- 1The Legacy Heritage Center & Dr. Larry Norton Institute, Soroka Medical Center and Ben Gurion University, Beer Sheva, Israel
- 2Medical School for International Health, Ben Gurion University of the Negev, Beer Sheva, Israel
- 3Internal Medicine Ward, Soroka Medical Center & Ben-Gurion University, Beer-Sheva, Israel
- 4Department of Dermatology and Venereology, Emek Medical Centre, Afula, Israel
This case report describes the occurrence of hyperbilirubinemia as a complication of metastatic melanoma. A 72-year-old male patient was diagnosed with BRAF V600E-mutated melanoma with metastases in the liver, lymph nodes, lungs, pancreas, and stomach. Due to a lack of clinical data and specific guidelines for the treatment of mutated metastatic melanoma patients with hyperbilirubinemia, a conference of specialists debated between initiating treatment or providing supportive care. Ultimately, the patient was started on the combination therapy of dabrafenib and trametinib. This treatment resulted in a significant therapeutic response via normalization of bilirubin levels and an impressive radiological response of metastases just one month post-treatment initiation.
1 Introduction
The incidence of melanoma in high-risk populations is increasing around the world (1). Approximately 4% of all patients diagnosed with the disease each year suffer from stage 4 metastatic melanoma (2), The averaged 3-year overall survival (OS) rates were 41.3% for BRAF plus MEK inhibitor, 49.9% for PD-1 inhibition, and 58.4% for CTLA-4 plus PD-1 inhibition, in first-line therapy (3), a highly concerning statistic.
Melanoma is an extremely diverse disease with a variable pathogenesis that depends on body location as well as individual risk factors, such as UV exposure. This is attested by the fact that melanoma contains one of the highest tumor mutational burdens of all cancers, with an average of more than 10 mutations per megabase (4, 5). This is especially true in cutaneous melanomas arising from UV exposed areas (4). The majority of melanomas present with an upregulation of the MAPK/ERK signaling cascade (5), which is involved in cell proliferation, differentiation, and suppression of inflammation (6, 7).
The Cancer Genome Atlas Network has divided melanoma mutations into four sub-types being BRAF, RAS, NF-1, and triple-wild type (8). This categorization aids in the targeted treatment of melanomas. With the BRAF gene being the most commonly mutated gene in melanoma, the BRAF subtype has the highest occurrence rates (5). The vast majority of BRAF mutations have been identified in amino acid 600 of the gene, leading to the discovery of the V600E, V600K, and V600R point mutations (8, 9). Patients with BRAF subtype melanomas have been reported to be younger on average as compared to patient with other subtypes (8). However, BRAF mutations are usually not enough to induce tumors on their own, where most benign nevi also harbor BRAF mutations. The development of a melanoma requires an accumulation of various mutations, such as those in the PI3 kinase pathway, TERT, or CDKN2A (5, 10, 11)
Due to the relatively high frequency of BRAF mutations in melanoma, several targeted treatments have been developed. These treatments include dabrafenib and vemurafenib, which directly inhibit BRAF; and cobimetinib and trametinib, which inhibit MEK, the downstream kinase to BRAF and encorafenib and binimetinib (11). Given as a BRAF and MEK inhibitor combination these treatments generally have a very high response rate (up to 70%), giving patients an additional year of life on average (11). Meta-static melanoma may cause elevated bilirubin levels in patients, with the majority of re-ported cases being due to metastasis in the liver, pancreas, or common bile duct (12–17). Data on the exact changes in bilirubin levels upon treatment of metastatic melanoma, however are lacking. Herein, we present the first reported case, to the best of our knowledge, of bilirubin elevation in a patient with aggressive metastatic melanoma treated with combination therapy of dabrafenib and trametinib. The patient exhibited significant improvement in the widespread disease, highlighting the safety and efficacy of the treatment despite high levels of bilirubin. We believe that this case report may provide new insight for the management and treatment of metastatic melanoma.
2 Case report
A 72 year-old male was referred to the emergency department in May 2021 by a family physician due to hematemesis, jaundice, and a decreased performance status. The patient was an active smoker (30 packs per year for the last 40 years), but was generally healthy, was on no medications, and had no family history of cancer. Upon admission, the patient reported an 8 kg weight loss over the past month. A physical examination revealed jaundice of the skin, sclera, and mucous membranes, with no other pathological findings.
