- Department of Gastrointestinal Surgery, Sichuan Cancer Hospital and Institute, Sichuan Cancer Center, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
Purpose: The study aims to assess the incidence of short-term patient-reported postoperative gastrointestinal symptoms (PGISs) after gastric cancer surgery and explore the relative risk factors for the symptoms.
Methods: Patients with radical gastrectomy were included for this retrospective and observational study. Symptoms extracted from the MD Anderson Symptom Inventory Gastrointestinal Cancer Module (MDASI-GI) were collected in postdischarge week (PDW) 1 and postoperative month (POM) 1. The distributing states of symptoms were analyzed in PDW1 and POM1. Logistic regression models were used to identify risk factors for PGISs.
Results: Among 356 patients with complete interviews, 156 (43.8%) patients reported abdominal distention in PDW1, which was significantly higher than patients in POM1 [103 (28.9%), p < 0.001]. Pain (15.2% vs. 9.8%), dysphagia (5.6% vs. 7.0%), diarrhea (3.7% vs. 3.4%), and vomiting (2.5% vs. 2.8%) had no significant differences between PDW1 and POM1. Logistic models found that risk factors for PGISs were total gastrectomy [odds ratio (OR): 1.948; 95% CI: 1.097–3.459; p = 0.023] and disturbed sleep (OR: 3.116; 95% CI: 1.831–5.303; p < 0.001) in PDW1 and female gender (OR: 1.726; 95% CI: 1.071–2.782; p = 0.025), total gastrectomy (OR: 1.729; 95% CI: 1.055–2.834; p = 0.030), and disturbed sleep (OR: 3.533; 95% CI: 1.757–7.106; p < 0.001) in POM1.
Conclusions: The main symptom after gastric cancer surgery was abdominal distention. The relative risk factors for gastrointestinal symptoms after gastric cancer surgery were total gastrectomy and disturbed sleep. Timely symptom intervention may improve the quality of life of postgastrectomy patients.
Introduction
Gastric cancer (GC) is the fifth most common type of cancer and the third leading cause of cancer deaths worldwide (1). In China, GC is still an upper gastrointestinal malignancy with high morbidity and mortality (2). At present, radical gastrectomy is the first-line treatment for GC that can prolong patients’ survival time (3, 4). As surgery for GC gradually shifts toward minimally invasive approaches that can minimize trauma and postoperative complications and accelerate recovery (5, 6), postoperative gastrointestinal symptoms (PGISs) such as abdominal distention, pain, and vomiting continue to exist that affect quality of life (7).
In recent years, more attention is being given to the patient’s subjective feelings as part of the recovery process (8). However, postoperative symptoms are not directly observable unless the patient reports them to the clinician (9). Patient-reported outcome (PRO) report is an important tool to assess the patient’s physical health, functional status, and posttreatment feeling without interpretation bias from the medical personnel (10, 11). Many studies assumed that PROs reflect more accurately on the patient’s perspective and assist clinicians to improve the quality of life of patients (12, 13).
PGIS is one of the common postoperative symptoms of GC. Gastrointestinal symptoms could increase patient distress and contribute to changes in functional status, treatment failure, depression, anxiety, and poor quality of life (14). In order to promote postoperative recovery and improve patients’ quality of life, this study aims to understand short-term patient-reported PGISs and explore relative risk factors of PGISs.
Methods
Participants
This study included patients with GC who underwent radical gastrectomy with D2 lymphadenectomy in the Department of Gastrointestinal Surgery of Sichuan Cancer Hospital in China between January 2020 and December 2021. Informed consent was taken from all of the participants in this study. The inclusion criteria were as follows: 1) age 18 year and above; 2) GC tumor–node–metastasis (TNM) stage c/pT1-4NxM0; 3) under standard radical gastrectomy (total, proximal, and distal gastrectomy). Exclusion criteria included: 1) previous abdominal radiotherapy; 2) preoperative chemotherapy; 3) pulmonary, cardiovascular, and renal disease; 4) mental illness; 5) diabetes.
Data collection
The electronic medical record system provided age, gender, surgical data, and TNM stage according to the eighth edition of “TNM Classification of Malignant Tumors” by the American Joint Committee on Cancer/Union for International Cancer Control (15). The double-tract reconstruction was used in proximal gastrectomy. Roux-en-Y anastomosis of the esophagus and jejunum was performed in total gastrectomy. Multiple anastomotic methods were applied to distal gastrectomy, such as Billroth I, Billroth II, and Billroth II plus Braun anastomosis and Roux-en-Y anastomosis of the remnant stomach and jejunum. PGISs and sleep status were reported by patients, and each patient was interviewed by a healthcare provider who was trained in qualitative interviewing. The interviews were qualitative one-to-one interviews, which were conducted postdischarge week (PDW) 1 and postoperative month (POM) 1. Each interview lasted for 20 min per patient. PGISs (pain, abdominal distention, diarrhea, vomiting, dysphagia) and disturbed sleep were asked during the interview. All of the symptoms were extracted from the MD Anderson Symptom Inventory Gastrointestinal Cancer Module (MDASI-GI), a valid reliable questionnaire for assessing symptom severity and interference with function in gastrointestinal cancer (16, 17).
