- 1Department of Critical Care Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China
- 2Department of Respiratory and Critical Care Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China
Background: Interstitial lung disease (ILD) is the most common and potentially most devastating manifestation of SSc in pulmonary involvement. However, the mechanism for systemic sclerosis-associated ILD (SSc-ILD) is unclear. This work aims to explore the potential candidates for SSc-ILD upon whole exome sequencing (WES) and attempts to analyze the possible pathogenesis of SSc-ILD from the perspective of the genetic level.
Materials: Variants were confirmed by whole exome sequencing (WES), and SKAT analysis was employed to explore the most differential variants. Targeted variants were performed in biological functions, associated with clinical manifestations, and the probable change of downstream.
Results: By WES and SKAT analysis of SSc with and without ILD, only the variants of RGPD4 achieved statistical power (P < 2.51 × 10-6, P-FDR = 0.025, OR = 15.95). A total of 20 rare functional variants (missense, truncating, splicing) were tested for the RGPD4 gene, and five truncating and damaging missense variants were identified. Carriers showed the older inclusion age (P = 0.02) and the higher frequency use of prednisone (P=0.02) compared to the non-carriers. Further analysis illustrated that carriers showed lower levels of TES in comparison to non-carriers but did not reach statistical difference (P = 0.08). In bivariate correlation analysis, we analyzed the relationship between the mutant status of RGPD4 and the levels of sex hormones after adjusting for age confounders. Only the level of TES showed a negative correlation with the mutant status (B = -0.509, P = 0.037).
Conclusion: The variants of RGPD4 might contribute to the ILD development of SSc and might also be a causative factor of lower TES among SSc-ILD, which provided insight to a better understanding of pathobiology of SSc-ILD, and androgen hormone supplement might be a therapeutic target in this debilitating disease.
Background
Systemic sclerosis (SSc) is a refractory connective tissue disease characterized by localized or diffuse skin thickening, progressive organic fibrosis, and ongoing vasculopathy (1). Pulmonary involvement is a common extra-skin manifestation of SSc, and interstitial lung disease (ILD) serves as the leading cause of death (2). However, the exact mechanisms of SSc-associated ILD (SSc-ILD) are unclear. Multiple genetic, epigenetic, and environmental factors contributed to the onset of SSc, and emerging evidence suggests that dysbiosis in the lung, skin, and gastrointestinal tract might trigger the chronic inflammation and autoimmunity in specific organs (3). Moreover, digital vasculopathy, known as Raynaud’s phenomenon, is the most common presenting feature and may precede diagnosis by many years (4).
A genetic component has been well established, with most risks attributed to variants in the immunological pathways including cell-mediated (via MHC) and innate (via TLRs) immune activation. Moreover, multiple studies suggest that genetic variations have been implicated in specific phenotypes of SSc-ILD besides disease susceptibility. The minor allele of IRF5 rs4728142:G>A was associated with better survival (P = 0.002) and mild ILD (P = 0.019) after adjusting age, gender, cutaneous involvement, and duration of disease (5). Some variants were reported to be correlated with specific clinical predictors, like STAT4 rs757486 with FVC% pred (6), CTGF G-945C with ATA positive, and SOX-5 rs11047102 with ACA positive (7). For these candidates, it is believed that loss-of-function (LoF) variants might be the culprit of genetic disorders.
To explore the genetic etiology of SSc-ILD and uncover a novel mechanism of pulmonary development, we have applied whole exome sequencing to search the potential candidates in 80 patients with or without ILD and 208 healthy controls. We initially provide evidence that RGPD4 variants might participate in the development of SSc-ILD in comparison to the other two groups upon SKAT analysis (P = 8.13 × 10-7, P-FDR = 0.015, OR = 17.23), especially LoF with a severe ILD phenotype (radiological pattern: UIP). Moreover, through biological function exploration of RGPD4, including the GTEx, GO, PPI, and GSCA Lite databases, it was reported that RGPD4 with the other four interactive proteins participated in the modulation of hormone receptors. Moreover, our data presented that RGPD4 variants might correlate with lower levels of testosterone in patients with SSc-ILD.
RANBP2-like and GRIP domain-containing 4 (RGPD4) is a protein-coding gene, and diseases associated with RGPD4 involve ovarian serous cystadenocarcinoma (OMIM 612707). Currently, few researchers explored the physiological function of this gene. A study in combination of the computational oncoproteomics approach and analysis of genetic expression with patient survival revealed that RGPD4 was a significantly mutated protein in OV (P = 0.0.002), and higher levels of RGPD4 protein indicated poor survival in LUAD (P = 0.048) (8). In the GeneCards database, GenomeRNAi human phenotypes for RGPD4 included G0/1 arrest, strongly decreased NFAT1 nuclear translocation, decreased viability, and increased gamma-H2AX phosphorylation (https://www.genecards.org). The nuclear factor of activated T cells (NFAT), a key regulator of T-cell development and function, could mediate the IL-2 related immunological pathway; meanwhile, its nuclear translocation was proved to be correlated with HIF-α-induced fibroblast fibrosis (9).
Women of childbearing age owned priority in the incidence of systemic sclerosis (10), and it is well known that sex hormone status regulated a few immunomodulatory functions (11). Luisa et al. (12) reported that an altered androgen could result in relative immunological hyperactivity contributing to enhance tissue damage and disease severity in SSc. Moreover, our team reported that testosterone showed a strong correlation with idiopathic pulmonary fibrosis (13). However, data on levels of sex hormones and their role in ILD are scare.
Thus, the objectives of this study were (i) to explore a pilot study that compares differential genes measured by WES and SKATO analysis in patients with SSc-ILD and patients with SSc without ILD and (ii) to present the biological function of potential candidates of SSc-ILD that compass the clinical relevance of patients with SSc-ILD.
Materials
Cohort collection
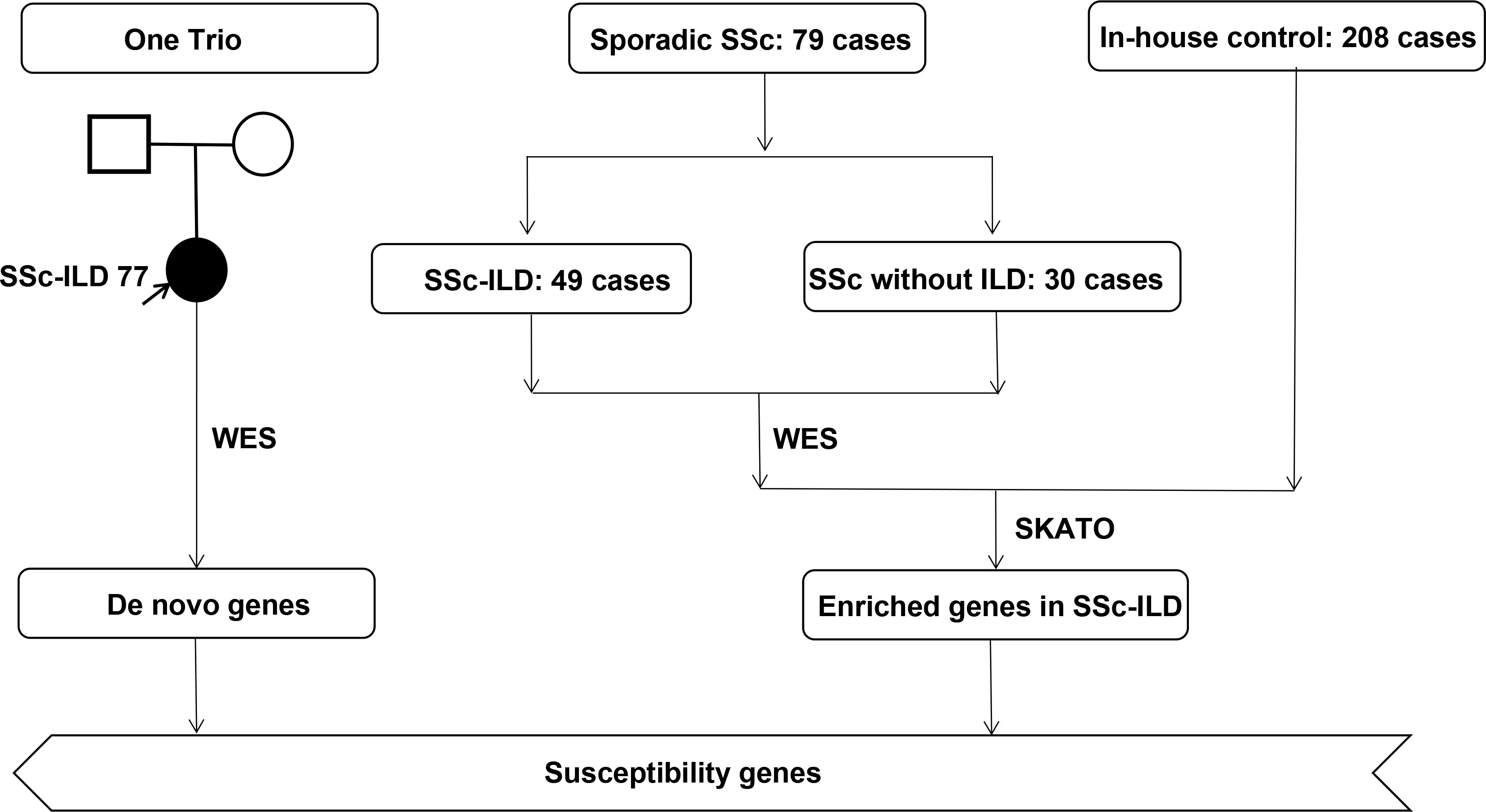
This was a retrospective, case–control study that was strictly carried out after getting the written informed consent from all participants and being approved by the ethics committee of Perking Union Medical College Hospital [JS1127]. Consecutive patients with SSc or SSc-ILD hospitalized in Perking Union Medical College Hospital (PUMCH) between January 2015 to January 2021 were retrospectively enrolled, and all the peripheral blood samples were collected at the time of hospitalization. A total of 80 cases, including 50 cases with SSc-ILD and 30 cases with SSc without ILD, and 208 healthy controls were enrolled in this study Figure 1. All included cases met the 2013 European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR), including immunological, fibrotic, and vascular features of the disease, and patients with a total of score greater than 9 are classified as having definite systemic sclerosis (1).
