- Department of Gastrocolorectal Surgery, General Surgery Center, The First Hospital of Jilin University, Changchun, China
Background: In recent years, the incidence of cancers of the digestive system has been increasing, posing a severe threat to the lives and health of people around the world, and has become one of the leading causes of cancer deaths worldwide. The three most common cancers of the digestive system include gastric, colorectal, and liver cancers, and attention has been paid to the role of diet in the progression of these cancers. However, the relationship between dietary factors and cancers of the digestive system remains to be investigated.
Methods: This study included 30,789 adults aged 20 years or older from the National Health and Nutrition Examination Survey (NHANES), conducted from 2007 to 2018. It assessed the association between 30 dietary factors and digestive system cancers. Descriptive analysis was used to explore the demographic characteristics of the participants and p-values were calculated using a weighted linear regression model. Categorical variables were described as percentages, and p-values were calculated using weighted chi-square tests.
Results: We found that protein, vitamin B1, calcium, and iron intake were positively associated with colorectal cancer; vitamin B2 and phosphorus intake were negatively related to colorectal cancer; dietary folate and vitamin B12 intake were negatively associated with gastric cancer; vitamin D and copper intake were positively associated with gastric cancer; vitamin E intake was negatively related to the development of hepatocellular carcinoma; and lycopene, vitamin B2, calcium, iron, and zinc intake was positively associated with the development of liver cancer. Other than that, we did not observe any correlation between other dietary factors and cancers of the digestive system.
Conclusion: Dietary intake is associated with digestive system cancers, and more epidemiologic studies are needed to validate our results.
1 Introduction
The incidence of digestive tumors is increasing year by year throughout the world. It is increasingly constituting a public health problem that threatens the world’s health and safety. Gastric, colorectal, and liver cancers are common digestive system tumors. Gastric cancer is the fourth leading cause of cancer deaths worldwide and the fifth most common cancer (1). Colorectal cancer, on the other hand, is the third most common malignancy and the second deadliest cancer globally. In contrast, the increase in the incidence of colorectal cancer is mainly attributed to the increased exposure to environmental risk factors due to westernization of lifestyles and diets (2–4). Although the therapeutic outlook for colorectal cancer is generally favorable, the increasing number of patients with colorectal cancer and the rising trend of younger incidence still pose a heavy economic burden and a significant public health challenge globally (3, 5, 6). A previous study of a U.S. population showed that people with high frequency and daily alcohol consumption had a greatly increased risk of colorectal cancer (7). The research of Li’s team suggests that heavy drinking, smoking and a sedentary lifestyle all increase the risk of colorectal cancer (8). A case–control study also elaborated that dairy consumption, obesity and red meat consumption increased the risk of colorectal cancer (9). Primary liver cancer, on the other hand, is the seventh most common cancer in the world and the second leading cause of cancer-related deaths globally (10). Primary liver cancer includes hepatocellular carcinoma, intrahepatic cholangiocarcinoma, and other types of liver cancer. They account for about 80, 15, and 5%, respectively (11). In 2018, 21.5% of gastrointestinal cancer cases worldwide were linked to dietary factors (12). To date, the relationship between dietary intake and cancers such as colorectal cancer, breast cancer, prostate cancer, and lung cancer has been extensively studied (13–16).
People have begun to examine the relationship between dietary intake and cancers of the digestive system in recent years. A pooled analysis of 14 prospective studies concluded that fruit and vegetable intake was not strongly associated with a reduced risk of colon cancer overall, but may be associated with a reduced risk of distal colon cancer (17). The World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) panel, in its Continuing Update Project (CUP), concluded that the evidence that foods high in dietary fiber protect against colorectal cancer and that consumption of red meat, processed meats, and alcohol (primarily in men) increases the risk of colorectal cancer is “compelling.” Milk and calcium may be associated with a lower risk of colorectal cancer, while there is limited evidence that folic acid, selenium, and vitamin D may be protective (18). Although there were significant differences in population characteristics, study design, and methodology across studies, plant-based diets (with some dairy and fish) were associated with a lower risk of colorectal cancer, whereas diets high in meat, refined grains, and added sugars appeared to increase risk (19–22). A prospective study found that alpha-carotene can effectively reduce the risk of liver cancer (23). A previous prospective study found that dietary fiber and coffee intake were negatively associated with developing hepatocellular carcinoma. In contrast, dietary glycemic index, sugar-sweetened beverages, and trans fats were positively related to the development of hepatocellular carcinoma (24). Different dietary factors have been associated with a higher risk of gastric cancer, such as low intake of fruits and vegetables, high intake of red and processed meat and preserved foods, and inadequate intake of several antioxidant minerals and vitamins (25–30).
Changes in diet structure often cause compensatory changes in other nutrients because the human body’s demand for nutrients is multifaceted; when the intake of a certain nutrient changes, it may affect the intake and utilization of other nutrients (31). Focusing on food groups or eating patterns allows for a more comprehensive assessment of the impact of diet on health. This approach takes into account the interactions between foods and overall dietary structure, rather than individual nutrients. By analyzing food groups or dietary patterns, researchers can avoid accidental associations due to nutrient covariation and more accurately assess the health effects of diet (32). Therefore, by including multiple dietary factors, this study can provide a comprehensive view of the potential relationship between diet and digestive system tumors.
The National Health and Nutrition Examination Survey (NHANES) is a research program led by the U.S. Centers for Disease Control and Prevention (CDC) to assess the health of adults and children nationwide (33). The study was designed to examine the relationship between dietary intake and cancer of the digestive system using a representative sample of Americans.
2 Methods
2.1 Study design and population
The population sample for this study was extracted from the NHANES database, a research program designed to assess the health and nutritional status of adults and children in the U.S. The survey is unique in that it combines interviews and physical examinations. It selected a representative noninstitutionalized U.S. population. Participants were interviewed at home, followed by various clinical and laboratory tests at a mobile examination center (MEC). The survey collected comprehensive data on demographics, socioeconomic status, diet and health. All the results of the survey have been weighted (34). We combined six consecutive NHANES surveys from 2007–2008, 2009–2010, 2011–2012, 2013–2014, 2015–2016, and 2017–2018 into one analytic sample.30789 adults aged 20 years or older were interviewed about dietary intake and medical conditions. NHANES was reviewed and approved by the NCHS Ethics Review Board, and all participants provided written informed consent by the Declaration of Helsinki.
2.2 Outcomes
The primary endpoint of the study was the diagnosis of cancer of the digestive tract. Cancer type was defined based on items on a medical status questionnaire, “Have you ever been told by a doctor or other health professional that you have cancer or a malignant tumor?” and “What type of cancer?” Answers indicating only stomach, colorectal, and liver cancers were categorized as outcome variables.
2.3 Dietary intakes
Trained interviewers conducted two consecutive 24-h dietary recalls to assess total nutritional intake by comprehensively referencing the NHANES. The first was conducted face-to-face during the MEC exam, and the second was collected by telephone 3–10 days later. Dietary intake was calculated from the average of the two dietary recall data (if available); otherwise, single nutritional recall data were used.