Data analysis
Demographic data, clinical data, and symptoms were presented as numbers and percentages. The t test and chi-square test were used to examine the quantitative and categorical data, respectively.
We performed univariate analyses using gender (women vs. men), approach of operations (open surgery vs. laparoscopic surgery), type of gastrectomy (total vs. proximal vs. distal), TNM stage (stages I–II vs. stage III), and sleep status (disturbed vs. non-disturbed), and PGISs were taken as the dependent variable. Those variables with p-values ≤0.05 were entered into the multivariate logistic analyses to determine the risk factors for PGISs.
Statistical analyses were performed using SPSS 20.0 software (IBM Corp., Armonk, NY, USA). A two-sided p-value <0.05 was considered significant.
Results
Patient characteristics
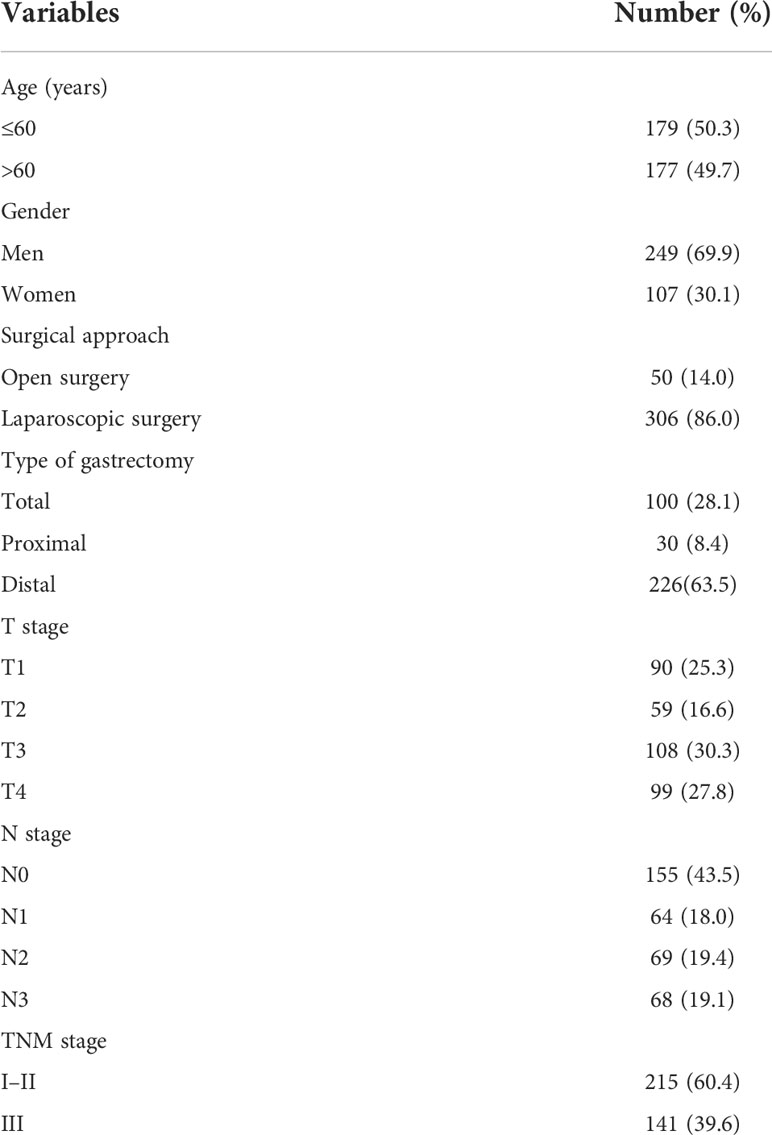
A total of 356 cases of postoperative patients with GC were involved in the study. There were 249 (69.9%) men and 107 (30.1%) women, and over 50% of the patients were aged ≤60 years (50.3%). Fifty (14%) patients underwent open surgery, and 306 (86%) patients underwent laparoscopic surgery. Of patients included in the study, 215 (60.4%) patients were staged with TNM I–II, and 141 (39.6%) patients were staged with TNM III. Baseline demographic and clinical features are summarized in Table 1.
