- 1Department of Neurological Surgery, University of California San Francisco, San Francisco, CA, United States
- 2Department of Radiation Oncology, University of California San Francisco, San Francisco, CA, United States
- 3Division of Neuro-Oncology, University of California San Diego, San Diego, CA, United States
- 4Department of Neuroscience, University of California San Diego, San Diego, CA, United States
- 5Division of Neuro-Oncology, University of California San Francisco, San Francisco, CA, United States
Meningiomas are the most common non-metastatic brain tumors, and although the majority are relatively slow-growing and histologically benign, a subset of meningiomas are aggressive and remain challenging to treat. Despite a standard of care that includes surgical resection and radiotherapy, and recent advances in meningioma molecular grouping, there are no systemic medical options for patients with meningiomas that are resistant to standard interventions. Misactivation of the cell cycle at the level of CDK4/6 is common in high-grade or molecularly aggressive meningiomas, and CDK4/6 has emerged as a potential target for systemic meningioma treatments. In this review, we describe the preclinical evidence for CDK4/6 inhibitors as a treatment for high-grade meningiomas and summarize evolving clinical experience with these agents. Further, we highlight upcoming clinical trials for patients meningiomas, and discuss future directions aimed at optimizing the efficacy of these therapies and selecting patients most likely to benefit from their use.
Introduction
Meningiomas are the most common primary intracranial tumor, and although the vast majority of meningiomas are considered Grade 1 tumors by the World Health Organization (WHO) and can be managed effectively, between 20-30% of cases are considered Grade 2 or 3 and prove challenging to treat. Surgery and radiotherapy are the therapeutic foundation of meningioma management, with no chemotherapeutic agents currently approved for these tumors (1). While there has been significant recent advances in the meningioma prognostication and classification using genomic and DNA methylation classifications, less progress has been made in their therapeutic treatment (2–9). Unfortunately, when these high-grade lesions recur and/or are found in regions along the skull base that make complete resection challenging, they often cause significant morbidity and ultimately prove to be fatal for patients. In this review, we describe the therapeutic rationale and preclinical/clinical evidence for small molecule inhibitors that target key cell cycle regulators, specifically cyclin dependent kinase (CDK) proteins, in the treatment of meningioma.
CDK 4/6 role in tumorigenesis
In non-pathological states, the process of cell division requires cells to progress through a series of highly regulated stages in sequential order, termed the cell cycle, and numerous checkpoints are present to prevent a cell from dividing in the absence of growth factors or in the presence of DNA damage (10). However, dysregulation of these cell division processes and uncontrolled cellular proliferation is a hallmark of cancer (10). CDKs interact with cyclin proteins to regulate this transition from one stage to the next, and, unsurprisingly, increased levels of these CDKs and their regulators, like FOXM1, are commonly observed in cancers such as meningiomas (11–14). CDK4 and 6 are two structurally similar cell cycle regulators that ultimately stimulate a cell forward in cell division to the S phase from G0/G1 (see Figure 1 for schematic of cyclin-CDK pathway). The downstream targets of CDK4/6 include the classic, canonical tumor suppressor protein, retinoblastoma (Rb), and following phosphorylation of Rb by CDK4/6, the transcription factor E2F is able to initiate DNA synthesis and the S phase of cell division (15). Inhibitors of CDK, termed cyclin-dependent kinase inhibitors (CKIs), regulate CDK activity and decreased expression of these regulatory proteins is frequently observed in many cancers, with p16, which is encoded by the gene CDKN2A, being the most well characterized CKI. Furthermore, dysregulation of p16, CDK6, and pRB protein have all been associated with recurrence in atypical meningiomas (16) and homozygous deletions of the CDKN2A/B gene has also been associated with early meningioma recurrence (17). Given their position as relatively upstream regulators of these crucial cell cycle pathways, CDK4/6 specific inhibitors have become very attractive cancer therapeutic agents.