All SSc patients underwent chest CT examination for the evaluation of ILD, which were classified as UIP, possible UIP, NSIP, or possible NSIP according to the international standard (2) (14, 15). UIP is defined as reticular, honeycombing, and traction bronchiectasis and architectural distortion that distributed in the peripheral, subpleural, and basal lobes. NSIP is featured by ground glass opacity (GGO), irregular lines, and consolidation that also distributed in the peripheral, subpleural, and basal lobes. Moreover, Orlandi and colleagues concluded that the features in CT images of SSc-ILD were fibrosis inside focal GGOs in the upper or lower lobes and reticulations in the lower lobes (especially if bilateral and symmetrical). Each diagnosis of ILD was combined with clinical characteristics, pulmonary function tests, and HRCT findings. HRCT findings were defined as ILD by consensus between radiologists and pulmonologists. Those with known environmental exposures, specific drug-induced pneumonia, or other known causes of ILD were excluded.
Whole exome sequencing
Peripheral blood samples were extracted from patients after obtaining informed consent. Every patient provided 1 ml of peripheral blood, and all the samples were stored at -80°C until further analysis. Exome sequencing was performed on DNA extracted from peripheral blood from 80 cases and 208 healthy controls, totaling 288 subjects. A series of quality control, DNA modification, end repair, adapter ligation, size selection, and PCR amplification were performed, and then high-throughput sequencing was performed on Illumina PE150 according to a previous study (16).
WES data interpretation
All tested SNPs and Indels needed quality control to ensure reliability, including GATK standard, Hardy–Weinberg equilibrium, and sample missing rate. Additionally, a principal component analysis was conducted to assess the effect of population stratification. Variants with high allele frequency (minor allele frequency >1%) were excluded from the analysis.
Sequence Kernel Association Test-Optional (SKAT-O) were performed assuming potential candidates among different groups. In our SKAT-O analysis, a total of 18,899 genes were included and genes with a P value of less than 2.51 × 10-5 were defined as the effective P value.
Sex hormone measurements
Under the condition of gender and age matching, a total of 96 postmenopausal women were enrolled to detect the concentration of sex hormones, including 17 patients with SSc-ILD, 9 patients with SSc without ILD, and 70 healthy people. All subjects provided 1 ml of peripheral blood, and plasma DS, TES, E2, and PRL levels were measured by enzyme-linked immunosorbent assay (ELISA) after centrifugation for plasma. All reagents used are commercially available (Novus Biologicals, Colorado, USA).
Statistical analysis
All data were analyzed using SPSS 25.0 software (IBM Statistics, Chicago, IL). Categorical variables were presented by percentages, and continuous data were presented as means and SD. Differences in categorical variables were analyzed using Fisher exact test, and differences in the continuous data were analyzed by Student’s t-test and ANOVA test. It was considered statistically significant when the P value was under 0.05.
Results
Cohort information
We enrolled a total of 30 cases with SSc without ILD, 49 cases with SSc-ILD, and one case with unaffected parent (trio) of Chinese Han ethnicity, consisting of 67 women and 13 men. Patients with SSc of late-onset year, longer duration, and positive anti-Scl-70 were of priority in suffering from interstitial lung disease. A total of 47 SSc patients suffered from microvascular damage, including Raynaud’s phenomenon (RP), pulmonary arterial hypertension (PAH), scleroderma renal crisis, and gastric antral vascular ectasia, and SSc-ILD patients had a higher risk than SSc patients without ILD [P = 0.03, OR 2.78, 95% CI (1.09-7.08)]. RP is the most common vascular damage of SSc patients, and ILD patients had a higher risk of PAH than patients without ILD among SSc patients [P = 0.02, OR 1.19, 95% CI (1.05-1.34)]. Moreover, higher levels of infectious and inflammatory indicators were found in patients with pulmonary involvements, like ESR (P = 0.004) and NLR (P < 0.001). Interstitial lung disease, as a serious complication, required a combination of hormone and immunosuppressive therapy in the clinical process compared to those without complications (P = 0.040). A total of six types of immunosuppressants were involved, and two antifibrotic agents were used among the 80 patients, and CTX is the most favorable immunosupressant. Detailed demographic information is presented in Table 1.
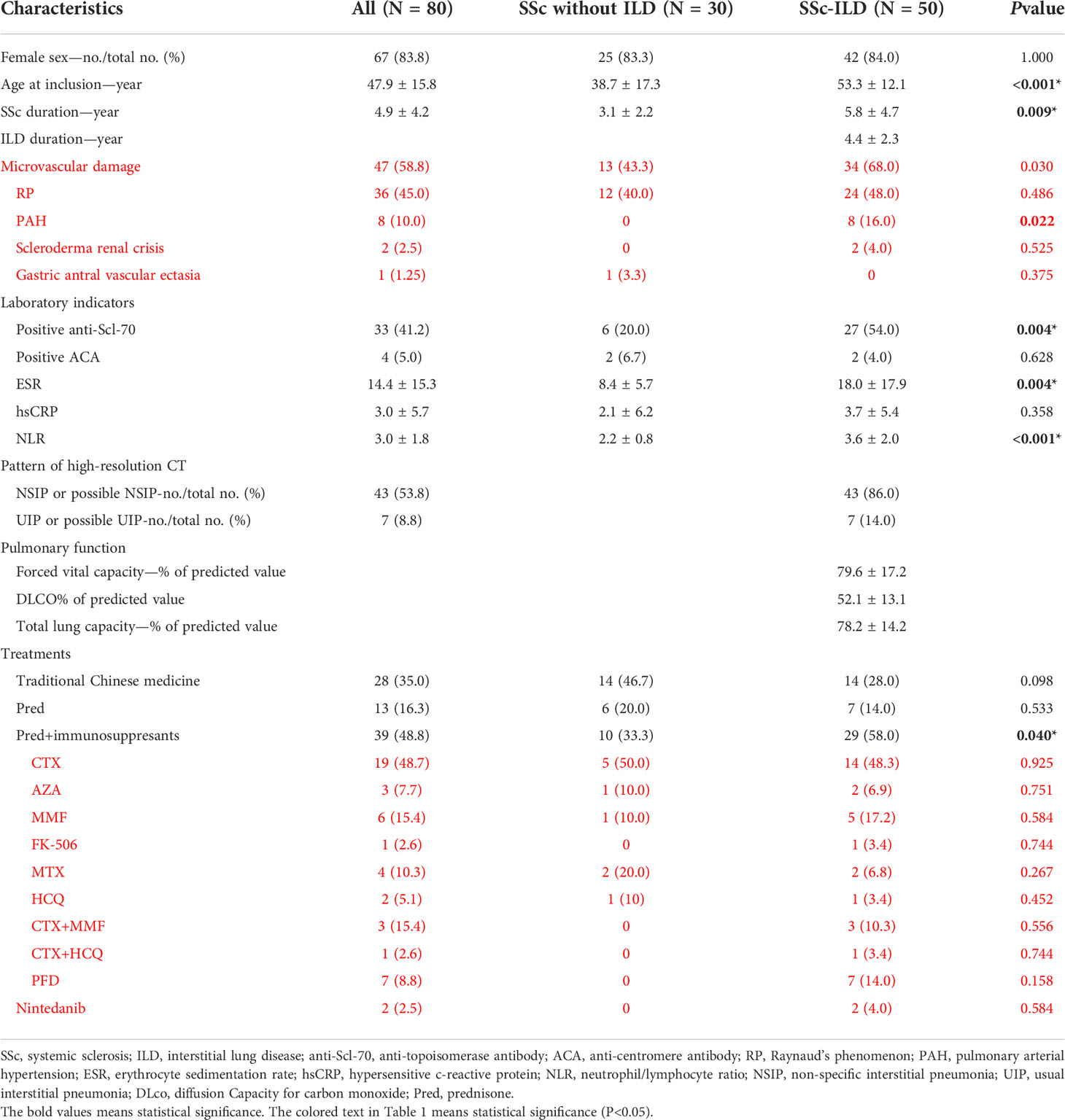
Table 1 Demographic and phenotypic characteristics of systemic sclerosis (SSc) vs. systemic sclerosis-associated interstitial lung disease (SSc-ILD).
