- 1Department of Oncology, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin, China
- 3The Genetic Analysis Department, YuceBio Technology Co., Ltd., Shenzhen, China
Patients with non-small cell lung cancer harboring the epidermal growth factor receptor (EGFR)-sensitive mutations are known to benefit significantly from EGFR tyrosine kinase inhibitors (TKIs), such as erlotinib, gefitinib, icotinib, or afatinib. However, the efficacy of EGFR-TKIs against rare mutations has not yet been well investigated. Here, we report a female patient with advanced lung adenocarcinoma (LUAD), carrying a rare mutation of EGFR Exon19 E746_L747delinsIP, who was administered first-generation EGFR-TKIs as the first-line treatment. The patient continued to progress slowly until peritoneal metastases have occurred. Subsequently, the patient was treated with anlotinib for 5 months until disease progression. Given the finding of the same EGFR rare mutation in peritoneal effusion without other EGFR-TKI resistance mutations, the patient received afatinib with a tremendous response. Our results may be of clinical relevance for patients with LUAD carrying this rare mutation, and these findings warrant further investigation.
Introduction
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) targeting therapy has shown promise in the treatment of non-small cell lung cancer (NSCLC) with common EGFR mutations, such as L858R and exon 19 deletion (exon 19del) (1). With the rapid development of next-generation sequencing (NGS) technology, many atypical mutations have been identified in EGFR exons 18, 19, 20, and 21, respectively, but their sensitivity to TKIs is unclear. In the current study, afatinib, in particular, has demonstrated clinical efficacy in the treatment of some uncommon mutations, compound mutations, and some exon 20 insertions (2, 3).
Previous studies have shown that NSCLC with peritoneal metastases is rare (approximately 2%) (4) and has a poor prognosis with a median overall survival (mOS) of less than 3 months (5). Peritoneal metastases from NSCLC are often complicated by peritoneal effusion. Once malignant ascites occurs, it usually means that patients are in poor physical condition and lose the opportunity for treatment (6). Owing to the lack of experience in treating peritoneal complications, oncologists face a great challenge in the treatment of NSCLC with peritoneal metastases (7). Here, we report the case of a patient carrying EGFR E746_L747delinsIP mutated lung adenocarcinoma (LUAD) with peritoneal metastases that was successfully treated with the second-generation EGFR TKI targeted therapy, afatinib.
Case presentation
A 57-year-old Chinese woman without a smoking history or family history of cancer suffered from a cough in January 2020. In April 2020, computed tomography (CT) scans revealed right atelectasis, lung neoplasms, and pleural effusion (Figure 1A). Whole-body bone scans and magnetic resonance imaging of the brain revealed no evidence of metastasis. Using immunohistochemistry, the patient was diagnosed with LUAD (stage IV). The E746_L747delinsIP (6.6%) at exon 19 and the S1190F (1.74%) at exon 28 of the EGFR were identified in the pleural effusion biopsy specimen by NGS sequencing with a panel covering 525 cancer-related genes. None of the mutations were associated with EGFR-TKI primary resistance. Based on this result, the patient started treatment with gefitinib (250 mg/day) in June 2020; however, no radiological response was observed during treatment. Unfortunately, she experienced slow progressive disease (PD) with increasing lung neoplasms and emerging left pleural effusion in December 2020 (Figures 1B–D). The patient was then switched to icotinib in February 2021, because EGFR E746_L747delinsIP was misunderstood to be an EGFR exon 19 del, which is sensitive to the first-generation of EGFR-TKIs. However, PD was confirmed by CT showing peritoneal metastases in March 2021 (Figures 1E, F).
