- 1Department of Abdominal Oncology, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Radiology, West China Hospital, Sichuan University, Chengdu, China
Background: Close to one third of colorectal cancer (CRC) patients are diagnosed with metastatic CRC (mCRC). Patients with wild-type RAS and BRAF usually receive anti-EGFR monoclonal antibody therapy containing cetuximab. Overall, 30–50% of mCRC patients are reported to harbor RAS mutations, and RAS mutation status should be assessed when considering EGFR inhibitor treatment according to mCRC biomarker guidelines. Of note, 0.67–2% of patients with CRC harbored a KRAS amplification. Here we reported a case of advanced rectal cancer with wild-type RAS and BRAF in a male patient who harbored a KRAS amplification during anti-EGFR treatment.
Case Presentation: A 46-year-old man was diagnosed with rectal adenocarcinoma with liver metastases (cT3NxM1a, stage IVA). After receiving first-line irinotecan- fluorouracil chemotherapy (FOLFIRI) plus cetuximab, second-line capecitabine- oxaliplatin chemotherapy (XELOX) plus bevacizumab, and third-line regorafenib, he rechallenged FOLFIRI and cetuximab for seven cycles, achieving a prolonged survival of at least 5 months. The KRAS copy number of circulating tumor DNA (ctDNA) was assessed during treatment. Notably, apart from serum carbohydrate antigen 199 (CA199) and carcinoembryonic antigen (CEA), the change of plasm Kirsten Rat Sarcoma Viral Oncogene Homolog (KRAS) copy number appeared to strongly correlate with treatment response.
Conclusion: Our findings suggest that the dynamic change of KRAS copy number on ctDNA during treatment might be a negative predictive biomarker. Additionally, RAS and BRAF wild-type mCRC patients who are resistant to first-line FOLFIRI plus cetuximab therapy may respond well to the FOLFIRI plus cetuximab “rechallenged” strategy.
Introduction
Colorectal cancer (CRC) is one of the most common malignancies, with more than 1.9 million new CRC cases and 935,000 deaths in 2020 (1). A total of 22% of patients with CRC have progressed to metastatic CRC (mCRC) at the time of diagnosis. And the prognosis of mCRC is dismal, with a 5-year survival rate of less than 20% (2). Systemic therapy (cytotoxic chemotherapy, biologic therapy, immunotherapy) is the primary treatment for unresectable mCRC (3, 4). Specifically, the combination of an anti-epidermal growth factor receptor (anti-EGFR) drug with chemotherapy is recommended as the first-line therapy for patients with RAS and BRAF wild-type mCRC (5, 6). After an initial response, secondary resistance to EGFR antibodies limits its application. Genes, including RAS, BRAF, and PI3K downstream of the EGFR signaling pathway, are key regulators of cell proliferation, differentiation, and division (7). And mutations of those genes may result in abnormal activation of the EGFR signaling pathway, contributing to anti-EGFR monoclonal antibody resistance (8–10). Furthermore, the curative effect of EGFR antibodies correlates with tumor sidedness (11, 12). In detail, the poor prognosis of right-sided CRC correlates with frequency alterations of the RAS, BRAF, PI3K, and TGF-β pathways. As for left-sided tumors, amplifications of receptor tyrosine kinases and mutations of APC and TP53 genes are more frequent; in addition, high sensitivity to EGFR antibodies is related to high expression of EGFR ligands amphiregulin (AREG) and epiregulin (EREG) (13–15). However, little is known regarding patients with CRC harboring KRAS copy number variation, and data on the response to anti-EGFR therapy are scarce. Here, we presented a case of an advanced CRC patient who harbored an elevated RAS copy number of ctDNA at the time of progressive disease, and experienced a favorable response to the rechallenge of irinotecan- and cetuximab-containing therapy after failure of multi-line treatment.
Case Presentation
In February 2018, a 46-year-old man was admitted to our center with chief complaints of increased stool frequency and occasional bloody stool lasting for over 1 month. A neoplasm with a distance of 4 to 7 cm from the anus was found in his colonoscopy, and liver multiple occupancies were detected in his abdominal computed tomography (CT). Subsequently, the patient was diagnosed with middle rectum adenocarcinoma with liver metastasis after conducting a pathological biopsy and magnetic resonance imaging (MRI) of the liver and rectum (Figure 1), accompanied with elevated CA199 (>1000.00 U/ml) and CEA (229.70 ng/ml), suggesting a stage of cT3NxM1a, stage IVA according to National Comprehensive Cancer Network (NCCN) staging criteria. He had been generally fit, except for 5 years of chronic gastritis.

Figure 1 Representative magnetic resonance imaging (MRI) images of lesions. (A–D) Representative images of T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and apparent diffusion coefficient (ADC) in the liver. (E–I) Representative images of rectum lesions in the sagittal position and coronal position (T1WI, T2WI, DWI and ADC), respectively. Red arrows indicate typical liver or rectum lesions.