Initial blood tests revealed low hemoglobin levels of 7.1 gm/dL (normal range: 14-16 gm/dL), accompanied by mild leukocytosis and thrombocytosis. Blood chemistry showed an elevation in both total and direct bilirubin of 11.05 and 6.97 gm/dL respectively (nor-mal range: 0.3-1.2 gm/dL), and elevated liver enzymes, especially in alkaline phosphatase and gamma-glutamyl transferase (GGT). The lactate dehydrogenase (LDH) level was 1193 (IU/L). These results were consistent with obstructive jaundice, with the elevated levels of alkaline phosphatase and GGT suggesting intrahepatic cholestasis. Chest radiography showed small nodules in the left lung that were suspected of being metastatic processes. A total body computed tomography (CT) scan (Figure 1) revealed hepatomegaly (25*19 cm in diameter), several nodules in both lungs suspected of being metastasis (the biggest was 15 mm in diameter), enlargement of the lymph nodes in the hilum of both lungs, a space occupying lesion (SOL) in the vertebral area (D10), a SOL in the fundus of the stomach (6 cm in diameter), multiple SOLs in the liver (the biggest was 5 cm in diameter), enlargement of the lymph nodes in the hilum of the liver (the biggest 5 cm in diameter, SOLs in the head of the pancreas, SOLs in the bilateral adrenals, and several enlarged lymph nodes in the abdominal space.
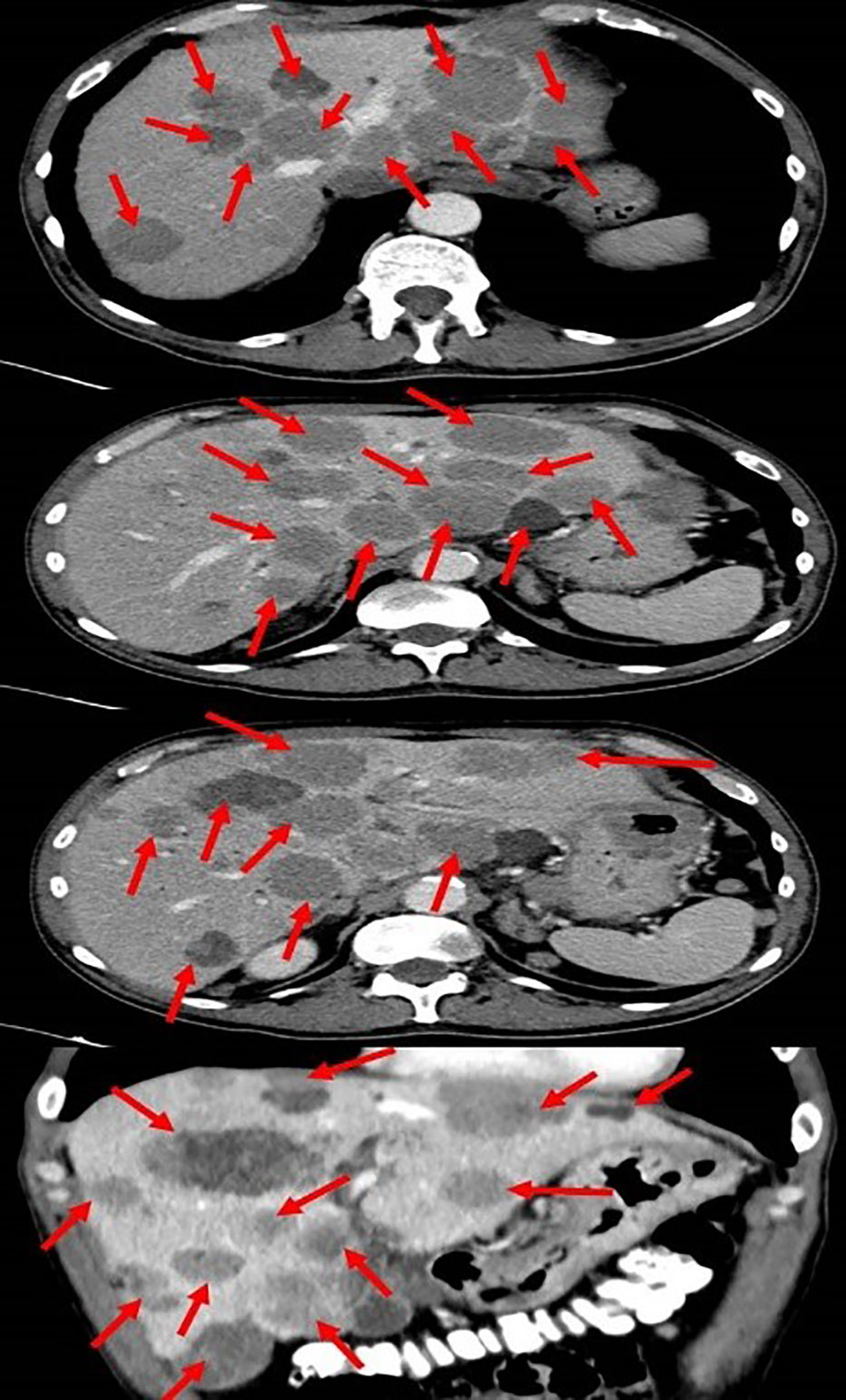
Figure 1 Total body computed tomography scan showing the metastatic melanoma in the liver (red arrows).