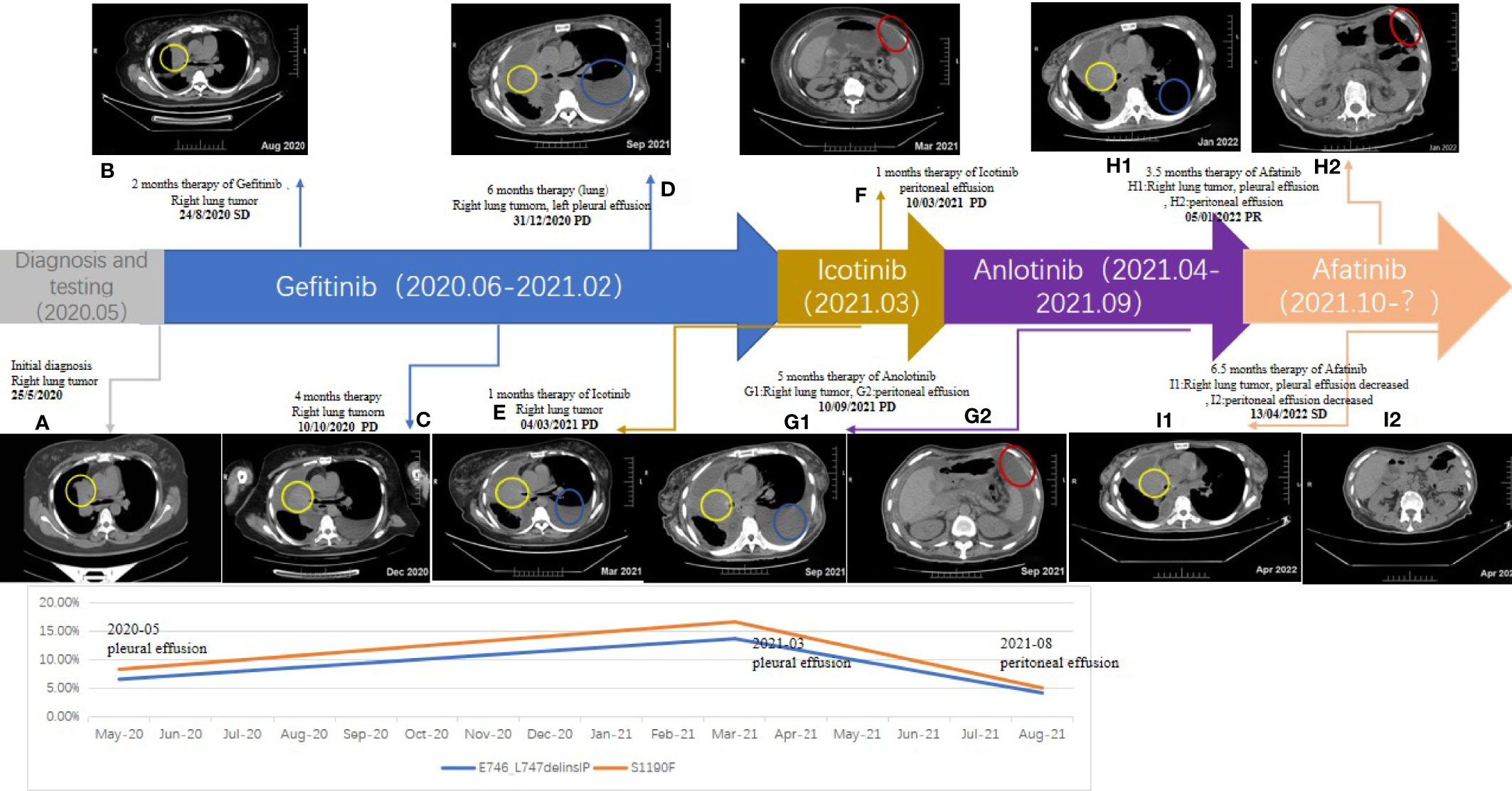
Figure 1 Timeline of the patient: Chest and Abdominal contrast-enhanced CT (A–F, G1, G2, H1, H2, I1, I2); yellow circle: right lung tumor; blue circle: left pleural effusion; red circle: peritoneal effusion.
