
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 27 June 2023
Sec. Pediatric Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1153128
This article is part of the Research Topic Advances in the Diagnosis, Molecular Classification, and Treatment of Lymphomas View all 7 articles
 Ahmed Mahdy1*
Ahmed Mahdy1* Asmaa Hamoda1,2
Asmaa Hamoda1,2 Ahmed Zaher3,4
Ahmed Zaher3,4 Eman Khorshed5,6
Eman Khorshed5,6 Madeha Elwakeel7,8
Madeha Elwakeel7,8 Omneya Hassanein9
Omneya Hassanein9 Iman Sidhom1,2*
Iman Sidhom1,2*Background: Pediatric classical Hodgkin lymphoma (CHL) is a curable disease; however, the optimal salvage regimen is unclear for relapsed/refractory (R/R) disease. This study aimed to compare response rates, toxicity, event-free survival (EFS), and overall survival (OS) of ifosfamide, carboplatin, and etoposide (ICE) with gemcitabine and vinorelbine (GV) regimen after first-line doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) in pediatric patients with R/R CHL.
Methods: This is a retrospective cohort study of 132 pediatric patients with R/R CHL treated from July 2012 to December 2020 with ICE (n = 82) or GV (n = 50).
Results: The median age at relapse was 13.9 years, and 68.2% were men. Rates of complete response, partial response, and progressive disease before consolidation were 50.6%, 3.7%, and 45.7% for ICE and 28.5%, 0%, and 71.5% for GV (P = 0.011). By multivariate analysis, regimen (P = 0.002), time to relapse (P = 0.0001), and B-symptoms (P = 0.002) were independent factors to lower response rates. Hematological toxicity, electrolyte disturbance, hemorrhagic cystitis, infectious complications, and hospital admission for fever neutropenia were statistically significant higher for the ICE regimen. Treatment-related mortalities were 2.4% for ICE and 2% for GV (P = 0.86). The 3-year EFS was 39.3% ± 11.4% for ICE and 24.9% ± 12.5% for GV (P = 0.0001), while 3-year OS was 69.3% ± 10.6% and 74% ± 12.9% (P = 0.3), respectively. By multivariate analysis, regimen (P = 0.0001), time to relapse (P = 0.011), B-symptoms (P = 0.001), and leukocytosis (P = 0.007) were significant for EFS, while anemia (P = 0.008), and progressive disease on early response evaluation (P = 0.022) were significant for OS.
Conclusions: The ICE regimen had a better overall response rate and EFS, but higher toxicity, than GV; however, OS and mortality were similar.
Relapsed or refractory (R/R) classical Hodgkin lymphoma (CHL) remains a clinical and therapeutic challenge. Approximately 10%–30% of patients relapse after first-line chemotherapy (1–3). The possibility of a cure after relapse depends on several prognostic factors, including duration of initial remission, stage, B-symptoms, and response to standard dose salvage chemotherapy (SDCT) (4–8). However, the duration of initial remission and response to pre-transplant SDCT remains the most important prognostic factors (7–11). The addition of brentuximab vedotin to upfront standard chemotherapy for newly diagnosed high-risk patients or those with R/R disease resulted in higher efficacy, without an increase in toxicity (12–14). Also, the combinations of brentuximab vedotin plus nivolumab, pembrolizumab, or nivolumab with conventional chemotherapy resulted in higher complete response rates and successful bridging to autologous stem cell transplant (ASCT) (15–17). However, the optimal pre-transplant SDCT for R/R CHL patients is unclear and needs more studies, as there are few studies in children addressing the question of what is the ‘best’ SDCT in terms of high efficacy and low toxicity (18). Therefore, we aimed to compare the outcome and short-term toxicities of an ifosfamide, carboplatin, and etoposide (ICE) regimen, with gemcitabine and vinorelbine (GV) in patients with R/R CHL.
This retrospective study included all pediatric patients, <18 years at initial diagnosis, with R/R CHL who received SDCT either an ICE or GV regimen after the first-line doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) in the pediatric oncology department, Children Cancer Hospital Egypt, from July 2012 to December 2020. All patients were followed until December 2021.
Patients with nodular lymphocyte–predominant HL (19) and patients with immunodeficiency were excluded as they were treated initially on different protocols other than the ABVD regimen. All the information concerning clinicopathological characteristics, time to relapse, response evaluations, toxicities, and survival were collected from the electronic medical records. The study was approved by the institutional review board.
The ABVD regimen was administered intravenous (IV) on days 1 and 15 as follows: vinblastine, 6 mg/m2; bleomycin, 10 international unit/m²; doxorubicin, 25 mg/m2; and dacarbazine, 375 mg/m2.
A total of 82 and 50 patients received ICE and GV regimens, respectively. ICE was the second-line SDCT for all relapsed or refractory cases from July 2012 to September 2017; then, the salvage was changed to the GV regimen. Treatment courses are repeated every 3–4 weeks upon hematological recovery for at least two courses in the absence of disease progression. The ICE regimen was administered as follows: ifosfamide, 1,800 mg/m2/day with an equal dose of MESNA (2-mercaptoethane sulfonate), IV on days 1–5; carboplatin 450 mg/m2/day IV on day 1; and etoposide, 100 mg/m2/day IV on days 1–5. The GV regimen was administered IV on days 1 and 8 as follows: vinorelbine, 25 mg/m2; and gemcitabine 1000 mg/m2. CMV (carboplatin, melphalan, and etoposide was the preparative regimen before ASCT before February 2017 and then changed to bendamustine, etoposide, cytarabine, and melphalan (BEAM) thereafter.
Based on time to relapse after ABVD therapy, patients were categorized into three groups; refractory defined as disease progression or relapse within 3 months after ABVD therapy, early relapse as a CR lasting for ≥3 months but <12 months from ABVD therapy, and late relapse as CR ≥12 months (1) (7) (20). Relapse was documented by tissue biopsy in 115 (87%) patients. In emergency situations necessitating urgent initiation of chemotherapy, or failure to document by tissue biopsy, unequivocal progressive or new radiological lesions were accepted as proof of relapse in the absence of another possible explanation.
All patients were staged according to the Ann Arbor staging system and underwent fluorodeoxyglucose positron emission tomography (FDG-PET) scan and bilateral bone marrow biopsies (BMB).
Patients with fevers ≥ (38.3°C), drenching night sweats, or unexplained weight loss > 10% of body mass (21) are defined to have B symptoms.
A single nodal mass of 10 cm or ≥⅓ of the transthoracic diameter at any level of thoracic vertebrae, recording the longest measurement by computed tomography (CT) scan (21).
Response to SDCT was decided based on PET/CT scans and BMB, if positive at relapse, after two to three courses (early response), fourth course, and before consolidation as an overall response rate (ORR) according to the standard response definition criteria by the Lugano Classification 2014 (21) using the 5-point scale and are defined as achieving a complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). Patients with PR or SD continued to receive SDCT for a maximum of six cycles or till consolidation with ASCT or radiotherapy, which comes earlier. Patients with PD at any time were off protocol study.
Grade III–IV acute toxicities and toxic death with a time frame of up to 4 weeks following each course of chemotherapy were collected and compared between both regimens according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v5.0 (22).
A spectrum of prognostic factors at relapse were evaluated for significance to the second-line ORR, event-free survival (EFS), and overall survival (OS) including the following; sex, age (<13 vs. ≥13 years), stage, histology, B-symptoms, bulky disease, leukocyte count (<13.5 vs. ≥13.5 × 103/mm3), hemoglobin (<10.5 vs. ≥10.5 gm/dl), erythrocyte sedimentation rate (ESR; <50 vs. ≥50 mm/h), lactate dehydrogenase (LDH; <700 vs. ≥700 IntUnit/L), albumin (<3.5 vs. ≥3.5 gm/dl), radiotherapy during first line, time to relapse, early response, and overall response. Cutoff values used to categorize these factors were based on values found to be predictive of outcome in previous studies (9) (23) (24).
