
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 06 March 2025
Sec. Clinical Nutrition
Volume 12 - 2025 | https://doi.org/10.3389/fnut.2025.1540933
This article is part of the Research Topic Nutritional Strategies for Enhancing Longevity and Healthy Aging View all 3 articles
Background: Chronic low-grade systemic inflammation plays a significant role in age-related macular degeneration (AMD) pathogenesis. The systemic inflammatory response index (SIRI), a novel inflammatory marker, may predict various diseases. However, data on the relationship between SIRI and AMD are limited. This study examines the relationship between SIRI and AMD and assesses its potential as a predictive biomarker.
Methods: A cross-sectional analysis of the National Health and Nutrition Examination Survey (NHANES) data from 2005 to 2008 was conducted on participants aged ≥40 years with SIRI and AMD status data. Multivariable logistic regression models adjusted for confounders were used to assess the association. Sensitivity and subgroup analyses, along with restricted cubic spline (RCS) curve analysis, were performed.
Results: Among 5,365 participants, 425 (7.9%) had AMD. The median SIRI was higher in AMD patients (1.23 vs. 1.04, p < 0.001). Higher SIRI was independently associated with increased odds (adjusted OR: 1.18, 95% CI:1.07–1.29, p = 0.001). RCS analyses revealed a dose–response relationship (p = 0.002). Subgroup analyses showed a positive association in male participants, individuals with hypertension, individuals with obesity, and non-smokers. Higher SIRI levels were independently associated with increased AMD risk (adjusted OR: 1.27, 95% CI: 1.03–1.56, p = 0.023).
Conclusion: Elevated SIRI is independently associated with increased AMD risk in the U.S. population. SIRI may serve as a biomarker for identifying high-risk individuals, enabling early intervention. The cross-sectional design limits causal inference, and unmeasured confounders may affect the results. SIRI could potentially serve as a non-invasive biomarker for AMD risk, pending further validation through longitudinal studies.
Age-related macular degeneration (AMD) is a progressive, inflammatory retinal disorder that primarily affects the macula (1), leading to severe vision impairment. Symptoms include difficulty performing tasks in low light or low contrast, visual distortion, and reduced visual acuity (2). AMD has become a significant public health concern due to the growing aging population and increasing life expectancy (3, 4). Globally, approximately 196 million people are affected by AMD (5). It is a leading cause of severe vision loss in individuals aged >55 years in high-income countries, and it imposes a significant psychosocial burden (6, 7). Late-stage AMD manifests as either geographic atrophy (dry AMD) or neovascularization (wet AMD), with 6–9% of cases leading to legal blindness worldwide (6). Established risk factors include age, genetic factors, and environmental factors (5).
The pathogenesis of AMD is complex and multifactorial, involving genetic susceptibility, aging-related disruption of retinal homeostasis, impaired lipid metabolism, immune activation, chronic inflammation, oxidative stress, and extracellular matrix (ECM) dysfunction (1). Among these factors, chronic inflammation plays a significant role. It promotes drusen formation, which can lead to severe vision loss (8). In AMD, inflammation is triggered by the adaptive immune response to accumulated molecular damage in the aging eye, leading to local immune activation (9). Chronic inflammation and tissue damage are associated with abnormal activation of the complement system (10, 11). Chronic inflammation also recruits microglia and macrophages to the subretinal and choroidal regions—key indicators of immune activation in AMD (8). Additionally, senescent cells resulting from chronic inflammation secrete pro-inflammatory cytokines and chemokines, further activating microglia, macrophages, and the complement system, contributing to the inflammatory response (8, 12).
Chronic inflammation induces oxidative stress (1), which damages retinal neurons and the retinal pigment epithelium (RPE), worsening inflammation and tissue damage (13, 14). It is also associated with lipid deposition and oxidation in drusen, generating pro-angiogenic signals that contribute to choroidal neovascularization, which is a late-stage manifestation of AMD (1, 15). Furthermore, chronic inflammation disrupts retinal homeostasis, impairing ECM maintenance and clearance of cellular debris, both of which contribute to AMD progression (16, 17). In summary, chronic inflammation is a central feature of AMD pathogenesis, contributing to tissue damage, immune system activation, and disease progression.
Early detection of AMD can leverage inflammatory biomarkers such as elevated interleukin-6, C-reactive protein, and white blood cell levels (18–22). Systemic inflammatory markers are advantageous for disease prediction due to their low cost and accessibility. They have recently gained popularity for predicting several diseases, including AMD (23–27). The systemic inflammatory response index (SIRI), identified by Qi et al. in 2016, is a novel marker that quantifies inflammation using peripheral blood parameters (28). It has been proposed as a predictor for various conditions, including cancer, cardiovascular diseases, and pneumonia (29–41). However, its relationship with AMD prevalence remains unclear. We hypothesize that higher SIRI levels are positively associated with AMD risk, given the role of systemic inflammation in AMD pathophysiology. Unlike neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), which are linked to other inflammatory conditions, SIRI may better capture a broad systemic inflammatory response by including lymphocyte, neutrophil, and monocyte counts. This population-based study aimed to investigate the association between SIRI and the risk of AMD.
This cross-sectional study primarily utilized data from the National Health and Nutrition Examination Survey (NHANES), a widely used dataset for analyzing the nutritional and health status of individuals in the United States. Data for this study were collected from 2005 to 2008. The analysis included 5,365 participants aged ≥40 years with valid data on SIRI and AMD status.
The primary outcome variable was AMD status, defined based on the standardized grading of retinal photographs. AMD was detected through the presence of (i) soft drusen and pigmented abnormalities, (ii) geographic atrophy or pigment epithelial detachment, (iii) subretinal hemorrhage or visible subretinal new vessels, and (iv) subretinal fibrous or laser treatment scars (42, 43).
SIRI was calculated using the formula:
as defined in previous studies (28).