We incorporated 30 dietary factors from the dietary questionnaire in the NHANES database. These factors contained four nutrients, 11 vitamins, four carotenoids and nine minerals, including protein (g), total sugars (g), total fat (g), cholesterol (mg), vitamin A (vitamin A, RAE(Retinol Activity Equivalents)) (ug), alpha-carotene (ug), beta-carotene (ug), beta-cryptoxanthin (ug), lycopene (ug), vitamin B1 (thiamine) (mg), vitamin B2 (riboflavin) (mg), niacin (mg), vitamin B6 (mg), dietary folate (ug), vitamin B12 (ug), vitamin C (ug), vitamin D (D2 + D3) (ug), vitamin E as alpha-tocopherol (mg), vitamin K (ug), calcium (mg), Phosphorus (mg), Magnesium (mg), Iron (mg), Zinc (mg), Copper (mg), Sodium (mg), Potassium (mg) and Selenium (mcg) as well as Caffeine (mg) and Alcohol (g).
2.4 Covariates
We included age, gender, race, energy intake (expressed in kilocalories), body mass index (BMI), diabetes mellitus, hypertension, poverty-to-income ratio (PIR), education level, and smoking as covariates in this study.
2.5 Statistical analysis
Continuous variables were described using descriptive analyses, and p values were calculated using weighted linear regression models. Categorical variables were described using percentages, and p values were calculated using weighted chi-square tests. Logistic regression analyses were performed using STATA software, and p values <0.05 were considered statistically significant. In calculating the weights, we divided the two-year cycle weights by six to reflect the six survey cycles.
3 Results
3.1 Characteristics of included participants
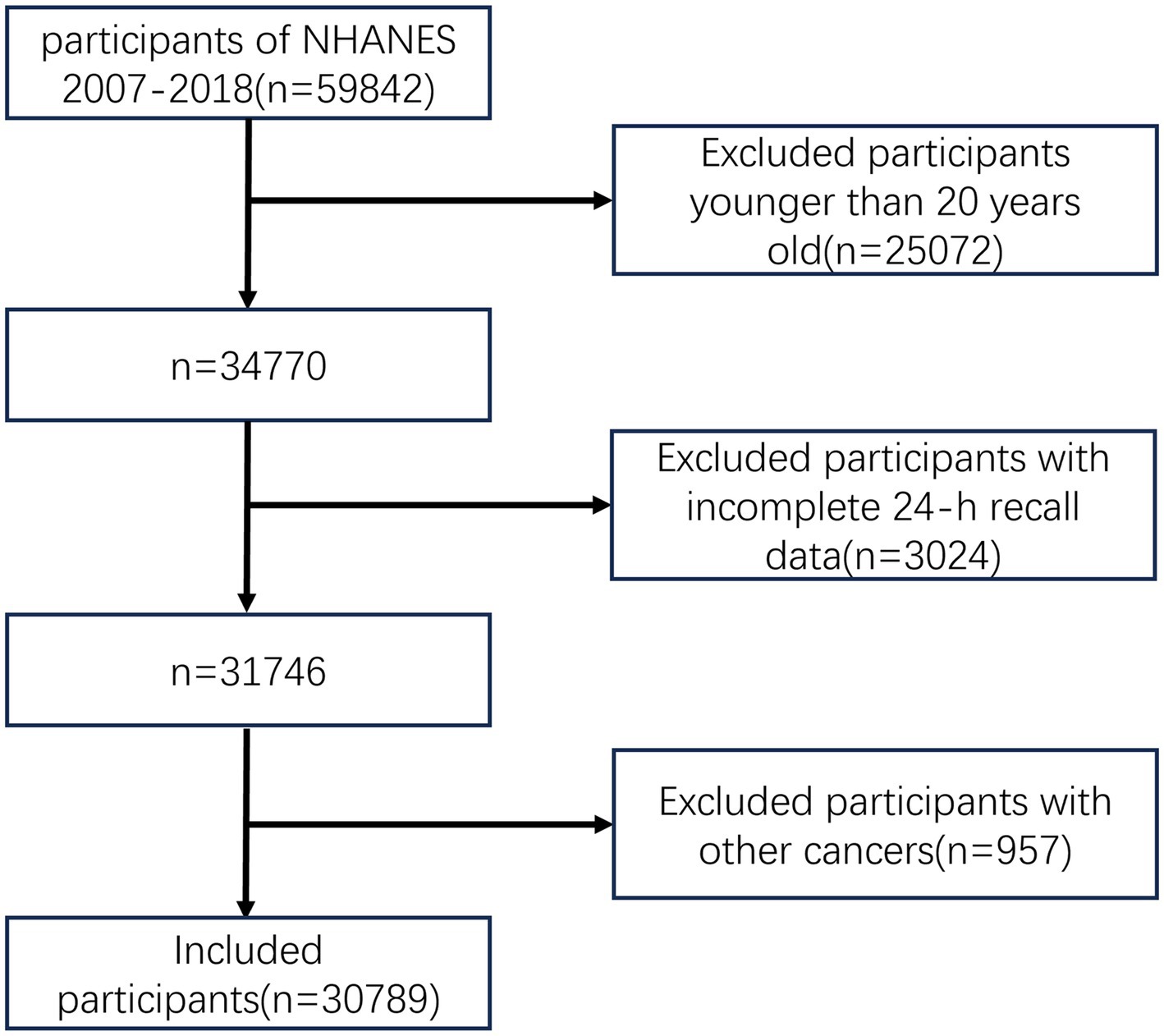
The flow chart of this study is shown in Figure 1. Compared with participants without cancer, colorectal cancer patients were older (p < 0.001), tended to be Non-Hispanic White (p < 0.001), and were predominately smokers (p = 0.007). Patients with gastric cancer were older (p < 0.001), tended to have higher levels of education (p = 0.003), and were predominantly smokers (p = 0.024). Patients with liver cancer were older (p < 0.001), had less daily energy intake (p = 0.013), and had higher BMI (p = 0.003). The baseline characteristics of participants in this study are shown in Table 1.
