
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 20 December 2023
Sec. Cancer Imaging and Image-directed Interventions
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1310452
This article is part of the Research TopicPrecision Medical Imaging for Cancer Diagnosis and Treatment - Vol. IIView all 35 articles
 Elisa Tassinari1†
Elisa Tassinari1† Nicole Conci1†
Nicole Conci1† Giacomo Battisti2
Giacomo Battisti2 Francesco Porta3
Francesco Porta3 Valerio Di Scioscio3
Valerio Di Scioscio3 Maria Giulia Pirini4
Maria Giulia Pirini4 Dario de Biase5
Dario de Biase5 Maria Concetta Nigro1
Maria Concetta Nigro1 Miriam Iezza1,6
Miriam Iezza1,6 Fausto Castagnetti1,6
Fausto Castagnetti1,6 Luigi Lovato3
Luigi Lovato3 Stefano Fanti2
Stefano Fanti2 Maria Abbondanza Pantaleo1,7
Maria Abbondanza Pantaleo1,7 Margherita Nannini1,7*
Margherita Nannini1,7*Background: Positron emission tomography (PET) with 18-fluorodeoxyglucose (18FDG) has proven to be highly sensitive in the early assessment of tumor response in gastrointestinal stromal tumors (GIST), especially in cases where there is doubt or when the early prediction of the response could be clinically useful for patient management. As widely known, kinase mutations have an undoubtful predictive value for sensitivity to imatinib, and the inclusion of KIT and PDGFRa mutational analysis in the diagnostic workup of all GIST is now considered standard practice.
Case presentation: Herein, we described in detail a case of an exon 11 KIT mutated-metastatic GIST patient, who presented an unexpected metabolic progression at the early 18FDG-PET evaluation after 1 month of first-line imatinib, unconfirmed at the liver biopsy performed near after, which has conversely shown a complete pathological response.
Conclusions: This report aims to highlight the existence of this metabolic pseudoprogression in GIST at the beginning of imatinib therapy in order to avoid early treatment discontinuation. Therefore, an early metabolic progression during a molecular targeted therapy always deserves to be evaluated in the context of the disease molecular profiling, and in case of a discordant finding between functional imaging and molecular background, a short-term longitudinal control should be suggested.
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract arising from the interstitial cells of Cajal (ICCs), with an incidence of approximately 10 to 15 cases per million population per year (1).
GISTs have always been considered the milestone of precision oncology, from when KIT mutations were recognized as the main pathogenetic driver, soon becoming the GIST therapeutic target (2–5). Since then, mutational analysis of KIT and PDGFRA has assumed a proven predictive value for sensitivity to molecular targeted therapies, and its inclusion in the diagnostic workup of all GISTs has become standard practice (6).
For sure, the advent of imatinib has drastically changed the GIST natural history, with an overall manageable toxicity profile and only rare serious adverse events (7). This treatment has also questioned the standard criteria of treatment response assessment, only based on uni- or bidimensional changes in tumor size (8, 9). As a matter of fact, imatinib-induced tumor necrosis has led to put greater relevance to tumor density and metabolism in treatment response assessment to all tyrosine kinase inhibitors (TKIs), becoming a new paradigm for imaging in the era of precision oncology (10). In particular, positron emission tomography (PET) with 18-fluorodeoxyglucose (18FDG) has proven to be highly sensitive in the early assessment of tumor response, especially in cases where there is doubt or when the early prediction of the response could be clinically useful for patient management (11–15).
However, even if 18FDG-PET is generally thought to be more sensitive than morphologic imaging modalities for assessing early therapy response, several questions remain unanswered, including the appropriate time to monitor a therapeutic protocol, the PET-CT protocol used, and the therapy response evaluation criteria that should be used (16). Indeed, although the majority of studies report a general decrease in FDG tumor uptake after imatinib therapy, the time interval between baseline and follow-up FDG-PET studies varies significantly, ranging from 1 week after imatinib onset to several months after treatment (17, 18).
Herein, we described in detail a case of an exon 11 KIT mutated-metastatic GIST patient, who presented an unexpected metabolic progression at the early 18FDG-PET evaluation after 1 month of first-line imatinib, unconfirmed at the liver biopsy performed near after, which has conversely shown a complete pathological response. This report aims to understand the existence of this metabolic pseudoprogression in GIST at the beginning of imatinib therapy in order to avoid early treatment discontinuation.