Covariates included demographic data (age, sex, ethnicity, and marital status), medical history (diabetes and hypertension), body mass index (BMI), obesity, and smoking status. Detailed information on variable collection methods is available in the NHANES Survey Methods and Analysis Guide. Age is a well-established risk factor for AMD, with its prevalence increasing significantly over time (4, 44). Women may have a higher risk due to hormonal factors and longer life expectancy (45). Racial and ethnic disparities exist, with Non-Hispanic White individuals at the highest risk (46). Marital status may influence health behaviors and access to healthcare, indirectly affecting AMD risk (47). Diabetes and hypertension contribute to AMD risk through mechanisms involving chronic inflammation, oxidative stress, and vascular health (48, 49). Obesity, linked to systemic inflammation and metabolic dysfunction, may exacerbate AMD development (50). Smoking is a well-documented risk factor due to its role in oxidative stress and inflammation (51, 52). These covariates were selected to account for potential confounders and ensure a comprehensive analysis of AMD risk determinants.
Baseline variables were characterized based on AMD presence. Continuous variables were expressed as mean ± standard deviation, while categorical variables were presented as numbers (%). Differences between groups were analyzed using analysis of variance for continuous variables and chi-square tests for categorical variables.
Multivariable logistic regression models were used to assess the association between SIRI and AMD risk, adjusting for potential confounders. SIRI was also categorized into tertiles based on the distribution of SIRI values in our study population to evaluate linear trends between SIRI and AMD risk. In the following extended models: (i) model 1 was unadjusted; (ii) model 2 was adjusted for sex, age, marital status, and race/ethnicity; and (iii) model 3 was further adjusted for obesity, hypertension, diabetes, and smoking status. A restricted cubic spline (RCS) curve was used to explore the relationship between SIRI and AMD risk.
Subgroup analyses were conducted with stratified factors comprising (i) sex (male/female), (ii) race (Mexican American, Non-Hispanic White, Non-Hispanic Black, or Other Races), (iii) hypertension (yes/no), (iv) diabetes (yes/no), (v) obesity (yes/no), and (vi) smoking status (non-, former, or current smokers).
Sensitivity analyses were conducted to assess the stability of the results. Participants with SIRI scores below the 5th percentile or above the 95th percentile were excluded, and multivariate logistic regression analyses were re-conducted.
Statistical significance was set at p < 0.05, and all analyses were conducted using R software (version 4.1.1).
A total of 5,365 participants (male (n) = 2,687 (50.1%), female (n) = 2,678 (49.9%)) with a median age of 59 years were included in the study. Among them, 425 (7.9%) participants had AMD. Participants with AMD were more likely to be older, Non-Hispanic White, of other marital status, and to have lower BMI, hypertension, and a history of former smoking compared to those without AMD (all p < 0.05). Additionally, the median SIRI was significantly higher among participants with AMD compared to those without AMD (1.23 vs. 1.04, p < 0.001). Table 1 presents participant characteristics categorized by AMD status.
The association between SIRI and AMD risk was assessed through multivariable logistic regression analyses (Table 2). When SIRI was analyzed as a continuous variable, a positive correlation with AMD was observed (p < 0.001). This positive correlation persisted after adjusting for potential confounders (p = 0.001).
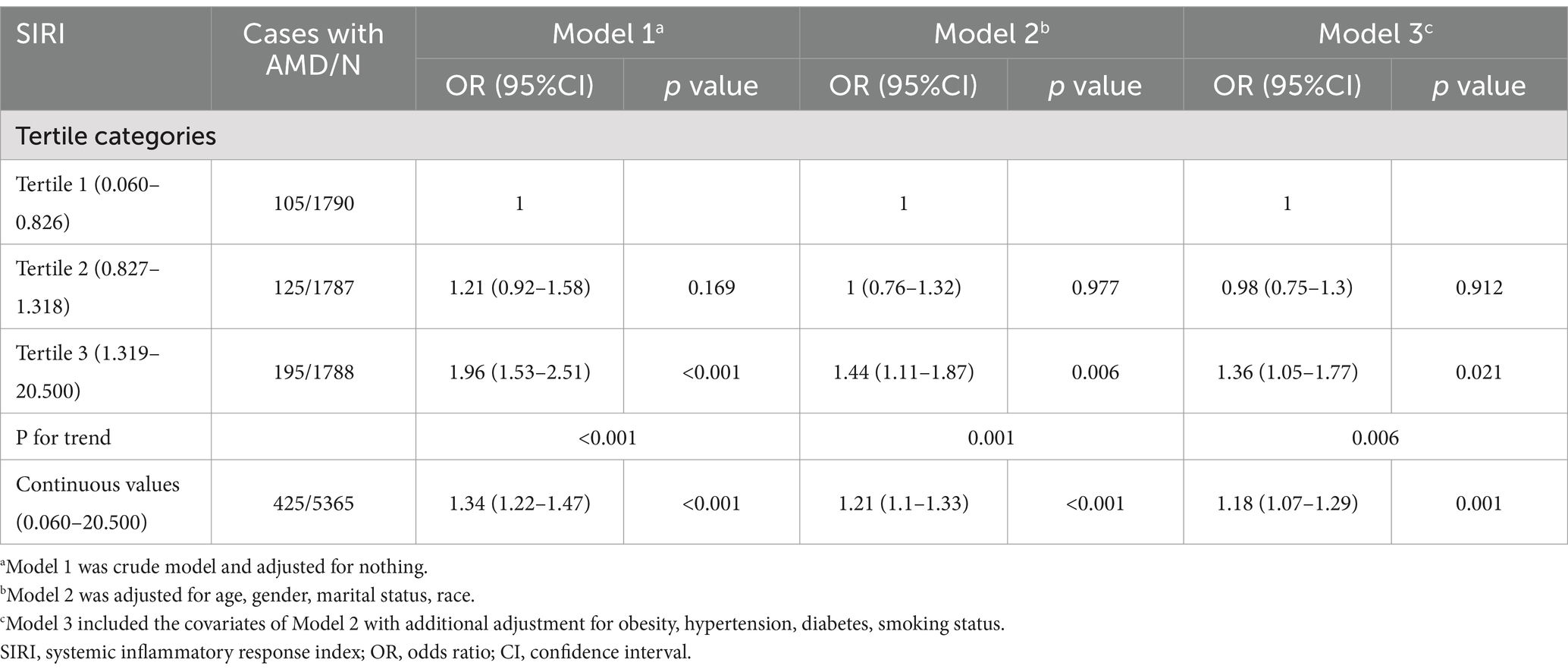
Table 2. Associations between the SIRI and age-related macular degeneration risk in the total cohort.
