- 1Third Surgery Department, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China
- 2Department of Gastrointestinal Surgery, Hengshui People’s Hospital, Hengshui, China
Objective: This study aimed to develop prognostic prediction models for patients with Siewert type II/III adenocarcinoma of the esophagogastric junction (AEG) who received neoadjuvant therapy (neoadjuvant chemoradiotherapy or neoadjuvant chemotherapy) and radical surgery. A baseline nomogram and a post-operative nomogram were constructed before neoadjuvant therapy and after surgery. The predictive performance of the constructed nomograms was internally validated and compared to the TNM staging system.
Materials and Methods: A total of 245 patients diagnosed with Siewert type II/III AEG and treated with neoadjuvant therapy followed by radical surgery at The Fourth Hospital of Hebei Medical University between January 2011 and December 2017 were enrolled. The variables before neoadjuvant therapy were defined as baseline factors, while the variables of baseline factors along with the variables of treatment and postoperative pathology were defined as post-operative factors. To construct the corresponding nomograms, independent predictors of baseline and post-operative factors were identified. The C-index and a time-dependent receiver operating characteristic curve were used to evaluate the model’s discrimination ability. The calibration ability of the model was determined by comparing the probability of predicted free-recurrence to the actual free-recurrence. Decision curve analysis (DCA) was used to determine the clinical usefulness of the nomogram.
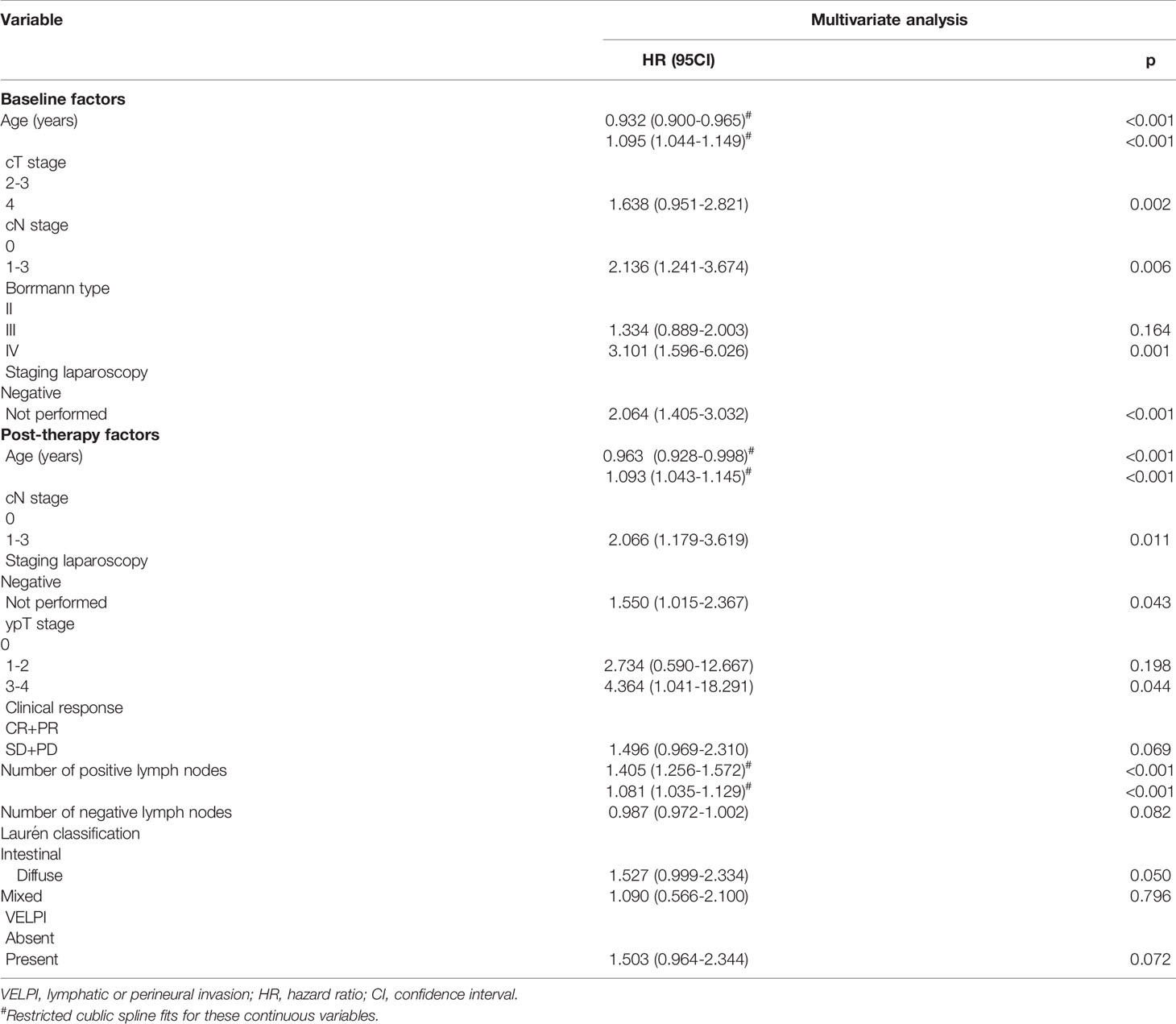
Results: Among the baseline factors, age, cT stage, cN stage, Borrmann type, and staging laparoscopy were independent prognostic predictors. In contrast, among the post-operative factors, age, cN stage, staging laparoscopy, ypT stage, clinical response, number of positive lymph nodes, number of negative lymph nodes, laurén classification, and lymphatic, or perineural invasion (VELPI) were independent prognostic predictors. The two nomograms were constructed using the independent predictors of prognosis. The C-indexes for the baseline and post-operative nomograms were 0.690 (95% CI, 0.644-0.736) and 0.817 (95% CI, 0.782-0.853), respectively. The AUCs of the baseline nomogram at 3 and 5 years were both greater than cTNM (73.1 vs 58.8, 76.1 vs 55.7). Similarly, the AUCs of the post-operative nomogram were both greater than ypTNM (85.2 vs 69.1, 88.2 vs 71.3) at 3 and 5 years. The calibration curves indicated that both models had a high degree of calibration ability. By comparing the DCA at 3 and 5 years, we determined that the two nomograms constructed had better clinical utility than the TNM staging system.
Conclusions: The constructed nomograms have a more accurate predictive ability than the eighth edition TNM staging system, which can be useful for treatment selection and follow-up monitoring of patients.
Introduction
Over the last few decades, the incidence of adenocarcinoma of esophagogastric junction (AEG) has increased globally, raising new concerns (1, 2). AEG is a term that refers to digestive tract malignancy that occurs in a special anatomical site within the epicenter located 5 cm above and below the esophagogastric junction (EGJ). Siewert typing is the world’s most widely used method. Siewert type II AEGs have an epicenter located between 1 cm above and 2 cm below the EGJ, and Siewert type III AEGSs have an epicenter located 2-5 cm below the EGJ (3). In patients with Siewert type II/III AEG, surgical resection is the primary treatment. However, because the majority of patients are in the advanced stage at the time of diagnosis, the 5-year survival rate is only 20%-30% even with radical resection (4–6). Neoadjuvant chemoradiotherapy and neoadjuvant chemotherapy are the primary components of neoadjuvant therapy for AEG. Neoadjuvant therapy has been shown to improve the prognosis of AEG patients by reducing the clinical stage of the tumor and increasing the rate of radical surgical resection. According to the current study, both methods of neoadjuvant therapy appear to have comparable survival benefits (7).