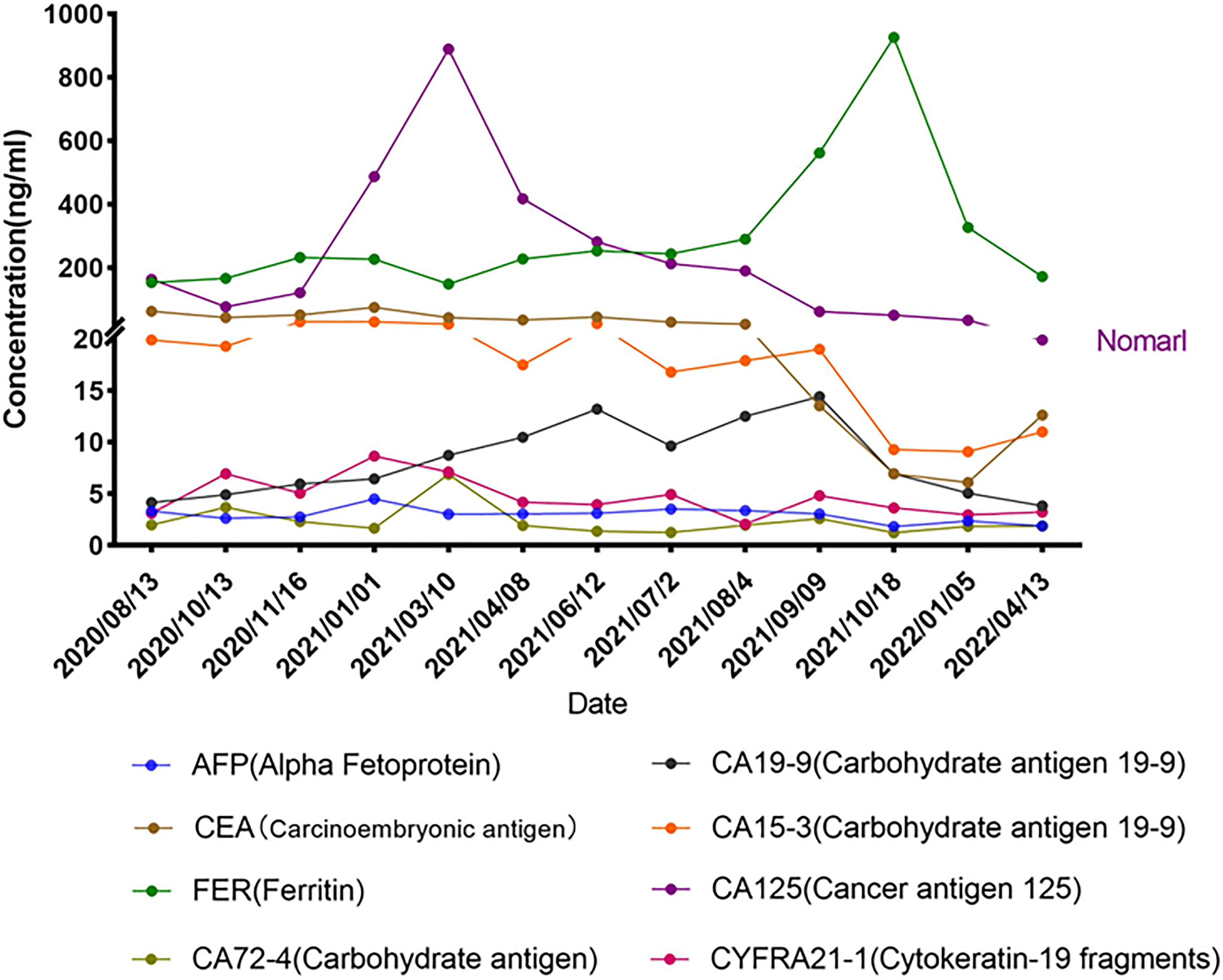
Repetitive NGS analysis using pleural effusion detected EGFR E746_L747delinsIP (13.7%) in exon 19 and S1190F (2.95%) in exon 28 with increasing variant allele frequencies (VAF) as per a small, customized panel covering 74 cancer target related genes in Yucebio Technology (Figure 2). A timeline illustrating the patient’s medical history and therapy is presented in Figure 1. The patient was currently exhibiting PD. Some studies have suggested that anlotinib may provide survival benefits to patients with NSCLC with abdominal or pleural effusion (6, 8). Considering that the patient may not tolerate the adverse effects of afatinib, as well as refractory ascites, pleural effusion, and an Eastern Cooperative Oncology Group (ECOG) Performance Status score of 3, she received anlotinib (12 mg/day) in April 2021. During the treatment, the patient’s chest symptoms were relieved, and simultaneously, CA125 and CYFRA21-1 showed a decreasing trend, especially CA125 (Figure 3); however, the peritoneal effusion with a chylous appearance continued to increase (Figure 1G2), requiring drainage of 1000–1500 ml per day. Anlotinib treatment was discontinued in September 2021 because of PD.