The endpoints analyzed were early response after two to three cycles, ORR, EFS, and OS. EFS was calculated from the date of start of SDCT until the date of progression, death, or last follow-up, and OS from the date of diagnosis until the date of death or last follow-up. EFS and OS were estimated using the Kaplan–Meier method (25). Univariate analyses of clinicopathological characteristics were performed to select covariates for multivariate analyses. The influence of these factors on the OS and EFS was estimated by Cox proportional hazards regression (26). The hazard ratio was calculated for each variable with 95% confidence intervals (95% CIs). All factors with a P-value ≤ 0.10 in univariate analysis were subject to stepwise Cox regression analysis. Effect of factors on the ORR was estimated using logistic regression. Numerical values were summarized using median and range. Categorical variables expressed as the number of cases and percentages, and groups were compared using the chi-square or Fisher’s exact test as appropriate. Analyses were performed using BM-SPSS Statistics 25.
As shown in Table 1, the median age at relapse or progression was 13.9 years (range, 3–24 years) and 68.2% were men. Clinicopathological characteristics were quite similar in the two protocols, except 47.6% of the ICE group did not receive radiotherapy versus 30% of the GV (P = 0.047). Furthermore, there was a trend of higher lung involvement in the ICE (29.3%), versus the GV group (16%), but it did not reach statistical significance (P = 0.06).
The median number of the ICE chemotherapy cycles was 4.74 (range, 2–6 cycles), and 4.7 cycles (range, 2–6 cycles) for the GV group. The early response could be evaluated in 131 (99.2%) and ORR before consolidation in 130 (98.5%) patients, as one died after the second-cycle ICE and one after the fourth-cycle GV. On early response evaluation, 54.3% of the ICE group achieved CR, 40.7% had PR, and 5% had PD versus 34%, 50%, and 16%, of the GV group, respectively (P = 0.024). Rates of CR, PR, and PD before consolidation were 50.6%, 3.7%, and 45.7%, respectively, for ICE and 28.5%, 0%, and 71.5%, respectively for GV (P = 0.011). There were 18 patients who achieved CR while receiving the ICE regimen but lost response and had PD. There were 12 of them who were found to have PD on routine evaluation after chemotherapy cycles and six patients on routine PET/CT assessment before ASCT with a mean of 127 days between the last ICE cycle and pre-transplant evaluation due to the waiting list for transplant. Eight patients of the GV regimen lost response and had PD after achieving CR. Six of them had PD on routine evaluation after chemotherapy cycles, and two patients had PD in pre-transplant assessment with a mean of 86.5 days between the last GV cycle and pre-transplant evaluation. Due to the higher remission status achieved by the ICE regimen, 46.3% of patients underwent ASCT as consolidation therapy after ICE chemotherapy versus 26% of the GV patients (P = 0.02).
As demonstrated in Table 2, by univariate analysis, the following factors were statistically significant for lower ORR before consolidation; the GV regimen (P = 0.004), female sex (P = 0.04), age ≥ 13 years (P = 0.01), refractory disease (P = 0.0001) on ABVD, higher stage (P = 0.01), lung involvement (P = 0.005), liver involvement (P = 0.01), B-symptoms (P=0.001), partial response on early evaluation, ESR ≥ 50, mm/h (P = 0.0001), hemoglobin < 10.5 gm/dl (P = 0.02), and leukocytes ≥ 13.5 × 10(3)/mcl (P = 0.01). By multivariate analysis, only regimen (P = 0.002), time to relapse (P = 0.002), and B-symptoms (P = 0.002) were statistically significant.
Consolidation therapy with ASCT or radiotherapy for responding patients and their outcome are shown in (Figure 1). For responding patients to ICE chemotherapy, the conditioning regimens used before stem cell rescue were CMV for 25 and BEAM for 13 patients, while 13 patients received BEAM, in the GV group. A total of 76% and 77% of patients maintained their CR after ASCT for the ICE, respectively, after CMV and BEAM versus 92% for the GV who received the BEAM preparative regimen.
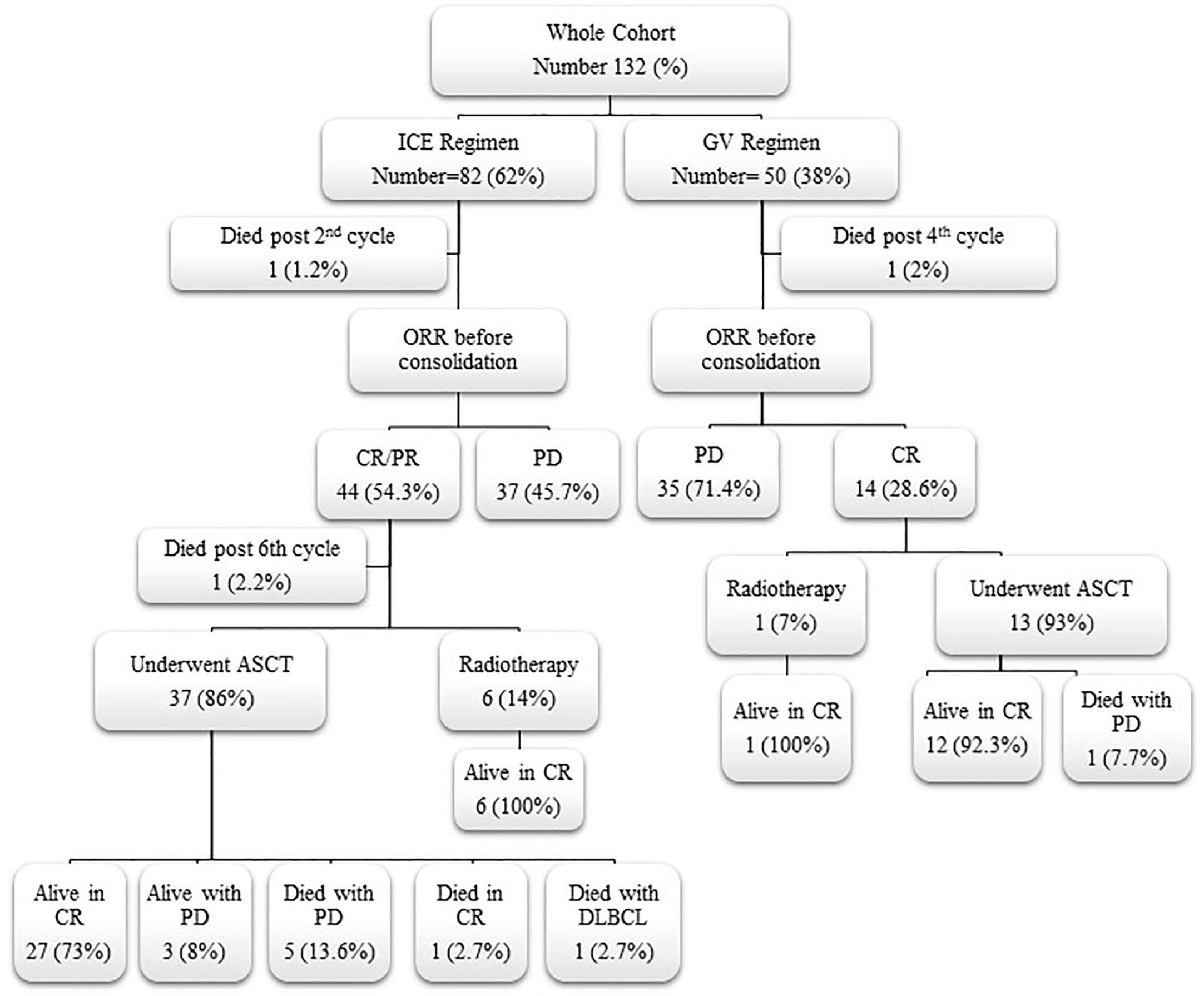
Figure 1 Flow chart for responding patients to second-line chemotherapy and their outcome. ICE, ifosfamide, carboplatin, and etoposide; GV, gemcitabine and vinorelbine; ORR, overall response rate; CR, complete response; PR, partial response; PD, progressive disease; ASCT, autologous stem cell transplantation; DLBCL, diffuse large B-cell lymphoma.
