
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 15 September 2021
Sec. Gastrointestinal Cancers
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.669078
 Susan A. Kennedy1†
Susan A. Kennedy1† Stephanie L. Annett2†
Stephanie L. Annett2† Margaret R. Dunne1
Margaret R. Dunne1 Fiona Boland3
Fiona Boland3 Linda M. O’Neill4
Linda M. O’Neill4 Emer M. Guinan5
Emer M. Guinan5 Suzanne L. Doyle6
Suzanne L. Doyle6 Emma K. Foley1
Emma K. Foley1 Jessie A. Elliott1
Jessie A. Elliott1 Conor F. Murphy1
Conor F. Murphy1 Annemarie E. Bennett4
Annemarie E. Bennett4 Michelle Carey7
Michelle Carey7 Daniel Hillary7
Daniel Hillary7 Tracy Robson2
Tracy Robson2 John V. Reynolds1
John V. Reynolds1 Juliette Hussey4
Juliette Hussey4 Jacintha O’Sullivan1*
Jacintha O’Sullivan1*Background: The Rehabilitation Strategies Following Esophagogastric cancer (ReStOre) randomized control trial demonstrated a significant improvement in cardiorespiratory fitness of esophagogastric cancer survivors. This follow-up, exploratory study analyzed the biological effect of exercise intervention on levels of 55 serum proteins, encompassing mediators of angiogenesis, inflammation, and vascular injury, from participants on the ReStOre trial.
Methods: Patients >6 months disease free from esophagogastric cancer were randomized to usual care or the 12-week ReStOre program (exercise training, dietary counselling, and multidisciplinary education). Serum was collected at baseline (T0), post-intervention (T1), and at 3-month follow up (T2). Serum biomarkers were quantified by enzyme-linked immunosorbent assay (ELISA).
Results: Thirty-seven patients participated in this study; 17 in the control arm and 20 in the intervention arm. Exercise intervention resulted in significant alterations in the level of expression of serum IP-10 (mean difference (MD): 38.02 (95% CI: 0.69 to 75.35)), IL-27 (MD: 249.48 (95% CI: 22.43 to 476.53)), and the vascular injury biomarkers, ICAM-1 (MD: 1.05 (95% CI: 1.07 to 1.66)), and VCAM-1 (MD: 1.51 (95% CI: 1.04 to 2.14)) at T1. A significant increase in eotaxin-3 (MD: 2.59 (95% CI: 0.23 to 4.96)), IL-15 (MD: 0.27 (95% CI: 0 to 0.54)) and decrease in bFGF (MD: 1.62 (95% CI: -2.99 to 0.26)) expression was observed between control and intervention cohorts at T2 (p<0.05).
Conclusions: Exercise intervention significantly altered the expression of a number of serum biomarkers in disease-free patients who had prior treatment for esophagogastric cancer.
Impact: Exercise rehabilitation causes a significant biological effect on serum biomarkers in esophagogastric cancer survivors.
Clinical Trial Registration: ClinicalTrials.gov (NCT03314311).
While the historical advice to cancer patients was rest and avoidance of physical activity, there is now compelling evidence which demonstrates that physical activity is not only safe for cancer survivors but extremely beneficial in improving health related quality of life, reducing levels of anxiety, fatigue, and depression (1). A significant rise in the number of cancer survivors worldwide is expected by 2040, which currently stands at approximately 44 million (1, 2). At the end of 2019, three seminal papers outlining the benefits of physical activity in cancer prevention, recurrence and treatment related side-effects were published following a roundtable review by the American College of Sports Medicine (ACSM) expert panel (1–3). As physical activity can impact cellular processes by altering the endogenous systemic milieu (3), this study examined a range of serum biomarkers in esophagogastric cancer survivors.
Prior to diagnosis, esophagogastric cancer patients may experience dysphagia and eating difficulties which can result in considerable weight loss and fatigue (4, 5). Moreover, chemotherapy, radiation and surgery have a detrimental effect on health related quality of life (HRQOL) and patients can struggle with loss of appetite, difficulty eating, sarcopenia, fatigue, and long standing post-operative weight loss (4, 6–9). Cardiorespiratory fitness is an important index of health and all-cause mortality and remains impaired in esophagogastric cancer survivorship (10, 11). Exercise rehabilitation strategies in esophagogastric cancer must aim to attenuate or avoid excess weight loss in these patients; however, there is a strong rationale to provide rehabilitation programmes for esophagogastric cancer patients.
The multidisciplinary ReStOre (Rehabilitation Strategies following Esophagogastric Cancer) program was developed to incorporate exercise rehabilitation with 1:1 dietary counselling and patient education sessions. A feasibility study of the ReStOre programme found high recruitment rates, adherence, acceptability and a lack of adverse events (12). A further randomized controlled trial (RCT) utilizing the ReStOre protocol demonstrated that structured exercise rehabilitation leads to meaningful improvements in cardiorespiratory fitness in esophagogastric cancer survivors (13). Following the ReStOre intervention participants experienced a clinically meaningful improvement in VO2 peak without weight loss occurring (13). The question remains however, whether these rehabilitation programs affect the serum inflammatory profiles of these patients pre, during, and post exercise intervention, and if so, how.
Esophageal cancer is an exemplar model of an inflammation-driven cancer characterized by the overproduction of cytokines and chemokines such as IL-6, IL-8, IL-10, IL-1β, TGF-α, and TGF-β in the tumor microenvironment driving carcinogenesis (14). In addition, inflammation is also associated with symptoms affecting HRQOL, such as fatigue, stress, and cachexia in cancer patients and survivors (15–17). A systematic review concluded that exercise interventions are beneficial in improving HRQOL in cancer survivors (18) and importantly this may be partially due to the ability of physical activity to attenuate inflammation (19). In the ReStOre feasibility study (n=22 control, n=21 intervention) a significant reduction in inflammatory status from baseline to post intervention, characterized by a decrease of 11.25% in IL-8 (p = 0.03) was observed (20). While not significant, there was also an overall pattern towards lower post-intervention inflammatory status (percentage change IL-1β = −5.83%; IL-6 = −3.89%; TNF-α = −10.87%) (20). Numerous studies have analyzed the expression levels of various biomarkers in a variety of oncology settings, mainly breast (21–23), colorectal, and prostate cancer (24, 25). However, to the best of our knowledge, a comprehensive assessment has not been conducted in the esophageal cancer setting. In order to gain a more comprehensive assessment of the impact of physical activity at a biological level, the aim of this exploratory study was to analyze the secretion levels of 55 angiogenic, inflammatory, chemokine, and cytokine mediators in the serum from esophagogastric cancer survivors participating in the ReStOre randomized clinical trial.
Study participants who had completed treatment with curative intent for esophageal, esophagogastric junction or gastric cancer were recruited from the Esophageal and Gastric Center, St. James’s Hospital (SJH), Dublin, Ireland as detailed in ‘The Rehabilitation Strategies Following Esophagogastric cancer’ (ReStOre) randomized control trial (1). Ethical approval was obtained [REC Reference: 2016-02 List 5 (6)] and the trial registered on ClinicalTrials.gov (NCT03314311). Briefly, disease-free patients treated for esophagogastric cancer were randomized into either usual care or the 12-week ReStOre program and outcomes measured included cardiorespiratory fitness, health related quality of life (HRQOL) and body composition, as reported previously (13). This current study includes a subset of patients from the previously reported ReStOre trial (13) in which serum samples were available for each of the measured time points [baseline (T0), post 12 week intervention (T1), and 24 week follow up (T2)]. The primary outcome measure of this study was blood biomarker analysis following exercise intervention.
The ReStOre program is a 12-week rehabilitation program consisting of supervised and home-based exercise, dietary counselling, and multidisciplinary counselling, as previously described (1). Briefly, aerobic exercise training commenced at a low intensity (30 - 40% heart rate reserve) and progressed weekly to a moderate intensity (45% - 60% heart rate reserve). Resistance exercise commenced at 2 sets of 12 repetition maximum and progressed to 6 sets of 17 repetition maximum. Adherence was monitored using polar heart rate monitors (Polar FT7, China) and exercise diaries. Participants received 1:1 dietary counselling from a registered dietitian. Dietary intake and gastrointestinal symptoms were assessed using a 24-hour dietary recall, semi-structured dietary interviewing, and validated scales of gastrointestinal symptoms. Participants attended seven group education sessions by the multidisciplinary team including a surgeon, dietitian, physiotherapist, occupational therapist and a psychotherapist specialized in mindfulness.