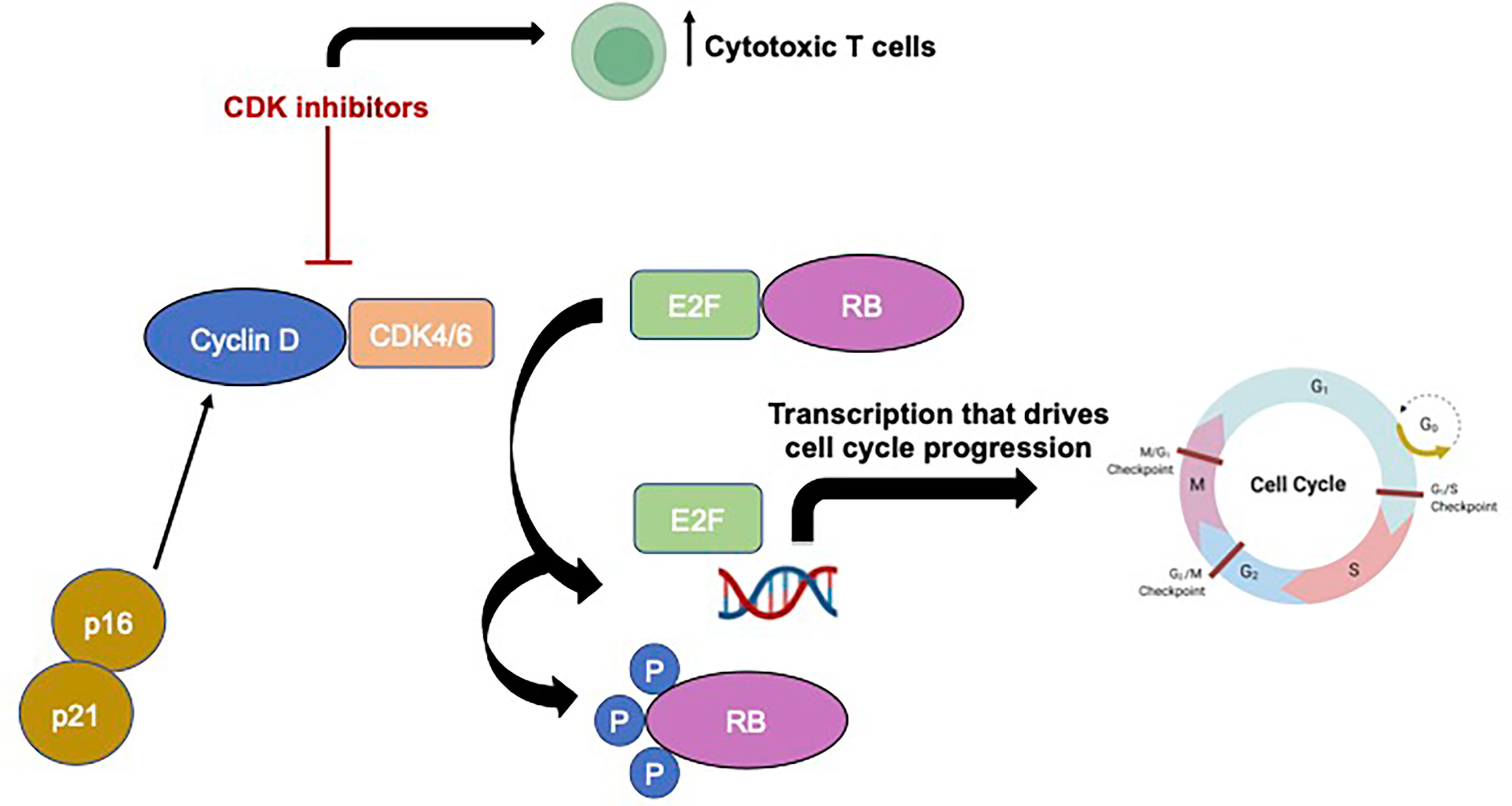
Figure 1 Schematic showing basic cyclin-CDK signaling pathway and mechanism of action of CDK inhibitors Made in BioRender.
Development of CDK inhibitors for treatment of malignancies
Pan-CDK inhibitors were first developed over three decades ago, but their therapeutic potential was thwarted by severe toxicities, and now more specific inhibitors have mostly replaced these early pan-CDK inhibitors (18). There are currently three FDA-approved CDK4/6 specific inhibitors available in the United States: Palbociclib, Ribociclib, and Abemaciclib, each with their own specific pharmacokinetics and toxicities. These agents have been used as monotherapy or in combinatorial approaches with other therapies for the treatment of various cancer types.
Breast cancer was one of the first malignancies where CDK inhibitors were utilized given promising preclinical data demonstrating reliance on CDK signaling during breast cancer tumorigenesis. All three specific inhibitors listed above demonstrated efficacy when used as treatment of estrogen receptor (ER)-positive breast cancer in combination with anti-estrogen therapy, replacing the previous gold standard of anti-estrogen therapy alone for ER-positive breast cancers (19–21). Palbociclib has been shown to be efficacious in other hormone receptor (HR)-positive breast cancer cell lines and is the only agent that can be used for perimenopausal and premenopausal women (22). When combined with an ER antagonist, Palbociclib significantly improved progression free survival, but not overall survival, in HR+ breast cancer (23, 24). Abemaciclib was also found to be safe and have some benefit as a single agent in HR+ breast cancer patients (25). Finally, Ribociclib may have a synergistic effect when used with an ER antagonist, and was found to improve PFS and overall response rate in patients with HR+/HER2- advanced breast cancer (26).
These examples of varying therapeutic efficacy to the different CDK4/6 inhibitors in breast cancer demonstrates the importance of finding biomarkers for tumor sensitivity to these agents. While hormone receptors may prove to be a powerful biomarker for breast cancer responsiveness, other markers are needed for other tumor types. CCND1 amplification and loss of p16 expression may indicate sensitivity to CDK inhibitors in breast cancer, although results are conflicting in the literature (19, 27). Another group of proteins, termed D-cyclin activating features (DCAFs), have also been associated with CDK4/6 inhibitor sensitivity (28). Furthermore, it is equally important to understand how resistance develops to CDK4/6 inhibitors, which seems to be common after prolonged treatment with these agents (29). As CDK4/6 inhibitors are trialed for patients with aggressive meningiomas, it will be important to design clinical trials incorporating window-of-opportunity strategies to obtain tissue for pharmacokinetics, pharmacodynamics, and biomarker analysis from treated patients.
There may be synergistic lethality in targeting CDK4/6 targets in combination with other signaling pathways, particularly those that interact with cell cycle regulation pathways. Other signaling pathways interact with CDK4/6 targets, such as the PI3K-AKT-mTOR and the RAS-RAF-MEK-ERK pathways, may also provide potential therapeutic targets that synergize with CDK4/6 inhibitors. For example, inhibitors of PI3K pathway proteins have been effective in preclinical breast cancer, mesothelioma, and head and neck cancer models when combined with CDK4/6 inhibition (30–32). Mutations in KRAS, NRAS, BRAF genes also lead to activation of the RAS-RAF-MEK-ERK pathway, and treatment with CDK4/6 inhibitors may have a synergistic effect when used with inhibitors of the RAS pathway (33). Like the PI3K pathway, RAS pathway inhibition alters mTOR levels to impact cell proliferation (34–36). Further investigation is needed to determine if inactivation of these overlapping signaling pathways will help prevent resistance to these agents and if there is a role for combinatorial strategies for the treatment of meningioma patients.