Thirty-five (70%) of the GV patients required salvage third-line chemotherapy after failure of the GV regimen versus 35 (42.7%) of the ICE regimen (P = 0.002). However, 34/35 (97%) patients of the GV group received the ICE regimen as a third line, and 15 of 32 evaluable patients achieved CR (46.9%). On the other hand, of the 35 patients who failed the ICE regimen, 27 of them received the GV as a salvage third-line chemotherapy, but only eight of them attained CR (29.6%). Again, it proves the superiority of the ICE regimen as salvage chemotherapy even as the third line in R/R HL patients. Eight patients of the ICE group received brentuximab vedotin with or without bendamustine during third or fourth lines, but only 2 patients responded versus 12 patients in the GV group with 50% of them achieving CR. Twenty-one (65.6%) patients of the GV group underwent ASCT post third, fourth, or fifth lines of chemotherapy versus 10 (30.3%) of the ICE group (P = 0.004). Detailed salvage chemotherapy regimens after second-line failure for both groups, response rates, ASCT, and survival are demonstrated in Table 3 and Figure 2.
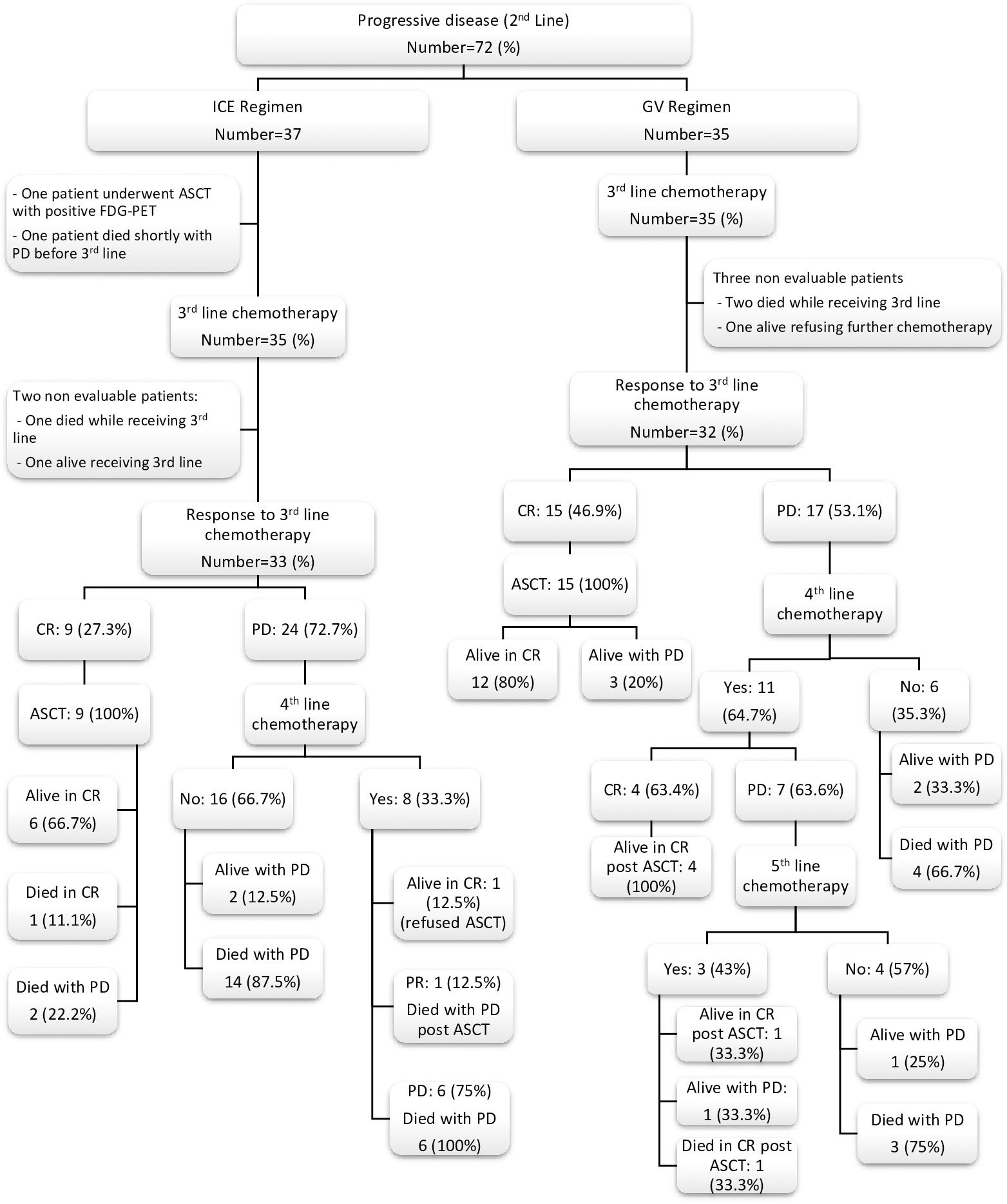
Figure 2 Flow chart for non-responding patients to second-line chemotherapy and their outcome after further salvage chemotherapy regimens. ICE, ifosfamide, carboplatin and etoposide; GV, gemcitabine and vinorelbine; CR, complete response; PR, partial response; PD, progressive disease; ASCT, autologous stem cell transplantation; FDG-PET, fluorodeoxyglucose (FDG)-positron emission tomography.
For responding patients to further salvage regimens after ICE failure, the conditioning regimens used prior to stem cell rescue were CMV for six and BEAM for four patients. There were 21 patients who received BEAM, as conditioning regimens before ASCT for responding patients after GV failure. A total of 67% and 60% of patients maintained their CR after ASCT for the ICE, respectively, after CMV and BEAM versus 90% for the GV who received the BEAM preparative regimen.
The main acute toxicities of the ICE regimen were hematological. Of the 389 ICE cycles given, grade 3/4 neutropenia were documented in 96.6%, grade 3/4 thrombocytopenia in 82%, and grade 3 anemia in 69.4% of all cycles. Sixty percent of patients required platelet transfusions and 61.3% red blood cell (RBC) transfusions. Hospital admissions for supportive care with fever neutropenia (FN) and/or hemorrhagic cystitis were required in 54.2% of cycles. Clinically documented infections (CDIs), bloodstream infection (BSI), and septic shock were reported in 17.5%, 4.8%, and 5.6% of ICE cycles, respectively; however, no patients were removed from the ICE regimen on account of persistent toxicities. Only two (0.26%) treatment-related deaths were reported. One died of a diffuse alveolar hemorrhage after the second cycle and one of sepsis after the sixth cycle.
