- 1Department of Food and Nutrition, Hanyang University, Seoul, Republic of Korea
- 2Department of Health Sciences and Technology, College of Medicine, Kyung Hee University, Seoul, Republic of Korea
- 3Department of Family Medicine, College of Medicine, Kyung Hee University, Seoul, Republic of Korea
Previous studies have suggested beneficial effects of n-3 polyunsaturated fatty acids on sarcopenia. However, the associations of dietary fish intake with the prevalence of sarcopenia are inconsistent, and those with the incidence of sarcopenia has not been studied. This study investigated the hypothesis that seafood and fish consumption is inversely associated with the subsequent incidence of sarcopenia. Using data from the Korean Frailty and Aging Cohort Study, 503 non-sarcopenic community-dwelling Korean adults aged 70–84 years were followed-up for 6 years. Sarcopenia was defined according to the Asian Working Group for Sarcopenia 2019 consensus. Dietary intake was assessed using two non-consecutive 24-h dietary recalls at baseline. The incidence of sarcopenia was 37.8% after the 6-year follow-up. The intake of oily fish was inversely associated with the incidence of sarcopenia (OR 0.99; 95% CI 0.98–1.00; p for trend = 0.046) and that of low gait speed (OR 0.98; 95% CI 0.97–1.00; p for trend = 0.016) after the 6-year follow-up, adjusting for confounding factors. Consumption of total seafood, fish, non-oily fish, or shellfish was not significantly associated with the incidence of sarcopenia or its parameters, such as muscle mass, handgrip strength, usual gait speed, 5-times sit-to-stand test, or the Short Physical Performance Battery. The findings demonstrate that the consumption of oily fish could be beneficial in preventing sarcopenia, particularly by improving usual gait speed in Korean community-dwelling older adults, suggesting oily fish as a strategy to reduce sarcopenia risk.
1 Introduction
Sarcopenia is a widespread geriatric condition characterized by progressive and systemic loss of skeletal muscle mass, strength, and function. Sarcopenia leads to adverse outcomes, such as falls, physical disability, frailty, and mortality (1). An estimated 10–16% of older adults suffer from sarcopenia globally, which is expected to increase significantly in the coming decades (2). Among the risk factors contributing to the development of sarcopenia, age-related chronic inflammation, commonly referred to as “inflammaging,” is increasingly recognized as being crucial in the pathogenesis of sarcopenia (3).
N-3 polyunsaturated fatty acids (PUFAs) are particularly abundant in seafood and fish. Their anti-inflammatory properties highlight their potential dietary role in the development and management of sarcopenia (3). Cross-sectional studies among older Chinese individuals have reported that the intake of seafood or fish is not associated with the prevalence of sarcopenia (4–6). However, oily fish intake is positively associated with muscle strength (7, 8) and physical performance (9) in older Europeans. Additionally, n-3 PUFA intake was reported to be inversely associated with the prevalence of sarcopenia in older Australian men (10), Japanese individuals with type 2 diabetes (11), and Brazilians who received a kidney transplant (12), and positively associated with physical performance in older Finnish women (13), Australian individuals with subjective memory complaints (14), and older British adults (15). The ratio of n-3 PUFA to energy intake was also inversely associated with the prevalence of sarcopenic obesity in older Korean women based on data from the Korean National Health and Nutrition Examination Survey (KNHANES) (16).
Blood levels of n-3 PUFA, an objective indicator of fish consumption, have been inversely associated with the prevalence of sarcopenia in older Koreans (17) and Japanese patients with liver cirrhosis and hepatocellular carcinoma (18), and positively associated with physical performance in older Europeans (15, 19, 20), Koreans (21), and Japanese (22). Meta-analyses of clinical trials showed that supplementation of n-3 PUFA can enhance muscle mass, muscle strength, and physical performance in older adults (23, 24). N-3 PUFA promoted muscle protein synthesis by activating the mammalian target of rapamycin (mTOR) signaling pathway and prevented muscle degradation by downregulating inflammatory cytokines, thereby mitigating inflammation-induced muscle loss in immobilized aged rodent models (25). In addition, n-3 PUFA enhanced muscle strength by improving membrane fluidity and modulating neuromuscular transmission (25).
To the best of our knowledge, no previous study has investigated the relationship between the incidence of sarcopenia and n-3 PUFA. Therefore, the aim of the present study was to investigate the hypothesis that the consumption of seafood and fish is inversely associated with the incidence of sarcopenia in community-dwelling older Koreans after a 6-year follow-up.
2 Methods
2.1 Participants
This longitudinal study was based on data from the Korean Frailty and Aging Cohort Study (KFACS), a nationwide multicenter cohort study of community-dwelling older adults aged 70–84 years (26). Surveys were performed across 10 centers, including eight medical hospitals and two public health centers in both urban and rural areas (Figure 1). In 2016, 1,559 participants were recruited, and dietary data and sarcopenia-related parameters were measured. At the baseline, 741 participants were included after exclusion owing to withdrawal of consent (n = 1), missing data for dual-energy X-ray absorptiometry (DXA) (n = 318), dietary intake (n = 439), educational level (n = 4), economic status (n = 6), comorbid status (n = 33), polypharmacy (n = 7), physical activity (n = 6), and sleep duration (n = 4). A total of 565 non-sarcopenic participants were followed up for 6 years in 2018, 2020, and 2022. At each follow-up period, sarcopenia-related parameters were measured to determine the incidence of sarcopenia, and non-sarcopenic participant diagnosed with sarcopenia was not followed up anymore. During follow-up, 62 participants were excluded since sarcopenia-related parameters were not measured at least once during any of the three follow-up assessments and thus sarcopenia status was unable to determine. The reasons for not determining sarcopenia were telephone survey (n = 16), proxy interview (n = 1), death (n = 18), refusal to participate (n = 17), relocation from the study area (n = 1), non-reachability (n = 6), inability to measure DXA for the survey at home (n = 2), or having a pacemaker (n = 1). Thus, 503 non-sarcopenic participants at the baseline were included in the final analysis. The present study was approved by the concerned Institutional Review Boards (KHUH-2015-12-103-107, 2021-05-081-039, and HYUIRB-202407-031), and written informed consent was obtained from all participants.