When SIRI was analyzed as a categorical variable, in model 3, participants in the highest SIRI tertile had an increased risk of AMD prevalence compared with those in the lowest SIRI quartile (p = 0.021). This association remained statistically significant across all three models. However, the second SIRI tertile did not exhibit a significant association with AMD risk in model 3. The trend test indicated a statistically significant association between SIRI and AMD risk (p for trend =0.006). Furthermore, RCS analyses demonstrated a dose–response relationship between SIRI and AMD risk (p = 0.002; Figure 1).
AMD is an age-related retinal disorder affecting the macula, with inflammation playing a pivotal role in its pathogenesis. Various cytokines and immune cells, including macrophages, dendritic cells, neutrophils, T-lymphocytes, and B-lymphocytes, contribute to both innate and adaptive immune responses involved in AMD progression (53). Elevated SIRI may reflect increased neutrophil-driven oxidative stress and monocyte-mediated chronic inflammation, both key contributors to AMD progression. Oxidative damage can initiate inflammation and lead to AMD-like lesions, as demonstrated in models where oxidative fragments elicit immune responses mimicking AMD pathology (54). Elevated NLR, a marker of systemic inflammation, has been linked with AMD, particularly its neovascular subtype (55). Activation of toll-like receptors (TLRs) in the RPE induces pro-inflammatory responses, contributing to chronic inflammation and AMD progression (56, 57). Additionally, genetic polymorphisms in complement system components, such as complement factor H (CFH), are strongly associated with AMD, with overactive complement pathways driving inflammation and disease progression (58, 59).
These findings suggest that inflammatory markers and cytokines could serve as potential diagnostic biomarkers for AMD (29). Already, anti-inflammatory therapies targeting specific pathways, such as the complement system and TLRs, are being explored as treatment options (53, 56). Thus, inflammation in AMD involves complex interactions between oxidative damage, immune responses, and genetic predispositions, offering potential opportunities for diagnostic and therapeutic strategies aimed at mitigating inflammation and managing AMD.
SIRI has been proposed as a predictor for various diseases, including cancers, cardiovascular diseases, and pneumonia (29–41). However, research examining its association with AMD risk remains limited. The existing literature presents conflicting perspectives on the relationship between leukocytes and AMD. While some studies suggest that elevated white blood cell counts are associated with an increased risk of AMD (22, 60), others report no significant correlation (52, 61). Recently, the use of various blood components from routine complete blood count (CBC) measurements to detect inflammatory markers in patients with AMD has garnered attention. For example, patients with AMD have been shown to have higher neutrophil and lower lymphocyte levels compared to controls (55, 62, 63). Furthermore, Karahan et al. reported that patients with wet AMD have significantly higher NLR and monocyte-to-lymphocyte ratio than those with dry AMD and healthy controls (64).
These studies highlight the potential usefulness of CBC parameters as markers of AMD-associated inflammation. Neutrophils, the most abundant type of leukocytes, play a significant role in both acute and chronic inflammation by mediating phagocytosis and releasing anti-inflammatory mediators (65, 66). Lymphocytes are involved in the onset and resolution of inflammation, responding to different activation or inhibition signals. Lymphocyte infiltration is associated with the initiation and progression of inflammatory responses, contributing to tissue damage and functional impairment in inflammatory diseases (66, 67). Similarly, monocytes contribute to inflammation (66, 68). Elevated PLR, often linked to thrombocytosis-induced lymphocytopenia, has been associated with poor prognoses in inflammatory disorders (69, 70). Among these leukocyte subtypes, neutrophils and lymphocytes appear to have a more significant role in AMD pathogenesis. Consequently, including other leukocyte types in SIRI calculations may detract from its usefulness as a focused inflammatory marker.
The results of the present study indicated that SIRI had a significant positive correlation with AMD risk. A recent retrospective study (71) reported no significant difference in SIRI scores between the AMD and control groups, suggesting that SIRI may be insufficient to assess AMD-related inflammation. However, this study had a major limitation, which was its small sample size (case group (n) = 90, control group (n) = 270), which limited its statistical power to identify certain trends or correlations. In contrast, the present study utilized a much larger sample of 5,365 participants and found a marked positive relationship between SIRI and AMD prevalence.
Subgroup analyses stratified by sex, ethnicity, hypertension, diabetes, obesity, and smoking status were conducted to investigate the relationship between SIRI and AMD in different populations (Table 3). The degree of association varied across subgroups.
SIRI was positively associated with AMD risk among male participants (p = 0.043). Similarly, significant associations were observed among participants with hypertension (p = 0.020), obesity (p = 0.040), and non-smokers (p = 0.009). These findings suggest that the association between SIRI and AMD risk was statistically significant in most subgroups. However, some subgroups did not exhibit significant association.
Previous studies have consistently shown that smoking significantly increases the risk of AMD and its subtypes. In comparison, current smokers had a significantly increased—approximately two-to three-fold—risk of AMD compared to non-smokers (51, 72–75), with geographic atrophy (GA) and neovascular AMD showing varying degrees of statistical significance in different studies (72, 74). Additionally, the AMD risk increases with cumulative smoking exposure, with individuals exceeding 40 pack years having a significantly higher risk (74). Genetic interactions, such as those involving the CFH and LOC387715 genes, further increase AMD risk in smokers, suggesting that individuals with certain genetic predispositions have higher risks when they smoke (76–79). Quitting smoking reduces this AMD, with former smokers showing a gradual decline in AMD prevalence over time (73–75). Smoking contributes to AMD pathogenesis through oxidative stress and exacerbation of ocular inflammation, both of which damage retinal tissues (78, 80).