The GV was well tolerated with less acute toxicity compared to the ICE regimen. Of the 235 GV cycles given, grade 3/4 neutropenia was documented in 18.7%, grade 3/4 thrombocytopenia in 4.6%, and grade 3 anemia in 8% of all courses. Only 5.1% of patients required RBC transfusions and 0.8% platelet transfusions. Each of the hospital admissions with FN and CDI were reported in 2.1% of cycles. Grade 3–4 electrolytes disturbance, BSI, and septic shock were not reported. Only one treatment-related death was documented with pneumonia and respiratory failure after the fourth cycle. Seizures, cardiac, and hepatic toxicities were low and not statistically different between both groups. A comparison of the main toxicities between both regimens is summarized in Table 4.
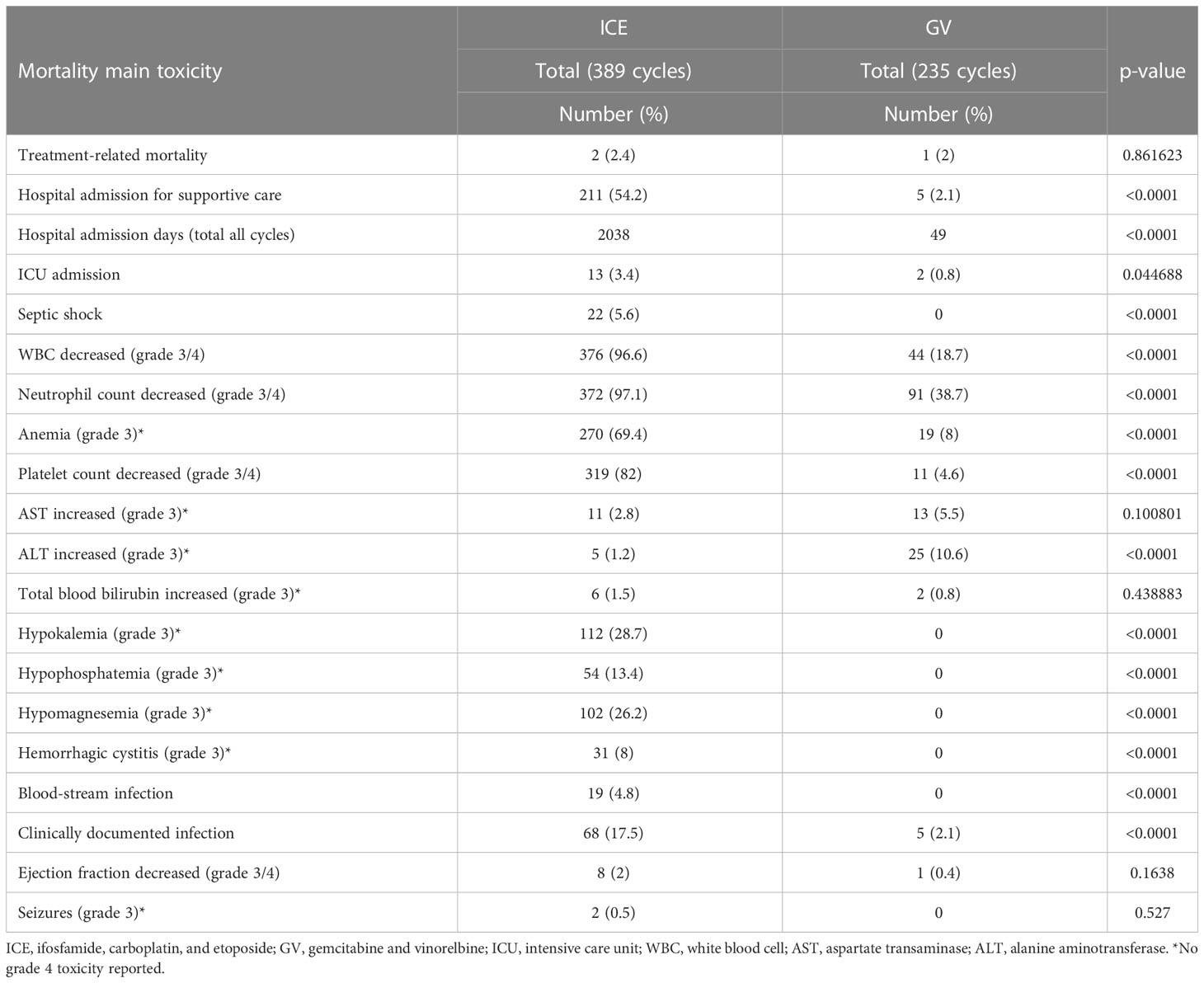
Table 4 Comparison of mortalities and main toxicities between both regimens according to the Common Terminology Criteria for Adverse Events Version 5.0.
The median follow-up time was 3.8 years (range, 0.1–8.8 years) for the ICE group and 2.6 years (range, 0.4–5.3 years) for the GV group. As shown in (Figures 3, 4) and Table 5, the 3-year EFS was 39.3% ± 11.4% for the ICE regimen versus 24.9% ± 12.5% for the GV (P = 0.0001).
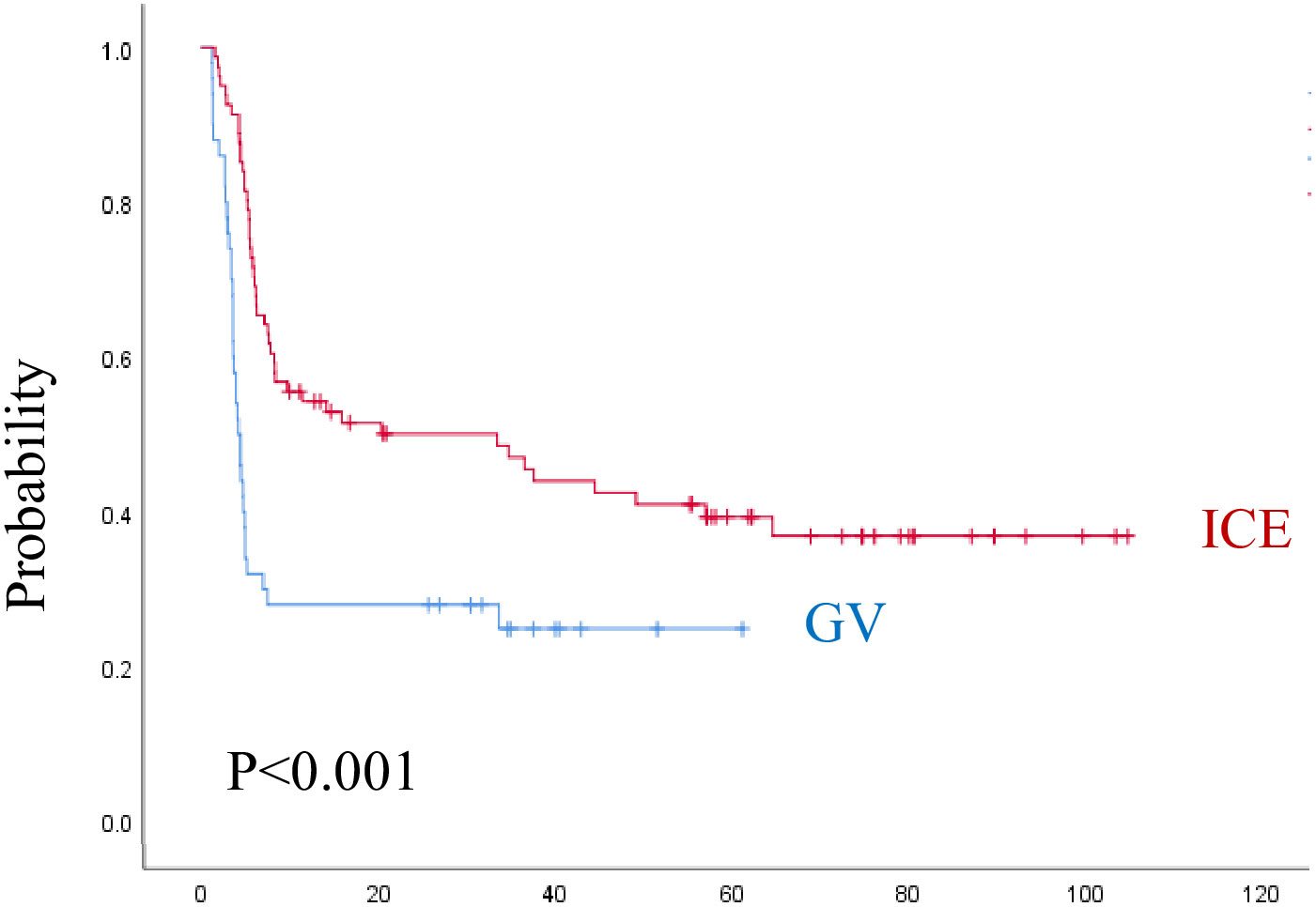
Figure 3 Event-free survival for both regimens. ICE, ifosfamide, carboplatin, and etoposide; GV, gemcitabine and vinorelbine.
