
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 09 May 2023
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1126890
This article is part of the Research Topic Biomarkers, Functional Mechanisms, and Therapeutic Potentials in Gastrointestinal Cancers View all 40 articles
 Mao-Yan Si1†
Mao-Yan Si1† Ding-Yu Rao2†
Ding-Yu Rao2† Yao Xia1
Yao Xia1 Cheng-Peng Sang3
Cheng-Peng Sang3 Kai-Yun Mao1
Kai-Yun Mao1 Xiang-Jin Liu1
Xiang-Jin Liu1 Zu-Xiong Zhang2*
Zu-Xiong Zhang2* Zhi-Xian Tang2*
Zhi-Xian Tang2*Esophageal cancer is a common malignant tumor with a high degree of malignancy. Understanding its pathogenesis and identifying early diagnostic biomarkers can significantly improve the prognosis of esophageal cancer patients. Exosomes are small double-membrane vesicles found in various body fluids containing various components (DNA, RNA, and proteins) that mediate intercellular signal communication. Non-coding RNAs are a class of gene transcription products that encode polypeptide functions and are widely detected in exosomes. There is growing evidence that exosomal non-coding RNAs are involved in cancer growth, metastasis and angiogenesis, and can also be used as diagnostic and prognostic markers. This article reviews the recent progress in exosomal non-coding RNAs in esophageal cancer, including research progress, diagnostic value, proliferation, migration, invasion, and drug resistance, provide new ideas for the precise treatment of esophageal cancer.
Esophageal cancer (EC) is one of the malignant tumors with high incidence and a serious threat to human health and life worldwide. Although significant progress has been made in the diagnosis, treatment, and pathogenesis of esophageal cancer for more than half a century, the five-year survival rate is still less than 20% (1). Moreover, its incidence and mortality continue to increase each year. The EC pathogenesis has not been clarified. The morbidity and mortality may be related to many factors. In recent years, many epidemiological studies have shown that esophageal cancer occurrence results from long-term interaction between genetic and environmental factors (2, 3). Because most esophageal cancer patients are in the middle and advanced stages of the disease when they are treated, the treatment methods are very limited (4). The primary treatment for esophageal cancer is surgery (5, 6). Even after rigorous surgical treatment, EC patients’ leading causes of death are tumor recurrence and distant metastases. New noninvasive biomarkers and therapeutic targets must be identified to improve esophageal cancer patients’ survival rate and quality of life.
Exosomes are a type of microvesicle with a size of 40~100 nm that are widely distributed in bodily fluids such as blood, saliva, and urine and are secreted into the circulatory system by cells. The vesicle carries various biological information molecules, such as mRNA, miRNA, and DNA fragments (7) which are often related to carcinogenesis, invasion, and metastasis (8). In recent years, detecting specific information molecules in exosomes for diagnosing malignant cancers have become a research priority.
Non-coding RNAs (ncRNAs) used to be considered as a class of gene transcription products that do not possess protein-coding functions, but more and more studies have shown that some ncRNAs can encode to produce functional polypeptides (9), and such ncRNAs and polypeptides can be confirmed to often have high conservation and homology by sequencing and mass spectrometry. Among them, lncRNAs and circRNAs can participate in regulating transcription and translation processes directly by performing functions such as protein scaffolding, regulatory splicing, and loop-roll translation, or indirectly regulating signaling pathways by influencing other RNAs as miRNA sponges, thus participating in regulating tumorigenesis and development (10–12). ncRNAs are widely found in exosomes (13–15). In addition, miRNAs do not circulate in the body in a free state; they generally bind to AGO2 or lipoproteins or are encapsulated by vesicles such as exosomes before entering the circulation (as shown in Figure 1) (5). Compared to miRNAs that bind to proteins and circulate in vivo, exosomal miRNAs may have better structural stability, target specificity and functional direction.