The present study’s findings indicated that individuals with AMD demonstrated a higher probability of lower BMI compared to those without AMD. However, the positive association between SIRI and AMD was more pronounced in participants with obesity. A meta-analysis (81) including 1,613 individuals from MEDLINE, EMBASE, and ISI Web databases reported a 32% increased risk of late AMD in obese participants (relative risk [RR]: 1.32, 95% CI: 1.11–1.53, p < 0.01), while no significant association was found for early AMD (RR: 0.91, 95% CI: 0.74–1.08; p = 0.67). These differences may be due to the varying impacts of obesity across different AMD stages. Additionally, the association between SIRI and AMD appeared stronger in males than in females, potentially due to higher antioxidant gene activity and enzyme levels in females, which could mitigate oxidative stress and reduce AMD risk (82, 83).
This study also revealed a statistically significant association between SIRI and AMD in participants with hypertension. Similar to previous studies, participants with AMD were more likely to develop hypertension than those without AMD (84, 85). Hypertension-related oxidative stress, pro-inflammatory factors, and other pathological mechanisms may underlie this relationship (86, 87). These findings emphasize the usefulness of SIRI for assessing inflammation in patients with hypertension, enabling early detection and prevention of AMD.
This study has several strengths. First, to the best of our knowledge, this is the first study to investigate the association between SIRI and AMD in a general population. Second, the use of the NHANES database, which has a large and representative sample that allows for the exclusion of possible interfering factors, enhances the reliability of the study’s findings. Third, by focusing on SIRI as a composite metric instead of its components, this study offers a comprehensive understanding of the complex interactions between inflammatory processes and AMD.
Despite its strengths, this study has several limitations. First, the cross-sectional design precludes the establishment of causal relationships between SIRI and AMD. Second, self-reported medical conditions introduce the potential for recall bias. Further prospective studies are needed to address these issues by clarifying the exact relationship between SIRI and AMD. Third, although multiple confounders were excluded, the influence of unmeasured confounders cannot be entirely ruled out. Finally, due to limitations in the NHANES dataset, this study focused on eligible adults, leaving gaps in understanding SIRI’s association with AMD in high-risk older populations. However, explanations on the association of SIRI with AMD in high-risk older populations are not provided because the usefulness of SIRI for detecting AMD is crucial in this demographic.
This study involved cross-sectional analyses of data from 5,365 individuals from the NHANES database to investigate the association between SIRI and AMD risk. The findings demonstrated a positive association between SIRI and AMD prevalence. Participants in the highest SIRI tertile demonstrated significantly higher odds of AMD compared to those in the lowest tertile, even after adjusting for potential confounders. Additionally, higher SIRI scores significantly increased the risk of AMD among males, participants with obesity, non-smokers, and those with hypertension. Overall, the findings demonstrated that elevated SIRI is independently associated with an increased risk of AMD in the US population.
These findings suggest that SIRI could be used as a biomarker for AMD risk assessment, which can greatly benefit patient care. Since it is derived from peripheral blood, its measurement is straightforward, cost-effective, and non-invasive. This makes it highly suitable for widespread use in routine screening, especially in primary healthcare settings. Such routine screening can lead to early diagnosis and intervention, which are key to improving the prognosis of AMD patients. For instance, closer eye monitoring can be arranged to detect any early-stage symptoms of AMD. Lifestyle changes, such as dietary adjustments and increased physical activity, can be recommended to mitigate the risk factors associated with AMD development. Additionally, preventive therapies, like antioxidant supplementation or anti-inflammatory medications, can be initiated. All these measures have the potential to either slow down the progression of AMD or, in the best-case scenario, prevent its onset altogether. This not only improves the quality of life for patients but also reduces the economic burden associated with long-term AMD management.
However, it is crucial to acknowledge the limitations of this study. The cross-sectional design of this study precludes the establishment of a causal relationship between SIRI and AMD. Although an association between them exists, it is possible that other unmeasured factors contribute simultaneously to the elevation of SIRI and the development of AMD.
To address the current knowledge gaps, further prospective studies are urgently needed. These studies should track large cohorts over an extended period, precisely measure SIRI levels at regular intervals, and closely monitor the development of AMD. By doing so, they can confirm the current results and, more importantly, explore the causal relationship between SIRI and AMD. Additionally, future research should focus on clarifying how the components of SIRI, such as the ratios of different immune cells, relate to the pathogenesis of AMD. This in-depth understanding will be instrumental in developing more targeted and effective prevention and treatment strategies for AMD, ultimately benefiting a large number of patients worldwide.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Ethics Committee of Tianjin Medical University Cancer Institute and Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
