- 1Department of Neurosurgery, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy
- 2University of Milan, Milan, Italy
- 3Alma Mater Studiorum University of Bologna, Bologna, Italy
- 4Neuropathology Unit, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy
- 5Radiotherapy Unit, Department of Radiosurgery, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy
- 6Neuroradiology Department, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy
- 7Department of Oncology and Hematology-Oncology, University of Milan, Milan, Italy
- 8Department of Neurological Surgery, Johns Hopkins Medical School, Baltimore, MD, United States
Angioleiomyoma (ALM) is a benign smooth muscle neoplasm that mainly occurs in lower extremities subcutaneous tissue and generally affects middle-aged adults. This tumor histotype may rarely localize intracranially, although only a few cases have been described in the literature. We report a case of intracranial ALM, whose differential diagnosis has been particularly challenging, and firstly provide a comprehensive radiological and intra-operative evaluation of a such rare entity. This represents also the first report of the use of intraoperative confocal microscopy in ALM and the first documented short-term recurrence. At this regard, a scoping literature review has been conducted with the aim of presenting the major clinical and diagnostic features along with the proposed therapeutic strategies.
Introduction
Angioleiomyoma (ALM), also called angiomyoma or vascular leiomyoma, is defined as a benign and indolent soft tissue neoplasm arising from smooth muscle cells. According to the 2016 central nervous system (CNS) tumors classification by World Health Organization (WHO), ALM is classified as a mesenchymal non-meningothelial brain tumor (1). On the contrary, in 2021 WHO classification of CNS tumors, the term “angioleiomyoma” is not mentioned because leiomyoma is now described in the soft tissue tumors category. Microscopically, the disease can be recognized by its pattern of intersecting fascicles, composed of eosinophilic spindle cells with blunt-ended nuclei (2). Considering the lack of mitotic activity and cytological atypia, ALM represents a benign neoplastic entity (2). Diffuse leptomeningeal leiomyoma and an angioleiomyomatous type represent disease variants and have been described in the literature (3). Epstein-Barr