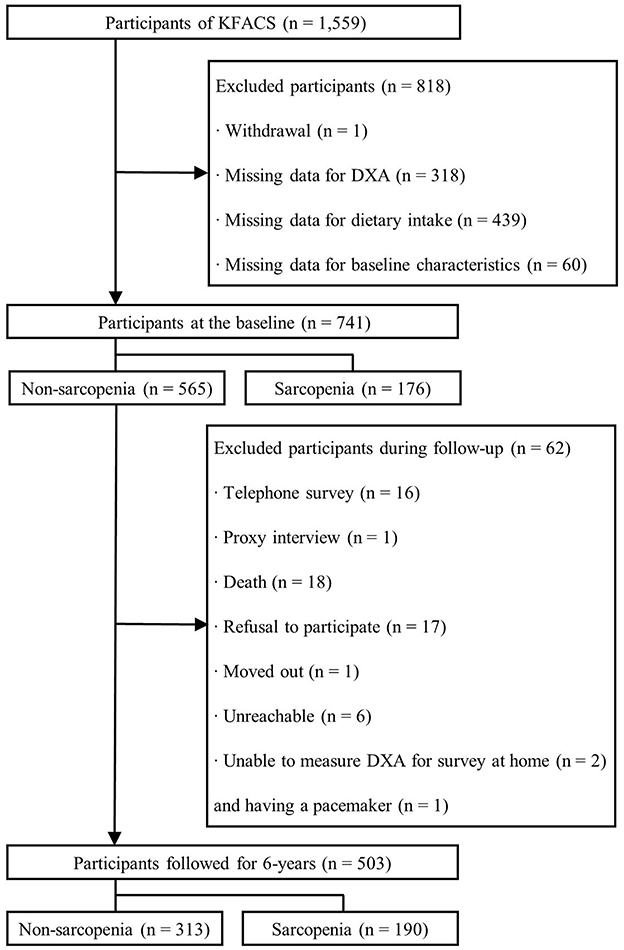
Figure 1. Flowchart of the participants for the 6-year follow-up. KFACS, Korean Frailty and Aging Cohort Study; DXA, dual-energy X-ray absorptiometry.
2.2 Assessment of sarcopenia
According to the 2019 consensus of the Asian Working Group for Sarcopenia (AWGS) (27), sarcopenia is defined as a low muscle mass with low muscle strength or physical performance. Appendicular skeletal muscle mass (ASM), calculated as the sum of the lean masses of both the arms and legs, was measured by DXA using either a Hologic DXA device (Hologic Inc., Bedford, MA, USA) or Lunar device (GE Healthcare, Madison, WI, USA). The ASM index was defined as ASM divided by height squared (kg/m2). Cut-off values for low muscle mass were ASM index <7.0 kg/m2 for men and <5.4 kg/m2 for women. Muscle strength was determined by measuring handgrip strength using a model TTK-5401 digital grip strength dynamometer (Takei Ltd., Tokyo, Japan). Each hand was alternately tested twice in the standing position and the highest value among the four grip strength measurements was obtained. The cut-off values for low muscle strength were <28 kg for men and <18 kg for women. Physical performance was assessed using the usual gait speed (UGS), 5-times sit-to-stand (5-STS) test, and Short Physical Performance Battery (SPPB). UGS was measured using a Gaitspeedmeter automatic gait speed timer (Dynamic Physiology, Daejeon, Korea). The participants were instructed to walk 4 m, with acceleration and deceleration phases of 1.5 m each. The average result of the two trials was used for the analysis. The 5-STS test measures the time taken to stand up five times from a sitting position, without depending on the chair arm. Participants were asked to perform the test as quickly as possible. The SPPB consists of standing balance tests (side-by-side, semi-tandem, and fully tandem), 5-STS test, and UGS assessment. Each SPPB test was scored from 0 to 4, with possible total scores ranging from 0 to 12 (28). Low physical performance was defined as meeting any of these three cut-offs: UGS <1.0 m/s, 5-STS test ≥ 12 s, and SPPB score ≤ 9 for both sexes.
2.3 Dietary assessment
Dietary data were obtained from trained interviewers using the 24-h dietary recall method twice, in spring and fall, at baseline. The mean values were used for analysis. The portion size of food was estimated using bowls and plates, as well as food pictures developed by the National Institute of Health (NIH) and the Korea Disease Control and Prevention Agency (KDCA). Total energy and seafood intakes were calculated using the NIH and KDCA dietary assessment systems based on the database of the National Rural Living Science Institute (29). Seafood consumption was defined as the combined intakes of fish and shellfish. Based on the Composition Table of Marine Products in Korea 2018 by the National Institute of Fisheries Science (30), fish includes raw fish, canned fish, fish paste, salted fish, and shellfish, including oysters, clams, mussels, shrimp, lobster, and crayfish. According to the UK Scientific Advisory Committee on Nutrition (SACN), oily fish are defined as any species that contains 5–20% fat (31). In the present study, 16 types of fishes, such as mackerel, anchovies, salmon, sardines, and eels were classified as oily fish.
2.4 Covariates
Data on age, sex, smoking status, years of education, economic status, living arrangements, and history of falls and fractures during the previous year were collected. Weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Low economic status refers to recipients of the National Basic Livelihood Security System or Medical Beneficial System (26). Comorbidities that were quantified included hypertension, dyslipidemia, myocardial infarction, congestive heart failure, angina, peripheral vascular disease, cerebrovascular disease, arthritis, osteoporosis, chronic obstructive pulmonary disease, asthma, diabetes mellitus, cancer, renal disease, and depression. Polypharmacy was defined as the use of five or more prescribed drugs in the previous 3 months (26). Sleep duration was categorized as <7, 7–8, or > 8 h according to the National Sleep Foundation's sleep duration recommendations for older adults (32). Low physical activity was measured using the International Physical Activity Questionnaire to calculate the energy expenditure, defined as ≤ 494.65 kcal/week for men and ≤ 283.50 kcal/week for women (26). Cognitive impairment was assessed using a Korean Mini-Mental State Examination (K-MMSE) score <24 (26), and nutritional status was evaluated using the Korean version of the Mini-Nutritional Assessment Short Form; a score ranging between 12 and 14 was defined to indicate normal nutritional status, that of 8–11 was associated with a risk of malnutrition, and <7 points indicated malnutrition (26).
2.5 Statistical analyses
The Kolmogorov–Smirnov test was used to confirm the normal distribution of the variables. Continuous variables were presented as mean ± standard deviation (SD). Statistical significance was verified using the independent t-test for parametric variables and the Mann–Whitney test for non-parametric variables. The proportions of categorical variables are presented as the number of participants and percentages using the chi-square test. In the multivariate models, covariates with a p-value < 0.20 were selected as confounding factors (33). Age, BMI, smoking status, living arrangements, number of comorbidities, cognitive impairment, fall experience, and sleep duration were included in the fully adjusted model. The associations between the intake of seafood, fish, and shellfish and the incidence of sarcopenia, as well as each sarcopenia diagnostic criterion, were assessed with odds ratios (ORs) and 95% confidence intervals (CIs) using multivariable logistic regression analysis. Participants were divided into tertiles of dietary intake, with the lowest tertile considered as the reference group. In addition, the p-values for the trends were calculated using the median value of each tertile. Analysis of covariance (ANCOVA) with Bonferroni correction was performed to evaluate the mean differences in sarcopenia parameters among the tertiles after adjusting for confounding variables. All statistical analyses were performed using the SPSS software (version 27.0; SPSS Inc., Chicago, IL, USA); p < 0.05 was considered statistically significant.