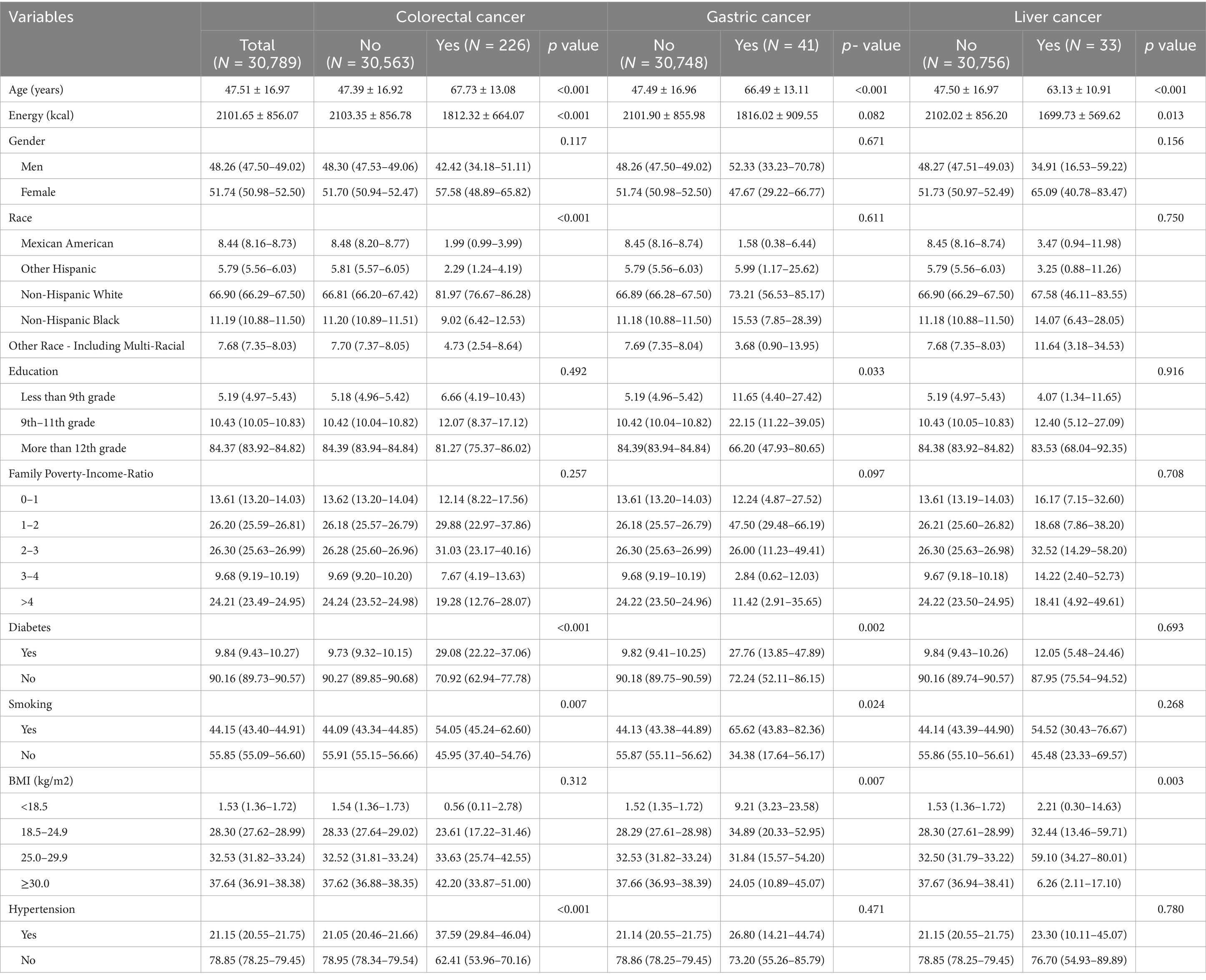
Table 1. Characteristics of participants with and without self-reported gastric cancer, colorectal cancer and liver cancer, separately.
3.2 Dietary intakes and digestive system risk
Table 2 lists the dietary intake of the study population in this study. Patients with colorectal cancer consumed less protein (p < 0.001), total sugars (p = 0.010), fat (p = 0.001), vitamin E (p = 0.002), lycopene (p = 0.008), vitamin B2 (p = 0.038), niacin (p = 0.001), vitamin B6 (p = 0.037), dietary folic acid (p < 0.001), vitamin C (p = 0.016), calcium (p = 0.018), phosphorus (p < 0.001), magnesium (p < 0.001), copper (p = 0.024), sodium (p < 0.001), potassium (p = 0.016), selenium (p < 0.001) and alcohol (p = 0.004). In addition, patients with gastric cancer consumed less protein (p = 0.009), vitamin B1 (p = 0.028), niacin (p = 0.003), vitamin B6 (p = 0.020), dietary folate (p = 0.010), vitamin C (p = 0.042), phosphorus (p = 0.030), and selenium (p = 0.021). Patients with liver cancer consumed less fat (p = 0.049), lycopene (p = 0.019) and selenium (p = 0.041). No relevant statistical differences were observed in other dietary factors in GI tumors (p > 0.05).
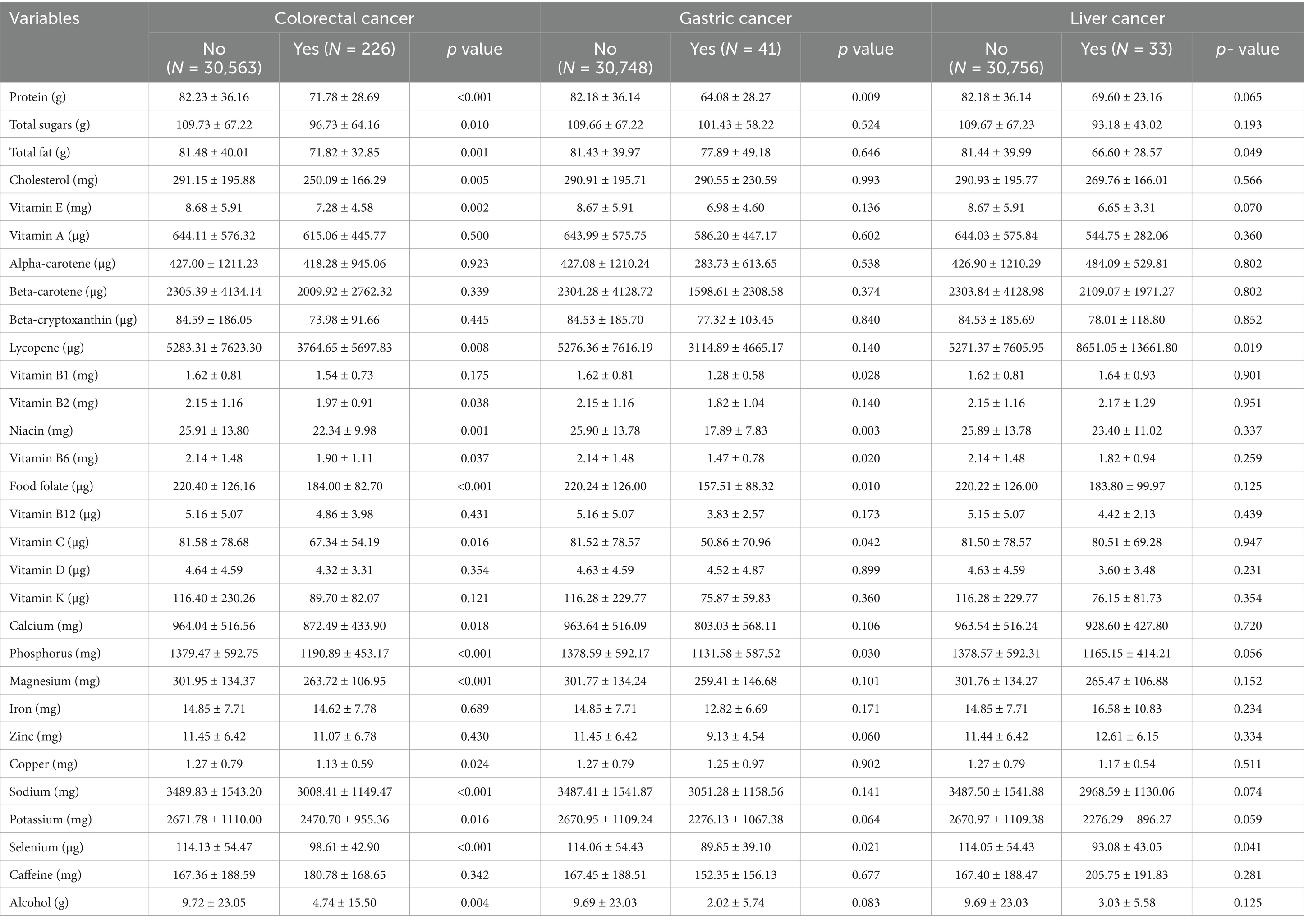
Table 2. Dietary intakes in participants with and without self-reported gastric cancer, colorectal cancer and liver cancer, separately.
