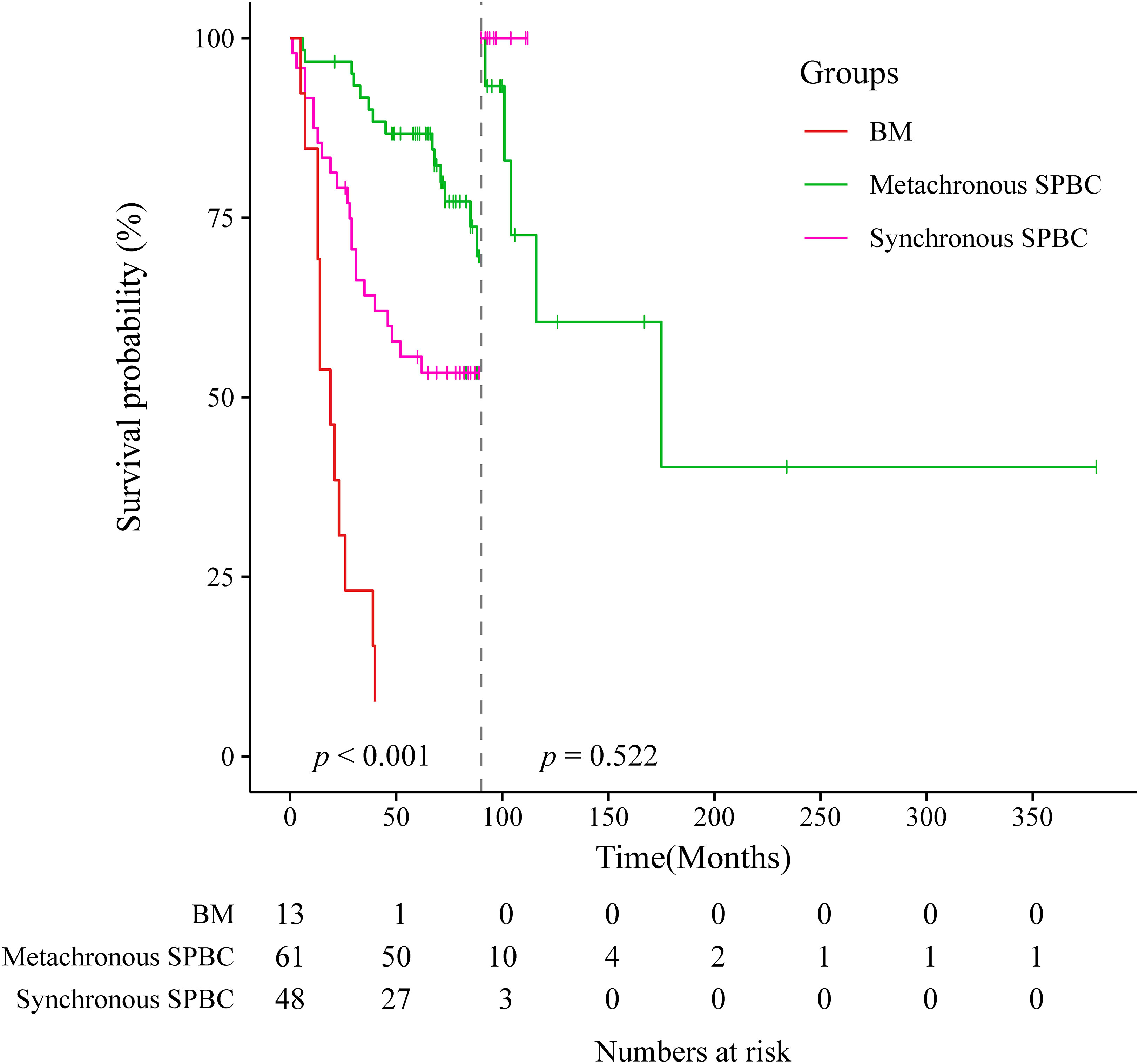
Figure 3.1 Landmark analysis at the 90-month landmark point of metachronous group, synchronous group and breast metastases group.
Univariate survival analysis showed that ER/PR -negative status, an interval of less than 6 months between the onset of two tumors, a late stage of first primary malignancy, and an age of diagnosis of first primary malignancy greater than 60 years predicted a worse prognosis for patients with SPBC. Multivariate analysis showed that OS improved in patients with ER/PR -positivity and at an early stage when diagnosed with extramammary malignancy (Table 4).