3 Results
3.1 Characteristics of participants
The incidence of sarcopenia was 37.8% after the 6-year follow-up. Participants with sarcopenia were older with a lower BMI and higher proportion of cognitive impairment than participants without sarcopenia at the 6-year follow-up (Table 1). Other characteristics were not significantly different between sarcopenic and non-sarcopenic participants. Participants with sarcopenia consumed less oily fish than non-sarcopenic participants. However, there were no differences in the intake of seafood, fish, non-oily fish, or shellfish between the two groups.
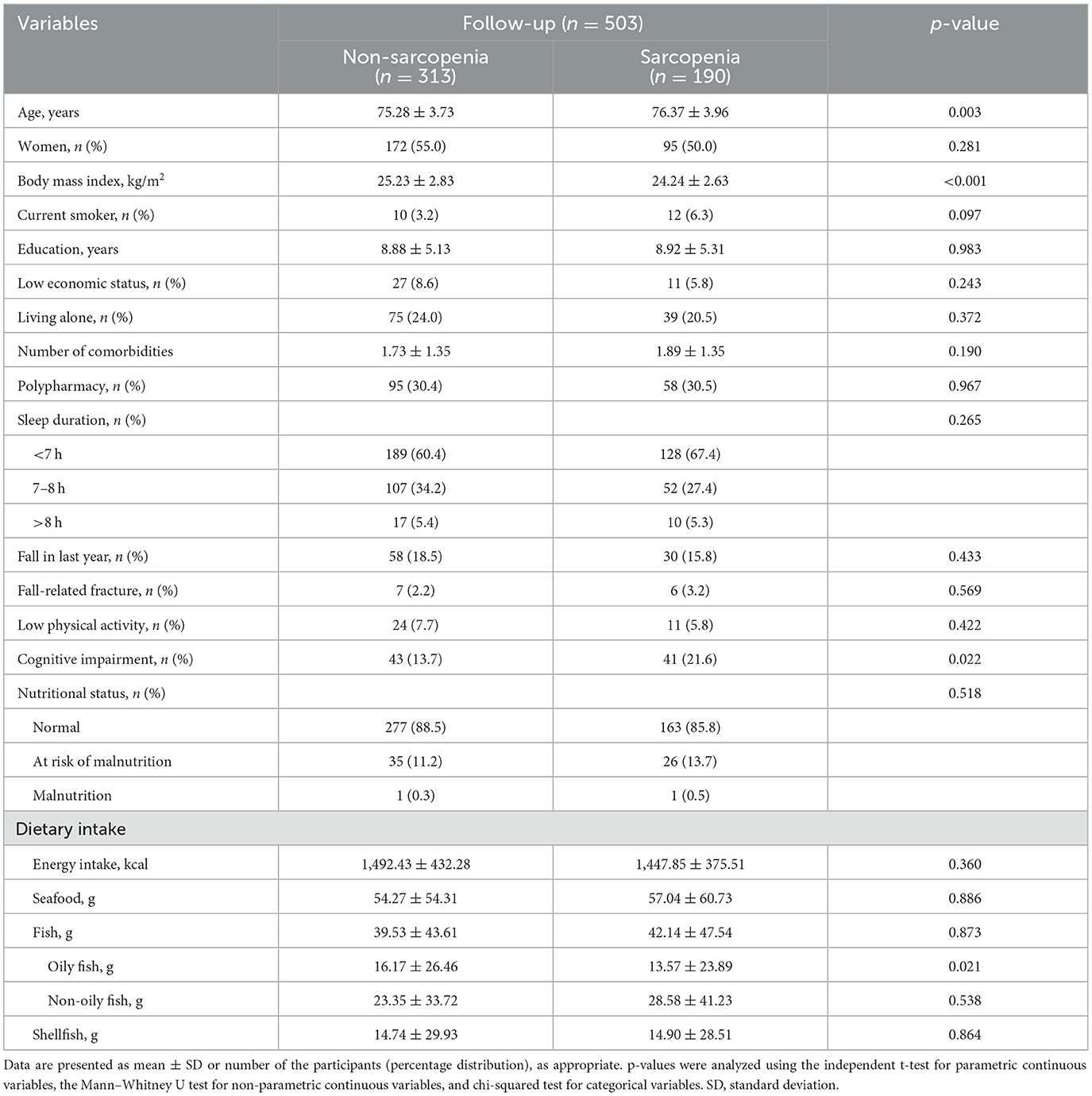
Table 1. Baseline characteristics of the participants according to the incidence of sarcopenia after the 6-year follow-up.
3.2 Association between sarcopenia and intake of seafood
Multivariate logistic regression analysis revealed an inverse association between the incidence of sarcopenia and oily fish intake at the 6-year follow-up (Table 2). There was no significant association between the incidence of sarcopenia and intake of seafood, fish, non-oily fish, or shellfish.
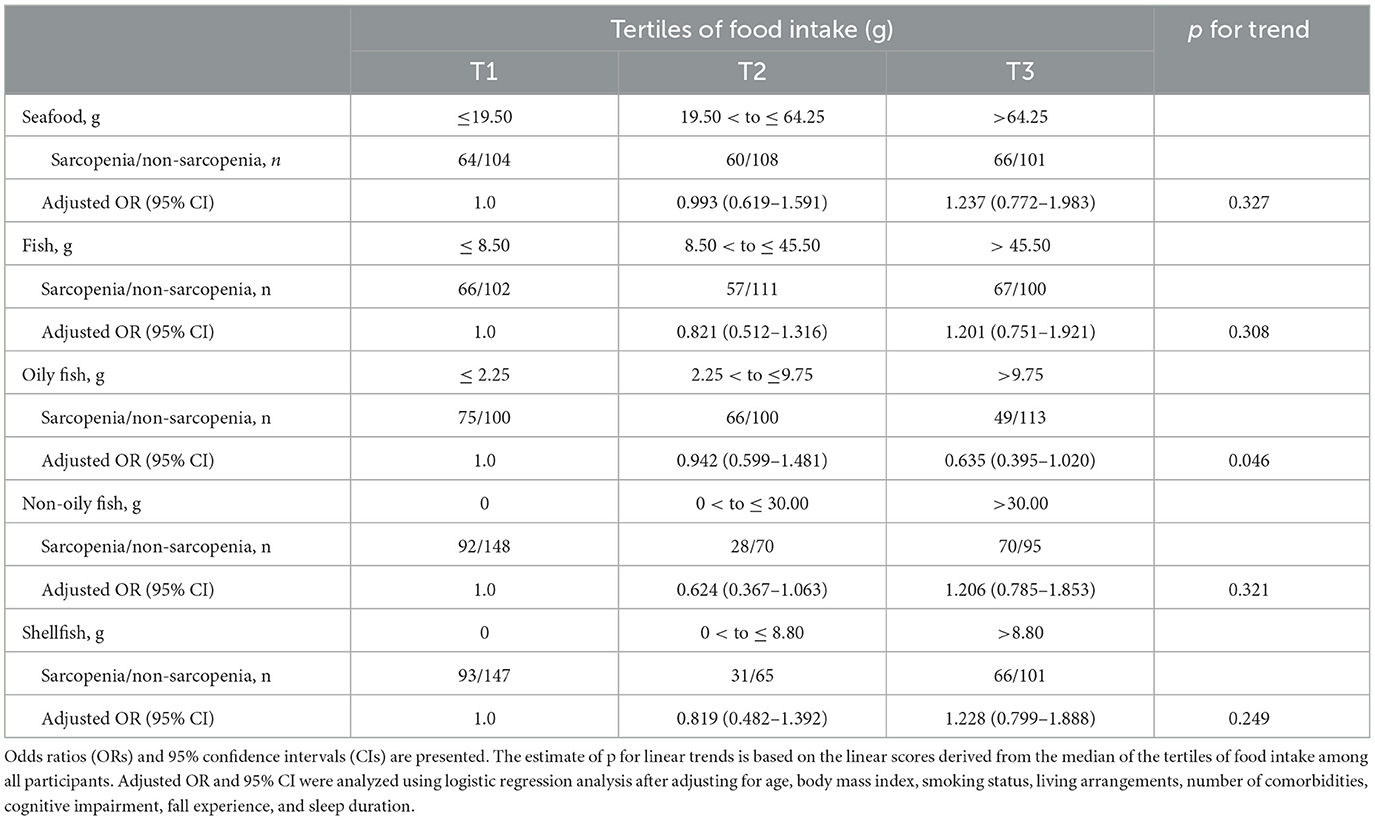
Table 2. Logistic regression of seafood, fish, and shellfish intake for the incidence of sarcopenia after the 6-year follow-up.