Association of RGPD4 mutation with risk of SSc-ILD
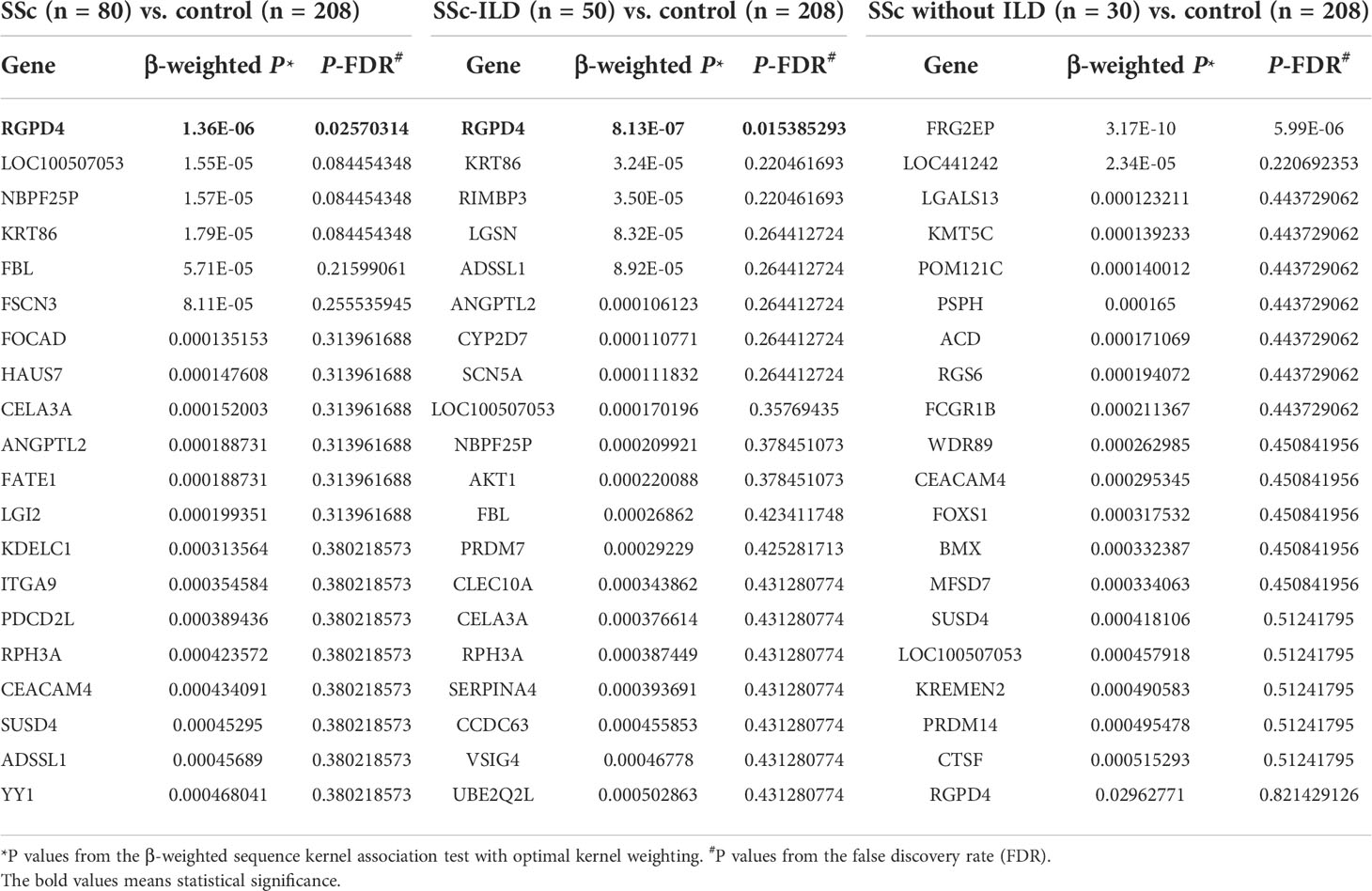
In order to explore novel pathogenic genes potentially contributing to the ILD of SSc, whole exome sequencing (WES) was performed. A total of 18,899 genes were screened to execute a SKAT-O analysis, and the top 20 genes identified with P < 1 × 10-3 were demonstrated in each group in Table 2. In the comparison of the SSc group (n = 80) and control group (n = 208), only RGPD4 on chromosome 2q12.3 met the P-value threshold criteria (0.05/18,899 = 2.51 × 10-6) (P = 1.36 × 10-6, P-FDR = 0.025, OR = 15.95). The patients were subsequently divided into patients with SSc-ILD and patients with SSc without ILD. Next, this outcome remained unchanged in the SSc-ILD group (n = 50) vs. control group (n = 208) (P = 8.13 × 10-7, P-FDR = 0.015, OR = 17.23) rather than the SSc without ILD group (n = 30) vs. control group (n = 208) (P = 2.96 × 10-2, P-FDR = 0.82, OR = 6.97) (Figure 2) , and the P-value and OR value showed the most significance in the SSc-ILD group.
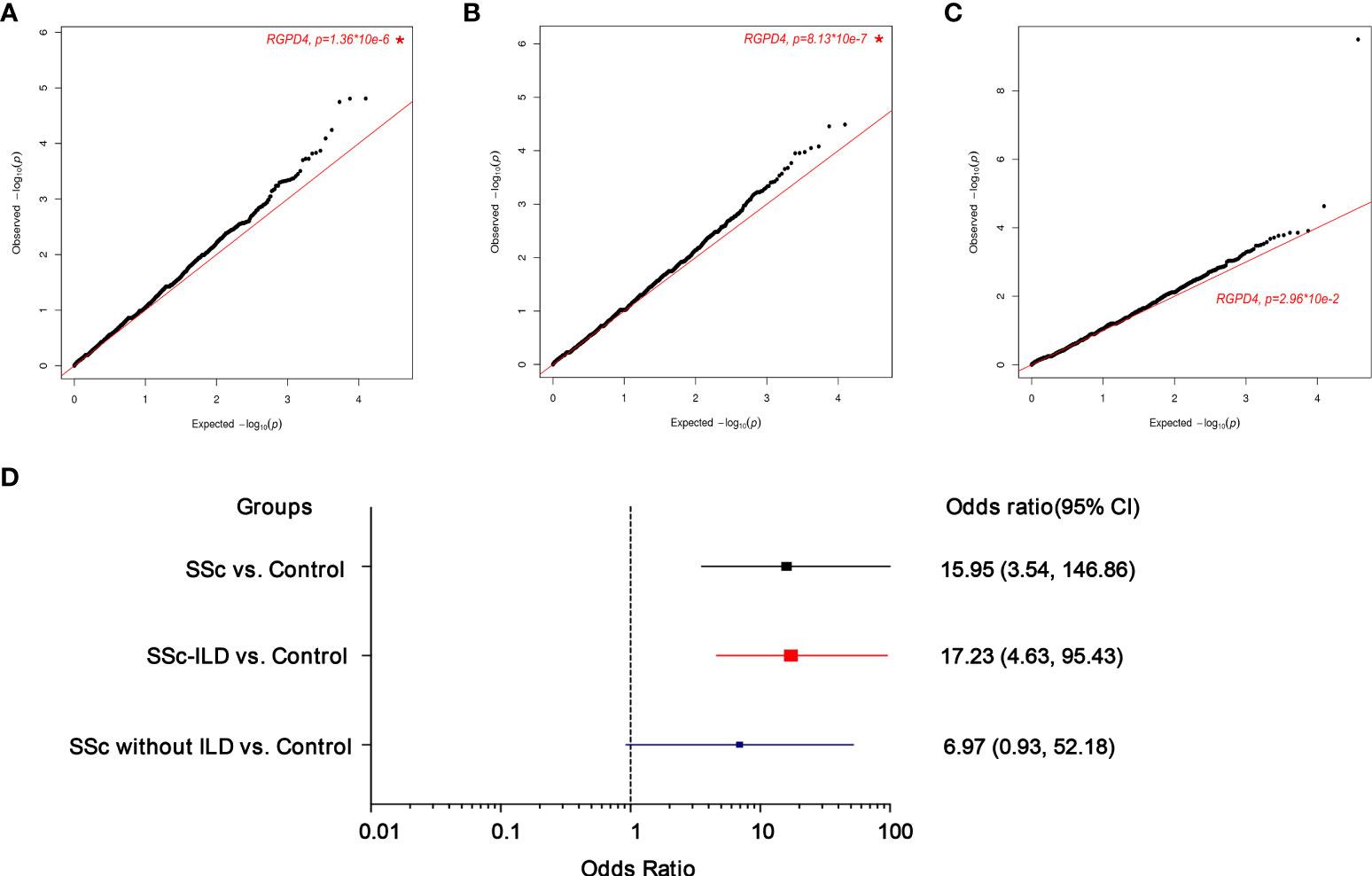
Figure 2 Quantile–quantile(q-q) plot of p values for rare variants test of association between each gene and the presence of the systemic sclerosis (SSc) phenotype upon SKATO analysis (cases=80, controls=208). A total of 18899 genes with at least 4 rare variants were included for correlation with a risk of SSc or ILD. The star indicates the most significant gene. (A) SSc vs. Control (B) SSc-ILD vs. Control (C) SSc without ILD vs. Control (D). The forest plots mainly shows the odds ratio(OR) and 95% confidence intervals (CI). The box indicates the OR value.