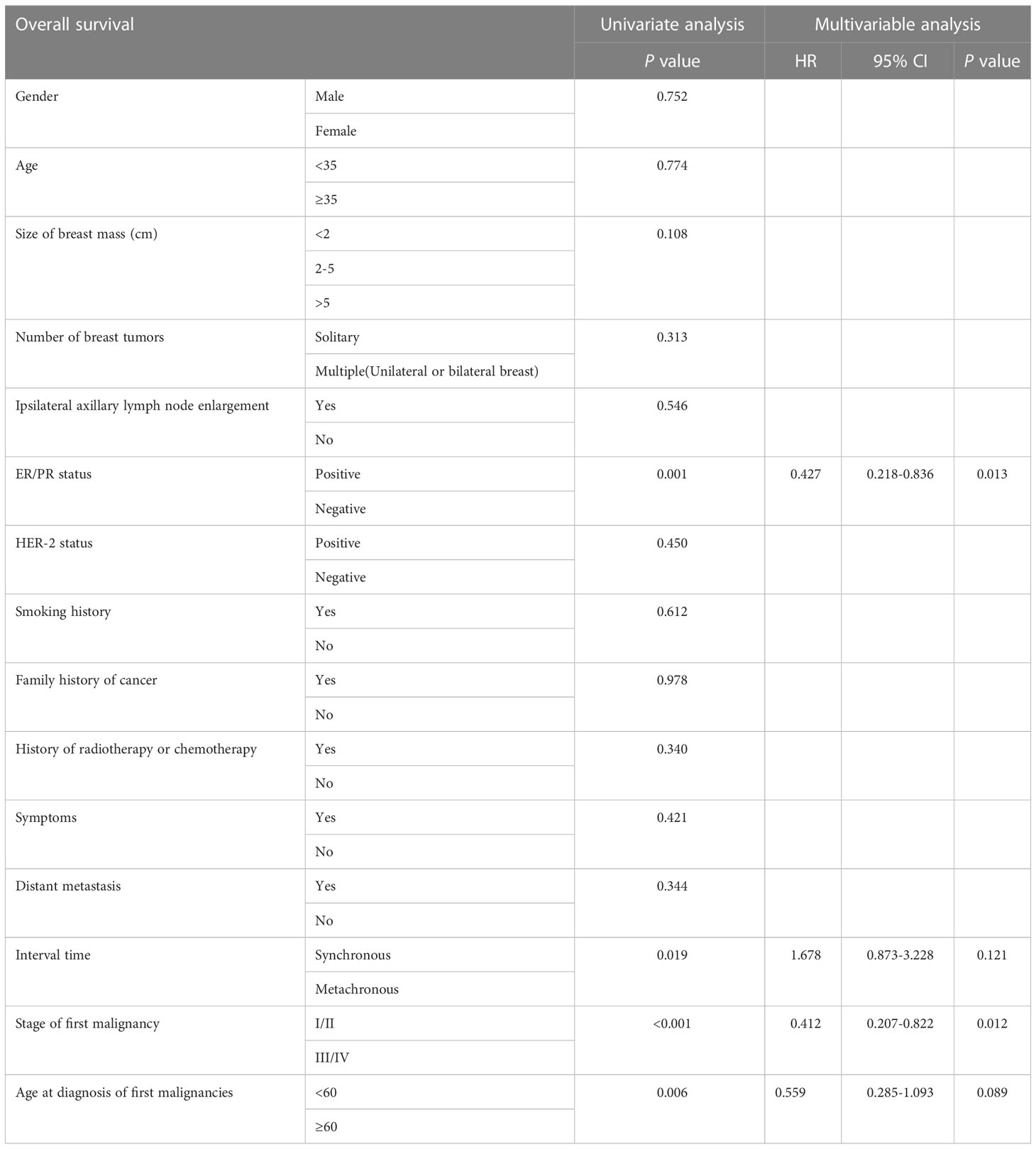
Table 4 Univariate survival analysis and multivariable analysis of patients with second primary breast carcinoma.
Differential diagnosis of SPBC and BM
A comparison of the clinical characteristics of SPBC and BM is shown in Table 1. Patients with SPBC were older than those with BM, with median ages of 55 and 44 years, respectively (p=0.018). The positive rate of ER/PR in SPBC patients was significantly higher than that in patients with BM (76.42% vs. 5.88%, p < 0.001). More patients with BM chose chemoradiotherapy than those with SPBC for the treatment of first primary cancers (76.47% vs. 47.15%, p=0.023).For the first primary malignant tumor, patients in stage III/IV were a small fraction (28.46%) in SPBC group, whereas they were the majority (88.24%) in BM group (p < 0.001). Of the BM patients, 88.24% (15/17) were found to have other organs or lymph node metastasis at the same time as breast metastasis, and only 0.81% (1/123) of SPBC patients had distant metastasis (p < 0.001). In terms of the age at diagnosis of first primary malignancy, the mean age of the patients in the SPBC group was older than that of patients in the BM group (53 years vs. 48.5 years, p<0.001).