3.3 Associations between sarcopenia parameters and intake of seafood
In the multivariate model, the incidence of low UGS was inversely associated with oily fish intake (Table 3). Additionally, the highest tertile of oily fish intake was inversely associated with the incidence of low UGS compared to the lowest tertile after adjusting for confounding factors. Consistently, ANCOVA showed a significant positive association between UGS and oily fish intake after adjusting for confounding factors (Table 4). There were no significant associations between the other sarcopenia parameters and the intake of seafood, fish, non-oily fish, or shellfish (Supplementary Tables S1, S2).
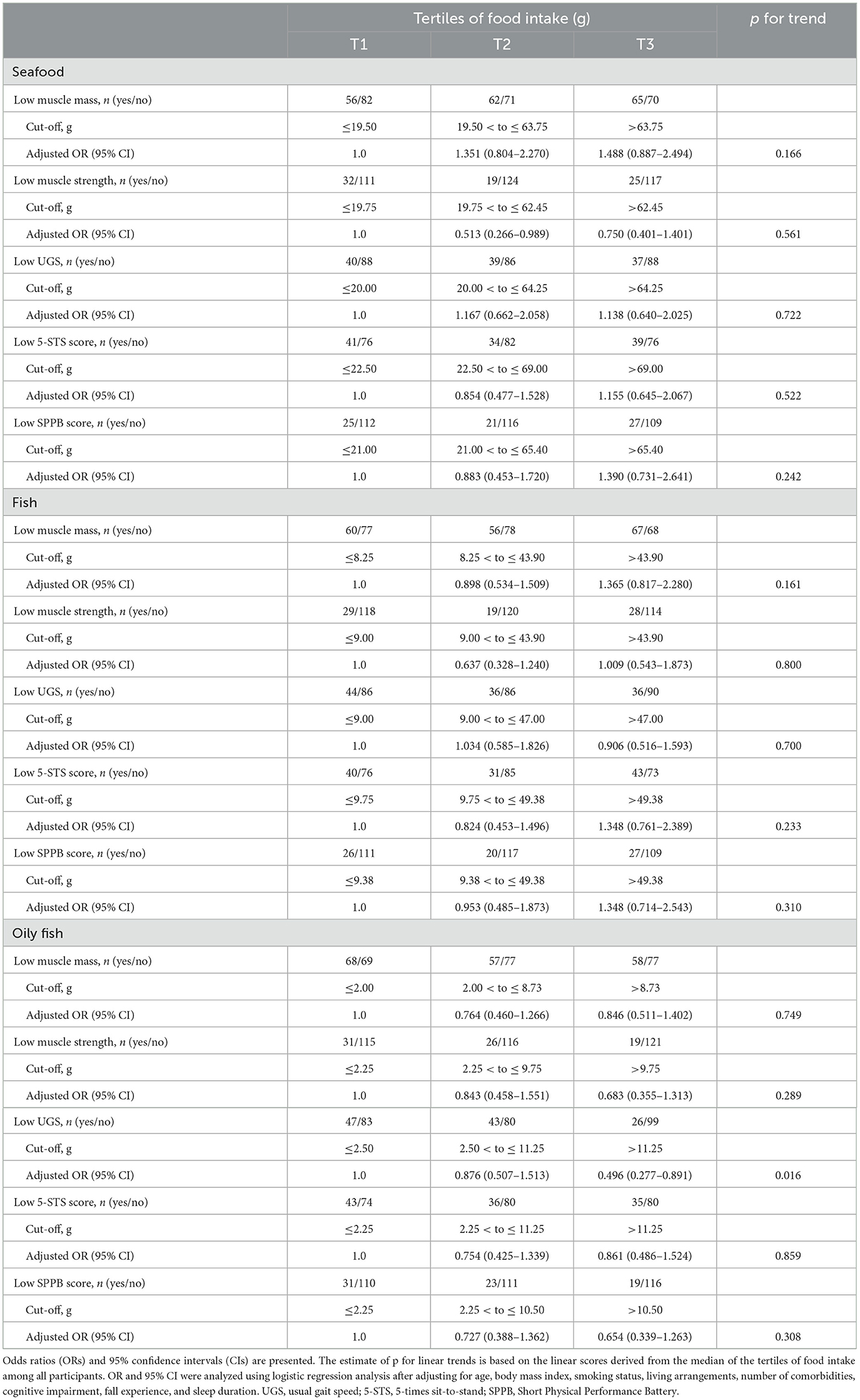
Table 3. Logistic regression of seafood, fish, and oily fish intake for the incidence of each sarcopenia component after the 6-year follow-up.