Measures were assessed at baseline (T0), immediately post 12-week intervention (T1), and at 3-month follow-up (T2, week 24) as outlined in the ReStOre randomized control trial (1). Cardiorespiratory fitness was determined using a maximal exercise test [cardiopulmonary exercise test (CPET)]. Body composition (anthropometric measurements and bioimpedance analysis), accelerometer measures of physical activity levels, and health related quality of life (HRQOL) questionnaires were recorded.
Non-fasting venous blood samples were collected from 37/43 ReStOre trial participants (17/22 control and 20/21 intervention, prior to exercise testing at baseline (T0), immediately post-intervention (T1), and at 3-month follow-up (T2). Following 30 min upright rest, samples were centrifuged at 2500 rpm for 10 min at 4°C and serum was cryopreserved at -80°C until analyzed. The V-PLEX Human Biomarker 54-Plex Kit (Meso-Scale Discovery) was used according to manufacturer’s instructions. The 54-plex kit is composed of 7 individual multiplex ELISA assays. The human angiogenesis panel contained: VEGF-A, VEGF-C, VEGF-D, Tie-2, Flt-1, PlGF, and bFGF. The human chemokine panel contained: Eotaxin, MIP-1β, Eotaxin-3, TARC, IP-10, MIP-1α, IL-8 (high abundance), MCP-1, MDC, and MCP-4. The human cytokine panel 1 contained: GM-CSF, IL-1α IL-5, IL-7, IL-12/IL-23p40, IL-15, IL-16, and TNF-β. The human cytokine panel 2 contained: IL-17A/F, IL-17B, IL-17C, IL-17D, IL-1RA, IL-3, IL-9, and TSLP. The human Th17 panel contained: IL-17A, IL-21, IL-22, IL-23, IL-27, IL-31, and MIP-3α. The human pro-inflammatory panel contained: IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, and TNF-α. The human vascular injury panel contained SAA, CRP, VCAM-1, and ICAM-1. All 54-plex assays were conducted as per manufacturer’s recommendations, with an alternative protocol of overnight supernatant incubation being used all assays except Vascular Injury and Angiogenesis, which were run in a single day. All results were reported in pg/ml. The anti-angiogenic protein FK506 binding protein like (FKBPL) was analyzed by ELISA (Cloud Clone, USA), as per manufacturer’s instructions. Serum samples were diluted two-fold using standard diluent and loaded as duplicates and FKBPL concentration was calculated using a 4-parameter fit standard curve.
Statistical analyses were performed using Stata v14 (Ref: StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP) or GraphPad Prism 8.0. Some observations were identified (from boxplots and summary statistics) as outliers and were removed prior to analysis. The exact number of values included in each summary measure and analysis are included in the tables. Descriptive statistics are presented as mean and standard deviation (SD) for continuous variables and frequency (percentage) for categorical data. Initially, associations between baseline serum biomarker and clinical measurements were assessed using Spearman’s rho correlation. Effect sizes were interpreted as 0.2 to 0.5 (weak), 0.51 to 0.80 (moderate), and more than 0.80 strong effect (26). Raw data, was visualized as dot plots, generated in GraphPad Prism (version 8.4.1). Data at time 1 (T1) and time 2 (T2) were analyzed using linear regression models, adjusting for baseline values (T0). When assumptions of linear regression were violated, data were transformed using a log transformation. P-values ≤0.05 were considered statistically significant. There was no adjustment for multiple comparisons.
Thirty-seven patients participated in this study; 17 in the control arm and 20 in the intervention arm. The mean age of participants was 63 years (SD: 10.1) in the control cohort, and 67 years (SD: 7.7) in the intervention cohort. Mean time post-surgery was in the control group was 37.6 (SD: 19.3) months and 22.6 (SD: 15) months in the intervention group. The majority of patients were diagnosed with adenocarcinoma and tumors were located in the esophagogastric junction (Table 1). In the control group, mean baseline BMI was 25.86 kg/m2 (SD: 5.11) with a majority (n=10) classed as having a healthy weight BMI, four classed as having an overweight BMI (25-30 kg/m2), and three as having an obese BMI (>30 kg/m2) (Table 1). In the intervention group, mean baseline BMI was 25.44 kg/m2 (SD: 3.95) with the majority classed as having a healthy weight BMI (n = 10), six classed as having an overweight BMI (25 -30 kg/m2), and four as having an obese BMI (>30 kg/m2). No participants were classed as having an underweight BMI (<18.5 kg/m2) (Table 1). At baseline (T0), the control group had a higher VO2 peak (mean 22.85, SD: 4.54 ml/min/kg) compared to the intervention group (mean 18.76, SD: 4.17 ml/min/kg) (Table 1). Compared to population norms (27), twenty-two participants had very poor cardiorespiratory fitness, eleven had poor fitness and four had a fair level of fitness at baseline (T0) (Table 1).
Some evidence of correlation was observed between serum biomarkers and BMI, namely IL-12/IL-23p40 and TNF-β. A moderate positive correlation was observed at baseline (T0) between BMI and the pro-inflammatory cytokines IL-12/IL-23p40 (rho=0.51, p=0.001) and TNF-β (rho=0.53, p=0.002), while a weak correlation was observed with IL-17A (rho=0.335, p=0.043) Table 2 and Supplementary Table 1. IL-1 receptor antagonist (IL-1RA) (rho=0.39, p=0.02) and IL-17A (rho=0.34, p=0.04) were weakly positively correlated to BMI (Table 2 and Supplementary Table 1). The angiogenic factor, VEGF, also weakly correlated to BMI (rho=0.34, p=0.04) (Table 2 and Supplementary Table 1). The 6-minute walk test (6-MWT) is a sub-maximal exercise test used to assess aerobic capacity and endurance (28). A weak positive correlation between 6-MWT and the anti-inflammatory cytokine IL-10 (rho=0.37, p=0.03) (Table 2 and Supplementary Table 2). Furthermore, a weak, negative correlation was observed with a greater 6-MWT distance and the pro-inflammatory cytokines IL-17A (rho= -0.34, p=0.04), IL-17D (rho=-0.33, p=0.05) and MIP-3α (rho=-0.37, p=0.02) (Table 2 and Supplementary Table 2). No further evidence of correlations were observed with 6-MWT.
The anaerobic threshold (AT), a measure of endurance which calculates the exertion level between aerobic and anaerobic training (3) showed a weak negative correlation with pro-inflammatory cytokines and chemokines including IFN-γ (rho=-0.34, p=0.04), IL-17A (rho=-0.35, p=0.03), IL-17B (rho=-0.43, p=0.01) and IP-10 (rho=-0.37, p=0.02) (Table 2 and Supplementary Table 3). VO2max is the maximum rate of oxygen consumption measured during incremental exercise and it is reflective of cardiorespiratory fitness (4). A larger VO2max was weakly negatively associated with the pro-inflammatory cytokine IL-17A (rho=-0.35, p=0.03) and VEGF-D (rho=-0.37, p=0.02); a mitogen important for both angiogenesis and lymphangiogenesis (Table 2 and Supplementary Table 4) (5). There was no correlation observed between fatigue score and any of the 55 serum biomarkers measured (Supplementary Table 5).
Linear regression analysis of the vascular injury panel which included ICAM-1 (Figure 1A) and VCAM-1 (Figure 1B) demonstrated significant differences between the control and intervention groups at T1 but not T2 (Supplementary Table 6). There was no evidence of significant differences in expression levels of serum amyloid A (SAA) and CRP observed at T1 or T2 (Supplementary Table 6). The expression level of ICAM-1 was significantly higher in the intervention compared to the control (ratio of means: 1.05 (95% confidence interval (CI): 1.07 to 1.66); p=0.02) at T1 (Figure 1A). Similarly, a significantly higher increase in VCAM-1 expression was observed in the intervention cohort compared to the control cohort [ratio of means: 1.51 (95% CI: 1.04 to 2.14); p=0.02] at T1 (Figure 1B).

