- 1Department of Medical Genetics, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Men’s Health and Reproductive Health Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 3Department of Pharmacognosy, College of Pharmacy, Hawler Medical University, Erbil, Iraq
- 4Center of Research and Strategic Studies, Lebanese French University, Erbil, Iraq
- 5Department of Biology, College of Education, Salahaddin University, Erbil, Iraq
- 6Urology and Nephrology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 7Institute of Human Genetics, Jena University Hospital, Jena, Germany
- 8Skull Base Research Center, Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 9Critical Care Fellowship, Department of Anesthesiology, Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
miR-15b-5p is encoded by MIR15B gene. This gene is located on cytogenetic band 3q25.33. This miRNA participates in the pathogenesis of several cancers as well as non-malignant conditions, such as abdominal aortic aneurysm, Alzheimer’s and Parkinson’s diseases, cerebral ischemia reperfusion injury, coronary artery disease, dexamethasone induced steatosis, diabetic complications and doxorubicin-induced cardiotoxicity. In malignant conditions, both oncogenic and tumor suppressor impacts have been described for miR-15b-5p. Dysregulation of miR-15b-5p in clinical samples has been associated with poor outcome in different kinds of cancers. In this review, we discuss the role of miR-15b-5p in malignant and non-malignant conditions.
Introduction
microRNAs (miRNAs) are a category of non-coding RNA with sizes about 20-24 nucleotide which participate in post-transcriptional control of gene expression (1). This effect is exerted through modulation of stability and translation of mRNAs. The primary transcripts produced by RNA polymerase II have 5’-cap and 3’-polyadenylated tail. Then, Drosha ribonuclease III enzyme cleaves this transcript to make the stem-loop precursor miRNA with an estimated size of 70 nucleotides (2). Finally, this transcript is processed by the Dicer ribonuclease to make the mature miRNA which can be combined into the RNA-induced silencing complex. Through incorporation into this complex, miRNAs can recognize their target transcript in a base pairing-dependent process resulting in suppression of translation or destabilization of transcript (3).
MIR15B gene is located on cytogenetic band 3q25.33 and encodes hsa-mir-15b. This miRNA participates in the pathogenesis of several cancers as well as non-malignant conditions, including cardiovascular disorders, neuropsychiatric diseases and metabolic conditions. This miRNA has been reported to exert oncogenic or tumor suppressor effects in different malignancies. We have searched the literature and discussed the role of miR-15b-5p in malignant and non-malignant conditions.
miR-15b-5p in Cancers
Cell Line Studies
In bladder cancer cell lines, the long non-coding RNA (lncRNA) MAGI2-AS3 acts as a molecular sponge for miR-15b-5p. In fact, MAGI2-AS3 exerts its tumor suppressor role in bladder cancer through decreasing level of this miRNA. Meanwhile, miR-15b-5p has been found to target the tumor suppressor gene CCDC19. Taken together, MAGI2-AS3/miR-15b-5p/CCDC19 axis has been revealed to regulate progression of bladder cancer (4).
An in vitro experiment in breast cancer cells has shown that miR-15b-5p silencing could restrain cell proliferation and invasiveness and induce apoptosis, while its up-regulation has exerted the opposite impacts. Notably, heparanase-2 (HPSE2) has been acknowledged as the target of miR-15b-5p in breast cancer cells, through which this miRNA applies its effect (5).
In cervical cancer cells, level of the tumor suppressor lncRNA FENDRR has been shown to be decreased. This lncRNA has binding sites for miR-15a-5p and miR-15b-5p, two miRNAs that can down-regulate expression of Tubulin alpha1A (TUBA1A). Taken together, FENDRR/miR-15a/b-5p/TUBA1A molecular route has been proved to regulate progression of cervical cancer (6).
Expression of miR-15b-5p has been reported to be surged in colon cancer cells. Treatment of HT-29 cells with a PNA against miR-15b-5p has been shown to reduce cell proliferation and activate the pro-apoptotic pathway (7). Another research in colon cancer cells has displayed that SIRT1 suppresses metastatic ability of cells through decreasing expression of miR-15b-5p. In fact, SIRT1 disrupts the regulatory effect of AP-1 on activation of expression of miR-15b-5p via deacetylating this activation factor. miR-15b-5p can target the transcript of a central enzyme in the fatty acid oxidation, namely acyl-CoA oxidase 1 (ACOX1). Taken together, SIRT1/miR-15b-5p/ACOX1 axis has been identified as a functional route in regulation of metastatic ability of colorectal cancer cells (8).
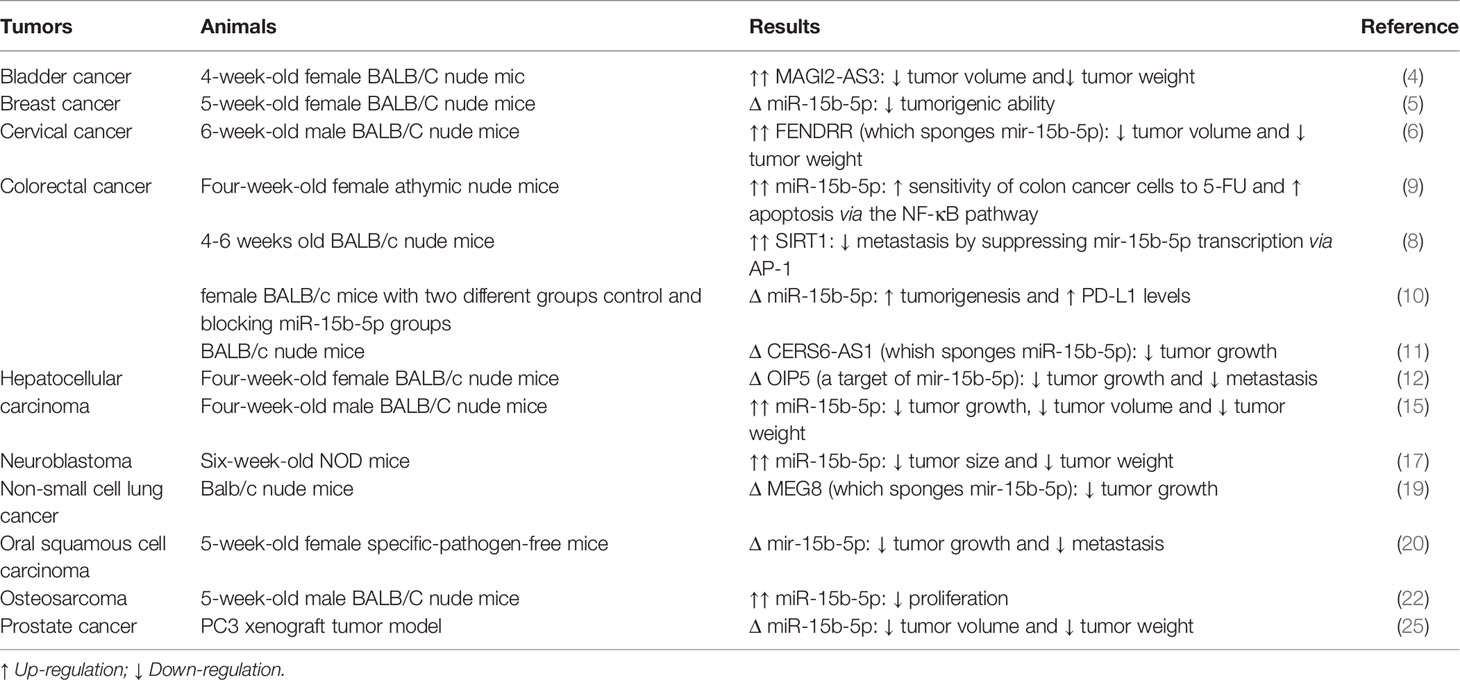
Figure 1 displays the oncogenic role of miR-15b-5p in bladder, breast, cervical, colorectal, liver, oral, ovarian, prostate and gastric cancers.