After adjusting for potential confounders, the associations between dietary intake and digestive system tumor cancers are shown in Table 3. After adjusting for covariates, protein [OR, 95%CI; 1.024(1.005–1.043), p = 0.013], vitaminB1 [OR, 95%CI; 1.480 (1.042–2.102), p = 0.029], calcium [OR, 95%CI; 1.001 (1.000–1.002), p = 0.001] and iron [OR, 95% CI; 1.042 (1.008–1.078), p = 0.015] intake were positively associated with colorectal cancer; vitamin B2 [OR, 95% CI; 0.560 (0.325–0.965), p = 0.037] and phosphorus [OR, 95% CI; 0.998 (0.997–1.000), p = 0.011] intake were negatively associated with colorectal cancer. Food folate [OR, 95% CI; 0.989 (0.981–0.997), p = 0.005] and vitamin B12 [OR, 95% CI; 0.837 (0.703–0.996), p = 0.045] intake were negatively associated with gastric cancer; vitamin D [OR, 95% CI; 1.048 (1.014–1.084), p = 0.005] and copper [OR, 95%CI; 3.489 (1.245–9.776), p = 0.017] intake were positively associated with gastric cancer. In addition, vitamin E [OR, 95% CI; 0.839 (0.741–0.949), p = 0.005] intake was negatively associated with liver cancer; lycopene [OR, 95%CI; 1.000 (1.000–1.000), p < 0.001], vitamin B2 [OR, 95%CI; 1.657 (1.058–2.595), p = 0.027], calcium [OR, 95%CI; 1.002 (1.001–1.003), p < 0.001], iron [OR, 95% CI; 1.095 (1.013–1.185), p = 0.023] and zinc [OR, 95% CI; 1.090 (1.014–1.171), p = 0.019] intake were positively associated with liver cancer.
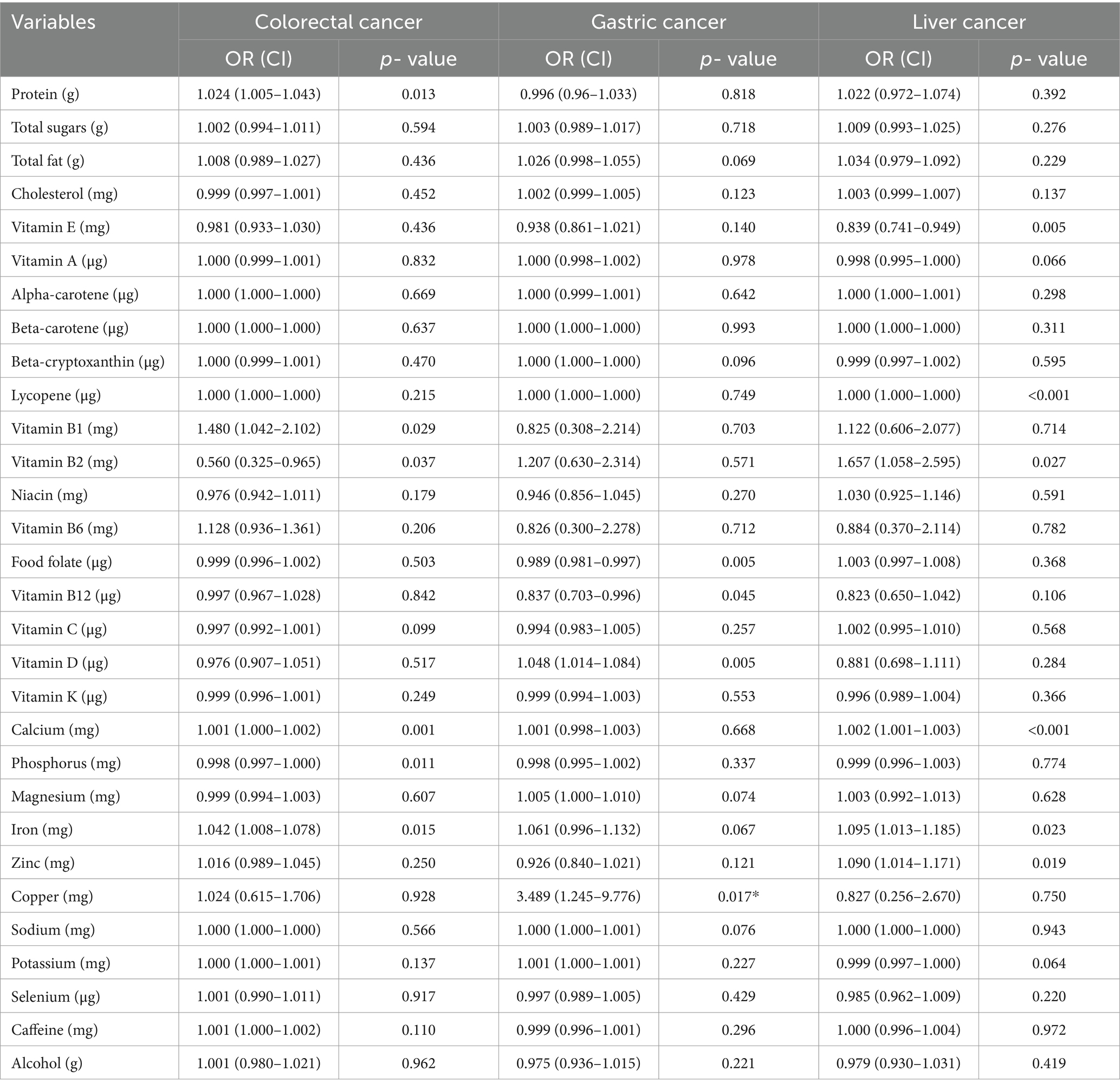
Table 3. ORs with 95% CIs of the associations between dietary intakes, gastric cancer, colorectal cancer and liver cancer, separately.
