
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 29 October 2020
Sec. Molecular and Cellular Oncology
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.574523
Esophageal squamous cell carcinoma (ESCC) is a deadly disease with a low 5-year survival rate. Anti-epidermal growth factor receptor (EGFR) therapy has been widely used in the treatment of malignancies, and chemotherapy regimens that include nimotuzumab have been confirmed to have satisfactory efficacy among esophageal carcinoma (EC) patients. However, a subpopulation of patients may develop resistance to nimotuzumab. Here, we report an advanced ESCC patient who experienced hyperprogressive disease induced by immune checkpoint inhibitors and was then treated with a chemotherapy regimen containing nimotuzumab. NGS examination of this patient demonstrated that PIK3CA mutation and a RICTOR amplification might participate in primary and acquired resistance to nimotuzumab, respectively, via the PI3K/AKT/mTOR signaling pathway.
Esophageal carcinoma (EC), which is the sixth leading cause of cancer-related mortality, affects more than 450,000 people worldwide. Patients suffering from EC may have a poor prognosis as a result of the stage at diagnosis and the metastatic properties of EC (1, 2). Esophageal squamous cell carcinoma (ESCC) is the predominant pathological type of EC (3), especially in eastern Asia, and is associated with a series of risk factors, including smoking status, alcohol consumption, improper eating habits, and poor nutrition (4).
ESCC is a deadly disease, with a 5-year survival rate of approximately 10% that requires treatment, including surgical operation, systemic chemotherapy, and radiotherapy. With the development of targeted sequencing, targeted therapy has been widely applied in the treatment of ESCC. Protein overexpression of epidermal growth factor receptor (EGFR) and EGFR amplification has been observed in many ESCC patients (5) and is related to a poor prognosis in both progression-free survival (PFS) and overall survival (OS) (6). Nimotuzumab (h-R3) is a humanized anti-EGFR monoclonal antibody that has demonstrated efficacy in EGFR-positive ESCC patients, as verified by multiple clinical trials (7–9). Nimotuzumab combined with paclitaxel plus cisplatin (TPN regimen), cisplatin plus 5-FU (PF + N) or concurrent chemoradiotherapy (nimotuzumab plus CCRT) all showed promising efficacy and tolerable safety in treating ESCC. In addition, monotherapy with nimotuzumab followed by radiotherapy yielded encouraging OS, PFS, and locoregional control (LC) (10). In another phase III clinical trial, Jing et al. (11) confirmed that nimotuzumab demonstrated better efficacy than did cetuximab (C225), a recombinant human/mouse chimeric EGFR monoclonal antibody. Nimotuzumab showed a significantly longer median PFS than cetuximab and a similar incidence of grade 3 or worse adverse events (AEs).
Regrettably, not all patients harboring EGFR amplification or EGFR protein overexpression respond well to nimotuzumab, while primary and acquired resistance to nimotuzumab from monotherapy or combined regimens can be observed in multiple studies on ESCC (7–10, 12). However, the mechanisms of primary and acquired resistance to nimotuzumab require further studies.
As described in our previous study (13), the tissue samples of the patient in this report were examined by next-generation sequencing (NGS) assay using three versions of a capture-based targeted sequencing panel. Tissue for the first NGS assay (2018-07) was acquired from the primary tumor during surgical resection, while metastatic tissue in the lung was acquired via percutaneous lung puncture and sent for the second NGS assay (2019-02). Blood samples were also acquired for each NGS assay. Genomic alterations were detected in 17 genes, including 16 genes altered before nimotuzumab treatment and 15 genes altered after the treatment. For disease evaluation, we used the Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1) to conduct the assessment of disease status via 64-slice spiral CT scan (HITACHI, JAPAN). Blood samples from the patient were sent for tumor marker examination in the clinical laboratory at our hospital. The patient in this report gave his consent for the publication of the report.