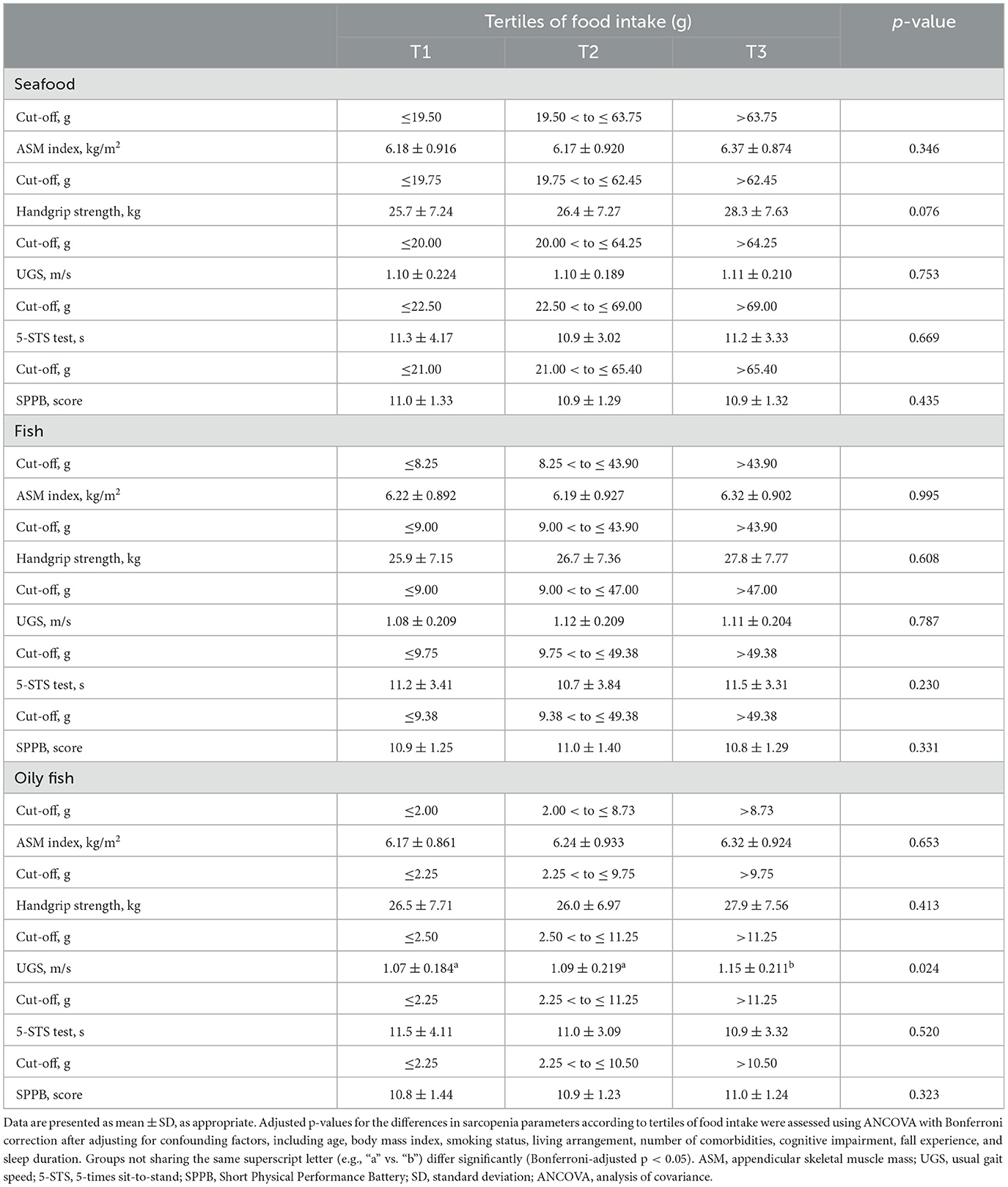
Table 4. Sarcopenia parameters at the 6-year follow-up according to tertiles of seafood, fish, and oily fish intake.
4 Discussion
In the present study, the intake of oily fish, but not seafood or other fish, was significantly associated with the incidence of sarcopenia in community-dwelling older Koreans after a 6-year follow-up period. Consistently, the prevalence of sarcopenia is not associated with the frequency of fish and shrimp intake, which ranges from 0 to 7 times per week in older Chinese individuals (4–6). The average daily fish intake of approximately 40 g is similar to the 35 g reported in older Chinese individuals (34). However, the frequency of oily fish intake was reportedly five times higher among Korean adults (35) compared with Chinese adults (36). Although no study has investigated the association between oily fish intake and the incidence or prevalence of sarcopenia, previous studies have reported the positive association of the intake of oily fish, but not non-oily fish, with muscle strength and physical performance in older British (7, 8) and Spanish populations (9). The beneficial effects of oily fish on sarcopenia could be attributed to the higher content of n-3 PUFA compared to non-oily fish. Oily fish contain approximately seven times more n-3 PUFA, especially eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), than non-oily fish (31). In the present study, the average daily intake of oily fish was approximately 15 g, similar to the positive associations between oily fish intake of 14–22 g and muscle strength (7) and physical performance (9). Furthermore, oily fish accounted for approximately 38% of the total fish intake in the present study, similar to the 39% reported in a Spanish study showing the beneficial effects of oily fish on physical performance (9). These previous studies support our finding that oily fish intake could affect sarcopenia, as achieving similar effects from non-oily fish would require substantially higher consumption.
Consistently, the intake of n-3 PUFA has been inversely associated with the prevalence of sarcopenia in older Australian men (10), Japanese individuals with type 2 diabetes (11), and Brazilian recipients of kidney transplant (12). Yang et al. (16) reported that the ratio of n-3 PUFA to energy intake was inversely associated with the prevalence of sarcopenic obesity in older Korean women based on data from KNHANES. Meta-analyses of clinical studies have indicated that supplementation of n-3 PUFA improves muscle mass, muscle strength, and physical performance in older adults (23, 24). In immobilized aged rodent models, n-3 PUFA reportedly increased muscle protein synthesis by activating the mTOR pathway, enhancing amino acid transport, and decreased muscle protein breakdown by reducing inflammatory signaling and inhibiting the ubiquitin-proteasome system (25).
Blood levels of n-3 PUFA, an objective biomarker of the dietary intake of n-3 PUFA, were inversely associated with the prevalence of sarcopenia in older Koreans (17) and Japanese patients with liver cirrhosis and hepatocellular carcinoma (18), but not in older Dutch adults (37). The erythrocyte levels of EPA and DHA were approximately 9% in Koreans and Japanese (38), but 4% in Dutch individuals (38). Additionally, the prevalence rate of sarcopenia was reportedly 13–20% in older Koreans and Japanese (39), but 22–32% in older Dutch individuals (40–42), which is higher than that in Asians. In the present study, the incidence of sarcopenia was 37.8% after the 6-year follow-up among older Koreans, aligning with the annual incidence estimate of 6.3% reported by the KFACS study (43).
