- 1Department of Pathology, Saitama Medical University Saitama Medical Center, Kawagoe, Japan
- 2Department of Orthopaedic Oncology and Surgery, Saitama Medical University International Medical Center, Hidaka, Japan
- 3Department of Pathology, Saitama Medical University International Medical Center, Hidaka, Japan
- 4Department of Orthopedic Surgery, Graduate School of Medical Science, University of Yamanashi, Chuo, Japan
- 5Department of Pathology, Dokkyo Medical University Nikko Medical Center, Nikko, Japan
Extraskeletal osteosarcoma (EO) is a soft tissue sarcoma characterized by the production of bone matrix by neoplastic cells. Benign osteoid in EO, leading to a diagnostic dilemma, is rarely encountered. Herein, for the first time, we present a case with cytogenetically confirmed EO combined with or preceding myositis ossificans (MO). A 21-year-old man had a mildly painful swelling in his left knee. Imaging studies demonstrated a 39-mm mass with peripheral mineralization and cystic change on the posterolateral side of the left fibular head. He was clinically suspected of having either MO or a malignancy, such that wide resection was performed. Macroscopically, the mass was grayish to brown. In the cut section, multiple cystic lesions in addition to solid components were noted. Histopathologically, the solid components demonstrated diffuse proliferation of pleomorphic tumor cells with osteoclast-like giant cells. The malignant tumor cells formed osteoid. In the periphery, the mass was benign, showing mature bone tissue and focally non-malignant woven bone with fibroblasts, compatible with zonation. Fluorescence in situ hybridization (FISH) demonstrated split signals of the USP6 gene. These findings suggested EO with preceding MO. Although the pathogenesis remains to be elucidated, the observed USP6 rearrangement might contribute to both the diagnosis of EO with preceding MO and an understanding of the underlying histopathology.
Introduction
Extraskeletal osteosarcoma (EO) is defined as soft tissue sarcoma with bone matrix production by malignant cells outside the skeletal system (1). The pathological diagnosis of EO is based solely on the identification of histological osteogenic differentiation. The presence of benign osteoid-simulating myositis ossificans (MO) is rarely encountered in EO cases, possibly presenting both diagnostic and etiologic dilemmas. EO has been recognized as having complex genomic signatures (2), but most of the genetic alterations discovered to date have been non-diagnostic in clinical practice. On the other hand, MO was recently found to harbor a USP6 rearrangement (3). However, unfortunately, the previous case reports of EO with MO lacked cytogenetic investigation. To our knowledge, this is the first report to describe a cytogenetically confirmed case in which EO was combined with or preceded MO. This is also only the second description of EO with distinct zonation of MO.
Clinical summary
A 21-year-old man consulted our hospital for a mildly painful swelling on his left knee that he had noticed 4 days earlier. A 40 × 40 × 30 mm subcutaneous mass was identified on the lateral side of the left fibular head. Laboratory test results were unremarkable. X-rays and computed tomography (CT) scans revealed a mass lesion on the posterolateral side of the left knee and the periphery of the mass showed mineralization (Figure 1). There was no continuity with the bone cortex. On magnetic resonance imaging (MRI), the mass was solid and cystic with intermediate signal intensity on T1-weighted imaging (Figure 2A), while showing heterogeneously high intensity with the fluid–fluid level on T2-weighted imaging (Figure 2B). The periphery was enhanced on FS-T1-weighted imaging with contrast (Figures 2C, D). His prior medical history, except for an injury to the same knee 10 years earlier, was unremarkable and he had never undergone radiation therapy. No evidence of metastasis was identified clinically. The core needle biopsy specimen was insufficient and did not yield a diagnosis. However, the cytology specimen suggested malignancy. Due to the tendency of the lesion to show growth and the positive cytology result, the tumor was clinically suspected of MO or hematoma with malignancy. The mass lesion was thus excised with a wide margin containing biopsy tract, without preoperative chemotherapy 6 weeks after the initial presentation. At the time of excision, the mass had not changed in size since the initial visit.