Postoperative gastrointestinal symptoms
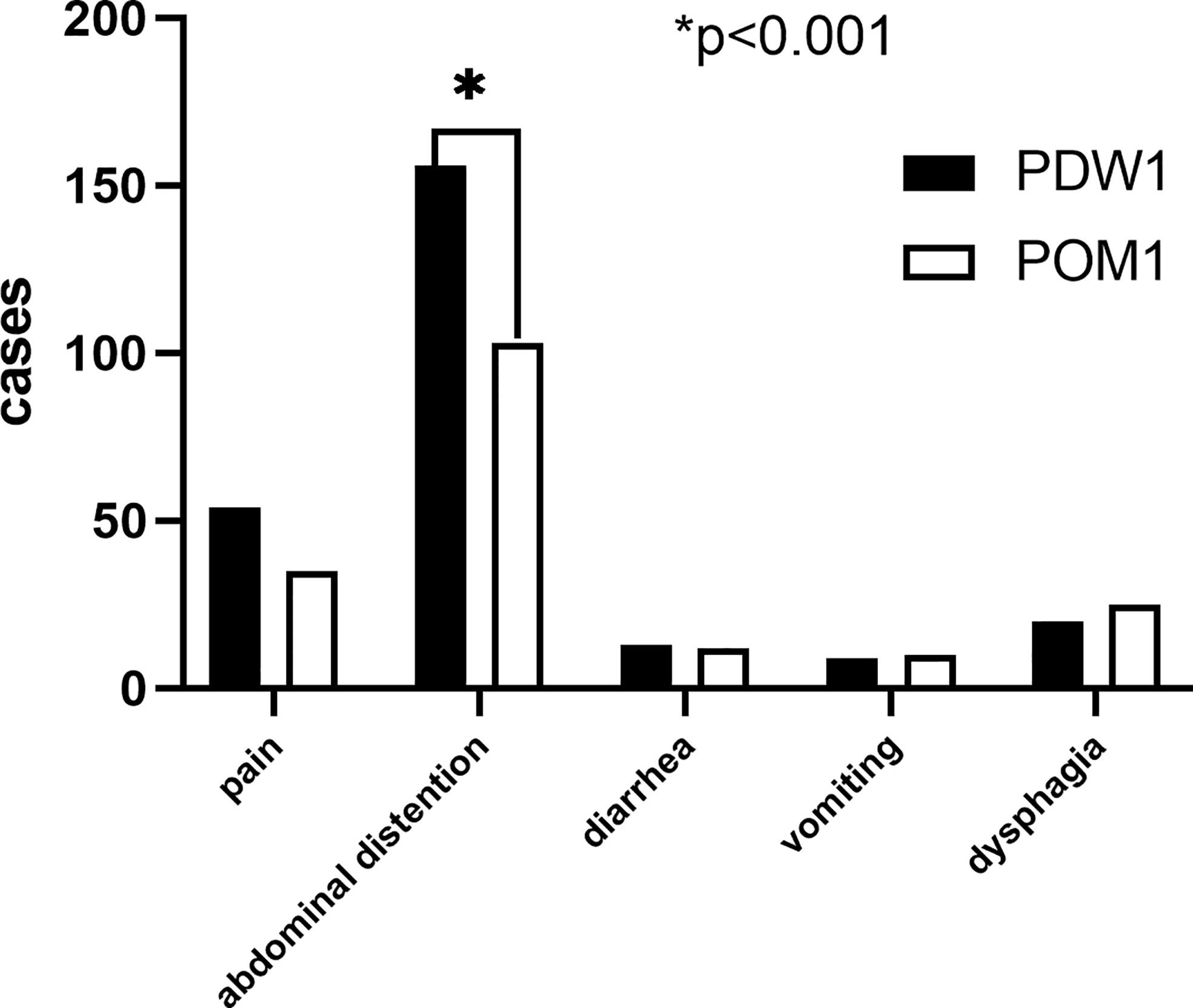
A total of 252 (70.8%) patients reported PGISs in PDW1, comprising abdominal distention (156, 43.8%), pain (54, 15.2%), dysphagia (20, 5.6%), diarrhea (13, 3.7%), and vomiting (9, 2.5%). Of the 356 patients, 185 (52.0%) patients reported PGISs about abdominal distention (103, 28.9%), pain (35, 9.8%), dysphagia (25, 7.0%), diarrhea (12, 3.4%), and vomiting (10, 2.8%) in POM1. The ratio of abdominal distention symptom reported in POM1 was lower than that in PDW1 (28.9% vs. 43.8%, p < 0.001). However, similar numbers of other PGISs were reported at two points in time (Figure 1).