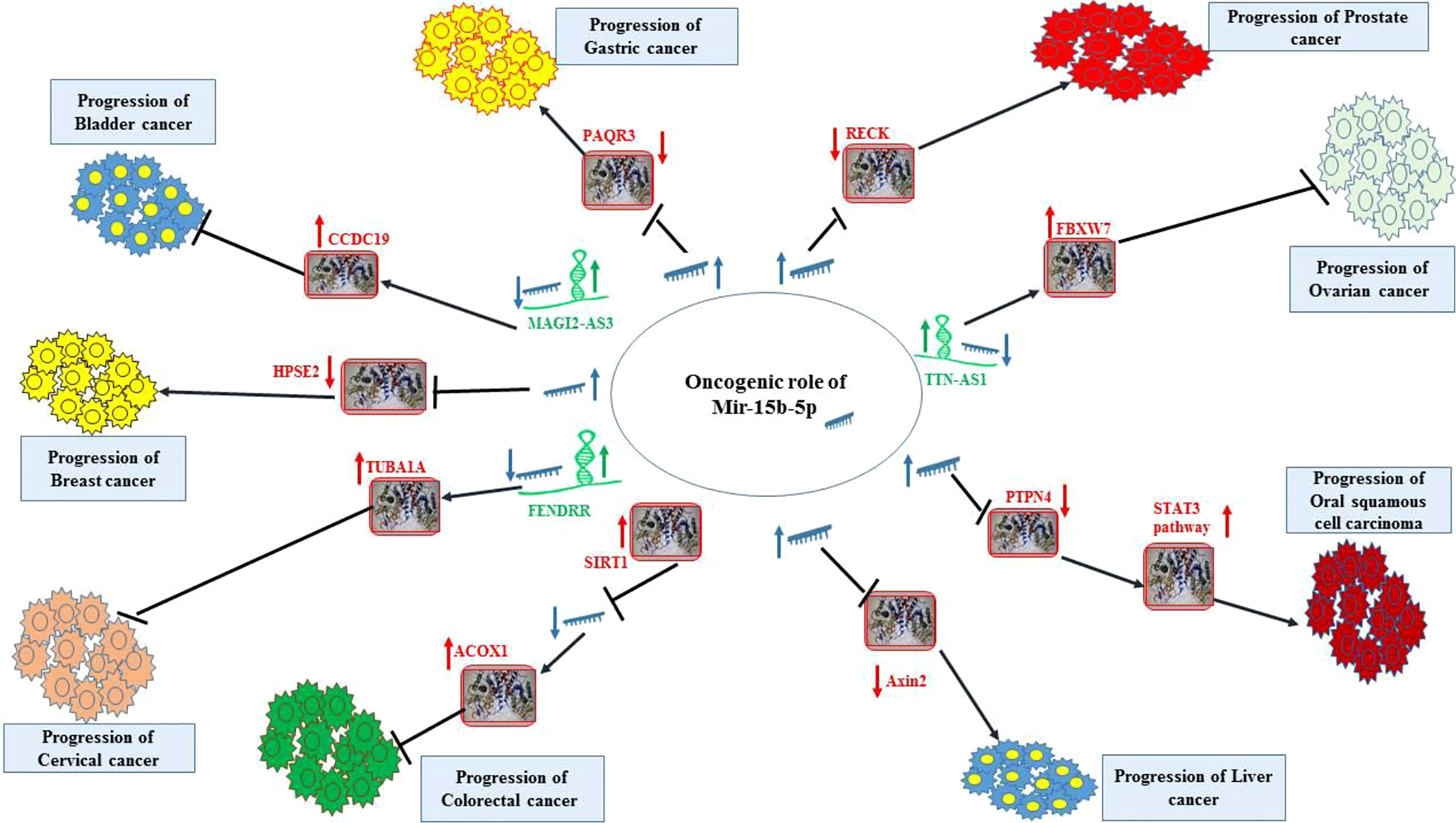
Figure 1 Oncogenic effect of miR-15b-5p in bladder, breast, cervical, colorectal, liver, oral, ovarian, prostate and gastric cancers. Detailed information about the conducted experiments is shown in Table 1.
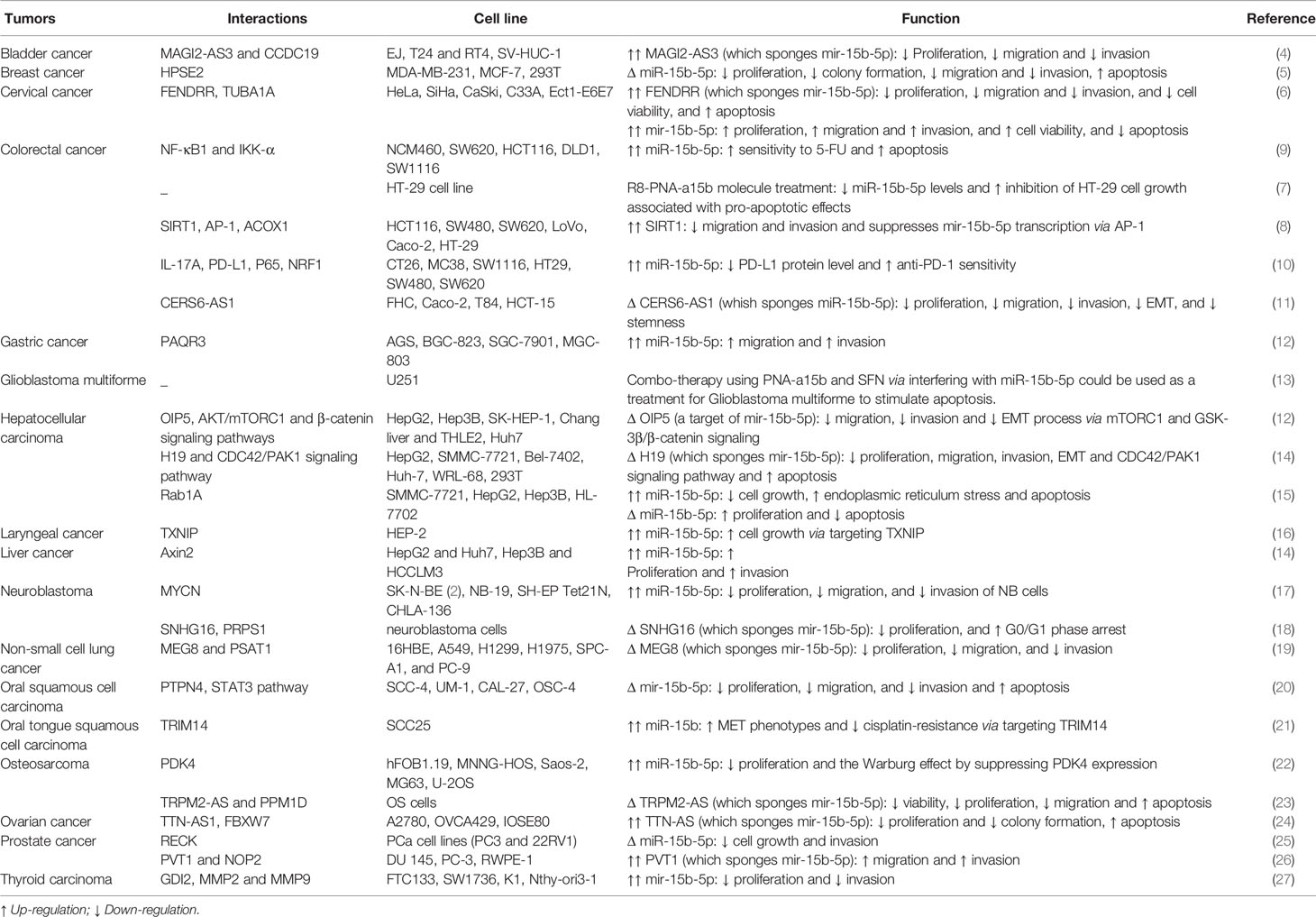
Table 1 Summary of cell line studies on the role of miR-15b-5p in cancers (Δ, knock-down or deletion; MET, mesenchymal-epithelial transition).
In contrast to the previously mentioned experiment in colorectal cancer cells (7), Zhao et al. have shown that miR-15b-5p has a tumor suppressor impact in this cancer. Notably, miR-15b-5p can enhance 5-fluorouracil (5-FU)-induced apoptosis in these cells and reversed the resistance of colorectal cancer cells to this therapeutic agent. Mechanistically, miR-15b-5p exerts this impact through modulating activity of the NF-κB signaling via decreasing NF-κB1 and IKK-α levels. miR-15b-5p has been found to target the anti-apoptosis transcript XIAP (9).
In vitro experiments in neuroblastoma cells have shown that up-regulation of miR-15a-5p, miR-15b-5p or miR-16-5p can reduce expression of MYCN transcript and N-Myc protein. On the other hand, suppression of these miRNAs could lead to enhancement of MYCN transcripts and N-Myc protein level, along with increasing half-life of its mRNA. The interaction between these miRNAs and MYCN mRNA has been proved through conducting immunoprecipitation and luciferase reporter assays. Notably, up-regulation of these miRNAs has diminished proliferation, migration, and invasiveness of neuroblastoma cells (17). Figure 2 shows tumor suppressor role of miR-15b-5p in thyroid cancer, hepatocellular carcinoma, neuroblastoma, osteosarcoma and prostate cancer.