In December 2020, a 78-year-old male patient, with a concomitant Philadelphia chromosome-positive chronic myeloproliferative neoplasm (MPN) treated with bosutinib, underwent duodenal resection and cholecystectomy, due to a duodenal GIST, diagnosed after an acute episode of melena and severe anemia.
The histologic examination confirmed the diagnosis of a predominantly epithelioid cell GIST at high risk of relapse according to the Miettinen criteria [site: duodenum; size: 5.5 cm; mitotic index: 6/50 high power field (HPF)]. The microscopic margins were negative and there was no evidence of tumor rupture. The molecular analysis performed with next-generation sequencing (NGS) analysis showed an exon 11 deletion of KIT (Gln556_Val559del) (Figure 1).
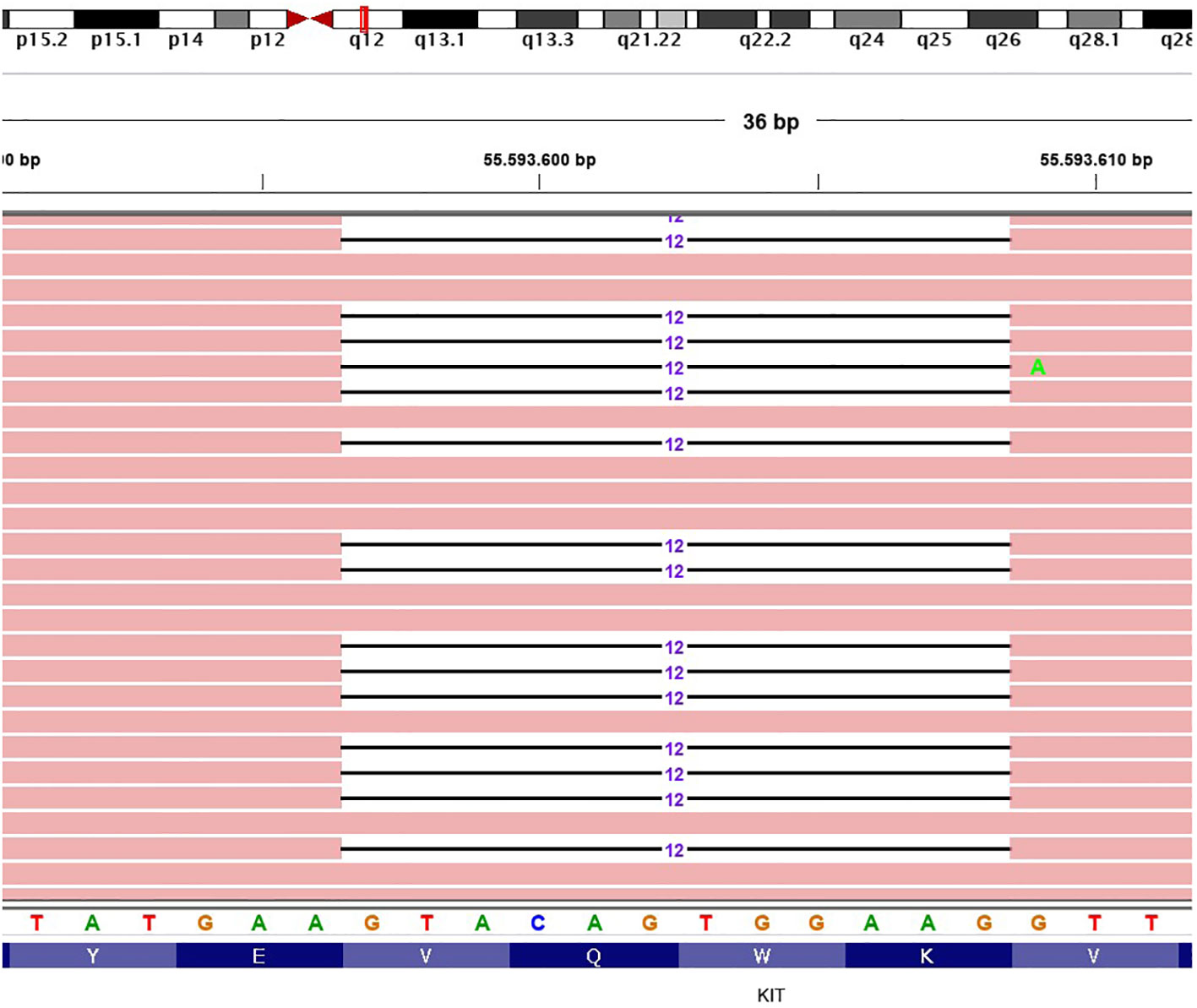
Figure 1 Representative graphical output of the next-generation sequencing analysis, showing an exon 11 deletion of KIT (Gln556_Val559del). The different colors are referred to the single DNA basis.