RJ: Conceptualization, Project administration, Validation, Writing – original draft. YY: Project administration, Writing – review & editing. HS: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We gratefully thank all the participants and staff of the National Health and Nutrition Examination Survey for their invaluable contributions to the collection, administration, and publication of the data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fleckenstein, M, Keenan, TDL, Guymer, RH, Chakravarthy, U, Schmitz-Valckenberg, S, Klaver, CC, et al. Age-related macular degeneration. Nat Rev Dis Primers. (2021) 7:31. doi: 10.1038/s41572-021-00265-2
2. Christen, WG, Glynn, RJ, Manson, JE, Ajani, UA, and Buring, JE. A prospective study of cigarette smokingand risk of age-related macular degeneration inmen. JAMA. (1996) 276:1147–51. doi: 10.1001/jama.1996.03540140035023
3. Guymer, RH, and Campbell, TG. Age-related macular degeneration. Lancet. (2023) 401:1459–72. doi: 10.1016/S0140-6736(22)02609-5
4. Wong, WL, Su, X, Li, X, Cheung, CM, Klein, R, Cheng, CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. (2014) 2:e106–16. doi: 10.1016/S2214-109X(13)70145-1
5. Fleckenstein, M, Schmitz-Valckenberg, S, and Chakravarthy, U. Age-related macular degeneration: a review. JAMA. (2024) 331:147–57. doi: 10.1001/jama.2023.26074
6. Jonas, JB, Cheung, CMG, and Panda-Jonas, S. Updates on the epidemiology of age-related macular degeneration. Asia Pac J Ophthalmol (Phila). (2017) 6:493–7. doi: 10.22608/APO.2017251
7. GBD 2019 Blindness and Vision Impairment Collaborators. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the right to sight: an analysis for the global burden of disease study. Lancet Glob Health. (2021) 9:e144–60. doi: 10.1016/S2214-109X(20)30489-7
8. Chen, M, and Xu, H. Parainflammation, chronic inflammation, and age-related macular degeneration. J Leukoc Biol. (2015) 98:713–25. doi: 10.1189/jlb.3RI0615-239R
9. Rozing, MP, Durhuus, JA, Krogh Nielsen, M, Subhi, Y, Kirkwood, TB, Westendorp, RG, et al. Age-related macular degeneration: a two-level model hypothesis. Prog Retin Eye Res. (2020) 76:100825. doi: 10.1016/j.preteyeres.2019.100825
10. Anderson, DH, Mullins, RF, Hageman, GS, and Johnson, LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. (2002) 134:411–31. doi: 10.1016/s0002-9394(02)01624-0
11. Fritsche, LG, Igl, W, Bailey, JN, Grassmann, F, Sengupta, S, Bragg-Gresham, JL, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. (2016) 48:134–43. doi: 10.1038/ng.3448
12. Kortvely, E, Hauck, SM, Behler, J, Ho, N, and Ueffing, M. The unconventional secretion of ARMS2. Hum Mol Genet. (2016) 25:3143–51. doi: 10.1093/hmg/ddw162
13. Jabbehdari, S, and Handa, JT. Oxidative stress as a therapeutic target for the prevention and treatment of early age-related macular degeneration. Surv Ophthalmol. (2021) 66:423–40. doi: 10.1016/j.survophthal.2020.09.002
14. Datta, S, Cano, M, Ebrahimi, K, Wang, L, and Handa, JT. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog Retin Eye Res. (2017) 60:201–18. doi: 10.1016/j.preteyeres.2017.03.002
15. Thompson, RB, Reffatto, V, Bundy, JG, Kortvely, E, Flinn, JM, Lanzirotti, A, et al. Identification of hydroxyapatite spherules provides new insight into subretinal pigment epithelial deposit formation in the aging eye. Proc Natl Acad Sci USA. (2015) 112:1565–70. doi: 10.1073/pnas.1413347112
16. Calippe, B, Augustin, S, Beguier, F, Charles-Messance, H, Poupel, L, Conart, JB, et al. Complement factor H inhibits CD47-mediated resolution of inflammation. Immunity. (2017) 46:261–72. doi: 10.1016/j.immuni.2017.01.006
17. Miller, JW. Age-related macular degeneration revisited--piecing the puzzle: the LXIX Edward Jackson memorial lecture. Am J Ophthalmol. (2013) 155:1–35.e13. doi: 10.1016/j.ajo.2012.10.018
18. Seddon, JM, Gensler, G, Milton, RC, Klein, ML, and Rifai, N. Association between C-reactive protein and age-related macular degeneration. JAMA. (2004) 291:704–10. doi: 10.1001/jama.291.6.704
19. Nahavandipour, A, Krogh Nielsen, M, Sørensen, TL, and Subhi, Y. Systemic levels of interleukin-6 in patients with age-related macular degeneration: a systematic review and meta-analysis. Acta Ophthalmol. (2020) 98:434–44. doi: 10.1111/aos.14402
20. Semba, RD, Moaddel, R, Cotch, MF, Jonasson, F, Eiriksdottir, G, Harris, TB, et al. Serum lipids in adults with late age-related macular degeneration: a case-control study. Lipids Health Dis. (2019) 18:7. doi: 10.1186/s12944-018-0954-7
21. Wu, S, Hsu, LA, Teng, MS, Lin, JF, Chou, HH, Lee, MC, et al. Interactive effects of C-reactive protein levels on the association between APOE variants and triglyceride levels in a Taiwanese population. Lipids Health Dis. (2016) 15:94. doi: 10.1186/s12944-016-0262-z
22. Shankar, A, Mitchell, P, Rochtchina, E, Tan, J, and Wang, JJ. Association between circulating white blood cell count and long-term incidence of age-related macular degeneration: the Blue Mountains eye study. Am J Epidemiol. (2007) 166:393–402. doi: 10.1093/aje/kwm096
23. Sengul, EA, Artunay, O, Kockar, A, Afacan, C, Rasier, R, Gun, P, et al. Correlation of neutrophil/lymphocyte and platelet/lymphocyte ratio with visual acuity and macular thickness in age-related macular degeneration. Int J Ophthalmol. (2017) 10:754–9. doi: 10.18240/ijo.2017.05.16
24. Suppiah, A, Malde, D, Arab, T, Hamed, M, Allgar, V, Smith, AM, et al. The prognostic value of the neutrophil-lymphocyte ratio (NLR) in acute pancreatitis: identification of an optimal NLR. J Gastrointest Surg. (2013) 17:675–81. doi: 10.1007/s11605-012-2121-1