In July 2018, a 52-year-old male with ESCC visited our hospital after multiple lines of treatment. The patient was first diagnosed with stage IIIB ESCC via postsurgical pathology on February 7th, 2017. The patient then received 2 cycles of the TP chemotherapy regimen and CCRT after surgery and remained stable until the first instance of recurrence on December 12th, 2017. First-line chemotherapy and further antiangiogenetic therapy were administered to the patient but were not effective. Therefore, the patient came to our hospital and received treatment, the detailed timeline of which is shown in Figure 1A. According to the CheckMate-032 study (14), nivolumab demonstrated clinically meaningful antitumor activity in patients with chemotherapy-refractory esophagogastric cancer. Considering that this patient had experienced PD after multiple lines treatment regimens, he was then treated with nivolumab after acquired consent. Therefore, the patient received 200 mg (3 mg/kg, q2w) of nivolumab on October 18th, 2018. However, the patients developed a hyperprogressive disease (HPD). Upon disease assessment, the metastatic lesions in the lung grew more than 50% compared with baseline within 1 month via the evaluation of CT scans, and the patient suffered severe immune-related pneumonia. After symptomatic and supportive treatment, the patient started a regimen of nimotuzumab combined with chemotherapy. After two cycles of the TN (nab-paclitaxel + nimotuzumab) regimen, the response was declared a partial response (PR), but after four cycles of this regimen, the patient showed progressive disease (PD). The sizes of two metastatic lesions in the lung increased more than 20% compared with the most recent CT scan (lesion 1: 23%; lesion 2: 24%; average: 23.4%), as shown in Figure 1B, and the levels of squamous cell carcinoma (SCC) antigen, a tumor marker, rose drastically, as shown in Figure 1C. Dynamic NGS was performed before and after nimotuzumab treatment, and the results are summarized in Table 1. NGS assays of this patient revealed that EGFR amplification and the p.E545K mutation of PIK3CA were present in his