According to the risk class of tumor, an adjuvant treatment with imatinib 400 mg daily for 3 years should have been considered; however, after a multidisciplinary discussion together with hematologists, it was deemed more proper to continue treatment with bosutinib and reserve the therapy for GIST in case of disease relapse. During the surveillance program, a computer tomography (CT) scan performed in July 2022 showed a wide liver lesion at II–III hepatic segments with metastatic features (Figure 2). The subsequent 18FDG-PET/CT confirmed the presence of a hypermetabolic liver lesion with a maximum standardized uptake value (SUVmax) of 9.4 (Figure 3). According to the molecular profile, in August 2022, a first-line treatment with imatinib 400 mg daily was started. The subsequent 18FDG-PET/CT performed 1 month later for the early treatment response evaluation, given the concomitant chronic MPN already previously treated with imatinib, showed a metabolic progression of the liver lesion, presenting an SUVmax of 20.1 (Figure 3). Given this unexpected result, as compared with the exon 11 KIT-mutant molecular profile, a liver biopsy was performed. The histological examination has displayed liver tissue associated with paucicellular lesion of collagenized neovascularized fibrous–edematous stroma with lymphogranulocytic inflammatory infiltrate, such as granulation tissue (Figure 4). Based on this finding, suggesting a likely complete pathological response, imatinib therapy has been continued. The subsequent abdominal CT scan and 18FDG-PET/CT, performed in October 2022, 2 months after the beginning of therapy, revealed both a morphologic and metabolic partial response of the liver lesion, with an SUVmax of 7.9 (Figures 5A, B).
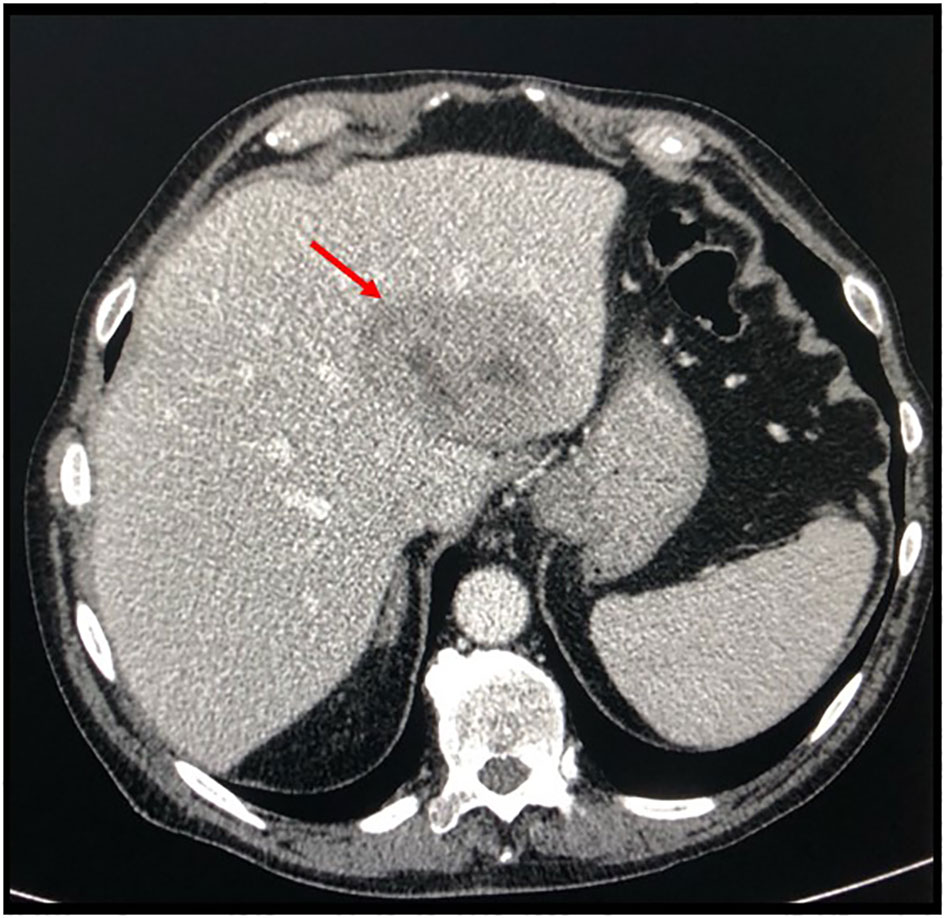
Figure 2 Basal axial CT scan evaluation, showing a hypodense and partially colliquated liver lesion at segments II–III (arrow).