When it comes to imaging differences between SPBC and BM patients, the number of breast tumors and the presence of microcalcifications are the main points of difference. As shown in Table 5, both SPBC and BM patients were more likely to show a solitary breast tumor. The difference is that patients with SPBC has a higher proportion of solitary breast tumors than BM patients (90.24% vs. 64.71%, p=0.01). Mammography was performed in 100 patients with SPBC and seven patients with BM. Microcalcifications were found in 75% (75/100) of the patients with SPBC, whereas in the BM group, only one patient with ovarian cancer reported the presence of microcalcifications (14.29%) (p=0.002). All 17 patients with BM underwent imaging studies, and four patients were diagnosed with breast metastasis; however, up to 70.59% were diagnosed with primary breast cancer. It was challenging to distinguish between primary breast cancer and metastatic breast cancer on imaging.
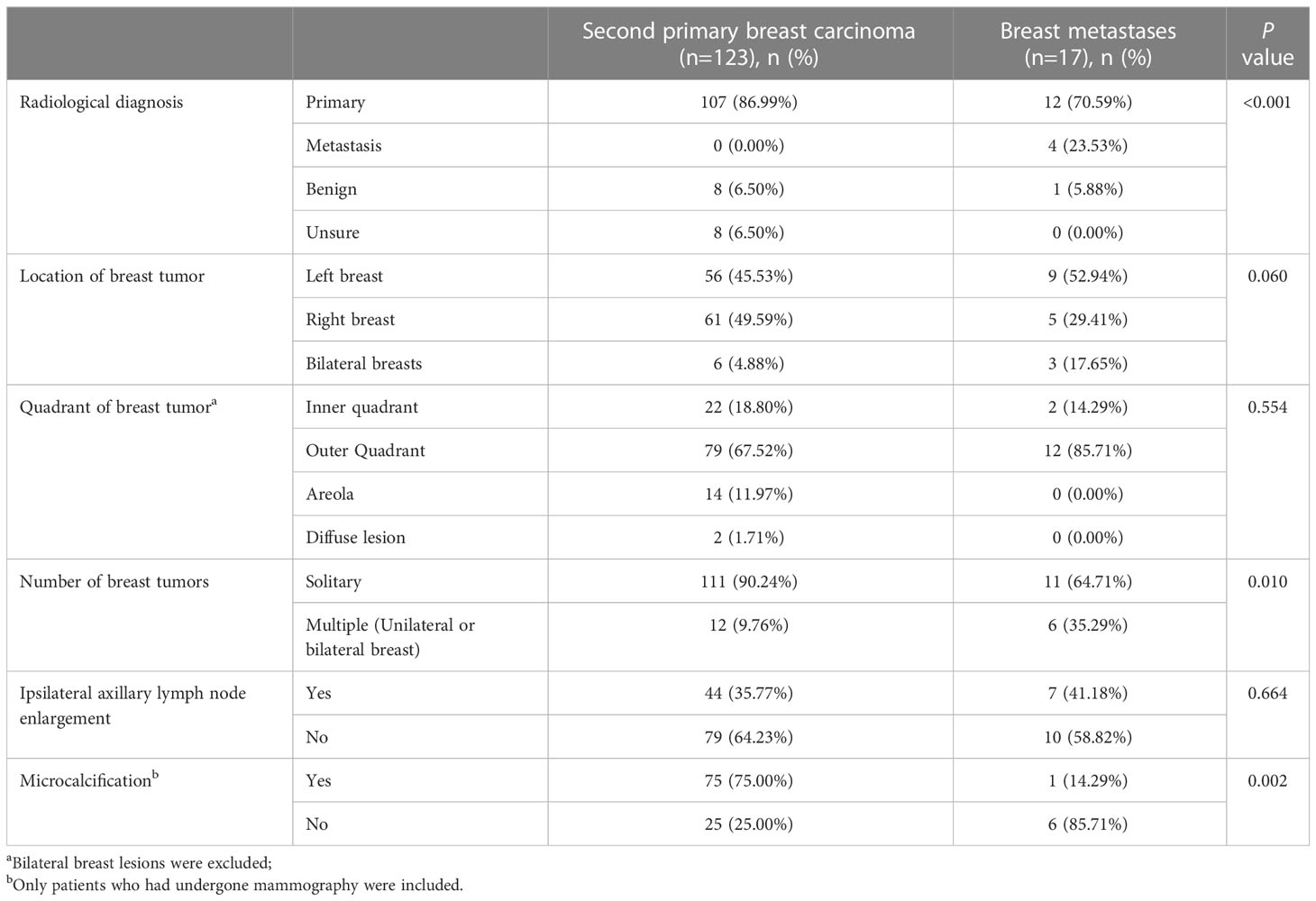
Table 5 Radiological characteristics of patients with second primary breast carcinoma and with those of breast metastases.
