- 1Department of Radiation Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Radiation Oncology, Beijing Shijitan Hospital, Capital Medical University, Beijing, China
Objective: To investigate whether radiation-induced lymphopenia (RIL) affects survival and identify the predictors of RIL in postoperative esophageal cancer.
Materials and methods: Post hoc analysis was conducted on data from 116 patients with esophageal cancer from a randomized controlled trial comparing adjuvant therapy with surgery alone. Doses of 54 Gy in 27 fractions was delivered in the postoperative radiotherapy (PORT) group and 50.4 Gy in 28 fractions combined with chemotherapy was delivered in postoperative concurrent chemoradiotherapy (POCRT) group. Blood counts were obtained before, during, and at first follow-up after treatment. Lymphopenia was graded per version 4.03 of the Common Terminology Criteria for Adverse Events. Disease-free survival (DFS) and overall survival (OS) were analyzed using the Kaplan-Meier method, and compared between groups using the log-rank test. Receiver operating characteristic curves identified thresholds for preventing grade 4 (G4) lymphopenia.
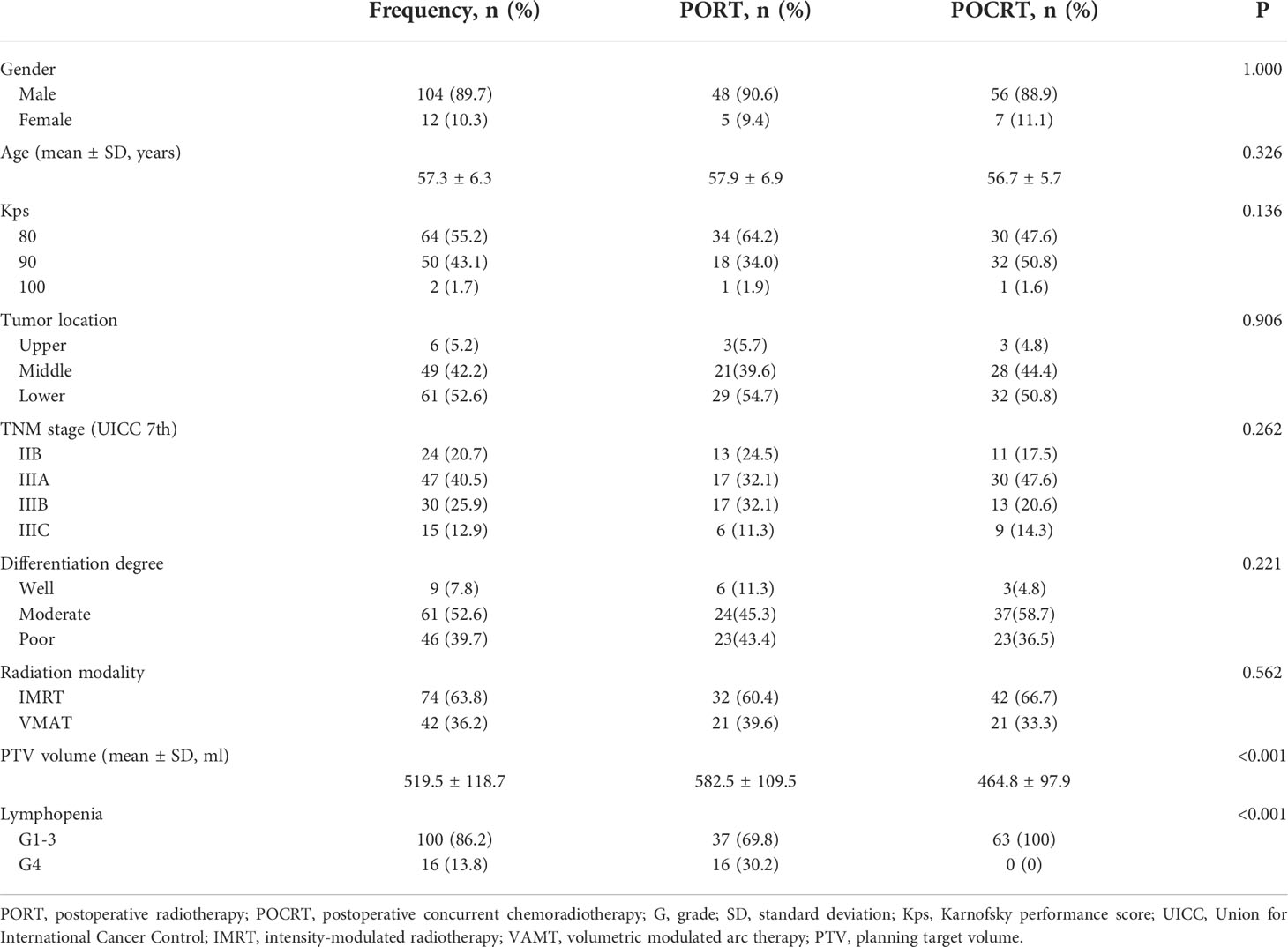
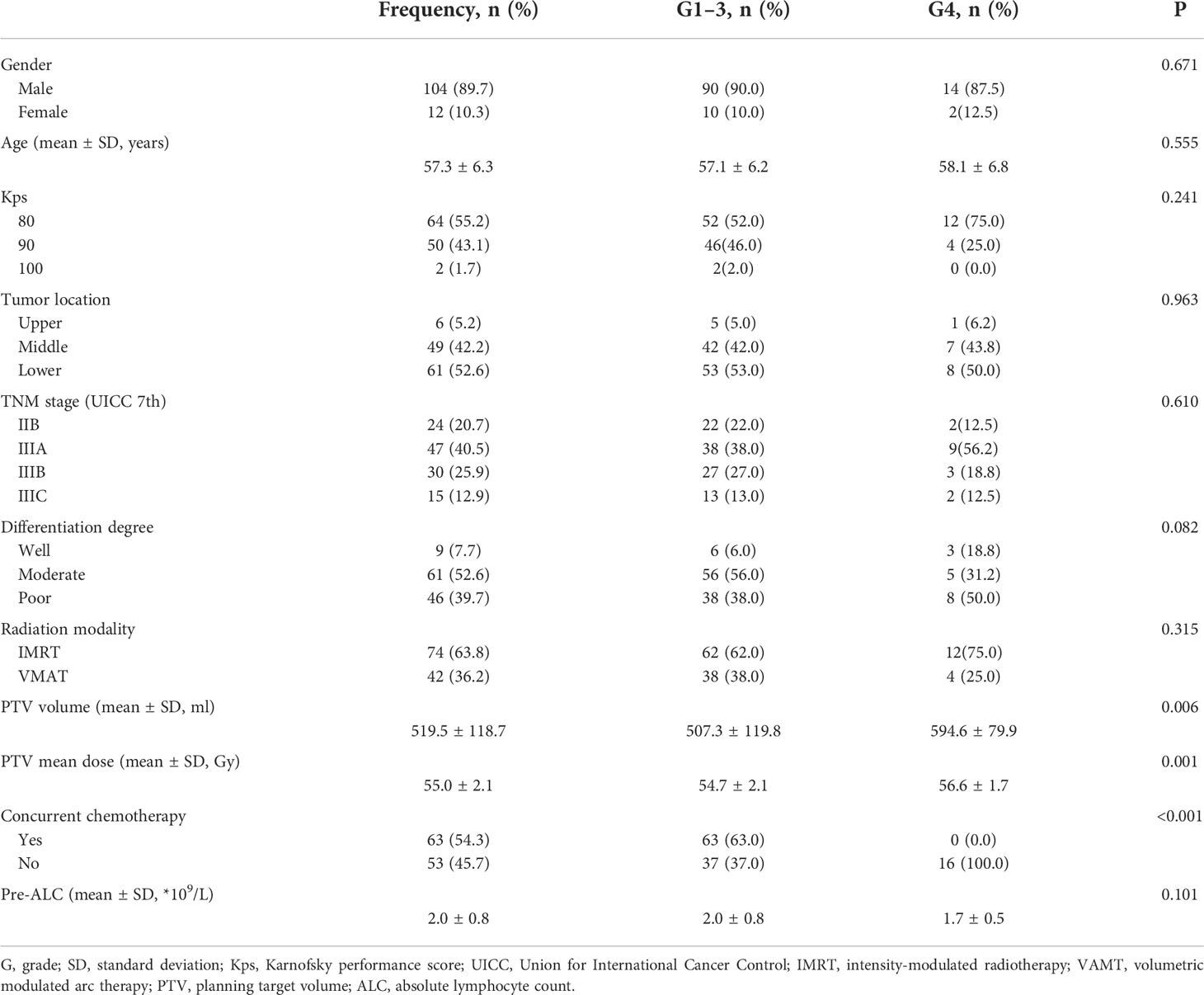
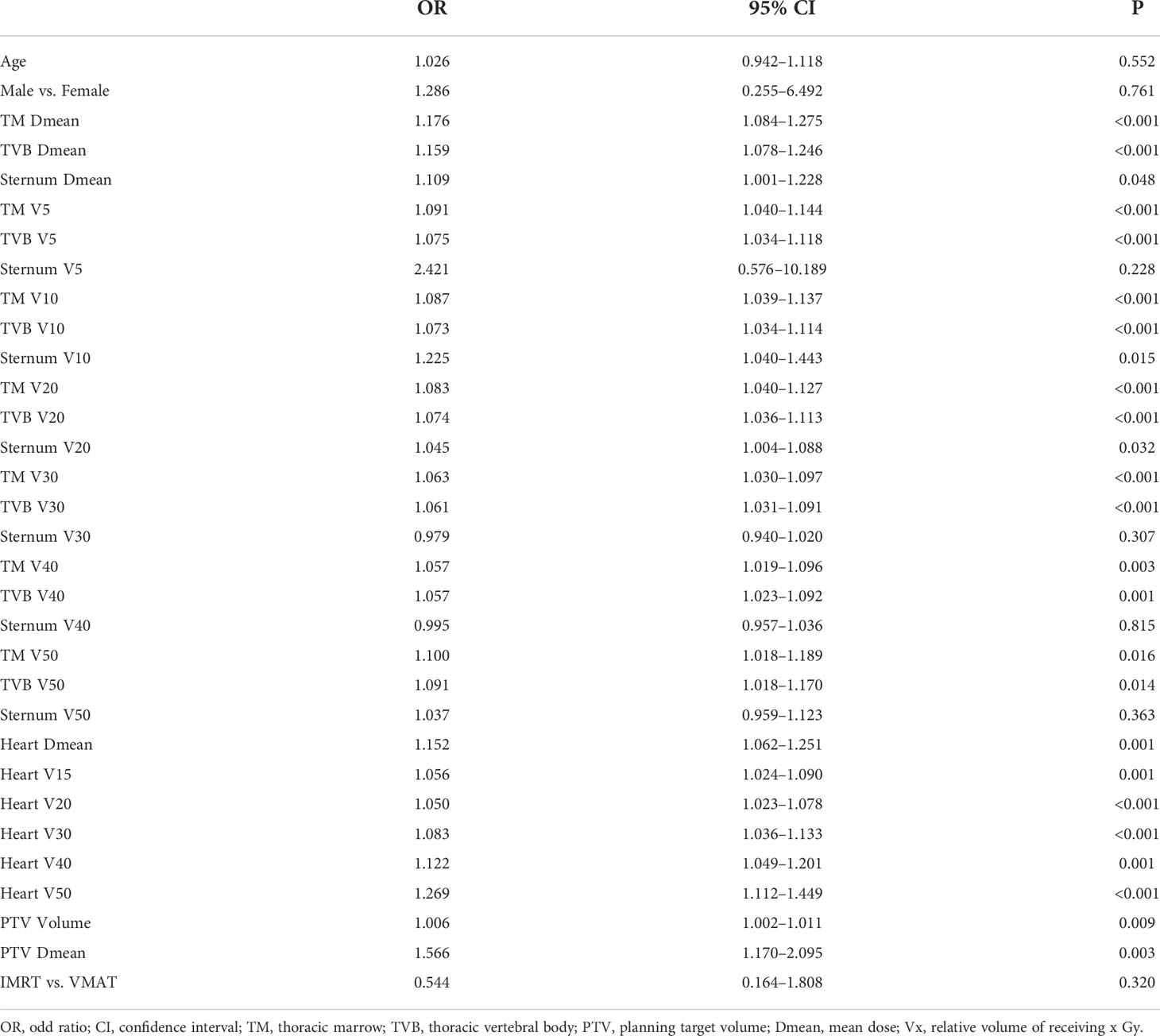
Results: Median follow-up duration was 56.0 months. During treatment, 16 patients (13.8%) had G4 lymphopenia. All cases of G4 lymphopenia occurred in group PORT (30.2% vs 0.0%, p<0.001). Baseline absolute lymphocyte count was comparable between G1-3 and G4 patients (2.0 ± 0.8 *109/L vs 1.7 ± 0.5 *109/L; p=0.101). The 3-year DFS was significantly lower in group G4 lymphopenia than that in group G1-3 (31.3% vs 57.6%, p=0.036). The 3-year OS was comparable between both groups (50.0% vs 66.5%, p=0.095). Logistic regression analysis revealed that exposed more thoracic marrow (TM V20 ≥75%; TVB V20 ≥71%), heart (V15 ≥40%) and PTV (volume ≥507 ml) were associated with G4 lymphopenia (p<0.05).