The clinical manifestation of patients carrying RGPD4 mutation
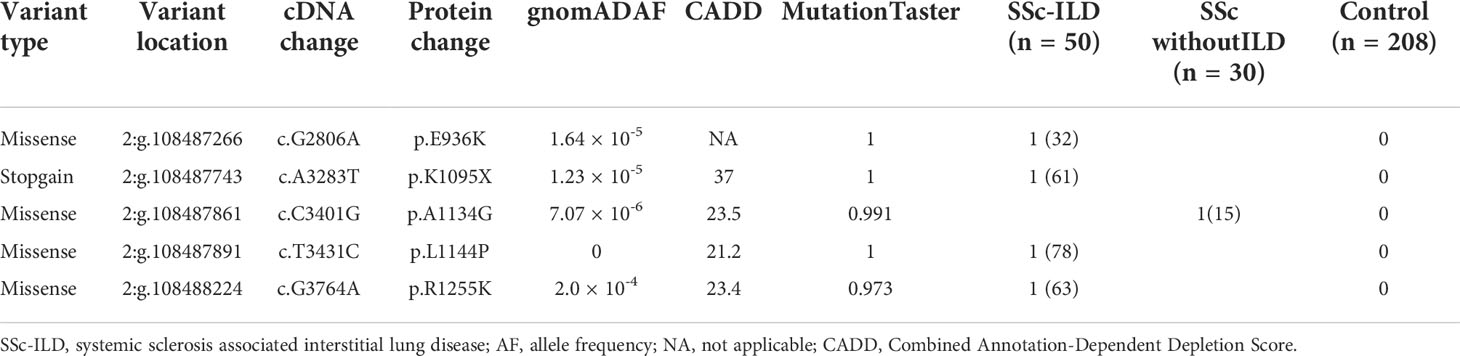
A total of 20 rare functional variants (missense, truncating, splicing) were tested for the RGPD4 gene, and five patients carried five truncating and damaging missense variants, of which three mutations are located in the RanBD1 domain (Table 3; Figure 3A). These five truncating and damaging missense variants owned well conservative except p.E936K. (Figures 3A, B). While the allele frequency of RGPD4 did not reach significant difference between the SSc-ILD group (8.0%) and the SSc without ILD group (3.3%) (P = 0.645), its highest ranking encouraged us to assess the corresponding phenotypes of carriers and additional datasets to determine its correlation with the development of SSc-ILD (Table 4).
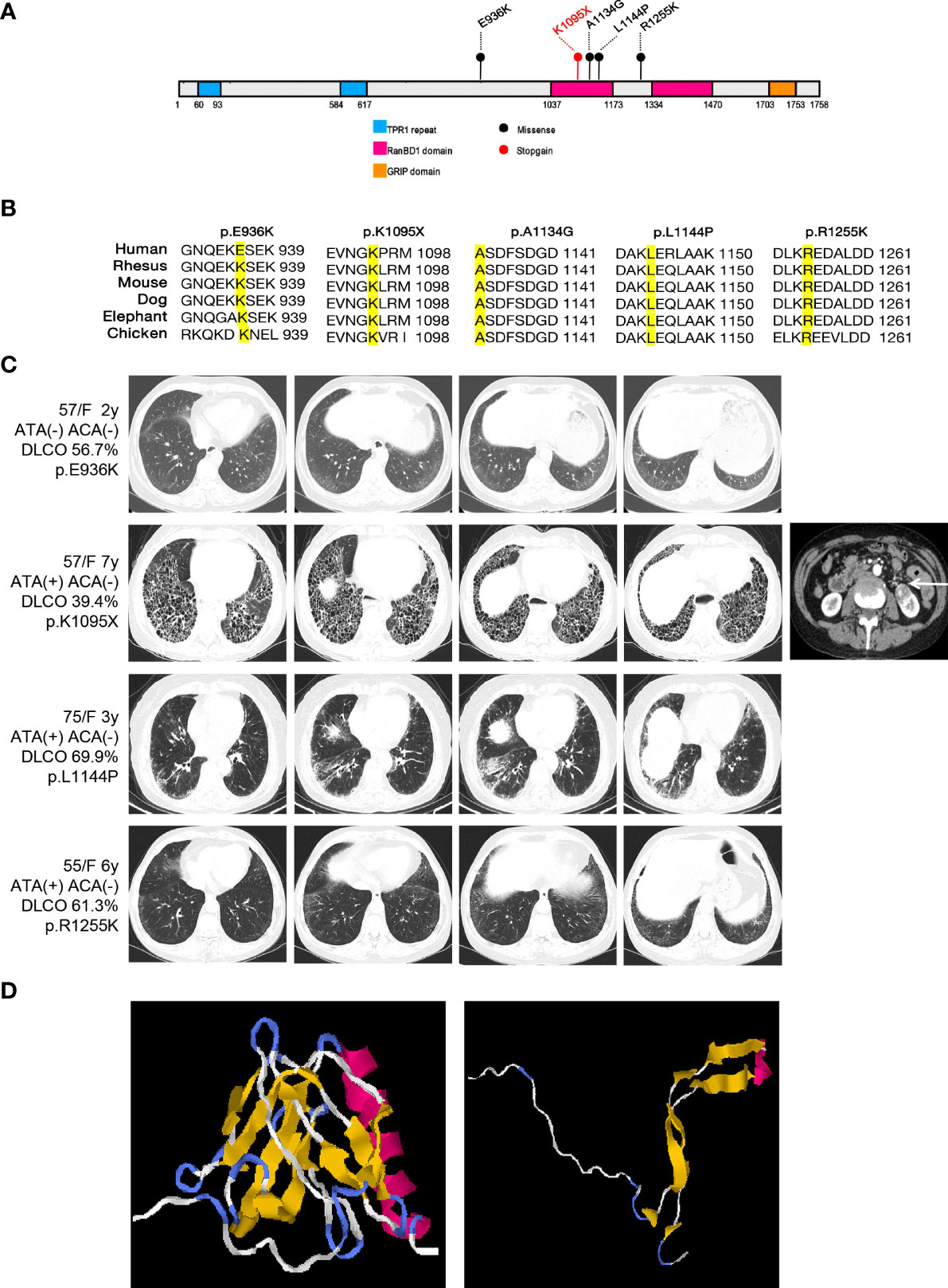
Figure 3 Overview of the damaging variants of RGPD4 and the radiological features of patients carrying the corresponding variants. (A) Schematic of the functional domains of RGPD4 protein with the damaging variants indicating by the circles. The blue rectangles indicate the TRP1 repeat domain. The red rectangles indicate the RanBD1 domain. The orange rectangle indicates the GRIP domain. The black indicates the missense and the red indicates the stopgain. (B) Alignments of RGPD4 amino acid sequences from Human, Rheusus, Mouse, Dog, Elephant and Chicken. (C) The radiological manifestation of the patients carrying the damaging variants of RGPD4. (D) Predicted 3D structure of normal and mutant RGPD4 protein. The left shows the normal structure and the right shows the structure caused by stopgain variation (p.K1095X).