Discussion
Cancer morbidity is on the rise, and it is estimated that by 2030, more than 21 million people will be diagnosed with malignancies, with morbidity increasing by 1.7% annually (12, 16, 17). Breast cancer is the most common malignancy among women in the United States (18).The number of cancer survivors is increasing owing to advances in diagnosis and treatment. By 2040, an estimated 26.1 million people in the United States will be cancer survivors (19).However, cancer survivors, who make up 3.5% of the U.S. population, make up 10% of newly diagnosed cancer patients, resulting in a growing number of MPMTs (9).A substantial number of patients newly diagnosed with breast carcinoma had different types of malignancies in the past (20).The development of MPMTs may be caused by a variety of genetic, therapeutic, extrinsic, and intrinsic factors (21, 22). Genome-wide association studies have identified 72 loci associated with breast cancer susceptibility, 17 of which are associated with MPMTs (23). Studies have shown that survivors of breast malignancy have a 25% increased risk of developing extramammary primary malignancies (10, 11). The association between malignant breast tumors and non-breast primary cancers has also been well documented (24–31). However, little attention has been paid to primary breast cancers secondary to non-breast primary malignancies.
Some studies have confirmed the pathogenesis of primary breast cancer secondary to primary extramammary malignancies. In terms of the type of first primary extramammary malignancy in patients with SPBC, we observed that thyroid cancer was the most common, accounting for 30.08% (37/123). In fact, several studies have reported elevated SPBC morbidity in thyroid cancer survivors (32, 33). The rising morbidity of thyroid cancer and the generally long survival time of thyroid cancer patients have led to a growing number of thyroid cancer survivors, whose long survival time also gives them enough time to develop a second primary malignancy, such as breast cancer. One of the molecular mechanisms underlying the increased risk of SPBC in thyroid cancer survivors could be the high ER levels in thyroid cancer patients, which are generally higher than those in the general population with sex steroid receptors in the human thyroid tissue (34, 35).In our study, there were 28 cases of gynecological tumors, which were the second most common extramammary malignancy after thyroid cancer, accounting for 22.76% of SPBC patients. It has been proven that gene mutation plays an important role in the occurrence of gynecologic tumors and breast cancer. Patients with BRCA or TP53 mutations have a significantly higher lifetime risk of both ovarian and breast cancers than the general population (36, 37), which is one of the reasons why patients with gynecologic neoplasms are more likely to develop SPBC. Unfortunately, due to the economic conditions of the patients, most of them were not tested for gene mutations, which made it impossible to obtain the mutation data of BRCA or TP53 in these patients. Furthermore, in addition to the same causative genes, another reason for the elevated risk of SPBC in gynecologic cancer survivors may be the higher survival rate of patients with gynecologic tumors, which has also been shown in previous studies conducted in Taiwan, China (38, 39).In our study, thyroid and gynecologic cancers accounted for more than half of the primary malignant tumor types in SPBC patients; therefore, understanding the potential association of these two tumor types with breast cancer could provide some clinical experience and evidence to us. Notably, a strong association between melanoma and breast cancer has been frequently reported. Both melanoma and breast cancer cells exhibit ERs, and hormonal factors in melanoma survivors may contribute to the development of SPBC (40–42). However, no patients with melanoma were observed in our study, possibly because of the high incidence of cutaneous melanoma in white women but less often in Asian populations (43). A previous study in Korea found an increased risk of SPBC in patients with primary bladder cancer (44), and it was hypothesized that germline mutations in fumarate hydratase might be associated with this phenomenon (45).Bluhm et al. found that the risk of SPBC was highest 5-9 years after NHL diagnosis (46), while another study showed that SPBC was significantly increased 10 years after NHL diagnosis (43).This may be due to the long-term effects of chemoradiotherapy in lymphoma patients.
An increased risk of breast cancer has been observed in many types of cancer within the first five years following diagnosis. Our study found that more than half of the patients (55.88%) in the metachronous group had two different types of primary malignancies within 5 years. Similar to our findings, Sandi et al., studying 10822 patients diagnosed between 2005 and 2015, found that 46.3% of the patients developed breast cancer within 5 years of the incident previous cancer diagnosis (47). Although the risk of breast cancer is high within 5 years of the occurrence of extramammary tumors, we found that the risk decreases over time.