peripheral blood before nimotuzumab treatment. According to the NGS examination after nimotuzumab treatment, amplification of rapamycin-insensitive companion of mammalian target of rapamycin (RICTOR) was detected, which indicated activation of the mTOR signaling pathway, while amplification of EGFR was not detected upon examination.
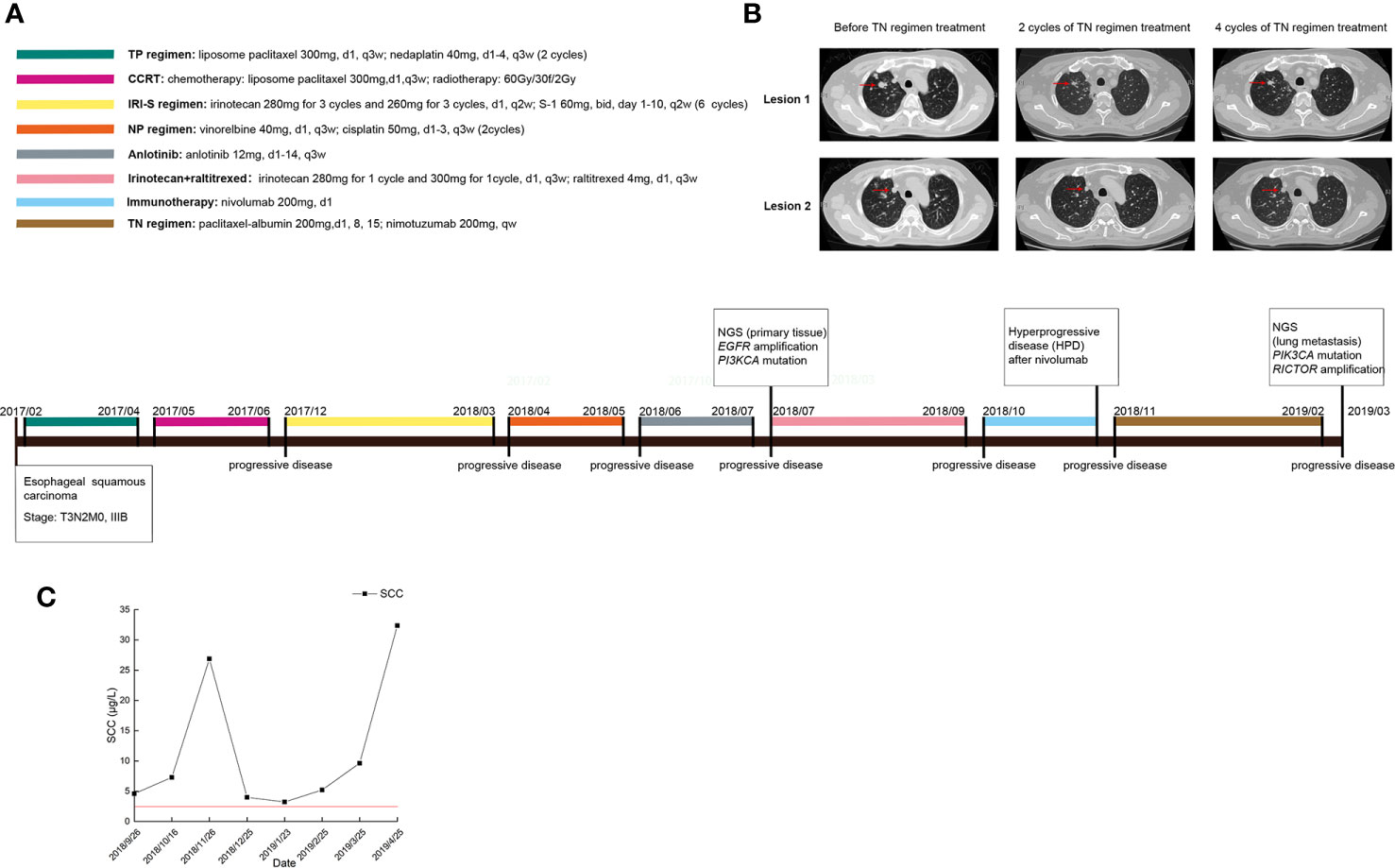
Figure 1 Clinical data of the patient during the treatment. (A) The timeline of treatments for a patient with advanced esophageal squamous carcinoma; (B) CT scans before and after nimotuzumab treatment; (C) Changes in tumor marker levels during treatment.
Anti-EGFR monoclonal antibodies (mAbs) have been widely used to treat malignancies, and patients have benefited from anti-EGFR mAbs such as cetuximab and nimotuzumab. However, resistance to anti-EGFR mAbs has diminished the available options for patients. Multiple studies have focused on the mechanisms of primary and acquired resistance to cetuximab. Since anti-EGFR mAbs block the activation of the EGFR signaling pathway, alterations in the expression and activity of EGFR signaling pathway-related genes may play important roles. PIK3CA mutations result in the persistent phosphorylation of Akt1 and overactivate the EGFR signaling pathway, which is related to primary resistance to cetuximab (15). Recently, studies have verified the relationship between PIK3CA mutation and primary cetuximab resistance (15–17). After phosphorylation of Akt1 and activation of the EGFR signaling pathway, PIK3CA mutations can lead to cetuximab resistance via activation of the downstream mTOR signaling pathway (18). Extraordinarily, the p.E545K mutation of PIK3CA enhances the binding ability to EGFR and significantly activates the downstream Akt signaling pathway (19). The mechanism of resistance to cetuximab seems clear, but no study has provided a clue as to whether nimotuzumab shares this resistance mechanism. We provided this case report to demonstrate the efficacy and safety of nimotuzumab in ESCC patients who experienced immunotherapy-related HPD and the potential mechanism of nimotuzumab resistance.