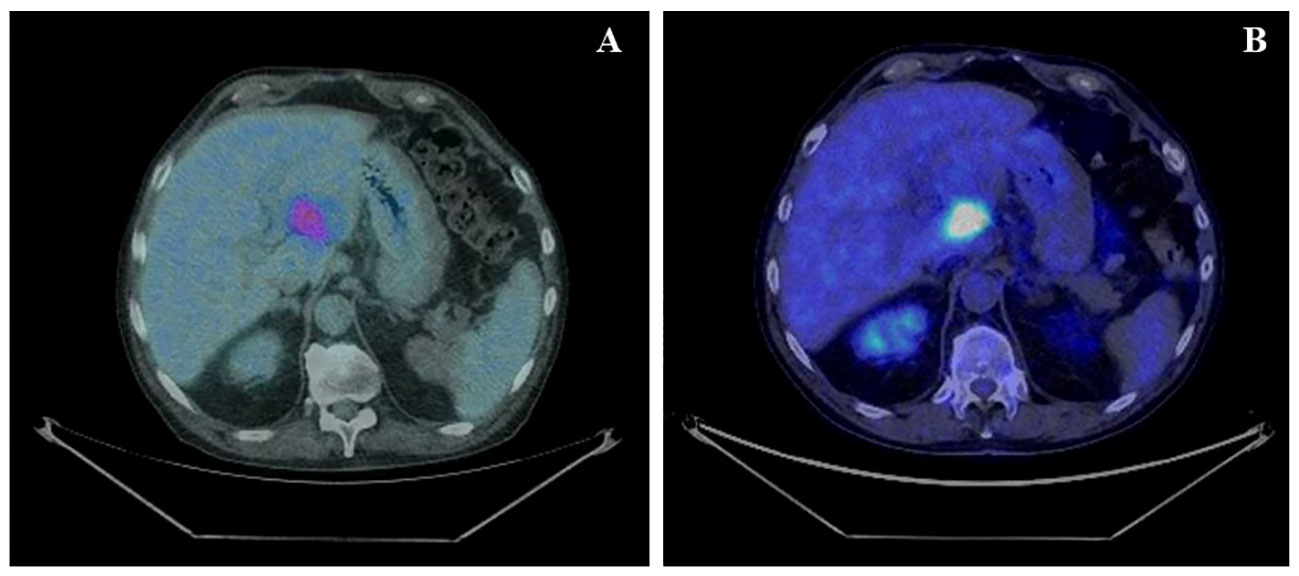
Figure 3 (A) Basal axial PET/CT fused images, showing a hypermetabolic lesion involving II–III liver segments (SUVmax 9.4). (B) Axial PET/CT fused images, restaging scan after a month of imatinib therapy: the hypermetabolic area appears increased in size and shows a greater 18F-FDG uptake: SUVmax 20.1 (vs. 9.4).