Based on the standard principles, the patient initially received first-line chemotherapy with FOLFIRI. No obvious adverse events were observed during chemotherapy. Then target region sequencing concluding 1406 targeted genes for his plasm ctDNA was conducted by NovaSeq, indicating that RAS and BRAF mutations were absent while the KRAS copy number of 3.31 copies was abnormal. Additionally, the status of microsatellite instability (MSI) or mismatch repair (MMR) was defined as proficient mismatch repair (pMMR). Therefore, one anti-EGFR monoclonal antibody (mAbs), cetuximab, was added. After two cycles of therapy, his tumor shrank remarkably, and partial response (PR) was assessed (Figures 2A, B), followed by stable disease (SD) based on Response Evaluation Criteria in Solid Tumors (RECIST); simultaneously accompanying with reduced CA199 (30.73 U/ml) and CEA values (21.18 ng/ml) in blood after four cycles. However, disease progressed in November 2018, mainly referring to the liver metastasis (Figure 2B). Of note, before CT scanning, the level of CA199 decreased while the copy number of wild-type KRAS and APC p.R499* (a gene encoding tumor suppressor adenomatous polyposis coli (APC) protein) mutation increased (Supplementary Table 1), indicating the potential resistance to cetuximab in combination with chemotherapy. From November 2018 to June 2019, the patient then received second-line XELOX chemotherapy plus bevacizumab. During six cycles, his metastasis lesion size in the liver remained stable, and even shrunk (Figure 2C), with the copy number of KRAS decreasing to 2.69 copies and mutant frequency of APC p.R499* decreasing to 8.9% (Supplementary Table 1). After finishing the second-line therapy, abdominal enhanced CT scanning showed that the size of the liver lesion had enlarged (Figure 2C). The patient was subsequently treated with third-line regorafenib. Unfortunately, it failed to control the tumor growth (Figure 2D). Then, we decided to choose FOLFIRI chemotherapy combined with cetuximab again after a discussion. On 4 October 2019, his last genetic testing reports showed the copy number of KRAS was 3.48 copies, and the mutant frequency of APC p.R499* was 15.1% (Supplementary Table 1). And tumor markers of CA199 and CEA decreased gradually. Although his liver lesions enlarged and the number of lesions also increased after six cycles, the physician considered that it might be due to the prolonged treatment interval (Figure 2E). Thus, he received one cycle of FOLFIRI plus cetuximab again. The patient decided to stop the treatment and never returned to the hospital after the last outpatient follow-up in March 2020. Figure 3 presents the whole process and dynamics in tumor-related markers.
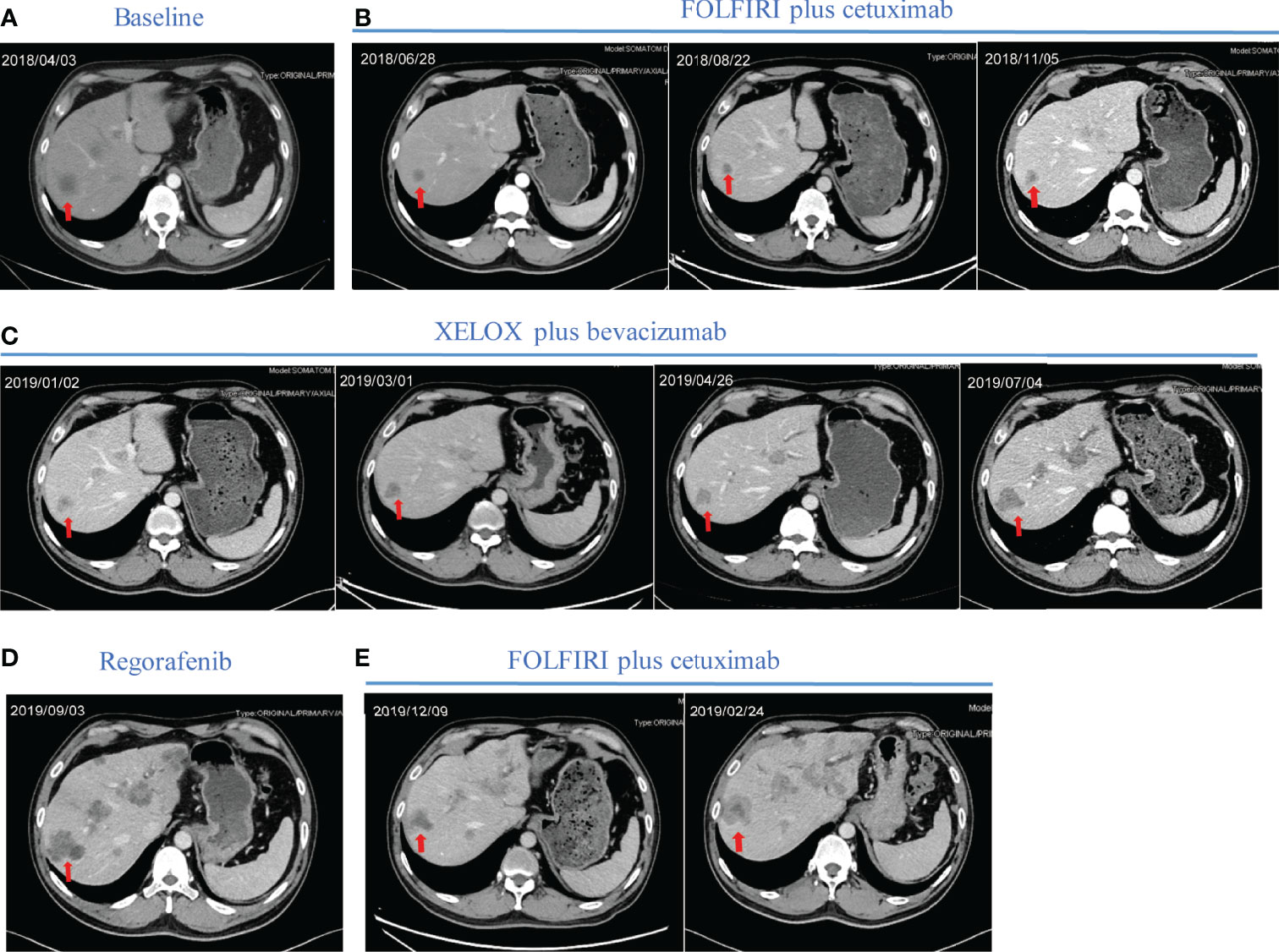
Figure 2 Representative computed tomography (CT) images of liver lesions during treatment. (A) Before first-line treatment, the representative CT image of the liver. (B) Regular CT was used to assess treatment efficacy during first-line treatment containing FOLFIRI plus cetuximab. (C) Regular revision CT was used to assess treatment efficacy during second-line treatment containing XELOX plus bevacizumab. (D) CT image of liver lesions after two cycles of regorafenib. (E) CT images of liver lesions after two cycles and four cycles of rechallenging FOLFIRI plus cetuximab. Red arrows indicate typical liver lesions. CT computed tomography; FOLFIRI, chemotherapy regimen containing irinotecan and fluorouracil; XELOX, chemotherapy regimen containing capecitabine and oxaliplatin.