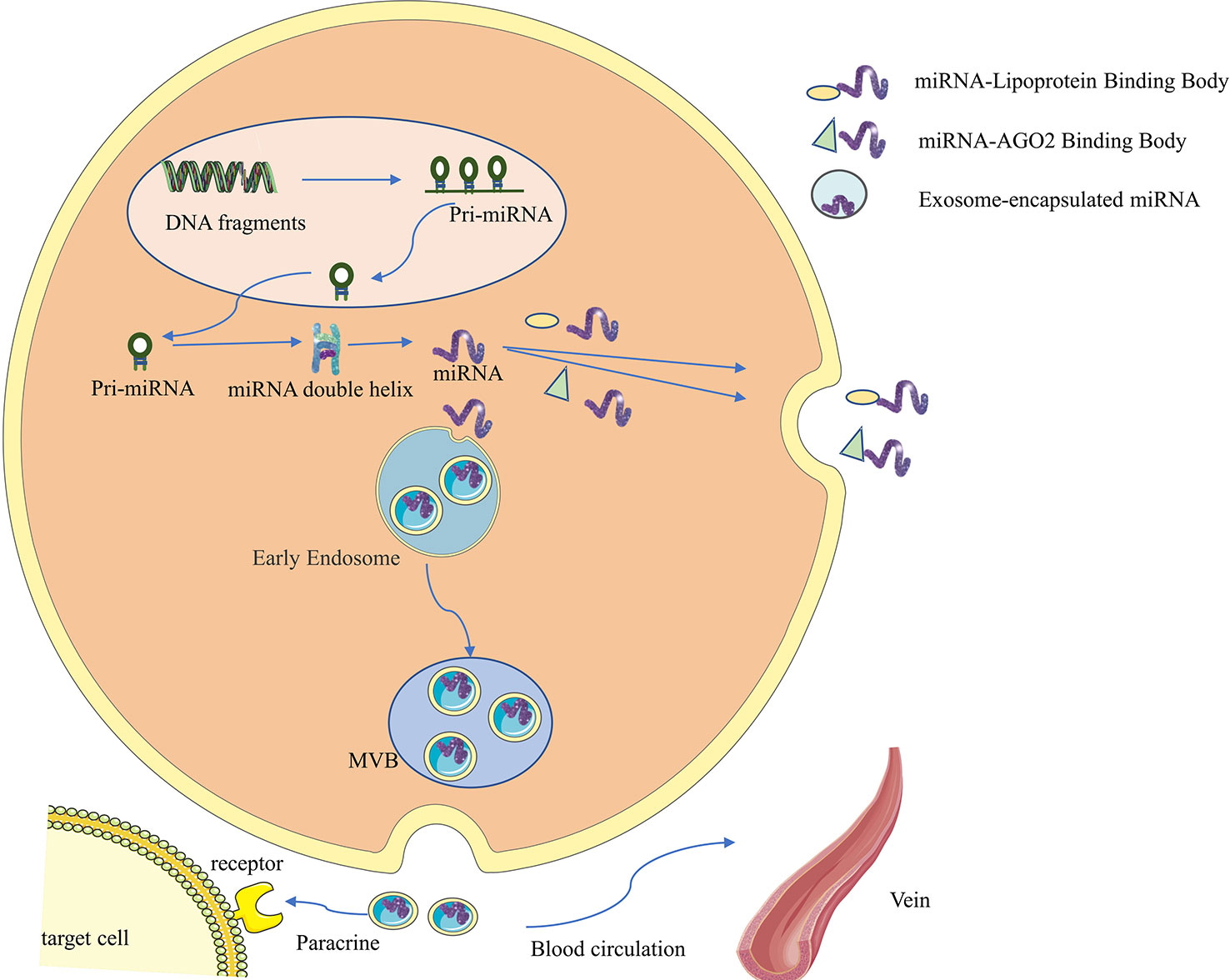
Figure 1 Synthesis, secretion and functional characteristics of exosomal miRNA. First, miRNA genes are transcribed into primary miRNAs (pri-miRNAs) in the nucleus, which range in length from hundreds to thousands of bases and contain one to several hairpin stem-loop structures with a 5’cap and a 3’polyA tail. The pri-miRNA is then further processed by the nuclease Drosha and its cofactor Pasha to form a precursor miRNA (pre-miRNA), consisting of 70 nucleotides, which is then transported into the cytoplasm via the GTP-dependent Exprotin-5 complex. Finally, the pre-miRNA is further cleaved by Dicer enzyme to form double-stranded miRNA:miRNA, then one miRNA chain is degraded, and the other mature miRNA chain binds to the 3’UTR of the target mRNA, so that the target mRNA is degraded or translation is inhibited, so as to achieve the purpose of regulating protein expression. miRNAs do not circulate in the body in a free state. They generally bind to AGO2 or lipoproteins, or are encapsulated by vesicles such as exosomes before entering the circulation.
Many studies have found tumor-derived exosomes in the circulating blood of esophageal cancer patients (16–18). These exosomes include numerous tumor-related specific molecules, such as mRNA, protein, lipid, and non-coding RNA., making them a powerful signal transmission function (19). Due to the necessity for numerous signal exchanges between the tumor and surrounding supporting cells, the exosome secretion increases significantly, participating in the regulation of tumor microenvironment and metastasis, and might play a key role in it. The analysis and detection of tumor exosomes can assist in early diagnosis, efficacy evaluation and prognosis analysis of tumors (20). For example, in esophageal squamous cell carcinoma, high miRNA-21 expression in exosomes can reflect 100% of the tumor level. Moreover, high miRNA-21 expression in exosomes often indicates extensive invasion and recurrence (21).
Studies of exosomes date back to 1946 when Chargaff and West reported that plasma clotting time increased after ultracentrifugation (22). The researchers attributed this phenomenon to subcellular procoagulant factors, which are small lipid-rich vesicles with 20 to 50 nm in diameter; in 1967, Wolf called it “platelet dust” (23). Endocytic vesicles were first identified in mature mammalian reticular cells (immature erythrocytes) in 1983 by Stahl’s (24) and Johnstone’s teams (25). In 1987, Johnstone et al. (26) defined vesicles formed in multivesicular bodies by endocytosis and released by the fusion of multivesicular bodies with plasma membrane as “exosomes”. In the following decade, exosomes were not taken seriously by researchers. In 1996, Raposo et al. (27) reported that exosomes secreted from B lymphocytes, which carry MHC class II molecules, costimulatory factors, and adhesion factors, could represent antigens. Studies have shown that these B cell-derived exosomes can directly stimulate the antitumor response of CD4+ cells. In 2007, Valadi et al. (28) found that RNA can be exchanged between different cells through exosomes and confirmed that tumor exosomes could promote or inhibit the growth and metastasis of tumor cells. The 2013 Nobel Prize in Physiology or Medicine was awarded by United States scientist James E. Rothman and Randy W.Schekman, Thomas C. Three scientists from Südho were awarded for their discovery of the regulatory mechanism of vesicular trafficking, the main transport system in cells, which pushed exosomes to a new climax. However, during the past decade, significant advancements have been achieved in this field of study, particularly the discovery of exosomal miRNA activity, which is crucial in cancer research. Therefore, exosomes can be used as diagnostic markers and prognostic indicators for tumors (29). Due to its characteristics can also be used as a carrier for drugs and functional molecules, providing a novel clinical therapeutic mode (30).