The TNM staging system issued by the American Joint Committee on Cancer (AJCC) is the primary tool for determining the patients’ treatment mode and prognosis. The 8th edition of the TNM staging system included clinical staging (cTNM) and post-neoadjuvant pathological staging (ypTNM), as well as the proposal to use the staging system for esophageal and gastric cancer for Siewert type II and III AEG, respectively (8). These updates provide more precise guidance for Siewert type II/III AEG patients receiving neoadjuvant therapy. However, because the TNM staging system is limited to anatomical variables such as tumor infiltration depth, lymph node invasion, and distant metastasis, the prognosis of patients with the same TNM staging remains heterogeneous. The integration of multiple factors, including patient demographic characteristics, treatment, and clinicopathological characteristics in the construction of a nomogram prediction model, has become a trend in tumor prognosis research and has been endorsed by the AJCC (9). The nomogram has been used to assist in the selection of individualized treatment and the development of follow-up strategies for patients with a variety of tumor types (10–12). Among gastrointestinal tumors, the nomogram is most widely used for colon cancer since it has been shown to be more clinically useful than the TNM staging system in predicting patient recurrence or survival (13–15). On the basis of molecular and clinicopathological features, a third-generation clinical calculator for colon cancer has been established (16). Numerous research has established nomograms for AEG. Gabriel E et al. (17) developed a prognostic calculator for patients with esophagus adenocarcinoma based on patient and treatment factors, which could predict the individual survival probability of patients receiving or not receiving neoadjuvant chemoradiotherapy before treatment. Zhou et al. (18) developed a nomogram for AEG patients using the Surveillance, Epidemiology, and End Results (SEER) database. Liu et al. (19) established Siewert II AEG patients undergoing preoperative radiotherapy based on the SEER database. Chen (20) established a nomogram for patients with Siewert type II/III AEG who did not receive preoperative treatment. Lemini R et al. (21) further externally validated several currently existing prognostic prediction models for the patients with esophageal adenocarcinoma. Unfortunately, none of these models showed satisfactory prediction accuracy. To our knowledge, we are the first to develop the prognostic prediction model for patients with Siewert type II/III AEG that includes both neoadjuvant chemoradiotherapy and neoadjuvant chemotherapy in combination with radical resection. To correspond to the TNM staging system, a baseline nomogram and a post-operative nomogram were constructed before the neoadjuvant therapy and after surgery to determine the probability of free-recurrence at 3 and 5 years, respectively. The predictive performance of the constructed nomograms was internally validated and compared to the TNM staging system.
Materials and Methods
Study Population
A total of 245 patients diagnosed with Siewert type II/III AEG who received neoadjuvant therapy followed by radical surgery at The Fourth Hospital of Hebei Medical University between January 2011 and December 2017 were included in the study. The following inclusion criteria were used (1): gastroscopy and histologically confirmed Siewert type II/III AEG; (2) patients treated with preoperative neoadjuvant chemoradiotherapy or neoadjuvant chemotherapy followed by R0 resection. The exclusion criterion was as follows:(1) absence of important clinicopathological or therapeutic data; (2) patients with multifocal gastric cancer or other malignancies. (3) the presence of distant metastases before neoadjuvant therapy, including patients with occult peritoneal metastases or positive laparoscopic cytology during staging laparoscopy. (4) death within 30 days after surgery. The study was reviewed and approved by the Ethics Committee of The Fourth Hospital of Hebei Medical University. The patients provided written informed consent.
Variables
Demographic, clinicopathological, and therapeutic data were retrospectively extracted from medical records. The variables included age at diagnosis, sex, clinical stage, Borrmann type, Siewert type, pre-treatment tumor markers, type of neoadjuvant therapy, surgical procedure (subtotal/total gastrectomy), and clinical response according to the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) [complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD)], laparoscopic cytology (negative/not performed), postneoadjuvant pathologic stage, AJCC tumor regression grade, number of positive lymph nodes, number of negative lymph nodes, Laurén classification, venous, lymphatic, or perineural invasion (VELPI), Histologic grade, and HER-2 expression.
The AJCC TNM staging system, 8th edition, was used in this study. In reference to previous studies, the tumor markers carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) were included (22, 23). Neoadjuvant therapies included neoadjuvant chemoradiotherapy and chemotherapy. Patients receiving neoadjuvant chemotherapy were preoperatively administered with two cycles of SOX (capecitabine plus oxaliplatin) or XELOX (S1 plus oxaliplatin) regimen. Surgery was performed 4 weeks after neoadjuvant chemotherapy, while the preoperative chemotherapy regimen was repeated 4 weeks after surgery (24). Additionally, for patients undergoing neoadjuvant radiotherapy, intensity-modulated radiation therapy (IMRT) (50.4Gy/25 fraction) was used in conjunction with an XELOX chemotherapy regimen. Surgery was performed 6-8 weeks following the final dose of radiotherapy. XELOX regimen was repeated as postoperative adjuvant chemotherapy 4 weeks after surgery (25). The majority of patients scheduled for neoadjuvant therapy at our center underwent staging laparoscopy prior to treatment. Staging laparoscopy aimed to look for occult peritoneal metastases that had not been detected by imaging and to perform concurrent laparoscopic cytology. All patients underwent radical resection involving total or subtotal gastrectomy combined with D2 lymphadenectomy.
Follow-up
Follow-up visits were conducted every 3 months for the first 2 years, every 6 months for the next 3 years, and annually thereafter. Disease-free survival (DFS) was defined as the time between the date of operation and the date of recurrence or the last follow-up if recurrence did not occur. The final follow-up was in November 2021.
Construction and Validation of the Nomogram
The variables before neoadjuvant therapy were defined as baseline factors, whereas the variables of baseline factors along with the variables of treatment and postoperative pathology were defined as post-operative factors. All variables were subjected to univariate Cox regressions. Subsequently, Multivariate Cox analyses were conducted separately for variables with a P<0.05 in the univariate Cox regression analysis for baseline and postoperative factors. The baseline and post-operative nomograms were constructed using multivariate Cox regression based on the independent risk factors identified. Harrell’s concordance index (c-index) and a time-dependent receiver operating characteristic (ROC) curve were used to assess the model’s discrimination ability. The C-index ranges from 0.5 to 1.0, and a higher C-index indicated better discrimination of the model. The calibration ability of the model was determined by comparing the predicted probability of free-recurrence to the actual free-recurrence. Finally, decision curve analysis (DCA) was used to determine the clinical usefulness of the nomogram.
Statistics Analysis
Categorical variables were expressed as frequency rates, while continuous variables were expressed as the median (interquartile range [IQR]). All continuous variables were subjected to linearity testing. Continuous variables with nonlinearity were modeled using restricted cubic splines. Univariate and multivariate analyses were performed using Cox regression models to determine the association between prognostic predictors and DFS, while hazard ratios (HRs) and 95% confidence intervals (CIs) were generated. To identify variables for multivariate Cox proportional hazards regression models, a backward stepwise selection method with the Akaike information criterion (AIC) was used. The nomograms were constructed based on the independent variables identified by multivariate analysis. Statistical analysis was performed using R software (version 4.0.3, http://www.R-project.org). The nomogram and calibration curve were generated using the Hmisc, rms, and ggplot2 packages, while riskRegression was used for receiver operating characteristic curve analysis (ROC), and “ggDCA” for decision curve analysis (DCA).
Results
Patients Characteristics and Univariate Cox Regression Analysis
Table 1 shows the demographic, clinicopathological, and treatment data of the 245 patients included in the study. The median follow-up time was 47 (IQR, 23-82) months. The median age of the patients was 62 (IQR, 57-66) years. There were 203 (82.9%) men and 42 (17.1%) women. 71 (29.0%) cases were diagnosed as Siewert type II and 174 (71.0%) cases as Siewert type III. For clinical stages, 65 patients were classified as cT2-3 stage and 180 patients as cT4 stage. Additionally, 54 patients were classified as cN0 stage and 191 patients as a cN1-3 stage. For Borrmann type, 5 patients were type I, 86 were type II, 139 were type III, and 15 were type IV. Among the pre-treatment tumor markers, 74 patients had elevated CEA and 64 patients had elevated CA199 levels. Pre-treatment laparoscopic cytology was performed and diagnosed as negative in 184 patients, while 61 patients were not examined. Neoadjuvant chemoradiotherapy was administered to 76 patients, neoadjuvant chemotherapy with the XELOX regimen was administered to 66 patients, and neoadjuvant chemotherapy with the SOX regimen was administered to 103 patients. Total gastrectomy was performed on 168 patients, whereas subtotal gastrectomy was performed on 77 individuals. 159 (64.9%) patients received neoadjuvant therapy and achieved objective remission (CR+PR). For the post neoadjuvant pathologic stage, there were 20, 35, and 190 cases of ypT0, ypT1-2, ypT3-4, respectively. There were 125, 59, 40, and 21 cases of ypTN0, ypTN1, ypTN2, ypTN3, respectively. There were 23 cases of AJCC-TRG grade 0, 62 cases of grade 1, 101 cases of grade 2, and 59 cases of grade 3. The median number of positive lymph nodes was 0 (0, 3), while negative lymph nodes were 24 (15, 33). For the Laurén classification, 118 (48.2%) were Intestinal, 75 (30.6%) were Diffuse, and 26 (10.6%) were mixed. A total of 139 patients presented with VELPI. 188 patients showed poor or undifferentiated histological grade. 38 patients showed a positive her2 status (immunohistochemistry 3+).