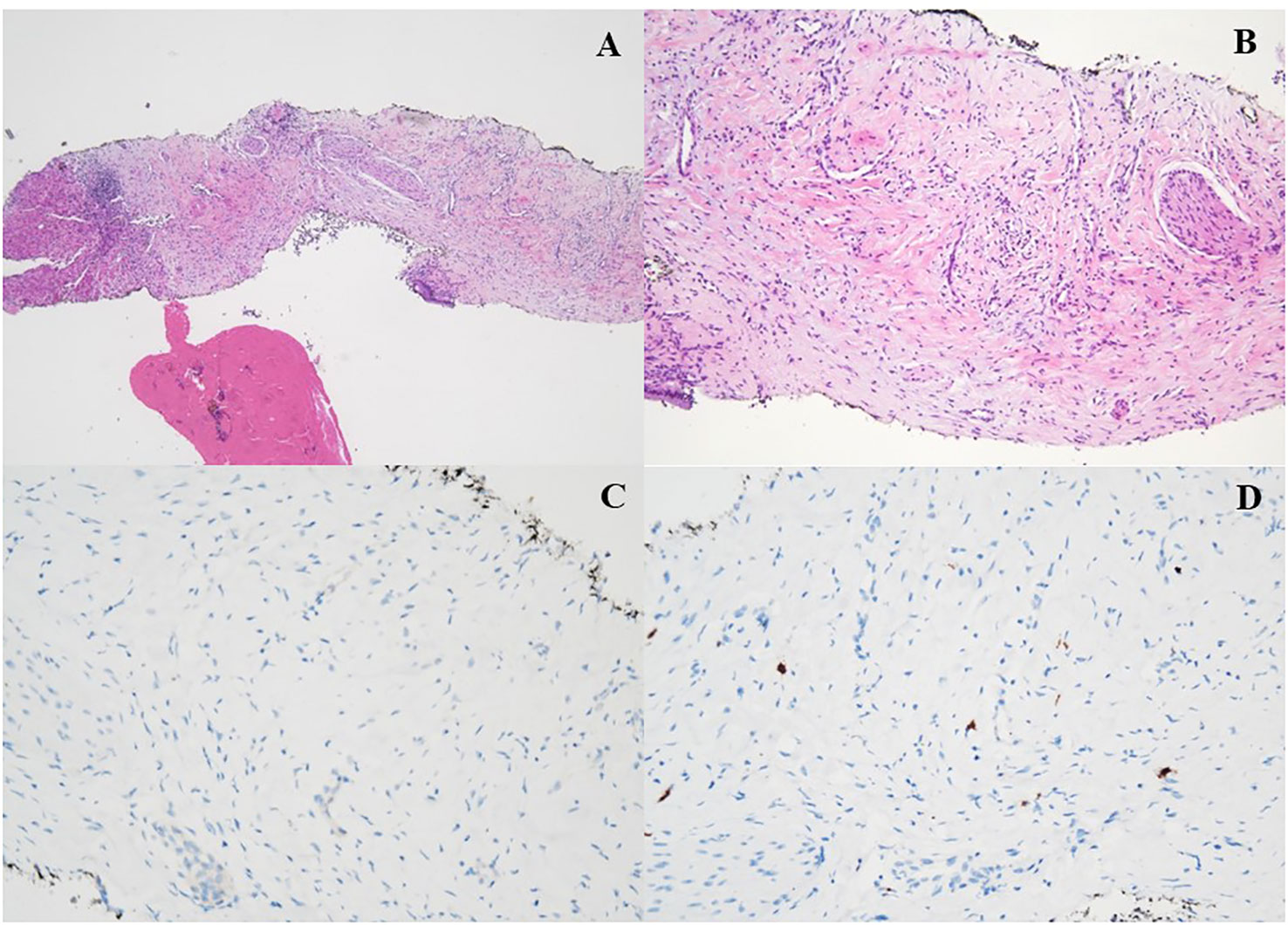
Figure 4 (A, B) H&E ×4: histologic features of the liver biopsy showing a vascular tissue with bland spindle cell fibroblast with collagenous stroma, admixed with a variable number of inflammatory elements (granulation tissue). (C, D) Immunohistochemical stain for DOG-1 (C) and CD117 (D) was all negative.