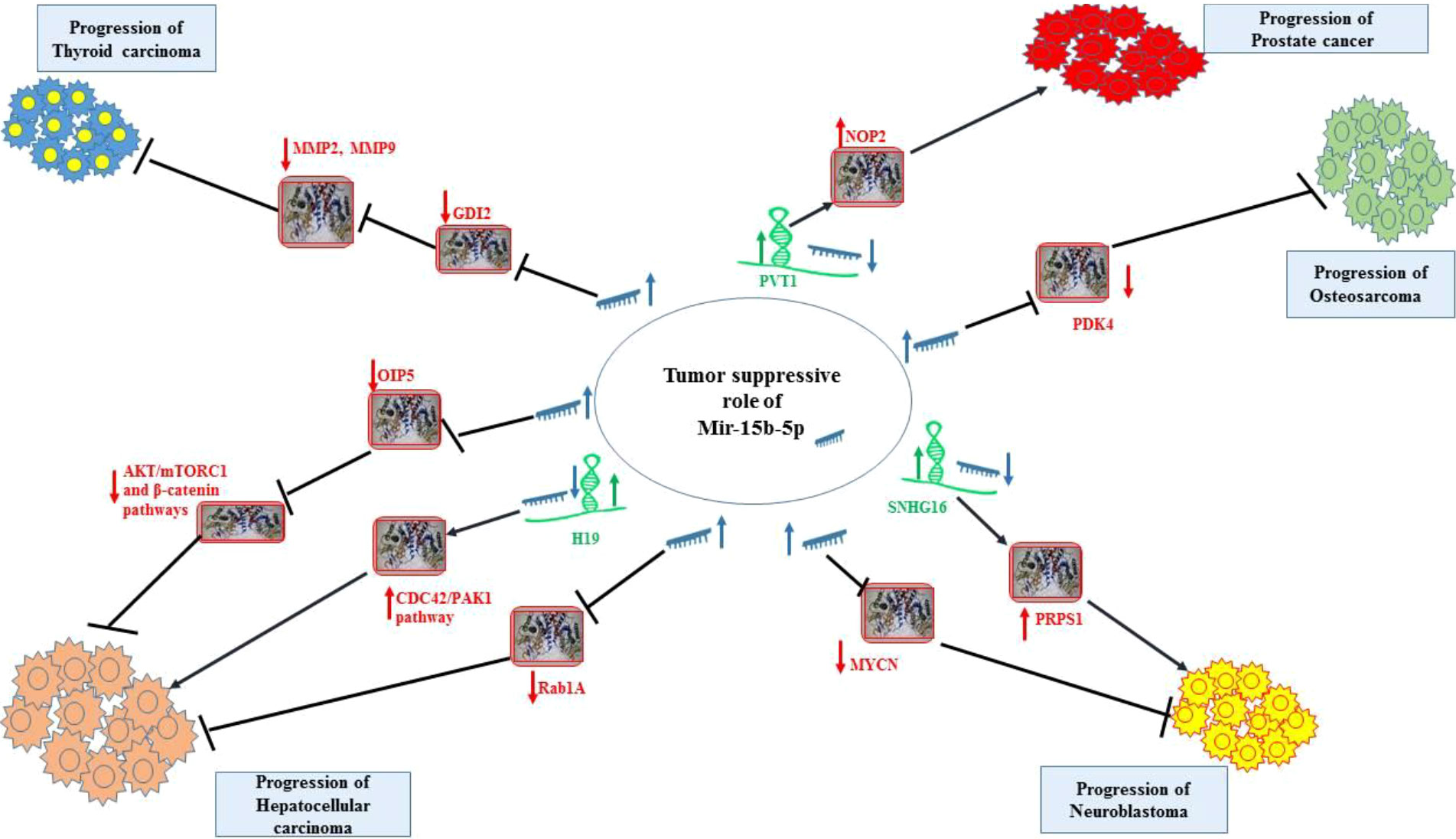
Figure 2 Tumor suppressor role of miR-15b-5p in thyroid cancer, hepatocellular carcinoma, neuroblastoma, osteosarcoma and prostate cancer. Detailed information about the conducted experiments is shown in Table 1.
Animal Studies
Lovat et al. have produced miR-15b/16-2 knockout mice for the purpose of identification of the role of this cluster. This intervention has led to development of B-cell lymphomas by age 15–18 month possibly though modulation of expression of Cyclins D2 and D1, and IGF1R. These genes participate in the regulation of proliferation and antiapoptotic pathways. Taken together, this cluster has been shown to have a tumor suppressor role in mice models of B-cell lymphoma (28).
In xenograft models of bladder cancer, up-regulation of MAGI2-AS3 has reduced tumor volume possibly through decreasing expression of miR-15b-5p (4). Up-regulation of FENDRR, another miR-15b-5p-sponging lncRNA has exerted similar effects in xenograft models of cervical cancer (6). In colorectal cancer cells, a single study has shown that over-expression of miR-15b-5p improves sensitivity of cells to 5-FU (9). On the other hand, another study has indicated that SIRT1 decreases metastasis through suppression of miR-15b-5p transcription (8). Moreover, miR-15b-5p has been demonstrated to decrease expression of PD-L1, suppress tumorigenic potential of colorectal cancer cells and increase anti-PD-1 sensitivity in colitis-associated cancer and APCmin/+ models of colorectal cancer (10).
In an animal model of osteosarcoma, over-expression of miR-15b-5p has been associated with reduced cell proliferation (22). Table 2 shows summary of animal studies on the role of miR-15b-5p in cancers.
Human Studies
Expression assays in clinical samples obtained from patients with bladder cancer, breast cancer, gastric cancer, oral squamous cell carcinoma and prostate cancer have shown up-regulation of miR-15b-5p. On the other hand, this miRNA has been found to be down-regulated in head and neck cancer squamous cell carcinomas, neublastoma and thyroid cancer samples. Different studies in colorectal cancer and hepatocellular carcinoma sample have shown contradictory expression patterns (Table 3). Moreover, dysregulation of expression of miR-15b-5p has been associated with poor clinical outcome in bladder cancer, breast cancer, head and neck/oral squamous cell carcinoma, hepatocellular carcinoma and neuroblastoma.
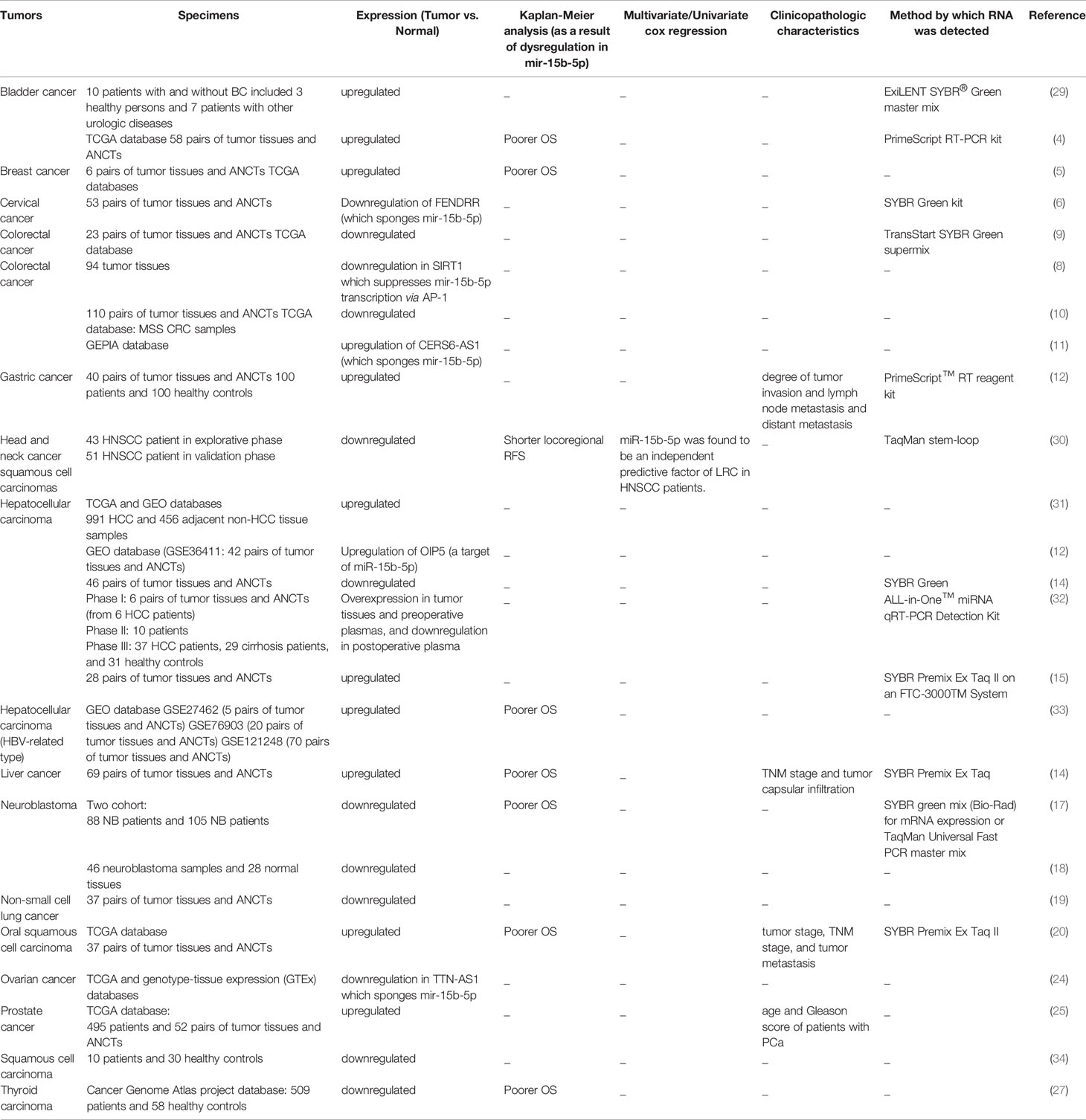
Table 3 Summary of human studies on the role of miR-15b-5p in cancers (NB, Neuroblastoma; OS, Overall survival; ANCTs, adjacent non-cancerous tissues; TNM, tumor‐node‐metastasis; MSS, microsatellite stable; CRC, colorectal cancer; RFS, relapse-free survival; HCC, Hepatocellular carcinoma).