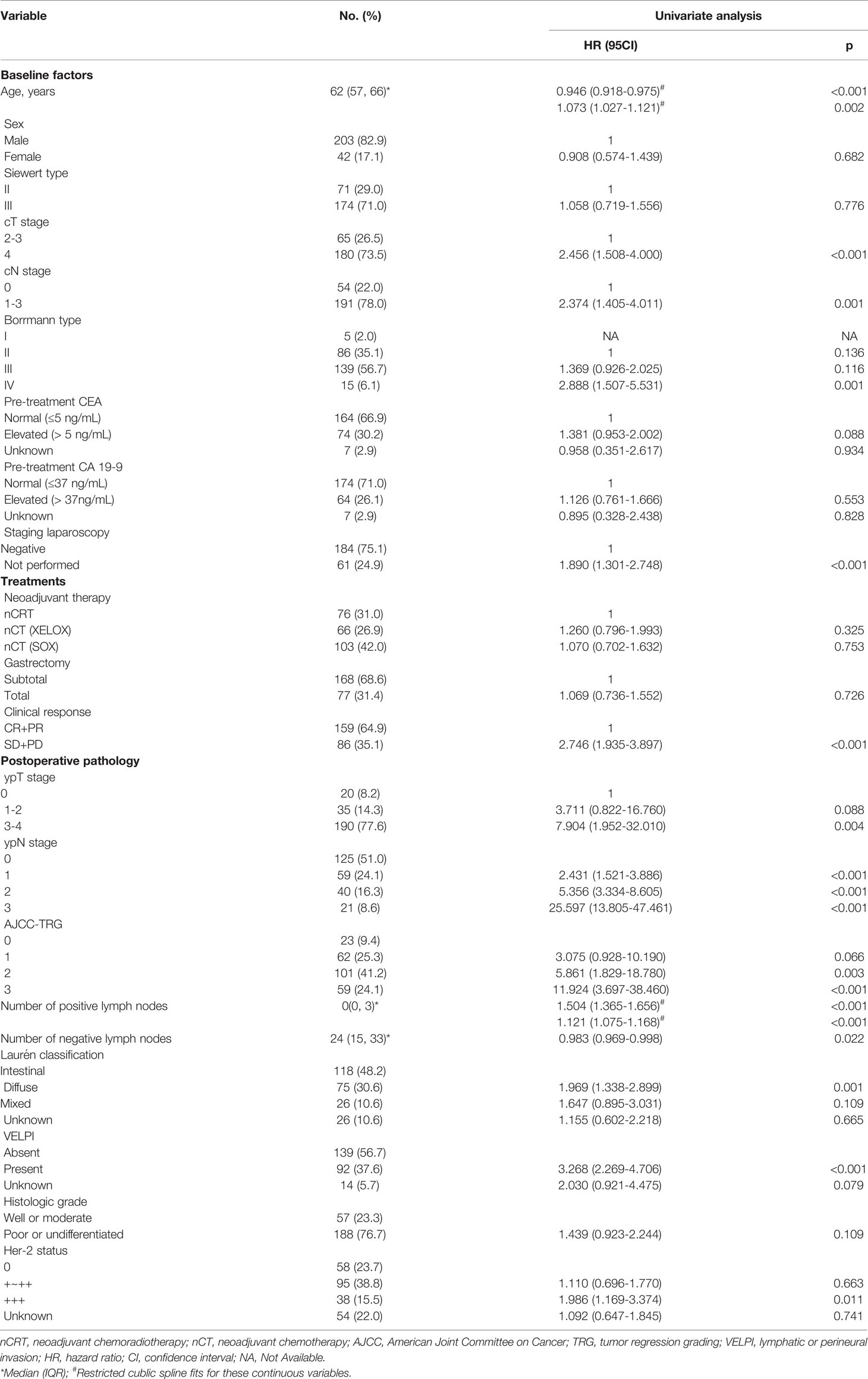
Table 1 Demographic, treatment, and clinicopathological characteristics of patients and univariate analyses for disease-free survival.
Due to the small number of patients with Borrmann type I, these individuals were excluded from the Cox analysis. Among the included continuous variables, age and the number of positive lymph nodes had a nonlinear relationship with recurrence, but the number of negative lymph nodes had a linear relationship with recurrence (Figure 1). Among the baseline factors, univariate cox analysis showed that age, cT stage, cN stage, Borrmann type, and laparoscopic cytology all influenced DFS (p=<0.05). Among the treatment factors, however, the only clinical response affected DFS (p=<0.05). Among the postoperative pathological factors, ypT stage, ypN stage, AJCC-TRG, number of positive lymph nodes, number of negative lymph nodes, Laurén classification, VELPI, and Her-2 status affected prognosis (p=<0.05), as shown in Table 1.
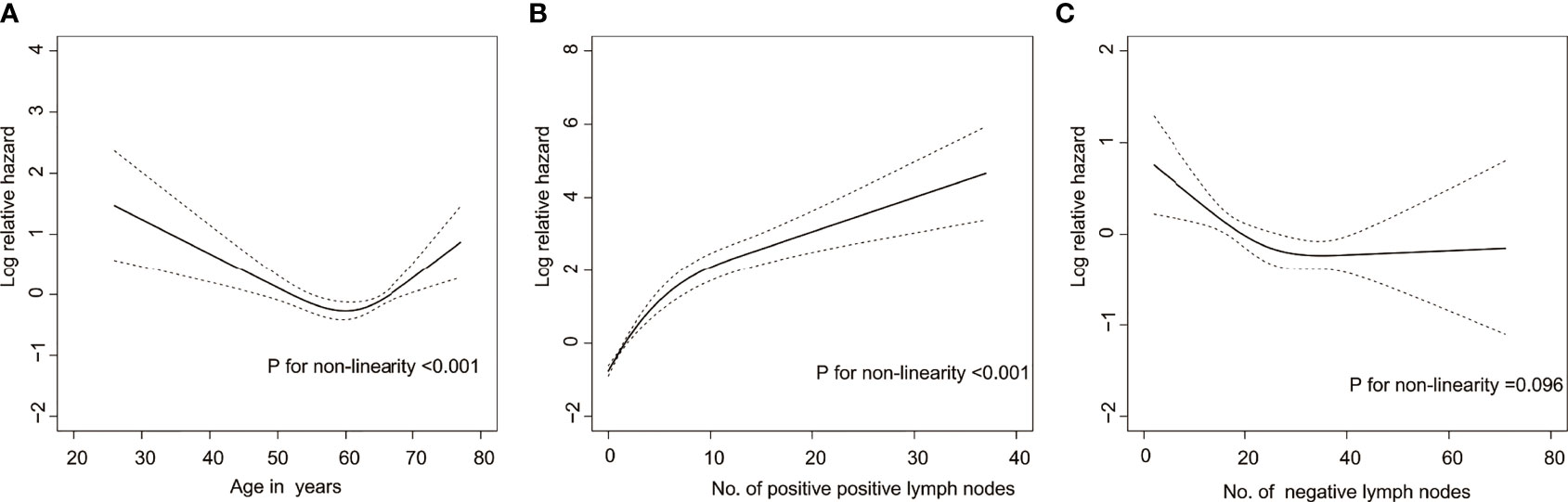
Figure 1 Risk of recurrence as a function of (A) age, (B) Number of positive lymph nodes, and (C) Number of negative lymph nodes. Solid line = risk function. Dashed lines = 95% confidence bands for the risk function.
Independent Prognostic Factors Among the Baseline and Postoperative Factors
In the multivariate analysis, age, cT stage, cN stage, Borrmann type, laparoscopic cytology all had an effect on DFS among the baseline factors (Table 2). Among the postoperative factors, Age, cN stage, Laparoscopic cytology, ypT stage, Clinical response, number of positive lymph nodes, number of negative lymph nodes, Laurén classification, VELPI all independently influenced DFS (Table 2).
Construction of Baseline and Post-Operative Nomograms
The baseline and post-operative nomograms were constructed using independent prognostic factors identified in cox regression. Each variable in the model was assigned a score, and the scores were summed to obtain a total score corresponding to the probability of free-recurrence at 3 and 5 years. The higher the total score, the lower the probability of free-recurrence for the patient (Figures 2A, B).