Figure 1 Multiplex ELISA analysis of Vascular injury panel. Data at immediately post 12-week intervention (Time 1; T1) and at 3-month follow-up (Time 2; T2) was analyzed using linear regression models, adjusting for baseline values (T0). A significant increase in serum (A) ICAM-1 and (B) VCAM-1 expression was observed at T1 (*p ≤ 0.05).
Of the panel of chemokine markers assessed, a significant increase in IP-10 expression was detected in the intervention group compared to the control group at T1 [mean difference (MD) 38.02 pg/ml (95% CI: 0.69 to 75.35); p=0.05] (Figure 2A and Supplementary Table 6). No significant difference in expression was observed at T2 (p=0.61) (Figure 2A and Supplementary Table 6). Furthermore, there was significant increase in eotaxin-3 in the intervention compared to the control at T2 (MD: 2.59 (95% CI: 0.23 to 4.96), p=0.03) (Figure 2B and Supplementary Table 6) at T2. No significant difference in expression levels was observed for the remaining chemokine panel members eotaxin, MIP-1β, TARC, IP-10, MIP-1α, IL-8, MCP-1, MDC and MCP-4 (Supplementary Table 6).

Figure 2 Exercise intervention increases IP-10 and Eotaxin–3 expression. Data at immediately post 12-week intervention (Time 1; T1) and at 3-month follow-up (Time 2; T2) was analyzed using linear regression models, adjusting for baseline values (T0). A significant increase in serum (A) IP-10 expression was observed at T1 between control and intervention cohorts while a significant increase in (B) Eotaxin-3 was observed at T2 (*p ≤ 0.05).
IL-27 expression was significantly increased in the intervention compared to the control cohort at T1 (MD: 249.48 (95% CI 22.43 to 476.53), p=0.03), at T1 p=0.03 (Figure 3 and Supplementary Table 6). There was no significant difference found between control and intervention cohorts for IL-17A, IL-22 and MIP-3α at T1 or T2 (Supplementary Table 6). IL-21 and IL-23 were below the level of detection. Statistical analysis could not be conducted for IL-31 as expression was only detected in a small number of patients.
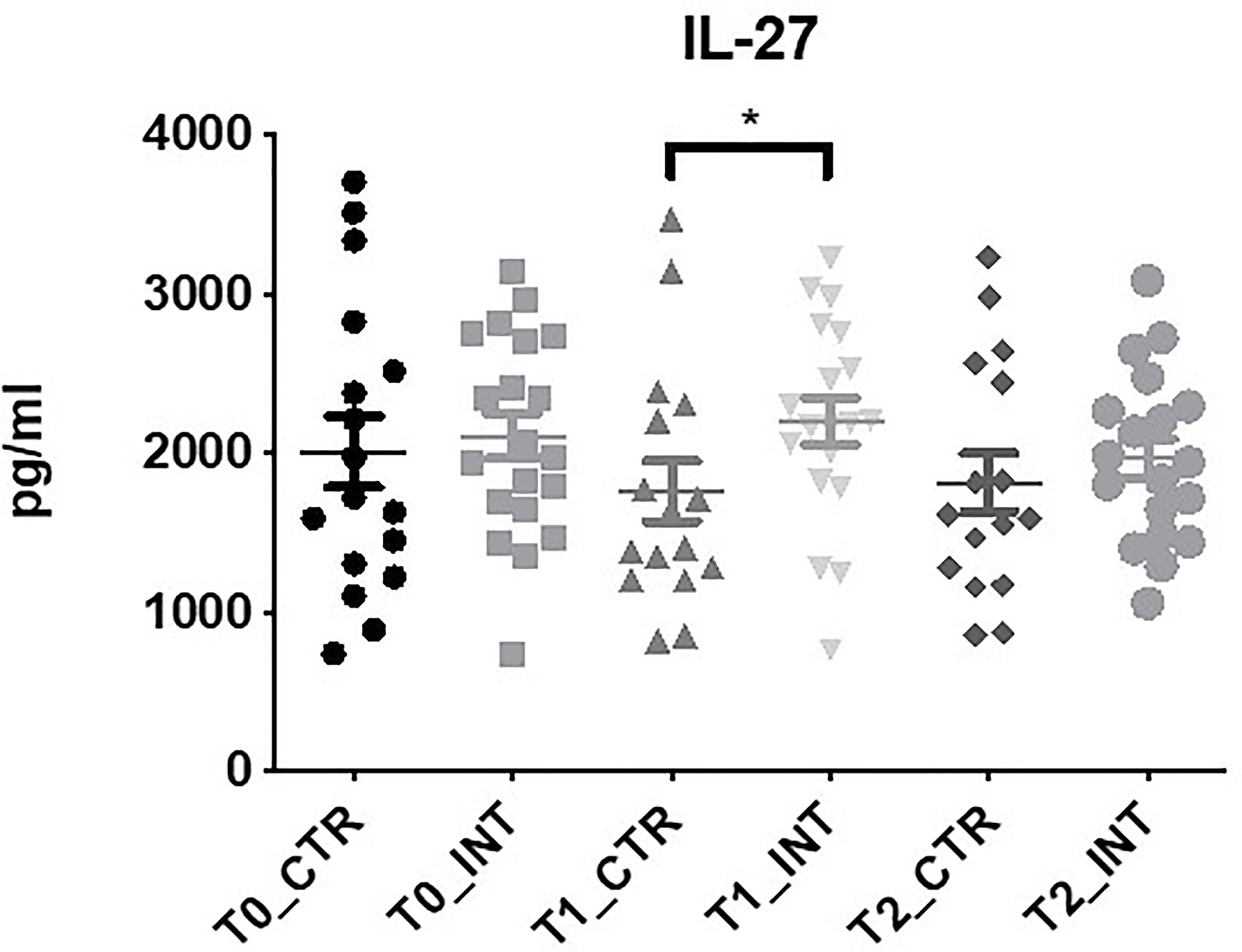
Figure 3 Exercise intervention increases IL-27 expression at T1. Data at immediately post 12-week intervention (Time 1; T1) and at 3-month follow-up (Time 2; T2) was analyzed using linear regression models, adjusting for baseline values (T0). A significant increase in serum IL-27 expression was observed at T1 between control and intervention cohorts (*p ≤ 0.05).
In the panel of angiogenesis markers bFGF expression was significantly decreased at 3-month follow-up (T2) in the intervention cohort compared to the control cohort (MD: -1.62 (95% CI -2.99 to -0.26), p=0.02), (Figure 4 and Supplementary Table 6). No significant difference in bFGF expression was observed at T1 (p=0.08) (Figure 4 and Supplementary Table 6). In addition, there was no significant difference found between control and intervention groups for angio VEGF, Flt-1, PlGF, VEGF, VEGF-C, VEGF-D, TIE-2, and FKBPL (Supplementary Table 6).