Role of miR-15b-5p in Non-Malignant Conditions
Cell Line Studies
In vitro experiments in vascular smooth muscle cells (VSMCs) have shown that up-regulation of miR-15b-5p suppresses cell proliferation and induces apoptosis, while its knock down leads to opposite results. These effects are possibly mediated through suppression of ACSS2. Transfection of these cells with miR-15b-5p mimic or inhibitor has led to down-regulation and up-regulation of ACSS2 and PTGS2, respectively. Taken together, miR-15b-5p may increase apoptosis of aortic VSMCs and suppress their proliferation through influencing ACSS2/PTGS2 axis, thus participating in the pathoetiology of abdominal aortic aneurysm (35).
miR-15b-5p has also been shown to mediate the anti-amyloid effect of curcumin in an in vitro model of Alzheimer’s disease through influencing expression of the amyloid precursor protein (36). Moreover, the antiangiogenic effect of isopimpinellin has been attributed to its impact on induction of miR-15b-5p expression and subsequent down-regulation of angiogenic stimulators (37).
In addition, miR-15b-5p has been shown to mediate the effects of LINC00473 in cerebral I/R injury. Experiments in a cellular model of cerebral I/R injury has shown down-regulation of LINC00473 in these cells. Up-regulation of this lncRNA has reversed the effects of oxygen glucose deprivation/reperfusion on cell viability and apoptosis as well as ROS levels. Mechanistically, LINC00473 acts as a molecular sponge for miR-15b-5p and miR-15a-5p and regulates expression of SRPK1 (38). Table 4 shows summary of cell line studies on the role of miR-15b-5p in non-malignant conditions.
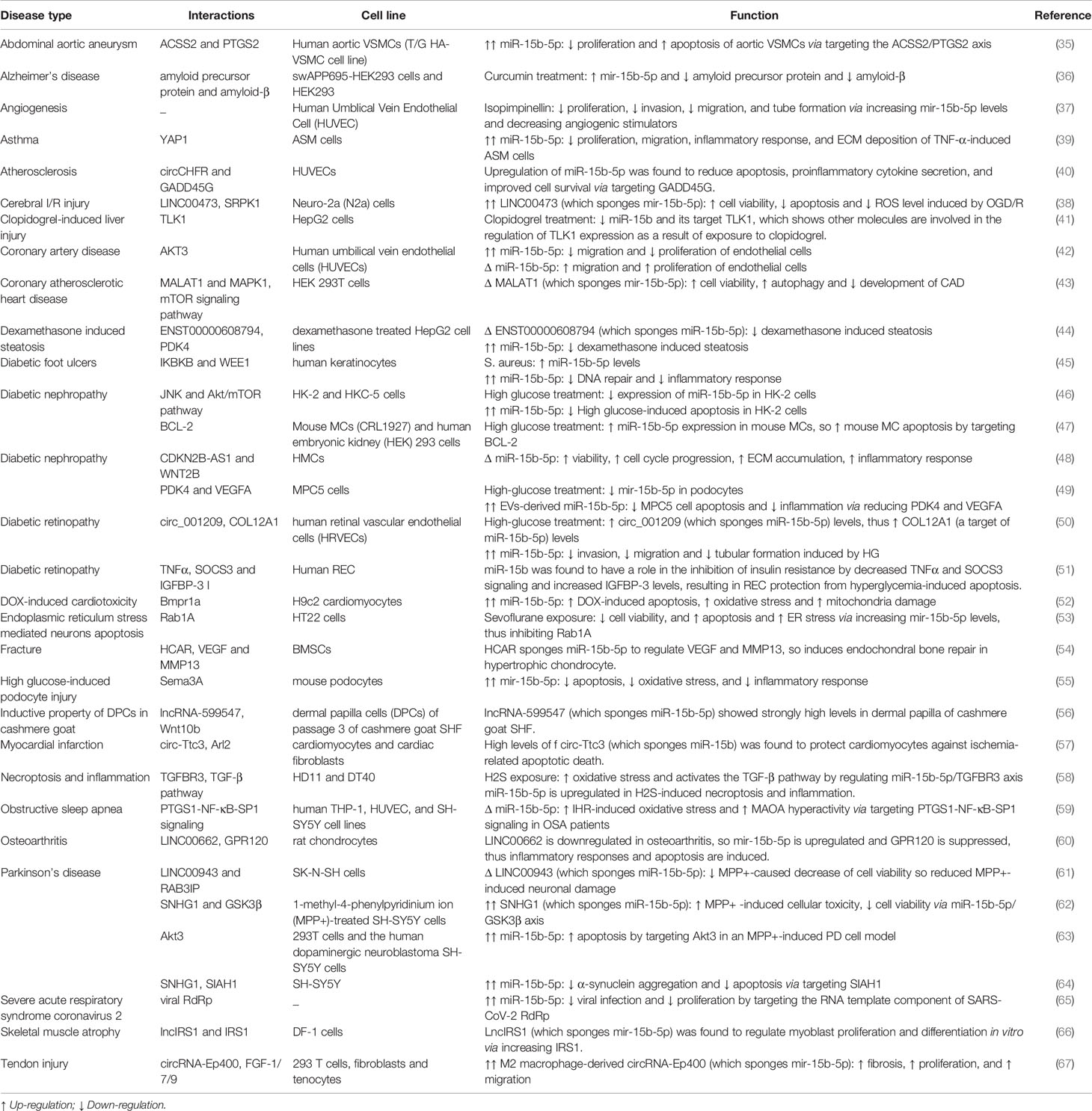
Table 4 Summary of cell line studies on the role of miR-15b-5p in non-malignant conditions (Δ, knock-down or deletion; DOX, doxorubicin; H2S, Hydrogen sulfide; HG, High glucose; SHF, secondary hair follicle; ER, endoplasmic reticulum; EVs, extracellular vesicles).
Animal Studies
Animal studies have highlighted the role of miR-15b-5p in different cellular processes and disorders such as angiogenesis, coronary artery disease, diabetic nephropathy, diabetic retinopathy, myocardial I/R injury, necroptosis and inflammation, Parkinson’s disease and trachea inflammatory injury (Table 5). For instance, overexpression of miR-15b-5p has considerably suppressed arteriogenesis and angiogenesis in animal models through targeting AKT3. Remarkably, siRNA-mediated silencing of AKT3 has inhibited arteriogenesis and the rescue of blood perfusion following femoral ligation in animals (42). Another animal study has shown that silencing of the miR-15b-5p-sponging lncRNA MALAT1 decreases atherosclerotic process (43). miR-15b-5p has also been shown to affect diabetic nephropathy and retinopathy in animals. Assessment of transcriptome of high glucose-exposed mouse mesangial cells has shown the effect of miR-15b-5p and its downstream target BCL-2 in regulation of high glocose-induced apoptosis. Besides, db/db mice has been shown to have higher levels of urinary miR-15b-5p (47).

Table 5 Summary of studies on the role of miR-15b-5p in non-malignant conditions (Δ, knock-down or deletion; MDA, malondialdehyde; ECs, endothelial cells; ACR, Albumin-to-Creatinine Ratio; H2S, Hydrogen sulfide).
Human Studies
Different experiments in human samples obtained from patients with acute mountain sickness, asthma-COPD overlap, coronary artery disease, diabetic foot ulcers, diabetic nephropathy, late pulmonary complications, obstructive sleep apnea and Parkinson’s disease have shown dysregulation of miR-15b-5p levels (Table 6).