Conclusions: G4 RIL had poor disease-free survival, which may be related to more dose exposure of thoracic marrow and heart due to larger PTV. Reasonably reducing the radiation field combined with concurrent chemotherapy, or radiation dose constraints for these normal tissues may be sufficient to decrease the incidence of G4 lymphopenia, but further prospective trials are needed to verify the results.
Clinical Trial Registration: clinicaltrials.gov, identifier NCT02279134
Introduction
According to the Worldwide Esophageal Cancer Collaboration Investigators in 2016, about 58.7% of patients with esophageal cancer underwent surgical resection first (1). About 20% of patients with R0 resection in our hospital received postoperative radiotherapy. Especially for pathological stage III or lymph node positive esophageal cancer, it is reported that postoperative radiotherapy can significantly reduce the local regional recurrence rate and improve the survival rate (2–5). Furthermore, our research group has always devoted to the esophageal cancer clinical research after surgery alone, and has conducted many data analyses and improvement on the postoperative radiation field. Radiation therapy is an essential component of the treatment of esophageal cancer. However, it is reported that radiation may suppress host immunity, manifesting as lymphopenia (6, 7). Lymphocytes are extremely radiosensitive; therefore, relatively low doses can result in significant depletion of lymphocyte number (8). Radiation-induced lymphopenia (RIL) has been reported to adversely affect survival of patients with solid malignancies, such as glioma, lung cancer, and breast cancer (9–11). Severe lymphopenia during chemoradiotherapy is a strong predictor of poor outcomes and pathologic response rates in esophageal cancer (12–14). However, to the best of our knowledge, no study has been conducted on postoperative radiation therapy in esophageal cancer. In this study, we aimed to investigate whether RIL could affect survival, and identify the predictors of severe lymphopenia in postoperative esophageal cancer.