The intake of other nutrients, vitamins, and minerals were not correlated with colorectal, gastric, or liver cancer.
4 Discussion
In this cross-sectional study, we reveal the relationship between dietary intake and digestive system cancers. To our knowledge, this is the first study to comprehensively examine the relationship between nutritional factors and digestive system cancers in the U.S. population using the NHANES database.
The prospect that intake of specific vitamins may have anticancer effects has received much attention in recent years (35–37). Our study found that vitamin B12 intake was negatively associated with the risk of gastric cancer. This may be attributed to the fact that vitamin B12 deficiency may contribute to cancer pathogenesis by reducing DNA synthesis, leading to altered expression of cancer-related genes (38).
In recent years, the relationship between vitamin D and cancer has received attention from many researchers (39–42). Epidemiologic findings seem to be inconsistent regarding whether vitamin D reduces the incidence of gastric cancer. Several meta-analyses have shown no significant relationship between gastric cancer incidence and vitamin D (43). However, other studies have shown a positive correlation between vitamin D intake and the incidence of gastric cancer, and the results of this paper are similar (44). In contrast, some studies have shown that insufficient vitamin D intake reduces the incidence and mortality of gastric cancer (45). Previous studies’ conflicting conclusions may be attributed to various factors involved in the vitamin D absorption pathway in the body, such as sunlight exposure and various hormones. In this study, food folate intake was negatively associated with stomach cancer. Folic acid plays a vital role in maintaining DNA stability, and if folic acid is deficient, it can cause DNA damage. At this point, inappropriate gene expression is caused by hypomethylation of DNA, which affects DNA repair and causes chromosomal breaks, leading to the development of cancer. Polymorphisms in methylenetetrahydrofolate reductase (MTHFR), a key enzyme in folate metabolism, may also play a role in cancer development (46, 47). In this study, vitamin B2 intake was negatively associated with colorectal cancer. Studies of vitamin B2 and the risk of colorectal carcinogenesis are limited. A prospective study on the role of vitamins in reducing the risk of colorectal cancer development found no evidence that a high intake of vitamin B2 reduces the risk of colorectal cancer (48). Xu’s team found that serum vitamin B2 levels were negatively correlated with the development of colorectal cancer in a study that included 1,009 cases of colorectal cancer patients (49). Interestingly, the results of previous research support the idea that high levels of vitamin B2 may play a role in promoting the development of colorectal cancer (50). Therefore, further studies are needed to investigate whether vitamin B2 intake is effective in preventing the development of colorectal cancer. Vitamin B2 intake was positively related to the development of liver cancer. A recent prospective study found no statistical effect when stratifying the study population by sex, BMI, alcohol intake, and physical activity to examine the association between vitamin B2 intake and hepatocellular carcinoma risk (51). Vitamin B1 intake is negatively associated with the risk of colorectal cancer. A recent study showed no evidence of a correlation between vitamin B1 intake and the development of colorectal cancer (52). Liu’s team conducted a Meta-analysis to come to the same conclusion as ours: vitamin B1 intake is a protective factor in reducing the incidence of colorectal cancer (53). Animal experiments have shown that vitamin B1 deficiency may be associated with the development of colorectal cancer. They found an increased number of ACF (abnormal crypt foci) in the colon of rats fed a slightly vitamin B1-deficient diet (54). A randomized controlled trial found that plasma thiamine concentrations were generally below the reference range in patients with prior colon polyps or intramucosal cancer (55). In this study, vitamin E intake was negatively associated with the risk of hepatocellular carcinoma. Vitamin E prevents the production of N-nitroso compounds and is also a protective antioxidant against cancer. In previous studies, people taking vitamin E supplements (200 UI/day) may have a reduced risk of colon cancer (56). But we did not find that connection. More epidemiological studies are needed to confirm the relationship between vitamin E and digestive cancers.
We found a positive correlation between copper intake and gastric cancer in dietary intake. According to previous studies, copper concentrations were reported to be elevated in some types of cancers, including gynecological cancers, breast cancer, lung cancer, and gastrointestinal cancers (57–59). The relationship between copper and carcinogenesis appears well established, as cancer cells may require more copper than non-dividing cells (60). The results of this study suggest that dietary phosphorus has a protective effect against colorectal cancer. Some recently conducted studies indicate that phosphorus has a protective effect on the development of cervical intraepithelial neoplasia and colorectal adenomas, similar to our findings (61). Yumie reported that a combination of low calcium and high phosphorus may play a role in cancer development (62). However, previous studies have focused only on the dietary calcium-phosphorus ratio, not dietary phosphorus intake. Phosphorus intake only reflects daily intake but may not accurately reflect cell phosphorus levels (61). More epidemiologic studies are needed to verify the association between dietary phosphorus intake and digestive cancers. In a Mendelian randomization study examining the effect of micronutrient levels on the risk of colorectal polyps in humans, no statistically significant association was observed between 11 micronutrients, including calcium, and the risk of developing colorectal polyps (63). In another study, a low-calcium diet was found to be a diet-related risk factor for colorectal cancer (64). However, our study reached the opposite conclusion that calcium intake was positively associated with colorectal cancer and the development of liver cancer. Iron intake is positively associated with liver and colorectal cancer. Iron-induced oxidative stress leads to two possible consequences: (1) failure of redox regulation leading to lipid peroxidation and oxidative damage to DNA and proteins, and (2) activation of multiple mechanisms of reductive and hypoxic protection of redox regulation through signal transduction. Both outcomes appear to play a role in iron-induced carcinogenesis. In our study, zinc intake was positively associated with liver cancer. However, the study by Liu et al. concluded that both dietary zinc intake and serum zinc levels were negatively related to the risk of hepatocellular carcinoma, and the same conclusion was obtained even after adjusting for other risk factors for hepatocellular carcinoma, including hepatitis B virus infection, which is contrary to our findings (65).
This study also showed a positive association between protein intake and colorectal cancer. A recent study reported that a diet high in animal protein was associated with a moderate increase in the risk of colon cancer (66). The increased risk of colon cancer with a high intake of animal proteins may be due to their composition of red or processed meats, the consumption of which increases the risk of colon cancer (67). Our results showed that lycopene intake was positively associated with liver cancer. Previous studies have suggested that lycopene has a preventive effect against liver cancer. Still, Yin et al. reported that in a meta-analysis of the results of Mendelian randomization of different tumors, little or no reduction in the risk of liver cancer was observed with elevated levels of circulating lycopene (68).
