
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 22 April 2021
Sec. Cancer Imaging and Image-directed Interventions
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.582847
This article is part of the Research Topic Breakthrough in Imaging-Guided Precision Medicine in Oncology View all 35 articles
Background: Our goal was to establish and verify a radiomics risk grading model for gastrointestinal stromal tumors (GISTs) and to identify the optimal algorithm for risk stratification.
Methods: We conducted a retrospective analysis of 324 patients with GISTs, the presence of which was confirmed by surgical pathology. Patients were treated at three different hospitals. A training cohort of 180 patients was collected from the largest center, while an external validation cohort of 144 patients was collected from the other two centers. To extract radiomics features, regions of interest (ROIs) were outlined layer by layer along the edge of the tumor contour on CT images of the arterial and portal venous phases. The dimensionality of radiomic features was reduced, and the top 10 features with importance value above 5 were selected before modeling. The training cohort used three classifiers [logistic regression, support vector machine (SVM), and random forest] to establish three GIST risk stratification prediction models. The receiver operating characteristic curve (ROC) was used to compare model performance, which was validated by external data.
Results: In the training cohort, the average area under the curve (AUC) was 0.84 ± 0.07 of the logistic regression, 0.88 ± 0.06 of the random forest, and 0.81 ± 0.08 of the SVM. In the external validation cohort, the AUC was 0.85 of the logistic regression, 0.90 of the random forest, and 0.80 of the SVM. The random forest model performed the best in both the training and the external validation cohorts and could be generalized.
Conclusion: Based on CT radiomics, there are multiple machine-learning models that can predict the risk of GISTs. Among them, the random forest algorithm had the highest prediction efficiency and could be readily generalizable. Through external validation data, we assume that the random forest model may be used as an effective tool to guide preoperative clinical decision-making.
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the digestive system, which occur in the stomach and small intestine. GISTs have a variety of biological characteristics and cannot be simply categorized as benign or malignant (1). For example, some small GISTs can progress rapidly and metastasize to the liver, while some large GISTs, even those not receiving the post-operative adjuvant treatment, present no long-term risk of recurrence or metastasis (2). Therefore, the preoperative evaluation of the malignant potential of GISTs is crucial for treatment decision-making.
Risk stratification is commonly applied to evaluate the biological behaviors and overall clinical outcome of GISTs. Currently, the most recognized criterion is the improved National Institutes of Health risk stratification standard introduced by Joensuu in 2008 (3), which is based on tumor maximum diameter and mitotic count and introduces two parameters: tumor site and tumor rupture. The risk of relapse is thereby divided into four categories: very low risk, low risk, intermediate risk, and high risk. Higher risk generally indicates a worse prognosis. Also, the introduction of imatinib mesylate has greatly changed the outcomes in high-risk GIST patients (4). The need for reliable preoperative risk stratification is of great significance for the development of treatment methods and prognostic evaluation. Most surgeries can completely remove the GISTs without first conducting a preoperative biopsy (5), which may cause tumor ulceration and bleeding, increasing the risk of tumor spread. Therefore, it is of great clinical value to explore non-invasive, reliable, and simple biomarkers for predicting the recurrence and metastasis risk of GISTs before surgery.
Previous GIST risk stratification research is largely based on analysis of computed tomography (CT) images (4, 6–9), which is likely influenced by the observer's subjective assessment. Therefore, an objective and quantitative technique is urgently needed for the accurate risk stratification of GISTs. Radiomics converts medical images into high-dimensional data that can be mined, which holds great potential for application in disease diagnosis, identification, and prognosis predictions (10–13).There are studies have examined the utility of radiomics in GIST risk stratification (14–16) and have achieved favorable results. However, most of these studies are single-center trials, whose prediction models have not been externally verified. Therefore, the generalizability of these models remains unclear. In addition, previous studies used a single classifier for modeling, due to the obvious differences in classifier algorithms (17), and such studies are unable to determine the classifier with the best performance in risk prediction.