Both primary and metastatic breast cancers should be considered when a cancer patient has a breast mass. Metastatic breast cancer is rare because the breast is rich in fibrous tissue and has a relatively poor blood supply. Williams et al. (48) reported data from 169 patients with breast metastases from solid tumors outside the breast, in which the most common primary tumor was malignant melanoma. Lee et al. (49) reported data from 33 patients with breast metastases from extramammary malignancies, in which the most common primary tumor was gastric cancer. In our study, the most common primary tumors in BM patients were lung cancer (35.29%), followed by ovarian cancer (11.76%), lymphoma (11.76%) and rectal cancer (11.76%), with rare primary tumors including hepatic carcinoma, gastric carcinoma, renal carcinoma, mucoepidermoid carcinoma, and malignant schwannoma in one case each. This result may reflect the different occurrences and treatments of malignant tumors in different races, regions, and hospitals. Due to the rarity of metastatic breast cancer, there are insufficient data to differentiate primary breast cancer from metastatic breast cancer. Both primary and metastatic breast cancers are characterized by painless solitary breast nodules, which are usually located in the outer quadrant of the breast because of the relatively abundant blood supply and glands in the outer quadrant. Metastatic breast cancer has a rapid nodule growth rate, and skin changes and nipple discharge are very rare (50–52). This finding is consistent with the results of the present study. McCrea et al. (53) suggested that microcalcifications are almost absent in patients with breast metastases, except in those whose primary tumor is ovarian cancer, where microcalcifications are mostly present. In this study, we found one case of metastatic breast cancer with microcalcification, the primary tumor of which was ovarian cancer. Therefore, it is inaccurate to distinguish breast nodules as primary or metastatic based on the presence or absence of microcalcifications. In this study, we found that patients with metastatic breast cancer were younger than those with SPBC, which may be due to the richer blood supply to the breast in younger patients. ER/PR-positive rates in patients with BM are usually low because these receptors are usually present in the breast tissue. Breast metastasis is mostly a part of systemic metastasis. In this study, most patients (88.24%) presented with additional systemic metastases when breast metastases were found. Patients with breast metastasis usually lose the chance of surgery and need systemic treatment for the primary tumor, while patients with SPBC are usually staged earlier and treated with comprehensive treatment based on surgery. Due to the different treatment methods, it is particularly important to make a good differential diagnosis between the two.
A previous study (43) has shown that the proportion of female patients with SPBC who survived for more than 5 years was approximately 43.82%, while in our study, the proportion was 66.06% (median OS:71 months), which may be due to the improvement of people’s health awareness and more targeted follow-up after the diagnosis of the first tumor. In addition, it may also reflect differences in the predilection type and malignancy of the first tumor in patients of different countries and races. Williams et al. (48) reported a median survival of 10 months for 169 cases of breast metastases from extramammary solid tumors; in our study, the median survival from the time BM was diagnosed was 19 months. Breast metastasis is rare, and we hope that more relevant studies will emerge in the future to provide more references.
Conclusions
In conclusion, SPBC is not uncommon, and survivors of extramammary malignancies should be followed-up. Therefore, it is important to make a differential diagnosis between SPBC and BM. The ER/PR status and the first primary malignancy may affect the prognosis of patients with SPBC. Identifying the characteristics of different coexisting primary cancers may increase the clinical vigilance of physicians and warrant new screening procedures to detect certain second primary malignancies at an earlier stage in patients with malignant tumors. However, there are still some limitations in this study. In this study, a number of factors that may affect the prognosis of SPBC patients were obtained through retrospective study, but the conclusions were not further verified. We look forward to developing a risk early warning system for cancer patients in the future by combining with engineers and artificial intelligence.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Tianjin Medical University Cancer Institute&Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YJ performed the analysis and wrote the manuscript. YJ modified the article. XW and BS designed and supervised the research. XW and BS contributed equally to this research. All authors examined and accredited the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by Individualized Medical Incubation Project of Precision Medicine Research and Industrial Innovation and Development Fund (SIMQ59-8), and Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-009A).