It is important to emphasize that this study also has some limitations. First, it relied on a single measure of diet from a dietary recall interview. Each respondent’s dietary behavior may have changed over time, and there may also be some bias in the information recalled by some respondents. Second, some respondents may not have reported accurate information about their cancer history. Third, there may be complex additive effects and biological interactions between the various dietary categories in the usual diet (69). Although we have adjusted for known covariates, some other minor factors may influence the final conclusions we reach. Finally, in this study, the interviews were conducted after the respondents were diagnosed with cancer, so the respondents’ dietary information could have been changed after cancer treatment. Because this study was a cross-sectional study, it is challenging to elucidate the causal relationship between nutritional factors and digestive system cancers.
5 Conclusion
In conclusion, this study found a potential relationship between protein, vitamin B1, vitamin E, vitamin B12, vitamin B2, vitamin D, folate, copper, calcium, iron, phosphorus, zinc, lycopene intake and digestive system tumors. The results may provide insights into preventing tumors of the digestive system. Further epidemiological studies are needed to confirm these results.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: The database reported in this study is accessible via https://www.cdc.gov/nchs/nhanes/index.htm.
Author contributions
XQ: Conceptualization, Data curation, Supervision, Validation, Writing – original draft. LG: Conceptualization, Investigation, Software, Writing – original draft. SW: Conceptualization, Data curation, Methodology, Writing – original draft. WL: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Jilin Province Special Project for Medical and Health Talents (No. JLSWSRCZX2021-094) and the Key Projects of Jilin Province Science and Technology Development Plan (No.20220203148SF).
Conflict of interest
The authors declare that the research was conducted without commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Xi, Y, and Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. (2021) 14:101174. doi: 10.1016/j.tranon.2021.101174
3. Keum, N, and Giovannucci, E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. (2019) 16:713–32. doi: 10.1038/s41575-019-0189-8
4. Murphy, N, Moreno, V, Hughes, DJ, Vodicka, L, Vodicka, P, Aglago, EK, et al. Lifestyle and dietary environmental factors in colorectal cancer susceptibility. Mol Asp Med. (2019) 69:2–9. doi: 10.1016/j.mam.2019.06.005
5. Campos, FG. Colorectal cancer in young adults: a difficult challenge. World J Gastroenterol. (2017) 23:5041–4. doi: 10.3748/wjg.v23.i28.5041
6. The Lancet Oncology. Colorectal cancer: a disease of the young? Lancet Oncol. (2017) 18:413. doi: 10.1016/S1470-2045(17)30202-4
7. Li, X, Hur, J, Zhang, Y, Song, M, Smith-Warner, SA, Liang, L, et al. Drinking pattern and time lag of alcohol consumption with colorectal cancer risk in US men and women. J Natl Cancer Inst. (2024):djae330. doi: 10.1093/jnci/djae330
8. Li, X, Chang, Z, Wang, J, Ding, K, Pan, S, Hu, H, et al. Unhealthy lifestyle factors and the risk of colorectal cancer: a Mendelian randomization study. Sci Rep. (2024) 14:13825. doi: 10.1038/s41598-024-64813-y
9. Bener, A, Öztürk, AE, Dasdelen, MF, Barisik, CC, Dasdelen, ZB, Agan, AF, et al. Colorectal cancer and associated genetic, lifestyle, cigarette, nargileh-hookah use and alcohol consumption risk factors: a comprehensive case-control study. Oncol Rev. (2024) 18:1449709. doi: 10.3389/or.2024.1449709
10. McGlynn, KA, Petrick, JL, and El-Serag, HB. Epidemiology of hepatocellular carcinoma. Hepatology. (2021) 73:4–13. doi: 10.1002/hep.31288
11. Chan, LK, Tsui, YM, Ho, DW, and Ng, IO. Cellular heterogeneity and plasticity in liver cancer. Semin Cancer Biol. (2022) 82:134–49. doi: 10.1016/j.semcancer.2021.02.015
12. Li, Y, Jia, X, Li, C, Sun, H, Nie, S, Giovannucci, EL, et al. The global incident gastrointestinal cancers attributable to suboptimal diets from 1990 to 2018. Gastroenterology. (2024) 167:1141–51. doi: 10.1053/j.gastro.2024.07.009
13. Thanikachalam, K, and Khan, G. Colorectal Cancer and nutrition. Nutrients. (2019) 11:164. doi: 10.3390/nu11010164
14. De Cicco, P, Catani, MV, Gasperi, V, Sibilano, M, Quaglietta, M, and Savini, I. Nutrition and breast Cancer: a literature review on prevention, treatment and recurrence. Nutrients. (2019) 11:1514. doi: 10.3390/nu11071514
15. Oczkowski, M, Dziendzikowska, K, Pasternak-Winiarska, A, Włodarek, D, and Gromadzka-Ostrowska, J. Dietary factors and prostate Cancer development, progression, and reduction. Nutrients. (2021) 13:496. doi: 10.3390/nu13020496
16. Sun, Y, Li, Z, Li, J, Li, Z, and Han, J. A healthy dietary pattern reduces lung Cancer risk: a systematic review and Meta-analysis. Nutrients. (2016) 8:134. doi: 10.3390/nu8030134
17. Koushik, A, Hunter, DJ, Spiegelman, D, Beeson, WL, van den Brandt, PA, Buring, JE, et al. Fruits, vegetables, and colon cancer risk in a pooled analysis of 14 cohort studies. J Natl Cancer Inst. (2007) 99:1471–83. doi: 10.1093/jnci/djm155
18. Moskal, A, Freisling, H, Byrnes, G, Assi, N, Fahey, MT, Jenab, M, et al. Main nutrient patterns and colorectal cancer risk in the European prospective investigation into Cancer and nutrition study. Br J Cancer. (2016) 115:1430–40. doi: 10.1038/bjc.2016.334
19. Randi, G, Edefonti, V, Ferraroni, M, La Vecchia, C, and Decarli, A. Dietary patterns and the risk of colorectal cancer and adenomas. Nutr Rev. (2010) 68:389–408. doi: 10.1111/j.1753-4887.2010.00299.x
20. Miller, PE, Lesko, SM, Muscat, JE, Lazarus, P, and Hartman, TJ. Dietary patterns and colorectal adenoma and cancer risk: a review of the epidemiological evidence. Nutr Cancer. (2010) 62:413–24. doi: 10.1080/01635580903407114