The “Asian esophageal cancer belt” extends from northern Iran through the Central Asian republics to Mongolia and north-central China. It is a special high-risk area for Esophageal squamous cell carcinoma (ESCC), with China alone accounting for more than half of the global cases (31). Because the early symptoms of EC are not obvious, patients are often in the middle and late stages when they are diagnosed, severely impacting their families and daily lives (32). Therefore, early detection and prompt treatment of esophageal cancer are of clinical importance (33). There is increasing evidence that early diagnosis and accurate prediction of treatment effects can significantly improve the ESCC patient’s prognosis (34). However, the specificity and sensitivity of its diagnostic and prognostic biomarkers remain unsatisfactory. Numerous studies have shown that cancer cell-derived exosomes contain specific nucleic acids and proteins that reflect the cancer cells’ origin (35). Therefore, exosomes are novel and potential biomarkers in many cancer types (36). Compared to other cancer biomarkers (such as circulating tumor cells (CTC) and circulating tumor DNA (ctDNA), exosomes have the advantages of sufficient quantity, strong stability, and strong accessibility (37, 38). Almost all cancer cell types can secrete numerous exosomes, and exosomes exist in almost all body fluids, such as blood, saliva, urine, tissue fluid and cerebrospinal fluid, which broadens the selection range of liquid biopsy sample sources (37, 39).
Lin et al. (40) showed the presence of miRNA in EC patients’ saliva and animal saliva exosomes. Furthermore, the chimeric RNA level in saliva exosomes can be used for the first time as a noninvasive biomarker for detecting early and late EC for postoperative monitoring, therapeutic response, and tumor recurrence (41).
The study of Li et al. (37), by comparing small RNAs in salivary exosomes from ESCC patients with RNA from healthy controls, a cancer-rich dual sesncRNA profile (i.e., tRNA-GlyGCC-5 and sRESE) was identified in salivary exosomes, which represents a non-invasive, convenient, and reliable biomarker for human ESCC diagnosis, prognosis, and especially prediction of preoperative patients who may benefit from adjuvant therapy.
Furthermore, samples from 51 patients with ESCC and 41 patients with benign illnesses were collected (i.e., the control group). Exosomal miR-21 levels were significantly increased in ESCC groups compared to controls (42). Exosome miR-21 may be a useful biomarker for detecting ESCC progression or efficacy of ESCC.
Yan et al. (43) found that serum exosomal lncRNA can be used as a biomarker for diagnosing and prognosis of EC. Furthermore, the lncRNA of four UCA1, ESCCAL-1, PEG10 and POU3F3 was most significantly up-regulated in EC exosomes. Similarly, increased expression levels are also observed in patients with advanced disease stages. Using ROC analysis, some lncRNAs showed high diagnostic values, e.g. AUCs for UCA1 and POU3F3 were 0.733 and 0.717, respectively. Based on these findings, the exosomal lncRNA combination provides a more sensitive diagnosis of ESCC, particularly for early disease. In Huang et al. (44), lncRNA PCAT1 was present in ESCC cell-derived exosomes and upregulated in the serum of ESCC patients. Further studies have shown that PCAT1 is an oncogene in ESCC and promotes ESCC progression by binding to miR-326. PCAT1 can be used as a therapeutic target and a potential non-invasive biomarker for ESCC patients.
In the field of tumor research, there have been considerable literature reports that exosomes can participate in many tumors’ progression, including promoting tumor proliferation, metastasis, and invasion (45, 46), inhibiting tumor cell apoptosis (47), regulating cell cycle (48, 49) and autophagy (50). The tumor-derived exosomal lncRNA ZFAS1 promotes proliferation. It inhibits apoptosis by up-regulating STAT3 and down-regulating miR-124, thus benefiting ESCC cells’ tumorigenesis (8). Tumor-derived exosomal miR-19b-3p can target Chromosome 10 promote EC cell invasion and inhibit apoptosis (51). Matsumoto et al. demonstrated that tumor-derived exosomes (52)could promote tumor progression and malignant transformation by altering gene expression and tumor cell phenotype (48). For example, Tumor-derived exosomal lncRNA PCAT1 (prostate cancer-associated transcript 1) promote ESCC cell proliferation via the sponge tumor suppressor miR-326 (44).
Cancer-associated fibroblasts (CAFs) are one of the most important components of the tumor microenvironment and play an essential role in tumor occurrence and development (53). Similarly, CAFs have been shown to contribute to tumor development and progression (52, 54). Zhao et al. found that CAF-derived exosomes could improve the ESCC cells’ growth and migration through the Hedgehog signaling pathway (55). Furthermore, CAFs use the exosomal miR-451 as a signaling molecule, providing a favorable niche for tumor cell migration and cancer progression (56).
Tumor-associated macrophages (TAM) are infiltrating macrophages in tumor tissues. The researchers found that exosomes secreted by ESCC cells can induce macrophage polarization to the M2 type through its content miR-301a-3p. Moreover, the TAMs proangiogenic switch is triggered by exosomes miR-301a-3p secreted by ESCC cells through PTEN/PI3K/AKT signaling pathway (57). These studies highlight the important role of exosomes in the growth and migration of EC.
Chemoradiotherapy is one of the most common treatments for advanced esophageal cancer, and chemotherapy resistance signifies chemotherapy failure. Therefore, the mechanism of human chemoradiotherapy resistance and how to reverse chemoradiotherapy resistance are pressing issues that must be resolved in tumor treatment. In tumors, most exosomes are tumor development promoters (58–60). Exosomes can serve as tumor signaling vectors. One of the adverse clinical impacts of exosomes as a source of tumors is their capacity to transfer resistance horizontally (61–63). Drug-resistant tumor cells can transfer drug resistance to sensitive cells through exosomes (18, 64), thus generating new anti-tumor cell reservoirs. Exosomes from drug-resistant tumor cells may confer resistance phenotype to sensitive cells through intercellular signaling.