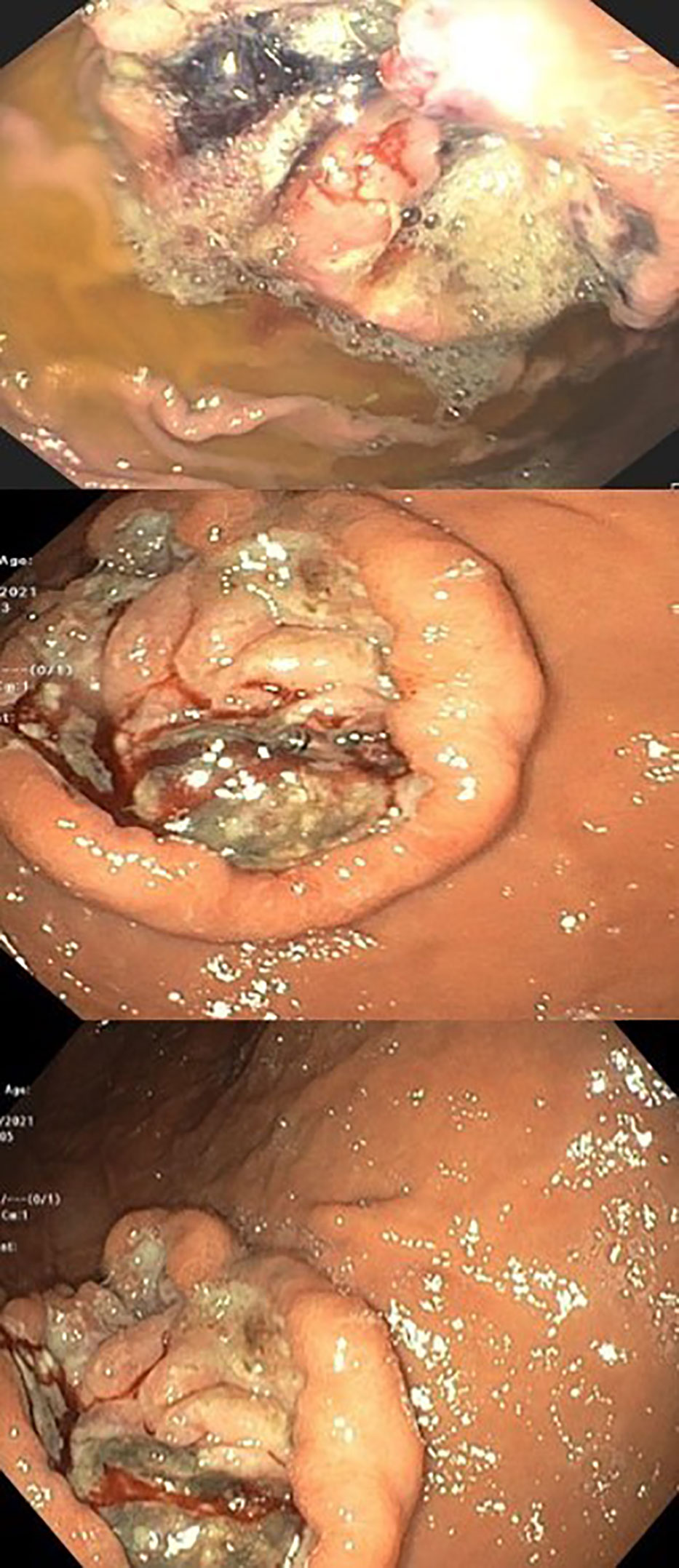
An ultrasound of the upper abdomen was performed and a common bile duct obstruction or dilatation were ruled out. The patient was sent for an urgent gastroscopy, which showed four peptic ulcers in the stomach (2 ulcers in the antrum, 1 ulcer in the body and 1 ulcer in the fundus) (Figure 2).