In response to these shortcomings, we conducted a multiclassifier and multicenter GISTs radiomics study, applying the three most commonly used machine-learning classifiers in radiomics to the same cohort of data to evaluate and compare the performance of the classifiers. Also, the model was tested with independent external data to further evaluate its generalizability to provide a reference for clinical treatment decisions.
Data from a total of 324 patients with GISTs presenting from January 1, 2016 to July 1, 2019 were collected retrospectively from three hospitals. Among them, 180 cases were analyzed from the First Affiliated Hospital of Zhejiang University School of Medicine, which was used as the training cohort, while 144 cases from another two hospitals (Zhejiang Cancer Hospital and the First Affiliated Hospital of Wannan Medical College) were used as the external validation cohort. The inclusion criteria were as follows: (1) surgical resection, negative margin, and a pathological diagnosis of GISTs, (2) abdominal enhancement CT examination within 15 days prior to surgery, and (3) pathological results with a clear risk assessment. Exclusion criteria were as follows: (1) patients receiving imatinib or other neoadjuvant therapy before surgery, and (2) those with poor CT image quality.
Clinical data, including age, gender, and tumor site, were derived from medical records. The National Institutes of Health's modified criteria were used to stratify the malignant potential of GISTs on the basis of the clinical and post-operative histological index. All patients were divided into two groups: high malignant potential group with intermediate risk and high risk; and low malignant potential group with very low risk and low risk. This study was a retrospective study, and the patient's informed consent was thereby waived, as approved by the hospital ethics committee.
All subjects received a default abdominal CT scan using one of the three multidetector CT (MDCT) systems with the scanning and reconstruction parameters used in daily clinical practice. See Table 1 for the detailed information of the CT protocol. Three-phase scans were unenhanced phase, arterial phase (25–30 s after injection), and portal vein phase (55–60 s after injection). The dose of iodine contrast agent was based on the patient's weight (1 mL/kg), and the flow rate was 2.5–3.5 mL/s.
Both tumor segmentation and radiomics feature extraction were performed using Matlab's IBEX software package (18). Two radiologists with a depth of experience delineated the regions of interest (ROIs) layer by layer along the edge of the tumor contour on the CT images of the arterial and portal venous phases.
All images were preprocessed with image resampling (voxel size of 1 × 1 × 1 mm3) and gray value homogenization (normalized to 1–256, fixed bin number method, 256 bins) before radiomics feature extraction. The radiological feature parameters involved six major categories: histogram parameters (n = 48), 2.5D and 3D gray level co-occurence matrix (n = 594, the 2.5D feature is computed from a single matrix after merging all 2D directional matrices, the 3D feature is computed from a single matrix after merging all 3D directional), gray level adjacent difference (n = 10), gray level run length matrix (n = 34), shape and size (n = 18). In each stage, we retrieved 704 parameters, and a total of 1,408 parameters were collected in the two stages.
During the early stage of the study, we randomly selected images from 40 patients, and two radiologists with more than 10 years of work experience performed ROI delineation independently. The blindness method was used to analyze the reliability and repeatability between observers. The consistency was evaluated using the intra-class correlation coefficient (ICC). There is a good agreement when the ICC is > 0.75. ROI extraction of the remaining images was performed by one of the radiologists.
Redundancy and overcorrelation in the characteristics of radiomics often lead to overfitting of the prediction model. In this study, we dimensionally reduced the radiomics features in two steps. First, multicollinearity of the features were analyzed by spearman correlation, and the correlation coefficient threshold was 0.8. Then, we used the boruta algorithm to iteratively assess the importance of features, and we removed the irrelevant features. Boruta algorithm can filter out all the characteristics related to the dependent variable and generate a ranking of importance. To achieve statistical significance, the top 10 features in importance ranking were selected for final modeling.