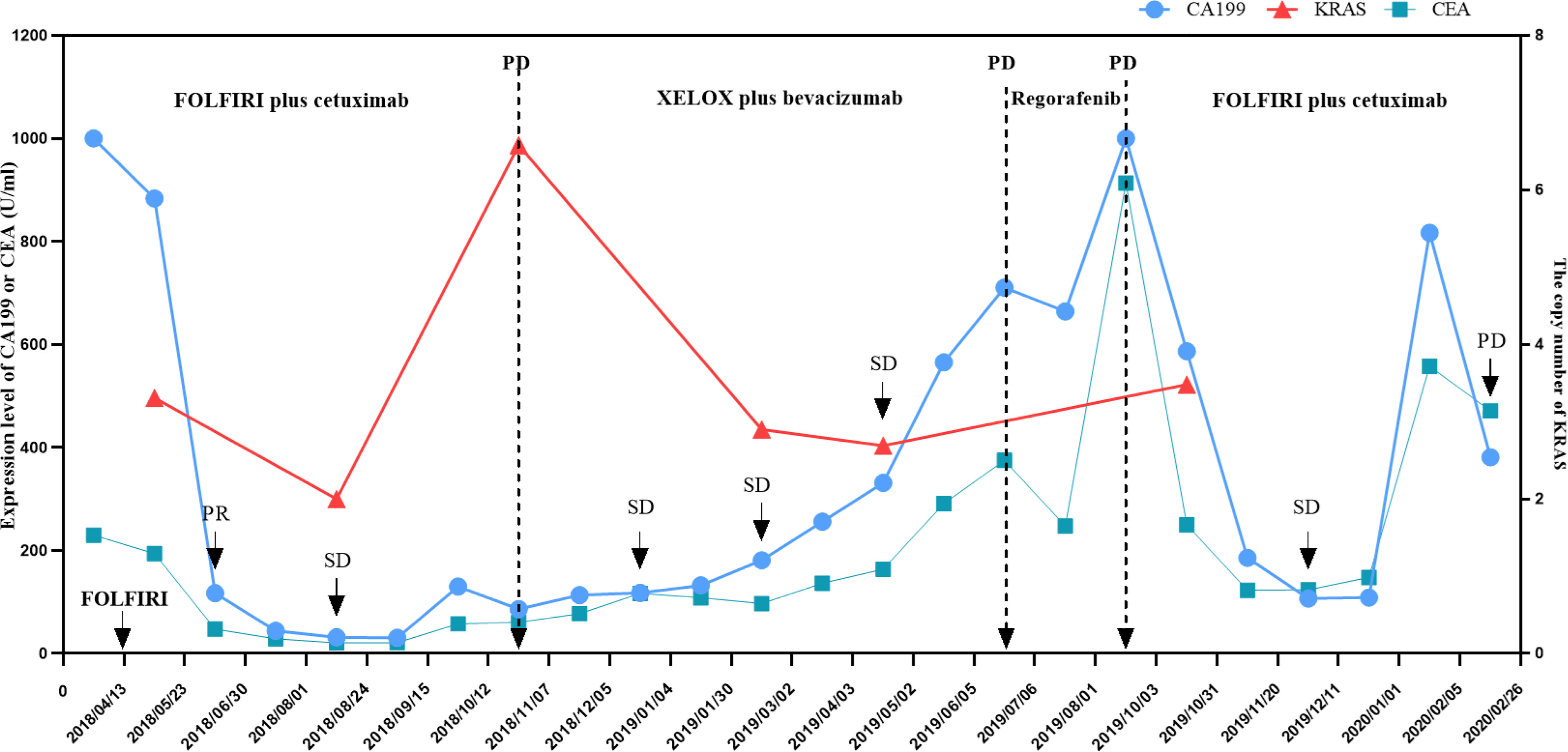
Figure 3 Changes in KRAS copy number, CA199, and CEA level during anti-tumor treatment and follow-up of the patient. The KRAS copy number was measured in the patient’s circulating tumor DNA. The CA199 and CEA tumor markers were measured in the patient’s serum at periodic intervals throughout the clinical course and annotated with the date, therapeutic approach, and treatment efficacy. CA199, cancer antigen 199; CEA, carcinoembryonic antigen; SD, stable response; PR, partial response; FOLFIRI, chemotherapy regimen containing irinotecan and fluorouracil; XELOX, chemotherapy regimen containing capecitabine and oxaliplatin.