In the present study, the consumption of oily fish, but not seafood or fish, was inversely associated with the incidence of low UGS at the 6-year follow-up. Our previous study also found that the consumption of oily fish was inversely associated with the prevalence of low UGS in older British individuals based on data from the UK Biobank (15). However, no significant association was observed between the intake of oily fish and UGS in older British individuals in the Hertfordshire Cohort Study (44). The intake of oily fish was similar in older British adults in the UK Biobank study and Hertfordshire Cohort Study (44, 45). However, the average UGS was 1.1 m/s among older British based on the UK Biobank (46), and 0.9 m/s from the Hertfordshire Cohort Study (44), suggesting that older British in Hertfordshire Cohort had slower UGS than those from UK Biobank. In the present study, the average UGS was 1.1 m/s, which is consistent with a previous KFACS study (47). Furthermore, n-3 PUFA intake has been positively associated with UGS in older Finnish women (13), Australian individuals with subjective memory complaints (14), and older British adults (15). Consistently, blood levels of n-3 PUFA have been positively associated with UGS in older British (15), French (19), Italian (20), Korean (21), and Japanese populations (22). A meta-analysis of clinical studies also showed that n-3 PUFA supplementation improved UGS in older Americans and Europeans (24).
There were no associations in the present study between seafood, fish, and oily fish consumption and muscle mass, handgrip strength, 5-STS test, and SPPB score. Previous studies consistently reported that the consumption of seafood or fish was not associated with muscle mass in older Chinese (48) and British women (49); handgrip strength in older Koreans (50), Norwegians (51), Finns (52), and Italian women (53); and the 5-STS test in older British individuals (44). Furthermore, the SPPB score was not associated with seafood consumption in older Norwegians (51) or Australian individuals with type 2 diabetes (54). Regarding oily fish consumption, there was no previous study investigating the association with muscle mass. Consistent with the present study, Martin et al. (44) also found no association between oily fish intake and 5-STS test in older British individuals. In the present study, there was no significant association between oily fish intake and handgrip strength, but the positive association was reported in older British individuals (7, 8, 15). These discrepancies could be due to the method of measuring handgrip strength, since the present study used a digital dynamometer but other studies used a hydraulic dynamometer. Savas et al. (55) reported that handgrip strength measured using a hydraulic dynamometer was lower than that measured using a digital dynamometer in adults, particularly in those >60 years of age. Unlike the present study, previous studies reported a positive association between oily fish intake and the SPPB score in Spanish population (9, 56). There were racial differences between the present study and the previous studies, and the cut-off score SPPB was also different; 6 in the previous studies and 9 in the present study. The cut-off score for SPPB is 9 according to AWGS (27), while 8 according to the European Working Group on Sarcopenia (57).
This is the first longitudinal study to show that the intake of oily fish, but not seafood or other fish, is inversely associated with the incidence of sarcopenia among older adults after a 6-year follow-up. However, this study had a few limitations. First, dietary data were obtained using two non-consecutive days of 24-h dietary recalls during two different seasons, which might not be sufficient to reflect the usual dietary intake. Second, although potential confounders were adjusted for in the analysis, residual confounding factors such as n-3 PUFA supplementation and other dietary influences may remain. Third, cooking methods such as frying or roasting could reduce the content of n-3 PUFA due to increased oxidation (58), but the study did not account for these cooking methods. Last, our findings were obtained only from Korean older adults, which might not be generalized to other populations.
5 Conclusion
The consumption of oily fish could have beneficial effects in preventing sarcopenia, partly by improving walking speed in community-dwelling older Koreans, suggesting oily fish as a strategy to reduce sarcopenia risk. Further clinical studies are required to confirm the preventive effects of n-3 PUFA against sarcopenia.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Kyung Hee University Hospital Institutional Review Board, Hanyang University Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SY: Formal analysis, Visualization, Writing – original draft. MK: Data curation, Writing – review & editing. CW: Data curation, Writing – review & editing. YP: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the BK21 Fostering Outstanding Universities for Research (FOUR) project of the National Research Foundation of Korea Grant, a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (grant number NRS RS-2024-00334109), and the “Korea National Institute of Health” (KNIH) research project (2024-ER0603-00).
Conflict of interest
The authors declare that this study was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1543290/full#supplementary-material
References
1. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9
2. Yuan S, Larsson SC. Epidemiology of sarcopenia: prevalence, risk factors, and consequences. Metabolism. (2023) 144:155533. doi: 10.1016/j.metabol.2023.155533
3. Dupont J, Dedeyne L, Dalle S, Koppo K, Gielen E. The role of omega-3 in the prevention and treatment of sarcopenia. Aging Clin Exp Res. (2019) 31:825–36. doi: 10.1007/s40520-019-01146-1
4. Hai S, Wang H, Cao L, Liu P, Zhou J, Yang Y, et al. Association between sarcopenia with lifestyle and family function among community-dwelling Chinese aged 60 years and older. BMC Geriatr. (2017) 17:187. doi: 10.1186/s12877-017-0587-0
5. Yang LJ, Wu GH, Yang YL, Wu YH, Zhang L, Wang MH, et al. Nutrition, physical exercise, and the prevalence of sarcopenia in elderly residents in nursing homes in China. Med Sci Monit. (2019) 25:4390–9. doi: 10.12659/MSM.914031