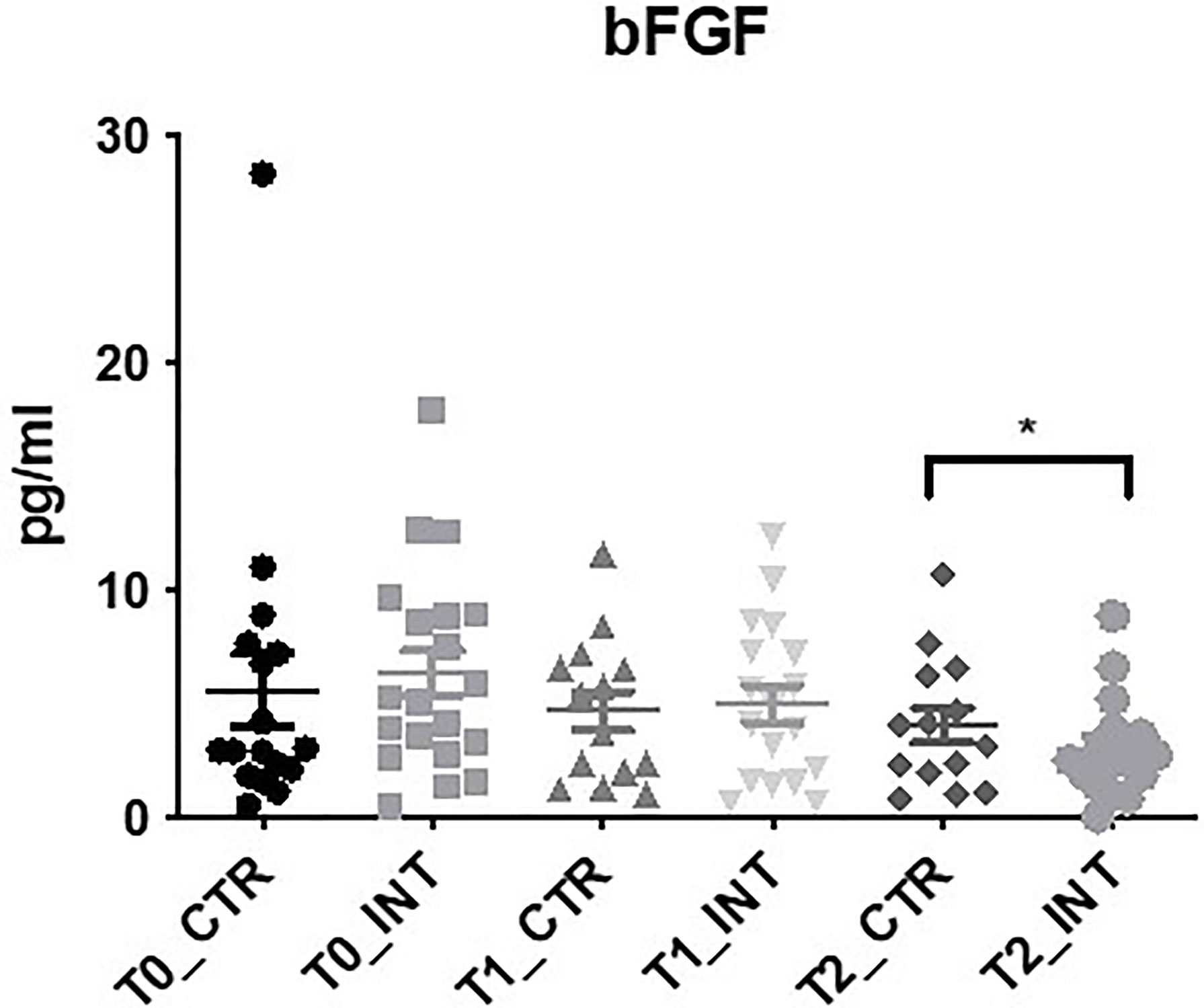
Figure 4 Exercise intervention decreases bFGF expression at T2. Data at immediately post 12-week intervention (Time 1; T1) and at 3-month follow-up (Time 2; T2) was analyzed using linear regression models, adjusting for baseline values (T0). A significant decrease in serum bFGF expression was observed at T2 between control and intervention cohorts (*p ≤ 0.05).
Eighteen cytokine makers were analyzed in this study. The expression level of IL-15 was the only cytokine found to be significantly altered following exercise intervention (Figure 5 and Supplementary Table 6). At T2, a significant increase (p=0.05) in expression was observed in the intervention cohort compared to the control cohort (MD: 0.27 (95% CI: <0.01 to 0.54), p=0.05). No significant change in the expression levels of the remaining cytokine markers were observed (Supplementary Table 6), or cytokine panel 2 (Supplementary Table 6). The expression levels of IL-3 and IL-17C were below the level of detection. Statistical analysis could not be conducted for GMCSF, IL-1α, IL-17A/F, and IL-31 as expression was only detected in a small number of patients.
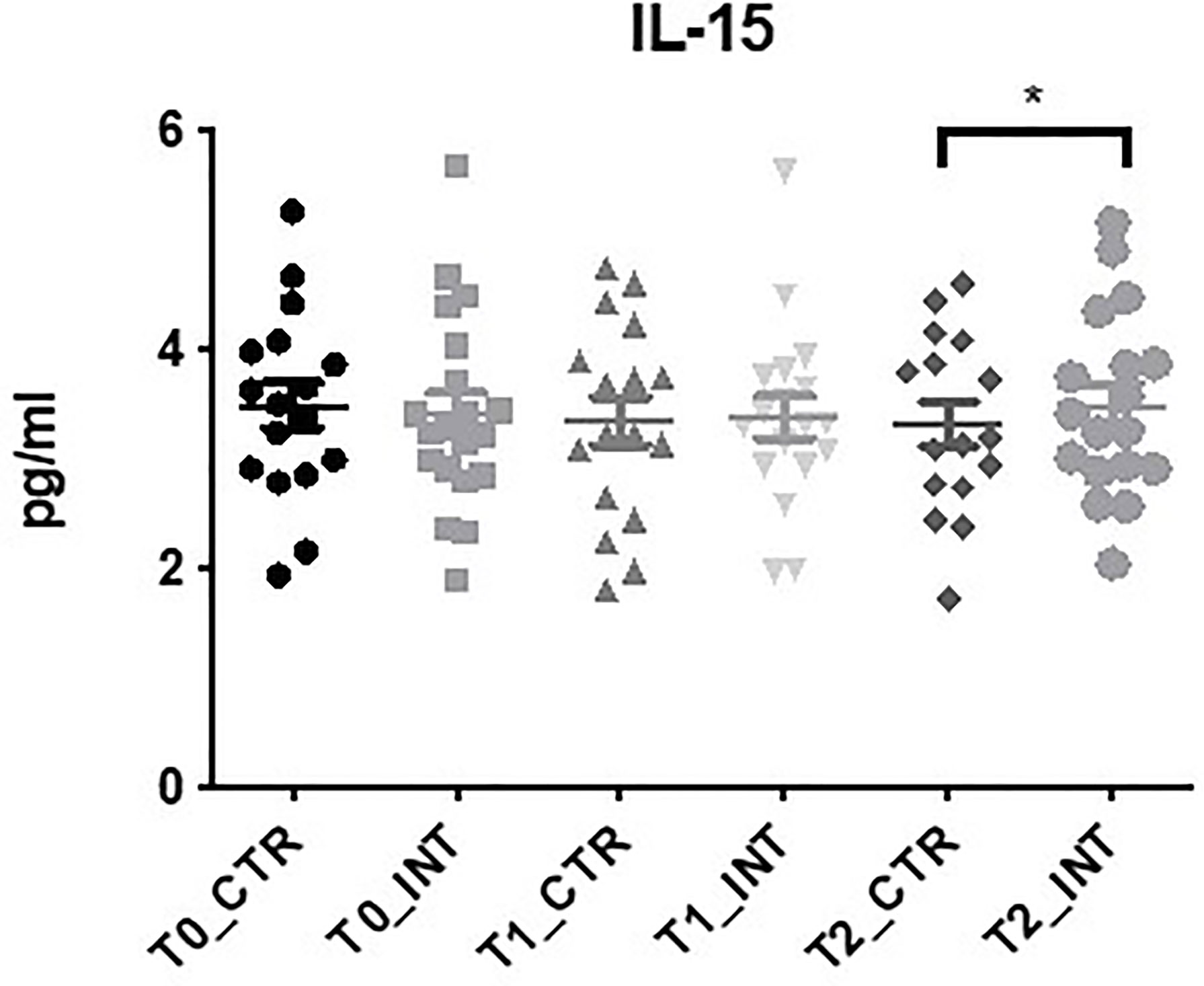
Figure 5 Exercise intervention increases IL-15 expression at T2. Data at immediately post 12-week intervention (Time 1; T1) and at 3-month follow-up (Time 2; T2) was analyzed using linear regression models, adjusting for baseline values (T0). A significant increase in serum IL-15 expression was observed at T2 between control and intervention cohorts (*p ≤ 0.05).