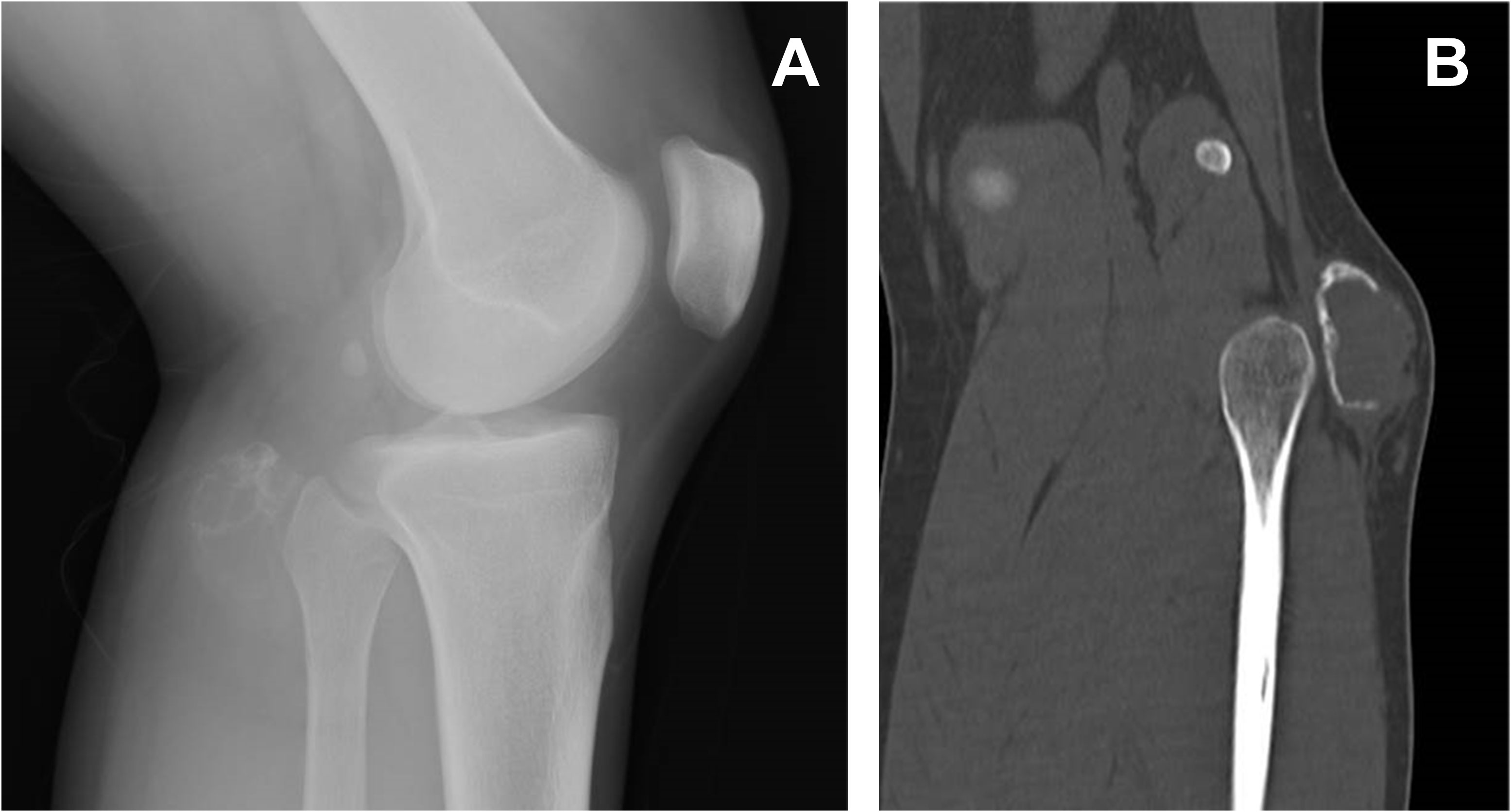
Figure 1 Lateral view x-ray of the left knee demonstrating a mass posterior to the fibular head (A). On reconstructed CT, the mass was calcified in the periphery, but the shell was not complete and showed focal interruption (B).