Discussion
Anti-EGFR mAbs involving cetuximab and panitumumab are critical and routine drugs for patients with RAS and BRAF wild-type mCRC, achieving a median overall survival (OS) of approximately 30 months (16–18). Following the initial response, secondary resistance invariably occurs, thereby limiting the clinical benefit of EGFR antibodies (10). To date, the mechanisms of acquired resistance have been extensively studied. In detail, several studies described that EGFR mutation (19, 20) or downregulation of signal pathways including KRAS-MAPK (21), Janus kinase (JAK)/signal transducer, and activator of transcription (STAT), might be the underlying mechanism of cetuximab resistance (22). Also, aberrant regulation of miRNAs (23) and several proteins (24, 25) has been found to be responsible for cetuximab-induced resistance in CRC. Resistance to first-line cetuximab plus chemotherapy in our case might be due to an elevated copy number of wild-type KRAS, which was consistent with previous studies (26–28).
KRAS and BRAF mutations in colorectal cancer have been widely considered to be associated with patient prognosis (29). Notably, copy number changes at critical regions have shown potential practical utilities predicting treatment response and clinical outcomes (30). Amplification of the KRAS gene has been identified as a crucial factor that leads to RAS/mitogen-activated protein kinase activation (31). Intriguingly, loss of KRAS gene copy number in tumor DNA is associated with better treatment response to anti-EGFR drugs even in the presence of KRAS mutation in the tumor, while amplification of KRAS predicts resistance to drugs independent of mutational status (32). In this paper, the KRAS copy number was elevated when PD was assessed during the first-line treatment of the patient, and vice versa, indicating that the dynamic trends of the KRAS copy number may be a negative predictive biomarker.
Furthermore, we found that mutations in the APC gene, a tumor suppressor that has a functional role in the typical (β-catenin-dependent) Wnt signaling pathway (33), was the most frequent in this case. APC mutations normally promote cell migration by reducing cell adhesion via deregulation of β-catenin and E-cadherin distributions among the cytoplasm and the cell membrane (34, 35). Previous research revealed that a portion of 80% of CRC tumors harbor somatic inactivating mutations in the APC gene during the early stages of non-hypermutated CRC (36, 37).
Recently, the potential efficacy of rechallenge with anti-EGFR mAbs in a later setting for patients who were previously treated with EGFR blockades has been suggested in retrospective and prospective studies (38–49) (Table 1). The CRICKET trial, a single-arm phase II trial of rechallenge with cetuximab in 28 patients with a response to previous EGFR inhibitions showed an objective response rate (ORR) of 21% and a median PFS of 3.4 months (44), while another phase II trials from Japan (JACCRO-CC-08 and -09) demonstrated limited efficacy of that similar regimen, with an ORR of 2.9-8.3% and median PFS of 2.4-3.1 months (50). Furthermore, D. Santini et al. reported a higher ORR (53.8%) and PFS (6.6 months) (39). And other clinical studies, such as REMARRY and PURSUIT, are ongoing (51). Altogether, rechallenge with anti-EGFR mAbs is feasible in patients with RAS and BRAF wild-type mCRC. In our study, the patient tolerated therapy well and obtained a favorable PFS with 5 months, without any severe adverse events (only red rashes appeared on his face and back). It should be noted that the PFS in our paper was superior to that in current trials (almost 2-4 months) as shown in Table 1.
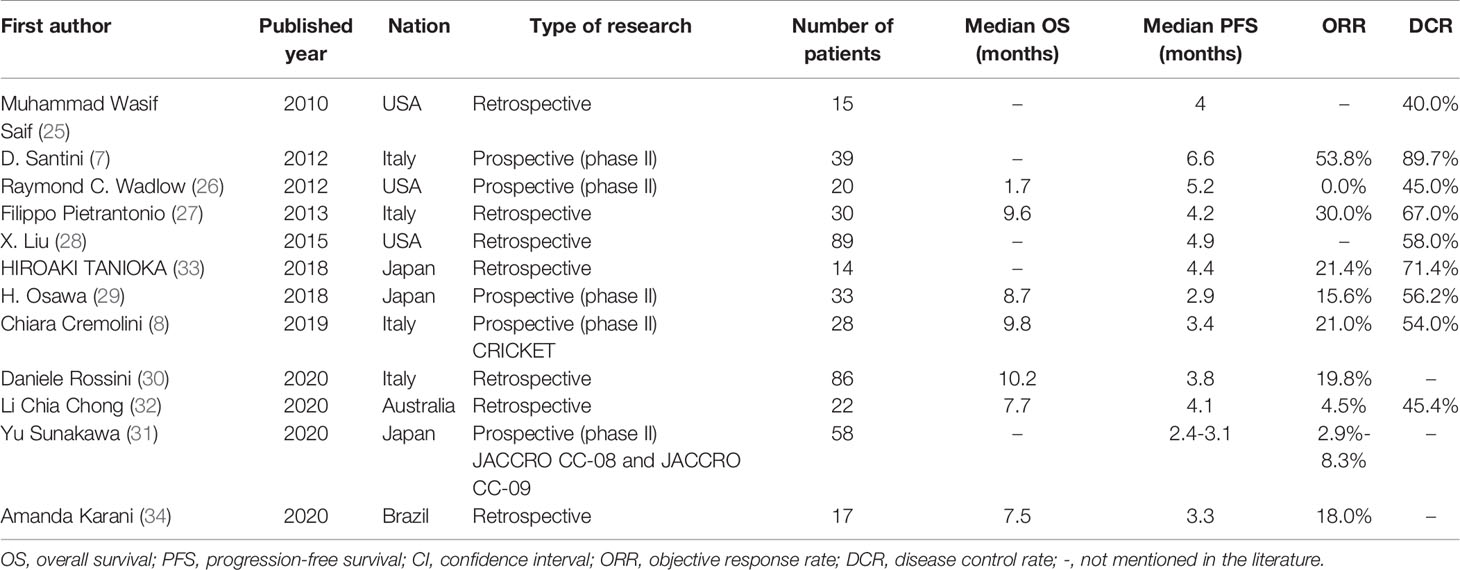
Table 1 Literature of reported clinical trials of rechallenge with anti-epidermal growth factor receptor (EGFR) therapy with panitumumab or cetuximab-based therapy for patients with metastatic colorectal cancer.