Materials and methods
Patients
This study was a post hoc analysis of data from a randomized controlled trial (NCT02279134) that was conducted from October 2014 through December 2019. The original trial recruited a total of 172 patients with esophageal cancer who had undergone radical esophagectomy. All patients were pathologically confirmed as stage IIB-III. The patients were randomly assigned to undergo surgery alone (SA group; n = 54), surgery and postoperative radiotherapy (PORT group; n = 54), or surgery and postoperative concurrent chemoradiotherapy (POCRT group; n = 64). The protocol has been described in detail elsewhere (15). Only patients who underwent PORT and POCRT were included in this study.
Laboratory data
For the present study, the absolute lymphocyte count (ALC) of patients at different time points were collected from the case report forms. The ALC values at baseline (pre-ALC; within 1 week before radiation therapy), during radiation therapy (tested once a week), and within 3 months after treatment were available. Lymphopenia was graded according to version 4.03 of the Common Terminology Criteria for Adverse Events. The nadir ALC during the course of radiation therapy was classified as grade 0 (G0, ALC ≥ 1.0 × 109/L), grade 1 (G1, 0.8 ≤ ALC < 1.0 × 109/L), grade 2 (G2, 0.5≤ ALC < 0.8 × 109/L), grade 3 (G3, 0.2 ≤ ALC < 0.5 × 109/L), or grade 4 (G4, ALC < 0.2 × 109/L).
Dose-volume parameters
Thoracic marrow (TM), including sternum and thoracic vertebral body (TVB; the superior margin was 1.0 cm above the planning target volume (PTV) dose line and the inferior margin was the lower margin of T12 or PTV dose line), was contoured with the heart, lung, and spinal cord (Figure 1A). The relative volume of normal tissues at risk of receiving x Gy (Vx) along with the mean dose (Dmean) was calculated from the dose volume histogram.
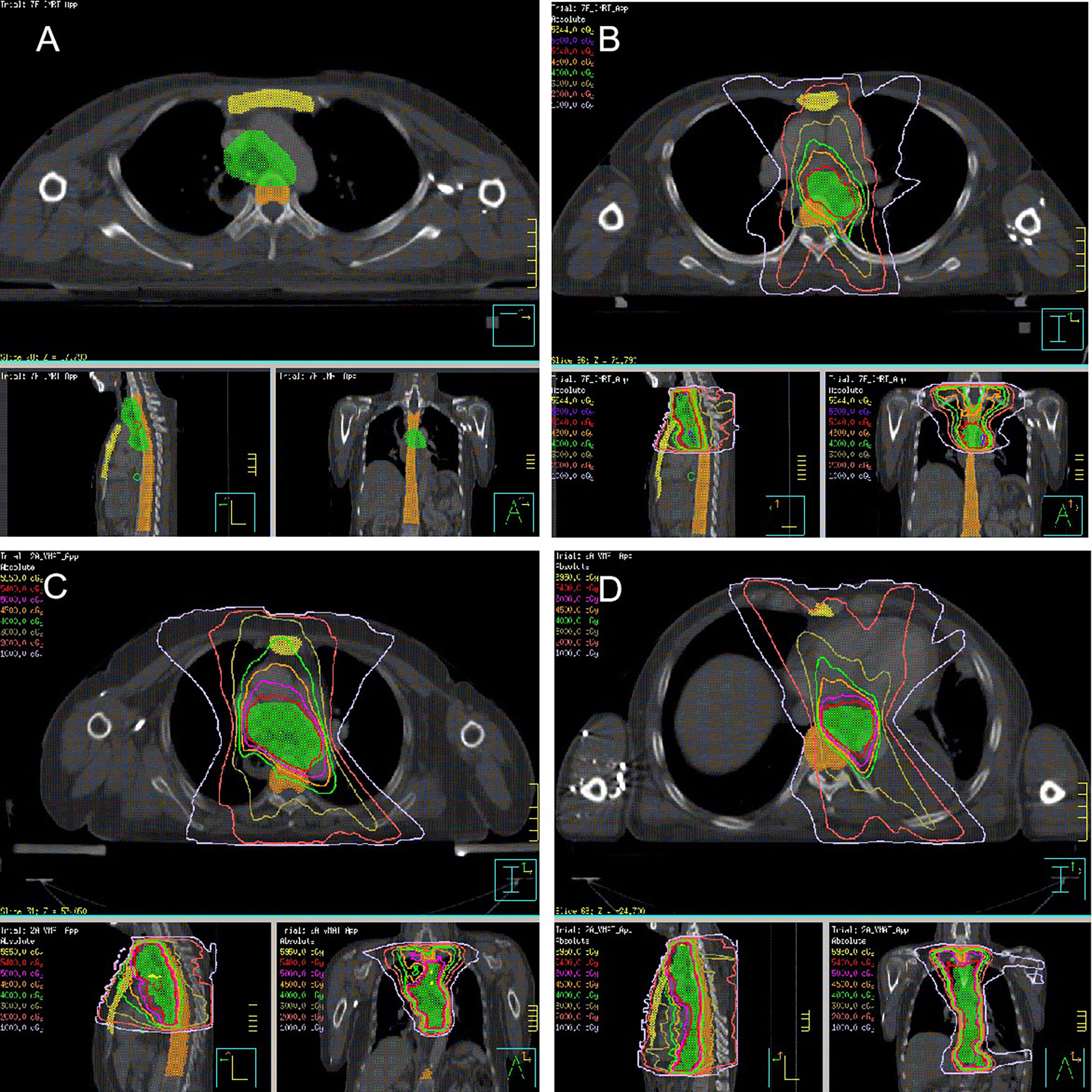
Figure 1 Radiation target (A. Thoracic marrow; yellow area, sternum; orange area, thoracic vertebral body; green area, PTV; (B) POCRT; (C) PORT, Upper-thoracic esophagus or Middle-thoracic esophagus with metastasis in 0 to 2 regional lymph nodes or metastasis in ≥ 3 regional lymph nodes in the mediastinum; (D) PORT, Lower-thoracic esophagus or middle-thoracic esophagus with metastasis in ≥ 3 regional lymph nodes distributed in two areas).
Treatment
Postoperative concurrent chemoradiotherapy
The borders of the clinical target volume (CTV) included the superior margin, which was the cricothyroid membrane for upper-thoracic tumors or the upper margin of the first thoracic vertebral body for middle-thoracic tumors. The inferior margin was 3.0 cm below the subcarina or the lower margin of the tumor bed (only for T4 lesions), including the lower cervical, bilateral supraclavicular region, and mediastinal stations 1R/L, 2R/L, 3p, 4R/L, 7, and part of 8 (Figure 1B). The prescription dose of PTV was 50.4 Gy (1.8 Gy/28 f). Paclitaxel (135-150 mg/m2) and cisplatin or nedaplatin (50-75 mg/m2) were administered concurrently. Chemotherapy was repeated every 28 days for two courses in the absence of disease progression or unacceptable toxicity.