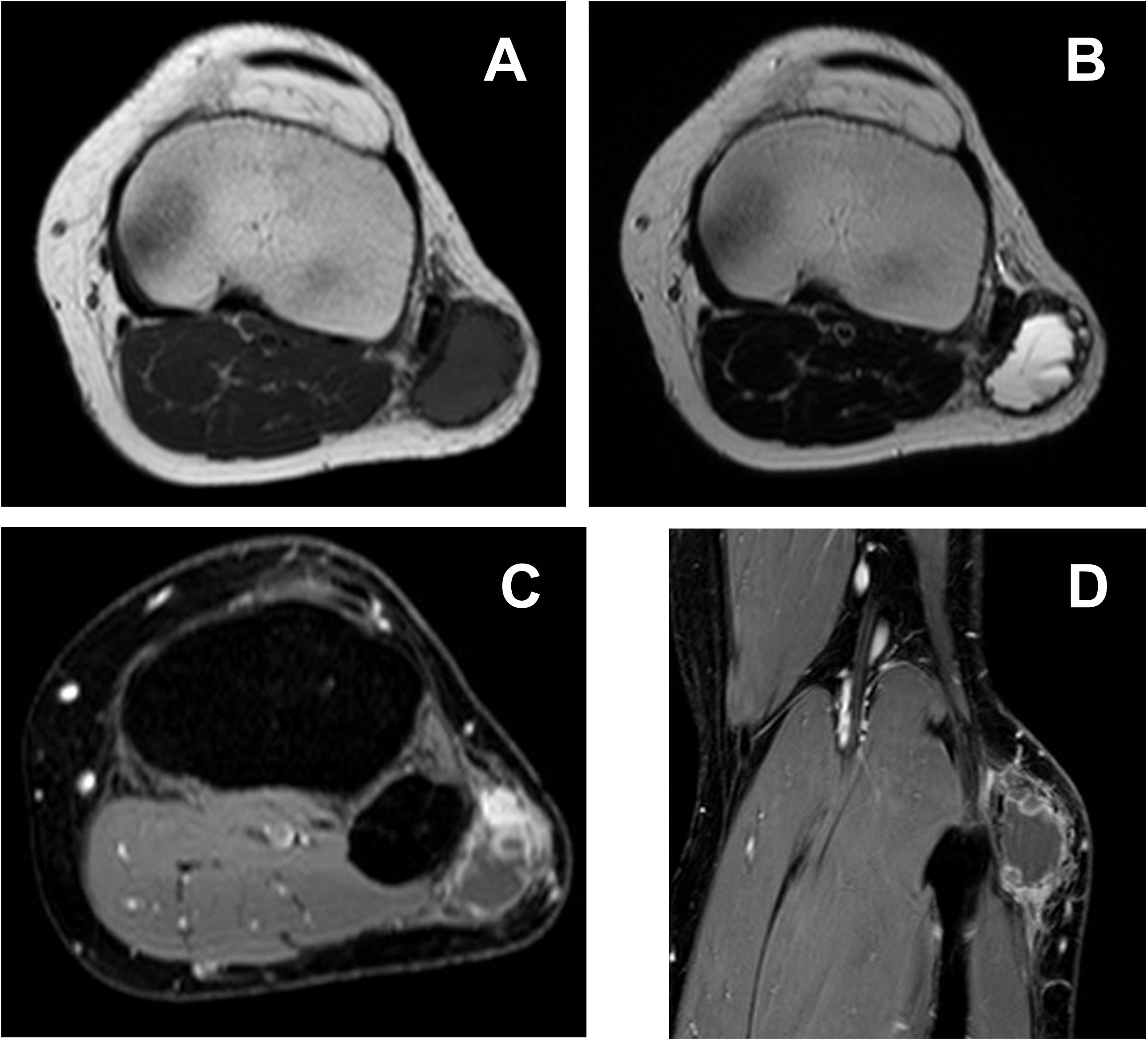
Figure 2 On T1-weighted imaging (A), the mass shows intermediate signal intensity, while heterogeneously high intensity with fluid–fluid level can be seen on T2-weighted imaging (B). Peripheral and nodular enhancement is recognizable on FS-T1-weighted imaging with contrast (C, D).
Pathological findings
Macroscopically, the gray-whitish or blackish-brown mass was located in the subcutaneous tissue, measuring 4.5 × 4 × 2.5 cm. On the cut surface, multiple cystic lesions filled with blood were noted with focal solid components (Figure 3). Histologically, the solid components were composed of diffuse proliferation of pleomorphic tumor cells, intermingled with abundant osteoclast-like giant cells (Figure 4A). Mitotic figures were numerous [22 per 10 high-power fields (HPFs)], with atypical mitoses. The tumor cells focally formed neoplastic osteoid (Figure 4B). No other morphological differentiation was confirmed. Cystic lesions included bloody materials and, internally, osteoclast-like giant cells (Figure 4C), compatible with telangiectatic or aneurysmal bone cyst-like change. The periphery of the mass consisted of benign mature bone with bland osteocytes (Figure 4D). The mature bone area was predominantly composed of lamellar bone trabeculae and focally woven bone with fibroblasts, and thus zonation was suggested (Figure 4E). EO components were adjacent to but discontinuous with mature bone lesions.