The benefits of physical activity are multi-factorial, from reducing cancer incidence, lowering the risk of recurrence and improving patient quality of life (29, 30). The ReStOre RCT has demonstrated that structured exercise rehabilitation resulted in improved confidence, physical, social and mental wellbeing of participants (13). This follow-up study analyzed the effect of physical activity on 55 serum biomarkers and enabled a more comprehensive assessment of angiogenic, inflammatory, cytokine and chemokine expression levels which may be altered following exercise intervention. Published research on the benefits of exercise in the cancer population has been dominated by breast cancer studies (1, 31, 32). To our knowledge, this is the largest screening analysis on the effect of exercise on serum inflammatory biomarkers in the esophageal cancer survivorship setting. In this study we have demonstrated that physical activity alters the secretion of a number of markers including eotaxin-3, IP-10, bFGF, IL-27, ICAM-1, and VCAM-1. Additional studies ongoing in the esophageal cancer setting include the PERFECT study, which included participants with esophageal cancer after surgery with curative intent in a 12-week exercise intervention. Secondary outcome measures of this study include blood marker analysis which at the time of writing have yet to be published (33). During the period between the intervention finishing (T1) and the 12 week post intervention follow up (T2), participants did not attend supervised exercise sessions, but a strong focus on self-management was instilled in participants to promote adherence to the exercise program in an unsupervised basis. Adherence to exercise was reflected in cardiorespiratory fitness levels measured by VO2 peak which were consistently higher in the intervention cohort compared to control at T1 and T2 (13).
A meta-analysis by Meneses-Echávez et al., has shown that circulating IL-6, which has been also associated with symptoms of fatigue, IL-8 and TNF-α were reduced in breast cancer survivors following regular exercise (23). While no significant alteration in the expression levels of IL-6, IL-8, TNF-α, or any of the pro-inflammatory’ cytokines were observed in this study (Supplementary Table 6), this could possibly be due to the duration of the exercise intervention. The mean exercise intervention in the meta-analysis conducted by Meneses-Echávez et al., was performed for 19 ± 13 weeks (23), indicating a longer intervention may have been required to observe significant changes in expression levels. Also, possibly the secretion of these inflammatory mediators may display cyclic profiles with time. Interestingly, our 12-week exercise intervention did significantly alter the expression levels of soluble intracellular adhesion molecular (ICAM-1) and soluble vascular adhesion molecule (VCAM-1), members of the vascular injury panel at T1 compared to controls. Both soluble ICAM-1 and VCAM-1 are reported to be increased following exercise and circulating levels of cells are important in skeletal muscle remodeling (34–36). Similarly, we also found the exercise intervention group had elevated ICAM-1 and VCAM-1 at T1 compared to controls (Figure 1). Other members of the vascular injury panel, CRP and SAA, demonstrated no significant difference in serum expression levels following exercise intervention. However, other clinical studies have shown that a longer intervention time (>16 weeks) is required to detect changes in the systemic levels of pro-inflammatory makers such as CRP, TNF-α and IL-6 which are shown to increase following physical inactivity or sedentary behavior (29, 37).
Basic fibroblast growth factor (bFGF) is a polypeptide growth factor which drives tumorigenesis through both the proliferation of tumor cells and the promotion of angiogenesis (38). Indeed, in esophageal cancer, high bFGF expression was associated with tumor progression and significantly correlated with depth of invasion, lymph-node metastasis, TNM stage and micro vessel density (39). A significant decrease in bFGF levels was identified at T2 (p=0.02), 24-weeks after commencing the exercise intervention, in the intervention group compared to the control group (Figure 4). To the best of our knowledge, this is the first study to investigate bFGF levels in cancer survivors following exercise intervention. Increased mRNA bFGF expression was previously observed in rat skeletal muscle following a single exercise bout (40), while serum bFGF levels significantly increased in overweight Japanese men 6-months after commencing an exercise intervention (41) Notably, serum bFGF levels were inversely correlated with both a reduction in BMI and improved exercise capacity (41). A key difference with the ReStOre RCT is that participants were monitored to ensure their BMI did not decrease, as unintentional weight loss is problematic in esophagogastric cancer survivors due to long-term nutritional challenges. Several studies have noted elevated levels of bFGF in inflammatory bowel conditions (42–44). In addition, inflammatory stimuli have been shown to increase bFGF levels (45, 46). One possible explanation for the decrease in bFGF at 3-month follow up (T2) is a decrease in systematic inflammation in the intervention cohort. A longer intervention and follow-up may be required to detect changes in other pro-inflammatory stimuli measured within the study. Moreover, bFGF levels are significantly upregulated in esophageal and gastroesophageal junction adenocarcinomas and Barrett’s esophagus; a precursor lesion in this cohort (47). Therefore, it is very encouraging to observe a decrease of serum bFGF in the intervention cohort.
Interferon gamma-induced protein (IP-10), also known as, CXCL10, is an immunomodulatory cytokine associated with lymphocytic infiltrate to the tumor site. High levels have been associated with poor survival in patients in breast (48), pancreatic (49), and esophageal (50) cancer. We found that baseline levels of IP-10 and aerobic threshold were negatively correlated (Supplementary Table 3). Additionally, a significant increase in IP-10 expression was observed in the intervention cohort at T1, following completion of the 12-week program, but not at the second 3-month follow up (T2), despite there being an enhancement in cardiovascular fitness at both time points (Figure 2). In contrast to our findings, a large RCT of healthy adults (n=413) reported that 8 weeks of exercise training was associated with a decrease in IP-10 and a 12-week exercise program in obese adolescents also observed a decrease in IP-10 serum concentration (51, 52). However, it is important to note that while a decrease in serum IP-10 was observed, both studies were conducted in a previously healthy or younger cohort with higher levels of elevated cardio respiratory fitness at enrolment in comparison to participants of the ReStOre RCT. It is well-reported that exercise may increase acute phase proteins and inflammatory markers, as observed in this study (Figure 1). Indeed inflammatory cytokines are released from skeletal muscle, resulting in enhanced plasma levels related to exercise duration, intensity and muscle mass (53, 54). However, after regular exercise, fewer inflammatory markers are released and long term adaption results in an increase release of anti-inflammatory substances (54). IP-10 (aka CXCL10) is an exercise controlled myokine (55) and therefore it is not surprising that changes in expression were observed in the intervention group. Further studies in a diverse range of subjects are required to precisely understand the role of IP-10 in physical activity.
IL-27 is a member of the IL-6/IL-27 family produced by antigen presenting cells with both pro and anti-tumorigenic properties (56, 57). We demonstrated an increase in serum IL-27 expression at T1 following exercise intervention (Figure 3). High serum IL-27 level is associated with cancer presence and lymph node metastases in gastroesophageal cancer (58). Little is known about how exercise impacts IL-27 in isolation, however, as a member of the subfamily of cytokines which signals through glycoprotein-130, it could play a role in the regulation of skeletal muscle remodeling (59, 60). Eotaxin-3, also known as CCL26, belongs to the CC cytokine family and it acts as a chemoattractant for eosinophils and basophils in allergic disorders (61). No studies to date have investigated the role of eotaxin-3 in esophageal cancer, however in colon cancer, eotaxin-3 induced tumor associated macrophages by binding to the CCR3 receptor and enhancing the invasiveness of tumor cells (62). Similarly, in prostate cancer mesenchymal stem cells modulate of the invasive potential of prostate cancer cells via the Eotaxin-3/CCR3 axis (63). We noted a significant increase in eotaxin-3 levels in the intervention group compared to the control group at T2 (Figure 2 and Supplementary Table 6). To our knowledge, no other study has investigated the effect of exercise intervention on eotaxin-3 levels, however the HEPAFIT RCT currently underway aims to access the effect of an exercise programme on overweight/obese adolescents and the study protocol states that eotaxin-3 will be measured (64).
Of the eleven cytokines analyzed in this study, IL-15 was the only cytokine family member observed to be significant. IL-15 is a member of the 4a-helix bundle cytokine, a myokine which is released by the muscle following exercise (65). The expression of IL-15 is high in skeletal muscle (66) and variability in plasma IL-15 expression has been shown following resistance training. In a 10-week acute resistance training program, Riechman et al., demonstrated increased plasma IL-15 expression in 127 participants post-training (67), however, this was not observed in a study by Nielson et al., which analyzed IL-15 mRNA, protein, and plasma expression levels in 8 healthy male subjects following heavy resistance training. While mRNA IL-15 expression levels increased in skeletal muscle, there was no significant difference in plasma IL-15 levels following resistance training (65). Interestingly, in this study we did not observe any significant changes in serum IL-15 expression immediately following the 12-week supervised exercise session (T1), however mean expression levels remained constant from T1 to T2 in the intervention cohort while a decrease of IL-15 expression was observed in the T2 control cohort leading to significance (Figure 5 and Supplementary Table 6).