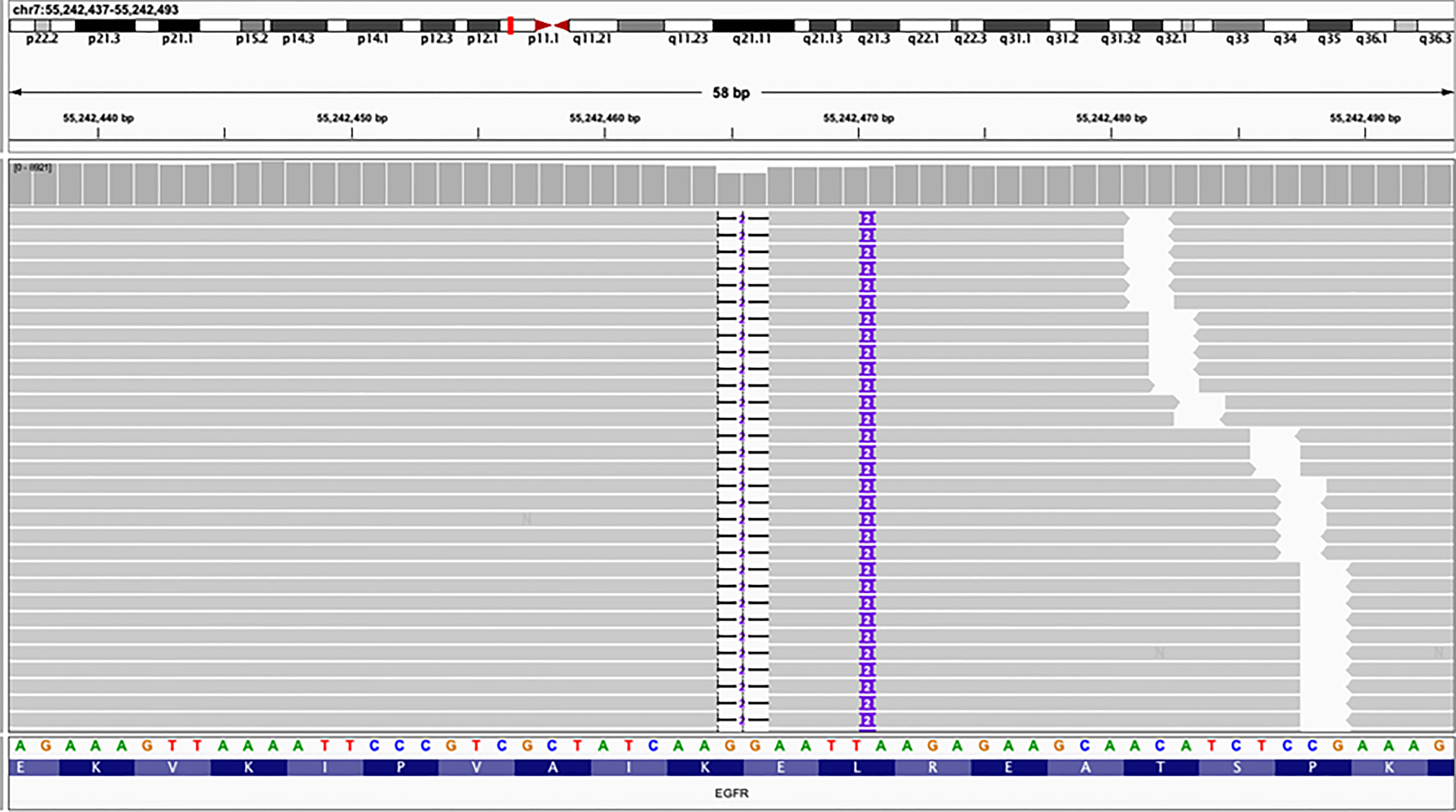
Figure 2 Next-generation sequencing (NGS) panel result showed an epidermal growth factor receptor (EGFR) mutation E746_L747delinsIP via simultaneous deletion and insertion of DNA fragments of 2 bp in exon 19.
Peritoneal effusions were collected for further genetic analysis. As shown in Figure 1, EGFR E746_L747delinsIP (4.2%) and S1190F (0.88%) were found again without other mutations, indicating that the LUAD metastasized to the peritoneum. Afatinib (40 mg/day) was administered in September 2021. The patient had received afatinib for approximately 3.5 months and achieved a partial response with a significant improvement in ascites and right pleural effusion. The ascites gradually diminished, and only a small amount of ascites was observed within the 1.5 months before the final test (Figures 1H1, H2). Serum tumor biomarkers showed a downward trend, especially CA125, decreasing to the average level for the first time (Figure 3). The treatment rapidly improved her clinical symptoms, including abdominal distension and appetite, despite the diarrhea. Her general condition improved from 3 to 2 according to the Eastern Cooperative Oncology Group Performance Status score. Since November 2021, the patient has had no further drainage of her ascites or pleural effusion, and the effusion showed a decreasing tendency. Follow-up CT until April 2022 showed that the pulmonary lesions were slightly enlarged, bilateral pulmonary nodules were increased, abdominal nodules continued to be stable, and the curative effect assessment of lesions primarily showed an increasing trend within the stable disease (SD) range (Figures 1I1, I2). Furthermore, the serum tumor biomarkers CEA and CYFRA21-1 showed an increase (Figure 3). In accordance with the patient’s and her family’s wishes, we performed a gene test using NGS again to explore if the patient developed drug-resistant mutations.