Acknowledgments
The authors gratefully acknowledge the financial supports by the Individualized Medical Incubation Project of Precision Medicine Research and Industrial Innovation and Development Fund under project number SIMQ59-8, as well as the Tianjin Key Medical Discipline (Specialty) Construction Project under project number TJYXZDXK-009A. We would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ikubo A, Matsufuji S, Morifuji Y, Koga H, Kobarai T, Kouya N, et al. Clinical features, prognosis, diagnostic approaches and treatment of multiple primary malignancies in the digestive system. Anticancer Res (2019) 39(12):6863–70. doi: 10.21873/anticanres.13904
2. Koubkova L, Hrstka R, Dobes P, Vojtesek B, Vyzula R. Second primary cancers - causes, incidence and the future. Klin Onkol (2014) 27(1):11–7. doi: 10.14735/amko201411
3. Coyte A, Morrison DS, McLoone P. Second primary cancer risk - the impact of applying different definitions of multiple primaries: results from a retrospective population-based cancer registry study. BMC Cancer (2014) 14:272. doi: 10.1186/1471-2407-14-272
4. Soerjomataram I, Coebergh JW. Epidemiology of multiple primary cancers. Methods Mol Biol (2009) 471:85–105. doi: 10.1007/978-1-59745-416-2_5
5. Irimie A, Achimas-Cadariu P, Burz C, Puscas E. Multiple primary malignancies–epidemiological analysis at a single tertiary institution. J Gastrointestin Liver Dis (2010) 19(1):69–73.
6. Chirila DN, Turdeanu NA, Constantea NA, Coman I, Pop T, Popp RA, et al. Multiple malignant tumors. Chirurgia (Bucur) (2013) 108(4):498–502.
7. Donin N, Filson C, Drakaki A, Tan HJ, Castillo A, Kwan L, et al. Risk of second primary malignancies among cancer survivors in the united states, 1992 through 2008. Cancer (2016) 122(19):3075–86. doi: 10.1002/cncr.30164
8. Liu H, Hemminki K, Sundquist J, Holleczek B, Katalinic A, Emrich K, et al. A population-based comparison of second primary cancers in Germany and Sweden between 1997 and 2006: clinical implications and etiologic aspects. Cancer Med (2013) 2(5):718–24. doi: 10.1002/cam4.116
9. Jo JH, Cho IR, Jung JH, Lee HS, Chung MJ, Bang S, et al. Clinical characteristics of second primary pancreatic cancer. PloS One (2017) 12(6):e0179784. doi: 10.1371/journal.pone.0179784
10. Mellemkjaer L, Christensen J, Frederiksen K, Pukkala E, Weiderpass E, Bray F, et al. Risk of primary non-breast cancer after female breast cancer by age at diagnosis. Cancer Epidemiol Biomarkers Prev (2011) 20(8):1784–92. doi: 10.1158/1055-9965.EPI-11-0009
11. Mellemkjaer L, Friis S, Olsen JH, Scelo G, Hemminki K, Tracey E, et al. Risk of second cancer among women with breast cancer. Int J Cancer (2006) 118(9):2285–92. doi: 10.1002/ijc.21651
12. Rubino C, Vathaire F, Diallo I, Shamsaldin A, Le MG. Increased risk of second cancers following breast cancer: role of the initial treatment. Breast Cancer Res Treat (2000) 61(3):183–95. doi: 10.1023/A:1006489918700
13. Bajdik CD, Abanto ZU, Spinelli JJ, Brooks-Wilson A, Gallagher RP. Identifying related cancer types based on their incidence among people with multiple cancers. Emerg Themes Epidemiol (2006) 3:17. doi: 10.1186/1742-7622-3-17
14. Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the surveillance, epidemiology, and end results (SEER) program. Oncologist (2007) 12(1):20–37. doi: 10.1634/theoncologist.12-1-20
15. Etiz D, Metcalfe E, Akcay M. Multiple primary malignant neoplasms: A 10-year experience at a single institution from Turkey. J Cancer Res Ther (2017) 13(1):16–20. doi: 10.4103/0973-1482.183219
16. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
17. Lee KD, Chen SC, Chan CH, Lu CH, Chen CC, Lin JT, et al. Increased risk for second primary malignancies in women with breast cancer diagnosed at young age: a population-based study in Taiwan. Cancer Epidemiol Biomarkers Prev (2008) 17(10):2647–55. doi: 10.1158/1055-9965.EPI-08-0109
18. Viale PH. The American cancer society's facts & figures: 2020 edition. J Adv Pract Oncol (2020) 11(2):135–6. doi: 10.6004/jadpro.2020.11.2.1
19. Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the "Silver tsunami": Prevalence trajectories and comorbidity burden among older cancer survivors in the united states. Cancer Epidemiol Biomarkers Prev (2016) 25(7):1029–36. doi: 10.1158/1055-9965.EPI-16-0133
20. Murphy CC, Gerber DE, Pruitt SL. Prevalence of prior cancer among persons newly diagnosed with cancer: An initial report from the surveillance, epidemiology, and end results program. JAMA Oncol (2018) 4(6):832–6. doi: 10.1001/jamaoncol.2017.3605
21. De Luca A, Frusone F, Vergine M, Cocchiara R, La Torre G, Ballesio L, et al. Breast cancer and multiple primary malignant tumors: Case report and review of the literature. In Vivo (2019) 33(4):1313–24. doi: 10.21873/invivo.11605
22. Yamamoto S, Yoshimura K, Ri S, Fujita S, Akasu T, Moriya Y. The risk of multiple primary malignancies with colorectal carcinoma. Dis Colon Rectum (2006) 49(10 Suppl):S30–6. doi: 10.1007/s10350-006-0600-8
23. Ghoussaini M, Pharoah PDP, Easton DF. Inherited genetic susceptibility to breast cancer: the beginning of the end or the end of the beginning? Am J Pathol (2013) 183(4):1038–51. doi: 10.1016/j.ajpath.2013.07.003
24. Bartal AH, Pinsky CM. Malignant melanoma appearing in a post-mastectomy lymphedematous arm: a novel association of double primary tumors. J Surg Oncol (1985) 30(1):16–8. doi: 10.1002/jso.2930300106
25. Kurul S, Akgun Z, Saglam EK, Basaran M, Yucel S, Tuzlali. S, et al. Successful treatment of triple primary tumor. Int J Surg Case Rep (2013) 4(11):1013–6. doi: 10.1016/j.ijscr.2013.08.010
26. Martin M, Sabari JK, Turashvili G, Halpenny DF, Rizvi H, Shapnik N, et al. Next-generation sequencing based detection of germline and somatic alterations in a patient with four metachronous primary tumors. Gynecol Oncol Rep (2018) 24:94–8. doi: 10.1016/j.gore.2018.04.004
27. Nyqvist J, Persson F, Parris TZ, Helou K, Kenne Sarenmalm E, Einbeigi Z, et al. Metachronous and synchronous occurrence of 5 primary malignancies in a female patient between 1997 and 2013: A case report with germline and somatic genetic analysis. Case Rep Oncol (2017) 10(3):1006–12. doi: 10.1159/000484403
28. Padmore RF, Lara JF, Ackerman DJ, Gales T, Sigurdson ER, Ehya H, et al. Primary combined malignant melanoma and ductal carcinoma of the breast. a report of two cases. Cancer (1996) 78(12):2515–25.
29. Petru G, Fuhrmann W, Borkenstein J. [Occurrence of 3 primary carcinomas within 26 months]. Wien Med Wochenschr (1990) 140(8):212–3.
30. Sini G, Colombino M, Lissia A, Maxia S, Gulino M, Paliogiannis P, et al. Primary dermal melanoma in a patient with a history of multiple malignancies: a case report with molecular characterization. Case Rep Dermatol (2013) 5(2):192–7. doi: 10.1159/000354032
31. Williamson CW, Paravati A, Ghassemi M, Lethert K, Hua P, Hartman P, et al. Five simultaneous primary tumors in a single patient: A case report and review of the literature. Case Rep Oncol (2015) 8(3):432–8. doi: 10.1159/000440799
32. Joseph KR, Edirimanne S, Eslick GD. The association between breast cancer and thyroid cancer: a meta-analysis. Breast Cancer Res Treat (2015) 152(1):173–81. doi: 10.1007/s10549-015-3456-6
33. Nielsen SM, White MG, Hong S, Aschebrook-Kilfoy B, Kaplan EL, Angelos P, et al. The breast-thyroid cancer link: A systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev (2016) 25(2):231–8. doi: 10.1158/1055-9965.EPI-15-0833
34. Manole D, Schildknecht B, Gosnell B, Adams E, Derwahl M. Estrogen promotes growth of human thyroid tumor cells by different molecular mechanisms. J Clin Endocrinol Metab (2001) 86(3):1072–7. doi: 10.1210/jc.86.3.1072
35. Saraiva PP, Figueiredo NB, Padovani CR, Brentani MM, Nogueira CR. Profile of thyroid hormones in breast cancer patients. Braz J Med Biol Res (2005) 38(5):761–5. doi: 10.1590/S0100-879X2005000500014
36. Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet (2003) 72(5):1117–30. doi: 10.1086/375033
37. Daly MB, Pilarski R, Axilbund JE, Buys SS, Crawford B, Friedman S, et al. Genetic/familial high-risk assessment: breast and ovarian, version 1.2014. J Natl Compr Canc Netw (2014) 12(9):1326–38. doi: 10.6004/jnccn.2014.0127