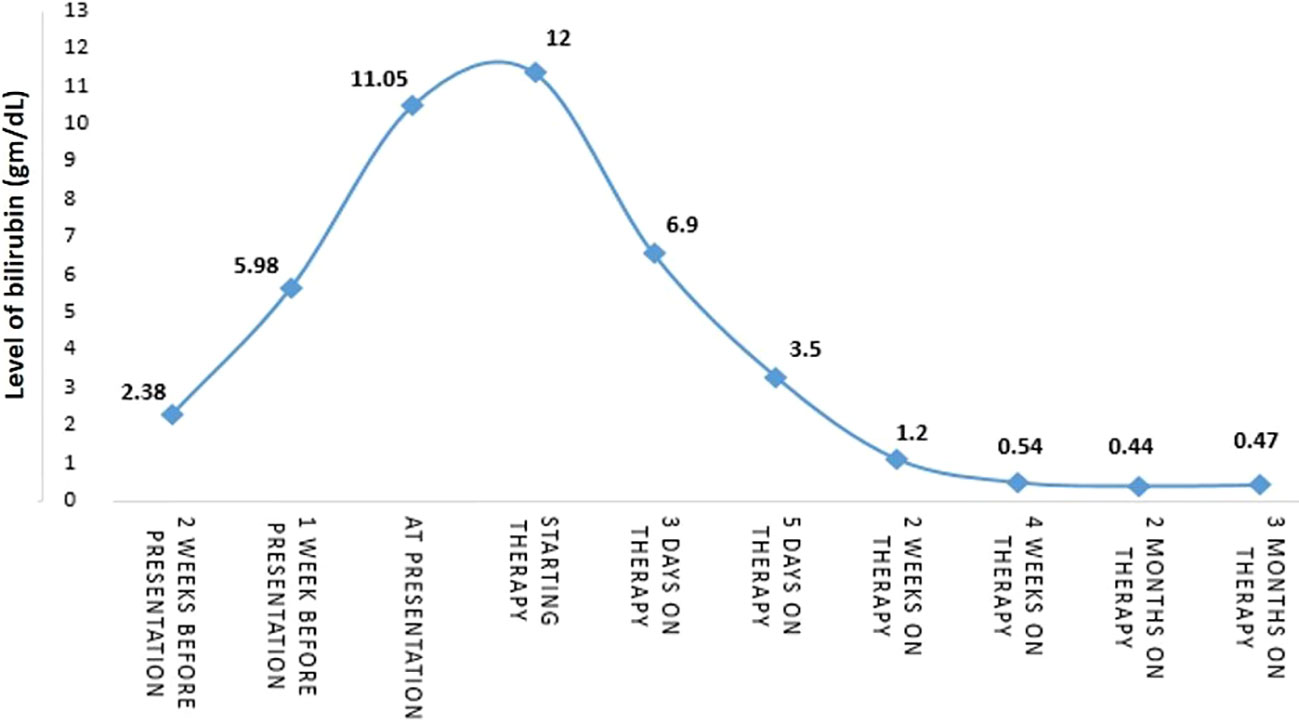
The biggest ulcer was 6 cm in diameter and was actively bleeding. A biopsy was taken from the ulcers, and histopathological results showed malignant melanoma. Molecular testing revealed the BRAF V600E mutation. The presumptive clinical diagnosis was stage 4 metastatic melanoma and a multidisciplinary conference was held which included an oncologist, hepatologist, and radiologist who all recommended systemic therapy for this extremely aggressive disease, despite very high bilirubin levels (had increased to 12 gm/dL). The possibility of stent insertion was discussed and rejected by the forum after careful assessment of imaging tests. The patient started combination therapy for his mutation (BRAF) with dabrafenib (150mg twice a day daily) and trametinib (2mg once a day daily). Three days after treatment initiation, bilirubin levels decreased to 6.9 gm/dL. Two days later levels decreased to 3.5 gm/dL, and one week later (2 weeks since treatment initiation), levels decreased to just above the normal range (1.2 gm/dL). After two weeks (al-most a month after starting treatment), bilirubin levels were within the normal range (0.54 gm/dL) (Figure 3).