The broad screen of angiogenic, inflammatory, chemokine and cytokine panels analyzed in this study has enabled us to assess serum markers in which (i) expression levels were undetectable, (ii) expression levels were identified in only a small number of patient samples and therefore could not be analyzed statistically, and (iii) expression levels were detected and warrant further investigation in a larger patient cohort. Of the 55 markers analyzed in our study, statistically significant results were seen for only 7. This could be due to the sample size and levels of biological heterogeneity observed in the control (n=17) and intervention (n=20) groups. One of the limitations of this study is the relatively small sample size given the number of tests and associations explored. The study was not powered to test for these associations, hence the exploratory nature of this study. Additionally, no adjustment for multiple comparisons was used. Hence, all results should be interpreted with caution. Furthermore, as stated previously, a longer exercise intervention (>12-weeks) may have yielded more significant variation in systemic markers (29, 68). While a longer exercise intervention period may be required to demonstrate changes in inflammatory markers, a single exercise session has been shown to reduce side effects of chemotherapy and reduce nausea in a cohort of women undergoing treatment for breast cancer (22). With the multitude of recent evidence, the benefits of exercise as an added therapeutic intervention in cancer treatment are clear, particularly in aiding and relieving treatment related side effects such as fatigue, nausea, and vomiting (21, 22). Of equal importance is the benefit of exercise to cancer survivors who experience deficits in cardiorespiratory fitness which impact daily activities. While there is insufficient literature at present on personalized exercise prescriptions, a future goal of exercise oncology is tailoring exercise programs specific to the individual patient’s cancer and their treatment type (1). The ReStOre RCT which combines both aerobic and resistance training, previously demonstrated the positive impacts of a 12-week supervised exercise intervention on participant physical, mental, and social well-being (13). In this study we demonstrate the biological effect of exercise intervention in significantly modulating a number of serum biomarkers involved in inflammation and metastasis. The ReStOre II clinical trial is currently underway and is recruiting a total of 120 patients who have had curative treatment for upper gastrointestinal (UGI) and hepatopancreaticobiliary (HPB) cancers (69). A secondary outcome of the trial is to establish an UGI cancer survivorship biobank for collaborative translational research studies (69) and therefore the results of this study will be validated in this larger cohort.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Esophageal and Gastric Center, St. James’s Hospital (SJH), Dublin, Ireland. The patients/participants provided their written informed consent to participate in this study.
Conception and design: JO’S, JH, and JR. Development of methodology: LO’N, EG, SD, EF, JE, CM, and AB. Acquisition of data: SK, SA, EF and MD. Analysis and interpretation of data: SK, SA, FB, MC, and DH. Writing, review, and/or revision of the manuscript: SK, SA, and JO’S. Study supervision: JO’S, JH, JR, and TR. All authors contributed to the article and approved the submitted version.
SK was funded by Science Foundation Ireland 17/TIDA/5053. SA and TR were awarded funding through the National Children’s Research Centre and the Children’s Medical & Research Foundation, Crumlin, Ireland, grant code (C/18/9). TR was also funded through the Science Foundation Ireland strategic partnership programme, Precision Oncology Ireland, grant code (18/SPP/352). LON, EG, SD, JH, and JOS were awarded funding by the Health Research Board Ireland (The ReStOre trial, grant number HRA-POR-2014-535).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.669078/full#supplementary-material
1. Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise Guidelines for Cancer Survivors: Consensus Statement From International Multidisciplinary Roundtable. Med Sci Sports Exerc (2019) 51(11):2375–90. doi: 10.1249/MSS.0000000000002116
2. Schmitz KH, Campbell AM, Stuiver MM, Pinto BM, Schwartz AL, Morris GS, et al. Exercise Is Medicine in Oncology: Engaging Clinicians to Help Patients Move Through Cancer. CA Cancer J Clin (2019) 69(6):468–84. doi: 10.3322/caac.21579
3. Patel AV, Friedenreich CM, Moore SC, Hayes SC, Silver JK, Campbell KL, et al. American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med Sci Sports Exerc (2019) 51(11):2391–402. doi: 10.1249/MSS.0000000000002117
4. Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, et al. Oesophageal Cancer. Nat Rev Dis Primers (2017) 3:17048. doi: 10.1038/nrdp.2017.48
5. Anandavadivelan P, Lagergren P. Cachexia in Patients With Oesophageal Cancer. Nat Rev Clin Oncol (2016) 13(3):185–98. doi: 10.1038/nrclinonc.2015.200
6. Blazeby JM, Sanford E, Falk SJ, Alderson D, Donovan JL. Health-Related Quality of Life During Neoadjuvant Treatment and Surgery for Localized Esophageal Carcinoma. Cancer (2005) 103(9):1791–9. doi: 10.1002/cncr.20980
7. Rees J, Hurt CN, Gollins S, Mukherjee S, Maughan T, Falk SJ, et al. Patient-Reported Outcomes During and After Definitive Chemoradiotherapy for Oesophageal Cancer. Br J Cancer (2015) 113(4):603–10. doi: 10.1038/bjc.2015.258
8. Rutegard M, Lagergren J, Rouvelas I, Lindblad M, Blazeby JM, Lagergren P. Population-Based Study of Surgical Factors in Relation to Health-Related Quality of Life After Oesophageal Cancer Resection. Br J Surg (2008) 95(5):592–601. doi: 10.1002/bjs.6021
9. Wainwright D, Donovan JL, Kavadas V, Cramer H, Blazeby JM. Remapping the Body: Learning to Eat Again After Surgery for Esophageal Cancer. Qual Health Res (2007) 17(6):759–71. doi: 10.1177/1049732307302021
10. Gannon JA, Guinan EM, Doyle SL, Beddy P, Reynolds JV, Hussey J. Reduced Fitness and Physical Functioning Are Long-Term Sequelae After Curative Treatment for Esophageal Cancer: A Matched Control Study. Dis Esophagus (2017) 30(8):1–7. doi: 10.1093/dote/dox018
11. Myers J, McAuley P, Lavie CJ, Despres JP, Arena R, Kokkinos P. Physical Activity and Cardiorespiratory Fitness as Major Markers of Cardiovascular Risk: Their Independent and Interwoven Importance to Health Status. Prog Cardiovasc Dis (2015) 57(4):306–14. doi: 10.1016/j.pcad.2014.09.011
12. O’Neill L GE, Doyle SL, Elliott JA, O’Sullivan J, Reynolds JV, Hussey J. Rehabilitation Strategies Following Esophageal Cancer (the ReStOre Trial): A Feasibility Study. Dis Esophagus (2017) 30(5):1–8. doi: 10.1093/dote/dow012
13. O’Neill LM, Guinan E, Doyle SL, Bennett AE, Murphy C, Elliott JA, et al. The RESTORE Randomized Controlled Trial: Impact of a Multidisciplinary Rehabilitative Program on Cardiorespiratory Fitness in Esophagogastric Cancer Survivorship. Ann Surg (2018) 268(5):747–55. doi: 10.1097/SLA.0000000000002895
14. O’Sullivan KE, Phelan JJ, O’Hanlon C, Lysaght J, O’Sullivan JN, Reynolds JV. The Role of Inflammation in Cancer of the Esophagus. Expert Rev Gastroenterol Hepatol (2014) 8(7):749–60. doi: 10.1586/17474124.2014.913478
15. Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and Their Relationship to the Symptoms and Outcome of Cancer. Nat Rev Cancer (2008) 8(11):887–99. doi: 10.1038/nrc2507
16. Alfano CM, Imayama I, Neuhouser ML, Kiecolt-Glaser JK, Smith AW, Meeske K, et al. Fatigue, Inflammation, and Omega-3 and Omega-6 Fatty Acid Intake Among Breast Cancer Survivors. J Clin Oncol (2012) 30(12):1280–7. doi: 10.1200/JCO.2011.36.4109
17. Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The Association Between Fatigue and Inflammatory Marker Levels in Cancer Patients: A Quantitative Review. Brain Behav Immun (2007) 21(4):413–27. doi: 10.1016/j.bbi.2006.11.004
18. Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, et al. Exercise Interventions on Health-Related Quality of Life for Cancer Survivors. Cochrane Database Syst Rev (2012) 8:CD007566. doi: 10.1002/14651858.CD007566.pub2
19. LaVoy EC, Fagundes CP, Dantzer R. Exercise, Inflammation, and Fatigue in Cancer Survivors. Exerc Immunol Rev (2016) 22:82–93.