Virus (EBV) and AIDS-associated ALMs have also been reported (4). ALM is commonly located in the lower extremities and affects middle-aged adults and usually manifests as an isolated, painful, and solid mass (5). Although ALM may originate from the subcutaneous tissue of the trunk (6), visceral and mucosal locations have been reported (7). On the contrary, primitive intracranial ALM represents an exceedingly rare tumor. Since the first reported case by Lach et al. in 1994, 57 cases have been reported in the literature (8). From these reports, it emerged that primitive CNS ALM is mostly observed in women, with an age peak around the fourth decade (9). Both the imaging features and the intraoperative surgical considerations have been analyzed in the present paper, in which we present the case of a middle-aged woman with a history of fatigue and weakness in the right limbs. Brain magnetic resonance imaging (MRI) revealed the presence of a lesion located in the free left edge of the tentorium, which posed an indication for surgical resection.
Case report
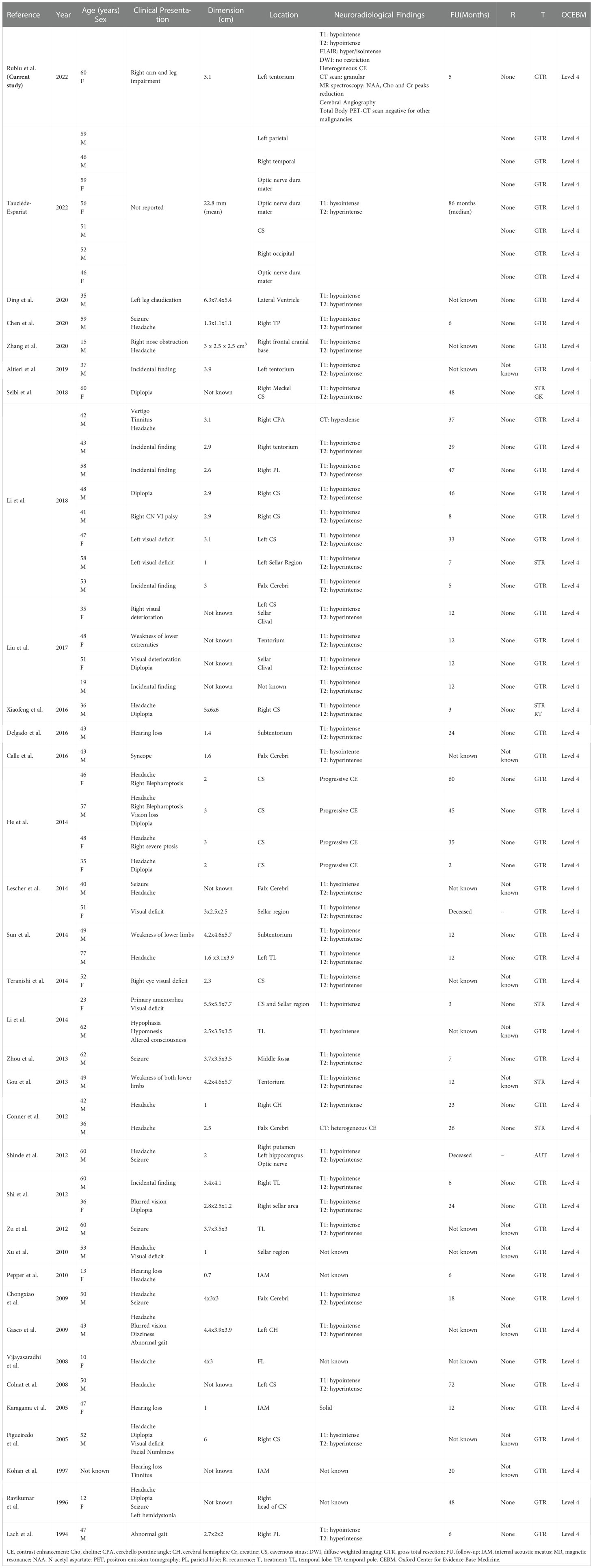
We present the case of a 60-year-old, right-handed, woman who suffered from a 9-months history of fatigue and weakness in the right limbs. Neurological examination revealed an ataxic gait, moderate right upper extremity dysmetria, and slight right hemiparesis. No previous history of CNS surgery or trauma was present, and no major comorbidities were reported. The patient underwent a preoperative brain MRI (Figures 1E-J) which disclosed a mass contiguous to the left tentorial free edge and extending into the ipsilateral thalamic and mesencephalic regions. The tumor was hypointense on T1-weighted images (WI) and on T2-WI (Figures 1H–J), and hyper/isointense on Fluid Attenuated Inversion Recovery (FLAIR) sequences. Postcontrast T1-WI showed heterogeneous enhancement of the lesion (Figures 1E–G), which was 31 millimeters in maximum diameter, and associated with moderate edema in the surrounding brain parenchyma. Magnetic Resonance spectroscopy (MRS) disclosed a reduction in N-acetyl-aspartate (NAA), and an increase in choline (Cho) and creatine (Cr) peaks with a reduction of Cho/NAA ratio in the tumor area, suggesting the glial nature of the lesion. In the suspicion of brain metastasis, a total body 18-Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography (18F-FDG PET-CT) scan was acquired, displaying an increased tracer metabolism in the lesion without evidencing any extracranial pathologic uptake. Moreover, a brain computed tomography (CT) scan showed granular calcifications inside the lesion. Taking into consideration the neuroradiological findings, surgery was thought to be appropriate to both reduce the mass effect and make a definitive histological diagnosis. In the operating room, the patient was positioned supine and, under microscopic guidance, we decided to approach the lesion through a transtemporal tranventricular route in order to gain a wider control on both the lesion and its vascularization. The lesion was encountered in the left tentorial hiatus and appeared as an extraparenchymal, red and capsular mass with an arterialized surface (Figure 2A). The mass displayed a dense consistency and extended into the mesencephalic-thalamic region, occupying both the crural and ambient cisterna. At the beginning of the resection procedure, an excessive bleeding from the vascularised surface occurred, thus leading to an immediate interruption of the procedure. Postoperatively a brain Digital Subtraction Angiography (DSA) was performed (Figures 1A–D) and the presence of a thrombosed giant aneurysm was excluded. Moreover, DSA showed a delayed and intense arterio-venous blush fed by the P2 tract of posterior cerebral artery (PCA) and by superior cerebellar artery (SCA) afferents. Given that embolization of the tumor was considered unsafe by our interventional neuroradiology team, we decided to proceed with a re-operation through the same previous surgical route. During the second surgical procedure, we achieved a subtotal removal of the tumor without any surgical complication. Although the tumor exhibited an intense and homogenous fluorescence, the use of the dedicated filter (YELLOW 560) was not necessary because the pathologic tissue was already recognizable for its high vascularization. On the contrary, In vivo intraoperative confocal microscopy was useful in confirming the presence of the pathologic tissue, showing the presence of high cellularity and vascularized lesion (Figure 2B). The tumor specimen underwent histological examination, which reported the presence of blood vessels, smooth muscle cells, and collagen tissue (Figure 3A). Immunochemistry disclosed