After dimensionality reduction of the radiomics features, the three most popular classifiers [logistic regression, support vector machine (SVM), and random forest] were applied to establish three risk stratification models for radiological prediction. We conducted holdout cross-validation for 30 times for each model in the training cohort (training: internal validation ratio is 4:1). Because each iteration is a resampling of the training cohort, each model yielded 30 different values of area under the curve (AUC), specificity, sensitivity, and accuracy, among which we used AUC as the standard to evaluate the effectiveness of the three models in the training cohort.
Subsequently, the three models were applied to the external validation cohort, and the effectiveness of the models were also evaluated through AUC, specificity, sensitivity, and accuracy.
All statistical analysis was performed using R software (version 3.4.1; http://www.Rproject.org). We performed descriptive statistical analysis for the training and external validation cohorts, and quantitative data was described as mean ± standard deviation (SD) and qualitative data was described by frequency (percent). Qualitative variables were compared using the chi-square test. Continuous variable data was evaluated using a two-sample t-test or Wilcoxon test. AUC was used as the evaluation standard for the comparison of the three classification algorithms in the training cohort. The Fridman test was used for the comparison among the three algorithms, and the Nemenyi test was used in post-hoc analysis. Two tailed p < 0.05 was considered statistically significant.
In total, 324 GIST patients were included in this study, of which 150 patients had low malignant potential and 174 patients had high malignant potential. Ninety-three men and 87 women were included in the training cohort, and 64 men and 80 women were included in the external validation cohort. Table 2 shows the baseline clinical data. Single factor analysis showed that there was no statistically significant difference between the low and the high malignant potential groups in terms of age, gender, and tumor site.
After dimension reduction by spearman correlation, we obtained 107 features, which through the dimension reduction by boruta algorithm, 25 parameters remained, from which we extracted the top 10 features, according to the built-in importance-ranking system. In the subset, parameters from the portal venous phase accounted for 80%. Morphology ranks the most important, although only one parameter was selected. See Table 3 for a list of specific parameters and their importance.
The specific performance of the three classifier prediction models is shown in Table 4 and Figures 1, 2. The Friedman test indicated that the AUC value of the three models in the training cohort was significantly different (p < 0.001). The Nemenyi test results show that the AUC of random forest was significantly higher than logistic regression (p = 0.001), significantly higher than SVM (p = 0.0103), and there was no significant statistics between logisitic regression and SVM (p = 0.09). The Friedman-Nemenyi test indicated that the AUC value of the random forest model was significantly higher than that of the other two prediction models. The random forest model achieved the most satisfactory results; the performance and generalizability were favorable. The performance of the SVM and logistic regression models were satisfactory, and the generalizability was acceptable, but the overall efficiency was not outstanding.
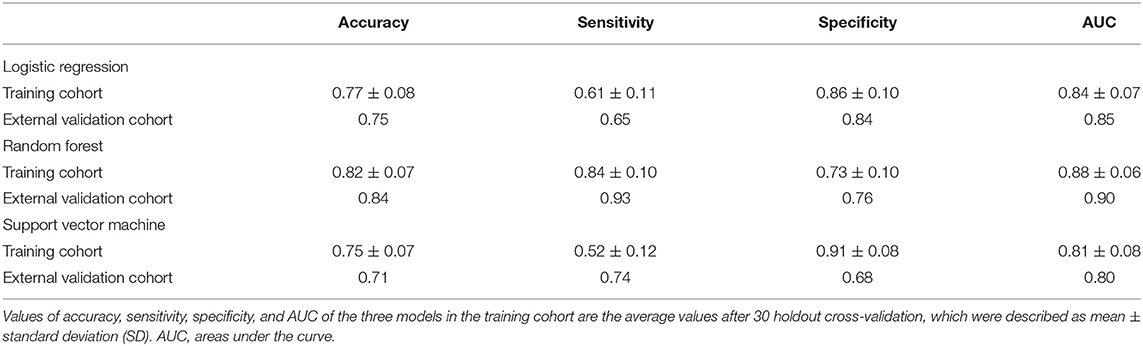
Table 4. A performance summary of the radiomics models in the training and external validation cohorts.