6. Wang B, Wei Y, Shao L, Li M, Zhang X, Li W, et al. Association of dietary patterns and sarcopenia in the elderly population: a cross-sectional study. Front Aging. (2023) 4:1239945. doi: 10.3389/fragi.2023.1239945
7. Robinson SM, Jameson KA, Batelaan SF, Martin HJ, Syddall HE, Dennison EM, et al. Diet and its relationship with grip strength in community-dwelling older men and women: the Hertfordshire cohort study. J Am Geriatr Soc. (2008) 56:84–90. doi: 10.1111/j.1532-5415.2007.01478.x
8. Gedmantaite A, Celis-Morales CA, Ho F, Pell JP, Ratkevicius A, Gray SR. Associations between diet and handgrip strength: a cross-sectional study from UK Biobank. Mech Ageing Dev. (2020) 189:111269. doi: 10.1016/j.mad.2020.111269
9. Vega-Cabello V, Caballero FF, Rodriguez-Artalejo F, Lopez-Garcia E, Struijk EA. Leucine intake and risk of impaired physical function and frailty in older adults. J Gerontol A Biol Sci Med Sci. (2023) 78:241–9. doi: 10.1093/gerona/glac191
10. Das A, Cumming RG, Naganathan V, Blyth F, Le Couteur DG, Handelsman DJ, et al. Associations between nutrient intakes and dietary patterns with different sarcopenia definitions in older Australian men: the concord health and ageing in men project. Public Health Nutr. (2021) 24:4490–505. doi: 10.1017/S1368980020003547
11. Okamura T, Hashimoto Y, Miki A, Kaji A, Sakai R, Iwai K, et al. Reduced dietary omega-3 fatty acids intake is associated with sarcopenia in elderly patients with type 2 diabetes: a cross-sectional study of KAMOGAWA-DM cohort study. J Clin Biochem Nutr. (2020) 66:233–7. doi: 10.3164/jcbn.19-85
12. Dos Reis AS, Limirio LS, Santos HO, de Oliveira EP. Intake of polyunsaturated fatty acids and ω-3 are protective factors for sarcopenia in kidney transplant patients. Nutrition. (2021) 81:110929. doi: 10.1016/j.nut.2020.110929
13. Isanejad M, Tajik B, McArdle A, Tuppurainen M, Sirola J, Kröger H, et al. Dietary omega-3 polyunsaturated fatty acid and alpha-linolenic acid are associated with physical capacity measure but not muscle mass in older women 65–72 years. Eur J Nutr. (2022) 61:1813–21. doi: 10.1007/s00394-021-02773-z
14. Erhardt R, Cardoso BR, Meyer BJ, Brownell S, O'Connell S, Mirzaee S, et al. Omega-3 long-chain polyunsaturated fatty acids: are they beneficial for physical and cognitive functioning in older adults? J Nutr Health Aging. (2021) 25:454–61. doi: 10.1007/s12603-020-1553-7
15. Kim J, Westra J, Tintle N, Harris WS, Park Y. Association of plasma n-3 polyunsaturated fatty acid levels and the prevalence of frailty in older adults: a cross-sectional analysis of UK Biobank. J Gerontol A Biol Sci Med Sci. (2024) 79:glae085. doi: 10.1093/gerona/glae085
16. Yang W, Lee JW, Kim Y, Lee JH, Kang HT. Increased omega-3 fatty acid intake is inversely associated with sarcopenic obesity in women but not in men, based on the 2014–2018 Korean National Health and Nutrition Examination Survey. J Clin Med. (2020) 9:3856. doi: 10.3390/jcm9123856
17. Jang IY, Jung HW, Park JH, Kim JH, Lee S, Lee E, et al. Lower serum n-3 fatty acid level in older adults with sarcopenia. Nutrients. (2020) 12:2959. doi: 10.3390/nu12102959
18. Sano A, Inoue J, Kakazu E, Ninomiya M, Tsuruoka M, Sato K, et al. Association of omega-3 polyunsaturated fatty acids with sarcopenia in liver cirrhosis patients with hepatocellular carcinoma. J Clin Transl Hepatol. (2024) 12:613–24. doi: 10.14218/JCTH.2024.00036
19. Frison E, Boirie Y, Peuchant E, Tabue-Teguo M, Barberger-Gateau P, Féart C. Plasma fatty acid biomarkers are associated with gait speed in community-dwelling older adults: the Three-City-Bordeaux study. Clin Nutr. (2017) 36:416–22. doi: 10.1016/j.clnu.2015.12.008
20. Abbatecola AM, Cherubini A, Guralnik JM, Andres Lacueva CA, Ruggiero C, Maggio M, et al. Plasma polyunsaturated fatty acids and age-related physical performance decline. Rejuvenation Res. (2009) 12:25–32. doi: 10.1089/rej.2008.0799
21. Kim D, Won CW, Park Y. Association between erythrocyte levels of n-3 polyunsaturated fatty acids and risk of frailty in community-dwelling older adults: the Korean frailty and aging cohort study. J Gerontol A Biol Sci Med Sci. (2021) 76:499–504. doi: 10.1093/gerona/glaa042
22. Kinoshita K, Otsuka R, Tange C, Nishita Y, Tomida M, Ando F, et al. Relationship between serum fatty acids and components of physical frailty in community-dwelling Japanese older adults. J Frailty Aging. (2021) 10:237–40. doi: 10.14283/jfa.2020.67
23. Cornish SM, Cordingley DM, Shaw KA, Forbes SC, Leonhardt T, Bristol A, et al. Effects of omega-3 supplementation alone and combined with resistance exercise on skeletal muscle in older adults: a systematic review and Meta-analysis. Nutrients. (2022) 14:2221. doi: 10.3390/nu14112221
24. Huang YH, Chiu WC, Hsu YP, Lo YL, Wang YH. Effects of omega-3 fatty acids on muscle mass, muscle strength and muscle performance among the elderly: a meta-analysis. Nutrients. (2020) 12:3739. doi: 10.3390/nu12123739
25. Therdyothin A, Phiphopthatsanee N, Isanejad M. The effect of omega-3 fatty acids on sarcopenia: mechanism of action and potential efficacy. Mar Drugs. (2023) 21:399. doi: 10.3390/md21070399
26. Won CW, Lee S, Kim J, Chon D, Kim S, Kim CO, et al. Korean frailty and aging cohort study (KFACS): cohort profile. BMJ Open. (2020) 10:e035573. doi: 10.1136/bmjopen-2019-035573
27. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–307.e2. doi: 10.1016/j.jamda.2019.12.012
28. Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. (2000) 55:M221–31. doi: 10.1093/gerona/55.4.M221
29. National Rural Resources Development Institute. Food Composition Table I. The 7th Revision, Korea (2006). Available at: https://www.fao.org/fileadmin/templates/food_composition/documents/regional/Korean_food_compositon_table_vol._1_7th__2006_.pdf (accessed on February 11, 2024).
30. National Institute of Fisheries Science. Composition Table of Marine Products in Korea. 8th Edn. Busan: National Institute of Fisheries Science (2018). p. 205–91.
31. Public Health England and the Food Standards Agency. SACN Advice on Fish Consumption: Benefits & Risks. London, UK (2004). Available at: https://assets.publishing.service.gov.uk/media/5a7dbedc40f0b65d88634277/SACN_Advice_on_Fish_Consumption.pdf (accessed on February 20, 2024).
32. Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National Sleep Foundation's sleep time duration recommendations: methodology and results summary. Sleep Health. (2015) 1:40–3. doi: 10.1016/j.sleh.2014.12.010
33. Greenland S, Pearce N. Statistical foundations for model-based adjustments. Annu Rev Public Health. (2015) 36:89–108. doi: 10.1146/annurev-publhealth-031914-122559
34. Wang Q, Liu S, Wang H, Su C, Liu A, Jiang L. Consumption of aquatic products and meats in Chinese residents: a nationwide survey. Front Nutr. (2022) 9:927417. doi: 10.3389/fnut.2022.927417
35. Kim KW, Sreeja SR, Kwon M, Yu YL, Kim MK. Association of blood mercury level with the risk of depression according to fish intake level in the general Korean population: findings from the Korean National Health and Nutrition Examination Survey (KNHANES) 2008–2013. Nutrients. (2020) 12:189. doi: 10.3390/nu12010189
36. Wang RZ, Zhang WS, Jiang CQ, Zhu F, Jin YL, Xu L. Association of fish and meat consumption with non-alcoholic fatty liver disease: Guangzhou biobank Cohort Study. BMC Public Health. (2023) 23:2433. doi: 10.1186/s12889-023-17398-6
37. Ter Borg S, Luiking YC, Van Helvoort A, Boirie Y, Schols JMGA, De Groot CPGM. Low levels of branched chain amino acids, eicosapentaenoic acid and micronutrients are associated with low muscle mass, strength and function in community-dwelling older adults. J Nutr Health Aging. (2019) 23:27–34. doi: 10.1007/s12603-018-1108-3
38. Schuchardt JP, Beinhorn P, Hu XF, Chan HM, Roke K, Bernasconi A, et al. Omega-3 world map: 2024 update. Prog Lipid Res. (2024) 95:101286. doi: 10.1016/j.plipres.2024.101286
39. Weng SE, Huang YW, Tseng YC, Peng HR, Lai HY, Akishita M, et al. The evolving landscape of sarcopenia in Asia: a systematic review and meta-analysis following the 2019 Asian working group for sarcopenia (AWGS) diagnostic criteria. Arch Gerontol Geriatr. (2025) 128:105596. doi: 10.1016/j.archger.2024.105596
40. Schaap LA, Van Schoor NM, Lips P, Visser M. Associations of sarcopenia definitions, and their components, with the incidence of recurrent falling and fractures: the Longitudinal Aging Study Amsterdam. J Gerontol A Biol Sci Med Sci. (2018) 73:1199–204. doi: 10.1093/gerona/glx245
41. Ter Borg S, de Groot LCPGM, Mijnarends DM, de Vries JHM, Verlaan S, Meijboom S, et al. Differences in nutrient intake and biochemical nutrient status between sarcopenic and nonsarcopenic older adults-results from the Maastricht sarcopenia Study. J Am Med Dir Assoc. (2016) 17:393–401. doi: 10.1016/j.jamda.2015.12.015
42. Verstraeten LMG, Mashni A, van Wijngaarden JP, Meskers CGM, Maier AB. Sarcopenia knowledge of geriatric rehabilitation patients is low while they are willing to start sarcopenia treatment: EMPOWER-GR. J Cachexia Sarcopenia Muscle. (2024) 15:352–60. doi: 10.1002/jcsm.13372
43. Choe HJ, Cho BL, Park YS, Roh E, Kim HJ, Lee SG, et al. Gender differences in risk factors for the 2 year development of sarcopenia in community-dwelling older adults. J Cachexia Sarcopenia Muscle. (2022) 13:1908–18. doi: 10.1002/jcsm.12993
44. Martin H, Aihie Sayer A, Jameson K, Syddall H, Dennison EM, Cooper C, et al. Does diet influence physical performance in community-dwelling older people? Findings from the Hertfordshire cohort study. Age Ageing. (2011) 40:181–6. doi: 10.1093/ageing/afq175
45. Foster HME, Celis-Morales CA, Nicholl BI, Petermann-Rocha F, Pell JP, Gill JMR, et al. The effect of socioeconomic deprivation on the association between an extended measurement of unhealthy lifestyle factors and health outcomes: a prospective analysis of the UK Biobank cohort. Lancet Public Health. (2018) 3:e576–85. doi: 10.1016/S2468-2667(18)30200-7
46. Chan LLY, Lord SR, Brodie MA. Daily-life walking speed, quality and quantity derived from a wrist motion sensor: large-scale normative data for middle-aged and older adults. Sensors. (2024) 24:5159. doi: 10.3390/s24165159
47. Jung HW, Kim S, Jang IY, Shin DW, Lee JE, Won CW. Screening value of timed up and go test for frailty and low physical performance in Korean older population: the Korean frailty and aging cohort study (KFACS). Ann Geriatr Med Res. (2020) 24:259–66. doi: 10.4235/agmr.20.0072
48. Tian HY, Qiu R, Jing LP, Chen ZY, Chen GD, Chen YM. Alternate Mediterranean diet score is positively associated with skeletal muscle mass index in middle-aged adults. Br J Nutr. (2017) 117:1181–8. doi: 10.1017/S0007114517001118
49. Kelaiditi E, Jennings A, Steves CJ, Skinner J, Cassidy A, MacGregor AJ, et al. Measurements of skeletal muscle mass and power are positively related to a Mediterranean dietary pattern in women. Osteoporos Int. (2016) 27:3251–60. doi: 10.1007/s00198-016-3665-9
50. Kim MH, Choi MK, Bae YJ. Relationship between protein intake and grip strength in qualitative and quantitative aspects among the elderly in Korea: results from the Korea National Health and Nutrition Examination Survey. BMC Geriatr. (2023) 23:330. doi: 10.1186/s12877-023-04016-8
51. Nygård LK, Dahl L, Mundal I, Šaltyte Benth J, Rokstad AMM. Protein intake, protein mealtime distribution and seafood consumption in elderly Norwegians: associations with physical function and strength. Geriatrics. (2020) 5:100. doi: 10.3390/geriatrics5040100
52. Perälä MM, von Bonsdorff MB, Männistö S, Salonen MK, Simonen M, Kanerva N, et al. The healthy Nordic diet predicts muscle strength 10 years later in old women, but not old men. Age Ageing. (2017) 46:588–94. doi: 10.1093/ageing/afx034
53. Barrea L, Muscogiuri G, Di Somma C, Tramontano G, De Luca V, Illario M, et al. Association between Mediterranean diet and hand grip strength in older adult women. Clin Nutr. (2019) 38:721–9. doi: 10.1016/j.clnu.2018.03.012
54. McClure R, Villani A. Greater adherence to a Mediterranean diet is associated with better gait speed in older adults with type 2 diabetes mellitus. Clin Nutr ESPEN. (2019) 32:33–9. doi: 10.1016/j.clnesp.2019.05.009
55. Savas S, Kilavuz A, Kayhan Koçak FÖ, Cavdar S. Comparison of grip strength measurements by widely used three dynamometers in outpatients aged 60 years and over. J Clin Med. (2023) 12:4260. doi: 10.3390/jcm12134260
56. Arias-Fernández L, Struijk EA, Rodríguez-Artalejo F, Lopez-Garcia E, Lana A. Habitual dietary fat intake and risk of muscle weakness and lower-extremity functional impairment in older adults: a prospective cohort study. Clin Nutr. (2020) 39:3663–70. doi: 10.1016/j.clnu.2020.03.018
57. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
Keywords: sarcopenia, oily fish, n-3 polyunsaturated fatty acids, seafood, usual gait speed, community-dwelling older adults
Citation: Yi S, Kim M, Won CW and Park Y (2025) Association between fish intake and incidence of sarcopenia in community-dwelling older adults after a 6-year follow-up: the Korean frailty and aging cohort study. Front. Nutr. 12:1543290. doi: 10.3389/fnut.2025.1543290
Received: 11 December 2024; Accepted: 13 January 2025;
Published: 28 January 2025.
Edited by:
Elma Izze da Silva Magalhães, Federal University of Rio Grande do Sul, BrazilReviewed by:
Aline Rezende Ribeiro De Abreu, Universidade Federal de Ouro Preto, BrazilCátia Ficagna, Federal University of Rio Grande do Sul, Brazil
Dafne Pavão Schattschneider, Federal University of Rio Grande do Sul, Brazil
Copyright © 2025 Yi, Kim, Won and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongsoon Park, eW9uZ3Nvb25AaGFueWFuZy5hYy5rcg==
 Seunghyun Yi1
Seunghyun Yi1 Chang Won Won
Chang Won Won Yongsoon Park
Yongsoon Park