positivity for actin protein on smooth muscle cells, for CD31 and CD34 on endothelial cells, and for vimentin on mesenchymal tissue. On the contrary, STAT6, GFAP, and EMA antigens were not detected. The Ki67 index ranged from 4 to 5% and no necrosis was identified. These characteristics were ultimately consistent with the diagnosis of leiomyoma. Postoperatively, the patient displayed mild expressive dysphasia and moderate right hemiparesis. (Figures 1K–M). The patient was then transferred to a rehabilitation setting on the fifth day after surgery and both speech disturbance and strength deficit gradually ameliorated. Considering the subtotal resection and the benign histopathological features, a 5-months brain MRI was programmed. When MRI was performed, a significant disease recurrence was disclosed (Figures 1N–P). At this regard, we think that the disease recurrence was mainly due to the presence of residual tumor left after the second surgical procedure. Therefore, after a multidisciplinary discussion and taking into consideration the risk associated with reoperation, the patient was referred to the radiation therapy specialist at our Institution. The patient has completed a full cycle of radiation therapy, without any other neurological deficit, and is now waiting for the follow-up MRI.
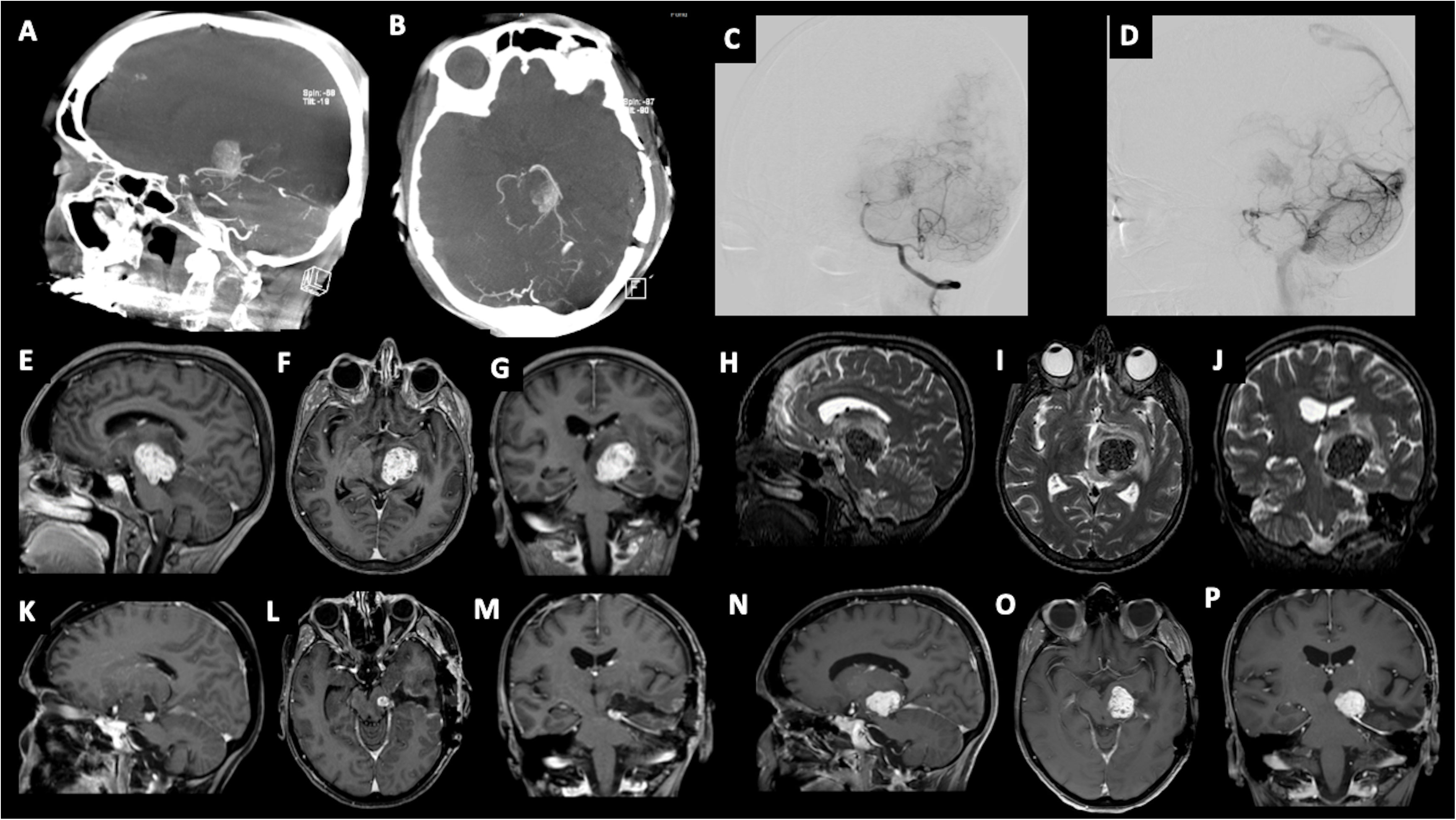
Figure 1 (A–D) Angiography imaging showing artero-venous blush lasting till late venous phase. The blush is fed by collaterals originating from P2 segment of the posterior cerebral artery (PCA), and left superior cerebellar artery (SCA). Preoperative post-contrast T1-weighted images in sagittal (E), axial (F), and coronal (G) views, showing the involvement of the tentorium hiatus and the extension to the left thalamo-mesencephalic region; Preoperative T2-weighted images in sagittal (H), axial (I), and coronal (J) views, showing the hypointensity of the tumor and the presence of perilesional edema. Post-operative post-contrast T1-weighted imaging showing residual tumor in sagittal (K), axial (L), and coronal views (M); (N-P) 5-month follow-up post-contrast T1-weighted imaging showing disease recurrence.
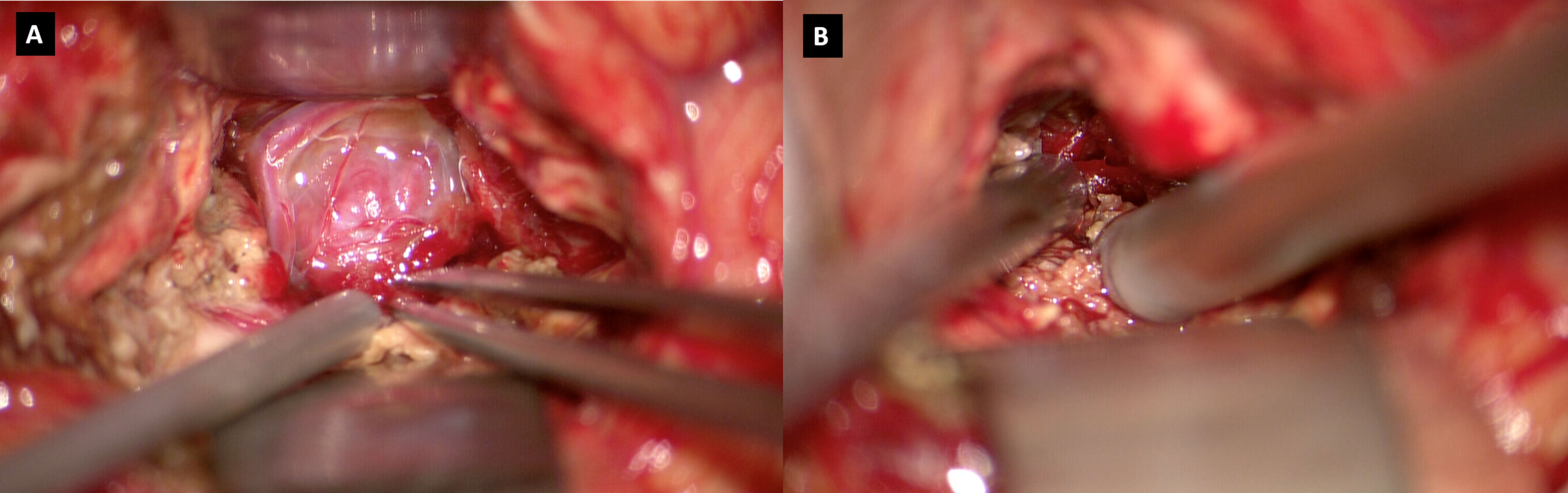
Figure 2 (A) Intraoperative visualization of the tumor, presenting as a hypervascularized mass in the tentorium hiatus; (B) Intraoperative use of in vivo confocal microscopy.