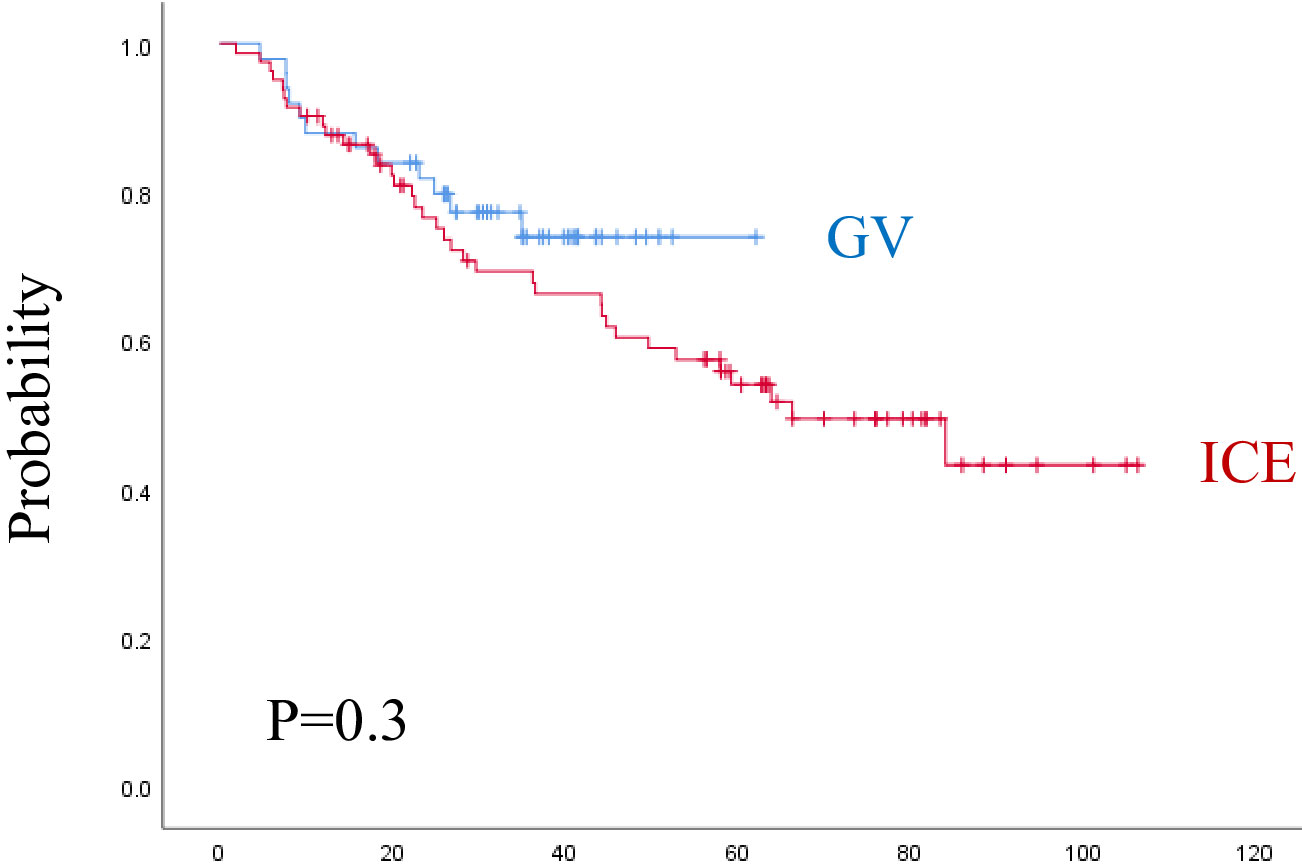
Figure 4 Overall survival for both regimens. ICE, ifosfamide, carboplatin, and etoposide; G: gemcitabine and vinorelbine.
When analyzing the prognostic factors by univariate analysis, regimen (P = 0.001), female sex (P = 0.03), age ≥ 13 years (P = 0.01), refractory disease during first line (P = 0.001), higher-stage (P = 0.008), liver involvement (P = 0.02), lung involvement (P = 0.001), B-symptoms (P = 0.001), ESR ≥ 50 mm/h (P = 0.001), leukocyte count ≥ 13.5 × 10(3)/mcl (P = 0.002), Hemoglobin < 10 gm/dl (P = 0.004), PD or PR on early evaluation (P = 0.0001) were statistically significant associated with lower EFS. By multivariate analysis, regimen (P = 0.0001), time to relapse (P = 0.011), B-symptoms (P = 0.001), leukocyte count ≥ 13.5 × 10(3)/mcl (P = 0.007), and radiotherapy during the first line (P = 0.025), were dominating over all other parameters. The 3-year OS was 69.3% ± 10.6% for the ICE regimen and 74% ± 12.9% for the GV (P = 0.3). The following factors were statistically significant for lower OS by univariate analysis, including, female sex (P = 0.02), RD during first-line (P = 0.001), higher-stage (P = 0.02), liver involvement (P = 0.004), lung involvement (P = 0.001), B-symptoms (P = 0.03), ESR ≥ 50 mm/h (P = 0.01), leukocyte count ≥ 13.5 × 10(3)/mcl (P = 0.002), hemoglobin < 10 gm/dl (P = 0.002), PD or PR at early evaluation (P = 0.0001), and PD before consolidation as ORR (P = 0.001). By multivariate analysis, only anemia (P = 0.008), and progressive disease on early response evaluation (P = 0.022) remain significant for OS.
The 3-year EFS for patients who underwent ASCT after second-line ICE was 79% ± 13.9% and 88.9% ± 20.6% after the second-line GV, while the 3-year EFS for patients who underwent ASCT after multiple lines in the ICE group was 71.4 ± 33.5% versus 70.5 ± 25.9% in the GV group.
Our report is a single-center retrospective study on relapsed or refractory pediatric CHL after the ABVD regimen who received either the GV or the ICE regimen. Apart from fewer patients numbers in the GV group (n = 50) compared to the ICE (n = 82), both groups had relatively comparable clinicopathological characteristics and received two uniform salvage regimens; thus, it was possible to compare efficacy and outcome because of the homogeneity of patients’ characteristics and previous treatment received by both groups.