The relations between postoperative gastrointestinal symtoms and clinical features
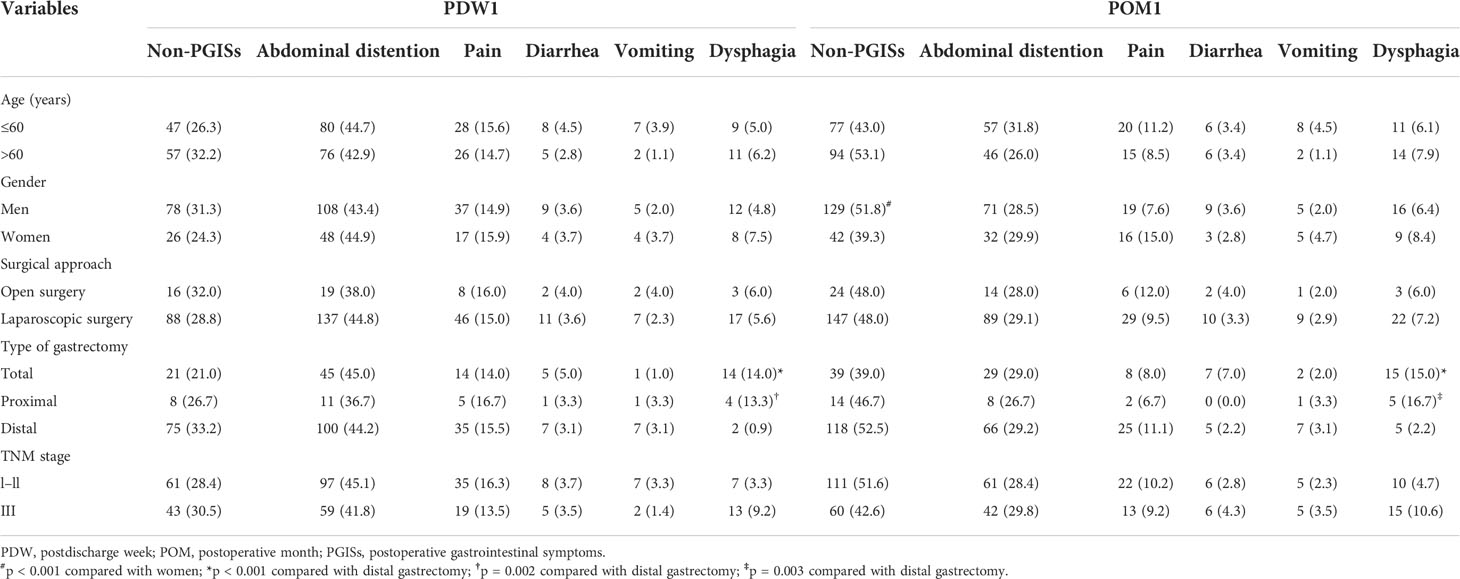
In POM1, the ratio of PGISs in men was less than that in women (48.2% vs. 60.7%, p < 0.001). In Table 2, compared with patients who underwent distal gastrectomy, the ratio of dysphagia was significantly higher in patients with total gastrectomy in PDW1 and POM1 (all p < 0.001) and the ratio of dysphagia was also significantly higher in patients with proximal gastrectomy in PDW1 (p = 0.002) and POM1 (p = 0.003).
The relations between postoperative gastrointestinal symtoms and sleep status
As Table 3 shows, patients with disturbed sleep reported PGISs more than patients with non-disturbed sleep in PDW1 and POM1 (all p < 0.001).