The patient here developed HPD and severe pneumonia and had poor fundamental pulmonary function, but nimotuzumab combined with nab-paclitaxel (TN regimen) demonstrated its advantage in safety. The four-cycle TN regimen was well tolerated by this patient, and the only Grade 3 AE reported was rash acneiform, which was located on his back and face. A PR was declared after the first two cycles of the TN regimen, demonstrating the efficacy of nimotuzumab combined with chemotherapy, even after HPD. However, the response to the TN regimen lasted only four cycles, which was less than 4 months. Given that nimotuzumab is a mAb for EGFR, the p.E545K mutation of PIK3CA in this patient might have led to constitutive Akt phosphorylation, and activation of the EGFR signaling pathway is strongly associated with primary resistance to nimotuzumab and a short time to treatment failure (TTF). Meanwhile, based on the NGS results before and after nimotuzumab treatment, we proposed that activation of the phosphoinositide-3-kinase (PI3K)/AKT/mTOR signaling pathway is the potential mechanism of primary and acquired resistance to nimotuzumab. Nimotuzumab combined with nab-paclitaxel might be an alternative choice for ESCC patients who experience HPD with tolerance safety. PIK3CA mutation and RICTOR amplification may participate in primary and acquired resistance to nimotuzumab, respectively, via the PI3K/AKT/mTOR signaling pathway. Further treatment with alpelisib (BYL719) (20) or rapalagos (21), which target the mTOR pathway, might benefit ESCC patients harboring mutations or genetic alterations that increase the activation of the PI3K–AKT/mTOR signaling pathway.
The studies involving human participants were reviewed and approved by the Affiliated Hospital of Qingdao University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Conception/Design: HH. Provision of study material or patients: HH, WY and HZ. Collection and/or assembly of data: DS, QL, and HZ. Data analysis and interpretation: DS, QL, and HZ. Manuscript writing: HH and DS. All authors contributed to the article and approved the submitted version.
Special funding from the Qilu Sanitation and Health Leading Talents Cultivation Project (to HH.)
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Lu Tian (Ocean University of China) and Tao Wang (Huaiyin Normal University) for their technical assistance in diagramming.
1. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet (2013) 381(9864):400–12. doi: 10.1016/S0140-6736(12)60643-6
2. Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol (2013) 19(34):5598–606. doi: 10.3748/wjg.v19.i34.5598
3. Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst (2005) 97(2):142–6. doi: 10.1093/jnci/dji024
4. Zhang HZ, Jin GF, Shen HB. Epidemiologic differences in esophageal cancer between Asian and Western populations. Chin J Cancer (2012) 31(6):281–6. doi: 10.5732/cjc.011.10390
5. Hanawa M, Suzuki S, Dobashi Y, Yamane T, Kono K, Enomoto N, et al. EGFR protein overexpression and gene amplification in squamous cell carcinomas of the esophagus. Int J Cancer (2006) 118(5):1173–80. doi: 10.1002/ijc.21454
6. Jia J, Cui Y, Lu M, Wang X, Li J, Li J, et al. The relation of EGFR expression by immunohistochemical staining and clinical response of combination treatment of nimotuzumab and chemotherapy in esophageal squamous cell carcinoma. Clin Transl Oncol (2016) 18(6):592–8. doi: 10.1007/s12094-015-1406-8
7. Lu M, Wang X, Shen L, Jia J, Gong J, Li J, et al. Nimotuzumab plus paclitaxel and cisplatin as the first line treatment for advanced esophageal squamous cell cancer: A single centre prospective phase II trial. Cancer Sci (2016) 107(4):486–90. doi: 10.1111/cas.12894
8. Zhao KL, Hu XC, Wu XH, Fu XL, Fan M, Jiang GL. A phase I dose escalation study of Nimotuzumab in combination with concurrent chemoradiation for patients with locally advanced squamous cell carcinoma of esophagus. Invest New Drugs (2012) 30(4):1585–90. doi: 10.1007/s10637-011-9735-0