Our whole cohort revealed a male-to-female ratio of 2.2:1; this male predominance and other patients and disease characteristics at relapse were reported in many other studies (27–29). The percentage of patients with first treatment failure whether refractory or early and late relapse was also comparable to what was reported in the literature (7) (9).
In the current study, the CR as the ORR in the GV regimen was 28.5%, which compares favorably with the COG phase II study of GV for children with R/R HL that reported a CR rate of 24% (30). Our results also documented the efficacy of the ICE regimen as 50.6% achieved CR, and 3.7% had PR, with an ORR of 54.3% at the time of consolidation. The published pediatric literature reported on the ICE response as a salvage regimen for pediatric R/R CHL are few; however, it was 33% as an ORR (CR + PR) in a study reported by Metzger et al. (9), and from 60% to 88% in other studies. These variations potentially were biased by the intensity of frontline therapy, timing of relapse, scheduling, and doses of ICE therapy, tools used in response assessment, and inclusion of adult patients in some studies (31) (32). In our results, it is obvious that the CR status at early evaluation and the ORR before consolidation were superior for the ICE regimen in comparison to the GV. Many factors were found to be significant for response by univariate analysis; however, aside from regimen, time to relapse and B-symptoms were significant in multivariate analysis. All these factors suggest that the response to salvage chemotherapy is highly influenced by the specific prognostic factors and tumor biology of CHL.
In the current study, the ICE regimen had a superior 3-year EFS in comparison to the GV; however, the 3-year OS was similar. Survival outcomes reported here were favorably similar to other children and adolescents series with R/R CHL (3) (9) (10) (33–35).
The duration of the first remission had a significant impact on the response and outcome. Children with refractory disease to the first line had significantly lower response rates when compared to those who relapsed later. In our analysis, only 10.3% of the patients with refractory disease achieved CR to SDCT, compared to 50% and 55.8% for those with early and late relapse, respectively. Also, the 3-year EFS was 6.5% ± 8.6%, 40.7% ± 20%, and 51% ± 11.6%, for refractory, early, and late relapsed patients, respectively (P = 0.011), confirming the poor response and survival for patients with refractory disease. This finding is in agreement with previous pediatric studies with relapsed HL indicating the prognostic significance of the length of time between primary diagnosis and treatment failure (7) (10) (30) (34).
Achieving complete metabolic remission (CMR) with a Deauville score of 1–3 on salvage SDCT is highly predictive for outcome. The best time point for FDG-PET response assessment is not clearly defined, but is it generally done after two cycles of SDCT. Failure to demonstrate an improvement (less than CR/PR) should prompt consideration of a switch to an alternative regimen. A third cycle may be considered in slow responders (PR) to try to achieve CR (36). In contrast, the EuroNet Pediatric Hodgkin Lymphoma Group recommends switching to second-line SDCT if failed to achieve CMR after two cycles of salvage chemotherapy (1). Similarly, in our analysis, only 26.3% of patients who had PR on early evaluation could achieve CR before consolidation. Furthermore, the 3-year EFS was 62.7% ± 12.5% of patients who achieved CR on early evaluation versus 21.9% ± 11%, who had PR and the 3-year OS was 94% ± 6.7% for patients who achieved CR/PR at early evaluation vs. 56% ± 12%, for PD. Obviously, patients with recurrent disease that is unresponsive to conventional SDCT have a poor prognosis.
Many other poor prognostic factors at relapse were reported in our study as well as others such as B-symptoms, female sex, older age, higher-stage, elevated ESR, leukocytes, and anemia (1) (7) (24) (37–39), (40), (41).
In the current study, patients who did not receive radiotherapy during first line had a lower response rate, and inferior EFS and OS, but this was confounded by the fact that patients who did not receive radiotherapy had worse prognostic factors as a higher percentage had PD during first line (42.5%), B-symptoms (57.6%), ESR ≥50, mm/h (70%), and hemoglobin <10.5 gm/dl (13%) versus (9%), (44%), (53.6%), and (6.4%), respectively, for patients who received radiotherapy.
Of interest in our study, despite the better response rates to the ICE, versus the GV regimen, the 3-year OS was not statistically significant between both regimens because a higher number of patients in the GV group could be salvaged and further achieved CR (46.9%) on third lines when compared to the ICE group (27.3%). Furthermore, patients who failed the third line in the GV group received further fourth and fifth lines more than the ICE group with a higher percentage of them who underwent ASCT (P = 0.004). Also, the time era was earlier when the ICE was given compared to the GV and the follow-up time for the ICE group was lengthier (3.8 years) than the GV (2.6 years). The efficacy of immune and targeted therapy for R/R patients is well documented (14); however, in our analysis, the use of brentuximab vedotin as a single agent or in combination was more commonly used for subsequent relapse in recent years with an overall CR rate of 40%.
Our study is not designed to test the efficacy of the condition pretransplant preparative regimen; however, in our whole cohort of both groups, 51 patients received BEAM, and 31 received the CMV regimen before stem cell rescue. Forty-three (84%) maintained their CR after ASCT after BEAM versus (74%) after the CMV preparative regimen. This could also explain the higher OS rates for the GV group; however, it should be noticed that the BEAM regimen was used recently and longer follow-up is needed to evaluate the remission status after ASCT as the relatively slow-progressing nature of HL warrants a much longer follow-up.
The GV was well tolerated with fewer toxicities as compared to the ICE regimen. Myelosuppression, grade III-IV hematological toxicities, transfusion support, infectious complications, grade III electrolytes disturbance secondary to ifosfamide, carboplatin renal affection, and hemorrhagic cystitis were significantly higher in the ICE group. Due to the predominance of all these toxicities, the hospital admissions for supportive care mainly with FN were higher in the ICE being 54.2% compared with 2.1% of the GV cycles, adding much burden and cost to the healthcare providers.
The hepatotoxicity was not significantly different between both regimens except for grade III elevation of alanine aminotransferase, which was higher in the GV regimen; however, in all patients, it was transient and no delay or modification of chemotherapy was needed.
The reported pediatric mortality when reviewing the published literature from salvage therapy is low, as expected in the young age of the patients and lack of comorbidities. In our study, the treatment-related mortality was low in both groups and not significant when comparing both regimens.
Although the ICE regimen has higher CR rates in comparison to the GV, it is not optimal since it is associated with an increased risk of short- and long-term toxicities as treatment-related secondary malignant neoplasm associated with the use of alkylators and epipodophyllotoxins (42).
The retrospective nature of our study, some missing data, shorter follow-up duration in the GV group, and not addressing late toxicities are considered limitations in our study.
Finally, despite the very low toxicity profile of GV, it is not recommended due to the low response rate. Based on our results, a chemotherapy combination with higher efficacy than the GV regimen and lower toxicity than ICE is needed. Further studies on the alternative salvage second line, such as ifosfamide, gemcitabine, vinorelbine (IGEV), and prednisone (IGEV), for pediatric patients with relapsed or refractory CHL, is recommended.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional review board of National cancer institute, Cairo university, Egypt, Organization No. IORG0003381 IRP No. IRB00004025, Study ID. PO1910-30702, IRB Review. 20 October 2019, IRB Review type. Full Board Review, IRB Review action. Approved, IRB Review No. 201920009.3. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
AM did the data collection, wrote the primary draft, and revised the final draft with the authors. AH was involved in planning and supervision and aided in interpreting the results and worked on the manuscript, ME and AZ revised PET/CT scans for staging and response evaluation, EK revised pathology data, OH performed data analysis and statistical work. IS contributed to the drafting, interpreting the results, and critical revision and helped in the writing of the manuscript. All authors discussed the results and approved the final manuscript.