Kang et al. found that exosomes from gefitinib-resistant cells containing the long non-coding RNA lncRNA PART1 promoted gefitinib resistance in ESCC via the miR-129/blc-2 axis (65). Exosomes containing miR-21 from cisplatin-resistant cells promote the development of cisplatin resistance in ESCC by targeting programmed cell death 4 (PDCD4) (66).Additionally, exosomal miR-193 delivery to ESCC cells increases cisplatin resistance by activating the janus kinase (JAK)-STAT signaling pathway (67). Furthermore, ESCC-derived exosomal lncRNA POU3F3 transforms fibroblasts (NF) into CAFs, and these CAFs can secreted IL-6 then enhances cisplatin resistance in ESCC cells (68). A recent study found that the hypoxic tumor cell-derived exosomal miR-340-5p confers radioresistance in ESCC by targeting KLF10/UVRAG (69). The miR-340-5p level in plasma exosomes is closely related to radiotherapy response and prognosis. MiR-340-5p may be a therapeutic target for overcoming radioresistance in ESCC. Luo et al. demonstrated that tumor-derived exosomal miR-339-5p enhanced the radiosensitivity of ESCC cells by targeting Cdc25A (70). These studies suggest that exosomes are vital in regulating resistance to EC therapy. It is believed that with further research, the role and mechanism of exosomes in esophageal cancer resistance will be gradually revealed and finally applied to clinical practice.
Exosomes are widely distributed and can shuttle freely in the body, known as the “Trojan Horse”. Exosomes have a role in various physiological and metabolic processes in the body, as they facilitate the flow of information between cells (71–73) (Figure 2). Meanwhile, exosomes also have the characteristics of non-immunity and easy penetration of cell membranes and can be specifically recognized by receptor cells (74). Therefore, exosomes have unique natural advantages as drug delivery vehicles (75). Research on drug delivery by exosomes has become a hot spot in recent years. Some small-molecule chemical and gene drugs have been successfully loaded into exosomes, showing great potential in treating various cancers (76, 77).
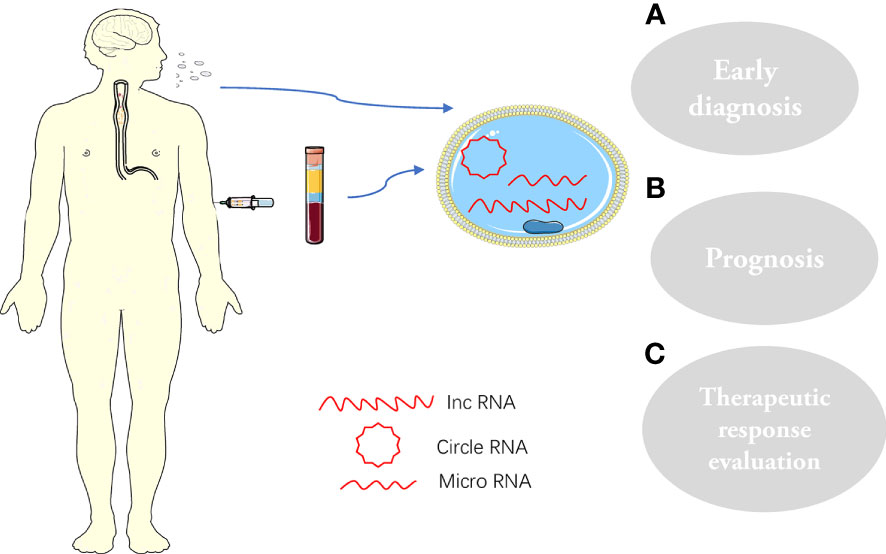
Figure 2 Exosomes and their cargoes, extracted from human plasma or saliva, are widely involved in the pathophysiology of ESCC: (A) Early diagnosis; (B) prognosis; (C) Therapeutic response evaluation.
Researchers at Oxford University have used exosomes as carriers to load therapeutic siRNA for treating Alzheimer’s disease. By modifying exosomes to have specific cell targeting, they not only successfully passed the blood-brain barrier but also accurately delivered therapeutic siRNA to target cells, reducing the mRNA and protein expression levels of corresponding genes in target cells, thus achieving the purpose of disease treatment (78, 79).
Exosomes are vital as drug carriers in enhancing anti-cancer response and targeted drug delivery (77, 80, 81). Exosomes can transport small molecules, such as nucleic acids, to target cells, and there are increasing studies using exosomes as vectors to deliver therapeutic nucleic acids for cancer treatment (82, 83). As a natural RNA vector, exosomes have high circulating stability and inherent homing ability, which has the advantage of simultaneous loading of multiple therapeutic nucleic acids compared with conventional antitumor delivery systems (84). Shtam et al. reported using exosomes to deliver latent therapeutic siRNAs against cancer cells to target cells. The successful delivery of siRNA to recipient cells was observed using confocal microscopy and flow cytometry. The significant reduction of oncogene protein levels and the mass death of cancer cells further proved that siRNA could be effectively delivered to target cells (85).In addition, Adriamycin-and paclitaxel-loaded exosomes have been used in cancer therapy with low immunogenicity and toxicity (86, 87). The utility of paclitaxel-loaded exosomes has improved efficacy in treating multidrug-resistant cancer cells (80). Cumulatively the above studies indicate that exosomes are an effective tool for carrying and delivering anticancer drugs.
In this article, we attempt to summarize the exosomal ncRNAs’ role in the diagnosis, growth, metastasis, drug resistance and targeted delivery of EC. Additionally, we discussed using exosomal ncRNA as biomarkers and therapeutic tools for the diagnosis, prognosis and prediction of EC.