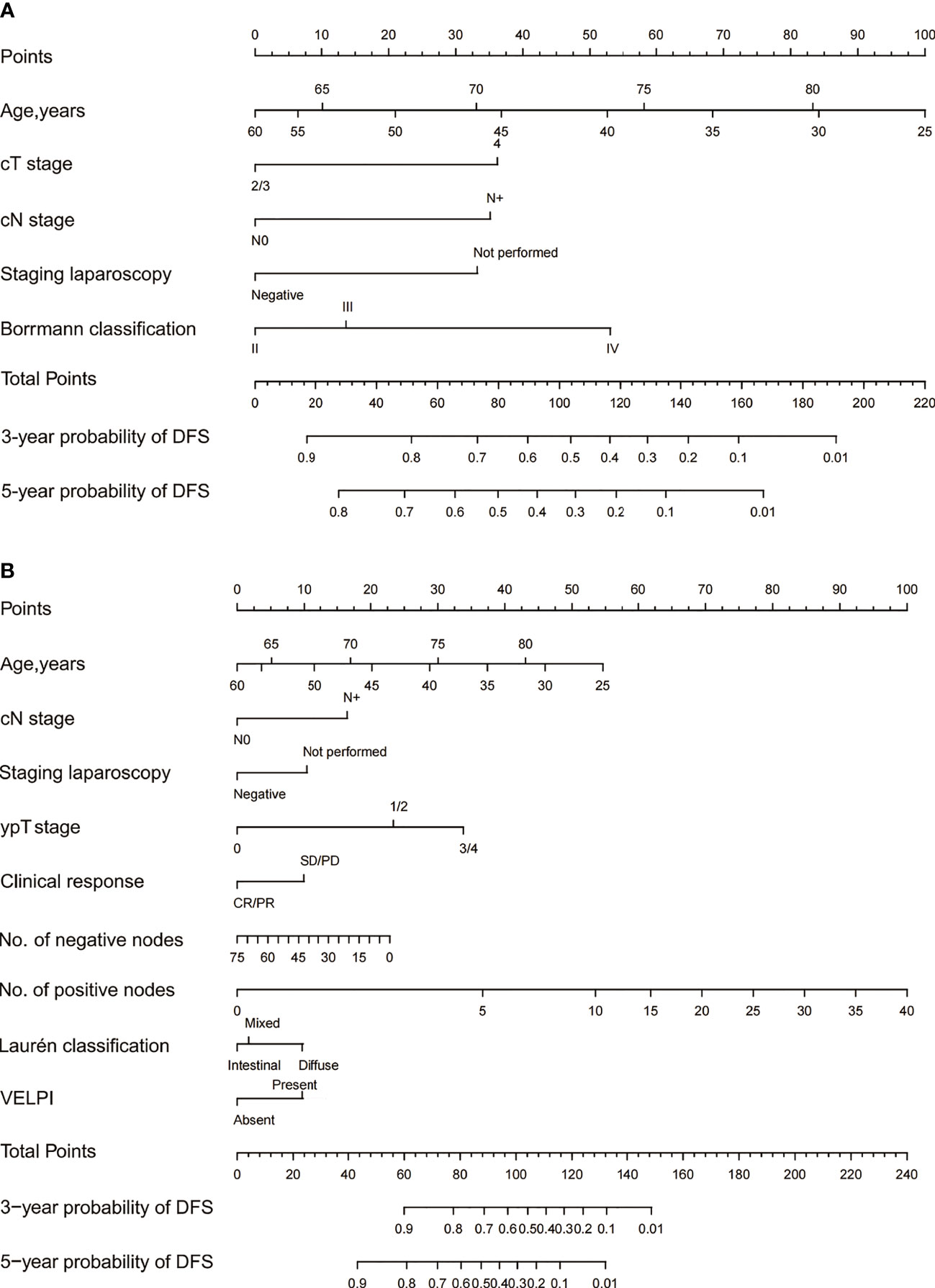
Figure 2 The 3- and 5-year DFS of Siewert Type II/III AEG patients were predicted by the baseline nomogram (A) and post-therapy nomogram (B). Each variable in the model corresponded to a score, and all the scores were summed to obtain a total score corresponding to the probability of free-recurrence at 3 and 5 years.
Internal Validation and Comparison With AJCC
The C-indexes for the model baseline and post-operative nomograms were 0.690(95% CI,0.644-0.736) and 0.817(95% CI,0.782-0.853), respectively. In the time-dependent ROC, the AUCs of the baseline nomogram at 3 and 5 years were both greater than cTNM (73.1 vs 58.8, 76.1 vs 55.7). Similarly, the AUCs of the postoperative nomogram at 3 and 5 years were both greater than ypTNM (85.2 vs 69.1, 88.2 vs 71.3), as shown in Figure 3. The calibration curves showed that both models had a good calibration ability (Figure 4). By comparing the DCA curves at 3 and 5 years, we found that the two nomograms had better clinical utility than the TNM staging system (Figure 5).
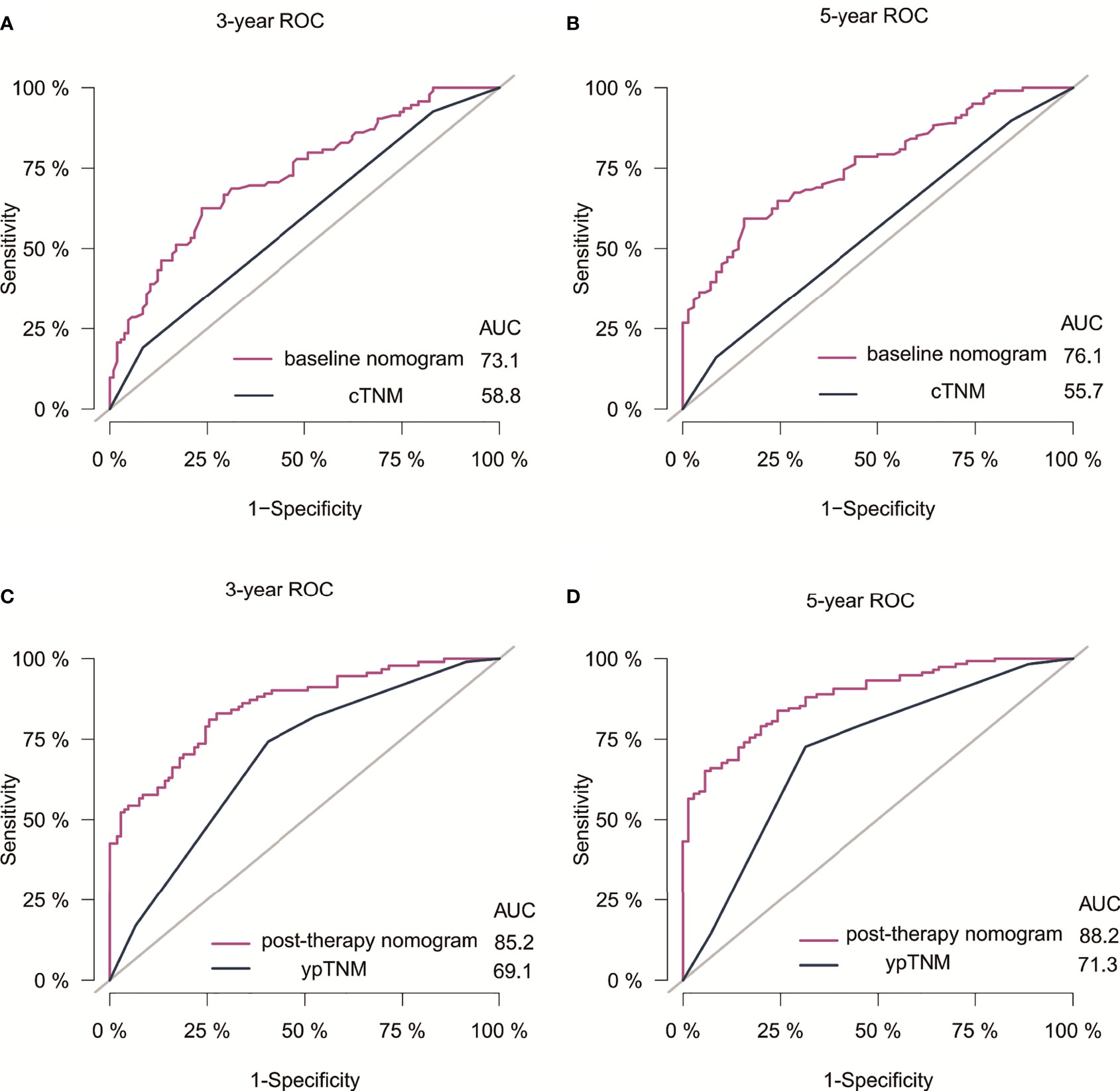
Figure 3 ROC of nomograms and the AJCC staging system for 3- and 5-year DFS prediction in Siewert Type II/III AEG patients. (A) 3-year ROC for baseline nomogram and cTNM staging. (B) 5-year ROC for baseline nomogram and cTNM staging. (C) 3-year ROC for post-therapy nomogram and ypTNM staging. (D) 5-year ROC for post-therapy nomogram and ypTNM staging. Receiver operating characteristic curves; AUC, Area under the curve.