Although retreatment with EGFR inhibitors seems to be beneficial, it is essential to select an appropriate biomarker to monitor disease progression and evaluate response to therapy, such as liquid biopsy. Given its feature of minimal invasiveness and convenience, liquid biopsy has recently attracted interest in the molecular cancer-diagnosis area. For example, the detection of ctDNA in peripheral blood has shown great potential in the clinic, particularly for those patients who cannot undergo biopsy (52–54). Recent research found that in patients with advanced non-small cell lung cancer, elevation of ctDNA preceded an abnormal radiographic finding and the frequency of mutated alleles in ctDNA increased consecutively for 3-5 months before clinical evidence of disease progression (55). Moreover, as for melanoma patients, an undetectable ctDNA at baseline or during treatment tended to correlate with a better objective response to therapy (56). Of note, scholars have identified the KRAS mutation as a driving factor of cetuximab-induced acquired resistance in CRC, which suggested that detecting KRAS mutant clones via a non-invasive method (generally peripheral blood sampling) could reflect true tumor performance before radiographic progression (26). However, we did not discover the relation between the KRAS mutant and radiographic progression due to limited and irregular gene testing. Interestingly, the KRAS copy number was elevated at the time of radiographic progression while the level of CA199 and CEA was still low (Figure 3), indicating that the status of RAS may be helpful to monitoring therapeutic efficacy. Nevertheless, more evidence is needed to support this phenomenon.
Conclusion
Our results demonstrated that the elevated KRAS copy number of ctDNA was correlated with progressive disease, and resistance to anti-EGFR treatment might be due to an increased KRAS gene copy number. If economic conditions permit, apart from RAS mutational testing, RAS copy number testing should be performed when considering EGFR inhibitor treatment, especially when the most commonly used clinical tumor markers including CA199 and CEA are at a low level. In addition, we reported an mCRC patient with wild-type KRAS, previously treated with an anti-epidermal growth factor receptor-based regimen, who obtained a good response after rechallenge with cetuximab-based therapy, and achieved a PFS of 5 months. This impressive response highlights the potential efficacy of reintroducing cetuximab for patients with acquired resistance to a previous treatment with chemotherapy plus cetuximab. We expect that our report can provide a reference for the systematic treatment, monitoring, and prognosis determination of patients with RAS and BRAF wild-type mCRC.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA790712.
Ethics Statement
Written informed consent was obtained from the individual for the publication of this case report and any potentially identifiable images or data included in this article.
Author Contributions
QX and ZZ conceived the study, performed the literature research, wrote the paper, and assessed figure and tables. YY, YW, and YX performed the literature research and critically reviewed the paper. YZ, JL, and ZZ collected the clinical data. MQ and QZ supervised the project. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by 1.3.5 project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYJC21042), Sichuan Science and Technology Programme (2019YFS0042) and the National Key Development Plan for Precision Medicine Research (2017YFC0910004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
All the authors gratefully acknowledge the patient and his family for allowing us to publish the clinical case.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.872630/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA-A Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Colorectal Cancer. Available at: https://seer.cancer.gov/statfacts/html/colorect.htm (Accessed January 28, 2021).
3. Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA (2021) 325(7):669–85. doi: 10.1001/jama.2021.0106
4. Brenner H, Kloor M, Pox CP. Colorectal Cancer. Lancet (2014) 383(9927):1490–502. doi: 10.1016/s0140-6736(13)61649-9
5. Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO Consensus Guidelines for the Management of Patients With Metastatic Colorectal Cancer. Ann Oncol (2016) 27(8):1386–422. doi: 10.1093/annonc/mdw235
6. Network NCC. NCCN Clinical Practice Guidelines in Oncology—Colon Cancer; Version 3 (2021). Available at: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1428.