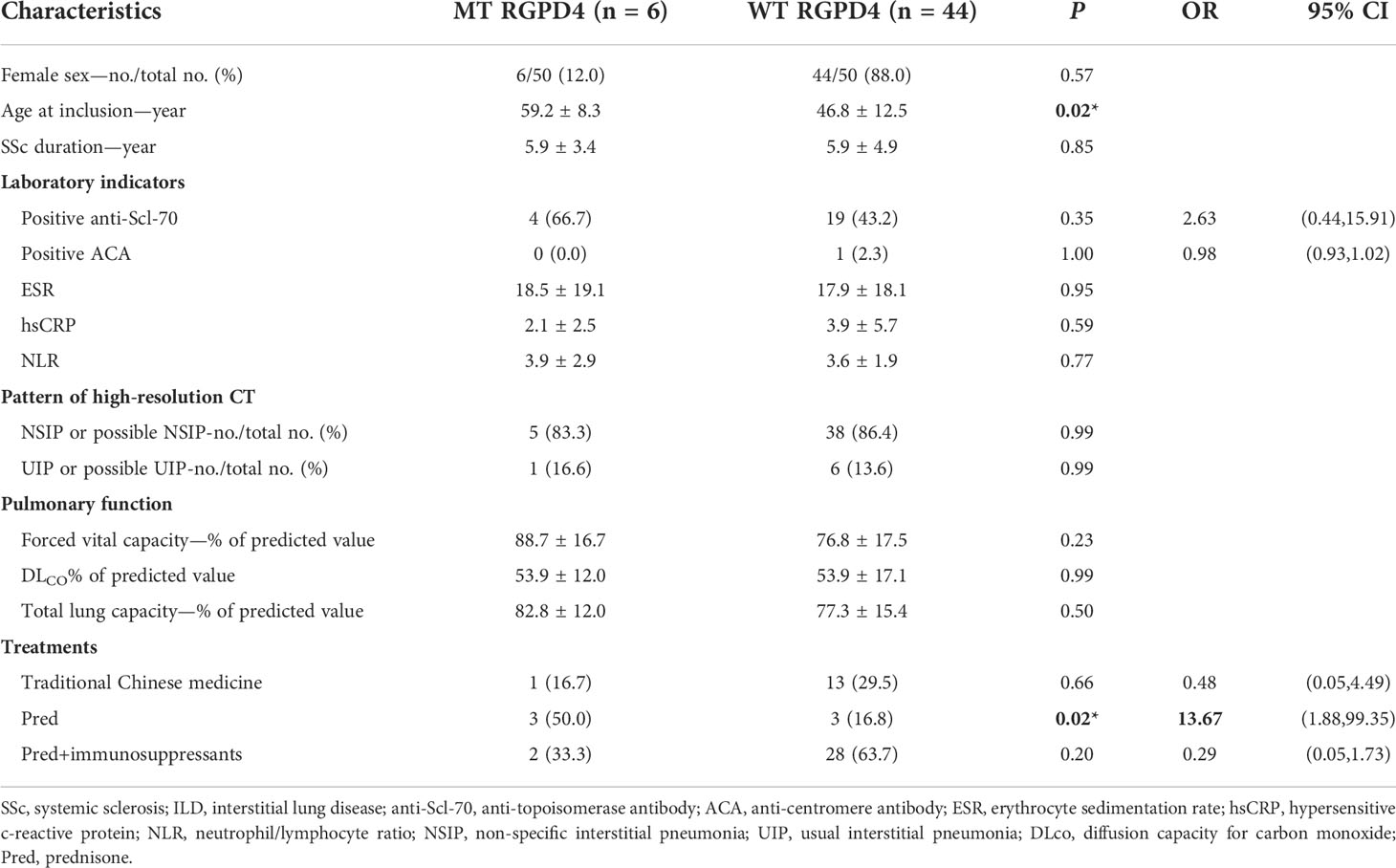
Table 4 Demographic and phenotypic characteristics of patients with SSc-associated interstitial lung disease (SSc-ILD) according to RGPD4-mutated status.
We observed that these RGPD4 variants were rare or completely absent from the gnomAD database. Carriers showed female predisposition (100%) and the NSIP radiological pattern (75%) (Figure 3C). Notably, a patient carrying the RGPD4 p.K1095X variant suffered from a severe phenotype, including dyspnea, Raynaud’s phenomenon, UIP radiological pattern, and severe dysfunction of DLCO. Unluckily, the patient suffered from left kidney clear cell carcinoma. In light of the severe phenotype and the stop–gain variation, we subsequently predicted the three-dimensional structure of normal and corresponding mutant RGPD4 proteins. As expected, the findings showed that the spatial structure caused by stop–gain variation was lost in comparison to the normal structure of the RGPD4 protein (Figure 3D).
The biological function of RGPD4
RGPD4 is a protein-coding gene and is proved to be a candidate gene for ovarian serous cystadenocarcinoma in the OMIM database (612707). It showed no distribution difference in RGPD4 expression among men and women in the GTEx database, except for testis (www.GTEx.org). The protein–protein interactions (PPI) of RGPD4 mainly involved four proteins, namely, SUMO1, UBE2I, RAN, and XPO1 (www.string-db.org). In the GSCALite database, we found that these five interacting proteins showed a strong correlation with malignant diseases, like tumors in the respiratory system (LUSC, LUAD, etc.) and tumors in the urinary system (KICH, KIRC, and KIRP). Furthermore, the mechanism involving such diseases contains the negative regulation of the androgen receptor.
Further analysis implied that RGPD4 also showed a correlation with the androgen biosynthesis and metabolism genes, including CYB5A, CYP3A5, COMT, and STS. Moreover, the correlation among these genes or proteins mainly involved the microRNAs (Figure 4). The abovementioned indirectly provided evidence that RGPD4 showed a correlation with androgen.
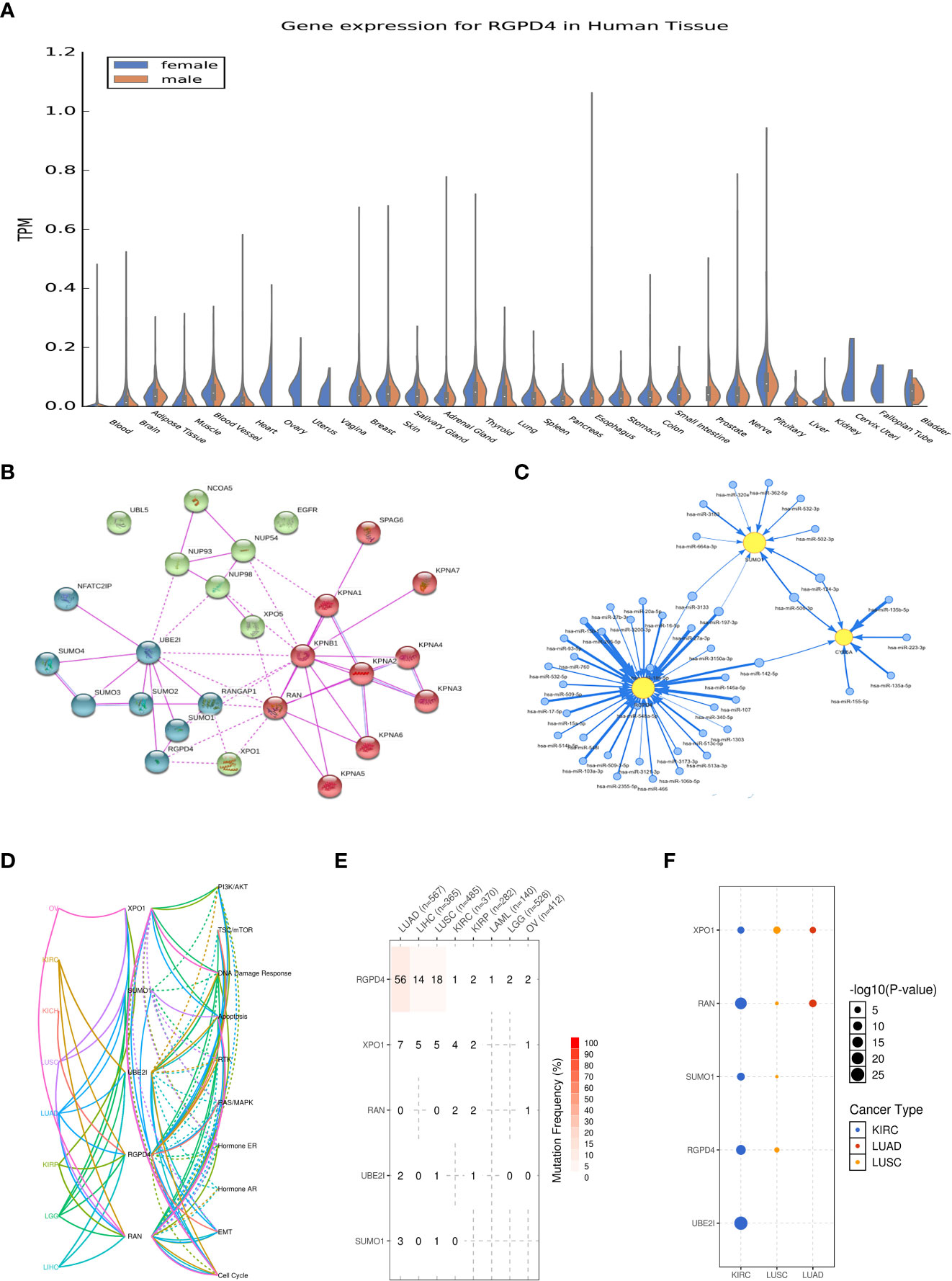
Figure 4 Summary of the biological function of RGPD4. (A) Gene expression(in TPM) of RGPD4 in different tissues amongst male and female (www.GTEx.org). (B) The protein-protein interactions (PPI) of RGPD4 (www.string-db.org). Line thickness indicates the strength of data support and the medium confidence was 0.4. All the interactions sources derive from experiments. (C) The interacted proteins commonly participate in the related pathways of different diseases. KICH, kidney chromophobe; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LGG, brain lower grade glioma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; OV, ovary serous cystadenocarcinoma. (D) The miRNA network among RGPD4 and the androgen metabolism genes. The blue circle indicates miRNA and the yellow circle indicates gene. (E) The reported SNV percentage profile of RGPD4, XPO1, RAN, UBE2I and SUMO1 in different tumors . SNV, single nucleotide variation. (F) The reported cancer types of RGPD4, XPO1, RAN, UBE2I and SUMO1. The size of the circle represents statistical difference and the color indicates different cancer.