Postoperative radiotherapy
The CTV was based on tumor and positive node location during surgery and pathological examination. The PTV was generated using a uniform 0.5 cm expansion around the CTV. Contouring of the CTV for tumors in different locations has been described in detail previously (15). Figures 1C, D illustrates the radiation target. The prescription dose was 54 Gy in 27 fractions of 2.0 Gy.
Follow-up
After treatment, patients were followed up every 3 months for the first 2 years, every 6 months for the next 2 years, and once a year thereafter. Recurrence was confirmed using diagnostic imaging or histopathology.
Tumor recurrence in regional lymph nodes was defined based on the Union for International Cancer Control (7th edition) criteria. The regional lymph node groups included supraclavicular, mediastinal, and celiac area. Distant metastasis was defined as spread of tumor to distant organs or non-regional lymph nodes.
Statistical analysis
Disease-free survival (DFS) was defined as the period from surgery to date of the first recurrence or death from any cause or censorship. Overall survival (OS) was defined as the interval from surgery to death from any cause or censorship. The Kaplan–Meier method was used to calculate DFS and OS, and the log-rank was used to determine the significance of differences. Logistic regression analysis was used to identify the factors of grade 4 lymphopenia. Receiver operating characteristic (ROC) curves identified thresholds to preventing G4 lymphopenia. All statistical analyses were performed using SPSS 20.0 (IBM Corp., Armonk, NY, USA). Two-tailed p<0.05 denoted statistically significant difference.
Results
Patients characteristics
Two patients lacking complete blood count data were excluded. Therefore, a total of 116 patients were included in the analysis. Table 1 shows the patients characteristics based on the treatment modality, 53 and 63 patients were assigned to the PORT and POCRT groups, respectively. The volume of PTV in the PORT and POCRT group were 582.5 ± 109.5 ml and 464.8 ± 97.9 ml, respectively (p < 0.001). Table 2 shows the demographic, tumor, and treatment characteristics between lymphopenic grades. Most of patients were male (89.7%); the average age was 57.3 years; and about half (44.8%) had a Karnofsky performance score of ≥90. Majority of patients (79.3%) had stage III disease. More patients underwent intensity-modulated radiotherapy (63.8%) rather than volumetric modulated arc therapy (36.2%). The ALC before treatment was comparable between patients in the G1-3 and G4 groups (2.0 ± 0.8 *109/L vs 1.7 ± 0.5 *109/L; p=0.101). All patients underwent different degrees of lymphopenia during treatment: G1 in 3 (2.6%) patients, G2 in 22 (19.0%) patients, G3 in 75 (64.7%) patients, and G4 in 16 (13.8%) patients. Patients with G4 lymphopenia only underwent PORT. The volume and mean dose of PTV were higher in group G4 (p < 0.05). All other characteristics were well balanced between the two groups.
Correlation between lymphopenia and survival
The median time of radiation therapy was 5.4 weeks. The ALC decreased gradually during treatment, and reached the nadir in the fifth week (Figure 2). The last follow-up date was January 25, 2021; the median follow-up period was 56.0 months. The median OS time was 33.2 months in group G4; however, the OS in group G1-3 was not reached. The 1-year, 3-year, and 5-year OS were 81.3%, 50.0%, and 30.0%, respectively, in the G4 group, compared with 92.0%, 66.5%, and 57.7%, respectively, in the G1-3 group (HR: 0.486, 95% CI: 0.208-1.133, p=0.095). The median DFS time was 17.4 months in group G4, but not attained in group G1-3. The 1-year, 3-year, and 5-year DFS were 62.5%, 31.3%, and 23.4%, respectively, in the G4 group, compared with 77.0%, 57.6%, and 52.2%, respectively, in the G1-3 group (HR: 0.425, 95% CI: 0.191-0.946, p=0.036) (Figure 3).
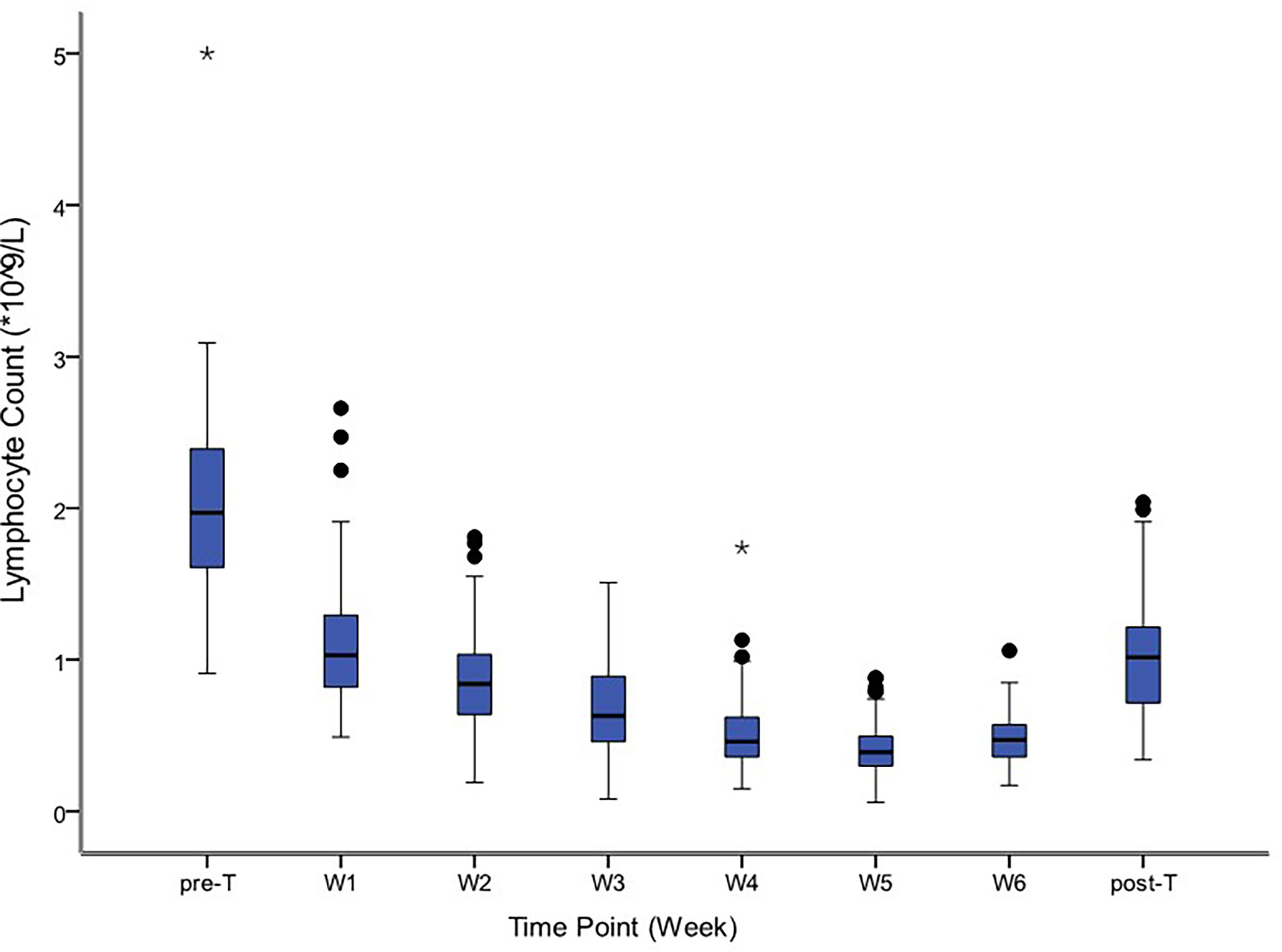
Figure 2 Distribution of absolute lymphocyte counts before, during and after treatment. The symbol * means outlier.