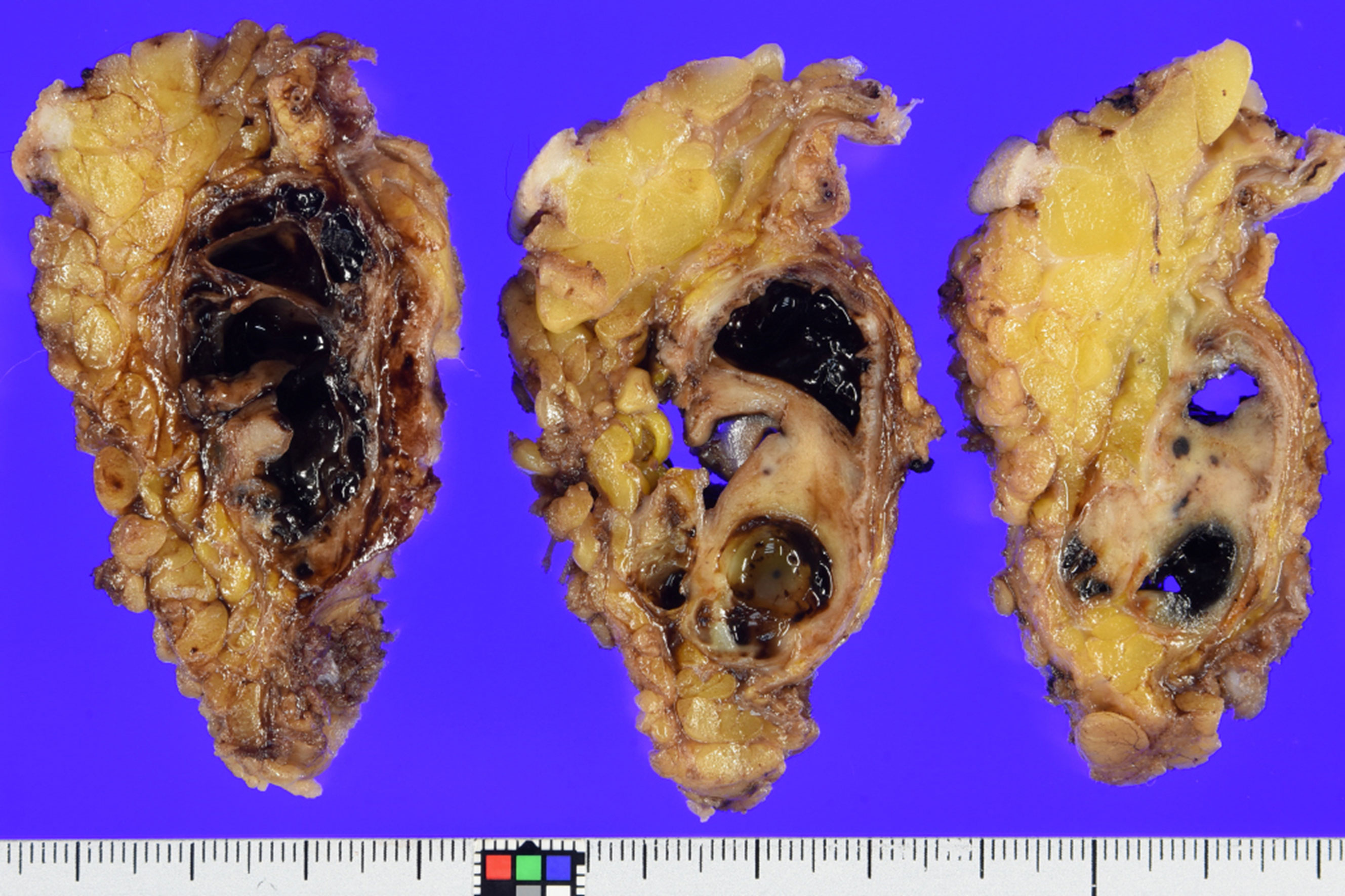
Figure 3 Grossly, on the cut section, the multicystic mass is seen to be subcutaneously located and mixed with hemorrhagic change and solid components. Mineralization was confirmed in the periphery, which corresponded to the imaging findings.