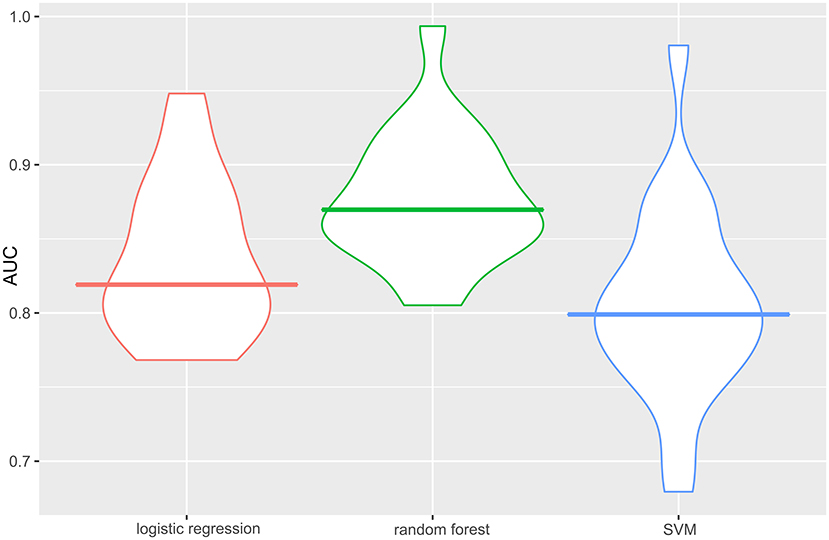
Figure 1. AUC of the three classifier prediction models performance in the training cohort. The random forest model achieved the best satisfactory results. The AUC is the average AUC obtained after 30 holdout cross-validation. The horizontal line of each diagram corresponds to the average AUC. AUC, the area under the curve; SVM, support vector machine.
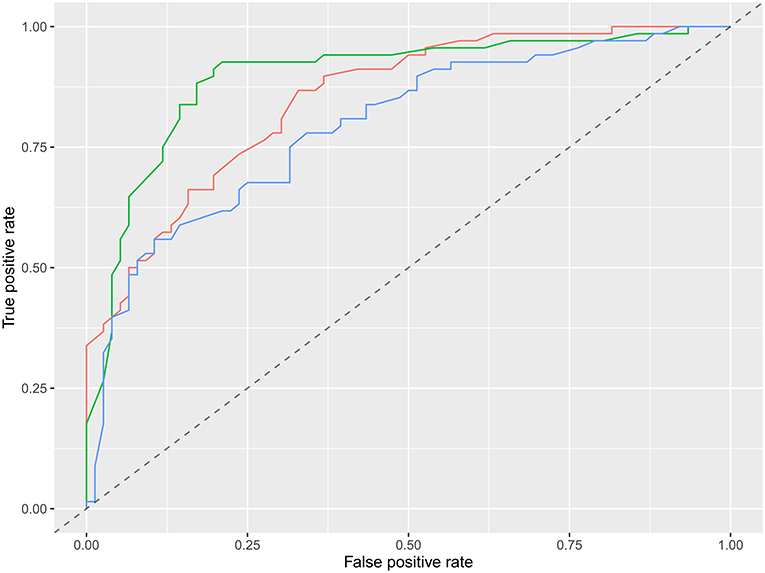
Figure 2. ROC diagram of multiple models in the external validation cohort. Red is logistic regression, green is random forest, and blue is support vector machine.
In this study, we built three prediction models based on CT radiomics for GIST risk stratification. After comparing the three most commonly used machine-learning models in radiomics, we found the random forest model showed the best performance in discriminating GISTs malignant potentials, and its generalizability is outstanding.