The Abstract has been accepted for POSTER VIEWING at the 54th Congress of the International Society of Paediatric Oncology (SIOP 2022), held in Barcelona, Spain on September 28 - October 1, 2022.
Abstract Number: 758
Abstract Title: GEMCITABINE AND VINORELBINE (GV) VERSUS IFOSFAMIDE, CARBOPLATIN, ETOPOSIDE (ICE) REGIMEN IN TREATING PEDIATRIC PATIENTS WITH RECURRENT OR PROGRESSIVE HODGKIN’S LYMPHOMA
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; ALT, alanine aminotransferase; ASCT, autologous stem cell transplant; AST, aspartate transaminase; BEAM, bendamustine, etoposide, cytarabine, and melphalan; BMB, bone marrow biopsy; BSI, blood stream infection; CDI, clinically documented infections; CHL, classical Hodgkin lymphoma; COG, Children’s Oncology Group; CMR, complete metabolic remission; CMV, carboplatin, melphalan, etoposide; CR, complete response; CT, computed tomography; CTCAE, Common Terminology Criteria For Adverse Events; DLBCL, diffuse large B-cell lymphoma; EFS, event-free survival; ESR, erythrocyte sedimentation rate; FDG/PET, fluorodeoxyglucose positron emission tomography; GV, gemcitabine and vinorelbine; ICE, ifosfamide, carboplatin, etoposide; ICU, intensive care unit; IGEV, ifosfamide, gemcitabine, vinorelbine, prednisone; IV, intravenous; LDH, lactate dehydrogenase; MESNA, 2-mercapto ethane sulfonate; NS, not significant; ORR, overall response rate; OS, overall survival; PD, progressive disease; PR, partial response; R/R, relapsed or refractory; RBC, red blood cell; RD, refractory disease; SD, stable disease; SDCT, standard-dose chemotherapy; WBC, white blood cells.
1. Daw S, Hasenclever D, Mascarin M, Fernández-Teijeiro A, Balwierz W, Beishuizen A, et al. Risk and response adapted treatment guidelines for managing first relapsed and refractory classical hodgkin lymphoma in children and young people. Recommendations from the EuroNet pediatric hodgkin lymphoma group. HemaSphere (2020) 4(1):e329. doi: 10.1097/HS9.0000000000000329
2. Smith CM, Friedman DL. Advances in Hodgkin lymphoma: including the patient’s voice. Front Oncol (2022) 12(February):1–8. doi: 10.3389/fonc.2022.855725
3. Garaventa A, Parodi S, Guerrini G, Farruggia P, Sala A, Pillon M, et al. Outcome of children and adolescents with recurrent classical Hodgkin lymphoma: the Italian experience. Cancers (Basel). (2022) 14(6):1–16. doi: 10.3390/cancers14061471
4. Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive hodgkin’s disease: a randomised trial. Lancet (2002) 359(9323):2065–71. doi: 10.1016/S0140-6736(02)08938-9
5. Magagnoli M, Balzarotti M, Castagna L, Demarco M, Santoro A. What is the best option to cure patients with Resistant/Relapsing hodgkins disease? Curr Stem Cell Res Ther (2012) 1(3):419–24. doi: 10.2174/157488806778226786
6. Bröckelmann PJ, von Tresckow B. Risk stratification and prognostic biomarkers in relapsed Hodgkin lymphoma. Br J Haematol (2020) 190(6):813–4. doi: 10.1111/bjh.16844
7. Schellong G, Dörffel W, Claviez A, Körholz D, Mann G, Scheel-Walter HG, et al. Salvage therapy of progressive and recurrent hodgkin’s disease: results from a multicenter study of the pediatric DAL/GPOH-HD study group. J Clin Oncol (2005) 23(25):6181–9. doi: 10.1200/JCO.2005.07.930
8. Friedmann AM, Wolfson JA, Hudson MM, Weinstein HJ, Link MP, Billett A, et al. Relapse after treatment of pediatric hodgkin lymphoma: outcome and role of surveillance after end of therapy. Pediatr Blood Cancer (2013) 60(9):1458–63. doi: 10.1002/pbc.24568
9. Metzger ML, Hudson MM, Krasin MJ, Wu J, Kaste SC, Kun LE, et al. Initial response to salvage therapy determines prognosis in relapsed pediatric hodgkin lymphoma patients. Cancer (2010) 116(18):4376–84. doi: 10.1002/cncr.25225
10. Gorde-Grosjean S, Oberlin O, Leblanc T, Pacquement H, Donadieu J, Lambilliotte A, et al. Outcome of children and adolescents with recurrent/refractory classical Hodgkin lymphoma, a study from the société française de lutte contre le cancer des enfants et des adolescents (SFCE). Br J Haematol (2012) 158(5):649–56. doi: 10.1111/j.1365-2141.2012.09199.x
11. Claviez A, Kabisch H, Suttorp M, Peters C, Hero B, Schiller I, et al. The impact of disease status at transplant and time to first relapse on outcome in children and adolescents with hodgkin’s lymphoma undergoing autologous stem cell transplantation. Blood (2004) 104(11). doi: 10.1182/blood.V104.11.1878.1878
12. Castellino SM, Pei Q, Parsons SK, Hodgson D, McCarten K, Horton T, et al. Brentuximab vedotin with chemotherapy in pediatric high-risk hodgkin’s lymphoma. N Engl J Med (2022) 387(18):1649–60. doi: 10.1056/NEJMoa2206660
13. Castellino SM, Pei Q, Parsons SK, Hodgson DC, McCarten K, Punnett A, et al. Brentuximab vedotin and association with event-free survival (EFS) in children with newly diagnosed high-risk Hodgkin lymphoma (HL): a report from the children’s oncology group phase 3 study AHOD1331 (NCT 02166463). J Clin Oncol (2022) 40(16_suppl):7504–7504. doi: 10.1200/JCO.2022.40.16_suppl.7504
14. Massano D, Carraro E, Mussolin L, Buffardi S, Barat V, Zama D, et al. Brentuximab vedotin in the treatment of paediatric patients with relapsed or refractory hodgkin’s lymphoma: results of a real-life study. Pediatr Blood Cancer (2022) 69(10):e29801. doi: 10.1002/pbc.29801
15. Moskowitz AJ, Schöder H, Yahalom J, McCall SJ, Fox SY, Gerecitano J, et al. PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosamide, carboplatin, and etoposide for patients with relapsed and refractory hodgkin’s lymphoma: a non-randomised, open-label, single-centre, phase 2 study. Lancet Oncol (2015) 16(3):284–92. doi: 10.1016/S1470-2045(15)70013-6
16. Herrera AF, Moskowitz AJ, Bartlett NL, Vose JM, Ramchandren R, Feldman TA, et al. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood (2018) 131(11):1183–94. doi: 10.1182/blood-2017-10-811224
17. Moskowitz AJ, Shah G, Schöder H, Ganesan N, Drill E, Hancock H, et al. Phase II trial of pembrolizumab plus gemcitabine, vinorelbine, and liposomal doxorubicin as second-line therapy for relapsed or refractory classical Hodgkin lymphoma. J Clin Oncol (2021) 39(28):3109–17. doi: 10.1200/JCO.21.01056
18. Daw S, Wynn R, Wallace H. Management of relapsed and refractory classical Hodgkin lymphoma in children and adolescents. Br J Haematol (2011) 152(3):249–60. doi: 10.1111/j.1365-2141.2010.08455.x
19. Swerdlow SH, Campo E, Pileri SA, Lee Harris N, Stein H, Siebert R, et al. The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood (2016) 127(20):2375–90. doi: 10.1182/blood-2016-01-643569
20. Josting A, Rueffer U, Franklin J, Sieber M, Diehl V, Engert A. Prognostic factors and treatment outcome in primary progressive Hodgkin lymphoma: a report from the German Hodgkin lymphoma study group. Blood (2000) 96(4):1280–6. doi: 10.1182/blood.V96.4.1280.h8001280_1280_1286
21. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of hodgkin and non-hodgkin lymphoma: the lugano classification. J Clin Oncol (2014) 32(27):3059–67. doi: 10.1200/JCO.2013.54.8800
22. National Cancer Institute. Common terminology criteria for adverse events (CTCAE) common terminology criteria for adverse events (CTCAE) v5.0. National Cancer Institute (2017).