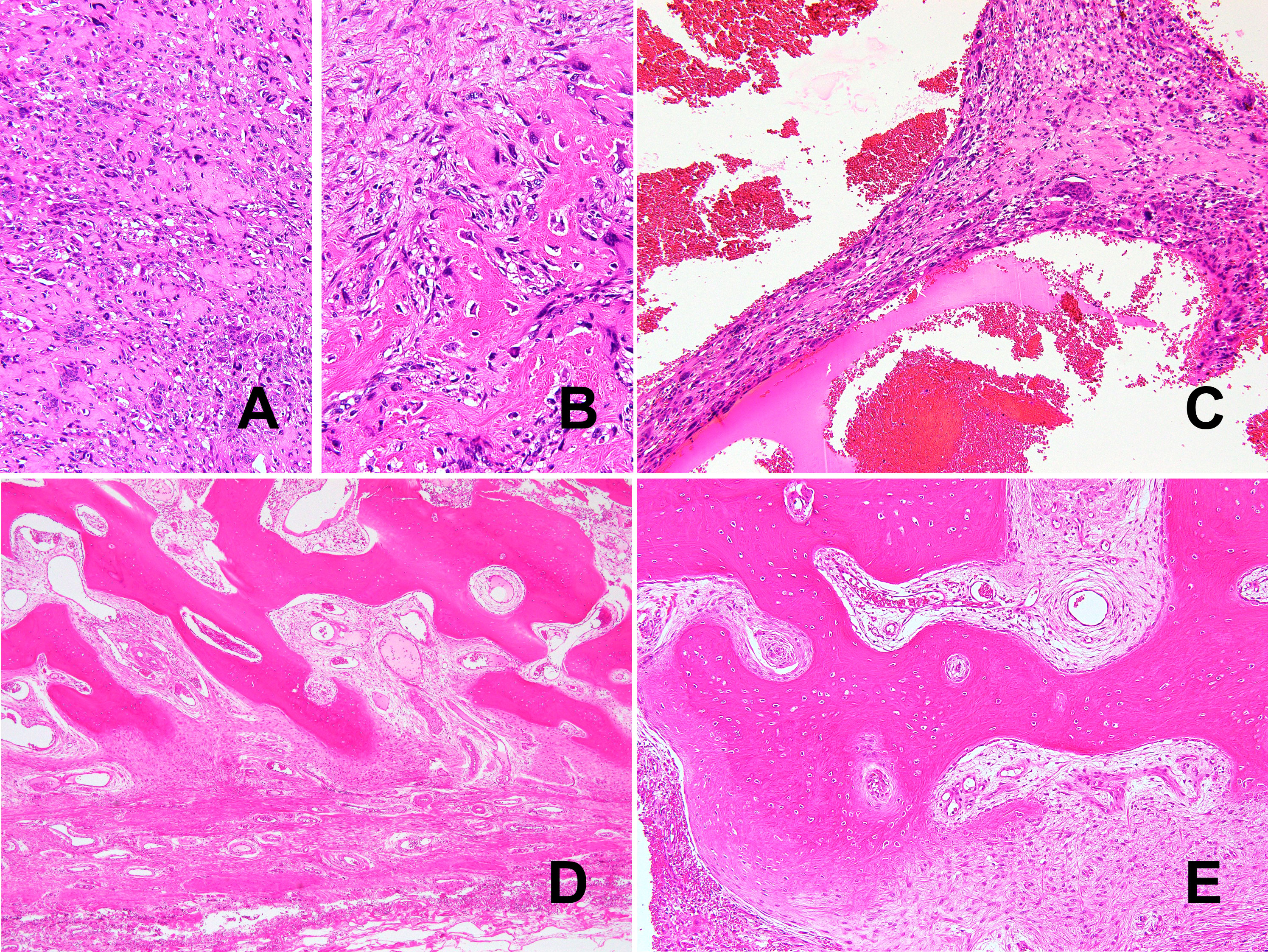
Figure 4 Histologically, the tumor was a high-grade pleomorphic sarcoma (A) with osteoid formation (B). Telangiectatic or aneurysmal bone cyst-like change with abundant osteoclast-like giant cells can also be seen (C). Morphologically bland-appearing, mature bone area in the periphery of the mass. (D). In addition to mature lamellar bone, woven bone and fibroblasts show a continuous pattern, consistent with zonation (E).
Immunohistochemically, the tumor cells showed no expression of MDM2 (Clone IF2, dilution 100×, ThermoFisher), CDK4 (Clone DCS-31, dilution 200×, Invitrogen), or H3.3 G34W (Clone RM263, dilution 800×, RevMAb Biosciences). Fluorescence in situ hybridization (FISH) revealed split signals of the USP6 gene in the tumor cells (Figure 5). Based on these pathological findings, we diagnosed EO with the preceding MO.