25. Torun, S, Tunc, BD, Suvak, B, Yildiz, H, Tas, A, Sayilir, A, et al. Assessment of neutrophil-lymphocyte ratio in ulcerative colitis: a promising marker in predicting disease severity. Clin Res Hepatol Gastroenterol. (2012) 36:491–7. doi: 10.1016/j.clinre.2012.06.004
26. Mertoglu, C, and Gunay, M. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as useful predictive markers of prediabetes and diabetes mellitus. Diabetes Metab Syndr. (2017) 11:S127–31. doi: 10.1016/j.dsx.2016.12.021
27. Ilhan, N, Daglioglu, MC, Ilhan, O, Coskun, M, Tuzcu, EA, Kahraman, H, et al. Assessment of neutrophil/lymphocyte ratio in patients with age-related macular degeneration. Ocul Immunol Inflamm. (2015) 23:287–90. doi: 10.3109/09273948.2014.921715
28. Qi, Q, Zhuang, L, Shen, Y, Geng, Y, Yu, S, Chen, H, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. (2016) 122:2158–67. doi: 10.1002/cncr.30057
29. Zhang, Y, Xing, Z, Zhou, K, and Jiang, S. The predictive role of systemic inflammation response index (SIRI) in the prognosis of stroke patients. Clin Interv Aging. (2021) 16:1997–2007. doi: 10.2147/CIA.S339221
30. Han, K, Shi, D, Yang, L, Wang, Z, Li, Y, Gao, F, et al. Prognostic value of systemic inflammatory response index in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Ann Med. (2022) 54:1667–77. doi: 10.1080/07853890.2022.2083671
31. Urbanowicz, T, Michalak, M, Olasińska-Wiśniewska, A, Rodzki, M, Witkowska, A, Gąsecka, A, et al. Neutrophil counts, neutrophil-to-lymphocyte ratio, and systemic inflammatory response index (SIRI) predict mortality after off-pump coronary artery bypass surgery. Cells. (2022) 11:1124–40. doi: 10.3390/cells11071124
32. Urbanowicz, T, Olasińska-Wiśniewska, A, Michalak, M, Perek, B, Al-Imam, A, Rodzki, M, et al. Pre-operative systemic inflammatory response index influences long-term survival rate in off-pump surgical revascularization. PLoS One. (2022) 17:e0276138. doi: 10.1371/journal.pone.0276138
33. Nascimento, MAL, Ferreira, LGR, Alves, TVG, and Rios, DRA. Inflammatory hematological indices, cardiovascular disease and mortality: a narrative review. Arq Bras Cardiol. (2024) 121:e20230752. doi: 10.36660/abc.20230752
34. Qin, Y, Liu, L, Zhao, S, Wang, W, Han, M, Dong, S, et al. Blood inflammatory biomarkers predict in-hospital pneumonia after endovascular treatment of aneurysm in patients with aneurysmal subarachoid hemorrhage. Neurosurg Rev. (2023) 46:171. doi: 10.1007/s10143-023-02082-5
35. Wang, L, Qin, X, Zhang, Y, Xue, S, and Song, X. The prognostic predictive value of systemic immune index and systemic inflammatory response index in nasopharyngeal carcinoma: a systematic review and meta-analysis. Front Oncol. (2023) 13:1006233. doi: 10.3389/fonc.2023.1006233
36. Topkan, E, Mertsoylu, H, Kucuk, A, Besen, AA, Sezer, A, Sezen, D, et al. Low systemic inflammation response index predicts good prognosis in locally advanced pancreatic carcinoma patients treated with concurrent chemoradiotherapy. Gastroenterol Res Pract. (2020) 2020:5701949. doi: 10.1155/2020/5701949
37. Sun, L, Hu, W, Liu, M, Chen, Y, Jin, B, Xu, H, et al. High systemic inflammation response index (SIRI) indicates poor outcome in gallbladder cancer patients with surgical resection: a single institution experience in China. Cancer Res Treat. (2020) 52:1199–210. doi: 10.4143/crt.2020.303
38. Li, S, Lan, X, Gao, H, Li, Z, Chen, L, Wang, W, et al. Systemic inflammation response index (SIRI), cancer stem cells and survival of localised gastric adenocarcinoma after curative resection. J Cancer Res Clin Oncol. (2017) 143:2455–68. doi: 10.1007/s00432-017-2506-3
39. Chen, L, Kong, X, Wang, Z, Wang, X, Fang, Y, and Wang, J. Pretreatment systemic inflammation response index in patients with breast cancer treated with neoadjuvant chemotherapy as a useful prognostic indicator. Cancer Manag Res. (2020) 12:1543–67. doi: 10.2147/CMAR.S235519
40. Chao, B, Ju, X, Zhang, L, Xu, X, and Zhao, Y. A novel prognostic marker systemic inflammation response index (SIRI) for operable cervical cancer patients. Front Oncol. (2020) 10:766. doi: 10.3389/fonc.2020.00766
41. Paliogiannis, P, Zinellu, A, Scano, V, Mulas, G, de Riu, G, Pascale, RM, et al. Laboratory test alterations in patients with COVID-19 and non COVID-19 interstitial pneumonia: a preliminary report. J Infect Dev Ctries. (2020) 14:685–90. doi: 10.3855/jidc.12879
42. Bird, AC, Bressler, NM, Bressler, SB, Chisholm, IH, Coscas, G, Davis, MD, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The international ARM epidemiological study group. Surv Ophthalmol. (1995) 39:367–74. doi: 10.1016/s0039-6257(05)80092-x
43. Klein, R, Davis, MD, Magli, YL, Segal, P, Klein, BE, and Hubbard, L. The Wisconsin age-related maculopathy grading system. Ophthalmology. (1991) 98:1128–34. doi: 10.1016/s0161-6420(91)32186-9
44. Klein, R, Klein, BE, and Linton, KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. (1992) 99:933–43. doi: 10.1016/s0161-6420(92)31871-7
45. Rudnicka, AR, Jarrar, Z, Wormald, R, Cook, DG, Fletcher, A, and Owen, CG. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta-analysis. Ophthalmology. (2012) 119:571–80. doi: 10.1016/j.ophtha.2011.09.027
46. Klein, R, Klein, BE, Knudtson, MD, Wong, TY, Cotch, MF, Liu, K, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology. (2006) 113:373–80. doi: 10.1016/j.ophtha.2005.12.013
47. Wang, JJ, Mitchell, P, Smith, W, Cumming, RG, and Attebo, K. Impact of visual impairment on use of community support services by elderly persons: the Blue Mountains eye study. Invest Ophthalmol Vis Sci. (1999) 40:12–9.9888427.