Discussion
With the increasing popularity of NGS, uncommon EGFR mutations have been detected. However, some rare mutations lack clinical efficacy data, leading to the demand for continuous clinical reporting. Our case may be able to illustrate the sensitivity of the rare EGFR E746_L747delinsIP mutation to the efficacy of the first- and the second-generation EGFR-TKIs.
Here, we report the first efficacy data of an NSCLC patient carrying EGFR E746_L747delinsIP using the NGS platform. This mutation induces a double amino acid substitution (E746I-L747P) via the simultaneous deletion and insertion of two nucleotides at different positions in EGFR exon 19 (Figure 2). According to the Human Genome Variation Society (HGVS), this mutation is named EGFR E746_L747delinsIP (9). We also found a co-mutation EGFR S1190F, an uncertain clinically significant mutation, without any functional studies to affect the kinase domain of the EGFR or the efficacy of EGFR-TKIs.
In our case, the patient received gefitinib but continued to progress slowly, and according to the NCCN, it is a viable strategy for limited metastatic NSCLC to continue taking the first-generation EGFR-TKI. Therefore, icotinib was administered. A high frequency of EGFR E746_L747delinsIP mutation was found in ascites without other known TKI resistance mutations, indicating that EGFR E746_L747delinsIP might be resistant to first-generation TKIs. Pleural and ascites metastases from LUAD are relatively rare in patients with NSCLC. It has an inferior prognosis, with a mOS of approximately 1.3 months in the best supportive care group (7). We detected the same rare EGFR mutations in the patient’s pleural fluid and ascites samples. It is plausible that EGFR E746_L747delinsIP is sensitive to afatinib owing to its remarkable polyserous effusions and abdominal response.
To date, no actual efficacy data have been reported for patients with NSCLC with EGFR E746_L747delinsIP. Koopman et al. (2021) considered it to be comparable to L747P and predicted it to be sensitive to EGFR TKI, and mentioned that EGFR E746_L747delinsIP was an uncommon, actionable mutation (10). Additionally, there are still some clinical case reports on L747P and found that the current assessment of L747P efficacy for the first- and third-generation EGFR-TKIs is inconsistent (11–13). Moreover, Coco et al. (2015) reported a similar case in which a patient carrying an EGFR E746V-L747P (E746_L747delinsVP) activating mutation caused by four continuous nucleotides (AATT > TTCC) was resistant to gefitinib. He predicted that EGFR E746V-L747P was resistant to gefitinib via structure prediction (14). The phenomenon presented in our case was similar to that described above. Therefore, we speculate that EGFR E746_L747delinsIP is a primary resistance variant for the first-generation EGFR-TKIs.
In addition, most case reports suggest that EGFR L747P is sensitive to afatinib via the Uncommon EGFR Mutations Database. Retrospective studies suggest that afatinib has clinical activity in NSCLC with uncommon EGFR mutations (3). A survey conducted by Robichaux et al. (2021) identified L747P as a PACC-class (P-loop and αC-helix compressing) variant, which was thought to be more effective against afatinib than any other TKI classes (15). Combined with the efficacy data of our case in the real world, we suggest that EGFR E746_L747delinsIP may be sensitive to afatinib, which is comparable to L747P.
In 2020, a retrospective study found that the NSCLC patients with uncommon EGFR exon 19 delins had significantly longer mPFS than those with the common exon 19 del with EGFR-TKI treatment (16). The patients in that study all presented EGFR exon19 in-frame deletion, which is unlike the complex variant in our case that did not cause any changes in amino acid length (Supplementary 1). EGFR exon 19 del at the K745-I759 position increases the kinase activity of EGFR, leading to the downstream pro-survival pathway hyperactivity, and consequently confers EGFR oncogenicity, which is sensitive to EGFR-TKIs (17, 18). Thus, the sensitivity of EGFR exon 19 rare complex delins mutation, in which the amino acid effect is missense mutations, to EGFR-TKIs warrants further investigation.