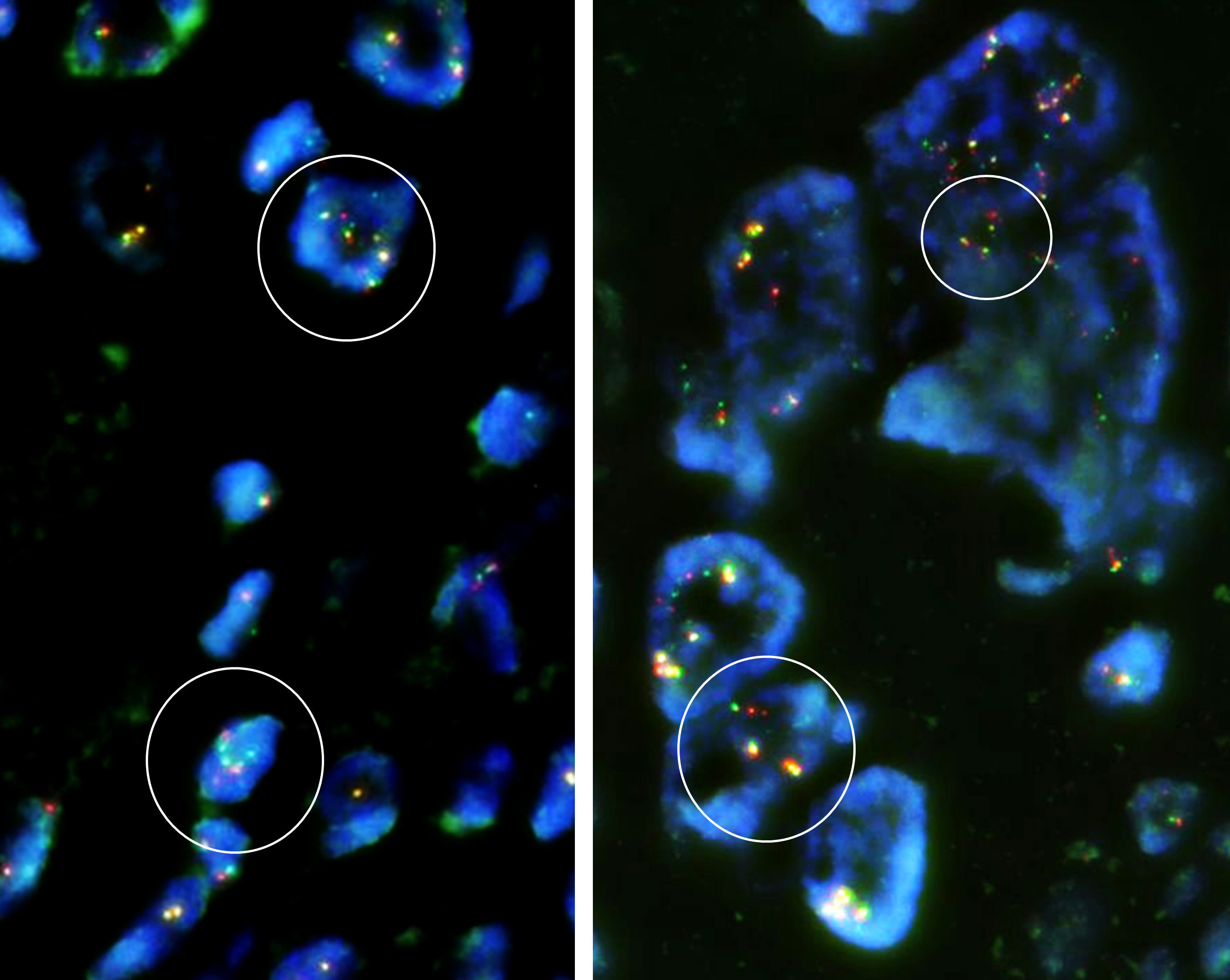
Figure 5 Fluorescence in situ hybridization (FISH) demonstrated break-apart signals of the USP6 gene (USP6 Split Dual Color FISH Probe, GSP Lab) in the EO component. Some of the tumor cells are polyploid, and focally split signals of the USP6 gene are identifiable in the tumor cells (circled in white), compatible with rearrangement of the USP6 gene.
The patient remains alive and limb function is completely preserved. Neither recurrence nor metastasis was recognized 16 months after surgery.