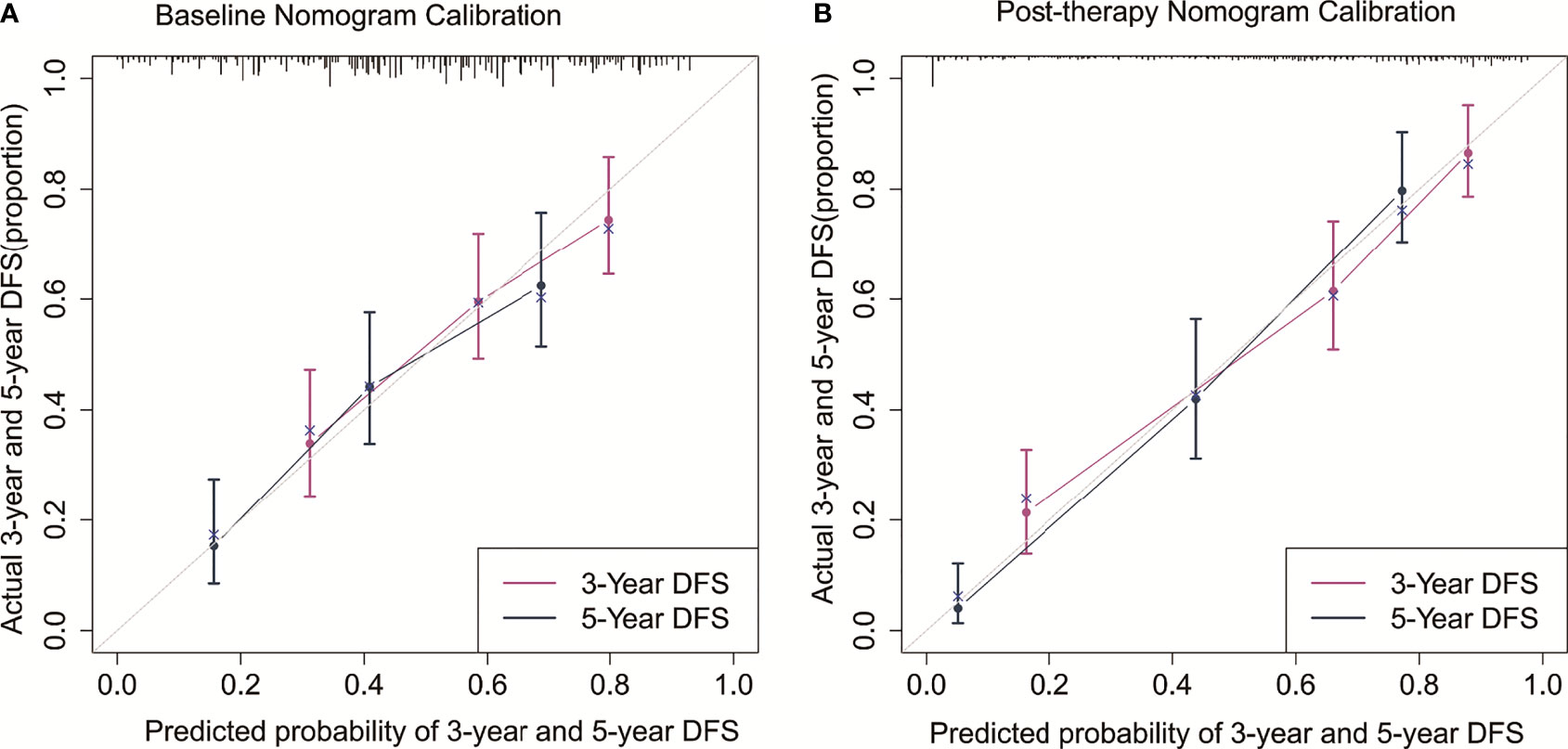
Figure 4 Calibration curves for the baseline nomogram (A) and post-therapy nomogram (B) predicted 3- and 5-year DFS.
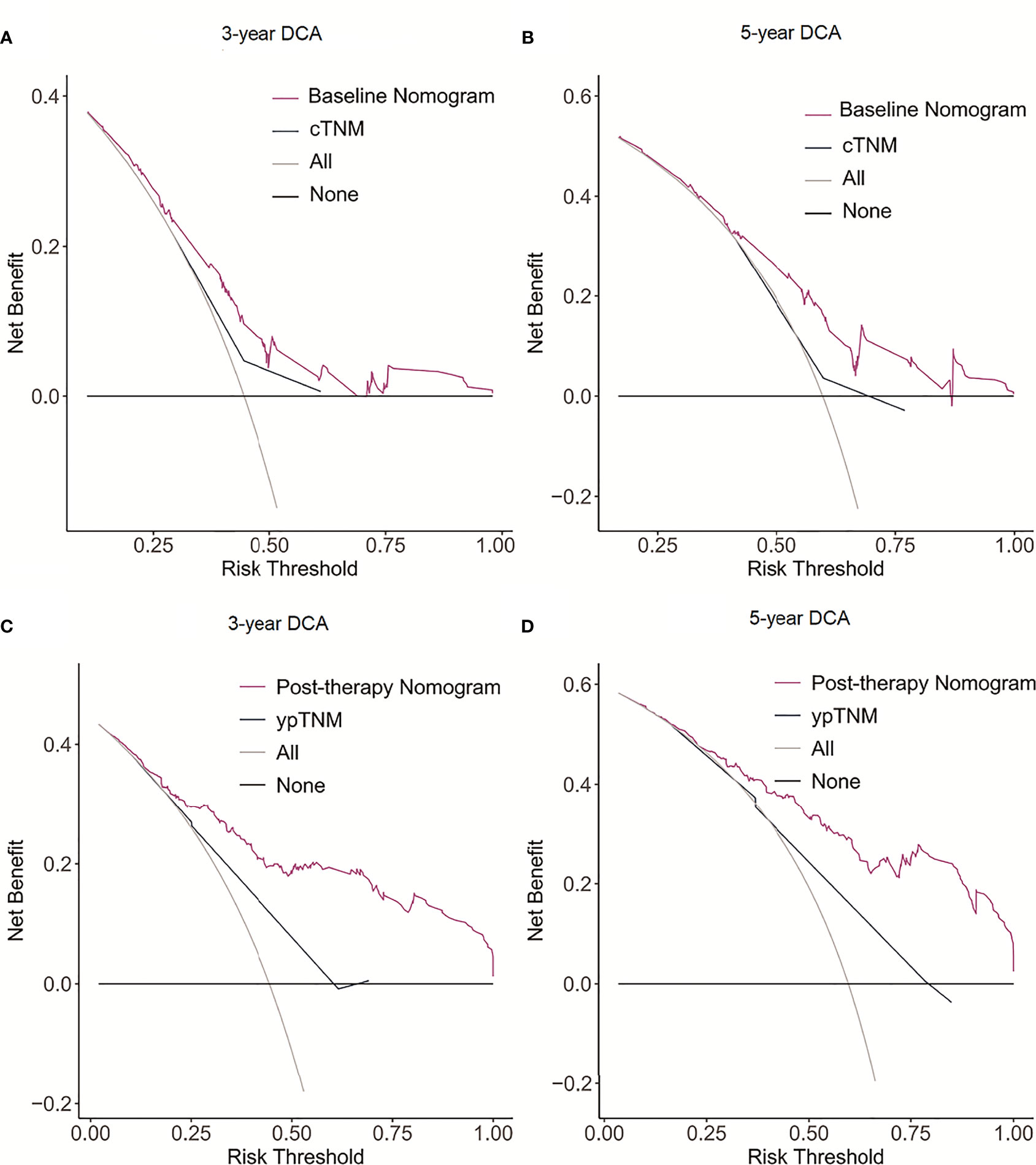
Figure 5 Decision curve analysis (DCA) of the nomogram model and AJCC staging model for predicting 3- and 5-year DFS. (A) 3-year DCA for baseline nomogram and cTNM staging. (B) 5-year DCA for baseline nomogram and cTNM staging. (C) 3-year DCA for post-therapy nomogram and ypTNM staging. (D) 5-year DCA for post-therapy nomogram and ypTNM staging.