Potential association between RGPD4 and sex hormone
Systemic sclerosis owns sex priority, as the incidence of the women is 3–14 times higher than the incidence of men (17), and androgen plays a vital role in the development of SSc (18). Additionally, proteomic analysis identified that RGPD4 determines the occurrence of ovarian serous cystadenocarcinoma, a type of cancer caused by high levels of androgen. Serum proteomic analysis also provided evidence that RGPD4 expression showed male specificity in Asperger’s syndrome (8). In summary, RGPD4 showed the sex-specific difference. Thus, we speculate whether RGPD4 showed a potential correlation with androgen concentration in patients with SSc.
The concentration of sex hormone in patients with SSc
We found that in comparison to healthy control, the TES concentration showed lower levels in patients with SSc-ILD (P = 0.03) and the E2 concentration showed higher levels (P < 0.001). However, the other two sex hormones, including DS and PRL, showed no significance between cases and control. Furthermore, only the E2 concentration showed higher levels in patients with SSc without ILD than that in healthy control (P < 0.001) (Figure 5).
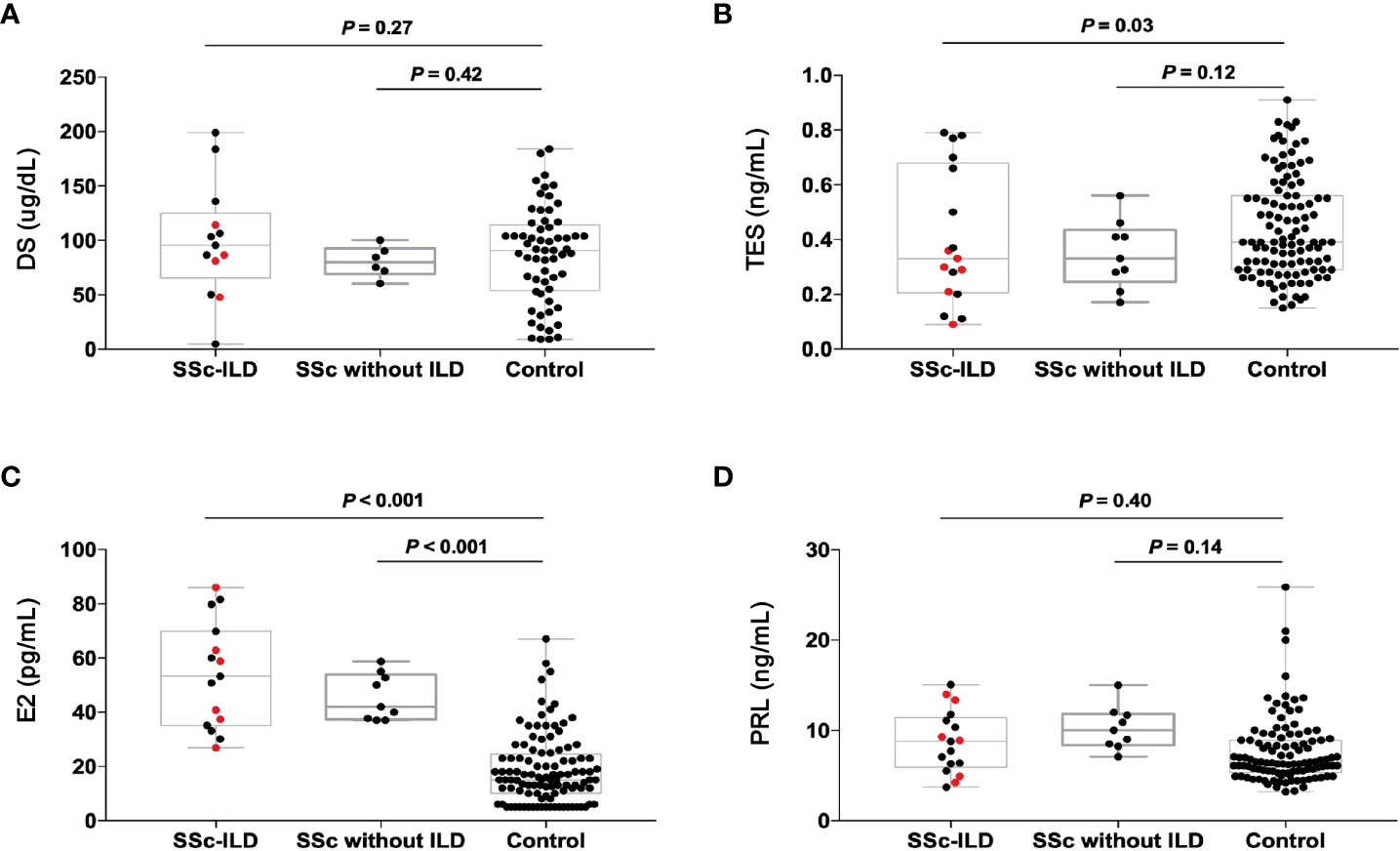
Figure 5 The concentration of sex hormones in the patients with SSc-ILD (n=17), SSc without ILD (n=9) and the healthy people (n=70). The red dots indicate the patients with RGPD4 mutation. (A) The DS concentration (B) The TES concentration (C) The E2 concentration (D) The PRL concentration. All the p values are calculated by general linear model after age adjustment. DS dehydroepiandrosterone sulfate, TES testosterone, E2 estrogen, PRL prolactin.
We further compare the concentrations of sex hormones in SSc-ILD patients carrying RGPD4 variants and those who are not. Surprisingly, the levels of TES concentration in carriers appeared to be lower than in non-carriers, but they did not reach statistical difference (0.26 ± 0.09 vs. 0.41 ± 0.25, P = 0.08), which might result from the low sample sizes.
Associations of variants in androgen biosynthesis and metabolism genes with androgen concentrations
To further explore the mechanism for the lower TES concentration in patients with SSc-ILD, we investigate the variants in androgen biosynthesis and metabolism genes with the levels of TES. A total of 11 SNPs in the coding sequences of androgen biosynthesis and metabolism genes were calculated. The levels of TES among different genotypes did not reach statistical difference after adjustment for age (all P > 0.05) (Table 5).