Figure 3 (A) H&H showing the presence of blood vessels, smooth muscle cells, and collagen tissue; (B) Confocal microscopy image showing neoplastic tissue characterized by significant neovascularization.
Material and methods
The case report has been described according to the CARE guidelines.
Surgery protocol
The standardized surgical protocol of fluorescein-guided technique is based on i.v. SF injection at standard dose of 5mg/kg, by a central or peripheral venous line, immediately upon completion of the induction of general anesthesia (10). The surgery was performed with the aid of a surgical microscope equipped with an integrated fluorescent filter tailored to the excitation and emission wavelength of sodium fluorescein (YELLOW 560 – Pentero 900; Carl Zeiss Meditec, Oberkochen, Germany). During resection, the microscope could be switched alternatively from fluorescent to white-light illumination. Intra-operative fluorescein-assisted miniatured confocal laser endomicroscopy (CONVIVO® system, Carl Zeiss, Meditec, Oberkochen, Germany) has been used.
Literature review search strategy
A literature review search has been performed with the aid of the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) statement guidelines, limited to the English language. SCOPUS, PubMed and Cochrane databases were queried using individual keywords and MeSH terms. A purposely defined search string was performed for PubMed, Scopus and Cochrane search: (“Leiomyoma”[Mesh]) AND “Central Nervous System”[Mesh], and for SCOPUS search: TITLE-ABS KEY (intracranial AND angioleiomyoma) AND (LIMIT TO (LANGUAGE, “English”)). The results were then limited to human subjects. After duplicate removal, title and abstracts were firstly screened and, for the papers deemed appropriate, full text was obtained and reviewed for appropriateness and extraction of data. Article references list was also examined to identify any other relevant study. Only studies dealing with the presence of intracranial ALM were included. Data from the included studies were extracted, organized, and analyzed. The qualitative assessment of the level of evidence of the papers extracted has been evaluated according to Oxford CEBM (11).
Results
The results of the scoping literature review are summarized in Table 1. The literature review was based on articles published between 1994 and 2020. Since Lach et al. first reported a case of ALM, in 1994, 57 cases of ALM have been described (8). Considering the reported cases, intracranial ALMs are more common in men, with a male/female ratio of 1.9:1 (37 males; 19 females). The average age was 45,6 years (range: from 10 to 62 years). The most frequent clinical presentation was headache, which was described in 40% of cases. 7 patients (14%) with ALM presented with seizures. In sellar or parasellar lesions (38%), diplopia (20%) and visual impairment (24%) represented other primary clinical manifestations. Other symptoms, according to the location of the tumor, were motor deficits (14%), hearing loss (8%), vertigo, and tinnitus (6%). 6 cases (12%) were asymptomatic. Concerning the neuroradiological findings, ALMs were described as hypointense on T1-WI and/or hyperintense in T2-WI in 34 patients (59%). The tumor appeared hysointense in T1WI in 11 cases (19%). Heterogeneous contrast enhancement on T1-WI was observed in 6 cases (10%). In one case the lesion was depicted as a “solid” mass (1%). Regarding disease location, the sites of occurrence in order of frequency were: cavernous sinus with invasion (30%), tentorium (14%), sellar region (10%), temporal lobe (12%), falx cerebri (10%), optic nerve dura mater (5%), parietal lobe (5%), frontal lobe (3%), lateral ventricle (2%), cerebellopontine angle (2%), and occipital region (1%). The mean period of follow-up was 21,4 months after treatment and recurrence was not reported in any case. The main treatment was gross total resection (GTR), which was performed in 43 cases (86%). GammaKnife and radiation therapy represented adjuvant therapies in one case (2%) of subtotal resection.