Discussion
MO has been regarded as an aggressively reactive process with bone formation (4). It commonly occurs in athletically active young men often with a prior history of trauma or surgery, and patients usually present with a painful mass (4). The precise etiology of MO has yet to be elucidated, but an inappropriate metaplastic change or osteogenic differentiation of fibroblasts has been proposed (4, 5). The presence of a zonal pattern of bone trabeculae is considered to be a distinctive feature of MO (4), but the zonation is ill-defined in some cases, especially in those with long-standing lesions. Rearrangement of the USP6 gene was recently reported in MO (3). MO has also been recategorized as one of the USP6-associated neoplasms (UAN), along with other tumorous lesions showing USP6 rearrangement, which suggests neoplastic change rather than a reactive process (6).
On the other hand, EO is a high-grade sarcoma with osteoid or bone matrix production by malignant neoplastic cells, and, by definition, the tumor has no connection to the skeletal system (1). EO is rare, comprising less than 1% of all soft tissue sarcomas and only about 4% of osteosarcomas overall (1, 7). The lower extremities, especially the thighs, are the most common site of involvement (1). Middle-aged to older patients, with a slight male predominance, tend to be affected (1). EO can show various histological subtypes as with conventional osteosarcoma, and telangiectatic or aneurysmal bone cyst-like change has been reported (8). EO is usually diagnosed based on the detection of malignant osteoid formation and by ruling out other sarcomas with osteogenic differentiation such as dedifferentiated liposarcoma. Although the exact etiology of EO has not been clarified, the genomic signature has revealed features overlapping those of conventional skeletal osteosarcoma (1, 2). Because EO is usually high-grade, mature bone is not a typical feature and can, therefore, pose a diagnostic dilemma as in our present case.
At the center of the tumor in this patient, there was a high-grade pleomorphic sarcoma with malignant osteoid formation and telangiectatic change. This telangiectatic change corresponded to the fluid–fluid level on MRI (Figure 2). In the periphery of the tumor, mature bone trabeculae surrounded the high-grade osteosarcoma component, which was recognized on x-ray and CT scans (Figure 1). Focal zonation was confirmed in the peripheral benign bone area.
Differential diagnoses in our case included ossifying fibromyxoid tumor (OFMT) (9), osteosarcoma arising from the tiny bone or ectopic/metaplastic bone tissue (4, 9), and EO with preceding MO. OFMT is characterized by the proliferation of uniform round cells with peripheral ossification and is characterized by a rearrangement of the PHF1 gene. The mass in our case was different from OFMT in that the tumor cells were pleomorphic. We were not able to identify either ectopic bone or continuity with the adjacent bone cortex. Therefore, OFMT and tiny bone osteosarcoma were ruled out, based on the factors discussed above. We confirmed the USP6 rearrangement in the tumor cells, a finding not previously reported in EO. Based on the USP6 rearrangement and peripheral mature bone tissue with focal zonation, we definitively diagnosed EO with preceding MO.
Malignant transformation of MO or osteosarcoma arising from preexisting MO is exceedingly rare, but several cases have been reported to date (4, 5, 7, 9). In these past reported cases, the ages ranged from 19 to 53 (mean, 37) years, and there was no gender predilection (4). The thigh was the location most commonly involved, though other areas including the jaw, retroperitoneum, wrist, and knee have also been reported (4). Benign bone tissue or MO component is commonly recognized in the periphery of the mass (7, 9). The prognosis is controversial but outcomes are generally dismal with most of the reported patients dying within 6 years of the initial presentation (4). The EO component is usually high-grade, but in one case report, a low-grade osteosarcoma with MO was described (10).
Most prior cases presented a diagnostic dilemma because they were diagnosed based on radiological and/or histological evidence of heterotopic benign bone tissue associated with EO (7, 9). The characteristic zonal distribution was confirmed in only one case (4). Furthermore, cytogenetic findings were not investigated in the previous cases, probably because osteosarcoma was long assumed to harbor no diagnostic cytogenetic abnormalities (1). As MO was known to harbor the USP6 rearrangement, we speculated that USP6 rearrangement could be detected in the sarcoma component and thus be a key factor in identifying EO arising from MO (3).
While there is no established criterion for diagnosing EO arising from MO, previously reported cases had difficulty proving the typical MO component and some of these cases are thus diagnostically questionable. In our current case, however, we identified a distinct zonation of the peripheral bone tissue that was etiologically significant in MO, as well as the USP6 rearrangement in the high-grade osteosarcoma component that existed in the center of the tumor. Our case is the first to provide confirmation of the rearrangement of USP6 and the second in which the zonation of the MO component was identified. These observations support the diagnosis of EO arising from MO and suggest that MO is a neoplastic process. Unusual USP6 rearrangement in EO may also be interpreted as MO with malignant transformation.