Our study had some limitations. First, the first-generation EGFR-TKI resistance mechanism of the EGFR E746_L747delinsIP should be supported by additional clinical data and functional studies. Second, large-scale data are required to support the use of afatinib to treat LUAD with EGFR E746_L747delinsIP, and a longer follow-up is needed to track the effect of afatinib on EGFR E746_L747delinsIP.
In conclusion, our case firstly showed that patients with rare EGFR E746_L747delinsIP mutated NSCLC-peritoneal metastases may be sensitive to afatinib and resistant to first-generation gefitinib and icotinib therapies. We suggest that first-generation EGFR-TKIs should be cautiously applied to patients with this EGFR exon 19 mutation, which causes amino acid substitution. Clinical trials are needed to develop treatment strategies for NSCLC harboring EGFR E746_L747delinsIP, and prospective or clinical studies are required to support these preliminary findings.
Data Availability Statement
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
FK and DW: Conceptualization, Methodology, and Review. LY, LZ, and BS: Data collection and analysis, Writing, and Editing. YD and JY: Literature research. All authors contributed to the article and approved the submitted version.
Funding
This work is supported by the National Natural Science Foundation of China (No. 81403220) and Tianjin Health and Family Planning-High Level Talent Selection and Training Project, National Key Research and Development (R&D) Plan (2018YFC1707400).
Conflict of Interest
Authors LY, YD, JY, and DW were employed by YuceBio Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.861271/full#supplementary-material
Supplementary 1 | Epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) efficacy in non-small cell lung cancer (NSCLC) with rare EGFR exon 19 mutation.
References
1. Li MM, Datto M, Duncavage EJ, Kulkarni S, Lindeman NI, Roy S, et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn (2017) 19(1):4–23. doi: 10.1016/j.jmoldx.2016.10.002
2. Beau-Faller M, Prim N, Ruppert AM, Nanni-Metellus I, Lacave R, Lacroix L, et al. Rare EGFR Exon 18 and Exon 20 Mutations in Non-Small-Cell Lung Cancer on 10 117 Patients: A Multicentre Observational Study by the French ERMETIC-IFCT Network. Ann Oncol (2014) 25(1):126–31. doi: 10.1093/annonc/mdt418
3. Yang JC, Schuler M, Popat S, Miura S, Heeke S, Park K, et al. Afatinib for the Treatment of NSCLC Harboring Uncommon EGFR Mutations: A Database of 693 Cases. J Thorac Oncol (2020) 15(5):803–15. doi: 10.1016/j.jtho.2019.12.126
4. Lurvink RJ, Rijken A, Bakkers C, Aarts MJ, Kunst PWA, van deBorne BE, et al. Synchronous Peritoneal Metastases From Lung Cancer: Incidence, Associated Factors, Treatment and Survival: A Dutch Population-Based Study. Clin Exp Metastasis (2021) 38(3):295–303. doi: 10.1007/s10585-021-10085-z
5. Patil T, Aisner DL, Noonan SA, Bunn PA, Purcell WT, Carr LL, et al. Malignant Pleural Disease is Highly Associated With Subsequent Peritoneal Metastasis in Patients With Stage IV Non-Small Cell Lung Cancer Independent of Oncogene Status. Lung Cancer (2016) 96:27–32. doi: 10.1016/j.lungcan.2016.03.007
6. Cao B, Liu Y, Yin W, Li Q, Liang L. [A Single Center, Retrospective Analysis of Prognosis in Non-Small Cell Lung Cancer Patients With Peritoneal Carcinomatosis]. Zhongguo Fei Ai Za Zhi (2019) 22(3):143–50. doi: 10.3779/j.issn.1009-3419.2019.03.0
7. Tani T, Nakachi I, Ikemura S, Nukaga S, Ohgino K, Kuroda A, et al. Clinical Characteristics and Therapeutic Outcomes of Metastatic Peritoneal Carcinomatosis in Non-Small-Cell Lung Cancer. Cancer Manag Res (2021) 13:7497–503. doi: 10.2147/CMAR.S330103