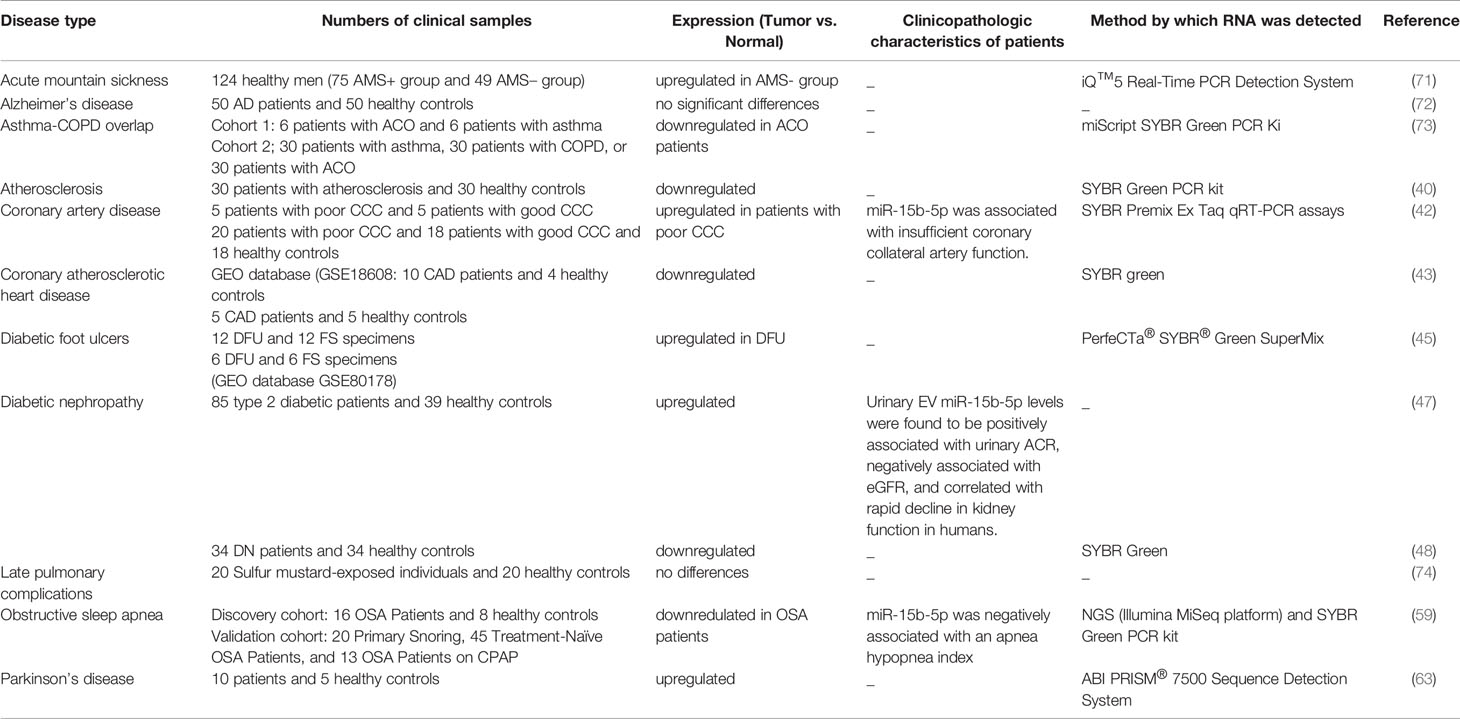
Table 6 Summary of human studies on the role of miR-15b-5p in non-malignant conditions (CAD, coronary atherosclerotic heart disease; CCC, coronary collateral circulation; ACR, albumin-to-creatinine ratio; eGFR, Estimated Glomerular Filtration Rate; AMS, Acute mountain sickness; COPD, chronic obstructive pulmonary disease; ACO, asthma-COPD overlap; DN, diabetic nephropathy; OSA, obstructive sleep apnea; CPAP, continuous positive airway pressure; DFU, Diabetic foot ulcers; FS, foot skin).
This miRNA might participate in the pathoetiology of acute mountain sickness. Levels of miR-15b-5p in the saliva have been found to be higher in individuals being resistant to this condition compared to susceptible ones. Combination of levels of miR-134-3p and miR-15b-5p could discriminate between these two groups. Thus, salivary levels of miR-134-3p and miR-15b-5p have been suggested as non-invasive markers for prediction of acute mountain sickness prior to exposure to high altitude (71).
Although in vitro studies indicated possible role of miR-15b-5p in the pathogenesis of Alzheimer’s disease (36), serum levels of miR-15b-5p were not significantly different between patients with Alzheimer’s disease and healthy subjects (72).
miR-15b-5p has been among miRNA having lower expression in asthma-COPD overlap patients. This miRNA can distinguish between asthma-COPD overlap patients and individuals with either asthma or COPD. In fact, miR-15b-5p has been shown to be superior to other miRNAs in separation of patients with asthma-COPD overlap from similar conditions (73).
In some conditions, dysregulation of this miRNA has been associated with clinicopathological parameters. For instance, in patients with coronary artery disease, dysregulation of miR-15b-5p has been associated with insufficient coronary collateral artery function (42). Moreover, in diabetic nephropathy, Urinary exosomal levels of miR-15b-5p have been positively associated with urinary albumin-to-creatinine ratio, negatively associated with eGFR, and correlated with speedy failure in kidney function (47).
Discussion
miR-15b-5p is an example of miRNAs with dual roels in the carcinogenesis. While it is a putative oncogenic miRNA in bladder cancer, breast cancer, gastric cancer, oral squamous cell carcinoma and prostate cancer, it has been found to be down-regulated in head and neck cancer squamous cell carcinomas, neublastoma and thyroid cancer samples as compared with corresponding non-cancerous samples (75). Moreover, in colorectal cancer and hepatocellular carcinoma, different studies have reported contradictory results.
This miRNA also participates in the pathogenesis of several non-malignant conditions, such as abdominal aortic aneurysm, Alzheimer’s disease, Parkinson’s disease, cerebral I/R injury, coronary artery disease, dexamethasone induced steatosis, diabetic complications and doxorubicin-induced cardiotoxicity.
miR-15b-5p has been shown to be sponged by several lncRNAs, namely MAGI2-AS3, H19, SNHG1, SNHG16, TTN‐AS1, PVT1, FENDRR, SSTR5−AS1, MALAT1, ENST00000608794, CDKN2B-AS1, LINC00473, LINC00662, LINC00943, LncRNA-599547 and CDKN2B-AS1 as well as the circular RNA Circ_001209. Thus, lncRNAs and circRNAs can affect expression of this miRNA. Other possible regulatory mechanisms for modulation of expression levels of miR-15b-5p should be clarified in future studies.
NF-κB, STAT3, AKT/mTORC1, CDC42/PAK1 and β-catenin signaling pathways are signaling pathways that mediate the effects of miR-15b-5p in the carcinogenesis. Notably, this miRNA could regulate response of cancer cells to 5-FU and anti-PD-1 drugs. Thus, therapeutics modalities affecting expression of miR-15b-5p can be considered as possible ways to combat resistance to anti-cancer agents. Evidence from in vitro and in vivo studies indicates that therapeutic intervention with miR-15-5p levels can significantly influence pathological processes. Moreover, disease-associated abnormal expression pattern of this miRNA in the affected tissues potentiates it as a diagnostic biomarkers. Particularly, in bladder cancer, breast cancer, head and neck cancers, liver cancer, neuroblastoma, oral squamous cell carcinoma and thyroid cancer, abnormal expression of miR-15-5p has been associated with poor clinical outcomes indicating the role of this miRNA as a prognostic biomarker. It is expected that therapeutic modalities affect expression of miR-15-5p and amend disease-associated dysregulation of this miRNA. Therefore, expression pattern of miR-15-5p can be used to monitor disease status and response to therapeutic options.
Since both oncogenic and tumor suppressor roles have been reported for miR-15-5p, different miR-15-5p-targeting therapeutic targets can been applied in the field of cancer therapy. In tissues that this miRNA exerts tumor suppressor roles, exogenous miR-15-5p can be used to inhibit cell proliferation or induce apoptosis. This goal can be achieved by administration of chemically synthesized miR-15-5p mimics to induce expression of endogenous mature double-stranded miR-15-5p to restore function of this miRNA. Viral vectors expressing miR-15-5p are appropriate vectors for delivery of this miRNA to tumor cells. On the other hand, when miR-15-5p exerts oncogenic roles, antisense oligonucleotides and miR-15-5p sponges can be used for suppression of level of this miRNA. Although these strategies are putative therapeutic modalities for treatment of cancer, they have not been applied in the clinical setting yet.
Conclusion
While the prognostic impact of dysregulation of miR-15b-5p has been confirmed in different types of cancer, there is no explicit evidence for application of this miRNA as a diagnostic marker in cancers. Since miRNAs dysregulation in the circulation provides a potential way for early non-invasive diagnosis of cancer, future studies should focus on evaluation of expression levels of miR-15b-5p in different biofluids during the course of cancer to provide insights into diagnostic role of this miRNA in cancer.
Author Contributions
SG-F wrote the manuscript and revised it. MT supervised and designed the study. TK, HJ, MH and BH collected the data and designed the figures and tables. All authors read and approved the submitted version.