Discussion
CNS ALM represents a rare disease, and no common agreement exists on its diagnostic and surgical management. We provide a case report with a short-term recurrence and a thorough pre-operative and intra-operative illustration, with the aid of confocal microscopy. A scoping literature review is also presented to summarize and augment the level of evidence for the management of CNS ALM. ALM is a grayish-brown soft tissue tumor composed of vascular channels and stroma, in which loose smooth muscle bundles and collagen are housed (8, 12). Microscopically, thick-walled vessels are surrounded by fascicles of eosinophilic spindle cells (12). These histological features are confirmed by immunostaining through positivity to alpha-actin and h-caldesmin, which represent specific markers for smooth muscle cells. Histologic features and immunostaining may facilitate differential diagnosis between ALM and meningiomas, arteriovenous malformations, and solitary fibrous tumors. Although Hachisuga et al. (6) found mature fat cells within a specimen of intracranial ALM, the present case was characterized by the presence of blood vessels, smooth muscle cells, and collagen tissue, without any evidence of fat tissue. CNS ALM usually increases in size over a period of months to years before causing any clinical manifestation. Even when present, clinical manifestations are nonspecific and mostly related to the space-occupying mass. In our case, the tumor was responsible for a slight right hemiparesis due to its proximity to the left cerebral peduncle. Because of their uncommon presentation and atypical neuroradiologic features on CT and MRI, intracranial ALMs are often misdiagnosed (9). Differential neuroradiological diagnosis includes meningiomas, schwannomas, cavernous hemangioma, solitary fibrous tumors, and dural metastasis (9). ALM usually appears as a hyperintense or isointense lesion on T1WI and shows hyperintensity on T2WI. Postgadolinium enhancement is also featured. In our case, the tumor appeared hypointense on T1WI and T2WI and hyper/isointense on FLAIR images. Post-gadolinium scan showed an intense and heterogeneous contrast enhancement with moderate perilesional brain edema (Figure 1). The maximal tumor diameter was 31 millimeters. MRS highlighted a low NAA/Cho ratio in the pathological area, mistakenly suggesting a glial nature of the lesion. Similarly to other intracranial tumors such as meningiomas and gliomas, in the case of ALM surgical resection represents the cornerstone of therapy. In our case, we decided to approach the lesion through a transtemporal tranventricular route in order to gain a wider control on the lesion. Although a sub-temporal intradural approach could have been performed, we decided to not choose it because of its associated need to retract the dominant temporal lobe. Moreover, the transtemporal tranventricular transchoroidal approach gave us the opportunity to violate only part of the inferior temporal gyrus. Finally, the lateral supracerebellar transtentorial approach was not performed because it could not allow a complete control of the vascular structures. The lesion was then identified in the left tentorial hiatus, appearing as an extraparenchymal, red and capsular mass with an arterialized surface (Figure 2A). The mass displayed a dense consistency and extended into the mesencephalic-thalamic region, occupying the crural and ambient cisterna. At the beginning of the surgical resection, an excessive bleeding from the vascularised lesion occurred, leading us to abort the procedure. At this regard, surgical resection may be challenging even to the most experienced surgeon, as reported by Gasco et al. (13, 14), because of the highly vascularization of intracranial ALMs. The bleeding propensity of ALM raises questions about the usefulness of preoperative embolization. At this regard, we suppose that preoperative embolization of the tumor may be considered in cases of complex vascular architecture lesions and proximity to large vessels (i.e., cavernous sinus), in which the embolization procedure may avert the burden of intraoperative bleeding. Nonetheless, despite the application of this procedure, significant bleeding from the tumor still represents a frequent complication (15, 16). After aborting the first surgical attempt, we performed a DSA that excluded the presence of any thrombosed aneurysm and provided us the needed information about vascular afferents to the tumor. The second surgical procedure was conducted through the same previous surgical route, achieving a subtotal removal of the tumor without any surgical complication. During the resection, the lesion displayed an intense and homogenous fluorescence, and the use of the dedicated filter (Yellow 560) was not necessary because the pathologic tissue was already recognizable for its high vascularization. Confocal laser endomicroscopy (CLE) was implemented and showed both abnormal vessels and neoplastic proliferation. At this regard, we would like to highlight the usefulness of the CLE in differentiating the neoplastic portion of the tumor from its vascular component. We also underline that CLE could have represented a useful intraoperative adjunct to exclude a vascular malformation in the first setting, thus preventing us from aborting the surgery. In patients with ALM, post-surgical complications such as hydrocephalus, seizure, and visual impairment (17–21) have been reported. Our patient developed mild expressive dysphasia and moderate right hemiparesis. Postoperative brain MRI showed a residual tumor located in the free tentorial edge and firmly attached to the left midbrain. Despite the presence of residual disease, considering its histological benignity and after a multidisciplinary neurooncological board, we decided for a follow-up with a brain MRI, which showed a significant recurrence at 5 months after surgery (Figures 1N–P). The patient was then referred to our radiation therapy specialist. The recurrence of the disease has not been described in the literature so far. As described by Xiaofeng et al., the postoperative residual disease can be treated by Cyber-knife (12). Such treatment should also be considered in cases at high risk for bleeding or when large vascular structures are involved. On the ground of our case, we may suggest a closer neuroradiological follow-up and, in selected cases, adjuvant radiation therapy in residual disease to prevent significant tumor recurrence. Because of the rarity of the lesion, a larger sample with multicentric collaborative studies is needed to reach more significant conclusions on the best adjuvant treatments. Moreover, given the fact that ALM usually does not exhibit any aggressive biological behavior, the identification of prognostic factors suggestive of disease recurrence is also needed. Immunotherapy has been proposed as a therapeutic option by Shinde et al., in their peculiar multifocal ALM report (20) but further clinical data are needed to confirm its clinical usefulness. On the other hand, Li et al. (20) opted for biopsy and radiosurgery in the case of an ALM located in the sellar region.