Risk factors for postoperative gastrointestinal symtoms
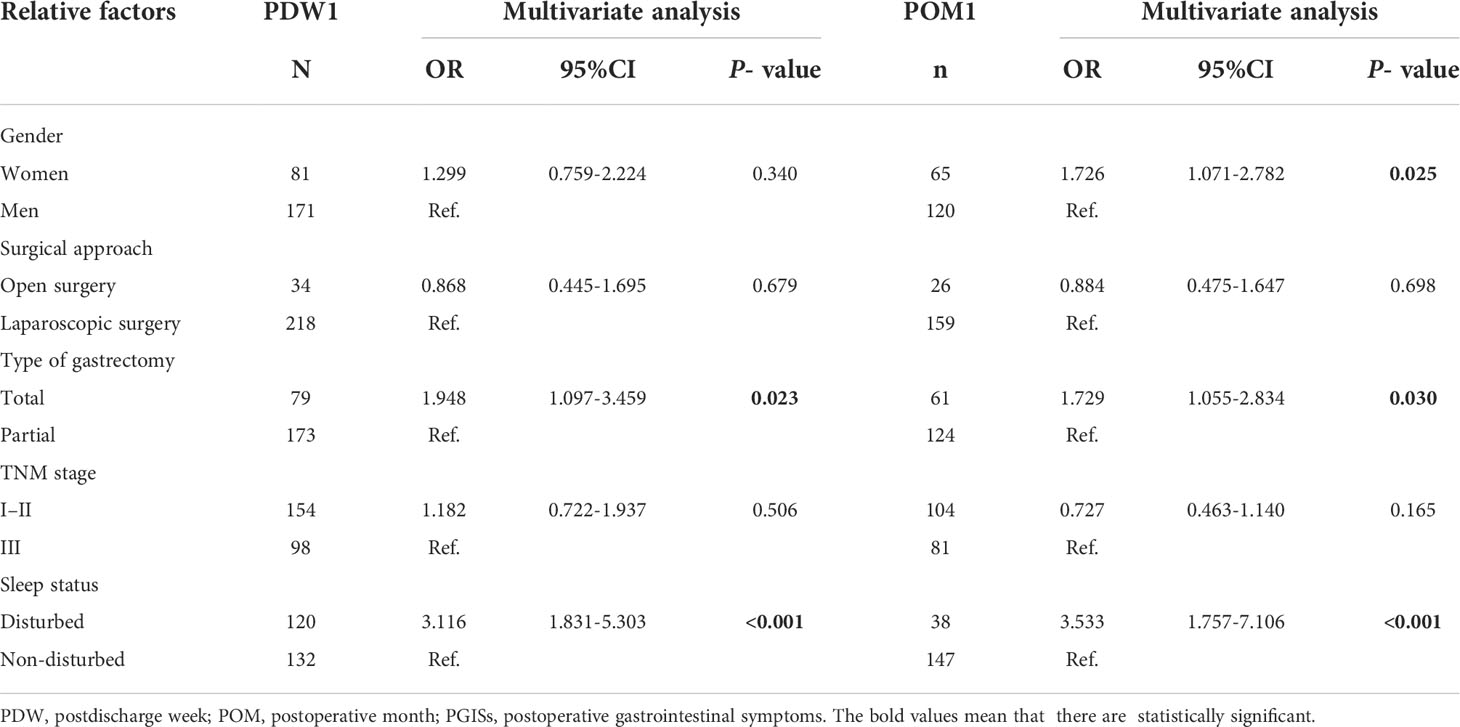
The multivariate logistic regression model identified that variables significantly associated with PGISs in PDW1 included total gastrectomy [odds ratio (OR): 1.948; 95% CI: 1.097–3.459; p = 0.023], disturbed sleep (OR: 3.116; 95% CI: 1.831–5.303; p < 0.001), and female gender (OR: 1.726; 95% CI: 1.071–2.782; p = 0.025) and total gastrectomy (OR: 1.729; 95% CI: 1.055–2.834; p = 0.030) and disturbed sleep (OR: 3.533; 95% CI: 1.757–7.106; p < 0.001) in POM1 (Table 4).
Discussion
Gastrointestinal symptoms are particularly common after abdominal surgery (18, 19). To our knowledge, this is the first study to alone observe short-term PGISs in GC. Five symptoms extracted from MDASI-GI were reported by patients through interviews. In this study, we found that abdominal distension is the main PGIS (43.8% vs. 28.9%), followed by pain (15.2% vs. 9.8%), dysphagia (5.6% vs. 3.4%), diarrhea (3.7% vs. 3.4%), and vomiting (2.5% vs. 2.8%) in PDW1 and POM1. Furthermore, with the recovery process, abdominal distension symptom was significantly relieved within 1 month after surgery, and other symptoms were also relieved but without statistical differences. Abdominal distension is the most common symptom after GC surgery and often associated with gastrointestinal dysfunction; studies found (18) that operation, analgesia, and inflammatory reaction could lead to the occurrence and development of gastrointestinal dysfunction. Therefore, the proportion of abdominal distension in early postoperative period was high, but with the recovery of gastrointestinal function, its ratio will decrease significantly. Previous studies showed that PGISs could increase the risk of postoperative complications, prolong the time of hospitalization, delay the follow-up adjuvant treatment, and reduce the quality of life (20, 21). PRO can provide the real feeling of patients after surgery, so that clinicians can carry out the necessary intervention to accelerate postoperative rehabilitation and improve the quality of life.