GISTs often exhibit complex and unpredictable biological behaviors. With the development of molecular pathology research, imatinib has emerged as a first-line molecular targeted drug, which has changed the treatment of GISTs and has become a successful model for the targeted diagnosis and treatment of solid tumors. The stratification of patients based on the risk of recurrence is a key issue in managing primary GISTs. The National Comprehensive Cancer Network guidelines recommend more than 3 years of post-operative imatinib be used as an adjuvant therapy for patients with a high recurrence risk (high-risk and intermediate-risk) (19, 20), while patients with a low recurrence risk (low-risk and very low-risk) that can be cured via surgical resection of the tumor should not receive adjuvant therapy with imatinib (21–23). Therefore, in this study, GIST patients were classified into low and high malignant potential groups according to the risk stratification. Because the clinical characteristics of GISTs lack specificity, the preoperative diagnosis and risk stratification of GISTs mainly rely on imaging examinations. Traditional imaging evaluates the risk of GISTs by observing the size, shape, presence or absence of necrosis, ulcers, and enhancement of GISTs, and the results depend much on the professional ability and subjective experience of radiologists (4, 6–9).
The rise in the use of radiomics in recent years has resulted in imaging studies to predict GISTs recurrence risks using objective and quantitative measures. Currently, most GISTs radiomics studies focus on risk prediction, and the AUC is relatively high at ~0.81–0.94 (15, 19, 24–27), demonstrating the superiority of radiomics over traditional methods in terms of prediction effectiveness. It also lays foundation for the future application of radiomics for GIST risk stratification. However, only one study has also conducted external data validation of the model (24). Its model efficiency was 0.87 in the training cohort and 0.85 in the external validation cohort. Although the performance of the model was not optimal, this study has published the most standardized and reliable results to date. There is no external validation for the other studies; the same data were used for the training and validation cohort, making the results less convincing (28). Studies have confirmed that equipment from different manufacturers results in differences in scanning parameter settings and post-processing reconstruction algorithms, resulting in significant differences in the radiomics parameters (29–31). Therefore, single-center research has its limitations (32). Multicenter research can provide diverse imaging data to better interpret tumor heterogeneity, which is also in line with the development of precision medicine (33). The highlight of this research lies in its multicenter design, which uses the largest amount of data among the three hospitals as the training cohort, while the data from the other two hospitals are fused into an independent external validation cohort. We found that the AUC of the random forest model in the training cohort was 0.88 ± 0.06, which was very good in both the training cohort and the validation cohort, indicating that the generalizability of the model is excellent. Our study confirms the potential of radiomics in GISTs diagnosis and prognosis, and it proposes that the predicted models must undergo multicenter testing before providing a reliable reference for clinical decision-making (34).
Different machine-learning algorithms have their own advantages and disadvantages. The performance of an algorithm in a specific machine-learning task cannot be predicted before research. Most previous radiomics studies used a single algorithm for modeling, and no specific reason was stated for choosing the model. Currently, the most common GIST risk stratification models are logistic regression, SVM, and random forest. Logistic regression is the most commonly used classification algorithm in the medical field (35) and in GISTs imaging histology. Wang et al. (26). collected 333 GISTs cases, and the AUC of the training cohort was 0.88. Ren et al. (27) also used logistic regression with 440 cases, and the final AUC of the training cohort was 0.93. SVM has many advantages in processing small samples and non-linear and high-dimensional data. Chen et al. chose SVM to build a prediction model, and the AUC was 0.86 in the training cohort and 0.85 in the external validation cohort. Random forest is a type of integrated machine learning, which is based on the decision tree method and can improve the prediction accuracy without significantly increasing the amount of calculation (36). Zhang et al. (19) used a random forest algorithm to predict GIST risk stratification, and achieved an AUC of 0.94 of the training cohort, which is the best performance among similar studies. These studies have their own advantages, but due to the heterogeneity between the data cohorts, the differences of the classifiers cannot be clarified. Hence, it is impossible to determine which classifier is the most suitable for stratifying the GIST risk. In this study, we conducted a multiclassification algorithm study on the same data and task and found that logistic regression and SVM performed stably, but the overall efficiency was not outstanding. Random forest performed the best in both the training and external validation cohorts, with the highest AUC and excellent generalizability, which indicated that this method is worthy of in-depth study and verification with a larger sample set and data from a multicenter study.