Conclusion
Intracranial ALM represents a rare and understudied CNS tumor. We report the first case of CNS ALM undergoing an intraoperative confocal endomicroscopy with the potential usefulness to discriminate an unexpected vascular malformation from a highly vascularized neoplastic lesion. We provide a comprehensive radiological and histopathological evaluation along with a literature review. Moreover, this case could suggest the need to consider radiation therapy as an adjuvant modality treatment in ALM subtotal removal.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
EC, FA and PF performed the clinical assessment. ER, EC and GB critically reviewed the literature and drafted the manuscript. All authors were responsible for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Italian Ministry of Health (RRC).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1072270/full#supplementary-material
Abbreviations
- ALM, angioleiomyoma; Cho, choline; CLE, confocal laser endomicroscopy; CNS, central nervous system; Cr, creatine; CT, computed tomography; DSA, digital subtraction angiography; EBV, Ebstein-Barr virus; 18F-FDG PET-CT, 18-fluoro-deoxy-glucose positron emission tomography-computed tomography; FLAIR, fluid-attenuated inversion recovery; GTR, gross total resection; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; NAA, N-acetyl aspartate; PCA, posterior cerebral artery; SCA, superior cerebellar artery; WHO, world health organization; WI, weighted imaging.
References
1. Wen PY, Huse JT. 2016 World health organization classification of central nervous system tumors. CONTINUUM Lifelong Learn Neurol (2017) 23. doi: 10.1212/CON.0000000000000536
2. Lin SL, Wang JS, Huang CS, Tseng HH. Primary intracerebral leiomyoma: A case with eosinophilic inclusions of actin filaments. Histopathology. (1996) 28. doi: 10.1046/j.1365-2559.1996.d01-440.x
3. Jänisch W, Janda J, Link I. Primary diffuse leptomeningeal leiomyomatosis. Zentralbl Pathol (1994) 140.
4. Jahnke K, Schilling A, Heidenreich J, Stein H, Brock M, Thiel E, et al. Radiologic morphology of low-grade primary central nervous system lymphoma in immunocompetent patients. Am J Neuroradiology. (2005) 26. doi: 10.1200/jco.2005.23.16_suppl.1536
5. Zhu G, Xiao D, Sun P. Expression of estrogen and progesterone receptors in angioleiomyoma of the nasal cavity of six patients. Oncol Lett (2016) 11. doi: 10.3892/ol.2016.4230
6. Hachisuga T, Hashimoto H, Enjoji M. Angioleiomyoma. a clinicopathologic reappraisal of 562 cases. Cancer. (1984) 54. doi: 10.1002/1097-0142(19840701)54:1<126::AID-CNCR2820540125>3.0.CO;2-F
7. Matsuyama A, Hisaoka M, Hashimoto H. Angioleiomyoma: a clinicopathologic and immunohistochemical reappraisal with special reference to the correlation with myopericytoma. Hum Pathol (2007) 38. doi: 10.1016/j.humpath.2006.10.012
8. Lach B, Duncan E, Rippstein P, Benoit BG. Primary intracranial pleomorphic angioleiomyoma–a new morphologic variant. an immunohistochemical and electron microscopic study. Cancer. (1994) 74. doi: 10.1002/1097-0142(19941001)74:7<1915::AID-CNCR2820740715>3.0.CO;2-1
9. Altieri R, Morrone A, Certo F, Parisi G, Buscema G, Broggi G, et al. Tentorial angioleiomyoma: A rare neurosurgical entity. case report and review of the literature. World Neurosurg (2019) 130. doi: 10.1016/j.wneu.2019.07.129
10. Xiaofeng L, Hongzhi X, Junrui C. Primary angioleiomyoma in the cavernous sinus: A case report. World Neurosurg (2016) 87. doi: 10.1016/j.wneu.2015.09.050
11. Falco J, Cavallo C, Vetrano IG, de Laurentis C, Siozos L, Schiariti M, et al. Fluorescein application in cranial and spinal tumors enhancing at preoperative MRI and operated with a dedicated filter on the surgical microscope: Preliminary results in 279 patients enrolled in the FLUOCERTUM prospective study. Front Surg (2019) 6:49. doi: 10.3389/fsurg.2019.00049
12. Gasco J, Franklin B, Rangel-Castilla L, Campbell GA, Eltorky M, Salinas P. Infratentorial angioleiomyoma: A new location for a rare neoplastic entity - case report. J Neurosurg (2009) 110. doi: 10.3171/2008.8.17645
13. Kohan D, Downey LL, Lim J, Cohen NL, Elowitz E. Uncommon lesions presenting as tumors of the internal auditory canal and cerebellopontine angle. Am J Otology. (1997) 18.