Although exosomal ncRNA has considerable application potential, challenges prevent its practicality. First, clinical samples require more accurate and standardized purification methods. Secondly, there are multiple bioactivators in exosomes and their main functional components still need further study. Currently, there is no standardized method for isolating and identifying exosome from biological body fluids. The methods used in the reported study lack repeatability and inconvenience, which limits their widespread use. Moreover, an ideal exosome enrichment strategy with high purity and efficiency cannot be obtained. Due to the lack of large-scale exosomes for clinical research, exosome-based engineering applications are limited to cellular or animal experiments. Finally, a systematic and in-depth study on the exocrine mechanism involved in tumor occurrence and development is lacking, Implementing exosomal ncRNA based diagnosis and treatment strategies still faces significant difficulties, but these tactics must be translated into practical application soon to assist EC patients.
M-YS searched for literature and wrote the first draft of this article. D-YR edited tables and figures. Z-XZ and Z-XT strictly reviewed the manuscript and polished the grammar. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Funds of China (Nos. 81860337, 81960326, 82060384); Natural Science Funds of Jiangxi Province (Nos. 20202ACBL206014, 20192BAB205009, 2020BABL206116); Science and Technology Program of Jiangxi Health Commission (Nos. 20201080, 202130660); National Health Commission Science and Technology Development Research Center (No. 2019ZH-07E-003); general projects of Jiangxi Traditional Chinese Medicine Science and Technology (No. 2020B0214).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yang J, Liu X, Cao S, Dong X, Rao S, Cai K. Understanding esophageal cancer: The challenges and opportunities for the next decade. Front Oncol (2020) 10:1727. doi: 10.3389/fonc.2020.01727
2. Hongo M, Nagasaki Y, Shoji T. Epidemiology of esophageal cancer: Orient to occident. effects of chronology, geography and ethnicity. J Gastroenterol Hepatol (2009) 24(5):729–35. doi: 10.1111/j.1440-1746.2009.05824.x
3. Zhang HZ, Jin GF, Shen HB. Epidemiologic differences in esophageal cancer between Asian and Western populations. Chin J Cancer (2012) 31(6):281–6. doi: 10.5732/cjc.011.10390
4. Hagymasi K, Tulassay Z. [Risk factors for esophageal cancer, and possible genetic background]. Orv Hetil (2009) 150(9):407–13.
5. Kato H, Nakajima M. Treatments for esophageal cancer: a review. Gen Thorac Cardiovasc Surg (2013) 61(6):330–5. doi: 10.1007/s11748-013-0246-0
6. Vaghjiani RG, Molena D. Surgical management of esophageal cancer. Chin Clin Oncol (2017) 6(5):47. doi: 10.21037/cco.2017.07.05
7. Simons M, Raposo G. Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol (2009) 21(4):575–81. doi: 10.1016/j.ceb.2009.03.007
8. Li Z, Qin X, Bian W, Li Y, Shan B, Yao Z, et al. Exosomal lncRNA ZFAS1 regulates esophageal squamous cell carcinoma cell proliferation, invasion, migration and apoptosis via microRNA-124/STAT3 axis. J Exp Clin Cancer Res (2019) 38(1):477. doi: 10.1186/s13046-019-1473-8
9. Guardia T, Zhang Y, Thompson KN, Lee SJ, Martin SS, Konstantopoulos K, et al. OBSCN restoration via OBSCN-AS1 long-noncoding RNA CRISPR-targeting suppresses metastasis in triple-negative breast cancer. Proc Natl Acad Sci U.S.A. (2023) 120(11):e2215553120. doi: 10.1073/pnas.2215553120
10. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell (2009) 136(2):215–33. doi: 10.1016/j.cell.2009.01.002
11. Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science (2013) 339(6121):819–23. doi: 10.1126/science.1231143
12. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet (2020) 395(10224):565–74. doi: 10.1016/S0140-6736(20)30251-8
13. Shi Y, Shao J, Zhang Z, Zhang J, Lu H.. Effect of condylar chondrocyte exosomes on condylar cartilage osteogenesis in rats under tensile stress. Front Bioeng Biotechnol (2022) 10:1061855. doi: 10.3389/fbioe.2022.1061855
14. Tu J, Zheng N, Mao C, Liu S, Zhang H, Sun L. UC-BSCs exosomes regulate Th17/Treg balance in patients with systemic lupus erythematosus via miR-19b/KLF13. Cells (2022) 11(24). doi: 10.3390/cells11244123
15. Luo B, Zhou K, Liufu Y, Huang X, Zeng H, Zhang Z. Novel insight into miRNA biology and its role in the pathogenesis of systemic lupus erythematosus. Front Immunol (2022) 13:1059887. doi: 10.3389/fimmu.2022.1059887
16. Xiao Z, Feng X, Zhou Y, Li P, Luo J, Zhang W, et al. Exosomal miR-10527-5p inhibits migration, invasion, lymphangiogenesis and lymphatic metastasis by affecting wnt/beta-catenin signaling via Rab10 in esophageal squamous cell carcinoma. Int J Nanomed (2023) 18:95–114. doi: 10.2147/IJN.S391173
17. Zhao L, Yu L, Wang X, He J, Zhu X, Zhang R, et al. Mechanisms of function and clinical potential of exosomes in esophageal squamous cell carcinoma. Cancer Lett (2023) 553:215993. doi: 10.1016/j.canlet.2022.215993
18. Miao N, Cai W, Ding S, Liu Y, Chen W, Sun T, et al. Characterization of plasma exosomal microRNAs in responding to radiotherapy of human esophageal squamous cell carcinoma. Mol Med Rep (2022) 26(3). doi: 10.3892/mmr.2022.12803
19. Lin M, Zhou C, He S, Yu H, Guo T, Ye J, et al. The research advances of exosomes in esophageal cancer. biomark Med (2019) 13(8):685–95. doi: 10.2217/bmm-2018-0314
20. Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta (2012) 1820(7):940–8. doi: 10.1016/j.bbagen.2012.03.017
21. Komatsu S, Ichikawa D, Takeshita H, Tsujiura M, Morimura R, Nagata H, et al. Circulating microRNAs in plasma of patients with oesophageal squamous cell carcinoma. Br J Cancer (2011) 105(1):104–11. doi: 10.1038/bjc.2011.198
22. Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem (1946) 166(1):189–97. doi: 10.1016/S0021-9258(17)34997-9
23. Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol (1967) 13(3):269–88. doi: 10.1111/j.1365-2141.1967.tb08741.x
24. Harding C, Stahl P. Transferrin recycling in reticulocytes: pH and iron are important determinants of ligand binding and processing. Biochem Biophys Res Commun (1983) 113(2):650–8. doi: 10.1016/0006-291X(83)91776-X
25. Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell (1983) 33(3):967–78. doi: 10.1016/0092-8674(83)90040-5
26. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. association of plasma membrane activities with released vesicles (exosomes). J Biol Chem (1987) 262(19):9412–20.
27. Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med (1996) 183(3):1161–72. doi: 10.1084/jem.183.3.1161
28. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol (2007) 9(6):654–9. doi: 10.1038/ncb1596
29. Tai YL, Chen KC, Hsieh JT, Shen TL. Exosomes in cancer development and clinical applications. Cancer Sci (2018) 109(8):2364–74. doi: 10.1111/cas.13697
30. Tomasetti M, Lee W, Santarelli L, Neuzil J. Exosome-derived microRNAs in cancer metabolism: possible implications in cancer diagnostics and therapy. Exp Mol Med (2017) 49(1):e285. doi: 10.1038/emm.2016.153
31. Harada K, Rogers JE, Iwatsuki M, Yamashita K, Baba H, Ajani JA. Recent advances in treating oesophageal cancer. F1000Res (2020) 9. doi: 10.12688/f1000research.22926.1
32. Watanabe M, Otake R, Kozuki R, Toihata T, Takahashi K, Okamura A, et al. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today (2020) 50(1):12–20. doi: 10.1007/s00595-019-01878-7
34. Vendrely V, Launay V, Najah H, Smith D, Collet D, Gronnier C. Prognostic factors in esophageal cancer treated with curative intent. Dig Liver Dis (2018) 50(10):991–6. doi: 10.1016/j.dld.2018.08.002
35. Kok VC, Yu CC. Cancer-derived exosomes: Their role in cancer biology and biomarker development. Int J Nanomed (2020) 15:8019–36. doi: 10.2147/IJN.S272378
36. Inamdar S, Nitiyanandan R, Rege K. Emerging applications of exosomes in cancer therapeutics and diagnostics. Bioeng Transl Med (2017) 2(1):70–80. doi: 10.1002/btm2.10059
37. Li K, Lin Y, Luo Y, Xiong X, Wang L, Durante K, et al. A signature of saliva-derived exosomal small RNAs as predicting biomarker for esophageal carcinoma: a multicenter prospective study. Mol Cancer (2022) 21(1):21. doi: 10.1186/s12943-021-01462-z
38. Yu D, Li Y, Wang M, Gu J, Xu W, Cai , et al. Exosomes as a new frontier of cancer liquid biopsy. Mol Cancer (2022) 21(1):56. doi: 10.1186/s12943-022-01509-9
39. Zheng X, Chen F, Zhang J, Zhang Q, Lin J. Exosome analysis: a promising biomarker system with special attention to saliva. J Membr Biol (2014) 247(11):1129–36. doi: 10.1007/s00232-014-9717-1
40. Lin Y, Dong H, Deng W, Lin W, Li K, Xiong X, et al. Evaluation of salivary exosomal chimeric GOLM1-NAA35 RNA as a potential biomarker in esophageal carcinoma. Clin Cancer Res (2019) 25(10):3035–45. doi: 10.1158/1078-0432.CCR-18-3169
41. Huang Z, Zhang L, Zhu D, Shan X, Zhou X, Qi LW, et al. A novel serum microRNA signature to screen esophageal squamous cell carcinoma. Cancer Med (2017) 6(1):109–19. doi: 10.1002/cam4.973
42. Tanaka Y, Kamohara H, Kinoshita K, Kurashige J, Ishimoto T, Iwatsuki M, et al. Clinical impact of serum exosomal microRNA-21 as a clinical biomarker in human esophageal squamous cell carcinoma. Cancer (2013) 119(6):1159–67. doi: 10.1002/cncr.27895
43. Yan S, Du L, Jiang X, Duan W, Li J, Xie Y, et al. Evaluation of serum exosomal lncRNAs as diagnostic and prognostic biomarkers for esophageal squamous cell carcinoma. Cancer Manag Res (2020) 12:9753–63. doi: 10.2147/CMAR.S250971
44. Huang L, Wang Y, Chen J, Wang Y, Zhao Y, Wang Y, et al. Long noncoding RNA PCAT1, a novel serum-based biomarker, enhances cell growth by sponging miR-326 in oesophageal squamous cell carcinoma. Cell Death Dis (2019) 10(7):513. doi: 10.1038/s41419-019-1745-4
45. Li P, Liu X, Xing W, Qiu H, Li R, Liu S, et al. Exosome-derived miR-200a promotes esophageal cancer cell proliferation and migration via the mediating Keap1 expression. Mol Cell Biochem (2022) 477(4):1295–308. doi: 10.1007/s11010-022-04353-z
46. Wu T, Hu H, Zhang T, Jiang L, Li X, Liu , et al. miR-25 promotes cell proliferation, migration, and invasion of non-Small-Cell lung cancer by targeting the LATS2/YAP signaling pathway. Oxid Med Cell Longev (2019) 2019:9719723. doi: 10.1155/2019/9719723
47. Zhang H, Zuo Z, Lu X, Wang L, Wang H, Zhu Z. MiR-25 regulates apoptosis by targeting bim in human ovarian cancer. Oncol Rep (2012) 27(2):594–8.