However, our study has the following limitations: (1) Our sample size was relatively small, and limited to Chinese people. As genetic mutations are the driving factors in the occurrence of GISTs, and the morbidity and mortality of GISTs varies among different races, it is necessary to conduct further in-depth studies on large samples of multinational and multiethnic populations, ideally in multicenter trials. (2) Because most of the previous articles suggested clinical parameters were not significant, this study used pure radiomic modeling and did not integrate clinical parameters for further analysis and comparison. (3) This study was a retrospective study, and the sample selection was biased, which requires further verification in prospective studies. (4) As the CT imaging protocols varies in different hospitals, radiomics features are affected by CT scanner parameters, such as reconstruction kernel or section thickness, thus obscuring underlying biologically important radiomics parameters. We did not process the data from multicenter with harmonization. Some features of IBEX are not compatible with IBSI (Image Biomarker Standardisation Initiative), which will affect the reproducibility of the results. (5) The algorithm of feature selection also affects the model performance. We did not compare the algorithms of dimensionality reduction; therefore, the final feature selection may not be the optimal.
In conclusion, this study predicts the risks of GISTs based on different machine-learning models of CT radiomics. After comparing the three most commonly used machine-learning algorithms in radiomics, a radiomics model of the random forest algorithm presents the most satisfactory prediction. The efficacy, optimal discrimination, strong generalizability, and confirmation in external validation data can be used as a more objective and non-invasive technique, which has the potential to become an effective tool for clinicians to predict the risk stratification of GISTs before surgery.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional review board of the Affiliated Hospital of College of Medicine Zhejiang University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
MW and ZF proposed the conception and design of this research and analyzed and interpreted the data. ZF and LZho developed methodology. MW, ZF, LZha, and XH collected data and performed preprocessing. MW, ZF, and JZ were major contributors in writing the manuscript. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Raut CP, Espat NJ, Maki RG, Araujo DM, Trent J, Williams TF, et al. Efficacy and tolerability of 5-year adjuvant imatinib treatment for patients with resected intermediate- or high-risk primary gastrointestinal stromal tumor: the PERSIST-5 clinical trial. JAMA Oncol. (2018) 4:e184060. doi: 10.1001/jamaoncol.2018.4060
2. Gill AJ, Angela C, Ricardo V, Adele C, Millie L, Jin R, et al. Immunohistochemistry for SDHB divides gastrointestinal stromal tumors (GISTs) into 2 distinct types. Am J Surg Pathol. (2010) 34:636–44. doi: 10.1097/PAS.0b013e3181d6150d
3. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. (2008) 39:1411–9. doi: 10.1016/j.humpath.2008.06.025
4. Maldonado FJ, Sheedy SP, Iyer VR, Hansel SL, Bruining DH, Mccollough CH, et al. Reproducible imaging features of biologically aggressive gastrointestinal stromal tumors of the small bowel. Abdom Radiol. (2017) 43:1567–74. doi: 10.1007/s00261-017-1370-6
5. Li J, Ye Y, Wang J, Zhang B, Qin S, Shi Y, et al. Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor. Chin J Cancer Res. (2017) 29:281–93. doi: 10.21147/j.issn.1000-9604.2017.04.01
6. Wang JK. Predictive value and modeling analysis of MSCT signs in gastrointestinal stromal tumors (GISTs) to pathological risk degree. Eur Rev Med Pharmacol Sci. (2017) 21:999–1005.