14. Sun L, Zhu Y, Wang H. Angioleiomyoma, a rare intracranial tumor: 3 case report and a literature review. World J Surg Oncol (2014) 12. doi: 10.1186/1477-7819-12-216
15. Figueiredo EG, Gomes M, Vellutini E, Rosemberg S, Marino R. Angioleiomyoma of the cavernous sinus: Case report. Neurosurgery. (2005) 56. doi: 10.1227/01.NEU.0000148003.42589.83
16. Karagama YG, Bridges LR, van Hille PT. Angioleiomyoma of the internal auditory meatus: A rare occurrence in the internal auditory canal. Ear Nose Throat J (2005) 84. doi: 10.1177/014556130508400413
17. Scherer K, Johnston J, Panda M. Dural based mass: Malignant or benign. J Radiol Case Rep (2009) 3. doi: 10.3941/jrcr.v3i11.189
18. Teranishi Y, Kohno M, Sora S, Sato H, Yokoyama M. Cavernous sinus angioleiomyoma: Case report and review of the literature. J Neurol Surg Rep (2014) 75. doi: 10.1055/s-0034-1376425
19. Shinde S v., Shah AB, Baviskar RB. Deshpande JR: Primary intracranial multicentric angioleiomyomas. Neurol India. (2012) 60. doi: 10.4103/0028-3886.93613
20. Li D, Hao SY, Tang J, Cao XY, Lin S, Wang JM, et al. Primary intracranial angioleiomyomas: Diagnosis, treatment, and literature review. Brain Tumor Pathol (2014) 31. doi: 10.1007/s10014-013-0150-4
Keywords: angiography, angioleiomyoma, CNS, fluorescein, intracranial, intraoperative confocal endomicroscopy, primitive
Citation: Rubiu E, La Corte E, Bonomo G, Restelli F, Falco J, Mazzapicchi E, Broggi M, Schiariti MP, Pollo B, Pinzi V, Bruzzone MG, Di Meco F, Acerbi F and Ferroli P (2022) Diagnostic and surgical management of primary central nervous system angioleiomyoma: A case report and literature review. Front. Oncol. 12:1072270. doi: 10.3389/fonc.2022.1072270
Received: 17 October 2022; Accepted: 01 December 2022;
Published: 16 December 2022.
Edited by:
Luigi Rigante, KBM Neurosurgery, GermanyReviewed by:
Paolo Palmisciano, University of Cincinnati, United StatesMirza Pojskic, University Hospital of Giessen and Marburg, Germany
Copyright © 2022 Rubiu, La Corte, Bonomo, Restelli, Falco, Mazzapicchi, Broggi, Schiariti, Pollo, Pinzi, Bruzzone, Di Meco, Acerbi and Ferroli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giulio Bonomo, ZG90dC5naXVsaW9ib25vbW9AZ21haWwuY29t
†ORCID: Giulio Bonomo, orcid.org/0000-0002-4749-7929
 Emanuele Rubiu
Emanuele Rubiu Emanuele La Corte
Emanuele La Corte Giulio Bonomo
Giulio Bonomo Francesco Restelli
Francesco Restelli Jacopo Falco
Jacopo Falco Elio Mazzapicchi
Elio Mazzapicchi Morgan Broggi
Morgan Broggi Marco Paolo Schiariti
Marco Paolo Schiariti Bianca Pollo
Bianca Pollo Valentina Pinzi5
Valentina Pinzi5 Francesco Di Meco
Francesco Di Meco Francesco Acerbi
Francesco Acerbi