Funding
This study was financially supported by Grant from Medical School of Shahid Beheshti University of Medical Sciences.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hussen BM, Hidayat HJ, Salihi A, Sabir DK, Taheri M, Ghafouri-Fard S. MicroRNA: A Signature for Cancer Progression. Biomed Pharmacother = Biomed Pharmacother (2021) 138:111528. doi: 10.1016/j.biopha.2021.111528
2. Ghafouri-Fard S, Shaterabadi D, Abak A, Shoorei H, Bahroudi Z, Taheri M, et al. An Update on the Role of miR-379 in Human Disorders. Biomed Pharmacother = Biomed Pharmacother (2021) 139:111553. doi: 10.1016/j.biopha.2021.111553
3. Ha M, Kim VN. Regulation of microRNA Biogenesis. Nat Rev Mol Cell Biol (2014) 15(8):509–24. doi: 10.1038/nrm3838
4. Wang F, Zu Y, Zhu S, Yang Y, Huang W, Xie H, et al. Long Noncoding RNA MAGI2-AS3 Regulates CCDC19 Expression by Sponging miR-15b-5p and Suppresses Bladder Cancer Progression. Biochem Biophys Res Commun (2018) 507(1-4):231–5. doi: 10.1016/j.bbrc.2018.11.013
5. Wu B, Liu G, Jin Y, Yang T, Zhang D, Ding L, et al. miR-15b-5p Promotes Growth and Metastasis in Breast Cancer by Targeting HPSE2. Front Oncol (2020) 10:108. doi: 10.3389/fonc.2020.00108
6. Zhu Y, Zhang X, Wang L, Zhu X, Xia Z, Xu L, et al. FENDRR Suppresses Cervical Cancer Proliferation and Invasion by Targeting miR-15a/B-5p and Regulating TUBA1A Expression. Cancer Cell Int (2020) 20(1):1–10. doi: 10.1186/s12935-020-01223-w
7. Gasparello J, Gambari L, Papi C, Rozzi A, Manicardi A, Corradini R, et al. High Levels of Apoptosis are Induced in the Human Colon Cancer HT-29 Cell Line by Co-Administration of Sulforaphane and a Peptide Nucleic Acid Targeting miR-15b-5p. Nucleic Acid Ther (2020) 30(3):164–74. doi: 10.1089/nat.2019.0825
8. Sun L-N, Zhi Z, Chen L-Y, Zhou Q, Li X-M, Gan W-J, et al. SIRT1 Suppresses Colorectal Cancer Metastasis by Transcriptional Repression of miR-15b-5p. Cancer Lett (2017) 409:104–15. doi: 10.1016/j.canlet.2017.09.001
9. Zhao C, Zhao Q, Zhang C, Wang G, Yao Y, Huang X, et al. miR-15b-5p Resensitizes Colon Cancer Cells to 5-Fluorouracil by Promoting Apoptosis via the NF-κb/XIAP Axis. Sci Rep (2017) 7(1):1–12. doi: 10.1038/s41598-017-04172-z
10. Liu C, Liu R, Wang B, Lian J, Yao Y, Sun H, et al. Blocking IL-17A Enhances Tumor Response to Anti-PD-1 Immunotherapy in Microsatellite Stable Colorectal Cancer. J Immunother Cancer (2021) 9(1):1–14. doi: 10.1136/jitc-2020-001895
11. Zhao SY, Wang Z, Wu XB, Zhang S, Chen Q, Wang DD, et al. CERS6-AS1 Contributes to the Malignant Phenotypes of Colorectal Cancer Cells by Interacting With miR-15b-5p to Regulate SPTBN2. Kaohsiung J Med Sci (2022) 38(5):403–414. doi: 10.1002/kjm2.12503.
12. Zhao C, Li Y, Chen G, Wang F, Shen Z, Zhou R. Overexpression of miR-15b-5p Promotes Gastric Cancer Metastasis by Regulating PAQR3. Oncol Rep (2017) 38(1):352–8. doi: 10.3892/or.2017.5673
13. Gasparello J, Papi C, Zurlo M, Gambari L, Rozzi A, Manicardi A, et al. Treatment of Human Glioblastoma U251 Cells With Sulforaphane and a Peptide Nucleic Acid (PNA) Targeting miR-15b-5p: Synergistic Effects on Induction of Apoptosis. Molecules (2022) 27(4):1299. doi: 10.3390/molecules27041299
14. Zhou Y, Fan R-G, Qin C-L, Jia J, Wu X-D, Zha W-Z. LncRNA-H19 Activates CDC42/PAK1 Pathway to Promote Cell Proliferation, Migration and Invasion by Targeting miR-15b in Hepatocellular Carcinoma. Genomics (2019) 111(6):1862–72. doi: 10.1016/j.ygeno.2018.12.009
15. Yang Y, Hou N, Wang X, Wang L, Se C, He K, et al. miR-15b-5p Induces Endoplasmic Reticulum Stress and Apoptosis in Human Hepatocellular Carcinoma, Both In Vitro and In Vivo, by Suppressing Rab1A. Oncotarget (2015) 6(18):16227. doi: 10.18632/oncotarget.3970
16. Yu F, Lin Y, Tan G, Ai M, Gong H, Liu W, et al. Tumor-Derived Exosomal microRNA-15b-5p Augments Laryngeal Cancer by Targeting TXNIP. Cell Cycle (2022) 7:1–11. doi: 10.1080/15384101.2021.2022845
17. Chava S, Reynolds CP, Pathania AS, Gorantla S, Poluektova LY, Coulter DW, et al. miR-15a-5p, miR-15b-5p, and miR-16-5p Inhibit Tumor Progression by Directly Targeting MYCN in Neuroblastoma. Mol Oncol (2020) 14(1):180–96. doi: 10.1002/1878-0261.12588
18. Ge Y, Tan S, Bi J, Rao M, Yu Y, Tian L. SNHG16 Knockdown Inhibits Tumorigenicity of Neuroblastoma in Children via miR-15b-5p/PRPS1 Axis. NeuroReport (2020) 31(17):1225–35. doi: 10.1097/WNR.0000000000001537
19. Guo K, Qi D, Huang B. LncRNA MEG8 Promotes NSCLC Progression by Modulating the miR-15a-5p-miR-15b-5p/PSAT1 Axis. Cancer Cell Int (2021) 21(1):1–16. doi: 10.1186/s12935-021-01772-8
20. Liu X, Dong Y, Song D. Inhibition of microRNA-15b-5p Attenuates the Progression of Oral Squamous Cell Carcinoma via Modulating the PTPN4/STAT3 Axis. Cancer Manage Res (2020) 12:10559. doi: 10.2147/CMAR.S272498
21. Wang X, Guo H, Yao B, Helms J. miR-15b Inhibits Cancer-Initiating Cell Phenotypes and Chemoresistance of Cisplatin by Targeting TRIM14 in Oral Tongue Squamous Cell Cancer. Oncol Rep (2017) 37(5):2720–6. doi: 10.3892/or.2017.5532
22. Weng Y, Shen Y, He Y, Pan X, Xu J, Jiang Y, et al. The miR-15b-5p/PDK4 Axis Regulates Osteosarcoma Proliferation Through Modulation of the Warburg Effect. Biochem Biophys Res Commun (2018) 503(4):2749–57. doi: 10.1016/j.bbrc.2018.08.035
23. Cai Y, Yang Y, Zhang X, Ma Q, Li M. TRPM2-AS Promotes the Malignancy of Osteosarcoma Cells by Targeting miR-15b-5p/PPM1D Axis. Cell Cycle (2022) 8:1–16. doi: 10.1080/15384101.2022.2033414
24. Miao S, Wang J, Xuan L, Liu X. LncRNA TTN-AS1 Acts as Sponge for miR-15b-5p to Regulate FBXW7 Expression in Ovarian Cancer. BioFactors (2020) 46(4):600–7. doi: 10.1002/biof.1622
25. Chen R, Sheng L, Zhang HJ, Ji M, Qian WQ. miR-15b-5p Facilitates the Tumorigenicity by Targeting RECK and Predicts Tumour Recurrence in Prostate Cancer. J Cell Mol Med (2018) 22(3):1855–63. doi: 10.1111/jcmm.13469
26. Sun F, Wu K, Yao Z, Mu X, Zheng Z, Sun M, et al. Long Noncoding RNA PVT1 Promotes Prostate Cancer Metastasis by Increasing NOP2 Expression via Targeting Tumor Suppressor MicroRNAs. OncoTargets Ther (2020) 13:6755. doi: 10.2147/OTT.S242441
27. Zou J, Qian J, Fu H, Yin F, Zhao W, Xu L. MicroRNA−15b−5p Exerts its Tumor Repressive Role via Targeting GDI2: A Novel Insight Into the Pathogenesis of Thyroid Carcinoma. Mol Med Rep (2020) 22(4):2723–32. doi: 10.3892/mmr.2020.11343
28. Lovat F, Fassan M, Gasparini P, Rizzotto L, Cascione L, Pizzi M, et al. miR-15b/16-2 Deletion Promotes B-Cell Malignancies. Proc Natl Acad Sci USA (2015) 112(37):11636–41. doi: 10.1073/pnas.1514954112