7. Zhou C, Duan X, Zhang X, Hu H, Wang D, Shen J. Predictive features of CT for risk stratifications in patients with primary gastrointestinal stromal tumour. Eur Radiol. (2016) 26:3086–93. doi: 10.1007/s00330-015-4172-7
8. Su Q, Wang Q, Zhang H, Yu D, Wang Y, Liu Z, et al. Computed tomography findings of small bowel gastrointestinal stromal tumors with different histologic risks of progression. Abdom Radiol. (2018) 43:2651–8. doi: 10.1007/s00261-018-1511-6
9. Xue A, Yuan W, Gao X, Fang Y, Shu P, Xu C, et al. Gastrointestinal stromal tumors (GISTs) with remarkable cystic change: a specific subtype of GISTs with relatively indolent behaviors and favorable prognoses. J Cancer Res Clin Oncol. (2019) 145:1559–68. doi: 10.1007/s00432-019-02853-y
10. Vivek V, Simone CB, Sunil K, Lin SH, Yang J, Hahn SM. The rise of radiomics and implications for oncologic management. J Natl Cancer Inst. (2017) 109:djx055. doi: 10.1093/jnci/djx055
11. Liang C, Huang Y, He L, Chen X, Ma Z, Dong D, et al. The development and validation of a CT-based radiomics signature for the preoperative discrimination of stage I-II and stage III-IV colorectal cancer. Oncotarget. (2016) 7:31401–12. doi: 10.18632/oncotarget.8919
12. Lu W, Chen W. Positron emission tomography/computerized tomography for tumor response assessment—a review of clinical practices and radiomics studies. Transl Cancer Res. (2016) 5:364–70. doi: 10.21037/tcr.2016.07.12
13. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. (2015) 278:151169. doi: 10.1148/radiol.2015151169
14. Choi IY, Yeom SK, Cha J, Cha SH, Lee SH, Chung HH, et al. Feasibility of using computed tomography texture analysis parameters as imaging biomarkers for predicting risk grade of gastrointestinal stromal tumors: comparison with visual inspection. Abdom Radiol. (2019) 44:2346–56. doi: 10.1007/s00261-019-01995-4
15. Feng C, Lu F, Shen Y, Li A, Yu H, Tang H, et al. Tumor heterogeneity in gastrointestinal stromal tumors of the small bowel: volumetric CT texture analysis as a potential biomarker for risk stratification. Cancer Imaging. (2018) 18:46. doi: 10.1186/s40644-018-0182-4
16. Yarandi RB, Panahi MH. Noninvasive prediction model for diagnosing gastrointestinal stromal tumors using contrast-enhanced harmonic endoscopic ultrasound: Methodological issues. Dig Liver Dis. (2019) 51:1355–6. doi: 10.1016/j.dld.2019.04.017
17. Demiar J, Schuurmans D. Statistical comparisons of classifiers over multiple data sets. J Mach Learn Res. (2006) 7:1–30. doi: 10.1007/s10846-005-9016-2
18. Zhang L, Fried DV, Fave XJ, Hunter LA, Yang J, Court LE. IBEX: An open infrastructure software platform to facilitate collaborative work in radiomics. Med Phys. (2015) 42:1341–53. doi: 10.1118/1.4908210
19. Zhang L, Kang L, Li G, Zhang X, Ren J, Shi Z, et al. Computed tomography-based radiomics model for discriminating the risk stratification of gastrointestinal stromal tumors. Radiol Med. (2020) 125:465–73. doi: 10.1007/s11547-020-01138-6
20. Blay JY, Levard A. Adjuvant imatinib treatment in gastrointestinal stromal tumor: which risk stratification criteria and for how long? A case report. Anti Cancer Drugs. (2016) 27:71–5. doi: 10.1097/CAD.0000000000000286
21. Rutkowski P, Przybył J, Zdzienicki M. Extended adjuvant therapy with imatinib in patients with gastrointestinal stromal tumors. Mol Diagn Ther. (2013) 17:9–19. doi: 10.1007/s40291-013-0018-7
22. Blay JY, Rutkowski P. Adherence to imatinib therapy in patients with gastrointestinal stromal tumors. Cancer Treat Rev. (2014) 40:242–7. doi: 10.1016/j.ctrv.2013.07.005