Preclinical evidence for CDK inhibitors in meningiomas
As mentioned above, cyclin overexpression has been associated with increased grade and risk of recurrence in meningioma (6, 37–40). Prior to the advent of CDK inhibitors, early preclinical studies utilized targeted small interfering RNA (siRNA) to inhibit CDK. Cheng et al. were one of the first groups to show that targeting cyclin D1 levels decreased cell proliferation, cell viability, and halted tumor cell invasion in malignant meningioma (41). Cyclin D1 knockdown was also shown to decrease antiapoptotic proteins such as survivin and Bcl-2, increasing time in G0/G1 phase and causing cell cycle arrest. siRNA targeting of cyclin D1 also diminished meningioma cell invasion via suppression of extracellular matrix metalloproteinases in vitro. This work opened the door for investigation of pharmacologic CDK inhibitors as therapeutic agents for meningioma.
Subsequent pre-clinical studies revealed anti-tumor effects for CDK inhibitors in various in vitro and in vivo meningioma models. The majority of studies utilized Palbociclib, which is the most frequently used CDK4/6 inhibitor in cancer clinical trials (42). Das et al. found Palbociclib induces G1 cell-cycle arrest and tumor cell apoptosis in a radiation-induced malignant meningioma model (43). Using Grade 1 and Grade 3 meningioma cell lines, Palbociclib treatment inhibited the expression of CDK4/6 and downstream E2F transcription factor, resulting in dramatic reduction of pRB and reduced cell proliferation. Treatment with 14 days of Palbociclib (10mg/kg) plus radiation (6 Gy) reduced total tumor volume in an in vivo subcutaneous mouse meningioma xenograft model. Work by Horbinski et al. further supported Palbociclib-induced suppression of pRb and cell proliferation in vitro, specifically in p16-/Rb+ meningioma cell lines (44). In contrast, p16+/Rb- cell lines were resistant to both radiation and CDK inhibition. This study also demonstrated combination therapy with radiation and Palbociclib significantly delayed tumor growth and prolonged overall survival in mouse xenograft models compared to ether treatment alone. Interestingly, this effect was primarily attributed to decreased cell proliferation, as histological analyses failed to demonstrate any difference in apoptosis or cell death.
Given CDK4/6 inhibitors are thought to be largely cytostatic (45), rather than cytotoxic when used as monotherapy, and there are still toxicities associated with these agents (46, 47), there is significant preclinical interest in combinatorial strategies and/or novel agents that may be cytotoxic. One example, TG02 (SB1317) is an orally available, multi-cyclin-dependent kinase inhibitor of CDK 1,2,5,7 and 9. As specific inhibition of CDK9 has been shown to induce downstream depletion of key oncoproteins including MCL-1 and c-MYC, targeting this CDK protein has also become of interest as a cancer therapy (48, 49). Von Achenbach et al. examined the effects of TG02 in primary patient-derived meningioma cell lines classified as benign, intermediate, or malignant by DNA methylation profiling and found dose-dependent inhibition of cell proliferation across cultures, without significant induction of apoptosis (50). Importantly, cell lines classified as malignant were overall more sensitive than those considered benign.
As mentioned above, there has significant interest in molecular profiling to improve patient selection and clinical response rates to CDK inhibition in patients with recurrent meningioma. Using DNA methylation profiling of 565 primary meningioma samples, Choudhury et al. identified three DNA methylation groups with distinct clinical outcomes and biological drivers: (A) Merlin-intact, (B) Immune-enriched, and (C) hypermitotic, and the latter group was notably had a loss of the endogenous CDK4/6 negative regulator, CDKN2A/B (51). Exposing patient cells from this group to the known CDK4/6 inhibitors Abemaciclib, Palbociclib, and Ribociclib resulted in growth attenuation across cell culture, organoid, and xenograft models. Specifically, in vivo, CDK4/6 blockade diminished pRb expression, inhibited cell proliferation, and prolonged overall survival. This study highlights the role DNA methylation profiling may play as a clinical tool to stratify meningioma patients for molecular treatments.
Agents that indirectly alter the CDK pathway are also being explored as potential meningioma therapies. For example, Negroni et al. found upregulation of the zinc finger transcription factor GATA binding protein 4 (GATA-4) in high grade meningioma primary patient samples, which resulted in overexpression of cyclin D (52). Accordingly, administration of NSC140905, a small molecule inhibitor of GATA-4 reduced expression of cyclin D1 and diminished meningioma cell viability in vitro. Another group is targeting the eukaryotic initiation factor 4F complex (eIF4F), which regulates the translation of many pro-oncogenic proteins like MYC and cyclins in various cancers (53). Oblinger et al. found elevated levels of eIF4A in primary meningioma samples and showed this protein to be a driver of tumor cell proliferation via induction of downstream cyclin-mediated signaling (54). Treatment of cells with silvestrol, an inhibitor of eIF4A, resulted in reduction of cyclins D1 and E1, and G2/M phase arrest. Although these inhibitors are further from clinical trials than the more established CDK inhibitors, these agents pose a novel and promising therapeutic possibility for targeting cyclin-mediated signaling in meningioma.
Meningioma tumor microenvironment on CDK inhibitors
The importance of the brain tumor microenvironment has blossomed in the era of immunotherapy, particularly for highly immunosuppressive tumors like glioblastoma. Given meningiomas ability to invade both brain and bone, early research investigating the meningioma microenvironment focused on specific extracellular matrix components, like matrix metalloproteinase expression (55). However, more recent research has begun to elucidate the importance of immune cells in the microenvironment. For example, new classification schema have emerged based on tumor DNA methylation signatures, with one category of meningiomas considered “immune-enriched” (56). Moreover, in addition to having more immunosuppressive infiltrating immune cells, higher-grade meningiomas appear to express more PD-L1 on tumor cells and tumor-infiltrating CD68+ macrophages (57, 58). Indeed, a large percentage of the meningioma microenvironment consists of CD45+ immune cells (59), with the macrophage population making up the largest percentage of this compartment (60).