29. Tölle A, Buckendahl L, Jung K. Plasma Mir−15b−5p and Mir−590−5p for Distinguishing Patients With Bladder Cancer From Healthy Individuals. Oncol Rep (2019) 42(4):1609–20. doi: 10.3892/or.2019.7247
30. Ahmad P, Sana J, Slávik M, Gurín D, Radová L, Gablo NA, et al. MicroRNA-15b-5p Predicts Locoregional Relapse in Head and Neck Carcinoma Patients Treated With Intensity-Modulated Radiotherapy. Cancer Genomics Proteomics (2019) 16(2):139–46. doi: 10.21873/cgp.20119
31. Pan WY, Zeng JH, Wen DY, Wang JY, Wang PP, Chen G, et al. Oncogenic Value of microRNA−15b−5p in Hepatocellular Carcinoma and a Bioinformatics Investigation. Oncol Lett (2019) 17(2):1695–713. doi: 10.3892/ol.2018.9748
32. Chen Y, Chen J, Liu Y, Li S, Huang P. Plasma miR-15b-5p, miR-338-5p, and miR-764 as Biomarkers for Hepatocellular Carcinoma. Med Sci Monit (2015) 21:1864. doi: 10.12659/MSM.893082
33. Xu J, Zhang J, Shan F, Wen J, Wang Y. SSTR5−AS1 Functions as a ceRNA to Regulate CA2 by Sponging Mir−15b−5p for the Development and Prognosis of HBV−Related Hepatocellular Carcinoma. Mol Med Rep (2019) 20(6):5021–31. doi: 10.3892/mmr.2019.10736
34. Jin X, Chen Y, Chen H, Fei S, Chen D, Cai X, et al. Evaluation of Tumor-Derived Exosomal miRNA as Potential Diagnostic Biomarkers for Early-Stage Non–Small Cell Lung Cancer Using Next-Generation Sequencing. Clin Cancer Res (2017) 23(17):5311–9. doi: 10.1158/1078-0432.CCR-17-0577
35. Gan S, Mao J, Pan Y, Tang J, Qiu Z. Hsa−Mir−15b−5p Regulates the Proliferation and Apoptosis of Human Vascular Smooth Muscle Cells by Targeting the ACSS2/PTGS2 Axis. Exp Ther Med (2021) 22(5):1–8. doi: 10.3892/etm.2021.10642
36. Liu H-Y, Fu X, Li Y-F, Li X-L, Ma Z-Y, Zhang Y, et al. miR-15b-5p Targeting Amyloid Precursor Protein Is Involved in the Anti-Amyloid Eflect of Curcumin in Swapp695-HEK293 Cells. Neural Regen Res (2019) 14(9):1603. doi: 10.4103/1673-5374.255979
37. Bhagavatheeswaran S, Ramachandran V, Shanmugam S, Balakrishnan A. Isopimpinellin Extends Antiangiogenic Effect Through Overexpression of miR-15b-5p and Downregulating Angiogenic Stimulators. Mol Biol Rep (2021) 49(1):279–91. doi: 10.1007/s11033-021-06870-4.
38. Yao B, Ye L, Chen J, Zhuo S, Lin H. LINC00473 Protects Against Cerebral Ischemia Reperfusion Injury via Sponging miR-15b-5p and miR-15a-5p to Regulate SRPK1 Expression. Brain Injury (2021) 35(11):1462–71. doi: 10.1080/02699052.2021.1972156
39. Zeng S, Cui J, Zhang Y, Zheng Z, Meng J, Du J. MicroRNA-15b-5p Inhibits Tumor Necrosis Factor Alpha-Induced Proliferation, Migration, and Extracellular Matrix Production of Airway Smooth Muscle Cells via Targeting Yes-Associated Protein 1. Bioengineered (2022) 13(3):5396–406. doi: 10.1080/21655979.2022.2036890
40. Li Y, Wang B. Circular RNA circCHFR Downregulation Protects Against Oxidized Low-Density Lipoprotein-Induced Endothelial Injury via Regulation of microRNA-15b-5p/Growth Arrest and DNA Damage Inducible Gamma. Bioengineered (2022) 13(2):4481–92. doi: 10.1080/21655979.2022.2032967
41. Freitas RC, Bortolin RH, Lopes MB, Tamborlin L, Meneguello L, Silbiger VN, et al. Modulation of miR-26a-5p and miR-15b-5p Exosomal Expression Associated With Clopidogrel-Induced Hepatotoxicity in HepG2 Cells. Front Pharmacol (2017) 8:906. doi: 10.3389/fphar.2017.00906
42. Zhu L-P, Zhou J-P, Zhang J-X, Wang J-Y, Wang Z-Y, Pan M, et al. MiR-15b-5p Regulates Collateral Artery Formation by Targeting AKT3 (Protein Kinase B-3). Arterioscler Thromb Vasc Biol (2017) 37(5):957–68. doi: 10.1161/ATVBAHA.116.308905
43. Zhu Y, Yang T, Duan J, Mu N, Zhang T. MALAT1/miR-15b-5p/MAPK1 Mediates Endothelial Progenitor Cells Autophagy and Affects Coronary Atherosclerotic Heart Disease via mTOR Signaling Pathway. Aging (Albany NY) (2019) 11(4):1089. doi: 10.18632/aging.101766
44. Liu F, Chen Q, Chen F, Wang J, Gong R, He B. The lncRNA ENST00000608794 Acts as a Competing Endogenous RNA to Regulate PDK4 Expression by Sponging miR-15b-5p in Dexamethasone Induced Steatosis. Biochim Biophys Acta (BBA)-Molecular Cell Biol Lipids (2019) 1864(10):1449–57. doi: 10.1016/j.bbalip.2019.07.003
45. Ramirez HA, Pastar I, Jozic I, Stojadinovic O, Stone RC, Ojeh N, et al. Staphylococcus Aureus Triggers Induction of miR-15B-5P to Diminish DNA Repair and Deregulate Inflammatory Response in Diabetic Foot Ulcers. J Invest Dermatol (2018) 138(5):1187–96. doi: 10.1016/j.jid.2017.11.038
46. Shen H, Fang K, Guo H, Wang G. High Glucose-Induced Apoptosis in Human Kidney Cells was Alleviated by miR-15b-5p Mimics. Biol Pharm Bull (2019) 42(5):758–63. doi: 10.1248/bpb.b18-00951
47. Tsai Y-C, Kuo M-C, Hung W-W, Wu L-Y, Wu P-H, Chang W-A, et al. High Glucose Induces Mesangial Cell Apoptosis Through miR-15b-5p and Promotes Diabetic Nephropathy by Extracellular Vesicle Delivery. Mol Ther (2020) 28(3):963–74. doi: 10.1016/j.ymthe.2020.01.014
48. Chang J, Yu Y, Fang Z, He H, Wang D, Teng J, et al. Long non-Coding RNA CDKN2B-AS1 Regulates High Glucose-Induced Human Mesangial Cell Injury via Regulating the miR-15b-5p/WNT2B Axis. Diabetol Metab Syndrome (2020) 12(1):1–11. doi: 10.1186/s13098-020-00618-z
49. Zhao T, Jin Q, Kong L, Zhang D, Teng Y, Lin L, et al. microRNA-15b-5p Shuttled by Mesenchymal Stem Cell-Derived Extracellular Vesicles Protects Podocytes From Diabetic Nephropathy via Downregulation of VEGF/PDK4 Axis. J Bioenerg Biomembr (2021) 54:17–30. doi: 10.1007/s10863-021-09919-y
50. Li B, Zhang G, Wang Z, Yang Y, Wang C, Fang D, et al. C-Myc-Activated USP2-AS1 Suppresses Senescence and Promotes Tumor Progression via Stabilization of E2F1 mRNA. Cell Death Dis (2021) 12(11):1–14. doi: 10.1038/s41419-021-04330-2
51. Ye E-A, Steinle JJ. miR-15b/16 Protects Primary Human Retinal Microvascular Endothelial Cells Against Hyperglycemia-Induced Increases in Tumor Necrosis Factor Alpha and Suppressor of Cytokine Signaling 3. J Neuroinflammation (2015) 12(1):1–8. doi: 10.1186/s12974-015-0265-0
52. Gao ZF, Ji XL, Gu J, Wang XY, Ding L, Zhang H. microRNA-107 Protects Against Inflammation and Endoplasmic Reticulum Stress of Vascular Endothelial Cells via KRT1-Dependent Notch Signaling Pathway in a Mouse Model of Coronary Atherosclerosis. J Cell Physiol (2019) 234(7):12029–41. doi: 10.1002/jcp.27864
53. Li Y, Xia H, Chen L, Zhang X. Sevoflurane Induces Endoplasmic Reticulum Stress Mediated Apoptosis Inmouse Hippocampal Neuronal HT22 Cells via Modulating miR-15b-5p/Rab1A Signaling Pathway. Int J Clin Exp Pathol (2017) 10(8):8270–80.