21. Yusof, AS, Isa, ZM, and Shah, SA. Dietary patterns and risk of colorectal cancer: a systematic review of cohort studies (2000-2011). Asian Pac J Cancer Prev. (2012) 13:4713–7. doi: 10.7314/apjcp.2012.13.9.4713
22. Fung, TT, and Brown, LS. Dietary patterns and the risk of colorectal Cancer. Curr Nutr Rep. (2013) 2:48–55. doi: 10.1007/s13668-012-0031-1
23. Ojobor, CC, O'Brien, GM, Siervo, M, Ogbonnaya, C, and Brandt, K. Carrot intake is consistently negatively associated with cancer incidence: a systematic review and meta-analysis of prospective observational studies. Crit Rev Food Sci Nutr. (2023) 1:1–13. doi: 10.1080/10408398.2023.2287176
24. Chen, Y, Zhao, L, Jung, SY, Pichardo, MS, Lopez-Pentecost, M, Rohan, TE, et al. Diabetes risk reduction diet and risk of liver cancer and chronic liver disease mortality: a prospective cohort study. J Intern Med. (2024) 296:410–21. doi: 10.1111/joim.20007
25. Vahid, F, and Davoodi, SH. Nutritional factors involved in the etiology of gastric Cancer: a systematic review. Nutr Cancer. (2021) 73:376–90. doi: 10.1080/01635581.2020.1756353
26. Bertuccio, P, Alicandro, G, Rota, M, Pelucchi, C, Bonzi, R, Galeone, C, et al. Citrus fruit intake and gastric cancer: the stomach cancer pooling (StoP) project consortium. Int J Cancer. (2019) 144:2936–44. doi: 10.1002/ijc.32046
27. Ferro, A, Costa, AR, Morais, S, Bertuccio, P, Rota, M, Pelucchi, C, et al. Fruits and vegetables intake and gastric cancer risk: a pooled analysis within the stomach cancer pooling project. Int J Cancer. (2020) 147:3090–101. doi: 10.1002/ijc.33134
28. Naemi Kermanshahi, M, Safaei, E, Tutunchi, H, Naghshi, S, Mobarak, S, Asadi, M, et al. Fruit and vegetable intake in relation to gastric cancer risk: a comprehensive and updated systematic review and dose-response meta-analysis of cohort studies. Front Nutr. (2023) 10:973171. doi: 10.3389/fnut.2023.973171
29. Ferro, A, Rosato, V, Rota, M, Costa, AR, Morais, S, Pelucchi, C, et al. Meat intake and risk of gastric cancer in the stomach cancer pooling (StoP) project. Int J Cancer. (2020) 147:45–55. doi: 10.1002/ijc.32707
30. Morais, S, Costa, A, Albuquerque, G, Araújo, N, Pelucchi, C, Rabkin, CS, et al. Salt intake and gastric cancer: a pooled analysis within the stomach cancer pooling (StoP) project. Cancer Causes Control. (2022) 33:779–91. doi: 10.1007/s10552-022-01565-y
31. Shanahan, ER, McMaster, JJ, and Staudacher, HM. Conducting research on diet-microbiome interactions: a review of current challenges, essential methodological principles, and recommendations for best practice in study design. J Hum Nutr Diet. (2021) 34:631–44. doi: 10.1111/jhn.12868
32. Zhu, K, Li, R, Yao, P, Yu, H, Pan, A, Manson, JE, et al. Proteomic signatures of healthy dietary patterns are associated with lower risks of major chronic diseases and mortality. Nat Food. (2024) 1:59. doi: 10.1038/s43016-024-01059-x
33. Chang, Y, Yu, C, Dai, X, Sun, H, and Tang, T. Association of dietary inflammatory index and dietary oxidative balance score with gastrointestinal cancers in NHANES 2005-2018. BMC Public Health. (2024) 24:2760. doi: 10.1186/s12889-024-20268-4
34. Laszkowska, M, Rodriguez, S, Kim, J, and Hur, C. Heavy alcohol use is associated with gastric Cancer: analysis of the National Health and nutrition examination survey from 1999 to 2010. Am J Gastroenterol. (2021) 116:1083–6. doi: 10.14309/ajg.0000000000001166
35. Muñoz, A, and Grant, WB. Vitamin D and Cancer: an historical overview of the epidemiology and mechanisms. Nutrients. (2022) 14:1448. doi: 10.3390/nu14071448
36. Venturelli, S, Leischner, C, Helling, T, Burkard, M, and Marongiu, L. Vitamins as possible Cancer biomarkers: significance and limitations. Nutrients. (2021) 13:3914. doi: 10.3390/nu13113914
37. Markowska, A, Antoszczak, M, Markowska, J, and Huczyński, A. Role of vitamin C in selected malignant neoplasms in women. Nutrients. (2022) 14:882. doi: 10.3390/nu14040882
38. Choi, SW, and Mason, JB. Folate and carcinogenesis: an integrated scheme. J Nutr. (2000) 130:129–32. doi: 10.1093/jn/130.2.129
39. Boughanem, H, Canudas, S, Hernandez-Alonso, P, Becerra-Tomás, N, Babio, N, Salas-Salvadó, J, et al. Vitamin D intake and the risk of colorectal Cancer: an updated Meta-analysis and systematic review of case-control and prospective cohort studies. Cancers (Basel). (2021) 13:2814. doi: 10.3390/cancers13112814
40. Brown, RB. Vitamin D, cancer, and dysregulated phosphate metabolism. Endocrine. (2019) 65:238–43. doi: 10.1007/s12020-019-01985-y
41. Pandolfi, F, Franza, L, Mandolini, C, and Conti, P. Immune modulation by vitamin D: special emphasis on its role in prevention and treatment of Cancer. Clin Ther. (2017) 39:884–93. doi: 10.1016/j.clinthera.2017.03.012
42. Mohr, SB. A brief history of vitamin d and cancer prevention. Ann Epidemiol. (2009) 19:79–83. doi: 10.1016/j.annepidem.2008.10.003
43. Khayatzadeh, S, Feizi, A, Saneei, P, and Esmaillzadeh, A. Vitamin D intake, serum vitamin D levels, and risk of gastric cancer: a systematic review and meta-analysis. J Res Med Sci. (2015) 20:790–6. doi: 10.4103/1735-1995.168404
44. Takasu, A, Gotoda, T, Suzuki, S, Kusano, C, Goto, C, Ishikawa, H, et al. Daily diet and nutrition risk factors for gastric Cancer incidence in a Japanese population. Gut Liver. (2024) 18:602–10. doi: 10.5009/gnl230354
45. Giovannucci, E, Liu, Y, Rimm, EB, Hollis, BW, Fuchs, CS, Stampfer, MJ, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. (2006) 98:451–9. doi: 10.1093/jnci/djj101
46. Boccia, S, Hung, R, Ricciardi, G, Gianfagna, F, Ebert, MP, Fang, JY, et al. Meta- and pooled analyses of the methylenetetrahydrofolate reductase C677T and A1298C polymorphisms and gastric cancer risk: a huge-GSEC review. Am J Epidemiol. (2008) 167:505–16. doi: 10.1093/aje/kwm344
47. Druesne-Pecollo, N, Tehard, B, Mallet, Y, Gerber, M, Norat, T, Hercberg, S, et al. Alcohol and genetic polymorphisms: effect on risk of alcohol-related cancer. Lancet Oncol. (2009) 10:173–80. doi: 10.1016/S1470-2045(09)70019-1