Interestingly, the mechanism of action of CDK inhibitors is likely not as simple as once thought. In addition to the direct effect on cycling tumor cells, CDK4 influences the composition of cells in tumor microenvironment and inhibition of this pathway results in changes in the tumor-infiltrating immune cell populations (61). In breast cancer models, CDK inhibition increased antigen presentation and increased the number of cytotoxic T cells in the tumor microenvironment while simultaneously reducing the number of immunosuppressive regulatory T cells (62). Currently, there is very little literature regarding the impact of CDK inhibition on the meningioma tumor microenvironment and even less is known how the meningioma microenvironment contributes to treatment resistance or efficacy.
Clinical trials using CDK inhibitors
To date, one clinical trial investigating CDK inhibitors for meningioma has been one completed and four additional trials are ongoing, for which results have yet to be published (Table 1). Many of these trials include multiple central nervous system (CNS) tumors, and the number of meningioma patients enrolled is currently unknown.
PBTC-042 was a phase I open-label dose-escalation trial to assess the maximum tolerated dose (MTD) and pharmacokinetics of daily oral PD-0332991 (Palbociclib isethionate) in Rb1+ recurrent, progressive, or refractory primary CNS tumors in young adults (NCT02255461). Secondary endpoints included evaluation of efficacy, genetic profiling of tumor samples, and further exploration of pharmacokinetic (PK) parameters. The study was terminated upon completion of primary endpoints and identification of the MTD, although detailed results have not yet been presented or published and it is unclear how many, if any, were meningioma patients. Outcomes data on ClinicalTrial.gov indicate a MTD of 75mg/m2 was identified, with hematologic toxicities, including anemia, neutropenia, and leukopenia predominantly being dose-limiting. Other common toxicities reported in this study included nausea, constipation, diarrhea, fatigue, and transaminitis, although these were not considered serious adverse events. There was also one serious non-hematologic adverse event of dehydration, and these non-hematologic toxicities are one reason these agents have been poorly tolerated by patients and are not more widely used clinically to date. No patients showed objective responses (defined as complete or partial response).
Currently recruiting trials have focused on the CDK4/6 inhibitors Ribociclib (LEE011) and Abemaciclib (LY2835219), the latter of which is distinguished by a shorter half-life and a slightly higher affinity for CDK4 (46). SJDAWN is a Phase 1 dose-escalation clinical trial exploring molecularly driven doublet (or combinatorial) therapies unique to a patient’s specific tumor type (NCT03434262). Patients who tolerate the drug combination are eligible for an expansion cohort to assess for early efficacy. Stratum B of this trial includes patients with recurrent or refractory anaplastic meningioma treated with combination Ribociclib and the MEK inhibitor Trametinib. Primary endpoints include determination of MTD and PK analysis and secondary outcomes include response rate and duration of objective response. The trial is currently ongoing, and no interim results have been reported to date.
Another ongoing study is investigating single-agent Ribociclib in the adult population as a phase 0/2 non-randomized open-label trial evaluating preoperative dosing of oral Ribociclib in patients with Rb+ or non-Rb-mutated recurrent WHO Grade 2/3 meningioma or high-grade glioma (NCT02933736). In this trial, patients receive 900mg of Ribociclib daily for 5 days prior to surgical resection and endpoints include evaluation of PK, PD, and tissue analyses for signs of any preliminary clinical response. PD analysis includes assessment of Rb and FOXM1 phosphorylation as markers of halted cellular progression from G1 to S phase (63). Interim results reported a median CSF concentration of ribociclib was 0.25 μM and tumor tissue concentration of unbound ribociclib 1.36 μM, and 4 out of 8 patients had a positive PK and PD tumor response (defined as unbound ribociclib concentration > 5-fold in vitro IC50 (0.04 μM) and >20% decrease in pRB levels, respectively) (64). These patients defined as PK/PD responders were subsequently enrolled in an exploratory Phase 2 cohort of continuous Ribociclib therapy (600mg daily for 3 weeks/1 week off). At 1 year on therapy, 2 of 4 patients were assessed to have a partial response (PR) by RANO criteria. Overall progression-free survival (PFS) was >12 months in 3 of 4 patients, and >23 months in the 4th patient. Given continuous Ribociclib in other solid tumors has been shown to have an acceptable safety profile, there is excitement for the final results of this ongoing study (25). Although the reThis study also showcases the importance of performing more Phase 0 and “window-of-opportunity” studies to confirm PK/PD for trials investigating CDK inhibitors for meningioma (65).
The remaining two ongoing studies aim to examine the efficacy of twice daily dosing of oral Abemaciclib. The only trial to enroll meningioma patients alone is A071401, a Phase 2 trial of SMO/AKT/NF2/CDK inhibitors in patients with progressive meningiomas harboring corresponding mutations in the respective signaling pathway (NCT02523014). Patients are considered eligible for Abemaciclib if molecular testing is positive for alterations in CDK4, CDK6, CDKN2A, CCND1, CCND2, CCND3, or CCNE1, with primary endpoints including PFS and response rate by Macdonald criteria. To date, interim results have only been reported for the FAK inhibitor cohorts but have not been described for the ongoing Abemaciclib group (66). The second investigational study testing this agent is MSK 17-261, a Phase 2 open-label, non-randomized study of Abemaciclib in patients with recurrent primary brain tumors (NCT03220646), including patients with recurrent meningiomas. Dosing is 200mg of Abemaciclib twice a day, which follows the MTD established in the Phase 1 trial which included patients with glioblastoma, breast cancer, non-small cell lung cancer, and other solid tumors (67). Recent interim results suggest promising early efficacy data for the subset of recurrent meningioma patients, although full results have yet to be published (68).