In this study, we found that the incidence of dysphagia symptom was higher in patients with total and proximal gastrectomy. This is consistent with studies from Lee et al. (22) and Choi et al. (23) suggesting that the dysphagia symptom was worse in the total gastrectomy group. Because of the disharmony and inconsistency of contraction and peristalsis between the esophagus and jejunum, patients with total or proximal gastrectomy sometimes suffer from dysphagia when eating. In addition, patients who underwent distal gastrectomy have a normal cardiac structure, so less dysphagia symptom is reported. According to multivariate models, total gastrectomy was considered as a risk factor for PGISs (p < 0.05). To reduce the burden of PGISs, surgeons should make the decision of total gastrectomy with caution and give professional dietary guidance after surgery.
The occurrence and development of PGISs are often related to many factors. Ana et al. (24) considered that gender is the influencing factor of gastrointestinal symptoms in patients with advanced cancer. Nolte et al. (25) found that the quality of life also varies by gender. In this study, we found that the ratio of women with PGISs was more than that of men in POM1 (60.7% vs. 48.2%, p < 0.001); this result clearly showed that the recovery of the gastrointestinal tract in women was slow after surgery. In fact, women are not only more emotionally sensitive than men but also more likely to think negatively (26, 27). In addition, Chinese male patients may show higher tolerance to their own symptoms due to the influence of traditional culture. Thus, our clinicians should keep in mind that gender will affect PROs and help them adjust their moods.
Moreover, we found that sleep status affected the occurrence of PGISs, and patients with disturbed sleep suffered from PGISs more than those without sleep disturbance (p < 0.001). Evidence has indicated that sleep disturbance occurred more frequently among cancer survivors (28), especially in GC (29), which reduces the quality of life and increases the incidence of complications (30). Patients who underwent gastrectomy always have psychosocial distresses such as fear of recurrence, difficulty in resuming social life, and concerns about family and finances (31), which can affect sleep status and then aggravate PGISs. In this study, we found that the risk of increased ratios of PGISs with disturbed sleep was 3.116 times that of patients with non-disturbed sleep in PDW1 (OR: 3.116; 95% CI: 1.831–5.303; p < 0.001) and 3.533 times in POM1 (OR: 3.533; 95% CI: 1.757–7.106; p < 0.001). Clinically, the sleep status and psychological status of patients are often ignored by surgeons. Therefore, clinicians need to pay more attention to sleep conditioning and psychological comfort.
In order to improve patients’ symptoms and enhance the quality of life of patients, timely intervention is very important. Comprehensive health education was proven to be an effective method, which could markedly improve patients’ quality of life through disease awareness-raising activities, guidance on behavior and lifestyle, rehabilitation management, and psychological counseling (32). Drug therapy is also an option. Previous studies have found that auricular-plaster therapy of traditional Chinese medicine can improve postoperative gastrointestinal dysfunction and the quality of life (33).
This study has several limitations. Firstly, patients and healthcare providers were from the same department; patients’ reported symptoms would be biased. Secondly, few patients underwent open surgery, which may lead us to underestimate the incidence of PGISs. However, minimally invasive surgical approaches to the treatment of GC have become increasingly more common (34). Finally, we only observed the main symptoms; other symptoms reported by patients were not included, which may lead to selection bias. Further research needs to be continued.
Conclusions
The main symptom after GC surgery was abdominal distention. The relative risk factors for gastrointestinal symptoms after GC surgery were total gastrectomy and disturbed sleep. Timely symptom intervention may improve the quality of life of postgastrectomy patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
RX and ZD designed the study; QG and SX collected the data; RX drafted the manuscript; PZ, ZD, and SX undertook the statistical analysis. All authors read and approved the final manuscript.