48. Matsumoto Y, Kano M, Murakami K, Toyozumi T, Suito H, Takahashi M, et al. Tumor-derived exosomes influence the cell cycle and cell migration of human esophageal cancer cell lines. Cancer Sci (2020) 111(12):4348–58. doi: 10.1111/cas.14660
49. Lu H, Zhang L, Lu S, Yang D, Ye J, Li M, et al. miR-25 expression is upregulated in pancreatic ductal adenocarcinoma and promotes cell proliferation by targeting ABI2. Exp Ther Med (2020) 19(5):3384–90. doi: 10.3892/etm.2020.8595
50. Khan T, Relitti N, Brindisi M, Magnano S, Zisterer D, Gemma S, et al. Autophagy modulators for the treatment of oral and esophageal squamous cell carcinomas. Med Res Rev (2020) 40(3):1002–60. doi: 10.1002/med.21646
51. Zeng Q, Zhu Z, Song L, He Z. Transferred by exosomes-derived MiR-19b-3p targets PTEN to regulate esophageal cancer cell apoptosis, migration and invasion. Biosci Rep (2020) 40(11).
52. Jang I, Beningo KA. Integrins, CAFs and mechanical forces in the progression of cancer. Cancers (Basel) (2019) 11(5).
53. Chen Y, McAndrews KM, Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol (2021) 18(12):792–804.
54. Ham IH, Lee D, Hur H. Role of cancer-associated fibroblast in gastric cancer progression and resistance to treatments. J Oncol (2019) 2019:6270784.
55. Zhao G, Li H, Guo Q, Zhou A, Wang X, Li P, et al. Exosomal sonic hedgehog derived from cancer-associated fibroblasts promotes proliferation and migration of esophageal squamous cell carcinoma. Cancer Med (2020) 9(7):2500–13.
56. Khazaei S, Nouraee N, Moradi A, Mowla SJ. A novel signaling role for miR-451 in esophageal tumor microenvironment and its contribution to tumor progression. Clin Transl Oncol (2017) 19(5):633–40. doi: 10.1007/s12094-016-1575-0
57. Shou Y, Wang X, Chen C, Liang Y, Yang C, Xiao Q, et al. Exosomal miR-301a-3p from esophageal squamous cell carcinoma cells promotes angiogenesis by inducing M2 polarization of macrophages via the PTEN/PI3K/AKT signaling pathway. Cancer Cell Int (2022) 22(1):153. doi: 10.1186/s12935-022-02570-6
58. Colak S, Medema JP. Cancer stem cells–important players in tumor therapy resistance. FEBS J (2014) 281(21):4779–91. doi: 10.1111/febs.13023
59. Christie EL, Bowtell DDL. Acquired chemotherapy resistance in ovarian cancer. Ann Oncol (2017) 28(suppl_8):viii13–5. doi: 10.1093/annonc/mdx446
60. Schram AM, Chang MT, Jonsson P, Drilon A. Fusions in solid tumours: diagnostic strategies, targeted therapy, and acquired resistance. Nat Rev Clin Oncol (2017) 14(12):735–48. doi: 10.1038/nrclinonc.2017.127
61. Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. J Control Release (2015) 219:278–94. doi: 10.1016/j.jconrel.2015.06.029
62. Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie G, et al. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res (2017) 36(1):53. doi: 10.1186/s13046-017-0528-y
63. Challagundla KB, Wise PM, Neviani P, Chava H, Murtadha M, Xu T, et al. Exosome-mediated transfer of microRNAs within the tumor microenvironment and neuroblastoma resistance to chemotherapy. J Natl Cancer Inst (2015) 107(7). doi: 10.1093/jnci/djv135
64. Sun Y, Wang J, Ma Y, Li J, Sun X, Zhao X, et al. Radiation induces NORAD expression to promote ESCC radiotherapy resistance via EEPD1/ATR/Chk1 signalling and by inhibiting pri-miR-199a1 processing and the exosomal transfer of miR-199a-5p. J Exp Clin Cancer Res (2021) 40(1):306. doi: 10.1186/s13046-021-02084-5
65. Kang M, Ren M, Li Y, Fu Y, Deng M, Li C. Exosome-mediated transfer of lncRNA PART1 induces gefitinib resistance in esophageal squamous cell carcinoma via functioning as a competing endogenous RNA. J Exp Clin Cancer Res (2018) 37(1):171. doi: 10.1186/s13046-018-0845-9
66. Yang YC, Liu GJ, Yuan DF, Li CQ, Xue M, Chen LJ. Influence of exosome-derived miR-21on chemotherapy resistance of esophageal cancer. Eur Rev Med Pharmacol Sci (2019) 23(4):1513–9.