Future directions
As mentioned, one concern with the use of CDK4/6 inhibitors is the development of resistance mechanisms to these therapies through quasi-redundant or alternative signaling pathways, which has been reported in breast cancer and medulloblastoma patients receiving CDK inhibitor monotherapy (12). Daggubati et al. found that in Hedgehog-associated medulloblastoma, decreased ribosomal protein expression in response to CDK inhibitor treatment caused ER stress and activated the unfolded protein response, which ultimately upregulated production of sterol lipids that activate the Smoothened (SMO) to sustain the Hedgehog signaling pathway despite cell cycle attenuation (69). Interestingly, the authors found that combinatorial therapies with CDK inhibitor and a small molecule that inhibited the production of these SMO-activating lipids was able to effectively block cancer cell growth and may help overcome resistance to monotherapy. Additional studies identifying resistance mechanisms to these inhibitors will be critical to translating preclinical successes to durable responses for patients in the clinic. Finally, given the difficulty patients have tolerating these agents, local delivery strategies such as convection enhanced delivery or approaches to improve drug concentration in the tumor such as blood brain barrier disruption via focused ultrasound should be explored for these therapies.
Conclusions
Patients with high-grade meningiomas face a difficult prognosis with no good systemic treatments available. Cell cycle regulators are commonly dysregulated in many cancers, including meningiomas, and represent a potential treatment strategy. Preclinical evidence supports the use of CDK4/6 specific inhibitors, Palbociclib, Abemaciclib, and Ribociclib, as potential therapeutic agents for meningioma patients and these agents are actively being explored in ongoing clinical trials. Future work identifying response biomarkers and mechanisms of resistance are needed to better select patients for these agents and improve their efficacy and durability.
Author contributions
JY, RK, and AZ drafted the manuscript. JY, JS, and NB organized and designed topic for review, all authors critically reviewed manuscript. All authors contributed to the article and approved the submitted version.
Funding
JY is supported by the Neurosurgery Research and Education Foundation Research Fellowship, the Glioblastoma Foundation Neil Peart Research Award, the Chan-Zuckerberg Biohub Physician Scientist Fellowship, and the ASCO Conquer Cancer Young Investigator Award in Drug Development. DR is supported by the UCSF Physician Scientist Scholar Program, the UCSF Wolfe Meningioma Program Project, and NIH grant R01 CA262311.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Brastianos PK, Galanis E, Butowski N, Chan JW, Dunn IF, Goldbrunner R. Advances in multidisciplinary therapy for meningiomas. Neuro Oncol (2019) 21(Suppl 1):I18–31. doi: 10.1093/NEUONC/NOY136
2. Youngblood MW, Duran D, Montejo JD, Li C, Omay SB, Özduman K, et al. Correlations between genomic subgroup and clinical features in a cohort of more than 3000 meningiomas. J Neurosurg (2019) 133(5):1345–54. doi: 10.3171/2019.8.JNS191266
3. Youngblood MW, Günel M. Molecular genetics of meningiomas. Handb Clin Neurol (2020) 169:101–19. doi: 10.1016/B978-0-12-804280-9.00006-8
4. Youngblood MW, Miyagishima DF, Jin L, Gupte T, Li C, Duran D, et al. Associations of meningioma molecular subgroup and tumor recurrence. Neuro Oncol (2021) 23(5):783–94. doi: 10.1093/NEUONC/NOAA226
5. Patel AJ, Wan YW, Al-Ouran R, Revelli JP, Cardenas MF, Oneissi M, et al. Molecular profiling predicts meningioma recurrence and reveals loss of DREAM complex repression in aggressive tumors. Proc Natl Acad Sci USA (2019) 116(43):21715–26. doi: 10.1073/PNAS.1912858116
6. Choudhury A, Magill ST, Eaton CD, Prager BC, Chen WC, Seo K, et al. Meningioma epigenetic grouping reveals biologic drivers and therapeutic vulnerabilities. medRxiv (2020) 2020:20237495. doi: 10.1101/2020.11.23.20237495
7. Driver J, Hoffman SE, Tavakol S, Woodward E, Maury EA, Bhave V. A molecularly integrated grade for meningioma. Neuro Oncol (2022) 24(5):796–808. doi: 10.1093/NEUONC/NOAB213
8. Sahm F, Schrimpf D, Stichel D, Jones DTW, Hielscher T, Schefzyk S., et al. DNA Methylation-based classification and grading system for meningioma: A multicentre, retrospective analysis. Lancet Oncol (2017) 18(5):682–94. doi: 10.1016/S1470-2045(17)30155-9
9. Nassiri F, Liu J, Patil V, Mamatjan Y, Wang JZ, Hugh-White R, et al. A clinically applicable integrative molecular classification of meningiomas. Nature (2021) 597(7874):119–25. doi: 10.1038/S41586-021-03850-3