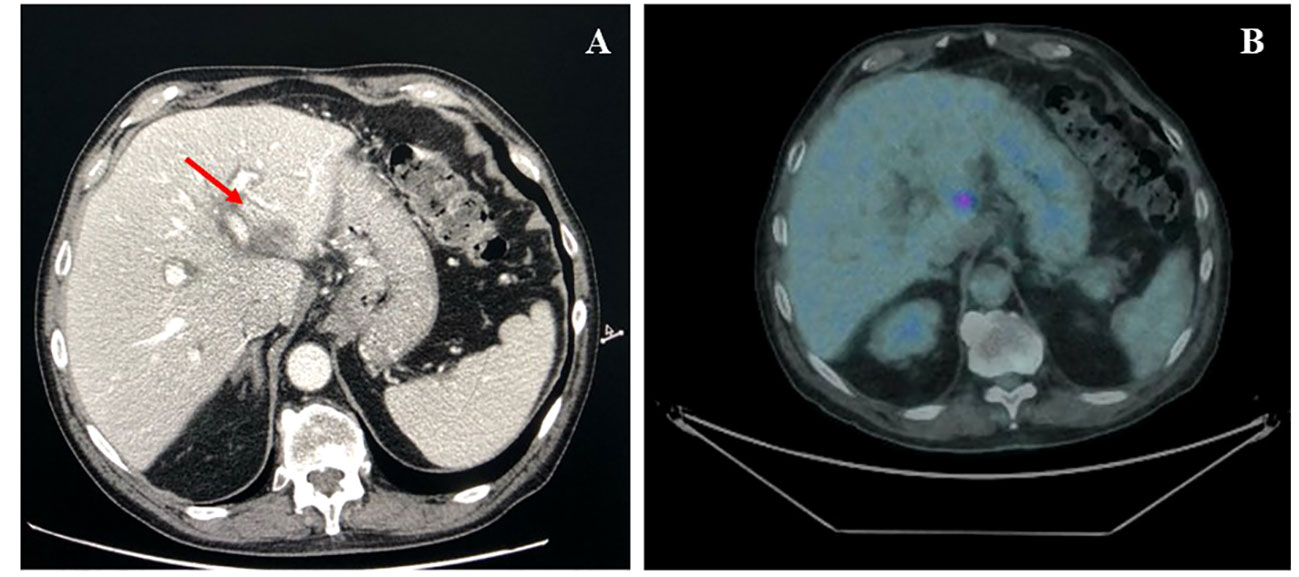
Figure 5 (A) Axial CT scan evaluation after 3 months of imatinib treatment (arrow), showing a partial response of liver lesion. (B) Axial PET/CT fused images scan, after 3 months of imatinib treatment, showing a marked decline in both lesion size and 18F-FDG uptake (SUVmax 7.9).
At present, the patient is still under treatment with imatinib at the standard dose, and at the last CT scan performed in July 2023, the liver lesion maintained the response, showing further dimensional reduction.
The role of 18FDG-PET in the early assessment of tumor response in GIST has soon become a model for functional imaging of all other oncogene-addicted solid tumors treated with TKIs because it allows selecting those patients who can really benefit from molecular targeted therapies and conversely identifying those who are primarily resistant, even if the correlation between 18FDG-PET response and progression-free survival (PFS) is still controversial (12, 17). This is extremely relevant in doubtful cases or especially in those in which molecular profiling is lacking.
As widely known, kinase mutations have an undoubtful predictive value for sensitivity to imatinib, and the inclusion of KIT and PDGFRa mutational analysis in the diagnostic workup of all GISTs is now considered standard practice (6). Primary exon 11 KIT mutations, the most common ones in the KIT gene (67%), especially deletions, are known to confer the highest sensitivity to imatinib, and primary resistance can be considered a rare event (19).