67. Shi S, Huang X, Ma X, Zhu X, Zhang Q. Research of the mechanism on miRNA193 in exosomes promotes cisplatin resistance in esophageal cancer cells. PloS One (2020) 15(5):e0225290. doi: 10.1371/journal.pone.0225290
68. Tong Y, Yang L, Yu C, Zhu W, Zhou X, Xiong Y, et al. Tumor-secreted exosomal lncRNA POU3F3 promotes cisplatin resistance in ESCC by inducing fibroblast differentiation into CAFs. Mol Ther Oncolytics (2020) 18:1–13. doi: 10.1016/j.omto.2020.05.014
69. Chen F, Xu B, Li J, Yang X, Gu J, Yao X, et al. Hypoxic tumour cell-derived exosomal miR-340-5p promotes radioresistance of oesophageal squamous cell carcinoma via KLF10. J Exp Clin Cancer Res (2021) 40(1):38. doi: 10.1186/s13046-021-01834-9
70. Luo A, Zhou X, Shi X, Zhao Y, Men Y, Chang X, et al. Exosome-derived miR-339-5p mediates radiosensitivity by targeting Cdc25A in locally advanced esophageal squamous cell carcinoma. Oncogene (2019) 38(25):4990–5006. doi: 10.1038/s41388-019-0771-0
71. Magoling BJA, Wu AY, Chen YJ, Wong WW, Chuo ST, Huang HC, et al. Membrane protein modification modulates big and small extracellular vesicle biodistribution and tumorigenic potential in breast cancers in vivo. Adv Mater (2023) e2208966. doi: 10.1002/adma.202208966
72. Guerra-Slompo EP, Cesaro G, Guimaraes BG, Zanchin NIT.. Dissecting trypanosoma brucei RRP44 function in the maturation of segmented ribosomal RNA using a regulated genetic complementation system. Nucleic Acids Res (2023). doi: 10.1093/nar/gkac1217
73. Wei F, Mu Y, Tan RP, Wise SG, Bilek MM, Zhou Y, et al. Osteo-immunomodulatory role of interleukin-4-Immobilized plasma immersion ion implantation membranes for bone regeneration. ACS Appl Mater Interfaces (2023). doi: 10.1021/acsami.2c17005
74. Wang J, Zheng Y, Zhao M. Exosome-based cancer therapy: Implication for targeting cancer stem cells. Front Pharmacol (2016) 7:533.
75. Srivastava A, Babu A, Filant J, Moxley KM, Ruskin R, Dhanasekaran D, et al. Exploitation of exosomes as nanocarriers for gene-, chemo-, and immune-therapy of cancer. J BioMed Nanotechnol (2016) 12(6):1159–73. doi: 10.1166/jbn.2016.2205
76. Su LL, Chang XJ, Zhou HD, Hou LB, Xue XY. Exosomes in esophageal cancer: A review on tumorigenesis, diagnosis and therapeutic potential. World J Clin cases (2019) 7(8):908–16. doi: 10.12998/wjcc.v7.i8.908
77. Bose RJC, Uday Kumar S, Zeng Y, Afjei R, Robinson E, Lau K, et al. Tumor cell-derived extracellular vesicle-coated nanocarriers: An efficient theranostic platform for the cancer-specific delivery of anti-miR-21 and imaging agents. ACS Nano (2018) 12(11):10817–32. doi: 10.1021/acsnano.8b02587
78. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol (2011) 29(4):341–5. doi: 10.1038/nbt.1807
79. Jiang L, Dong H, Cao H, Ji X, Luan S, Liu J. Exosomes in pathogenesis, diagnosis, and treatment of alzheimer's disease. Med Sci Monit (2019) 25:3329–35. doi: 10.12659/MSM.914027
80. Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine (2016) 12(3):655–64. doi: 10.1016/j.nano.2015.10.012
81. Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett (2016) 371(1):48–61. doi: 10.1016/j.canlet.2015.10.020
82. Seow Y, Wood MJ. Biological gene delivery vehicles: beyond viral vectors. Mol Ther (2009) 17(5):767–77. doi: 10.1038/mt.2009.41
83. Camussi G, Quesenberry PJ. Perspectives on the potential therapeutic uses of vesicles. Exosomes Microvesicles (2013) 1(6). doi: 10.5772/57393
84. Shao J, Zaro J, Shen Y. Advances in exosome-based drug delivery and tumor targeting: From tissue distribution to intracellular fate. Int J Nanomed (2020) 15:9355–71. doi: 10.2147/IJN.S281890
85. Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal (2013) 11:88. doi: 10.1186/1478-811X-11-88
86. Schindler C, Collinson A, Matthews C, Pointon A, Jenkinson L, Minter RR, et al. Exosomal delivery of doxorubicin enables rapid cell entry and enhanced in vitro potency. PloS One (2019) 14(3):e0214545. doi: 10.1371/journal.pone.0214545
87. Saari H, Lazaro-Ibanez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M., et al. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of paclitaxel in autologous prostate cancer cells. J Control Release (2015) 220(Pt B):727–37. doi: 10.1016/j.jconrel.2015.09.031
Keywords: esophageal cancer, exosomes, IncRNA, miRNA, prospects
Citation: Si M-Y, Rao D-Y, Xia Y, Sang C-P, Mao K-Y, Liu X-J, Zhang Z-X and Tang Z-X (2023) Role of exosomal noncoding RNA in esophageal carcinoma. Front. Oncol. 13:1126890. doi: 10.3389/fonc.2023.1126890
Received: 18 December 2022; Accepted: 31 March 2023;
Published: 09 May 2023.
Edited by:
Zequn Li, The Affiliated Hospital of Qingdao University, ChinaReviewed by:
Furong Huang, Duke University, United StatesCopyright © 2023 Si, Rao, Xia, Sang, Mao, Liu, Zhang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Xian Tang, dHpoaXhpYW4yMDIwQGdtdS5lZHUuY24=; Zu-Xiong Zhang, d3oxOTg1MDkyMUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.