We have presented an exceptionally rare case of EO with preceding MO, and USP6 rearrangement appears to reinforce the concept of malignant transformation of MO. Although the precise etiology of EO accompanied by MO remains as yet poorly understood, our current case provides the first confirmation of the rearrangement of USP6 and is the second demonstration of zonation of the MO component.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not provided for this study on human participants because Our hospital does not require ethical approval in case reports. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conception and design of the study: HI and TK. Acquisition and analysis of data: HI, TK, TT, YY and SK. Drafting the manuscript or figures: HI, TK, JI and TY. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Joint Research Support Grant based on the Comprehensive Agreement between Saitama University and Saitama Medical University.
Acknowledgments
We are grateful to Mr. Nobuyuki Suzuki and Mr. Kouichi Kamada for performing immunohistochemistry and FISH analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. WHO Classification of Tumours Editorial Board. WHO classification of tumours: Soft tissue and bone tumours. 5th ed. Lyon: WHO Classification of Tumours Editorial Board (2020).
2. Jour G, Wang L, Middha S, Zehir A, Chen W, Sadowska J, et al. The molecular landscape of extraskeletal osteosarcoma: A clinicopathological and molecular biomarker study. J Pathol Clin Res (2016) 2:9–20. doi: 10.1002/cjp2.29
3. Sukov WR, Franco MF, Erickson-Johnson M, Chou MM, Unni KK, Wenger DE, et al. Frequency of USP6 rearrangements in myositis ossificans, brown tumor, and cherubism: molecular cytogenetic evidence that a subset of "myositis ossificans-like lesions" are the early phases in the formation of soft-tissue aneurysmal bone cyst. Skeletal Radiol (2008) 37:321–7. doi: 10.1007/s00256-007-0442-z
4. Savant D, Kenan S, Kenan S, Kahn L. Extraskeletal osteosarcoma arising in myositis ossificans: a case report and review of the literature. Skeletal Radiol (2017) 46:1155–61. doi: 10.1007/s00256-017-2674-x
5. Walczak BE, Johnson CN, Howe BM. Myositis ossificans. J Am Acad Orthop Surg (2015) 23:612–22. doi: 10.5435/JAAOS-D-14-00269
6. Hiemcke-Jiwa LS, van Gorp JM, Fisher C, Creytens D, van Diest PJ, Flucke U. USP6-associated neoplasms: A rapidly expanding family of lesions. Int J Surg Pathol (2020) 28:816–25. doi: 10.1177/1066896920938878
7. Agarwal K, Agarwal S, Kumar L. Retroperitoneal extraskeletal osteosarcoma with cystic change arising from heterotopic ossification: A rare entity. Annal Pathol Lab Med (2015) 2:42–6.
8. Mirra JM, Fain JS, Ward WG, Eckardt JJ, Eilber F, Rosen G. Extraskeletal telangiectatic osteosarcoma. Cancer (1993) 71:3014–9. doi: 10.1002/1097-0142(19930515)71:10<3014::AID-CNCR2820711021>3.0.CO;2-8
9. Konishi E, Kusuzaki K, Murata H, Tsuchihashi Y, Beabout JW, Unni KK. Extraskeletal osteosarcoma arising in myositis ossificans. Skeletal Radiol (2001) 30:39–43. doi: 10.1007/s002560000298
Keywords: extraskeletal osteosarcoma, myositis ossificans, USP6, high-grade sarcoma, malignant transformation
Citation: Imada H, Torigoe T, Yazawa Y, Kanno S, Ichikawa J, Yamaguchi T and Kawasaki T (2023) Case Report: Extraskeletal osteosarcoma with preceding myositis ossificans. Front. Oncol. 13:1024768. doi: 10.3389/fonc.2023.1024768
Received: 24 August 2022; Accepted: 13 February 2023;
Published: 24 February 2023.
Edited by:
Athina Markou, National and Kapodistrian University of Athens, GreeceReviewed by:
Vijay Goni, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaVeera Venkat Shivaji Ramarao Edupuganti, National Cancer Institute (NIH), United States
Copyright © 2023 Imada, Torigoe, Yazawa, Kanno, Ichikawa, Yamaguchi and Kawasaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomonori Kawasaki, tomo.kawasaki.14@gmail.com
 Hiroki Imada
Hiroki Imada