One month later a positron emission tomography PET– CT scan (Figure 4), showed a significant improvement with the disappearance of the lung nodules, lymph nodes in the hilum of both lungs being of normal size, the disappearance of the SOL in the D10 area, decreased diameters of the SOLs in the head of the pancreas, a decreased diameter of the SOL in the fundus of the stomach (to 2.8cm), the disappearance of some of the SOLs in the liver, and decreased diameters of the lymph nodes in the liver hilum. No SOLs or enlargement in bilateral adrenals or lymph nodes of the abdominal space were seen.
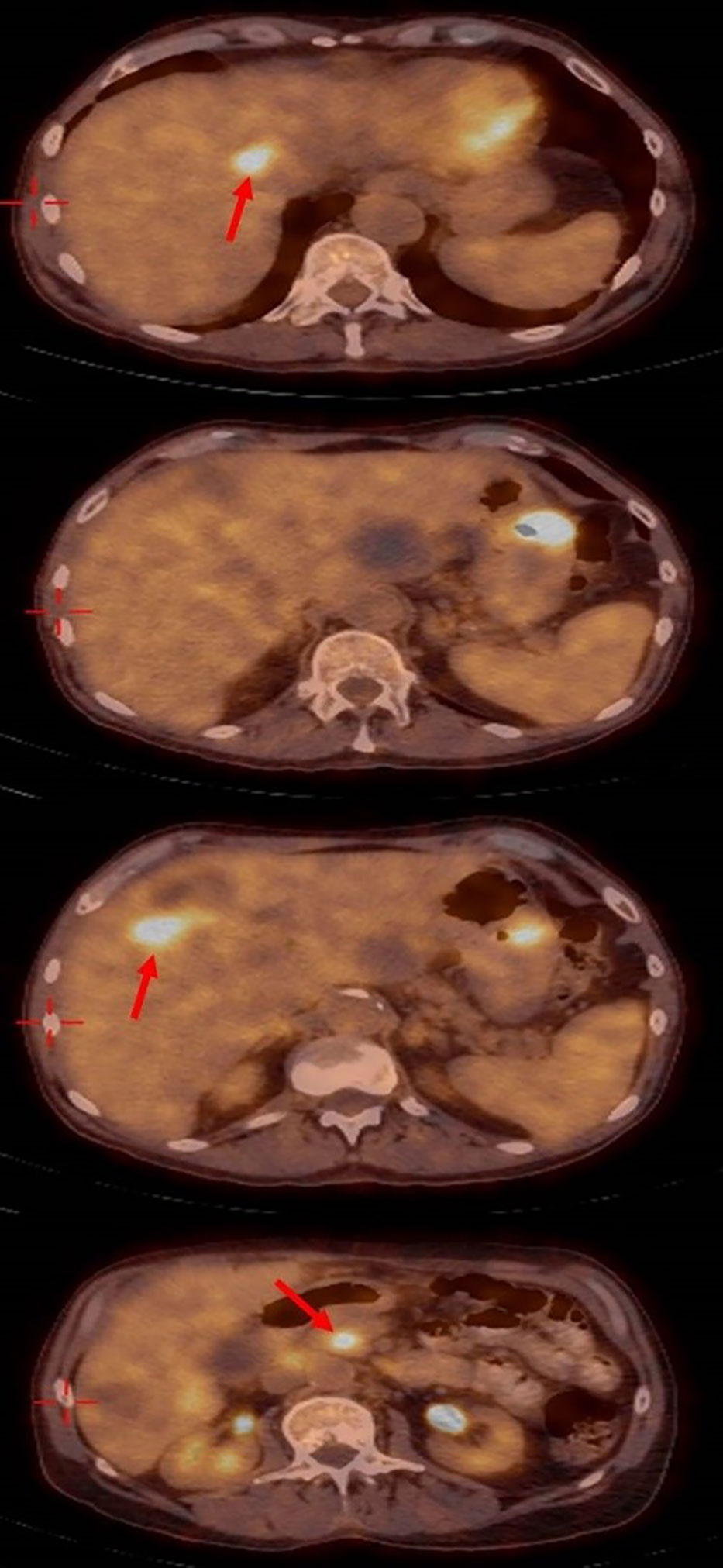
Figure 4 Positron emission tomography– computed tomography showing the significant response with disappearing of most liver metastasis.
The patient remained on follow-up status with routine blood tests, which remained within normal range 6 months from treatment initiation. Unfortunately he died due to disease progression in February 2022 without additional follow-ups.