23. López RL, Del Muro XG. Management of localized gastrointestinal stromal tumors and adjuvant therapy with imatinib. Anti Cancer Drugs. (2012) 23:S3–6. doi: 10.1097/CAD.0b013e3283559fab
24. Chen T, Ning Z, Xu L, Feng X, Han S, Roth HR, et al. Radiomics nomogram for predicting the malignant potential of gastrointestinal stromal tumours preoperatively. Eur Radiol. (2019) 29:1074–82. doi: 10.1007/s00330-018-5629-2
25. Liu S, Pan X, Liu R, Zheng H, Chen L, Guan W, et al. Texture analysis of CT images in predicting malignancy risk of gastrointestinal stromal tumours. Clin Radiol. (2017) 73:266–74. doi: 10.1016/j.crad.2017.09.003
26. Wang C, Li H, Jiaeken Y, Huang P, Sun L, Dong F, et al. Building CT radiomics-based models for preoperatively predicting malignant potential and mitotic count of gastrointestinal stromal tumors. Transl Oncol. (2019) 12:1229–36. doi: 10.1016/j.tranon.2019.06.005
27. Ren C, Wang S, Zhang S. Development and validation of a nomogram based on CT images and 3D texture analysis for preoperative prediction of the malignant potential in gastrointestinal stromal tumors. Cancer Imaging. (2020) 20:5. doi: 10.1186/s40644-019-0284-7
28. Hunter LA, Krafft S, Stingo F, Choi H, Martel MK, Kry SF, et al. High quality machine-robust image features: identification in nonsmall cell lung cancer computed tomography images. Med Phys. (2013) 40:121916. doi: 10.1118/1.4829514
29. Mackin D, Fave X, Zhang L, Fried D, Yang J, Taylor B, et al. Measuring computed tomography scanner variability of radiomics features. Invest Radiol. (2015) 50:757–65. doi: 10.1097/RLI.0000000000000180
30. Parmar C, Grossmann P, Bussink J, Lambin P, Aerts HJWL. Machine learning methods for quantitative radiomic biomarkers. Sci Rep. (2015) 5:13087. doi: 10.1038/srep13087
31. Balagurunathan Y, Gu Y, Wang H, Kumar V, Grove O, Hawkins S, et al. Reproducibility and prognosis of quantitative features extracted from CT images. Transl Oncol. (2014) 7:72–87. doi: 10.1593/tlo.13844
32. Orlhac F, Frouin F, Nioche C, Ayache N, Buvat I. Validation of a method to compensate multicenter effects affecting CT radiomics. Radiology. (2019) 291:53–9. doi: 10.1148/radiol.2019182023
33. Zhao B, Tan Y, Tsai WY, Schwartz LH, Lu L. Exploring variability in CT characterization of tumors: a preliminary phantom study. Transl Oncol. (2014) 7:88–93. doi: 10.1593/tlo.13865
34. Caramella C, Allorant A, Orlhac F, Bidault F, Asselain B, Ammari S, et al. Can we trust the calculation of texture indices of CT images? A phantom study. Med Phys. (2018) 45:1529. doi: 10.1002/mp.12809
35. Nakasone Y, Ikeda O, Yamashita Y, Kudoh K, Shigematsu Y, Harada K. Shock index correlates with extravasation on angiographs of gastrointestinal hemorrhage: a logistics regression analysis. Cardiovasc Intervent Radiol. (2007) 30:861–5. doi: 10.1007/s00270-007-9131-5
Keywords: gastrointestinal stromal tumor, risk classification, radiomics, X-ray computer, multi-classifier
Citation: Wang M, Feng Z, Zhou L, Zhang L, Hao X and Zhai J (2021) Computed-Tomography-Based Radiomics Model for Predicting the Malignant Potential of Gastrointestinal Stromal Tumors Preoperatively: A Multi-Classifier and Multicenter Study. Front. Oncol. 11:582847. doi: 10.3389/fonc.2021.582847
Received: 13 July 2020; Accepted: 19 February 2021;
Published: 22 April 2021.
Edited by:
Romain-David Seban, Institut Curie, FranceCopyright © 2021 Wang, Feng, Zhou, Zhang, Hao and Zhai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Zhai, 1181366339@qq.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.