Discussion
Although the optimal multidisciplinary treatment strategy for patients with advanced Siewert type II/III AEG remains controversial, neoadjuvant therapy in combination with surgical resection continues to be the primary approach. The most often used treatment modality is neoadjuvant chemotherapy or neoadjuvant radiotherapy. However, with the advent of targeted drugs and immune checkpoint inhibitors, preoperative therapies have become more diverse (26). The purpose of constructing a baseline nomogram was mainly to estimate the prognosis of individual patients before they are scheduled to receive neoadjuvant chemoradiotherapy or neoadjuvant chemotherapy and to provide a basis for an individualized therapeutic approach.
Age, cT stage, cN stage, Borrmann type, and staging laparoscopy were the predictors in the baseline nomogram. Some studies have shown that age is an independent risk factor affecting the prognosis of patients with AEG, and the prognosis worsens with increasing age (27–29). Nevertheless, other researchers have demonstrated that age is not a factor contributing to prognosis (30–32). The explanation for this variation between research could be because different studies used different subjects, treatment, and age cut-off values. In this study, age was found to be nonlinearly related to patient prognosis, with the risk of recurrence decreasing with age in patients under 60 years old and increasing with age above 60 years old. The advanced cT and cN stages were associated with a worse prognosis, which was consistent with previous studies (33, 34). The accuracy of clinical staging has significantly improved as a result of advances in imaging technologies and standardization of operations. Multi-detector computed tomography (MDCT) is the most commonly used imaging technique for the comprehensive assessment of clinical staging of AEG. MDCT has a high accuracy for cT staging of gastric cancer (35–37), but its accuracy for cN staging is relatively limited (38–40). Therefore, in this study, cN staging was divided into cN0 or cN+ to reduce bias in cN staging. Peritoneal metastasis is one of the common distant metastases sites for AEG. The presence or absence of peritoneal metastasis has a significant impact on the treatment strategy is chosen and patient prognosis (41). MDCT is currently the main method for evaluating distant metastases, with a high degree of accuracy in evaluating liver and lung metastasis. However, its assessment of peritoneal metastases is unsatisfactory, particularly for occult peritoneal metastasis. MDCT has high specificity but low sensitivity in diagnosing and evaluating peritoneal metastases (42–44). Sarela AI et al. (45) conducted a retrospective assessment of 657 patients with gastric cancer and AEG who underwent CT evaluation for M0. Peritoneal metastases were detected in 149 patients (23%) and significantly correlated with tumor location after laparoscopy, with AEG patients being more likely to have occult peritoneal metastases. In patients with progressive gastric cancer and AEG, staging laparoscopy can improve the accuracy of peritoneal metastasis diagnosis (46). In our study, 75.1% of patients underwent staging laparoscopy before neoadjuvant therapy, and the results indicated that patients who had a negative laparoscopy had a better prognosis than those who did not undergo laparoscopy. According to certain studies, diagnostic laparoscopy and cytology should be performed on all patients with Siewert II/III AEG with cT3/T4 before neoadjuvant chemotherapy to determine the presence of occult peritoneal metastases (47). Additionally, Borrmann type is a predictor in the baseline nomogram. Borrmann type I patients was excluded from this study due to the small sample size. Borrmann type III/IV patients have a worse prognosis than Borrmann type II patients, consistent with previous studies (48).
After neoadjuvant therapy and surgical resection, we added treatment and postoperative pathology variables to the baseline variables and identified independent risk factors among them to construct a post-operative nomogram. Age and laparoscopic staging were also significant predictive factors of prognosis. Among the anatomical variables of T and N elements, the number of positive lymph nodes was chosen to replace ypN staging and the number of negative lymph nodes was added to improve the predictive efficacy of the model. Additionally, the prognostic assessment of ypT staging in patients with AEG is controversial. Sisic L et al. (49) demonstrated that survival curves for patients with AEG and gastric cancer treated with neoadjuvant therapy at ypT0-2 staging were overlapping, with ypT3-4 staging showing prognostic stratification. They hypothesized that residual tumor cells following neoadjuvant therapy may remain in any layer of the GI tract, and therefore ypT staging did not provide a good stratification of prognosis. In this study, ypT staging was further optimized into ypT0, ypT1/2, and ypT3/4 categories. The results indicated that this prediction model included the four anatomical variables of T and N elements, cN staging, ypT staging, number of positive lymph nodes, and number of negative lymph nodes, but not cT staging. It has been demonstrated that the ypTNM stage rather than the initial cTNM stage is the main determinant of prognosis in neoadjuvant-treated AEG patients (50). The present findings indicate that lymph node status including the presence of preoperative lymph node metastases or more numbers of positive lymph nodes postoperatively indicated a poor prognosis, but a greater number of negative lymph nodes dissection improved prognosis. Optimized ypT staging was also found to be an independent risk factor for prognosis. In patients with AEG, clinical response was an independent risk factor for prognosis which is consistent with our previous study (24). Patients whose tumors were effectively controlled locally (CR+PR) had a better prognosis. To simplify the model, we used VELPI as a reference from a previous study on colon cancer prognosis (16). Additionally, it is an independent factor affecting prognosis in the model. Lymphovascular invasion (LVI) has been reported to be more prevalent in AEG patients than in esophageal and gastric cancers, particularly in Siewert type III. It may be associated with the development of this type of AEG in patients with chronic atrophic gastritis, a condition in which the mucosa is thin and tumor cells are more likely to invade the lymph vessels. AEGs with LVI have a worse prognosis (51). Additionally, Lauren classification is a significant independent factor affecting prognosis, with patients with a diffuse prognosis having a worse prognosis, which is consistent with previous reports (52).
The C-indexes of model baseline and post-operative nomograms are 0.690 and 0.817, respectively, indicating that both models have good predictive performance, while the postoperative nomogram model performed better. The prognosis of patients is dynamic. Both the patient’s response to neoadjuvant therapy and post-operative clinicopathological features can affect their prognosis. Therefore, the post-therapy nomogram has a better predictive performance. Additionally, the constructed nomogram had higher predictive efficiency than TNM when the AUC and DCA curves were compared. Unlike the TNM staging system, which categorizes AEG patients with Siewert type II/III into distinct staging sites, the model we constructed integrated these patients into a single model with high predictive performance. Therefore, it will be more practical and convenient for individualized patient management. Just as the clinical significance of cTNM staging and ypTNM staging in the 8th edition of the TNM staging system, we developed corresponding baseline and post-operative prediction models respectively. The models demonstrated higher prognostic predictive efficacy than the TNM staging system which can provide useful information for patient individualized treatment and follow-up.
There are some limitations to this study. First, this single-center retrospective analysis may have introduced bias in patient selection. Second, because the majority of patients with Siewert type II/III AEG are treated with general surgery in China, only patients with transabdominal radical resection were included in this study. Patients undergoing transthoracic resection were excluded due to the large variation in treatment modalities among thoracic clinicians. Third, patients with neoadjuvant targeted or immunotherapy were excluded due to the lack of sufficient follow-up time and inconsistent clinical response assessment criteria.
In conclusion, the nomogram we constructed has a more accurate predictive ability than the TNM staging system, which can provide useful information for patient treatment selection and follow-up monitoring.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The study was reviewed and approved by the Ethics Committee of The Fourth Hospital of Hebei Medical University. The patients provided written informed consent.