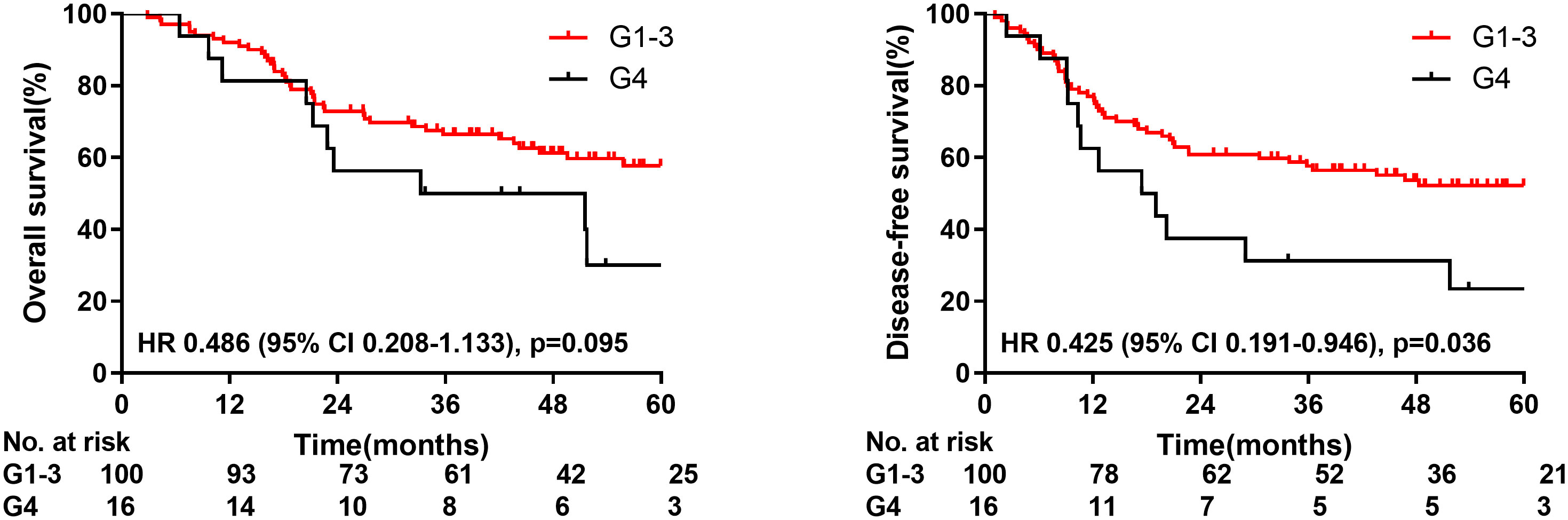
Figure 3 Overall survival and disease-free survival for patients with radiation induced lymphopenia.
Predictors of lymphopenia
Table 3 shows the relationship between lymphopenia during treatment and different clinical characteristics. Patients age, gender, and radiation technique were not significantly associated with the risk of G4 lymphopenia. In terms of dosimetric predictors, the radiation dose of TM, TVB, Heart, PTV, and PTV volume were all associated with higher rates of G4 lymphopenia (all p<0.05). Sternum Dmean, V10, and V20 were predictors of G4 lymphopenia (p<0.05).
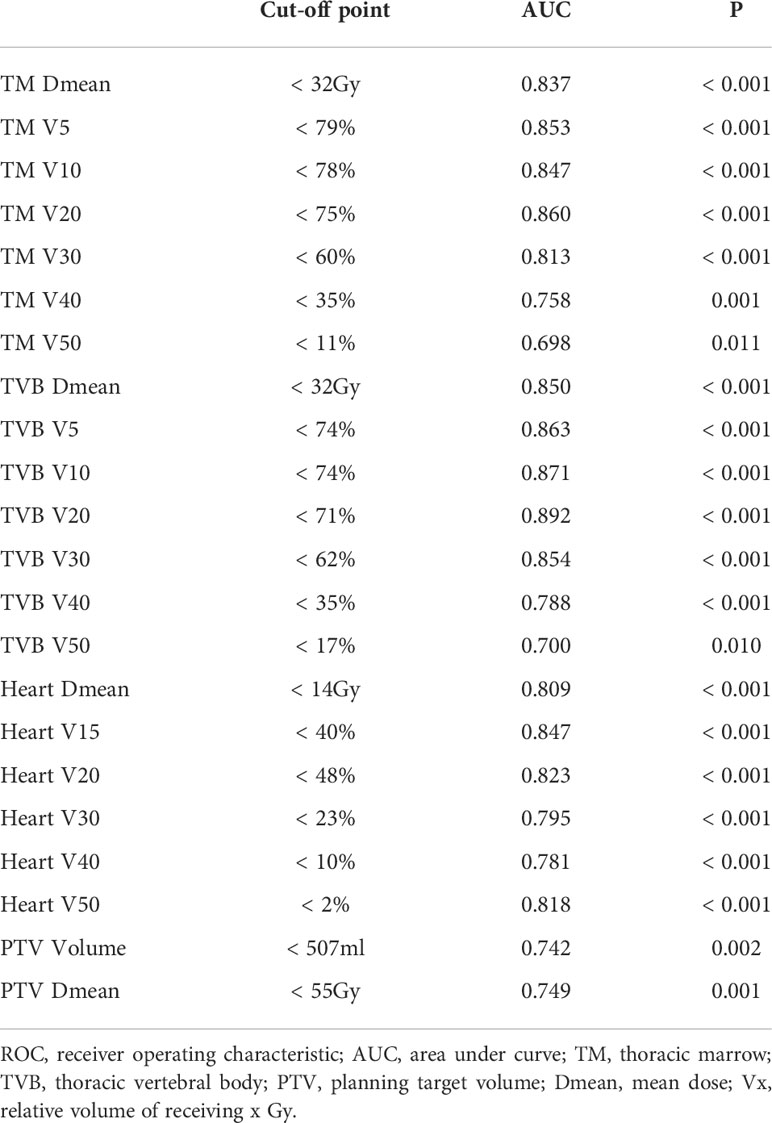
We further explored the optimal cut-off points of the dosimetric variables significantly associated with G4 lymphopenia (TM, TVB, Heart, PTV Volume, PTV Dmean) using ROC curve analysis (Table 4). The ROC curves for partial important variables were available on line (Supplementary Figures 1–4).
Discussion
As we all know, the lymph node metastasis of esophageal cancer occurs early and widely, and the recurrence of lymph nodes after radical resection is the main reason, accounting for 23.8%-58% (16), especially for patients with pathological positive lymph nodes. Therefore, how to balance the effective radiation field is the focus of our research. This post hoc analysis is from a prospective randomized controlled trial after the third modified radiation field, which showed that postoperative adjuvant therapy could improve the survival rate compared with surgery alone.