In the presented case, the increase of FDG uptake after 1 month of imatinib was unexpected as compared with the molecular profile of the primary GIST. This has been the reason why tumor sampling of the metastatic lesion has been performed in order to confirm the GIST’s diagnosis of the hepatic lesion and exclude the presence of a resistant secondary mutation. The histological finding has conversely shown the presence of collagenous stroma strongly suggestive of a pathological complete response, and the surrounding abundant inflammatory infiltrate could explain the transient increased FDG uptake we found. Indeed, it is well established that activated inflammatory cells, especially those involved in inflammatory foci, show increased glucose metabolism, leading to a high FDG uptake (20). This evidence represents the pathophysiologic basis for the possible increase in FDG uptake in the case of a complete pathologic response, thus leading to false positivity on PET examination. This phenomenon referred to as the “flare effect” represents the underlying mechanism of pseudoprogression.
To our knowledge, this is the first case describing a metabolic pseudoprogression during the early assessment of tumor response in a metastatic KIT exon 11 mutant-GIST patient after 1 month of first-line imatinib. Even if extremely rare, clinicians should be aware of the possibility of this event, which should be interpreted according to the molecular profile if known, in order to avoid early treatment discontinuation. Therefore, an early metabolic progression during a molecular targeted therapy always deserves to be evaluated in the context of the disease molecular profiling, and in case of a discordant finding between functional imaging and molecular background, a short-term longitudinal control should be suggested.
Once again, GISTs have shown to be a model for functional imaging in the era of precision oncology, highlighting how all metabolic findings should be firstly interpreted together with the molecular data available.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
ET: Conceptualization, Writing – original draft, Writing – review & editing. NC: Conceptualization, Writing – original draft, Writing – review & editing. GB: Data curation, Resources, Writing – original draft, Writing – review & editing. FP: Data curation, Resources, Software, Writing – review & editing. VS: Data curation, Resources, Software, Writing – review & editing. MGP: Data curation, Resources, Software, Writing – review & editing. DB: Writing – review & editing, Resources. MCN: Conceptualization, Writing – original draft, Writing – review & editing. MI: Data curation, Resources, Writing – review & editing. FC: Data curation, Resources, Supervision, Writing – review & editing. LL: Data curation, Resources, Supervision, Writing – review & editing. SF: Data curation, Resources, Software, Supervision, Writing – review & editing. MAP: Conceptualization, Data curation, Methodology, Supervision, Writing – review & editing. MN: Conceptualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work reported in this publication was funded by the Italian Ministry of Health, RC-2022-2773452.
The authors declare that the content of the manuscript has previously appeared online as a preprint on Research Square (DOI: 10.21203/rs.3.rs-2812984/v1) and Europe PMC (DOI: 10.21203/rs.3.rs-2812984/v1).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
GIST, gastrointestinal stromal tumors; ICCs, interstitial cells of Cajal; TKIs, tyrosine kinase inhibitors; PET, positron emission tomography; 18FDG, 18-fluorodeoxyglucose; MPN, Philadelphia chromosome-positive chronic myeloproliferative neoplasm; SUVmax, maximum standardized uptake value; CT, computer tomography; PFS, progression-free survival.
1. Joensuu H. Gastrointestinal stromal tumor (GIST). Ann Oncol (2006) 17 Suppl 10:x280–6. doi: 10.1093/annonc/mdl274
2. Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol (2004) 22(18):3813–25. doi: 10.1200/JCO.2004.05.140
3. Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science (1998) 279(5350):577–80. doi: 10.1126/science.279.5350.577
4. Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med (2002) 347(7):472–80. doi: 10.1056/NEJMoa020461
5. Benjamin RS, Choi H, Macapinlac HA, Burgess MA, Patel SR, Chen LL, et al. We should desist using RECIST, at least in GIST. J Clin Oncol (2007) 25(13):1760–4. doi: 10.1200/JCO.2006.07.3411
6. Casali PG, Blay JY, Abecassis N, Bajpai J, Bauer S, Biagini R, et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2022) 33(1):20–33. doi: 10.1016/j.annonc.2021.09.005
7. Bongiovanni A, Ricci M, Riva N, Calpona S, Oboldi D, Pieri F, et al. Pleural effusion in a patient with metastatic gastrointestinal stromal tumor treated with imatinib: case report. Future Oncol (2014) 10(15):2423–7. doi: 10.2217/fon.14.159
8. Van den Abbeele AD. The lessons of GIST–PET and PET/CT: a new paradigm for imaging. Oncologist (2008) 13 Suppl 2:8–13. doi: 10.1634/theoncologist.13-S2-8
9. Dimitrakopoulou-Strauss A, Ronellenfitsch U, Cheng C, Pan L, Sachpekidis C, Hohenberger P, et al. Imaging therapy response of gastrointestinal stromal tumors (GIST) with FDG PET, CT and MRI: a systematic review. Clin Transl Imaging (2017) 5(3):183–97. doi: 10.1007/s40336-017-0229-8
10. Van den Abbeele AD, Badawi RD. Use of positron emission tomography in oncology and its potential role to assess response to imatinib mesylate therapy in gastrointestinal stromal tumors (GISTs). Eur J Cancer (2002) 38 Suppl 5:S60–5. doi: 10.1016/s0959-8049(02)80604-9
11. Antoch G, Kanja J, Bauer S, Kuehl H, Renzing-Koehler K, Schuette J, et al. Comparison of PET, CT, and dual-modality PET/CT imaging for monitoring of imatinib (STI571) therapy in patients with gastrointestinal stromal tumors. J Nucl Med (2004) 45(3):357–65.