23. Smith RS, Chen Q, Hudson MM, Link MP, Kun L, Weinstein H, et al. Prognostic factors for children with hodgkin’s disease treated with combined-modality therapy. J Clin Oncol (2003) 21(10):2026–33. doi: 10.1200/JCO.2003.07.124
24. Josting A, Franklin J, May M, Koch P, Beykirch MK, Heinz J, et al. New prognostic score based on treatment outcome of patients with relapsed hodgkin’s lymphoma registered in the database of the German hodgkin’s lymphoma study group. J Clin Oncol (2002) 20(1):221–30. doi: 10.1200/JCO.2002.20.1.221
25. Stel VS, Dekker FW, Tripepi G, Zoccali C, Jager KJ. Survival analysis I: the Kaplan-Meier method. Nephron - Clin Pract (2011) 119(1):c83–8. doi: 10.1159/000324758
26. Bender R, Augustin T, Blettner M. Generating survival times to simulate cox proportional hazards models. Stat Med (2005) 24(11):1713–23. doi: 10.1002/sim.2059
27. Kelly KM. Hodgkin Lymphoma in children and adolescents: improving the therapeutic index. Hematol (United States). (2015) 2015(1):514–21. doi: 10.1182/blood-2015-07-641035
28. Alkhayat N, Alshahrani M, Elyamany G, Sedick Q, Ibrahim W, Hamzi H, et al. Clinicopathologic features and therapy outcome in childhood hodgkin’s lymphoma: a report from tertiary care center in Saudi Arabia. J Egypt Natl Canc Inst (2021) 33(1):21. doi: 10.1186/s43046-021-00078-0
29. Aquino S, Clavio M, Rossi E, Vignolo L, Miglino M, Spriano M, et al. Therapy of hodgkin’s lymphoma in clinical practice: a retrospective long-term follow-up analysis. Oncol Lett (2011) 2(2):289–95. doi: 10.3892/ol.2011.255
30. Cole PD, Schwartz CL, Drachtman RA, De Alarcon PA, Chen L, Trippett TM. Phase II study of weekly gemcitabine and vinorelbine for children with recurrent or refractory hodgkin’s disease: a children’s oncology group report. J Clin Oncol (2009) 27(9):1456–61. doi: 10.1200/JCO.2008.20.3778
31. Moskowitz CH, Nimer SD, Zelenetz AD, Trippett T, Hedrick EE, Filippa DA, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood (2001) 97(3):616–23. doi: 10.1182/blood.V97.3.616
32. Karuturi M, Younes A, Fayad L, Kwak L, Pro B, Shah J, et al. Ifosfamide, carboplatin, etoposide with or without bortezomib in patients with relapsed/refractory Hodgkin lymphoma: results of a randomized phase II trial. Leuk Lymph (2016) 57(2):445–7. doi: 10.3109/10428194.2015.1032966
33. Claviez A, Canals C, Dierickx D, Stein J, Badell I, Pession A, et al. Allogeneic hematopoietic stem cell transplantation in children and adolescents with recurrent and refractory Hodgkin lymphoma: an analysis of the European group for blood and marrow transplantation. Blood (2009) 114(10):2060–7. doi: 10.1182/blood-2008-11-189399
34. Shankar A, Hayward J, Kirkwood A, Mccarthy K, Hewitt M, Morland B, et al. Treatment outcome in children and adolescents with relapsed Hodgkin lymphoma - results of the UK HD3 relapse treatment strategy. Br J Haematol (2014) 165(4):534–44. doi: 10.1111/bjh.12768
35. Talleur AC, Flerlage JE, Shook DR, Chilsen AM, Hudson MM, Cheng C, et al. Autologous hematopoietic cell transplantation for the treatment of relapsed/refractory pediatric, adolescent, and young adult Hodgkin lymphoma: a single institutional experience. Bone Marrow Transplant [Internet] (2020) 55(7):1357–66. doi: 10.1038/s41409-020-0879-4
36. Collins GP, Parker AN, Pocock C, Kayani I, Sureda A, Illidge T, et al. Guideline on the management of primary resistant and relapsed classical Hodgkin lymphoma. Br J Haematol (2014) 164(1):39–52. doi: 10.1111/bjh.12582
37. Akhtar S, El Weshi A, Rahal M, Abdelsalam M, Al Husseini H, Maghfoor I. High-dose chemotherapy and autologous stem cell transplant in adolescent patients with relapsed or refractory hodgkin’s lymphoma. Bone Marrow Transplant (2010) 45(3):476–82. doi: 10.1038/bmt.2009.197
38. Shah GL, Moskowitz CH. Transplant strategies in relapsed/refractory Hodgkin lymphoma. Blood (2018) 131(15):1689–97. doi: 10.1182/blood-2017-09-772673
39. Lieskovsky YYE, Donaldson SS, Torres MA, Wong RM, Amylon MD, Link MP, et al. High-dose therapy and autologous hematopoietic stem-cell transplantation for recurrent or refractory pediatric hodgkin’s disease: results and prognostic indices. J Clin Oncol (2004) 22(22):4532–40. doi: 10.1200/JCO.2004.02.121
40. Harker-Murray PD, Drachtman RA, Hodgson DC, Chauvenet AR, Kelly KM, Cole PD. Stratification of treatment intensity in relapsed pediatric Hodgkin lymphoma. Pediatr Blood Cancer (2014) 61(4):579–86. doi: 10.1002/pbc.24851
41. Wolden SL, Chen L, Kelly KM, Herzog P, Gilchrist GS, Thomson J, et al. Long-term results of CCG 5942: a randomized comparison of chemotherapy with and without radiotherapy for children with hodgkin’s lymphoma - a report from the children’s oncology group. J Clin Oncol (2012) 30(26):3174–80. doi: 10.1200/JCO.2011.41.1819
Keywords: relapsed/refractory pediatric CHL, ICE, GV, toxicity, outcome
Citation: Mahdy A, Hamoda A, Zaher A, Khorshed E, Elwakeel M, Hassanein O and Sidhom I (2023) Outcome and toxicity of ifosfamide, carboplatin, and etoposide versus gemcitabine and vinorelbine regimen for pediatric patients with relapsed or refractory Hodgkin’s lymphoma. Front. Oncol. 13:1153128. doi: 10.3389/fonc.2023.1153128
Received: 28 January 2023; Accepted: 01 June 2023;
Published: 27 June 2023.
Edited by:
Andrea Di Cataldo, University of Catania, ItalyReviewed by:
Matthew Barth, University at Buffalo, United StatesCopyright © 2023 Mahdy, Hamoda, Zaher, Khorshed, Elwakeel, Hassanein and Sidhom. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed Mahdy, ahmed.mahdy@57357.org; Iman Sidhom, ImanSidhom@cu.edu.eg
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.