Funding
This work was supported by the Sichuan Provincial Science and Technology Project (No. 2020YFS0430; No. 2018SZ0263).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PGISs, postoperative gastrointestinal symptoms; MDASI-GI, MD Anderson Symptom Inventory Gastrointestinal Cancer Module; PDW, postdischarge week; POM, postoperative month; GC, gastric cancer; PRO, patient-reported outcome; TNM, tumor–node–metastasis.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries]. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Cui H, Cao B, Liu G, Xi H, Chen Z, Liang W, et al. Comparison of short-term outcomes and quality of life in totally laparoscopic distal gastrectomy and totally robotic distal gastrectomy for clinical stage I-III gastric cancer: study protocol for a multi-institutional randomised clinical trial. BMJ Open (2021) 11:e043535. doi: 10.1136/bmjopen-2020-043535
3. Xu R, Xiao S, Ding Z, Zhao P. Does early postoperative enteral ecoimmunonutrition enhance intestinal function in gastric cancer? Asia Pac J Clin Nutr (2020) 29:469–75. doi: 10.6133/apjcn.202009_29(3).0004
4. Zheng J, Xue Y, Li C. Short-term efficacy and quality of life of gastric cancer patients undergoing radical gastrectomy assisted by external vision. Comput Math Methods Med (2021) 2021:4256347. doi: 10.1155/2021/4256347
5. Li GX. Shifting paradigm toward minimally invasive gastrectomy for gastric cancer–china's contribution. Zhonghua Wei Chang Wai Ke Za Zhi (2021) 24:657–61. doi: 10.3760/cma.j.cn.441530-20210728-00301
6. Berlth F, Knospe L, Jansen-Winkeln B, Hadzijusufovic E, Tagkalos E, Niebisch S, et al. Status of minimally invasive gastrectomy: Current advancements: robotic surgery and intraoperative imaging for gastric cancer]. Chirurg (2021) 92:528–34. doi: 10.1007/s00104-021-01391-z
7. Eom BW, Lee J, Lee IS, Son YG, Ryu KW, Kim SG, et al. Development and validation of a symptom-focused quality of life questionnaire (KOQUSS-40) for gastric cancer patients after gastrectomy. Cancer Res Treat (2021) 53:763–72. doi: 10.4143/crt.2020.1270
8. Hu X, Zhao F, Yu H, Luo Y, Liu J, Zhang Y. GC-PROM: validation of a patient-reported outcomes measure for Chinese patients with gastric cancer. BMC Cancer (2020) 20:41. doi: 10.1186/s12885-020-6518-z
9. Lee L, Tran T, Mayo NE, Carli F, Feldman LS. What does it really mean to "recover" from an operation? Surgery (2014) 155:211–6. doi: 10.1016/j.surg.2013.10.002
10. Fiore JF Jr, Figueiredo S, Balvardi S, Lee L, Nauche B, Landry T, et al. How do we value postoperative recovery?: a systematic review of the measurement properties of patient-reported outcomes after abdominal surgery. Ann Surg (2018) 267:656–69. doi: 10.1097/SLA.0000000000002415
11. US Department of Health and Human Services FDA Center for Drug Evaluation and Research, US Department of Health and Human Services FDA Center for Biologics Evaluation and Research, US Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes (2006) 4:79. doi: 10.1186/1477-7525-4-79
12. Schütte K, Schulz C, Middelberg-Bisping K. Impact of gastric cancer treatment on quality of life of patients. Best Pract Res Clin Gastroenterol (2021) 50-51:101727. doi: 10.1016/j.bpg.2021.101727
13. Hu Y, Zaydfudim VM. Quality of life after curative resection for gastric cancer: Survey metrics and implications of surgical technique. J Surg Res (2020) 251:168–79. doi: 10.1016/j.jss.2020.02.005
14. Cherwin CH. Gastrointestinal symptom representation in cancer symptom clusters: a synthesis of the literature. Oncol Nurs Forum (2012) 39:157–65. doi: 10.1188/12.ONF.157-165
15. Ji X, Bu ZD, Yan Y, Li ZY, Wu AW, Zhang LH, et al. The 8th edition of the American joint committee on cancer tumor-node-metastasis staging system for gastric cancer is superior to the 7th edition: results from a Chinese monoinstitutional study of 1663 patients. Gastric Cancer (2018) 21:643–52. doi: 10.1007/s10120-017-0779-5
16. Wang XS, Williams LA, Eng C, Mendoza TR, Shah NA, Kirkendoll KJ, et al. Validation and application of a module of the m. d. Anderson symptom inventory for measuring multiple symptoms in patients with gastrointestinal cancer (the MDASI-GI). Cancer (2010) 116:2053–63. doi: 10.1002/cncr.24920
17. Li N, Lu J, Xia D, Jiang X, Wen X, Qin X, et al. Serum biomarkers predict adjuvant chemotherapy-associated symptom clusters in radical resected colorectal cancer patients. J Gastrointest Oncol (2022) 13:197–209. doi: 10.21037/jgo-21-904