8. Liu Y, Cheng Y, Wang Q, Li K, Shi J, Wu L, et al. 1787p Effect of Anlotinib in Advanced Small Cell Lung Cancer Patients With Pleural Metastases/Pleural Effusion: A Subgroup Analysis From a Randomized, Double-Blind Phase II Trial (ALTER1202). Ann Oncol (2020) 31:S1036. doi: 10.1016/j.annonc.2020.08.1548
9. den Dunnen JT, Dalgleish R, Maglott DR, Hart RK, Greenblatt MS, McGowan-Jordan J, et al. HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Hum Mutat (2016) 37(6):564–9. doi: 10.1002/humu.22981
10. Koopman B, Garcia BNC, Kuijpers C, Damhuis RAM, van derWekken AJ, Groen HJM, et al. A Nationwide Study on the Impact of Routine Testing for EGFR Mutations in Advanced NSCLC Reveals Distinct Survival Patterns Based on EGFR Mutation Subclasses. Cancers (Basel) (2021) 13(14):3641. doi: 10.3390/cancers13143641
11. Huang J, Wang Y, Zhai Y, Wang J. Non-Small Cell Lung Cancer Harboring a Rare EGFR L747P Mutation Showing Intrinsic Resistance to Both Gefitinib and Osimertinib (AZD9291): A Case Report. Thorac Cancer (2018) 9(6):745–9. doi: 10.1111/1759-7714.12637
12. Huang X, Yang Y, Wang P, Wang J, Chen S, Mao X, et al. A Rare EGFR Mutation L747P Conferred Therapeutic Efficacy to Both Ge Fi Tinib and Osimertinib: A Case Report. Lung Cancer (2020) 150:9–11. doi: 10.1016/j.lungcan.2020.09.017
13. van der Wekken AJ, Stigt JA, A'T Hart N. A Novel EGFR Mutation in Exon 19 Showed Stable Disease After TKI Treatment. J Thorac Oncol (2012) 7(8):e8. doi: 10.1097/JTO.0b013e31825ccae8
14. Coco S, Truini A, Vanni I, Genova C, Rosano C, Dal Bello MG, et al. Uncommon EGFR Exon 19 Mutations Confer Gefitinib Resistance in Advanced Lung Adenocarcinoma. J Thorac Oncol (2015) 10(6):e50–2. doi: 10.1097/JTO.0000000000000538
15. Robichaux JP, Le X, Vijayan RSK, Hicks JK, Heeke S, Elamin YY, et al. Structure-Based Classification Predicts Drug Response in EGFR-Mutant NSCLC. Nature (2021) 597(7878):732–7. doi: 10.1038/s41586-021-03898-1
16. Peng X, Long X, Liu L, Zeng L, Yang H, Jiang W, et al. Clinical Impact of Uncommon Epidermal Growth Factor Receptor Exon 19 Insertion-Deletion Variants on Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitor Efficacy in Non-Small-Cell Lung Cancer. Eur J Cancer (2020) 141:199–208. doi: 10.1016/j.ejca.2020.10.005
17. Zhang Z, Stiegler AL, Boggon TJ, Kobayashi S, Halmos B. EGFR-Mutated Lung Cancer: A Paradigm of Molecular Oncology. Oncotarget (2010) 1(7):497–514. doi: 10.18632/oncotarget.186
Keywords: lung adenocarcinoma, peritoneal carcinomatosis, sensitive, afatinib, EGFR E746_L747delinsIP
Citation: Zhang L, Yang L, Sun B, Deng Y, Yang J, Wu D and Kong F (2022) Case Report: Afatinib Sensitivity in Rare EGFR E746_L747delinsIP Mutated LUAD With Peritoneal Metastases. Front. Oncol. 12:861271. doi: 10.3389/fonc.2022.861271
Received: 24 January 2022; Accepted: 27 April 2022;
Published: 31 May 2022.
Edited by:
Zhi Li, The First Affiliated Hospital of China Medical University, ChinaReviewed by:
Haruhiko Sugimura, Hamamatsu University School of Medicine, JapanJunichi Shimizu, Aichi Cancer Center, Japan
Copyright © 2022 Zhang, Yang, Sun, Deng, Yang, Wu and Kong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fanming Kong, a29uZ2Zhbm1pbmcwOEAxNjMuY29t; Dongfang Wu, d3VkZkB5dWNlYmlvLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Lili Zhang
Lili Zhang Lu Yang
Lu Yang Binxu Sun
Binxu Sun Yixiao Deng
Yixiao Deng Jie Yang
Jie Yang Dongfang Wu
Dongfang Wu Fanming Kong
Fanming Kong