Author Contributions
ZG was involved in final data analysis and manuscript writing. HG, YT, and ZZ were assisted in the data collection and analysis. QZ was involved in study design and responsible for the entire research project. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Imamura Y, Watanabe M, Toihata T, Takamatsu M, Kawachi H, Haraguchi I, et al. Recent Incidence Trend of Surgically Resected Esophagogastric Junction Adenocarcinoma and Microsatellite Instability Status in Japanese Patients. Digestion (2019) 99(1):6–13. doi: 10.1159/000494406
2. Steevens J, Botterweck AA, Dirx MJ, van den Brandt PA, Schouten LJ. Trends in Incidence of Oesophageal and Stomach Cancer Subtypes in Europe. Eur J Gastroenterol Hepatol (2010) 22(6):669–78. doi: 10.1097/MEG.0b013e32832ca091
3. Siewert JR, Stein HJ. Classification of Adenocarcinoma of the Oesophagogastric Junction. Br J Surg (1998) 85(11):1457–9. doi: 10.1046/j.1365-2168.1998.00940.x
4. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative Chemotherapy Versus Surgery Alone for Resectable Gastroesophageal Cancer. N Engl J Med (2006) 355(1):11–20. doi: 10.1056/NEJMoa055531
5. Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, et al. Perioperative Chemotherapy Compared With Surgery Alone for Resectable Gastroesophageal Adenocarcinoma: An Fnclcc and Ffcd Multicenter Phase Iii Trial. J Clin Oncol (2011) 29(13):1715–21. doi: 10.1200/jco.2010.33.0597
6. Shapiro J, van Lanschot JJB, Hulshof M, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant Chemoradiotherapy Plus Surgery Versus Surgery Alone for Oesophageal or Junctional Cancer (Cross): Long-Term Results of a Randomised Controlled Trial. Lancet Oncol (2015) 16(9):1090–8. doi: 10.1016/s1470-2045(15)00040-6
7. Laxague F, Schlottmann F. Esophagogastric Junction Adenocarcinoma: Preoperative Chemoradiation or Perioperative Chemotherapy? World J Clin Oncol (2021) 12(7):557–64. doi: 10.5306/wjco.v12.i7.557
8. Daiko H, Kato K. Updates in the 8th Edition of the Tnm Staging System for Esophagus and Esophagogastric Junction Cancer. Japanese J Clin Oncol (2020) 50(8):847–51. doi: 10.1093/jjco/hyaa082
9. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition Ajcc Cancer Staging Manual: Continuing to Build a Bridge From a Population-Based to a More "Personalized" Approach to Cancer Staging. CA: Cancer J Clin (2017) 67(2):93–9. doi: 10.3322/caac.21388
10. Zhuang A, Zhuang A, Wu Q, Lu W, Tong H, Zhang Y. Prognostic Factor Analysis and Nomogram Construction of Primary Retroperitoneal Liposarcoma: A Review of 10 Years of Treatment Experience in a Single Asian Cohort of 211 Cases. Front Oncol (2021) 11:777647. doi: 10.3389/fonc.2021.777647
11. Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, et al. Prognostic Nomogram for Intrahepatic Cholangiocarcinoma After Partial Hepatectomy. J Clin Oncol (2013) 31(9):1188–95. doi: 10.1200/jco.2012.41.5984
12. Wu C, Hou SZ, Wu Z, Huang X, Wang Z, Tian B. Prognostic Nomogram for Patients Undergoing Radical Pancreaticoduodenectomy for Adenocarcinoma of the Pancreatic Head. BMC Cancer (2021) 21(1):624. doi: 10.1186/s12885-021-08295-5
13. Valentini V, van Stiphout RG, Lammering G, Gambacorta MA, Barba MC, Bebenek M, et al. Nomograms for Predicting Local Recurrence, Distant Metastases, and Overall Survival for Patients With Locally Advanced Rectal Cancer on the Basis of European Randomized Clinical Trials. J Clin Oncol (2011) 29(23):3163–72. doi: 10.1200/jco.2010.33.1595
14. Renfro LA, Grothey A, Xue Y, Saltz LB, André T, Twelves C, et al. Accent-Based Web Calculators to Predict Recurrence and Overall Survival in Stage III Colon Cancer. J Natl Cancer Inst. (2014) 106:dju333. doi: 10.1093/jnci/dju333
15. Weiser MR, Gönen M, Chou JF, Kattan MW, Schrag D. Predicting Survival After Curative Colectomy for Cancer: Individualizing Colon Cancer Staging. J Clin Oncol (2011) 29(36):4796–802. doi: 10.1200/jco.2011.36.5080
16. Weiser MR, Hsu M, Bauer PS, Chapman WC Jr, González IA, Chatterjee D, et al. Clinical Calculator Based on Molecular and Clinicopathologic Characteristics Predicts Recurrence Following Resection of Stage I-III Colon Cancer. J Clin Oncol (2021) 39(8):911–9. doi: 10.1200/jco.20.02553
17. Gabriel E, Attwood K, Shah R, Nurkin S, Hochwald S, Kukar M. Novel Calculator to Estimate Overall Survival Benefit From Neoadjuvant Chemoradiation in Patients With Esophageal Adenocarcinoma. J Am Coll Surg (2017) 224(5):884–94.e1. doi: 10.1016/j.jamcollsurg.2017.01.043
18. Zhou Z, Zhang H, Xu Z, Li W, Dang C, Song Y. Nomogram Predicted Survival of Patients With Adenocarcinoma of Esophagogastric Junction. World J Surg Oncol (2015) 13:197. doi: 10.1186/s12957-015-0613-7
19. Liu F, Zhou R, Jiang F, Liu G, Li K, Zhu G. Proposal of a Nomogram for Predicting Survival in Patients With Siewert Type Ii Adenocarcinoma of the Esophagogastric Junction After Preoperative Radiation. Ann Surg Oncol (2019) 26(5):1292–300. doi: 10.1245/s10434-019-07237-7
20. Chen J, Xia YJ, Liu TY, Lai YH, Yu JS, Zhang TH, et al. Development and Validation of a Survival Nomogram for Patients With Siewert Type Ii/Iii Adenocarcinoma of the Esophagogastric Junction Based on Real-World Data. BMC Cancer (2021) 21(1):532. doi: 10.1186/s12885-021-08249-x
21. Lemini R, Díaz Vico T, Trumbull DA, Attwood K, Spaulding AC, Elli EF, et al. Prognostic Models for Stage I-Iii Esophageal Cancer: A Comparison Between Existing Calculators. J Gastrointest Oncol (2021) 12(5):1963–72. doi: 10.21037/jgo-20-337
22. Tokunaga R, Imamura Y, Nakamura K, Uchihara T, Ishimoto T, Nakagawa S, et al. Carbohydrate Antigen 19-9 Is a Useful Prognostic Marker in Esophagogastric Junction Adenocarcinoma. Cancer Med (2015) 4(11):1659–66. doi: 10.1002/cam4.514
23. Sert OZ, Bozkurt H, Olmez T, Aray E, Uzun O, Gulmez S, et al. Clinical Importance of Serum Cea and Ca-19-9 Levels in Oesophagogastric Junction Adenocarcinomas. Przeglad gastroenterologiczny (2021) 16(3):240–7. doi: 10.5114/pg.2020.101911
24. Zhao Q, Lian C, Huo Z, Li M, Liu Y, Fan L, et al. The Efficacy and Safety of Neoadjuvant Chemotherapy on Patients With Advanced Gastric Cancer: A Multicenter Randomized Clinical Trial. Cancer Med (2020) 9(16):5731–45. doi: 10.1002/cam4.3224
25. Tian Y, Wang J, Qiao X, Zhang J, Li Y, Fan L, et al. Long-Term Efficacy of Neoadjuvant Concurrent Chemoradiotherapy for Potentially Resectable Advanced Siewert Type Ii and Iii Adenocarcinomas of the Esophagogastric Junction. Front Oncol (2021) 11:756440. doi: 10.3389/fonc.2021.756440
26. Nakamura Y, Kawazoe A, Lordick F, Janjigian YY, Shitara K. Biomarker-Targeted Therapies for Advanced-Stage Gastric and Gastro-Oesophageal Junction Cancers: An Emerging Paradigm. Nat Rev Clin Oncol (2021) 18(8):473–87. doi: 10.1038/s41571-021-00492-2
27. Wang T, Wu Y, Zhou H, Wu C, Zhang X, Chen Y, et al. Development and Validation of a Novel Competing Risk Model for Predicting Survival of Esophagogastric Junction Adenocarcinoma: A Seer Population-Based Study and External Validation. BMC Gastroenterol (2021) 21(1):38. doi: 10.1186/s12876-021-01618-7