54. Bai Y, Gong X, Dong R, Cao Z, Dou C, Liu C, et al. Long non-Coding RNA HCAR Promotes Endochondral Bone Repair by Upregulating VEGF and MMP13 in Hypertrophic Chondrocyte Through Sponging miR-15b-5p. Genes Dis (2020). doi: 10.1016/j.gendis.2020.07.013
55. Fu Y, Wang C, Zhang D, Chu X, Zhang Y, Li J. miR-15b-5p Ameliorated High Glucose-Induced Podocyte Injury Through Repressing Apoptosis, Oxidative Stress, and Inflammatory Responses by Targeting Sema3A. J Cell Physiol (2019) 234(11):20869–78. doi: 10.1002/jcp.28691
56. Yin RH, Zhao SJ, Wang ZY, Zhu YB, Yin RL, Bai M, et al. LncRNA-599547 Contributes the Inductive Property of Dermal Papilla Cells in Cashmere Goat Through miR-15b-5p/Wnt10b Axis. Anim Biotechnol (2020) 14:1–15. doi: 10.1080/10495398.2020.1806860
57. Cai L, Qi B, Wu X, Peng S, Zhou G, Wei Y, et al. Circular RNA Ttc3 Regulates Cardiac Function After Myocardial Infarction by Sponging miR-15b. J Mol Cell Cardiol (2019) 130:10–22. doi: 10.1016/j.yjmcc.2019.03.007
58. Qianru C, Xueyuan H, Bing Z, Qing Z, Kaixin Z, Shu L. Regulation of H2S-Induced Necroptosis and Inflammation in Broiler Bursa of Fabricius by the miR-15b-5p/TGFBR3 Axis and the Involvement of Oxidative Stress in This Process. J Hazard Mater (2021) 406:124682. doi: 10.1016/j.jhazmat.2020.124682
59. Chen Y-C, Hsu P-Y, Su M-C, Chen T-W, Hsiao C-C, Chin C-H, et al. MicroRNA Sequencing Analysis in Obstructive Sleep Apnea and Depression: Anti-Oxidant and MAOA-Inhibiting Effects of miR-15b-5p and miR-92b-3p Through Targeting PTGS1-NF-κb-SP1 Signaling. Antioxidants (2021) 10(11):1854. doi: 10.3390/antiox10111854
60. Lu M, Zhou E. Long Noncoding RNA LINC00662-miR-15b-5p Mediated GPR120 Dysregulation Contributes to Osteoarthritis. Pathol Int (2020) 70(3):155–65. doi: 10.1111/pin.12875
61. Meng C, Gao J, Ma Q, Sun Q, Qiao T. LINC00943 Knockdown Attenuates MPP+-Induced Neuronal Damage via miR-15b-5p/RAB3IP Axis in SK-N-SH Cells. Neurol Res (2021) 43(3):181–90. doi: 10.1080/01616412.2020.1834290
62. Xie N, Qi J, Li S, Deng J, Chen Y, Lian Y. Upregulated lncRNA Small Nucleolar RNA Host Gene 1 Promotes 1-Methyl-4-Phenylpyridinium Ion-Induced Cytotoxicity and Reactive Oxygen Species Production Through miR-15b-5p/GSK3β Axis in Human Dopaminergic SH-SY5Y Cells. J Cell Biochem (2019) 120(4):5790–801. doi: 10.1002/jcb.27865
63. Zhu J, Xu X, Liang Y, Zhu R. Downregulation of microRNA-15b-5p Targeting the Akt3-Mediated GSK-3β/β-Catenin Signaling Pathway Inhibits Cell Apoptosis in Parkinson’s Disease. BioMed Res Int (2021) 2021:8814862. doi: 10.1155/2021/8814862
64. Cory-Slechta D, Allen J, Conrad K, Marvin E, Sobolewski M. Developmental Exposure to Low Level Ambient Ultrafine Particle Air Pollution and Cognitive Dysfunction. Neurotoxicology (2018) 69:217–31. doi: 10.1016/j.neuro.2017.12.003
65. Sato A, Ogino Y, Tanuma S-i, Uchiumi F. Human microRNA hsa-miR-15b-5p Targets the RNA Template Component of the RNA-Dependent RNA Polymerase Structure in Severe Acute Respiratory Syndrome Coronavirus 2. Nucleosides Nucleotides Nucleic Acids (2021) 40(8):790–7. doi: 10.1080/15257770.2021.1950759
66. Li Z, Cai B, Abdalla BA, Zhu X, Zheng M, Han P, et al. LncIRS1 Controls Muscle Atrophy via Sponging miR-15 Family to Activate IGF1-PI3K/AKT Pathway. J Cachexia Sarcopenia Muscle (2019) 10(2):391–410. doi: 10.1002/jcsm.12374
67. Yu Y, Sun B, Wang Z, Yang M, Cui Z, Lin S, et al. Exosomes From M2 Macrophage Promote Peritendinous Fibrosis Posterior Tendon Injury via the MiR-15b-5p/FGF-1/7/9 Pathway by Delivery of circRNA-Ep400. Front Cell Dev Biol (2021) 1557. doi: 10.3389/fcell.2021.595911
68. Wang F, Zhang M. Circ_001209 Aggravates Diabetic Retinal Vascular Dysfunction Through Regulating miR-15b-5p/COL12A1. J Trans Med (2021) 19(1):1–12. doi: 10.1186/s12967-021-02949-5
69. Niu S, Xu L, Yuan Y, Yang S, Ning H, Qin X, et al. Effect of Down-Regulated miR-15b-5p Expression on Arrhythmia and Myocardial Apoptosis After Myocardial Ischemia Reperfusion Injury in Mice. Biochem Biophys Res Commun (2020) 530(1):54–9. doi: 10.1016/j.bbrc.2020.06.111
70. Song N, Wang W, Wang Y, Guan Y, Xu S, Guo M-y. Hydrogen Sulfide of Air Induces Macrophage Extracellular Traps to Aggravate Inflammatory Injury via the Regulation of miR-15b-5p on MAPK and Insulin Signals in Trachea of Chickens. Sci Total Environment (2021) 771:145407. doi: 10.1016/j.scitotenv.2021.145407
71. Huang H, Dong H, Zhang J, Ke X, Li P, Zhang E, et al. The Role of Salivary miR-134-3p and miR-15b-5p as Potential Non-Invasive Predictors for Not Developing Acute Mountain Sickness. Front Physiol (2019) 10:898. doi: 10.3389/fphys.2019.00898
72. Poursaei E, Abolghasemi M, Bornehdeli S, Shanehbandi D, Asadi M, Sadeghzadeh M, et al. Evaluation of Hsa-Let-7d-P, Hsa-Let-7g-5p and hsa-miR-15b-5p Level in Plasma of Patients With Alzheimer’s Disease Compared to Healthy Controls. Psychiatr Genet (2022). doi: 10.1097/YPG.0000000000000303
73. Hirai K, Shirai T, Shimoshikiryo T, Ueda M, Gon Y, Maruoka S, et al. Circulating microRNA-15b-5p as a Biomarker for Asthma-COPD Overlap. Allergy (2021) 76(3):766–74. doi: 10.1111/all.14520
74. Salimi S, Noorbakhsh F, Faghihzadeh S, Ghaffarpour S, Ghazanfari T. Expression of miR-15b-5p, miR-21-5p, and SMAD7 in Lung Tissue of Sulfur Mustard-Exposed Individuals With Long-Term Pulmonary Complications. Iranian J Allergy Asthma Immunol (2019) 8:332–9. doi: 10.18502/ijaai.v18i3.1126
Keywords: miR-15b-5p, cancer, biomarker, expression, malignance
Citation: Ghafouri-Fard S, Khoshbakht T, Hussen BM, Jamal HH, Taheri M and Hajiesmaeili M (2022) A Comprehensive Review on Function of miR-15b-5p in Malignant and Non-Malignant Disorders. Front. Oncol. 12:870996. doi: 10.3389/fonc.2022.870996
Received: 07 February 2022; Accepted: 05 April 2022;
Published: 02 May 2022.
Edited by:
Ondrej Slaby, Central European Institute of Technology (CEITEC), CzechiaReviewed by:
Francesca Lovat, The Ohio State University, United StatesJie Li, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2022 Ghafouri-Fard, Khoshbakht, Hussen, Jamal, Taheri and Hajiesmaeili. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Taheri, bW9oYW1tYWQudGFoZXJpQHVuaS1qZW5hLmRl; Mohammadreza Hajiesmaeili, bXJoYWppZXNtYWVpbGlAc2JtdS5hYy5pcg==
 Soudeh Ghafouri-Fard
Soudeh Ghafouri-Fard Tayyebeh Khoshbakht
Tayyebeh Khoshbakht Bashdar Mahmud Hussen
Bashdar Mahmud Hussen Hazha Hadayat Jamal
Hazha Hadayat Jamal Mohammad Taheri
Mohammad Taheri Mohammadreza Hajiesmaeili8,9*
Mohammadreza Hajiesmaeili8,9*