9. Ling Y, Chen J, Tao M, Chu X, Zhang X. A pilot study of nimotuzumab combined with cisplatin and 5-FU in patients with advanced esophageal squamous cell carcinoma. J Thorac Dis (2012) 4(1):58–62. doi: 10.3978/j.issn.2072-1439.2011.08.02
10. Ma NY, Cai XW, Fu XL, Li Y, Zhou XY, Wu XH, et al. Safety and efficacy of nimotuzumab in combination with radiotherapy for patients with squamous cell carcinoma of the esophagus. Int J Clin Oncol (2014) 19(2):297–302. doi: 10.1007/s10147-013-0564-3
11. Jing W, Yan W, Liu Y, Li J, Yu J, Zhu H. Slight advantages of nimotuzumab versus cetuximab plus concurrent chemoradiotherapy in locally advanced esophageal squamous cell carcinoma. Cancer Biol Ther (2019) 14:1–6. doi: 10.1080/15384047.2019.1598760
12. Lai X, Gu Q, Zheng X, Liu G, Feng W, Lin X, et al. Combined nimotuzumab with chemoradiotherapy in patients with locally advanced or metastatic esophageal squamous cell carcinoma: A retrospective study. J Cancer Res Ther (2016) 12(Supplement):89–95. doi: 10.4103/0973-1482.191612
13. Hou H, Zhu H, Zhao H, Yan W, Wang Y, Jiang M, et al. Comprehensive molecular characterization of young chinese patients with lung adenocarcinoma identified a distinctive genetic profile. Oncologist (2018) 23(9):1008–15. doi: 10.1634/theoncologist.2017-0629
14. Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, et al. CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer. J Clin Oncol (2018) 36(28):2836–44. doi: 10.1200/JCO.2017.76.6212
15. Sartore-Bianchi A, Martini M, Molinari F, Veronese S, Nichelatti M, Artale S, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res (2009) 69(5):1851–7. doi: 10.1158/0008-5472.CAN-08-2466
16. Xu JM, Wang Y, Wang YL, Wang Y, Liu T, Ni M, et al. PIK3CA Mutations Contribute to Acquired Cetuximab Resistance in Patients with Metastatic Colorectal Cancer. Clin Cancer Res (2017) 23(16):4602–16. doi: 10.1158/1078-0432.CCR-16-2738
17. Napolitano S, Martini G, Rinaldi B, Martinelli E, Donniacuo M, Berrino L, et al. Primary and Acquired Resistance of Colorectal Cancer to Anti-EGFR Monoclonal Antibody Can Be Overcome by Combined Treatment of Regorafenib with Cetuximab. Clin Cancer Res (2015) 21(13):2975–83. doi: 10.1158/1078-0432.CCR-15-0020
18. Wang Z, Martin D, Molinolo AA, Patel V, Iglesias-Bartolome R, Degese MS, et al. mTOR co-targeting in cetuximab resistance in head and neck cancers harboring PIK3CA and RAS mutations. J Natl Cancer Inst (2014) 106(9):dju215. doi: 10.1093/jnci/dju215
19. Zhao S, Cao Y, Liu SB, Wang XA, Bao RF, Shu YJ, et al. The E545K mutation of PIK3CA promotes gallbladder carcinoma progression through enhanced binding to EGFR. J Exp Clin Cancer Res (2016) 35(1):97–109. doi: 10.1186/s13046-016-0370-7
20. O’Brien NA, McDermott MSJ, Conklin D, Luo T, Ayala R, Salgar S, et al. Targeting activated PI3K/mTOR signaling overcomes acquired resistance to CDK4/6-based therapies in preclinical models of hormone receptor-positive breast cancer. Breast Cancer Res (2020) 22(1):89. doi: 10.1186/s13058-020-01320-8
Keywords: nimotuzumab, resistance, PIK3CA, mTOR, PD-L1, esophageal squamous cell carcinoma
Citation: Sun D, Yan W, Zhu H, Liu Q and Hou H (2020) Case Report: Primary and Acquired Resistance Mechanisms of Nimotuzumab in Advanced Esophageal Squamous Cell Carcinoma Revealed by Targeted Sequencing. Front. Oncol. 10:574523. doi: 10.3389/fonc.2020.574523
Received: 20 June 2020; Accepted: 05 October 2020;
Published: 29 October 2020.
Edited by:
Roger Chammas, University of São Paulo, BrazilReviewed by:
Fuming Li, University of Pennsylvania, United StatesCopyright © 2020 Sun, Yan, Zhu, Liu and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Helei Hou, aG91aGVsZWlAcWR1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.