Our study revealed that DFS was worse in patients with G4 lymphopenia during PORT for esophageal cancer. The predictors of G4 RIL include the radiation volume of PTV and the adjacent hematopoietic system, such as sternum, thoracic vertebral body and heart, however it seems to have little relationship with chemotherapy. Lymphopenia is known to be one of the manifestations of immunosuppression. Many clinical studies have shown that it is a predictor of poor prognosis in pancreatic cancer, brain tumor, non-small cell lung cancer, and nasopharyngeal carcinoma (17–20). According to recent studies, patients with lymphopenia during radical or neoadjuvant chemoradiotherapy of esophageal cancer have a poor prognosis and low complete pathologic response rate (12–14). Our study showed that lymphocytes were extremely sensitive to radiation. Lymphocytes decreased at the beginning of radiotherapy and sharply with the accumulation of radiation dose. Radiation doses, as low as 2 Gy, can inactivate about 50% of circulating lymphocytes in the radiation field, resulting in RIL during radiotherapy (21, 22). In a malignant glioma model, 60 Gy prescription dose irradiates the brain at a dose of 2 Gy per fraction, resulting in an average dose of 2 Gy for circulating lymphocytes, and almost all circulating blood is at least irradiated 0.5 Gy (8). T lymphocytes are an important part of cellular immunity. Cytotoxic CD8+ T lymphocytes act as effector cells; they directly kill abnormal cells and secrete proinflammatory cytokines (23). Therefore, radiation-induced reduction of CD8+ T lymphocytes may have a negative effect on cell-mediated immunity, because even if the number of lymphocytes recovers after radiotherapy, the newly produced immature T lymphocytes cannot produce antitumor effects. Regulatory T cells (Tregs), another T cell subtype, are known to be involved in immunosuppression (24). Muroyama et al. (25) found that the phenotypic and functional inhibitory Treg cells number increases in a tumor microenvironment after irradiation of tumor with 10 Gy in mice. According to Oweida et al. (26, 27), the combination of radiotherapy and immunotherapy with Treg targeted inhibitors can inhibit tumor growth. Since Treg is relatively resistant to radiation, surviving Treg cells are usually assumed to have the ability to inhibit the recovery of effector T cells during lymphocyte recovery (28). Clinical study findings also showed that a high proportion of CD8+ T/Treg cells predicted a better therapeutic response (29). Therefore, the effect of lymphopenia on the survival of patients could be mainly due to the extensive effect of radiotherapy on the number and function of effector T lymphocytes in blood circulation. Moreover, Treg cells are radiation-resistant and affect the recovery of effector T lymphocytes after radiotherapy, resulting in the decline of cellular immune function, early recurrence, and worse prognosis.
Lymphopenia is mainly due to the reduction in number of mature lymphocytes in peripheral blood and the production of lymphocytes in hematopoietic organs after radiation. The heart is highly vascularized. The thoracic marrow is the main hematopoietic organ of adults. The heart and sternum are located in front of the esophagus and the thoracic vertebra is located behind the esophagus. In this study, we found that the irradiated volume and dose of thoracic vertebra, heart, and PTV during postoperative radiotherapy of esophageal cancer were the main factors causing G4 lymphopenia. Fang (13, 30) and van Rossum (31) reported that G4 lymphocytes decreased more significantly in patients with larger PTV in radical chemoradiotherapy of esophageal cancer, which is consistent with the results of our study. Davuluri (12) reported that the incidence of G4 lymphopenia in patients with lesions in the lower sections of the esophagus is higher than that in patients with lesions in the middle and upper sections of the esophagus. Considering that the lesions in the lower sections of the esophagus are adjacent to the heart and spleen, which are rich in blood, a large number of lymphocytes are irradiated. Saito (32) previously reported that the average irradiation dose of spleen in chemoradiotherapy of esophageal cancer can predict G4 lymphopenia. Besides, the exposure of thoracic vertebra in esophageal cancer radiotherapy has been reported to be related to more grade 3 hematological toxicity (33–35). According to Newman (36), lymphopenia during chemoradiotherapy of esophageal cancer is closely related to the volume of irradiated thoracic vertebral body, which is consistent with our findings.
Proton radiotherapy in malignant tumors has been more widely used than photon radiotherapy for its physical advantages. Mohan (37) reported that proton radiotherapy could better reduce the incidence of G3 lymphopenia in glioblastoma than photon radiotherapy. Nichols (38) also revealed that proton radiotherapy could better reduce the mean radiation dose of the lungs by 33% and bone marrow V10 by 30% than photon radiotherapy. Shiraishi (39) and Liu (40) reported that proton radiation to the heart has lower doses than photon radiation. Several studies have reported that proton radiotherapy has a lower incidence of G4 lymphopenia than photon radiotherapy during chemoradiotherapy in esophageal cancer (30, 41, 42). Our study reveals that a greater volume and dose of PTV has a higher irradiation dose of thoracic marrow and heart, which results in a more obvious decrease in the number of peripheral blood lymphocytes.
Our study reported the cut-off values of PTV, heart, and thoracic marrow necessary to prevent the incidence of G4 lymphopenia. According to the prospective randomized controlled trial by Ni et al. (43), for patients with pathological stage IIB–III esophageal squamous cell carcinoma after radical surgery, POCRT, which reduced the radiation field to 3 cm below the carina and reduced the radiation dose to 50.4 Gy, did not increase the in- or out-of-field recurrence. Additionally, the survival rate was more comparable than with the PORT. POCRT appears to be an effective and safe treatment. Based on findings from the previous and present trials, POCRT can be considered for these patients to ensure a smaller PTV volume and dose to reduce the exposure of the heart and thoracic marrow and prevent severe lymphopenia. In the event where POCRT cannot be performed, attention should be paid to the protection of the heart and thoracic marrow, and corresponding radiation dose constraints should be given to prevent lymphopenia. Therefore, under the condition of reasonably reducing the postoperative irradiation field, synchronous chemotherapy should be strengthened to reduce the impact on lymphocytes and reduce the impact on survival. Of course, the postoperative irradiation field should be designed according to the recurrence sites and rates after esophagectomy, and the irradiation dose of normal tissue should be considered at the same time, so as to reduce the recurrence rate and convert it into the benefit of survival without increasing toxic and side effects.
In addition, actively search for drugs to enhance immunity or promote lymphocyte recovery is the direction of future research. Zheng (44) found that after a single low-dose whole-body irradiation in the mouse lung melanoma model, cinnamon effectively improved the imbalance of T cell subsets and promoted effective antitumor immunity by promoting the proliferation of Th1 and inhibiting the expansion of Th17 and Treg cells. In addition, an experimental study has also shown that exogenous IL-7 delivered to the irradiated animal model can not only restore the lymphocyte count but also enhance the antitumor effect. Exogenous IL-7 is helpful to overcome RIL and improve the therapeutic effect combined with radiotherapy (45). However, these findings need to be verified by future clinical studies.
The limitation of this study is that the sample size is relatively small. We expect to continue accumulating more cases and prolong the follow-up time.
Conclusion
G4 lymphopenia had poor DFS, and the radiation volume of thoracic marrow, heart, and PTV may predict G4 lymphopenia in postoperative esophageal cancer. Radiation dose constraints for these normal tissues may be sufficient to decrease G4 lymphopenia, but further prospective trials are needed to verify the results.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Cancer Institute and Hospital, Chinese Academy of Medical Sciences, Beijing. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization: ZX. Project planning: WN, ZX. Writing: WN. Statistical counseling: WN. Editing: ZX, ZZ, DC, QF, JL, JML. All authors provided review of the manuscript. All authors read and approved the final manuscript.