18. Tan S, Wu G, Yu W, Li N. Research advance in causes of postoperative gastrointestinal dysfunction. Zhonghua Wei Chang Wai Ke Za Zhi (2016) 19:351–5. doi: 10.3760/cma.j.issn.1671-0274.2016.03.029
19. Wang L, Ding K, Yang D, Zhao X. Management strategies of postoperative gastrointestinal tract dysfunction:a review of 210 cases. Asian J Surg (2022) 45:479–80. doi: 10.1016/j.asjsur.2021.08.065
20. Zhang S, Guo W, Jiao Y, Guo X, Xu L, Gao H. Systematic review and meta-analysis of the effect of transcutaneous electrical acupoint stimulation on gastrointestinal function after laparoscopic surgery. Ann Palliat Med (2021) 10:11840–8. doi: 10.21037/apm-21-3046
21. Chen Y, Dong C, Lian G, Li D, Yin Y, Yu W, et al. Dexamethasone on postoperative gastrointestinal motility: A placebo-controlled, double-blinded, randomized controlled trial. J Gastroenterol Hepatol (2020) 35:1549–54. doi: 10.1111/jgh.15020
22. Lee JH, Lee HJ, Choi YS, Kim TH, Huh YJ, Suh YS, et al. Postoperative quality of life after total gastrectomy compared with partial gastrectomy: Longitudinal evaluation by European organization for research and treatment of cancer-OG25 and STO22. J Gastric Cancer (2016) 16:230–9. doi: 10.5230/jgc.2016.16.4.230
23. Choi JH, Han SU, Yang HK, Kim YW, Ryu KW, Park JM, et al. The pattern of postoperative quality of life following minimally invasive gastrectomy for gastric cancer: a prospective cohort from Korean multicenter robotic gastrectomy trial. Ann Surg Treat Res (2020) 99:275–84. doi: 10.4174/astr.2020.99.5.275
24. Ana J, Rosario M, Alberto A, Virginia M, Yolanda V, Beatriz M, et al. Symptom clusters in advanced cancer. J Pain Symptom Manage (2011) 42(1):24–31. doi: 10.1016/J.JPAINSYMMAN.2010.10.266
25. Nolte S, Liegl G, Petersen MA, Aaronson NK, Costantini A, Fayers PM, et al. General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the unites states. Eur J Cancer (2019) 107:153–63. doi: 10.1016/j.ejca.2018.11.024
26. Linden W, Vodermaier A, Mackenzie R, Greig D. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord (2012) 141:343–51. doi: 10.1016/j.jad.2012.03.025
27. Belov OO, Dronenko VG, Rybinska VA, Tkach AA, Shevchuk TV. Gender features of depressive and anxious manifestations of the lung cancer patients. Wiad Lek (2022) 75:393–6. doi: 10.36740/WLek202202112
28. Garland SN, Johnson JA, Savard J, Gehrman P, Perlis M, Carlson L, et al. Sleeping well with cancer: a systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr Dis Treat (2014) 10:1113–24. doi: 10.2147/NDT.S47790
29. Yoon HS, Yang JJ, Song M, Lee HW, Lee Y, Lee KM, et al. Short sleep duration and its correlates among cancer survivors in Korea: the Korea national health and nutrition examination surveys. Asian Pac J Cancer Prev (2015) 16:4705–10. doi: 10.7314/apjcp.2015.16.11.4705
30. Schulte FSM, Chalifour K, Eaton G, Garland SN. Quality of life among survivors of adolescent and young adult cancer in Canada: A young adults with cancer in their prime (YACPRIME) study. Cancer (2021) 127:1325–33. doi: 10.1002/cncr.33372
31. Rastogi S, Tevaarwerk AJ, Sesto M, Van Remortel B, Date P, Gangnon R, et al. Effect of a technology-supported physical activity intervention on health-related quality of life, sleep, and processes of behavior change in cancer survivors: A randomized controlled trial. Psychooncology (2020) 29:1917–26. doi: 10.1002/pon.5524
32. Gao Q, Li H, Zou Y, Hou B, Liu L. Effectiveness of a comprehensive post-operative health education program in improving quality of life after gastric cancer surgery. Ann Palliat Med (2020) 9:921–6. doi: 10.21037/apm.2020.04.14
33. Zhang Y, Wang X, Yang H. Effect of traditional Chinese medicine nursing on postoperative patients with gastric cancer and its impact on quality of life. Am J Transl Res (2021) 13(5):5589–95.
Keywords: surgery, gastrectomy, gastric cancer, symptom, risk factor
Citation: Xu R, Gu Q, Xiao S, Zhao P and Ding Z (2022) Patient-reported gastrointestinal symptoms following surgery for gastric cancer and the relative risk factors. Front. Oncol. 12:951485. doi: 10.3389/fonc.2022.951485
Received: 24 May 2022; Accepted: 23 August 2022;
Published: 14 September 2022.
Edited by:
Shantibhusan Senapati, Institute of Life Sciences (ILS), IndiaReviewed by:
Ilker Sengul, Giresun University, TurkeyKeduovinuo Keditsu, Putuonuo Nursing Home, India
Alessandro Puzziello, University of Salerno, Italy
Copyright © 2022 Xu, Gu, Xiao, Zhao and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi Ding, ZHpzY2NoQHNpbmEuY29t
 Rui Xu
Rui Xu Shuomeng Xiao
Shuomeng Xiao