48. Ajebli, M, Meretsky, CR, Akdad, M, Amssayef, A, and Hebi, M. The role of dietary vitamins and antioxidants in preventing colorectal Cancer: a systematic review. Cureus. (2024) 16:e64277. doi: 10.7759/cureus.64277
49. Xu, L, Wu, QX, Li, X, Fang, YJ, Zhou, RL, Che, MM, et al. Serum flavin mononucleotide but not riboflavin is inversely associated with the risk of colorectal cancer. Food Funct. (2022) 13:12246–57. doi: 10.1039/d2fo02580a
50. Ma, Y, Huangfu, Y, Deng, L, Wang, P, Shen, L, and Zhou, Y. High serum riboflavin is associated with the risk of sporadic colorectal cancer. Cancer Epidemiol. (2023) 83:102342. doi: 10.1016/j.canep.2023.102342
51. Antwi, SO, Petrick, JL, Campbell, PT, Norez, DA, Stevens, VL, Liao, LM, et al. One-carbon metabolism-related micronutrients intake and risk for hepatocellular carcinoma: a prospective cohort study. Int J Cancer. (2020) 147:2075–90. doi: 10.1002/ijc.33007
52. Gholamalizadeh, M, Behrad Nasab, M, Ahmadzadeh, M, Doaei, S, Jonoush, M, Shekari, S, et al. The association among calorie, macronutrient, and micronutrient intake with colorectal cancer: a case-control study. Food Sci Nutr. (2022) 10:1527–36. doi: 10.1002/fsn3.2775
53. Liu, Y, Xiong, WJ, Wang, L, Rang, WQ, and Yu, C. Vitamin B1 intake and the risk of colorectal Cancer: a systematic review of observational studies. J Nutr Sci Vitaminol (Tokyo). (2021) 67:391–6. doi: 10.3177/jnsv.67.391
54. Bruce, WR, Furrer, R, Shangari, N, O'Brien, PJ, Medline, A, and Wang, Y. Marginal dietary thiamin deficiency induces the formation of colonic aberrant crypt foci (ACF) in rats. Cancer Lett. (2003) 202:125–9. doi: 10.1016/j.canlet.2003.08.005
55. Bruce, WR, Cirocco, M, Giacca, A, Kim, YI, Marcon, N, and Minkin, S. A pilot randomised controlled trial to reduce colorectal cancer risk markers associated with B-vitamin deficiency, insulin resistance and colonic inflammation. Br J Cancer. (2005) 93:639–46. doi: 10.1038/sj.bjc.6602770
56. Albanes, D, Malila, N, Taylor, PR, Huttunen, JK, Virtamo, J, Edwards, BK, et al. Effects of supplemental alpha-tocopherol and beta-carotene on colorectal cancer: results from a controlled trial (Finland). Cancer Causes Control. (2000) 11:197–205. doi: 10.1023/a:1008936214087
57. Adeoti, ML, Oguntola, AS, Akanni, EO, Agodirin, OS, and Oyeyemi, GM. Trace elements; copper, zinc and selenium, in breast cancer afflicted female patients in LAUTECH Osogbo, Nigeria. Indian J Cancer. (2015) 52:106–9. doi: 10.4103/0019-509X.175573
58. Jin, Y, Zhang, C, Xu, H, Xue, S, Wang, Y, Hou, Y, et al. Combined effects of serum trace metals and polymorphisms of CYP1A1 or GSTM1 on non-small cell lung cancer: a hospital based case-control study in China. Cancer Epidemiol. (2011) 35:182–7. doi: 10.1016/j.canep.2010.06.004
59. Sohrabi, M, Gholami, A, Azar, MH, Yaghoobi, M, Shahi, MM, Shirmardi, S, et al. Trace element and heavy metal levels in colorectal Cancer: comparison between cancerous and non-cancerous tissues. Biol Trace Elem Res. (2018) 183:1–8. doi: 10.1007/s12011-017-1099-7
60. Lopez, J, Ramchandani, D, and Vahdat, L. Copper depletion as a therapeutic strategy in Cancer. Met ions. Life Sci. (2019) 19:18. doi: 10.1515/9783110527872-018
61. Kesse, E, Boutron-Ruault, MC, Norat, T, Riboli, E, and Clavel-Chapelon, F. Dietary calcium, phosphorus, vitamin D, dairy products and the risk of colorectal adenoma and cancer among French women of the E3N-EPIC prospective study. Int J Cancer. (2005) 117:137–44. doi: 10.1002/ijc.21148
62. Takata, Y, Shu, XO, Yang, G, Li, H, Dai, Q, Gao, J, et al. Calcium intake and lung cancer risk among female nonsmokers: a report from the Shanghai Women's health study. Cancer Epidemiol Biomarkers Prev. (2013) 22:50–7. doi: 10.1158/1055-9965.EPI-12-0915-T
63. Lv, S, Ding, Y, Huang, J, He, Y, Xie, R, Shi, X, et al. Genetic prediction of micronutrient levels and the risk of colorectal polyps: a mendelian randomization study. Clin Nutr. (2024) 43:1405–13. doi: 10.1016/j.clnu.2024.04.019
64. Su, J, Liang, Y, and He, X. The global burden and trends analysis of early-onset colorectal cancer attributable to dietary risk factors in 204 countries and territories, 1990-2019: a secondary analysis for the global burden of disease study 2019. Front Nutr. (2024) 11:1384352. doi: 10.3389/fnut.2024.1384352
65. Liu, X, Zhang, Y, Yishake, D, Luo, Y, Liu, Z, Chen, Y, et al. Dietary intake and serum levels of copper and zinc and risk of hepatocellular carcinoma: a matched case-control study. Chin Med J. (2024) 137:596–603. doi: 10.1097/CM9.0000000000002761
66. Yu, YC, Paragomi, P, Jin, A, Wang, R, Schoen, RE, Koh, WP, et al. Low-carbohydrate diet score and the risk of colorectal Cancer: findings from the Singapore Chinese health study. Cancer Epidemiol Biomarkers Prev. (2023) 32:802–8. doi: 10.1158/1055-9965.EPI-22-0683
67. Chao, A, Thun, MJ, Connell, CJ, McCullough, ML, Jacobs, EJ, Flanders, WD, et al. Meat consumption and risk of colorectal cancer. JAMA. (2005) 293:172–82. doi: 10.1001/jama.293.2.172
68. Yin, L, Yan, H, Chen, K, Ji, Z, Zhang, X, Ji, G, et al. Diet-derived circulating antioxidants and risk of digestive system tumors: a Mendelian randomization study. Nutrients. (2022) 14:3274. doi: 10.3390/nu14163274
Keywords: dietary factors, NHANES, gastric cancer, liver cancer, colorectal cancer, nutrients
Citation: Qin X, Ge L, Wu S and Li W (2025) Association of dietary intake with cancer of the digestive system: a cross-sectional study. Front. Nutr. 12:1539401. doi: 10.3389/fnut.2025.1539401
Edited by:
Mireille Serhan, University of Balamand, LebanonReviewed by:
Maud Rizk, University of Balamand, LebanonPatricia Dahdah, University of Sassari, Italy
Copyright © 2025 Qin, Ge, Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Li, d2VpbGk4MzA4QGpsdS5lZHUuY24=
 Xinxin Qin
Xinxin Qin Litao Ge
Litao Ge Wei Li
Wei Li