10. Hanahan D. Hallmarks of cancer: new DimensionsHallmarks of cancer: new dimensions. Cancer Discov (2022) 12(1):31–46. doi: 10.1158/2159-8290.CD-21-1059
11. Vasudevan HN, Braunstein SE, Phillips JJ, Pekmezci M, Tomlin BA, Wu A, et al. Comprehensive molecular profiling identifies FOXM1 as a key transcription factor for meningioma proliferation. Cell Rep (2018) 22(13):3672–83. doi: 10.1016/j.celrep.2018.03.013
12. O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol (2016) 13(7):417–30. doi: 10.1038/nrclinonc.2016.26
13. Kim H, Park K-J, Ryu B-K, Park D-H, Kong D-S, Chong K, et al. Forkhead box M1 (FOXM1) transcription factor is a key oncogenic driver of aggressive human meningioma progression. Neuropathol Appl Neurobiol (2020) 46(2):125–41. doi: 10.1111/nan.12571
14. Pereira BJA, de Santana Júnior PA, de Almeida AN, Cavalcante SG, de Melo KCM, de Aguiar PHP, et al. Cyclin E1 expression and malignancy in meningiomas. Clin Neurol Neurosurg (2020) 190:105647. doi: 10.1016/j.clineuro.2019.105647
15. Kent LN, Leone G. The broken cycle: E2F dysfunction in cancer. Nat Rev Cancer (2019) 19(6):326–38. doi: 10.1038/s41568-019-0143-7
16. Kim MS, Kim KH, Lee EH, Lee YM, Lee S-H, Kim HD, et al. Results of immunohistochemical staining for cell cycle regulators predict the recurrence of atypical meningiomas. J Neurosurg (2014) 121(5):1189–200. doi: 10.3171/2014.7.JNS132661
17. Sievers P, Hielscher T, Schrimpf D, Stichel D, Reuss DE, Berghoff AS, et al. CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol (2020) 140(3):409–13. doi: 10.1007/s00401-020-02188-w
18. Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov (2015) 14(2):130–46. doi: 10.1038/NRD4504
19. Finn RS, Martin M, Rugo HS, Jones S, Im S-A, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med (2016) 375(20):1925–36. doi: 10.1056/NEJMOA1607303/SUPPL_FILE/NEJMOA1607303_DISCLOSURES.PDF
20. Hortobagyi GN, Stemmer SM, Burris HA, Yap Y-S, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med (2016) 375(18):1738–48. doi: 10.1056/NEJMOA1609709/SUPPL_FILE/NEJMOA1609709_DISCLOSURES.PDF
21. Goetz MP, Toi M, Campone M, Trédan O, Bourayou N, Sohn J, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol (2017) 35(32):3638–46. doi: 10.1200/JCO.2017.75.6155
22. Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, et al. PD 0332991, a selective cyclin d kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res (2009) 11(5). doi: 10.1186/BCR2419
23. Turner NC, Ro J, André F, Loi S, Verma S, Iwata H, et al. Palbociclib in Hormone-Receptor–positive advanced breast cancer. N Engl J Med (2015) 373(3):209–19. doi: 10.1056/NEJMOA1505270/SUPPL_FILE/NEJMOA1505270_DISCLOSURES.PDF
24. Turner NC, Slamon DJ, Ro J, Bondarenko I, Im S-A, Masuda N, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med (2018) 379(20):1926–36. doi: 10.1056/NEJMOA1810527/SUPPL_FILE/NEJMOA1810527_DATA-SHARING.PDF
25. Infante JR, Cassier PA, Gerecitano JF, Witteveen PO, Chugh R, Ribrag V, et al. A phase I study of the cyclin-dependent kinase 4/6 inhibitor ribociclib (LEE011) in patients with advanced solid tumors and lymphomas. Clin Cancer Res (2016) 22(23):5696–705. doi: 10.1158/1078-0432.CCR-16-1248
26. Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol (2018) 36(24):2465–72. doi: 10.1200/JCO.2018.78.9909
27. Finn RS, Liu Y, Zhu Z, Martin M, Rugo HS, Dieras V, et al. Biomarker analyses of response to cyclin-dependent kinase 4/6 inhibition and endocrine therapy in women with treatment-naïve metastatic breast cancer. Clin Cancer Res (2020) 26(1):110–21. doi: 10.1158/1078-0432.CCR-19-0751
28. Gong X, Litchfield LM, Webster Y, Chio LC, Wong SS, Stewart TR, et al. Genomic aberrations that activate d-type cyclins are associated with enhanced sensitivity to the CDK4 and CDK6 inhibitor abemaciclib. Cancer Cell (2017) 32(6):761–76.e6. doi: 10.1016/J.CCELL.2017.11.006
29. Garrido-Castro AC, Goel S. CDK4/6 inhibition in breast cancer: Mechanisms of response andTreatment failure. Curr Breast Cancer Rep (2017) 9(1):26. doi: 10.1007/S12609-017-0232-0
30. Vora SR, Juric D, Kim N, Mino-Kenudson M, Huynh T, Costa C, et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell (2014) 26(1):136–49. doi: 10.1016/J.CCR.2014.05.020
31. Bonelli MA, Digiacomo G, Fumarola C, Alfieri R, Quaini F, Falco A, et al. Combined inhibition of CDK4/6 and PI3K/AKT/mTOR pathways induces a synergistic anti-tumor effect in malignant pleural mesothelioma cells. Neoplasia (2017) 19(8):637–48. doi: 10.1016/J.NEO.2017.05.003
32. Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by akt and suppresses mTOR signalling. Nat Cell Biol (2002) 4(9):648–57. doi: 10.1038/ncb839