3 Discussion
This case report describes a patient with serious complications related to metastatic melanoma. The patient suffered from widespread metastatic disease, accompanied by very high levels of bilirubin due to intrahepatic cholestasis caused by liver metastases. Elevated bilirubin levels are due to one of three main causes: 1. Pre-hepatic complications such as a massive breakdown of red blood cells (hemolysis); 2. Hepatic causes, such as direct damage of the liver cells (toxins, viral infections and metastatic disease); and 3. Post-hepatic causes such as gallstones and locally advanced cancers (18). In addition, the patient suffered from elevated LDH levels, seven organ sites with metastases, and a very poor ECOG score of 4. Without immediate intervention, it was expected that this patient would not survive. Expected treatment outcomes remained unknown.
According to the ASCO guidelines on systemic treatment of melanoma, patients with BRAF-mutant melanoma stage III should be treated with either nivolumab, pembrolizumab or a combination dabrafenib and trametinib therapy (19). For patients with stage IV unresectable or metastatic melanoma, more options are available, including immunotherapy (ipilimumab plus nivolumab, nivolumab or pembrolizumab) or targeted therapy for BRAF-positive patients (dabrafenib plus trametinib, encorafenib plus binimetinib or vemurafenib plus cobimetinib) (19).
Dabrafenib is an inhibitor of the associated enzyme BRAF. Primary routes of elimination of dabrafenib are hepatic metabolism and biliary secretion. Whereas mild hepatic impairment is not a contraindication for administration of dabrafenib, there is no available data regarding the safety and efficacy of this therapy among patients with severe hepatic impairment.
Trametinib is a MEK inhibitor drug with anti-cancer activity. It inhibits MEK1 and MEK2. According to the manufacturer, moderate or severe hepatic impairment had no significant effect on trametinib exposure or apparent clearance.
The combination of dabrafenib and trametinib was approved for use in BRAF V600-mutant melanoma in 2013 (20). It is known to have a fast response with improved survival rates among patients compared to that of BRAF inhibitor monotherapy (21). However, during trials, these treatments did not include patients with high levels of bilirubin, and thus patient response in such an instance is relatively unknown. This combination however has been shown to be more efficacious in the treatment of melanoma than dabrafenib alone (22). During clinical trials, several side effects were reported, including pyrexia, chills, fatigue, nausea, vomiting, diarrhea, headache, rash, arthralgia, and myalgia (20, 23).
Due to the aggressive nature of metastatic melanoma disease and this specific patient’s poor performance status, with a terminal prognosis without intervention. It was therefore decided that the best treatment option in this case would be combination target therapy with dabrafenib and trametinib. This treatment option was very effective, and the patient showed rapid improvements in bilirubin levels and in radiographic findings of metastases.
One initial issue with BRAF-mutation-specific treatment is that resistance to single drug regimens may develop quickly, usually within 6-7 months of beginning treatment (9, 24). Therefore, regimens consisting of two drugs, i.e., a BRAF inhibitor and a MEK inhibitor, have been recommended and approved. Although such combinations do not eliminate the progression of resistance, they do delay it (24).
Jason et al. showed that patients with BRAF mutant melanoma with liver metastases treated with targeted therapy, had higher proportion of response in the liver metastases (46.3%) compared with patients treated with immunotherapy (35.0%) (25).
Recently several clinical trials evaluated patients with unresectable or metastatic BRAF-mutant melanoma with the combination of BRAF/MEK inhibitors with anti-PD-(L)1 therapy known as triplet therapy (KEYNOTE-022, IMspire150 and COMBI-i). These trials showed that the objective response rate of KEYNOTE-022 was surprisingly lower (63%) compared to the control arm (72%), and unsatisfactory results were seen in the IMspire150 (66.5% for triplet therapy compared to the control arm 65%). COMBI-i showed better results but not convincingly (68.5% for triplet therapy compared to the control arm 64.2%). However, all three trials showed a trend of improved overall survival (26–29). Furthermore, all three trials showed a significant increase in toxicity that were reported in the triplet arms compared with the combination arms, especially with higher rates of treatment-related toxicity- grades 3–5, such as treatment-related death, elevated creatine phosphokinase, rate of treatment discontinuation, hepatitis, pneumonitis, fever, rash, diarrhea and liver transaminase elevation (27–29).