48. Cheung, N, and Wong, TY. Diabetic retinopathy and systemic vascular complications. Prog Retin Eye Res. (2007) 26:189–203. doi: 10.1016/j.preteyeres.2006.12.001
49. Klein, R, Klein, BE, Tomany, SC, and Cruickshanks, KJ. The association of cardiovascular disease with the long-term incidence of age-related maculopathy: the beaver dam eye study. Ophthalmology. (2003) 110:1273–80. doi: 10.1016/S0161-6420(03)00599-2
50. Seddon, JM, George, S, Rosner, B, and Rifai, N. Progression of age-related macular degeneration: prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers. Arch Ophthalmol. (2005) 123:774–82. doi: 10.1001/archopht.123.6.774
51. Thornton, J, Edwards, R, Mitchell, P, Harrison, RA, Buchan, I, and Kelly, SP. Smoking and age-related macular degeneration: a review of association. Eye (Lond). (2005) 19:935–44. doi: 10.1038/sj.eye.6701978
52. Gopinath, B, Flood, VM, Rochtchina, E, Wang, JJ, and Mitchell, P. Homocysteine, folate, vitamin B-12, and 10-y incidence of age-related macular degeneration. Am J Clin Nutr. (2013) 98:129–35. doi: 10.3945/ajcn.112.057091
53. Tan, W, Zou, J, Yoshida, S, Jiang, B, and Zhou, Y. The role of inflammation in age-related macular degeneration. Int J Biol Sci. (2020) 16:2989–3001. doi: 10.7150/ijbs.49890
54. Hollyfield, JG, Bonilha, VL, Rayborn, ME, Yang, X, Shadrach, KG, Lu, L, et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. (2008) 14:194–8. doi: 10.1038/nm1709
55. Niazi, S, Krogh Nielsen, M, Sørensen, TL, and Subhi, Y. Neutrophil-to-lymphocyte ratio in age-related macular degeneration: a systematic review and meta-analysis. Acta Ophthalmol. (2019) 97:558–66. doi: 10.1111/aos.14072
56. Klettner, A, and Roider, J. Retinal pigment epithelium expressed toll-like receptors and their potential role in age-related macular degeneration. Int J Mol Sci. (2021) 22:8387. doi: 10.3390/ijms22168387
57. Zareparsi, S, Buraczynska, M, Branham, KE, Shah, S, Eng, D, Li, M, et al. Toll-like receptor 4 variant D299G is associated with susceptibility to age-related macular degeneration. Hum Mol Genet. (2005) 14:1449–55. doi: 10.1093/hmg/ddi154
58. Liu, B, Wei, L, Meyerle, C, Tuo, J, Sen, HN, Li, Z, et al. Complement component C5a promotes expression of IL-22 and IL-17 from human T cells and its implication in age-related macular degeneration. J Transl Med. (2011) 9:1–12. doi: 10.1186/1479-5876-9-111
59. Telander, DG. Inflammation and age-related macular degeneration (AMD). Semin Ophthalmol. (2011) 26:192–7. doi: 10.3109/08820538.2011.570849
60. Yasuda, M, Kiyohara, Y, Hata, Y, Arakawa, S, Yonemoto, K, Doi, Y, et al. Nine-year incidence and risk factors for age-related macular degeneration in a defined Japanese population. The Hisayama study. Ophthalmology. (2009) 116:2135–40. doi: 10.1016/j.ophtha.2009.04.017
61. Wu, KHC, Tan, AG, Rochtchina, E, Favaloro, EJ, Williams, A, and Mitchell, P. Circulating inflammatory markers and hemostatic factors in age-related maculopathy: a population-based case-control study. Invest Ophthalmol Vis Sci. (2007) 48:1983–8. doi: 10.1167/iovs.06-0223
62. Pinna, A, Porcu, T, D’Amico-Ricci, G, Dore, S, Boscia, F, Paliogiannis, P, et al. Complete blood cell count-derived inflammation biomarkers in men with age-related macular degeneration. Ocul Immunol Inflamm. (2019) 27:932–6. doi: 10.1080/09273948.2018.1485960
63. Naif, S, Majed, R, Mohieldin, E, Hanan, A, Lamis, A, and Maha, A. Neutrophil-lymphocyte ratios in dry age-related macular degeneration. Ocul Immunol Inflamm. (2023) 31:1647–52. doi: 10.1080/09273948.2022.2092752
64. Karahan, M, Hazar, L, Erdem, S, Ava, S, Dursun, ME, Demirtaş, AA, et al. Is there a relationship between hematological inflammatory parameters and age-related macular degeneration? Ther Adv Ophthalmol. (2021) 13:251584142110105. doi: 10.1177/25158414211010550
65. Herrero-Cervera, A, Soehnlein, O, and Kenne, E. Neutrophils in chronic inflammatory diseases. Cell Mol Immunol. (2022) 19:177–91. doi: 10.1038/s41423-021-00832-3
66. Kauppinen, A, Paterno, JJ, Blasiak, J, Salminen, A, and Kaarniranta, K. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci. (2016) 73:1765–86. doi: 10.1007/s00018-016-2147-8
67. Sakai, Y, and Kobayashi, M. Lymphocyte “homing” and chronic inflammation. Pathol Int. (2015) 65:344–54. doi: 10.1111/pin.12294
68. Shi, C, and Pamer, EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. (2011) 11:762–74. doi: 10.1038/nri3070
69. Akboga, MK, Canpolat, U, Yayla, C, Ozcan, F, Ozeke, O, Topaloglu, S, et al. Association of Platelet to lymphocyte ratio with inflammation and severity of coronary atherosclerosis in patients with stable coronary artery disease. Angiology. (2016) 67:89–95. doi: 10.1177/0003319715583186
70. Lian, L, Xia, YY, Zhou, C, Shen, XM, Li, XL, Han, SG, et al. Application of platelet/lymphocyte and neutrophil/lymphocyte ratios in early diagnosis and prognostic prediction in patients with resectable gastric cancer. Cancer Biomark. (2015) 15:899–907. doi: 10.3233/CBM-150534