33. Chen SH, Gong X, Zhang Y, Van Horn RD, Yin T, Huber L, et al. RAF Inhibitor LY3009120 sensitizes RAS or BRAF mutant cancer to CDK4/6 inhibition by abemaciclib via superior inhibition of phospho-RB and suppression of cyclin D1. Oncogene (2018) 37(6):821–32. doi: 10.1038/ONC.2017.384
34. Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by erk implications for tuberous sclerosis and cancer pathogenesis. Cell (2005) 121(2):179–93. doi: 10.1016/J.CELL.2005.02.031
35. Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J. Tumor-promoting phorbol esters and activated ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci USA (2004) 101(37):13489–94. doi: 10.1073/PNAS.0405659101
36. Lee MS, Helms TL, Feng N, Gay J, Chang QE, Tian F, et al. Efficacy of the combination of MEK and CDK4/6 inhibitors in vitro and in vivo in KRAS mutant colorectal cancer models. Oncotarget (2016) 7(26):39595–608. doi: 10.18632/ONCOTARGET.9153
37. Bi WL, Greenwald NF, Abedalthagafi M, Wala J, Gibson WJ, Agarwalla PK, et al. Genomic landscape of high-grade meningiomas. NPJ Genom Med (2017) 2(1). doi: 10.1038/S41525-017-0014-7
38. Boström J, Meyer-Puttlitz B, Wolter M, Blaschke B, Weber RG, Lichter P, et al. Alterations of the tumor suppressor genes CDKN2A (p16(INK4a)), p14(ARF), CDKN2B (p15(INK4b)), and CDKN2C (p18(INK4c)) in atypical and anaplastic meningiomas. Am J Pathol (2001) 159(2):661–9. doi: 10.1016/S0002-9440(10)61737-3
39. Maxwell M, Galanopoulos T, Antoniades HN. Expression of cyclin D1 proto-oncogene mRNA in primary meningiomas may contribute to tumorigenesis. Int J Oncol (1996) 9(6):1213–7. doi: 10.3892/IJO.9.6.1213
40. Alama A, Barbieri F, Spaziante R, Bruzzo C, Dadati P, Dorcaratto A, et al. Significance of cyclin D1 expression in meningiomas: A preliminary study. J Clin Neurosci (2007) 14(4):355–8. doi: 10.1016/J.JOCN.2006.04.001
41. Cheng G, Zhang L, Lv W, Dong C, Wang Y, Zhang J. Overexpression of cyclin D1 in meningioma is associated with malignancy grade and causes abnormalities in apoptosis, invasion and cell cycle progression. Med Oncol (2015) 32(1):1–8. doi: 10.1007/S12032-014-0439-0
42. Finn R, Hurvitz S, Allison M, Applebaum S, Glaspy J, DiCarlo B, et al. Phase I study of PD 0332991, a novel, oral, cyclin-d kinase (CDK) 4/6 inhibitor in combination with letrozole, for first-line treatment of metastatic post-menopausal, estrogen receptor-positive (ER+), human epidermal growth factor receptor 2 (HER2)-negati. Cancer Res (2009) 69(Suppl 24):5069–9. doi: 10.1158/0008-5472.SABCS-09-5069
43. Das A, Alshareef M, Martinez Santos JL, Porto GBF, McDonald DG, Infinger LK, et al. Evaluating anti-tumor activity of palbociclib plus radiation in anaplastic and radiation-induced meningiomas: Pre-clinical investigations. Clin Transl Oncol (2020) 22(11):2017–25. doi: 10.1007/S12094-020-02341-7
44. Horbinski C, Xi G, Wang Y, Hashizume R, Gopalakrishnan M, Phillips JJ, et al. The effects of palbociclib in combination with radiation in preclinical models of aggressive meningioma. Neurooncol Adv (2021) 3(1). doi: 10.1093/NOAJNL/VDAB085
45. McClendon AK, Dean JL, Rivadeneira DB, Yu JE, Reed CA, Gao E, et al. CDK4/6 inhibition antagonizes the cytotoxic response to anthracycline therapy. Cell Cycle (2012) 11(14):2747. doi: 10.4161/CC.21127
46. Spring LM, Zangardi ML, Moy B, Bardia A. Clinical management of potential toxicities and drug interactions related to cyclin-dependent kinase 4/6 inhibitors in breast cancer: Practical considerations and recommendations. Oncologist (2017) 22(9):1039–48. doi: 10.1634/THEONCOLOGIST.2017-0142
47. Thill M, Schmidt M. Management of adverse events during cyclin-dependent kinase 4/6(CDK4/6) inhibitor-based treatment in breast cancer. Ther Adv Med Oncol (2018) 10. doi: 10.1177/1758835918793326
48. Goh KC, Novotny-Diermayr V, Hart S, Ong LC, Loh YK, Cheong A, et al. TG02, a novel oral multi-kinase inhibitor of CDKs, JAK2 and FLT3 with potent anti-leukemic properties. Leukemia (2012) 26(2):236–43. doi: 10.1038/LEU.2011.218
49. Juric V, Murphy B. Cyclin-dependent kinase inhibitors in brain cancer: Current state and future directions. Cancer Drug Resist (2020) 3(1):48–62. doi: 10.20517/CDR.2019.105
50. von Achenbach C, Le Rhun E, Sahm F, Wang SS, Sievers P, Neidert MC, et al. Sensitivity of human meningioma cells to the cyclin-dependent kinase inhibitor, TG02. Transl Oncol (2020) 13(12):100852. doi: 10.1016/j.tranon.2020.100852
51. Choudhury A, Magill S, Eaton CD, Prager BC, Chen WC, Seo K, et al. Meningioma DNA methylation grouping reveals biologic drivers and therapeutic vulnerabilities. Int J Radiat Oncol (2021) 111(3):e558–9. doi: 10.1016/J.IJROBP.2021.07.1513