4 Conclusions
In summary, we report a case of a patient with complicated hyperbilirubinemia, anemia and hypertransaminasemia related to metastatic melanoma, the patient had BRAF mutant (V600E), because of his aggressive hyperbilirubinemia and performance status it was very hard to choose. However, for a lifesaving situation we decided an important strategy for treatment decision-making, therefore, the patient was treated with BRAF and MEK inhibitor combination (dabrafenib and trametinib). The patient achieved significantly radiological and blood test response. Overall, our case demonstrates the importance and potential of the safety for treating hyperbilirubinemia and hypertransaminasemia in patients with metastatic melanoma with BRAF mutant.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization, WS and AY; methodology, WS; software, WS; validation, WS, AY, RS, AC, KR, MT, AO, AA, IP, and KS; formal analysis, WS; investigation, WS, AY, RS, AC, KR, MT, IP, AO, AA, and KS; resources, WS; data curation, WS; writing—original draft preparation, WS, and RS,; writing—review and editing, WS, AY, RS, AC, KR, MT, AO, AA, and KS; visualization, WS; supervision, WS; project administration, WS; funding acquisition, WS. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank our social worker Lena Turky for her help regarding social conditions. In addition, we wish to thank David B. Geffen, for his critical review of the manuscript; also, we thank the patient and the patient’s family for their cooperation during the whole treatment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Whiteman DC, Green AC, Olsen CM. The growing burden of invasive melanoma: Projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Invest Dermatol (2016) 136(6):1161–71. doi: 10.1016/j.jid.2016.01.035
2. American Cancer Society’s. Melanoma: Statistics. Cancer.Net [Internet] (2022). Available at: https://www.cancer.net/cancer-types/melanoma/statistics.
3. Ugurel S, Röhmel J, Ascierto PA, Becker JC, Flaherty KT, Grob JJ, et al. Survival of patients with advanced metastatic melanoma: The impact of MAP kinase pathway inhibition and immune checkpoint inhibition - update 2019. Eur J Cancer. (2020) 130:126–38. doi: 10.1016/j.ejca.2020.02.021
4. Davis EJ, Johnson DB, Sosman JA, Chandra S. Melanoma: What do all the mutations mean? Cancer (2018) 124(17):3490–9. doi: 10.1002/cncr.31345
5. Turner N, Ware O, Bosenberg M. Genetics of metastasis: melanoma and other cancers. Clin Exp Metastasis. (2018) 35(5-6):379–91. doi: 10.1007/s10585-018-9893-y
6. Seger R, Krebs EG. The MAPK signaling cascade. FASEB J (1995) 9(9):726–35. doi: 10.1096/fasebj.9.9.7601337
7. Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med (2006) 203(7):1651–6. doi: 10.1084/jem.20051848
8. Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell (2015) 161(7):1681–96. doi: 10.1016/j.cell.2015.05.044
9. Yang K, Oak ASW, Slominski RM, Brożyna AA, Slominski AT. Current molecular markers of melanoma and treatment targets. Int J Mol Sci (2020) 21(10):3535. doi: 10.3390/ijms21103535
10. Cheng L, Lopez-Beltran A, Massari F, MacLennan GT, Montironi R. Molecular testing for BRAF mutations to inform melanoma treatment decisions: a move toward precision medicine. Mod Pathol (2018) 31(1):24–38. doi: 10.1038/modpathol.2017.104
11. Leonardi GC, Falzone L, Salemi R, Zanghì A, Spandidos DA, Mccubrey JA, et al. Cutaneous melanoma: From pathogenesis to therapy (Review). Int J Oncol (2018) 52(4):1071–80. doi: 10.3892/ijo.2018.4287
12. Rothschild PS, Subramaniam A, Chakrabarti R. Jaundice and haematemesis: An unusual presentation of metastatic malignant melanoma. Cureus (2020) 12(5):e8035. doi: 10.7759/cureus.8035
13. Liu X, Feng F, Wang T, Qin J, Yin X, Meng G, et al. Laparoscopic pancreaticoduodenectomy for metastatic pancreatic melanoma: A case report. Med (Baltimore). (2018) 97(44):e12940. doi: 10.1097/MD.0000000000012940