28. Cao J, Yang T, Wang G, Zhang H, You Y, Chen J, et al. Analysis of the Clinicopathological Features and Prognostic Factors in 734 Cases of Chinese Hui and Han Patients With Adenocarcinoma of the Esophagogastric Junction. Surg Oncol (2018) 27(3):556–62. doi: 10.1016/j.suronc.2018.07.008
29. Yoon HH, Khan M, Shi Q, Cassivi SD, Wu TT, Quevedo JF, et al. The Prognostic Value of Clinical and Pathologic Factors in Esophageal Adenocarcinoma: A Mayo Cohort of 796 Patients With Extended Follow-Up After Surgical Resection. Mayo Clinic Proc (2010) 85(12):1080–9. doi: 10.4065/mcp.2010.0421
30. Feng Y, Jiang Y, Zhao Q, Liu J, Zhang H, Chen Q. Long-Term Outcomes and Prognostic Factor Analysis of Resected Siewert Type Ii Adenocarcinoma of Esophagogastric Junction in China: A Seven-Year Study. BMC Surg (2020) 20(1):302. doi: 10.1186/s12893-020-00926-1
31. Matsuda T, Kurokawa Y, Yoshikawa T, Kishi K, Misawa K, Ohi M, et al. Clinicopathological Characteristics and Prognostic Factors of Patients With Siewert Type Ii Esophagogastric Junction Carcinoma: A Retrospective Multicenter Study. World J Surg (2016) 40(7):1672–9. doi: 10.1007/s00268-016-3451-z
32. Cheng YX, Tao W, Liu XY, Yuan C, Zhang B, Zhang W, et al. The Outcome of Young Vs. Old Gastric Cancer Patients Following Gastrectomy: A Propensity Score Matching Analysis. BMC Surg (2021) 21(1):399. doi: 10.1186/s12893-021-01401-1
33. Wang S, Feng C, Dong D, Li H, Zhou J, Ye Y, et al. Preoperative Computed Tomography-Guided Disease-Free Survival Prediction in Gastric Cancer: A Multicenter Radiomics Study. Med Phys (2020) 47(10):4862–71. doi: 10.1002/mp.14350
34. Feng C, Cheng J, Zeng X, Zhang Y, Hong N, Ye Y, et al. Development and Evaluation of a Cemdct-Based Preoperative Risk Stratification Model to Predict Disease-Free Survival After Radical Surgery in Patients With Gastric Cancer. Abdominal Radiol (New York) (2021) 46(9):4079–89. doi: 10.1007/s00261-021-03049-0
35. Kwee RM, Kwee TC. Imaging in Local Staging of Gastric Cancer: A Systematic Review. J Clin Oncol (2007) 25(15):2107–16. doi: 10.1200/jco.2006.09.5224
36. Seevaratnam R, Cardoso R, McGregor C, Lourenco L, Mahar A, Sutradhar R, et al. How Useful Is Preoperative Imaging for Tumor, Node, Metastasis (Tnm) Staging of Gastric Cancer? A Meta-Analysis. Gastric Cancer (2012) 15 Suppl 1:S3–18. doi: 10.1007/s10120-011-0069-6
37. Kim JW, Shin SS, Heo SH, Choi YD, Lim HS, Park YK, et al. Diagnostic Performance of 64-Section Ct Using Ct Gastrography in Preoperative T Staging of Gastric Cancer According to 7th Edition of Ajcc Cancer Staging Manual. Eur Radiol (2012) 22(3):654–62. doi: 10.1007/s00330-011-2283-3
38. Kwee RM, Kwee TC. Imaging in Assessing Lymph Node Status in Gastric Cancer. Gastric Cancer (2009) 12(1):6–22. doi: 10.1007/s10120-008-0492-5
39. Wani AH, Parry AH, Feroz I, Choh NA. Preoperative Staging of Gastric Cancer Using Computed Tomography and Its Correlation With Histopathology With Emphasis on Multi-Planar Reformations and Virtual Gastroscopy. J Gastrointest Cancer (2021) 52(2):606–15. doi: 10.1007/s12029-020-00436-6
40. Jiang ZY, Kinami S, Nakamura N, Miyata T, Fujita H, Takamura H, et al. Diagnostic Ability of Multi-Detector Spiral Computed Tomography for Pathological Lymph Node Metastasis of Advanced Gastric Cancer. World J Gastrointest Oncol (2020) 12(4):435–46. doi: 10.4251/wjgo.v12.i4.435
41. Yonemura Y, Endou Y, Sasaki T, Hirano M, Mizumoto A, Matsuda T, et al. Surgical Treatment for Peritoneal Carcinomatosis From Gastric Cancer. Eur J Surg Oncol (2010) 36(12):1131–8. doi: 10.1016/j.ejso.2010.09.006
42. Kwee RM, Kwee TC. Modern Imaging Techniques for Preoperative Detection of Distant Metastases in Gastric Cancer. World J Gastroenterol (2015) 21(37):10502–9. doi: 10.3748/wjg.v21.i37.10502
43. Wang Z, Chen JQ. Imaging in Assessing Hepatic and Peritoneal Metastases of Gastric Cancer: A Systematic Review. BMC Gastroenterol (2011) 11:19. doi: 10.1186/1471-230x-11-19
44. Burbidge S, Mahady K, Naik K. The Role of Ct and Staging Laparoscopy in the Staging of Gastric Cancer. Clin Radiol (2013) 68(3):251–5. doi: 10.1016/j.crad.2012.07.015
45. Sarela AI, Lefkowitz R, Brennan MF, Karpeh MS. Selection of Patients With Gastric Adenocarcinoma for Laparoscopic Staging. Am J Surg (2006) 191(1):134–8. doi: 10.1016/j.amjsurg.2005.10.015
46. Ikoma N, Blum M, Chiang YJ, Estrella JS, Roy-Chowdhuri S, Fournier K, et al. Yield of Staging Laparoscopy and Lavage Cytology for Radiologically Occult Peritoneal Carcinomatosis of Gastric Cancer. Ann Surg Oncol (2016) 23(13):4332–7. doi: 10.1245/s10434-016-5409-7
47. Cordin J, Lehmann K, Schneider PM. Clinical Staging of Adenocarcinoma of the Esophagogastric Junction. Recent Results Cancer Res Fortschr der Krebsforschung Progres dans les Recherches sur le Cancer (2010) 182:73–83. doi: 10.1007/978-3-540-70579-6_6
48. Zhai Z, Zhu ZY, Zhang Y, Yin X, Han BL, Gao JL, et al. Prognostic Significance of Borrmann Type Combined With Vessel Invasion Status in Advanced Gastric Cancer. World J Gastrointestinal Oncol (2020) 12(9):992–1004. doi: 10.4251/wjgo.v12.i9.992
49. Sisic L, Blank S, Nienhüser H, Dorr S, Haag GM, Jäger D, et al. Prognostic Differences in 8th Edition Tnm Staging of Esophagogastric Adenocarcinoma After Neoadjuvant Treatment. Eur J Surg Oncol (2018) 44(10):1646–56. doi: 10.1016/j.ejso.2018.06.030
50. Davies AR, Gossage JA, Zylstra J, Mattsson F, Lagergren J, Maisey N, et al. Tumor Stage After Neoadjuvant Chemotherapy Determines Survival After Surgery for Adenocarcinoma of the Esophagus and Esophagogastric Junction. J Clin Oncol (2014) 32(27):2983–90. doi: 10.1200/jco.2014.55.9070
51. Zheng C, Feng X, Zheng J, Yan Q, Hu X, Feng H, et al. Lymphovascular Invasion as a Prognostic Factor in Non-Metastatic Adenocarcinoma of Esophagogastric Junction After Radical Surgery. Cancer Manag Res (2020) 12:12791–9. doi: 10.2147/cmar.S286512
Keywords: nomogram, disease-free survival, neoadjuvant radiotherapy, prognosis, esophagogastric junction adenocarcinoma
Citation: Guo Z, Guo H, Tian Y, Zhang Z and Zhao Q (2022) Nomograms for Predicting Disease-Free Survival in Patients With Siewert Type II/III Adenocarcinoma of the Esophagogastric Junction Receiving Neoadjuvant Therapy and Radical Surgery. Front. Oncol. 12:908229. doi: 10.3389/fonc.2022.908229
Received: 30 March 2022; Accepted: 19 May 2022;
Published: 08 June 2022.
Edited by:
Emmanuel Gabriel, Mayo Clinic, United StatesReviewed by:
Liming Jiang, Ningbo University, ChinaXiaofeng Duan, Tianjin Medical University Cancer Institute and Hospital, China
Copyright © 2022 Guo, Guo, Tian, Zhang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qun Zhao, emhhb3F1bkBoZWJtdS5lZHUuY24=
 Zhenjiang Guo
Zhenjiang Guo Honghai Guo
Honghai Guo Yuan Tian
Yuan Tian Ze Zhang1
Ze Zhang1 Qun Zhao
Qun Zhao