Funding
This work were supported by the Capital Fund for Health Improvement and Research [grant number 2016-2-4021] and Youth Fund of Beijing Shijitan Hospital (grant number 2020-q12). The manuscript has been peer reviewed by the funding body. The funding source has no role in study design, data collection, analysis, interpretation, the writing of the manuscript, or the decision to submit the current study.
Acknowledgments
We thank all participants of this trial, and all investigators who devote their time and passion in the implementation of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.936684/full#supplementary-material
References
1. Rice TW, Ishwaran H, Hofstetter WL, Kelsen DP, Apperson-Hansen C, Blackstone EH. Recommendations for pathologic staging (pTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus (2016) 29(8):897–905. doi: 10.1111/dote.12533
2. Xu Y, Liu J, Du X, Sun X, Zheng Y, Chen J, et al. Prognostic impact of postoperative radiation in patients undergoing radical esophagectomy for pathologic lymph node positive esophageal cancer. Radiat Oncol (2013) 8:116. doi: 10.1186/1748-717X-8-116
3. Shridhar R, Weber J, Hoffe SE, Almhanna K, Karl R, Meredith K. Adjuvant radiation therapy and lymphadenectomy in esophageal cancer: A SEER database analysis. J Gastrointest Surg (2013) 17(8):1339–45. doi: 10.1007/s11605-013-2192-7
4. Zhang W, Liu X, Xiao Z, Zhang H, Chen D, Feng Q, et al. Postoperative intensity-modulated radiotherapy improved survival in lymph node-positive or stage III thoracic esophageal squamous cell carcinoma. Oncol Res Treat (2015) 38(3):97–102. doi: 10.1159/000375391
5. Yu S, Zhang W, Ni W, Xiao Z, Wang Q, Zhou Z, et al. A propensity-score matching analysis comparing long-term survival of surgery alone and postoperative treatment for patients in node positive or stage III esophageal squamous cell carcinoma after R0 esophagectomy. Radiother Oncol (2019) 140:159–66. doi: 10.1016/j.radonc.2019.06.020
6. Santin AD, Hermonat PL, Ravaggi A, Bellone S, Roman J, Pecorelli S, et al. Effects of concurrent cisplatinum administration during radiotherapy vs. radiotherapy alone on the immune function of patients with cancer of the uterine cervix. Int J Radiat Oncol Biol Phys (2000) 48(4):997–1006. doi: 10.1016/S0360-3016(00)00769-0
7. Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res (2011) 17(16):5473–80. doi: 10.1158/1078-0432.CCR-11-0774
8. Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: Modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest (2013) 31(2):140–4. doi: 10.3109/07357907.2012.762780
9. Grossman SA, Ellsworth S, Campian J, Wild AT, Herman JM, Laheru D, et al. Survival in patients with severe lymphopenia following treatment with radiation and chemotherapy for newly diagnosed solid tumors. J Natl Compr Canc Netw (2015) 13(10):1225–31. doi: 10.6004/jnccn.2015.0151
10. Tang CMM, Liao ZM, Gomez DM, Levy LM, Zhuang YM, Gebremichael RAB, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys (2014) 89(5):1084–91. doi: 10.1016/j.ijrobp.2014.04.025
11. Sun GY, Wang SL, Song YW, Jin J, Wang WH, Liu YP, et al. Radiation-induced lymphopenia predicts poorer prognosis in patients with breast cancer: A Post hoc analysis of a randomized controlled trial of postmastectomy hypofractionated radiation therapy. Int J Radiat Oncol Biol Phys (2020) 108(1):277–85. doi: 10.1016/j.ijrobp.2020.02.633
12. Davuluri R, Jiang W, Fang P, Xu C, Komaki R, Gomez DR, et al. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys (2017) 99(1):128–35. doi: 10.1016/j.ijrobp.2017.05.037
13. Fang P, Jiang W, Davuluri R, Xu C, Krishnan S, Mohan R, et al. High lymphocyte count during neoadjuvant chemoradiotherapy is associated with improved pathologic complete response in esophageal cancer. Radiother Oncol (2018) 128(3):584–90. doi: 10.1016/j.radonc.2018.02.025
14. Li Q, Zhou S, Liu S, Liu S, Yang H, Zhao L, et al. Treatment-related lymphopenia predicts pathologic complete response and recurrence in esophageal squamous cell carcinoma undergoing neoadjuvant chemoradiotherapy. Ann Surg Oncol (2019) 26(9):2882–9. doi: 10.1245/s10434-019-07334-7
15. Ni W, Yu S, Zhang W, Xiao Z, Zhou Z, Chen D, et al. A phase-II/III randomized controlled trial of adjuvant radiotherapy or concurrent chemoradiotherapy after surgery versus surgery alone in patients with stage-IIB/III esophageal squamous cell carcinoma. BMC Cancer (2020) 20(1):130. doi: 10.1186/s12885-020-6592-2
16. Ni W, Yang J, Deng W, Xiao Z, Zhou Z, Zhang H, et al. Patterns of recurrence after surgery and efficacy of salvage therapy after recurrence in patients with thoracic esophageal squamous cell carcinoma. BMC Cancer (2020) 20(1):144–52. doi: 10.1186/s12885-020-6622-0
17. Wild AT, Ye X, Ellsworth SG, Smith JA, Narang AK, Garg T, et al. The association between chemoradiation-related lymphopenia and clinical outcomes in patients with locally advanced pancreatic adenocarcinoma. Am J Clin Oncol (2015) 38(3):259–65. doi: 10.1097/COC.0b013e3182940ff9
18. Damen P, Kroese TE, van Hillegersberg R, Schuit E, Peters M, Verhoeff J, et al. The influence of severe radiation-induced lymphopenia on overall survival in solid tumors: A systematic review and meta-analysis. Int J Radiat Oncol Biol Phys (2021) 111(4):936–948. doi: 10.1016/S0167-8140(21)07966-4
19. Upadhyay R, Venkatesulu BP, Giridhar P, Kim BK, Sharma A, Elghazawy H, et al. Risk and impact of radiation related lymphopenia in lung cancer: A systematic review and meta-analysis. Radiother Oncol (2021) 157:225–33. doi: 10.1016/j.radonc.2021.01.034
20. Xie X, Gong S, Jin H, Yang P, Xu T, Cai Y, et al. Radiation-induced lymphopenia correlates with survival in nasopharyngeal carcinoma: Impact of treatment modality and the baseline lymphocyte count. Radiat Oncol (2020) 15(1):65. doi: 10.1186/s13014-020-01494-7
21. Nakamura N, Kusunoki Y, Akiyama M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat Res (1990) 123(2):224–7. doi: 10.2307/3577549
22. Yovino S, Grossman SA. Severity, etiology and possible consequences of treatment-related lymphopenia in patients with newly diagnosed high-grade gliomas. CNS Oncol (2012) 1(2):149–54. doi: 10.2217/cns.12.14
23. Newell EW, Sigal N, Bendall SC, Nolan GP, Davis MM. Cytometry by time-of-Flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity (2012) 36(1):142–52. doi: 10.1016/j.immuni.2012.01.002
24. Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: Impact on clinical outcome. Nat Rev Cancer (2012) 12(4):298–306. doi: 10.1038/nrc3245
25. Muroyama Y, Nirschl TR, Kochel CM, Lopez-Bujanda Z, Theodros D, Mao W, et al. Stereotactic radiotherapy increases functionally suppressive regulatory T cells in the tumor microenvironment. Cancer Immunol Res (2017) 5(11):992–1004. doi: 10.1158/2326-6066.CIR-17-0040
26. Oweida A, Hararah MK, Phan A, Binder D, Bhatia S, Lennon S, et al. Resistance to radiotherapy and PD-L1 blockade is mediated by TIM-3 upregulation and regulatory T-cell infiltration. Clin Cancer Res (2018) 24(21):5368–80. doi: 10.1158/1078-0432.CCR-18-1038
27. Oweida AJ, Darragh L, Phan A, Binder D, Bhatia S, Mueller A, et al. STAT3 modulation of regulatory T cells in response to radiation therapy in head and neck cancer. JNCI: J Natl Cancer Inst (2019) 111(12):1339–49. doi: 10.1093/jnci/djz036
28. Baba J, Watanabe S, Saida Y, Tanaka T, Miyabayashi T, Koshio J, et al. Depletion of radio-resistant regulatory T cells enhances antitumor immunity during recovery from lymphopenia. Blood (2012) 120(12):2417–27. doi: 10.1182/blood-2012-02-411124
29. Shinto E, Hase K, Hashiguchi Y, Sekizawa A, Ueno H, Shikina A, et al. CD8+ and FOXP3+ tumor-infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann Surg Oncol (2014) 21 Suppl 3:S414–21. doi: 10.1245/s10434-014-3584-y
30. Fang P, Shiraishi Y, Verma V, Jiang W, Song J, Hobbs BP, et al. Lymphocyte-sparing effect of proton therapy in patients with esophageal cancer treated with definitive chemoradiation. Int J Particle Ther (2018) 4(3):23–32. doi: 10.14338/IJPT-17-00033.1
31. van Rossum P, Deng W, Routman DM, Liu AY, Xu C, Shiraishi Y, et al. Prediction of severe lymphopenia during chemoradiation therapy for esophageal cancer: Development and validation of a pretreatment nomogram. Pract Radiat Oncol (2020) 10(1):e16–26. doi: 10.1016/j.prro.2019.07.010
32. Saito T, Toya R, Yoshida N, Shono T, Matsuyama T, Ninomura S, et al. Spleen dose-volume parameters as a predictor of treatment-related lymphopenia during definitive chemoradiotherapy for esophageal cancer. In Vivo (Athens) (2018) 32(6):1519–25. doi: 10.21873/invivo.11409
33. Lee J, Lin J, Sun F, Lu K, Lee C, Chen Y, et al. Dosimetric predictors of acute haematological toxicity in oesophageal cancer patients treated with neoadjuvant chemoradiotherapy. Brit J Radiol (2016) 89(1066):20160350. doi: 10.1259/bjr.20160350
34. Zhang A, Deek MP, Kim S, Sayan M, Grann A, Wagman RT, et al. Vertebral body irradiation during chemoradiation therapy for esophageal cancer contributes to acute bone marrow toxicity. J Gastrointest Oncol (2019) 10(3):513–22. doi: 10.21037/jgo.2019.01.20
35. Fabian D, Ayan A, DiCostanzo D, Barney CL, Aljabban J, Diaz DA, et al. Increasing radiation dose to the thoracic marrow is associated with acute hematologic toxicities in patients receiving chemoradiation for esophageal cancer. Front Oncol (2019) 9:147. doi: 10.3389/fonc.2019.00147
36. Newman NB, Anderson JL, Sherry AD, Osmundson EC. Dosimetric analysis of lymphopenia during chemoradiotherapy for esophageal cancer. J Thorac Dis (2020) 12(5):2395–405. doi: 10.21037/jtd.2020.03.93
37. Mohan R, Liu AY, Brown PD, Mahajan A, Dinh J, Chung C, et al. Proton therapy reduces the likelihood of high-grade radiation-induced lymphopenia in glioblastoma patients: Phase II randomized study of protons vs photons. Neuro-oncol (Charlottesville Va.) (2021) 23(2):284–94. doi: 10.1093/neuonc/noaa182
38. Nichols RC, Huh SN, Henderson RH, Mendenhall NP, Flampouri S, Li Z, et al. Proton radiation therapy offers reduced normal lung and bone marrow exposure for patients receiving dose-escalated radiation therapy for unresectable stage iii non-small-cell lung cancer: A dosimetric study. Clin Lung Cancer (2011) 12(4):252–7. doi: 10.1016/j.cllc.2011.03.027
39. Shiraishi Y, Xu C, Yang J, Komaki R, Lin SH. Dosimetric comparison to the heart and cardiac substructure in a large cohort of esophageal cancer patients treated with proton beam therapy or intensity-modulated radiation therapy. Radiother Oncol (2017) 125(1):48–54. doi: 10.1016/j.radonc.2017.07.034
40. Liu C, Bhangoo RS, Sio TT, Yu NY, Shan J, Chiang JS, et al. Dosimetric comparison of distal esophageal carcinoma plans for patients treated with small-spot intensity-modulated proton versus volumetric-modulated arc therapies. J Appl Clin Med Phys (2019) 20(7):15–27. doi: 10.1002/acm2.12623
41. Shiraishi Y, Fang P, Xu C, Song J, Krishnan S, Koay EJ, et al. Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: A propensity matched analysis of the relative risk of proton versus photon-based radiation therapy. Radiother Oncol (2018) 128(1):154–60. doi: 10.1016/j.radonc.2017.11.028
42. Routman DM, Garant A, Lester SC, Day CN, Harmsen WS, Sanheuza CT, et al. A comparison of grade 4 lymphopenia with proton versus photon radiation therapy for esophageal cancer. Adv Radiat Oncol (2019) 4(1):63–9. doi: 10.1016/j.adro.2018.09.004
43. Ni W, Yu S, Xiao Z, Zhou Z, Chen D, Feng Q, et al. Postoperative adjuvant therapy versus surgery alone for stage IIB–III esophageal squamous cell carcinoma: A phase III randomized controlled trial. Oncologist (2021) 26(12):e2151–60. doi: 10.1002/onco.13914
44. Zheng XM, Guo YM, Wang LM, Zhang HM, Wang SM, Wang LM, et al. Recovery profiles of T-cell subsets following low-dose total body irradiation and improvement with cinnamon. Int J Radiat Oncol Biol Phys (2015) 93(5):1118–26. doi: 10.1016/j.ijrobp.2015.08.034
Keywords: esophageal cancer, postoperative radiotherapy, lymphopenia, thoracic marrow, survival
Citation: Ni W, Xiao Z, Zhou Z, Chen D, Feng Q, Liang J and Lv J (2022) Severe radiation-induced lymphopenia during postoperative radiotherapy or chemoradiotherapy has poor prognosis in patients with stage IIB-III after radical esophagectomy: A post hoc analysis of a randomized controlled trial. Front. Oncol. 12:936684. doi: 10.3389/fonc.2022.936684
Received: 05 May 2022; Accepted: 22 August 2022;
Published: 08 September 2022.
Edited by:
Dinesh Thotala, Washington University in St. Louis, United StatesReviewed by:
Hsin-Hua Lee, Kaohsiung Medical University, TaiwanYu-Jie Huang, Kaohsiung Chang Gung Memorial Hospital, Taiwan
Copyright © 2022 Ni, Xiao, Zhou, Chen, Feng, Liang and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zefen Xiao, xiaozefen@sina.com
 Wenjie Ni
Wenjie Ni