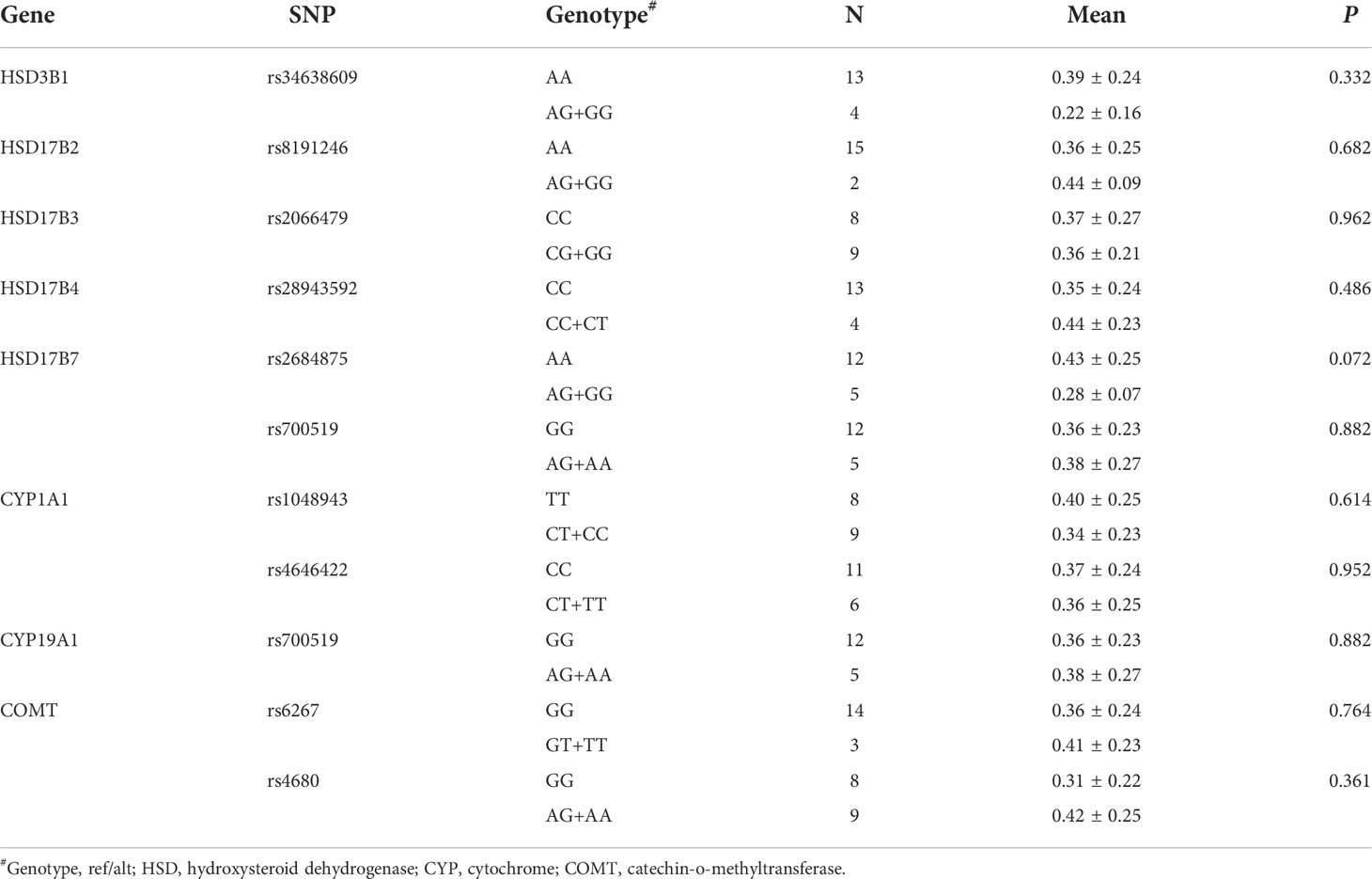
Table 5 The associations of SNPs in the coding exon of androgen-metabolizing genes with testosterone concentration in patients with SSc-ILD.
Potential associations between RGPD4 and androgen concentrations
In bivariate correlation analysis, we analyzed the relationship between the mutant status of RGPD4 and the levels of sex hormones after adjusting for age confounders. Only the level of TES showed a negative correlation with the mutant status (B = -0.509, P = 0.037). Next, after adjusting the mutant status of RGPD4 and age confounders, the level of TES showed no correlation with the levels of the other three hormones in partial correlation analysis (all P > 0.05) (Table 6).
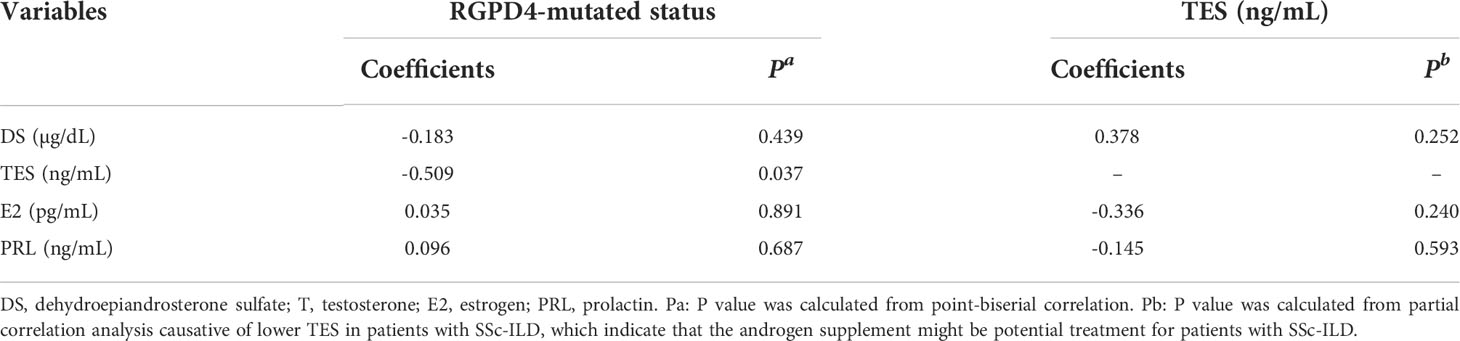
Table 6 Correlation coefficients between sex hormone and the mutated status of RGPD4 gene as well as correlation coefficients between TES and other hormone after age adjustment.
Discussion
Immune-mediated pathways are key in driving the fibrotic process, but how this translates into genetic predisposition is unclear so far. International large cohorts of SSc with and without ILD demonstrated that few specific genetic variants are associated with the development and rate of progression of SSc-ILD. The single-nucleotide polymorphisms (SNPs) of IRF5, IRAK1, CTGF, and CD247 were proved to be the potential candidate genes of SSc-ILD (19). Moreover, the minor allele of IRF5 rs4728142 G>A was proved as a better survival indicator of SSc-ILD (5). Our study introduces the new gene RGPD4 as a candidate contributing to SSc-ILD upon evaluating SSc patients with and without an ILD phenotype with WES, and five of 80 patients were identified with deleterious or LoF variants of RGPD4. Patients carrying those variants showed predominance of lower levels of testosterone than those without variants. Our study provides the potential candidates contributing to SSc-ILD with new insight, which might aid the discovery of new pathogenesis or clinical management for disease.
Interstitial lung disease is a common pulmonary manifestation in patients with SSc and has been the leading cause of death in recent years (20). The 10-year survival rate of SSc was 74.9%, while the 10-year survival rate of SSC-ILD was only 29%–69% (21). It remains an urgent issue to improve clinical outcomes among patients with SSc-ILD. Although new anti-fibrosis drugs, autologous stem cell transplantation, and lung transplantation have been used for treatment, the overall prognosis is not optimistic (22, 23). It still owns great challenge to explore effective and simple treatments. Our team aimed to explore the potential candidates contributing to the pathogenesis of SSc-ILD. The published studies had mostly been executed in subjects of European descent who possess a lower prevalence of ILD than those of African descent (19), and the reported genetic predictors of SSc-ILD mostly derive from common variants; however, the rare variants (minor allele frequency <0.01 or 0.05) are more likely to modulate protein function and result in clinically relevant consequences (24). Our team recently found that the mutant of the gene RANBP2-like and GRIP domain-containing 4 (RGPD4, 2q12.3), one of the members of the RGP family, might be correlated with the pulmonary involvement in SSc patients. Moreover, the variant showed lower levels of testosterone among SSc patients with ILD. In the database of Mouse Genome Information (MGI) upon the mouse model, the phenotypes for the RGPD4 gene involved the reproductive system phenotype and respiratory disease (http://www.informatics.jax.org). However, these data were proved upon the model of mice rather than human beings and a detailed mechanism is needed.
Another significant question that should be addressed is whether RGPD4 mutation was related to the level of testosterone or the disease course. Data in the literature demonstrated that androgen steroids play an immunosuppressive role upon the T-cell and B-cell subsets in multiple autoimmune diseases (25). Early studies have found a decreased concentration of DHEAS in SSc patients (16). and Mirone et al. (12)also confirmed that the levels of testosterone and DHEAS correlated with the disease duration and disease severity score among SSc patients. In other autoimmune diseases, including rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), the level of androgen reflected the severity of systemic damage (26). Our research included the previous research; furthermore, our results implicated that the lower status of testosterone might be modulated by the genetic mutant status in SSc patients with ILD. Our team previously demonstrated that androgen-related pathway genes were associated with the development of IPF by modulating the leukocyte telomere length (13). Whether the lung involvement of SSc patients is related to hormone regulation of leucocyte telomere length needs to be further explored. Interestingly, it had been reported that testosterone supplementation in male SLE patients with genetic hypogonadism alleviated and testosterone protects against the development of joint and lung involvement in male SKG mice (27). The above data, to some extent, provide the hope that androgen replacement therapy may be the new treatment for patients with SSc-ILD.
The major limitation of our study is the small sample size. It was unfortunate that there was insufficient power to test the association between the polymorphism and each of the ILD subsets individually. Next, the animal model of SSc will be built upon the mouse, and the conditional knockout of RGPD4 in the mouse will be explored to confirm the pathogenicity to interstitial lung disease and detect the level of testosterone. Upon that, the extra testosterone supplement will be administered to evaluate the severity of SSc-ILD. In addition, this research was single-center, and more validation should be performed in the other centers. Lastly, this research mainly attempts to explore the perspective of genomics; the perspective of transcriptome and proteomics further will be explored in the future. Nevertheless, despite these limitations, our study provides important information regarding the potentially distinct pathogenic mechanism involved in patients with SSc-ILD.