12. Stroobants S, Goeminne J, Seegers M, Dimitrijevic S, Dupont P, Nuyts J, et al. 18FDG-Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec). Eur J Cancer (2003) 39(14):2012–20. doi: 10.1016/s0959-8049(03)00073-x
13. Gayed I, Vu T, Iyer R, Johnson M, Macapinlac H, Swanston N, et al. The role of 18F-FDG PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors [published correction appears in J Nucl Med. J Nucl Med (2004) 45(1):17–21.
14. Farag S, Geus-Oei LF, van der Graaf WT, van Coevorden F, Grunhagen D, Reyners AKL, et al. Early evaluation of response using 18F-FDG PET influences management in gastrointestinal stromal tumor patients treated with neoadjuvant imatinib. J Nucl Med (2018) 59(2):194–6. doi: 10.2967/jnumed.117.196642
15. Yokoyama K, Tsuchiya J, Nakamoto Y, Tateishi U. Additional value of [18F]FDG PET or PET/CT for response assessment of patients with gastrointestinal stromal tumor undergoing molecular targeted therapy: A meta-analysis. Diagn (Basel) (2021) 11(3):475. doi: 10.3390/diagnostics11030475
16. Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol (2007) 25(13):1753–9. doi: 10.1200/JCO.2006.07.3049
17. Chacón M, Eleta M, Espindola AR, Roca E, Méndez G, Rojo S, et al. Assessment of early response to imatinib 800 mg after 400 mg progression by 18F-fluorodeoxyglucose PET in patients with metastatic gastrointestinal stromal tumors. Future Oncol (2015) 11(6):953–64. doi: 10.2217/fon.14.292
18. Altini C, Mammucci P, Pisani AR, D'Alò C, Sardaro A, Rubini D, et al. 18F-FDG PET/CT in GIST treatment response evaluation beyond Imatinib. Hell J Nucl Med (2021) 24(3):239–46. doi: 10.1967/s002449912407
19. Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol (2003) 21(23):4342–9. doi: 10.1200/JCO.2003.04.190
Keywords: gastrointestinal stromal tumors, GIST, functional imaging, FDG-PET, imatinib
Citation: Tassinari E, Conci N, Battisti G, Porta F, Di Scioscio V, Pirini MG, de Biase D, Nigro MC, Iezza M, Castagnetti F, Lovato L, Fanti S, Pantaleo MA and Nannini M (2023) Metabolic pseudoprogression in a patient with metastatic KIT exon 11 GIST after 1 month of first-line imatinib: a case report. Front. Oncol. 13:1310452. doi: 10.3389/fonc.2023.1310452
Received: 09 October 2023; Accepted: 27 November 2023;
Published: 20 December 2023.
Edited by:
David Aebisher, University of Rzeszow, PolandReviewed by:
Alessandro De Vita, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyCopyright © 2023 Tassinari, Conci, Battisti, Porta, Di Scioscio, Pirini, de Biase, Nigro, Iezza, Castagnetti, Lovato, Fanti, Pantaleo and Nannini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Margherita Nannini, bWFyZ2hlcml0YS5uYW5uaW5pQHVuaWJvLml0
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.