14. Tung CC, Chang MC, Chang YT. An unusual case of obstructive jaundice. diagnosis: Metastatic melanoma. Gastroenterology (2011) 141(1):e12–3. doi: 10.1053/j.gastro.2010.04.063
15. van Bokhoven MM, Aarntzen EH, Tan AC. Metastatic melanoma of the common bile duct and ampulla of vater. Gastrointest Endosc. (2006) 63(6):873–4. doi: 10.1016/j.gie.2005.12.023
16. Colovic RB, Grubor NM, Jovanovic MD, Micev MT, Colovic NR. Metastatic melanoma to the common bile duct causing obstructive jaundice: a case report. World J Gastroenterol (2007) 13(5):813–5. doi: 10.3748/wjg.v13.i5.813
17. Dhaliwal A, McKeown J, Bhat I. Malignant melanoma: a rare cause of obstructive jaundice. J Gastrointest Surg (2021) 25(4):1076–7. doi: 10.1007/s11605-020-04805-1
18. Subbiah V, West H. Jaundice (Hyperbilirubinemia) in cancer. JAMA Oncol (2016) 2(8):1103. doi: 10.1001/jamaoncol.2016.1236
19. Seth R, Messersmith H, Kaur V, Kirkwood JM, Kudchadkar R, McQuade JL, et al. Systemic therapy for melanoma: ASCO guideline. J Clin Oncol (2020) 38(33):3947–70. doi: 10.1200/JCO.20.00198
20. Menzies AM, Long GV. Dabrafenib and trametinib, alone and in combination for BRAF-mutant metastatic melanoma. Clin Cancer Res (2014) 20(8):2035–43. doi: 10.1158/1078-0432.CCR-13-2054
21. Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med (2019) 381(7):626–36. doi: 10.1056/NEJMoa1904059
22. Long GV, Flaherty KT, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study [published correction appears in Ann oncol. Ann Oncol (2017) 28(7):1631–9. doi: 10.1093/annonc/mdx176. 2019 Nov 1;30(11):1848].
23. Long GV, Hauschild A, Santinami M, Atkinson V, Mandalà M, Chiarion-Sileni V, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med (2017) 377(19):1813–23. doi: 10.1056/NEJMoa1708539
24. Patel H, Yacoub N, Mishra R, White A, Long Y, Alanazi S, et al. Current advances in the treatment of BRAF-mutant melanoma. Cancers (Basel). (2020) 12(2):482. doi: 10.3390/cancers12020482
25. Luke JJ, Ghate SR, Kish J, Lee CH, McAllister L, Mehta S, et al. Targeted agents or immuno-oncology therapies as first-line therapy for BRAF-mutated metastatic melanoma: a real-world study. Future Oncol (2019) 15(25):2933–42. doi: 10.2217/fon-2018-0964
26. Dixon-Douglas JR, Patel RP, Somasundram PM, McArthur GA. Triplet therapy in melanoma - combined BRAF/MEK inhibitors and anti-PD-(L)1 antibodies. Curr Oncol Rep (2022) 24(8):1071–9. doi: 10.1007/s11912-022-01243-x
27. Ferrucci PF, Di Giacomo AM, Del Vecchio M, Atkinson V, Schmidt H, Schachter J, et al. KEYNOTE-022 part 3: a randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer. (2020) 8(2):e001806. doi: 10.1136/jitc-2020-001806
28. Gutzmer R, Stroyakovskiy D, Gogas H, Robert C, Lewis K, Protsenko S, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (2020) 395(10240):1835–44. doi: 10.1016/S0140-6736(20)30934-X
Keywords: dabrafenib, trametinib, hyperbilirubinemia, metastatic melanoma, BRAF mutation (V600E), safety
Citation: Shalata W, Steckbeck R, Polishchuk I, Cohen AY, Rouvinov K, Tokar M, Abu Jama A, Abu Saleh O, Sheva K and Yakobson A (2023) Safety and rapid response of dabrafenib and trametinib therapy during hyperbilirubinemia in metastatic melanoma. Front. Oncol. 13:1102330. doi: 10.3389/fonc.2023.1102330
Received: 18 November 2022; Accepted: 01 February 2023;
Published: 14 February 2023.
Edited by:
Jinbao Liu, Guangzhou Medical University, ChinaCopyright © 2023 Shalata, Steckbeck, Polishchuk, Cohen, Rouvinov, Tokar, Abu Jama, Abu Saleh, Sheva and Yakobson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Walid Shalata, d2FsaWQuc2hhbGF0YUBnbWFpbC5jb20=
†These authors have contributed equally to this work
 Walid Shalata
Walid Shalata Rachel Steckbeck2
Rachel Steckbeck2 Ashraf Abu Jama
Ashraf Abu Jama Alexander Yakobson
Alexander Yakobson