38. Hung YP, Liu CJ, Hu YW, Chen MH, Li CP, Yeh CM, et al. Secondary primary malignancy risk in patients with ovarian cancer in Taiwan: A nationwide population-based study. Med (Baltimore) (2015) 94(38):e1626. doi: 10.1097/MD.0000000000001626
39. Lee KD, Chen CY, Huang HJ, Wang TY, Teng D, Huang SH, et al. Increased risk of second primary malignancies following uterine cancer: a population-based study in Taiwan over a 30-year period. BMC Cancer (2015) 15:393. doi: 10.1186/s12885-015-1426-3
40. Borg A, Sandberg T, Nilsson K, Johannsson O, Klinker M, Masback A, et al. High frequency of multiple melanomas and breast and pancreas carcinomas in CDKN2A mutation-positive melanoma families. J Natl Cancer Inst (2000) 92(15):1260–6. doi: 10.1093/jnci/92.15.1260
41. Stedman KE, Moore GE, Morgan RT. Estrogen receptor proteins in diverse human tumors. Arch Surg (1980) 115(3):244–8. doi: 10.1001/archsurg.1980.01380030004002
42. Clegg LX, Reichman ME, Miller BA, Hankey BF, Singh GK, Lin YD, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National longitudinal mortality study. Cancer Causes Control (2009) 20(4):417–35. doi: 10.1007/s10552-008-9256-0
43. Cheng Y, Huang Z, Liao Q, Yu X, Jiang H, He Y, et al. Risk of second primary breast cancer among cancer survivors: Implications for prevention and screening practice. PLoS One (2020) 15(6):e0232800. doi: 10.1371/journal.pone.0232800
44. Kwon WA, Joung JY, Lim J, Oh CM, Jung KW, Kim SH, et al. Risk of second primary cancer among bladder cancer patients: a population-based cohort study in Korea. BMC Cancer (2018) 18(1):617. doi: 10.1186/s12885-018-4530-3
45. Lehtonen HJ, Kiuru M, Ylisaukko-Oja SK, Salovaara R, Herva R, Koivisto PA, et al. Increased risk of cancer in patients with fumarate hydratase germline mutation. J Med Genet (2006) 43(6):523–6. doi: 10.1136/jmg.2005.036400
46. Bluhm EC, Ronckers C, Hayashi RJ, Neglia JP, Mertens AC, Stovall M, et al. Cause-specific mortality and second cancer incidence after non-Hodgkin lymphoma: a report from the childhood cancer survivor study. Blood (2008) 111(8):4014–21. doi: 10.1182/blood-2007-08-106021
47. Pruitt SL, Zhu H, Heitjan DF, Rahimi A, Maddineni B, Tavakkoli A, et al. Survival of women diagnosed with breast cancer and who have survived a previous cancer. Breast Cancer Res Treat (2021) 187(3):853–65. doi: 10.1007/s10549-021-06122-w
48. Williams SA, Ehlers RA. 2nd, Hunt KK, Yi M, Kuerer HM, Singletary SE, et al. Metastases to the breast from nonbreast solid neoplasms: presentation and determinants of survival. Cancer (2007) 110(4):731–7. doi: 10.1002/cncr.22835
49. Lee SK, Kim WW, Kim SH, Hur SM, Kim S, Choi JH, et al. Characteristics of metastasis in the breast from extramammary malignancies. J Surg Oncol (2010) 101(2):137–40. doi: 10.1002/jso.21453
50. Vergier B, Trojani M, Mascarel I, Coindre JM, Treut Le A. Metastases to the breast: differential diagnosis from primary breast carcinoma. J Surg Oncol (1991) 48(2):112–6. doi: 10.1002/jso.2930480208
51. Yeh CN, Lin CH, Chen MF. Clinical and ultrasonographic characteristics of breast metastases from extramammary malignancies. Am Surg (2004) 70(4):287–90. doi: 10.1177/000313480407000402
52. Toombs BD, Kalisher L. Metastatic disease to the breast: clinical, pathologic, and radiographic features. AJR Am J Roentgenol (1977) 129(4):673–6. doi: 10.2214/ajr.129.4.673
Keywords: second primary breast carcinoma, multiple primary malignant tumors, breast metastases, prognosis, survival
Citation: Jing Y, Wang X and Sun B (2023) Clinical characteristics and survival of second primary breast carcinoma with extramammary malignancies. Front. Oncol. 13:1160370. doi: 10.3389/fonc.2023.1160370
Received: 07 February 2023; Accepted: 06 March 2023;
Published: 17 March 2023.
Edited by:
Wenwen Zhang, Nanjing Medical University, ChinaCopyright © 2023 Jing, Wang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaofang Wang, eGlhb2Zhbmd3YW5nMjAwNUAxNjMuY29t; Bei Sun, c3VucGVpMDAzQHNpbmEuY29t
†These authors have contributed equally to this work
 Yaoyao Jing
Yaoyao Jing Xiaofang Wang
Xiaofang Wang Bei Sun
Bei Sun