52. Negroni C, Hilton DA, Ercolano E, Adams CL, Kurian KM, Baiz D, et al. GATA-4, a potential novel therapeutic target for high-grade meningioma, regulates miR-497, a potential novel circulating biomarker for high-grade meningioma. EBioMedicine (2020) 59. doi: 10.1016/J.EBIOM.2020.102941
53. Kong T, Xue Y, Cencic R, Zhu X, Monast A, Fu Z, et al. eIF4A inhibitors suppress cell-cycle feedback response and acquired resistance to CDK4/6 inhibition in cancer. Mol Cancer Ther (2019) 18(11):2158–70. doi: 10.1158/1535-7163.MCT-19-0162
54. Oblinger JL, Burns SS, Huang J, Pan L, Ren Y, Shen R, et al. Overexpression of eIF4F components in meningiomas and suppression of meningioma cell growth by inhibiting translation initiation. Exp Neurol (2018) 299(Pt B):299–307. doi: 10.1016/J.EXPNEUROL.2017.06.015
55. Sahab-Negah S, Gorji A. Meningioma tumor microenvironment. Adv Exp Med Biol (2020) 1296:33–48. doi: 10.1007/978-3-030-59038-3_3/TABLES/3
56. Choudhury A, Magill ST, Eaton CD, Prager BC, Chen WC, Cady MA, et al. Meningioma DNA methylation groups identify biological drivers and therapeutic vulnerabilities. Nat Genet (2022) 54(5). doi: 10.1038/S41588-022-01061-8
57. Han SJ, Reis G, Kohanbash G, Shrivastav S, Magill ST, Molinaro AM, et al. Expression and prognostic impact of immune modulatory molecule PD-L1 in meningioma. J Neurooncol (2016) 130(3):543–52. doi: 10.1007/S11060-016-2256-0
58. Du Z, Abedalthagafi M, Aizer AA, Shrivastav S, Magill ST, Molinaro AM, et al. Increased expression of the immune modulatory molecule PD-L1 (CD274) in anaplastic meningioma. Oncotarget (2015) 6(7):4704–16. doi: 10.18632/ONCOTARGET.3082
59. Domingues PH, Teodósio C, Ortiz J, Sousa P, Otero Á, Maillo A, et al. Immunophenotypic identification and characterization of tumor cells and infiltrating cell populations in meningiomas. Am J Pathol (2012) 181(5):1749–61. doi: 10.1016/J.AJPATH.2012.07.033
60. Garzon-Muvdi T, Bailey DD, Pernik MN, Pan E. Basis for immunotherapy for treatment of meningiomas. Front Neurol (2020) 11:945/BIBTEX. doi: 10.3389/FNEUR.2020.00945/BIBTEX
61. Klein ME, Kovatcheva M, Davis LE, Tap WD, Koff A. CDK4/6 inhibitors: The mechanism of action may not be as simple as once thought. Cancer Cell (2018) 34(1):9–20. doi: 10.1016/J.CCELL.2018.03.023
62. Goel S, Decristo MJ, Watt AC, Brinjones H, Sceneay J, Li BB, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature (2017) 548(7668):471–5. doi: 10.1038/nature23465
63. Sanai N, Tien A-C, Li J, Bao X, DeRogatis A, Fujita Y, et al. 392O - a phase 0/II clinical trial of a CDK4/6 inhibitor in aggressive meningioma patients. Ann Oncol (2019) 30:v144. doi: 10.1093/annonc/mdz243.002
64. Tien A-C, Li J, Bao X, DeRogatis A, Fujita Y, Pennington-Krygier C, et al. Mngi-01. a phase 0 trial of ribociclib in aggressive meningioma patients incorporating a tumor pharmacodynamic- and pharmacokinetic-guided expansion cohort. Neuro Oncol (2019) 21(Suppl 6):vi139. doi: 10.1093/NEUONC/NOZ175.583
65. Vogelbaum MA, Krivosheya D, Borghei-Razavi H, Sanai N, Weller M, Wick W, et al. Phase 0 and window of opportunity clinical trial design in neuro-oncology: A RANO review. Neuro Oncol (2020) 22(11):1568–79. doi: 10.1093/NEUONC/NOAA149
66. Brastianos PK, Twohy E, Gerstner ER, Kaufmann TJ, Iafrate AJ, Jeyapalan SA, et al. Alliance A071401: Phase II trial of FAK inhibition in meningiomas with somatic NF2 mutations. J Clin Oncol (2020) 38(Suppl 15):2502–2. doi: 10.1200/JCO.2020.38.15_SUPPL.2502
67. Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov (2016) 6(7):740–53. doi: 10.1158/2159-8290.CD-16-0095
68. Coffee E, Panageas K, Young R, Morrison T, Daher A, Grommes C, et al. Ctni-55. the cdk4/6 inhibitor abemaciclib in patients with recurrent meningioma and other primary cns tumors. Neuro Oncol (2021) 23(Supplement_6):vi72–3. doi: 10.1093/NEUONC/NOAB196.280
Keywords: CDK inhibitor, meningioma, cell cycle dysregulation, clinical trials, molecular profiling and subtyping
Citation: Young JS, Kidwell RL, Zheng A, Haddad AF, Aghi MK, Raleigh DR, Schulte JD and Butowski NA (2022) CDK 4/6 inhibitors for the treatment of meningioma. Front. Oncol. 12:931371. doi: 10.3389/fonc.2022.931371
Received: 28 April 2022; Accepted: 27 June 2022;
Published: 22 July 2022.
Edited by:
Hailiang Tang, Fudan University, ChinaReviewed by:
Regina M. Graham, University of Miami Health System, United StatesMirna Lechpammer, Foundation Medicine Inc., United States
Copyright © 2022 Young, Kidwell, Zheng, Haddad, Aghi, Raleigh, Schulte and Butowski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jacob S. Young, Jacob.young@ucsf.edu; Nicholas A. Butowski, Nicholas.butowski@ucsf.edu
 Jacob S. Young
Jacob S. Young