Conclusion
Interstitial lung disease is a common and devastating manifestation of SSc patients and is the leading cause of death in SSc. In order to initiate early recognition and treatment regimens to preclude progressive fibrosis, genetic susceptibility might be involved in the development and rate of progression of SSc-ILD. By searching the potential genetic candidates upon WES and gene burden analysis of SSc with and without ILD, only the gene RGPD4 variants satisfied the statistical power. Correlation analysis of clinical outcomes and biological function demonstrated that carriers were more prone to suffer from dyspnea, NSIP pattern, and lower TES, which provided insight to a better understanding of the pathobiology of SSc-ILD and androgen hormone supplement might be a therapeutic target in this debilitating disease.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving human participants were reviewed and approved by The Institutional Review Board (IRB) of Peking Union Medical College Hospital (PUMCH). The patients/participants provided their written informed consent to participate in this study.
Author contributions
ZX contributed to the concept and design of this article. NW mainly participated in the manuscript writing. QZ, Xiaoyan Jing, Jian Guo, and NW participated in the sample collection, sample processing, clinical information collection, and data analysis. ZX and HH analyzed and interpreted the data. All authors read and approved the final manuscript.
Funding
This work was funded by the National Key Research and Development Plan of Precision Medicine Medical Research (Grant number: 2016YFC0905700). The funding body has no role in the design of the study and collection, in the analysis and interpretation of data, and in the writing of the manuscript. We thank all your support.
Acknowledgments
We thank all the patients and their families for participation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. Classification criteria for systemic sclerosis: an American college of Rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum (2013) 65:2737–47. doi: 10.1002/art.38098
2. Travis WD, Costabel U, Hansell DM, King TE Jr., Lynch DA, Nicholson AG, et al. Pneumonias, an official American thoracic Society/European respiratory society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med (2013) 188:733–48. doi: 10.1164/rccm.201308-1483ST
3. Alexander T, Snowden JA, Burman J, Chang HD, Del Papa N, Farge D, et al. Intestinal microbiome in hematopoietic stem cell transplantation for autoimmune diseases: Considerations and perspectives on behalf of autoimmune diseases working party (ADWP) of the EBMT. Front Oncol (2021) 11:722436. doi: 10.3389/fonc.2021.722436
4. Ruaro B, Smith V, Sulli A, Pizzorni C, Tardito S, Patane M, et al. Innovations in the assessment of primary and secondary raynaud’s phenomenon. Front Pharmacol (2019) 10:360. doi: 10.3389/fphar.2019.00360
5. Sharif R, Mayes MD, Tan FK, Gorlova OY, Hummers LK, Shah AA, et al. IRF5 poly- morphism predicts prognosis in patients with systemic sclerosis. Ann Rheum Dis (2012) 71(7):1197–202. doi: 10.1136/annrheumdis-2011-200901
6. Stock CJW, De Lauretis A, Visca D, Daccord C, Kokosi M, Kouranos V, et al. Defining genetic risk factors for scleroderma-associated interstitial lung disease: IRF5 and STAT4 gene variants are associated with scleroderma while STAT4 is protective against scleroderma-associated interstitial lung disease. Clin Rheumatol (2020) 39:1173–9. doi: 10.1007/s10067-019-04922-6
7. Tochimoto A, Kawaguchi Y, Yamanaka H. Genetic susceptibility to interstitial lung disease associated with systemic sclerosis. Clin Med Insights Circ Respir Pulm Med (2015) 9:135–40. doi: 10.4137/CCRPM.S23312
8. Zhao J, Cheng F, Zhao Z. Tissue-specific signaling networks rewired by major somatic mutations in human cancer revealed by proteome-wide discovery. Cancer Res (2017) 7:2810–21. doi: 10.1158/0008-5472.CAN-16-2460
9. Senavirathna LK, Huang C, Yang X, Munteanu MC, Sathiaseelan R, Xu D, et al. Hypoxia induces pulmonary fibroblast proliferation through NFAT signaling. Sci Rep (2018) 8:2709. doi: 10.1038/s41598-018-21073-x
10. White B. Immunopathogenesis of systemic sclerosis. Rheum Dis Clin North Am (1996) 22:695–708. doi: 10.1016/S0889-857X(05)70296-9
11. Cutolo M, Capellino S, Sulli A, Serioli B, Secchi ME, Villaggio B, et al. Estrogens and autoimmune diseases. Ann NY Acad Sci (2006) 1089:538–47. doi: 10.1196/annals.1386.043
12. Mirone L, Barini A, Barini A. Androgen and prolactin (Prl) levels in systemic sclerosis (SSc): relationship to disease severity. Ann NY Acad Sci (2006) 1069:257–62. doi: 10.1196/annals.1351.023
13. Fang C, Huang H, Zhang Q, Wang N, Jing X, Guo J, et al. Relation between sex hormones and leucocyte telomere length in men with idiopathic pulmonary fibrosis. Respirology (2020) 25:1265–73. doi: 10.1111/resp.13871
14. Orlandi M, Landini N, Sambataro G, Nardi C, Tofani L, Bruni C, et al. The role of chest CT in deciphering interstitial lung involvement: systemic sclerosis versus COVID-19. Rheumatol (Oxf) (2022) 61:1600–9. doi: 10.1093/rheumatology/keab615
15. Nihtyanova SI, Denton CP. Pathogenesis of systemic sclerosis associated interstitial lung disease. J Scleroderma Relat Disord (2020) 5:6–16. doi: 10.1177/2397198320903867
16. Mak AC, Tang PL, Cleveland C, Smith MH, Kari Connolly M, Katsumoto TR, et al. Brief report: Whole-exome sequencing for identification of potential causal variants for diffuse cutaneous systemic sclerosis. Arthritis Rheumatol (2016) 68:2257–62. doi: 10.1002/art.39721
17. Cottin V, Brown KK. Interstitial lung disease associated with systemic sclerosis (SSc-ILD). Respir Res (2019) 20:13. doi: 10.1186/s12931-019-0980-7
18. .2La Montagna G, Baruffo A, Buono G, Valentini G. Dehydroepiandrosterone sulphate serum levels in systemic sclerosis. Clin Exp Rheumatol (2001) 19:21–6.
19. Stock CJW, Renzoni EA. Genetic predictors of systemic sclerosis-associated interstial lung disease: a review of recent literature. Eur J Hum Genet (2018) 26:765–77. doi: 10.1038/s41431-018-0104-8
20. Perelas A, Silver RM, Arrossi AV, Highland KB. Systemic sclerosis-associated interstitial lung disease. Lancet Respir Med (2020) 8:304–20. doi: 10.1016/S2213-2600(19)30480-1
21. Khanna D, Tashkin DP, Denton CP, Renzoni EA, Desai SR, Varga J. Etiology, risk factors, and biomarkers in systemic sclerosis with interstitial lung disease. Am J Respir Crit Care Med (2020) 201:650–60. doi: 10.1164/rccm.201903-0563CI
22. Yanaba K. Strategy for treatment of fibrosis in systemic sclerosis: Present and future. J Dermatol (2016) 43:46–55. doi: 10.1111/1346-8138.13026
23. Amjadi S, Roofeh D, Khanna D. Management of systemic sclerosis-associated lung disease in the current era. Int J Rheum Dis (2020) 23:137–9. doi: 10.1111/1756-185X.13799
24. Toyoda Y, Mancikova A, Krylov V, Morimoto K, Pavelcova K, Bohata J, et al. Functional characterization of clinically-relevant rare variants in ABCG2 identified in a gout and hyperuricemia cohort. Cells (2019) 8:363. doi: 10.3390/cells8040363
25. Tanriverdi F, Silveira LF, MacColl GS, Bouloux PM. The hypothalamic-pituitary--gonadal axis: immune function and autoimmunity. J Endocrinol (2003) 176:293–304. doi: 10.1677/joe.0.1760293
26. Islander U, Jochems C, Lagerquist MK, Forsblad-d’Elia H, Carlsten H. Estrogens in rheumatoid arthritis; the immune system and bone. Mol Cell Endocrinol (2011) 335:14–29. doi: 10.1016/j.mce.2010.05.018
Keywords: RGPD4, systemic sclerosis, interstitial lung disease, testosterone, whole exosome sequence
Citation: Wang N, Zhang Q, Sun W, Yang X, Huang H and Xu Z (2022) Exome sequencing link mutation in RGPD4 with systemic sclerosis-associated interstitial lung disease and the low level of testosterone—an exploration study. Front. Oncol. 12:956552. doi: 10.3389/fonc.2022.956552
Received: 30 May 2022; Accepted: 15 August 2022;
Published: 08 September 2022.
Edited by:
Helmut H. Popper, Medical University of Graz, AustriaReviewed by:
Barbara Ruaro, University of Trieste, ItalyMohit Kumar Rai, Sanjay Gandhi Post Graduate Institute of Medical Sciences (SGPGI), India
Copyright © 2022 Wang, Zhang, Sun, Yang, Huang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zuojun Xu, eHV6akBob3RtYWlsLmNvbQ==
 Na Wang
Na Wang Qian Zhang2
Qian Zhang2