20. Guinan EM, Doyle SL, O’Neill L, Dunne MR, Foley EK, O’Sullivan J, et al. Effects of a Multimodal Rehabilitation Programme on Inflammation and Oxidative Stress in Oesophageal Cancer Survivors: The ReStOre Feasibility Study. Support Care Cancer (2017) 25(3):749–56. doi: 10.1007/s00520-016-3455-0
21. van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, et al. Effect of Low-Intensity Physical Activity and Moderate- to High-Intensity Physical Exercise During Adjuvant Chemotherapy on Physical Fitness, Fatigue, and Chemotherapy Completion Rates: Results of the PACES Randomized Clinical Trial. J Clin Oncol (2015) 33(17):1918–27. doi: 10.1200/JCO.2014.59.1081
22. Johnsson A, Demmelmaier I, Sjovall K, Wagner P, Olsson H, Tornberg AB. A Single Exercise Session Improves Side-Effects of Chemotherapy in Women With Breast Cancer: An Observational Study. BMC Cancer (2019) 19(1):1073. doi: 10.1186/s12885-019-6310-0
23. Meneses-Echavez JF, Correa-Bautista JE, Gonzalez-Jimenez E, Schmidt Rio-Valle J, Elkins MR, Lobelo F, et al. The Effect of Exercise Training on Mediators of Inflammation in Breast Cancer Survivors: A Systematic Review With Meta-Analysis. Cancer Epidemiol Biomarkers Prev (2016) 25(7):1009–17. doi: 10.1158/1055-9965.EPI-15-1061
24. Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical Activity, Biomarkers, and Disease Outcomes in Cancer Survivors: A Systematic Review. J Natl Cancer Inst (2012) 104(11):815–40. doi: 10.1093/jnci/djs207
25. Jones LW, Alfano CM. Exercise-Oncology Research: Past, Present, and Future. Acta Oncol (2013) 52(2):195–215. doi: 10.3109/0284186X.2012.742564
27. Wilkins LW. ACSM’s Guidelines for Exercise Testing and Prescription, 8 ed. Lippincott Williams and Wilkins (2010).
28. Laboratories ATSCoPSfCPF. ATS Statement: Guidelines for the Six-Minute Walk Test. Am J Respir Crit Care Med (2002) 166(1):111–7. doi: 10.1164/ajrccm.166.1.at1102
29. Hojman P, Gehl J, Christensen JF, Pedersen BK. Molecular Mechanisms Linking Exercise to Cancer Prevention and Treatment. Cell Metab (2018) 27(1):10–21. doi: 10.1016/j.cmet.2017.09.015
30. Schwartz AL, de Heer HD, Bea JW. Initiating Exercise Interventions to Promote Wellness in Cancer Patients and Survivors. Oncol (Williston Park) (2017) 31(10):711–7.
31. Dethlefsen C, Pedersen KS, Hojman P. Every Exercise Bout Matters: Linking Systemic Exercise Responses to Breast Cancer Control. Breast Cancer Res Treat (2017) 162(3):399–408. doi: 10.1007/s10549-017-4129-4
32. Garcia DO, Thomson CA. Physical Activity and Cancer Survivorship. Nutr Clin Pract (2014) 29(6):768–79. doi: 10.1177/0884533614551969
33. van Vulpen JK, Siersema PD, van Hillegersberg R, Nieuwenhuijzen GAP, Kouwenhoven EA, Groenendijk RPR, et al. Physical ExeRcise Following Esophageal Cancer Treatment (PERFECT) Study: Design of a Randomized Controlled Trial. BMC Cancer (2017) 17(1):552. doi: 10.1186/s12885-017-3542-8
34. Stromberg A, Rullman E, Jansson E, Gustafsson T. Exercise-Induced Upregulation of Endothelial Adhesion Molecules in Human Skeletal Muscle and Number of Circulating Cells With Remodeling Properties. J Appl Physiol (1985) (2017) 122(5):1145–54. doi: 10.1152/japplphysiol.00956.2016
35. Rader EP, Naimo MA, Ensey J, Baker BA. VCAM-1 Upregulation Accompanies Muscle Remodeling Following Resistance-Type Exercise in Snell Dwarf (Pit1(dw/dw)) Mice. Aging Cell (2018) 17(5):e12816. doi: 10.1111/acel.12816
36. Brevetti G, De Caterina M, Martone VD, Ungaro B, Corrado F, Silvestro A, et al. Exercise Increases Soluble Adhesion Molecules ICAM-1 and VCAM-1 in Patients With Intermittent Claudication. Clin Hemorheol Microcirc (2001) 24(3):193–9.
37. Friedenreich CM, O’Reilly R, Shaw E, Stanczyk FZ, Yasui Y, Brenner DR, et al. Inflammatory Marker Changes in Postmenopausal Women After a Year-Long Exercise Intervention Comparing High Versus Moderate Volumes. Cancer Prev Res (Phila) (2016) 9(2):196–203. doi: 10.1158/1940-6207.CAPR-15-0284
38. Babina IS, Turner NC. Advances and Challenges in Targeting FGFR Signalling in Cancer. Nat Rev Cancer (2017) 17(5):318–32. doi: 10.1038/nrc.2017.8
39. Han B, Liu J, Ma MJ, Zhao L. Clinicopathological Significance of Heparanase and Basic Fibroblast Growth Factor Expression in Human Esophageal Cancer. World J Gastroenterol (2005) 11(14):2188–92. doi: 10.3748/wjg.v11.i14.2188
40. Breen EC, Johnson EC, Wagner H, Tseng HM, Sung LA, Wagner PD. Angiogenic Growth Factor mRNA Responses in Muscle to a Single Bout of Exercise. J Appl Physiol (1985) (1996) 81(1):355–61. doi: 10.1152/jappl.1996.81.1.355
41. Seida A, Wada J, Kunitomi M, Tsuchiyama Y, Miyatake N, Fujii M, et al. Serum bFGF Levels Are Reduced in Japanese Overweight Men and Restored by a 6-Month Exercise Education. Int J Obes Relat Metab Disord (2003) 27(11):1325–31. doi: 10.1038/sj.ijo.0802408
42. Thorn M, Raab Y, Larsson A, Gerdin B, Hallgren R. Intestinal Mucosal Secretion of Basic Fibroblast Growth Factor in Patients With Ulcerative Colitis. Scand J Gastroenterol (2000) 35(4):408–12. doi: 10.1080/003655200750023985
43. Bousvaros A, Zurakowski D, Fishman SJ, Keough K, Law T, Sun C, et al. Serum Basic Fibroblast Growth Factor in Pediatric Crohn’s Disease. Implications for Wound Healing. Dig Dis Sci (1997) 42(2):378–86. doi: 10.1023/a:1018882322566
44. Di Sabatino A, Ciccocioppo R, Armellini E, Morera R, Ricevuti L, Cazzola P, et al. Serum bFGF and VEGF Correlate Respectively With Bowel Wall Thickness and Intramural Blood Flow in Crohn’s Disease. Inflammation Bowel Dis (2004) 10(5):573–7. doi: 10.1097/00054725-200409000-00011
45. Wiktorowska-Owczarek A. The Effect of Diclofenac on Proliferation and Production of Growth Factors by Endothelial Cells (HMEC-1) Under Hypoxia and Inflammatory Conditions. Acta Pharm (2014) 64(1):131–8. doi: 10.2478/acph-2014-0006
46. Zittermann SI, Issekutz AC. Basic Fibroblast Growth Factor (bFGF, FGF-2) Potentiates Leukocyte Recruitment to Inflammation by Enhancing Endothelial Adhesion Molecule Expression. Am J Pathol (2006) 168(3):835–46. doi: 10.2353/ajpath.2006.050479
47. Lord RV, Park JM, Wickramasinghe K, DeMeester SR, Oberg S, Salonga D, et al. Vascular Endothelial Growth Factor and Basic Fibroblast Growth Factor Expression in Esophageal Adenocarcinoma and Barrett Esophagus. J Thorac Cardiovasc Surg (2003) 125(2):246–53. doi: 10.1067/mtc.2003.203
48. Mulligan AM, Raitman I, Feeley L, Pinnaduwage D, Nguyen LT, O’Malley FP, et al. Tumoral Lymphocytic Infiltration and Expression of the Chemokine CXCL10 in Breast Cancers From the Ontario Familial Breast Cancer Registry. Clin Cancer Res (2013) 19(2):336–46. doi: 10.1158/1078-0432.CCR-11-3314
49. Lunardi S, Jamieson NB, Lim SY, Griffiths KL, Carvalho-Gaspar M, Al-Assar O, et al. IP-10/CXCL10 Induction in Human Pancreatic Cancer Stroma Influences Lymphocytes Recruitment and Correlates With Poor Survival. Oncotarget (2014) 5(22):11064–80. doi: 10.18632/oncotarget.2519
50. Blank S, Nienhuser H, Dreikhausen L, Sisic L, Heger U, Ott K, et al. Inflammatory Cytokines Are Associated With Response and Prognosis in Patients With Esophageal Cancer. Oncotarget (2017) 8(29):47518–32. doi: 10.18632/oncotarget.17671
51. Mendelson M, Michallet AS, Monneret D, Perrin C, Esteve F, Lombard PR, et al. Impact of Exercise Training Without Caloric Restriction on Inflammation, Insulin Resistance and Visceral Fat Mass in Obese Adolescents. Pediatr Obes (2015) 10(4):311–9. doi: 10.1111/ijpo.255
52. Meyer JD, Hayney MS, Coe CL, Ninos CL, Barrett BP. Differential Reduction of IP-10 and C-Reactive Protein via Aerobic Exercise or Mindfulness-Based Stress-Reduction Training in a Large Randomized Controlled Trial. J Sport Exerc Psychol (2019) 41(2):96–106. doi: 10.1123/jsep.2018-0214
53. Kazeem A, Olubayo A, Ganiyu A. Plasma Nitric Oxide and Acute Phase Proteins After Moderate and Prolonged Xercises. Iran J Basic Med Sci (2012) 15(1):602–7.