7. Sabbah DA, Hajjo R, Sweidan K. Review on Epidermal Growth Factor Receptor (EGFR) Structure, Signaling Pathways, Interactions, and Recent Updates of EGFR Inhibitors. Curr Topics Medicinal Chem (2020) 20(10):815–34. doi: 10.2174/1568026620666200303123102
8. De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA Mutations on the Efficacy of Cetuximab Plus Chemotherapy in Chemotherapy-Refractory Metastatic Colorectal Cancer: A Retrospective Consortium Analysis. Lancet Oncol (2010) 11(8):753–62. doi: 10.1016/S1470-2045(10)70130-3
9. Bertotti A, Papp E, Jones S, Adleff V, Anagnostou V, Lupo B, et al. The Genomic Landscape of Response to EGFR Blockade in Colorectal Cancer. Nature (2015) 526(7572):263–7. doi: 10.1038/nature14969
10. Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, et al. K-Ras Mutations and Benefit From Cetuximab in Advanced Colorectal Cancer. N Engl J Med (2008) 359(17):1757–65. doi: 10.1056/NEJMoa0804385
11. Brule SY, Jonker DJ, Karapetis CS, O'Callaghan CJ, Moore MJ, Wong R, et al. Location of Colon Cancer (Right-Sided Versus Left-Sided) as a Prognostic Factor and a Predictor of Benefit From Cetuximab in NCIC CO.17. Eur J Cancer (2015) 51(11):1405–14. doi: 10.1016/j.ejca.2015.03.015
12. Moretto R, Cremolini C, Rossini D, Pietrantonio F, Battaglin F, Mennitto A, et al. Location of Primary Tumor and Benefit From Anti-Epidermal Growth Factor Receptor Monoclonal Antibodies in Patients With RAS and BRAF Wild-Type Metastatic Colorectal Cancer. Oncologist (2016) 21(8):988–94. doi: 10.1634/theoncologist.2016-0084
13. Seligmann JF, Elliott F, Richman SD, Jacobs B, Hemmings G, Brown S, et al. Combined Epiregulin and Amphiregulin Expression Levels as a Predictive Biomarker for Panitumumab Therapy Benefit or Lack of Benefit in Patients With RAS Wild-Type Advanced Colorectal Cancer. JAMA Oncol (2016) 2(5):633–42. doi: 10.1001/jamaoncol.2015.6065
14. Zarkavelis G, Boussios S, Papadaki A, Katsanos KH, Christodoulou DK, Pentheroudakis G. Current and Future Biomarkers in Colorectal Cancer. Ann Gastroenterol (2017) 30(6):613–21. doi: 10.20524/aog.2017.0191
15. Boussios S, Ozturk MA, Moschetta M, Karathanasi A, Zakynthinakis-Kyriakou N, Katsanos KH, et al. The Developing Story of Predictive Biomarkers in Colorectal Cancer. J Pers Med (2019) 9(1):12. doi: 10.3390/jpm9010012
16. Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 Treatment and RAS Mutations in Colorectal Cancer. N Engl J Med (2013) 369(11):1023–34. doi: 10.1056/NEJMoa1305275
17. Van Cutsem E, Lenz HJ, Kohne CH, Heinemann V, Tejpar S, Melezinek I, et al. Fluorouracil, Leucovorin, and Irinotecan Plus Cetuximab Treatment and RAS Mutations in Colorectal Cancer. J Clin Oncol (2015) 33(7):692–700. doi: 10.1200/JCO.2014.59.4812
18. Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, et al. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA (2017) 317(23):2392–401. doi: 10.1001/jama.2017.7105
19. Bagchi A, Haidar JN, Eastman SW, Vieth M, Topper M, Iacolina MD, et al. Molecular Basis for Necitumumab Inhibition of EGFR Variants Associated With Acquired Cetuximab Resistance. Mol Cancer Ther (2018) 17(2):521–31. doi: 10.1158/1535-7163.MCT-17-0575
20. Bray SM, Lee J, Kim ST, Hur JY, Ebert PJ, Calley JN, et al. Genomic Characterization of Intrinsic and Acquired Resistance to Cetuximab in Colorectal Cancer Patients. Sci Rep (2019) 9(1):15365. doi: 10.1038/s41598-019-51981-5
21. Diaz LA Jr., Williams RT, Wu J, Kinde I, Hecht JR, Berlin J, et al. The Molecular Evolution of Acquired Resistance to Targeted EGFR Blockade in Colorectal Cancers. Nature (2012) 486(7404):537–40. doi: 10.1038/nature11219
22. Zhao B, Wang L, Qiu H, Zhang M, Sun L, Peng P, et al. Mechanisms of Resistance to Anti-EGFR Therapy in Colorectal Cancer. Oncotarget (2017) 8(3):3980–4000. doi: 10.18632/oncotarget.14012
23. Lu Y, Zhao X, Liu Q, Li C, Graves-Deal R, Cao Z, et al. lncRNA MIR100HG-Derived miR-100 and miR-125b Mediate Cetuximab Resistance via Wnt/beta-Catenin Signaling. Nat Med (2017) 23(11):1331–41. doi: 10.1038/nm.4424
24. Leonard B, Brand TM, O'Keefe RA, Lee ED, Zeng Y, Kemmer JD, et al. BET Inhibition Overcomes Receptor Tyrosine Kinase-Mediated Cetuximab Resistance in HNSCC. Cancer Res (2018) 78(15):4331–43. doi: 10.1158/0008-5472.CAN-18-0459
25. Liu Q, Xin C, Chen Y, Yang J, Chen Y, Zhang W, et al. PUM1 Is Overexpressed in Colon Cancer Cells With Acquired Resistance to Cetuximab. Front Cell Dev Biol (2021) 9:696558. doi: 10.3389/fcell.2021.696558
26. Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, et al. Emergence of KRAS Mutations and Acquired Resistance to Anti-EGFR Therapy in Colorectal Cancer. Nature (2012) 486(7404):532–6. doi: 10.1038/nature11156
27. Favazza LA, Parseghian CM, Kaya C, Nikiforova MN, Roy S, Wald AI, et al. KRAS Amplification in Metastatic Colon Cancer Is Associated With a History of Inflammatory Bowel Disease and may Confer Resistance to Anti-EGFR Therapy. Modern Pathol (2020) 33(9):1832–43. doi: 10.1038/s41379-020-0560-x
28. Fang T, Liang T, Wang Y, Wu H, Liu S, Xie L, et al. An Early-Onset Advanced Rectal Cancer Patient With Increased KRAS Gene Copy Number Showed A Primary Resistance to Cetuximab in Combination With Chemotherapy: A Case Report. Front Oncol (2021) 11:755578. doi: 10.3389/fonc.2021.755578
29. Gallo G, Sena G, Vescio G, Papandrea M, Sacco R, Trompetto M, et al. The Prognostic Value of KRAS and BRAF in Stage I-III Colorectal Cancer. A System Review Ann Ital Chir (2019) 90:127–37.