71. Sannan, NS. Assessment of aggregate index of systemic inflammation and systemic inflammatory response index in dry age-related macular degeneration: a retrospective study. Front Med (Lausanne). (2023) 10:1143045. doi: 10.3389/fmed.2023.1143045
72. Cong, R, Zhou, B, Sun, Q, Gu, H, Tang, N, and Wang, B. Smoking and the risk of age-related macular degeneration: a meta-analysis. Ann Epidemiol. (2008) 18:647–56. doi: 10.1016/j.annepidem.2008.04.002
73. Neuner, B, Komm, A, Wellmann, J, Dietzel, M, Pauleikhoff, D, Walter, J, et al. Smoking history and the incidence of age-related macular degeneration--results from the muenster aging and retina study (MARS) cohort and systematic review and meta-analysis of observational longitudinal studies. Addict Behav. (2009) 34:938–47. doi: 10.1016/j.addbeh.2009.05.015
74. Khan, JC, Thurlby, DA, Shahid, H, Clayton, DG, Yates, JR, Bradley, M, et al. Smoking and age related macular degeneration: the number of pack years of cigarette smoking is a major determinant of risk for both geographic atrophy and choroidal neovascularisation. Br J Ophthalmol. (2006) 90:75–80. doi: 10.1136/bjo.2005.073643
75. Evans, JR, Fletcher, AE, and Wormald, RP. 28, 000 cases of age related macular degeneration causing visual loss in people aged 75 years and above in the United Kingdom may be attributable to smoking. Br J Ophthalmol. (2005) 89:550–3. doi: 10.1136/bjo.2004.049726
76. Baird, PN, Robman, LD, Richardson, AJ, Dimitrov, PN, Tikellis, G, McCarty, CA, et al. Gene-environment interaction in progression of AMD: the CFH gene, smoking and exposure to chronic infection. Hum Mol Genet. (2008) 17:1299–305. doi: 10.1093/hmg/ddn018
77. Schmidt, S, Hauser, MA, Scott, WK, Postel, EA, Agarwal, A, Gallins, P, et al. Cigarette smoking strongly modifies the association of LOC387715 and age-related macular degeneration. Am J Hum Genet. (2006) 78:852–64. doi: 10.1086/503822
78. Cai, B, Zhang, Z, Sun, S, Lin, T, Ke, Y, Li, Z, et al. A pilot application of an iTRAQ-based proteomics screen estimates the effects of cigarette Smokers’ serum on RPE cells with AMD high-risk alleles. Transl Vis Sci Technol. (2022) 11:15. doi: 10.1167/tvst.11.2.15
79. Scott, WK, Schmidt, S, Hauser, MA, Gallins, P, Schnetz-Boutaud, N, Spencer, KL, et al. Independent effects of complement factor H Y402H polymorphism and cigarette smoking on risk of age-related macular degeneration. Ophthalmology. (2007) 114:1151–6. doi: 10.1016/j.ophtha.2006.08.054
80. Velilla, S, García-Medina, JJ, García-Layana, A, Dolz-Marco, R, Pons-Vázquez, S, Pinazo-Durán, MD, et al. Smoking and age-related macular degeneration: review and update. J Ophthalmol. (2013) 2013:895147. doi: 10.1155/2013/895147
81. Zhang, QY, Tie, LJ, Wu, SS, Lv, PL, Huang, HW, Wang, WQ, et al. Overweight, obesity, and risk of age-related macular degeneration. Invest Ophthalmol Vis Sci. (2016) 57:1276–83. doi: 10.1167/iovs.15-18637
82. Borras, C, Sastre, J, Garcia-Sala, D, Lloret, A, Pallardo, FV, and Vina, J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med. (2003) 34:546–52. doi: 10.1016/s0891-5849(02)01356-4
83. Bhatia, K, Elmarakby, AA, El-Remessey, A, and Sullivan, JC. Oxidative stress contributes to sex differences in angiotensin ii-mediated hypertension in spontaneously hypertensive rats. Am J Phys Regul Integr Comp Phys. (2012) 302:R274–82. doi: 10.1152/ajpregu.00546.2011
84. Zhao, S, Dong, S, Qin, Y, Wang, Y, Zhang, B, and Liu, A. Inflammation index SIRI is associated with increased all-cause and cardiovascular mortality among patients with hypertension. Front Cardiovasc Med. (2023) 9:1066219. doi: 10.3389/fcvm.2022.1066219
85. Ou-Yang, H, Fu, HY, Luo, Y, Xu, ZY, Liu, J, Gao, R, et al. Inflammation markers and the risk of hypertension in people living with HIV. Front Immunol. (2023) 14:1133640. doi: 10.3389/fimmu.2023.1133640
86. Katsi, VK, Marketou, ME, Vrachatis, DA, Manolis, AJ, Nihoyannopoulos, P, Tousoulis, D, et al. Essential hypertension in the pathogenesis of age-related macular degeneration: a review of the current evidence. J Hypertens. (2015) 33:2382–8. doi: 10.1097/HJH.0000000000000766
Keywords: association, systemic inflammatory response index, risk, age-related macular degeneration, NHANES
Citation: Jia R, Yin Y and Shan H (2025) Systemic inflammatory response index as a novel biomarker for age-related macular degeneration: a cross-sectional study from NHANES (2005–2008). Front. Nutr. 12:1540933. doi: 10.3389/fnut.2025.1540933
Received: 06 December 2024; Accepted: 24 February 2025;
Published: 06 March 2025.
Edited by:
Pintu Choudhary, CBL Government Polytechnic, IndiaReviewed by:
M. N. Lavanya, University of Horticultural Sciences, Bagalkot, IndiaCopyright © 2025 Jia, Yin and Shan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huimin Shan, c2hhbmh1aW1pbjIwMjBAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.