54. Khazaei M. Chronic Low-Grade Inflammation After Exercise: Controversies. Iran J Basic Med Sci (2012) 15(5):1008–9.
55. Crescioli C, Sottili M, Bonini P, Cosmi L, Chiarugi P, Romagnani P, et al. Inflammatory Response in Human Skeletal Muscle Cells: CXCL10 as a Potential Therapeutic Target. Eur J Cell Biol (2012) 91(2):139–49. doi: 10.1016/j.ejcb.2011.09.011
56. Fabbi M, Carbotti G, Ferrini S. Dual Roles of IL-27 in Cancer Biology and Immunotherapy. Mediators Inflamm (2017) 2017:3958069. doi: 10.1155/2017/3958069
57. Kourko O, Seaver K, Odoardi N, Basta S, Gee K. IL-27, IL-30, and IL-35: A Cytokine Triumvirate in Cancer. Front Oncol (2019) 9:969. doi: 10.3389/fonc.2019.00969
58. Diakowska D, Lewandowski A, Markocka-Maczka K, Grabowski K. Concentration of Serum Interleukin-27 Increase in Patients With Lymph Node Metastatic Gastroesophageal Cancer. Adv Clin Exp Med (2013) 22(5):683–91.
59. Villarino AV, Huang E, Hunter CA. Understanding the Pro- and Anti-Inflammatory Properties of IL-27. J Immunol (2004) 173(2):715–20. doi: 10.4049/jimmunol.173.2.715
60. Fix DK, Hardee JP, Gao S, VanderVeen BN, Velazquez KT, Carson JA. Role of Gp130 in Basal and Exercise-Trained Skeletal Muscle Mitochondrial Quality Control. J Appl Physiol (1985) (2018) 124(6):1456–70. doi: 10.1152/japplphysiol.01063.2017
61. Zajkowska M, Mroczko B. Eotaxins and Their Receptor in Colorectal Cancer-A Literature Review. Cancers (Basel) (2020) 12(6):1383. doi: 10.3390/cancers12061383
62. Lan Q, Lai W, Zeng Y, Liu L, Li S, Jin S, et al. CCL26 Participates in the PRL-3-Induced Promotion of Colorectal Cancer Invasion by Stimulating Tumor-Associated Macrophage Infiltration. Mol Cancer Ther (2018) 17(1):276–89. doi: 10.1158/1535-7163.MCT-17-0507
63. Ishida Y, Kido A, Akahane M, Kishi S, Tsukamoto S, Fujii H, et al. Mesenchymal Stem Cells Up-Regulate the Invasive Potential of Prostate Cancer Cells via the Eotaxin-3/CCR3 Axis. Pathol Res Pract (2018) 214(9):1297–302. doi: 10.1016/j.prp.2018.06.012
64. Gonzalez-Ruiz K, Correa-Bautista JE, Izquierdo M, Garcia-Hermoso A, Dominguez-Sanchez MA, Bustos-Cruz RH, et al. Effects of an Exercise Program on Hepatic Metabolism, Hepatic Fat, and Cardiovascular Health in Overweight/Obese Adolescents From Bogota, Colombia (the HEPAFIT Study): Study Protocol for a Randomized Controlled Trial. Trials (2018) 19(1):330. doi: 10.1186/s13063-018-2721-5
65. Nielsen AR, Mounier R, Plomgaard P, Mortensen OH, Penkowa M, Speerschneider T, et al. Expression of Interleukin-15 in Human Skeletal Muscle Effect of Exercise and Muscle Fibre Type Composition. J Physiol (2007) 584(Pt 1):305–12. doi: 10.1113/jphysiol.2007.139618
66. Ficek K, Cieszczyk P, Leznicka K, Kaczmarczyk M, Leonska-Duniec A. Novel Associations Between Interleukin-15 Polymorphisms and Post-Training Changes of Body Composition Parameters in Young Nonobese Women. Front Physiol (2019) 10:876. doi: 10.3389/fphys.2019.00876
67. Riechman SE, Balasekaran G, Roth SM, Ferrell RE. Association of Interleukin-15 Protein and Interleukin-15 Receptor Genetic Variation With Resistance Exercise Training Responses. J Appl Physiol (1985) (2004) 97(6):2214–9. doi: 10.1152/japplphysiol.00491.2004
68. Friedenreich CM, Neilson HK, Woolcott CG, Wang Q, Stanczyk FZ, McTiernan A, et al. Inflammatory Marker Changes in a Yearlong Randomized Exercise Intervention Trial Among Postmenopausal Women. Cancer Prev Res (Phila) (2012) 5(1):98–108. doi: 10.1158/1940-6207.CAPR-11-0369
Keywords: esophagogastric cancer, exercise training, cardiorespiratory fitness, biomarker analysis, multidisciplinary rehabilitation treatment, cancer survivorship, cancer rehabilitation
Citation: Kennedy SA, Annett SL, Dunne MR, Boland F, O’Neill LM, Guinan EM, Doyle SL, Foley EK, Elliott JA, Murphy CF, Bennett AE, Carey M, Hillary D, Robson T, Reynolds JV, Hussey J and O’Sullivan J (2021) Effect of the Rehabilitation Program, ReStOre, on Serum Biomarkers in a Randomized Control Trial of Esophagogastric Cancer Survivors. Front. Oncol. 11:669078. doi: 10.3389/fonc.2021.669078
Received: 17 February 2021; Accepted: 17 August 2021;
Published: 15 September 2021.
Edited by:
Ziwen Liu, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Rodolfo Paula Vieira, Brazil University, BrazilCopyright © 2021 Kennedy, Annett, Dunne, Boland, O’Neill, Guinan, Doyle, Foley, Elliott, Murphy, Bennett, Carey, Hillary, Robson, Reynolds, Hussey and O’Sullivan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jacintha O’Sullivan, b3N1bGxpajRAdGNkLmll
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.