30. Wang H, Liang L, Fang JY, Xu J. Somatic Gene Copy Number Alterations in Colorectal Cancer: New Quest for Cancer Drivers and Biomarkers. Oncogene (2016) 35(16):2011–9. doi: 10.1038/onc.2015.304
31. Soh J, Okumura N, Lockwood WW, Yamamoto H, Shigematsu H, Zhang W, et al. Oncogene Mutations, Copy Number Gains and Mutant Allele Specific Imbalance (MASI) Frequently Occur Together in Tumor Cells. PloS One (2009) 4(10):e7464. doi: 10.1371/journal.pone.0007464
32. Mekenkamp LJ, Tol J, Dijkstra JR, de Krijger I, Vink-Borger ME, van Vliet S, et al. Beyond KRAS Mutation Status: Influence of KRAS Copy Number Status and microRNAs on Clinical Outcome to Cetuximab in Metastatic Colorectal Cancer Patients. BMC Cancer (2012) 12:292. doi: 10.1186/1471-2407-12-292
33. Zhan T, Rindtorff N, Boutros M. Wnt Signaling in Cancer. Oncogene (2017) 36(11):1461–73. doi: 10.1038/onc.2016.304
34. Aoki K, Taketo MM. Adenomatous Polyposis Coli (APC): A Multi-Functional Tumor Suppressor Gene. J Cell Sci (2007) 120(Pt 19):3327–35. doi: 10.1242/jcs.03485
35. Fodde R, Kuipers J, Rosenberg C, Smits R, Kielman M, Gaspar C, et al. Mutations in the APC Tumour Suppressor Gene Cause Chromosomal Instability. Nat Cell Biol (2001) 3(4):433–8. doi: 10.1038/35070129
36. Basu S, Haase G, Ben-Ze'ev A. Wnt Signaling in Cancer Stem Cells and Colon Cancer Metastasis. F1000Res (2016) 5:699. doi: 10.12688/f1000research.7579.1
37. Armaghany T, Wilson JD, Chu Q, Mills G. Genetic Alterations in Colorectal Cancer. Gastrointest Cancer Res (2012) 5(1):19–27.
38. Saif MW, Kaley K, Chu E, Copur MS. Safety and Efficacy of Panitumumab Therapy After Progression With Cetuximab: Experience at Two Institutions. Clin Colorectal Cancer (2010) 9(5):315–8. doi: 10.3816/CCC.2010.n.046
39. Santini D, Vincenzi B, Addeo R, Garufi C, Masi G, Scartozzi M, et al. Cetuximab Rechallenge in Metastatic Colorectal Cancer Patients: How to Come Away From Acquired Resistance? Ann Oncol (2012) 23(9):2313–8. doi: 10.1093/annonc/mdr623
40. Wadlow RC, Hezel AF, Abrams TA, Blaszkowsky LS, Fuchs CS, Kulke MH, et al. Panitumumab in Patients With KRAS Wild-Type Colorectal Cancer After Progression on Cetuximab. Oncologist (2012) 17(1):14. doi: 10.1634/theoncologist.2011-0452
41. Pietrantonio F, Perrone F, Biondani P, Maggi C, Lampis A, Bertan C, et al. Single Agent Panitumumab in KRAS Wild-Type Metastatic Colorectal Cancer Patients Following Cetuximab-Based Regimens: Clinical Outcome and Biomarkers of Efficacy. Cancer Biol Ther (2013) 14(12):1098–103. doi: 10.4161/cbt.26343
42. Liu X, George GC, Tsimberidou AM, Naing A, Wheler JJ, Kopetz S, et al. Retreatment With Anti-EGFR Based Therapies in Metastatic Colorectal Cancer: Impact of Intervening Time Interval and Prior Anti-EGFR Response. BMC Cancer (2015) 15:713. doi: 10.1186/s12885-015-1701-3
43. Henricks LM, Lunenburg CATC, de Man FM, Meulendijks D, Frederix GWJ, Kienhuis E, et al. DPYD Genotype-Guided Dose Individualization of Fluoropyrimidine Therapy: A Prospective Safety and Cost-Analysis on DPYD Variants DPYD*2A, C.2846A>T, C.1679t>G and C.1236G>a. Ann Oncol (2018) 29:150–204. doi: 10.1093/annonc/mdy281
44. Cremolini C, Rossini D, Dell'Aquila E, Lonardi S, Conca E, Del Re M, et al. Rechallenge for Patients With RAS and BRAF Wild-Type Metastatic Colorectal Cancer With Acquired Resistance to First-Line Cetuximab and Irinotecan: A Phase 2 Single-Arm Clinical Trial. JAMA Oncol (2019) 5(3):343–50. doi: 10.1001/jamaoncol.2018.5080
45. Rossini D, Germani MM, Pagani F, Pellino A, Dell'Aquila E, Bensi M, et al. Retreatment With Anti-EGFR Antibodies in Metastatic Colorectal Cancer Patients: A Multi-Institutional Analysis. Clin Colorectal Cancer (2020) 19(3):191–9.e6. doi: 10.1016/j.clcc.2020.03.009
46. Sunakawa Y, Nakamura M, Ishizaki M, Kataoka M, Satake H, Kitazono M, et al. RAS Mutations in Circulating Tumor DNA and Clinical Outcomes of Rechallenge Treatment With Anti-EGFR Antibodies in Patients With Metastatic Colorectal Cancer. Jco Precis Oncol (2020) 4:898–911. doi: 10.1200/PO.20.00109
47. Chong LC, Hardingham JE, Townsend AR, Piantadosi C, Rico GT, Karapetis C, et al. Rechallenge With Anti-EGFR Therapy in Metastatic Colorectal Cancer (mCRC): Results From South Australia mCRC Registry. Targeted Oncol (2020) 15(6):751–7. doi: 10.1007/s11523-020-00760-8
48. Tanioka H, Asano M, Yoshida R, Waki N, Uno F, Ishizaki M, et al. Cetuximab Retreatment in Patients With Metastatic Colorectal Cancer Who Exhibited a Clinical Benefit in Response to Prior Cetuximab: A Retrospective Study. Oncol Lett (2018) 16(3):3674–80. doi: 10.3892/ol.2018.9127
49. Karani A, Felismino TC, Diniz L, Macedo MP, Silva VSE, Mello CA. Is There a Role for Rechallenge and Reintroduction of Anti-EGFR Plus Chemotherapy in Later Lines of Therapy for Metastatic Colorectal Carcinoma? A Retrospective Analysis. Ecancermedicalscience (2020) 14:1069. doi: 10.3332/ecancer.2020.1069
50. Sunakawa Y, Nakamura M, Ishizaki M, Kataoka M, Satake H, Kitazono M, et al. RAS Mutations in Circulating Tumor DNA and Clinical Outcomes of Rechallenge Treatment With Anti-EGFR Antibodies in Patients With Metastatic Colorectal Cancer. Jco Precis Oncol (2020) 4:898–911. doi: 10.1200/PO.20.00109
51. Nakajima H, Kotani D, Bando H, Kato T, Oki E, Shinozaki E, et al. REMARRY and PURSUIT Trials: Liquid Biopsy-Guided Rechallenge With Anti-Epidermal Growth Factor Receptor (EGFR) Therapy With Panitumumab Plus Irinotecan for Patients With Plasma RAS Wild-Type Metastatic Colorectal Cancer. BMC Cancer (2021) 21(1):674. doi: 10.1186/s12885-021-08395-2
52. Kilgour E, Rothwell DG, Brady G, Dive C. Liquid Biopsy-Based Biomarkers of Treatment Response and Resistance. Cancer Cell (2020) 37(4):485–95. doi: 10.1016/j.ccell.2020.03.012
53. De Rubis G, Rajeev Krishnan S, Bebawy M. Liquid Biopsies in Cancer Diagnosis, Monitoring, and Prognosis. Trends Pharmacol Sci (2019) 40(3):172–86. doi: 10.1016/j.tips.2019.01.006
54. Martins I, Ribeiro IP, Jorge J, Goncalves AC, Sarmento-Ribeiro AB, Melo JB, et al. Liquid Biopsies: Applications for Cancer Diagnosis and Monitoring. Genes (Basel) (2021) 12(3):349. doi: 10.3390/genes12030349
55. Jiang J, Adams HP, Lange M, Siemann S, Feldkamp M, McNamara S, et al. Plasma-Based Longitudinal Mutation Monitoring as a Potential Predictor of Disease Progression in Subjects With Adenocarcinoma in Advanced non-Small Cell Lung Cancer. BMC Cancer (2020) 20(1):885. doi: 10.1186/s12885-020-07340-z
Keywords: Kirsten Rat Sarcoma Viral Oncogene Homolog (KRAS), gene copy number, biomarker, colorectal cancer, cetuximab, anti-EGFR monoclonal antibody
Citation: Xiong Q, Zeng Z, Yang Y, Wang Y, Xu Y, Zhou Y, Liu J, Zhang Z, Qiu M and Zhu Q (2022) KRAS Gene Copy Number as a Negative Predictive Biomarker for the Treatment of Metastatic Rectal Cancer With Cetuximab: A Case Report. Front. Oncol. 12:872630. doi: 10.3389/fonc.2022.872630
Received: 15 February 2022; Accepted: 14 April 2022;
Published: 26 May 2022.
Edited by:
Maen Abdelrahim, Houston Methodist Research Institute, United StatesReviewed by:
Nicholas Pavlidis, University of Ioannina, GreeceGiuseppe Sena, Azienda Ospedaliera Pugliese Ciaccio, Italy
Copyright © 2022 Xiong, Zeng, Yang, Wang, Xu, Zhou, Liu, Zhang, Qiu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meng Qiu, cWl1bWVuZzMzQGhvdG1haWwuY29t; Qing Zhu, bmV3emh1cWluZzE5NzJAeWFob28uY29t
 Qunli Xiong
Qunli Xiong Zhu Zeng
Zhu Zeng Yang Yang1
Yang Yang1 Yongfeng Xu
Yongfeng Xu Jinlu Liu
Jinlu Liu Meng Qiu
Meng Qiu Qing Zhu
Qing Zhu