- 1Department of Hepatobiliary Surgery, Guangxi Medical University Cancer Hospital, Nanning, China
- 2Department of Gastroenterology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 3Guangxi Medical University Cancer Hospital, Nanning, China
- 4Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, Ministry of Education, Nanning, China
- 5Guangxi Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, Nanning, China
Backgrounds: Adherence to the Planetary Health Diet Index (PHDI) has been shown to benefit both individual health and the planet. However, its impact on Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) remains unclear. This study aimed to investigate the relationship between PHDI adherence and the MASLD risk.
Methods: We analyzed a cohort of 15,865 adults (aged ≥18 years) using data from the National Health and Nutrition Examination Survey (NHANES, 2005–2018). The PHDI was derived from 24-h dietary assessments and comprised the scores of 15 food groups. Multivariate logistic regression was used to investigate the association between PHDI and MASLD, while restricted cubic spline (RCS) regression and threshold analysis were employed to explore potential non-linear relationship. Subgroup analyses were conducted to assess the influence of various demographic and clinical characteristics on the observed associations. Mediation analysis was performed to evaluate the indirect effect of PHDI on MASLD, and weighted quantile sum (WQS) regression was used to assess the influence of individual PHDI nutrients on MASLD.
Results: Among the cohort, 6,125 individuals were diagnosed with MASLD. Multivariate logistic regression revealed that a higher quintile of PHDI was significantly associated with reduced MASLD risk in the fully adjusted model (OR = 0.610, 95%CI 0.508–0.733, p < 0.001). Notably, nonlinear relationships between PHDI and MASLD risk were observed through RCS analysis (p = 0.002). Subgroup analyses indicated that PHDI was particularly effective in reducing MASLD risk among females, those with higher education attainment, and those living with a partner. WQS regression identified saturated fatty acids as the most significant factor contributing to MASLD risk (weight = 0.313). Additionally, BMI and waist circumference (81.47 and 87.66%, respectively) partially mediated the association between PHDI and MASLD risk, suggesting that the effect of PHDI on MASLD operates, in part, through its impact on BMI and waist circumference. The association between PHDI and MASLD remained robust across multiple sensitivity analyses.
Conclusion: Our findings indicate that adherence to PHDI is linked to a lower risk of MASLD, providing crucial insights for strategies aimed at mitigating the MASLD epidemic while simultaneously fostering environmental sustainability.
1 Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD), previously known as nonalcoholic fatty liver disease (NAFLD), affects approximately one-third of the global adult population (1). MASLD can progress to metabolic dysfunction-associated steatohepatitis (MASH), advanced fibrosis, cirrhosis, and even hepatocellular carcinoma (HCC), imposing a significant social burden (2). In addition, MASLD is associated with an increased risk of various extrahepatic conditions, including cardiovascular disease, diabetes, and chronic kidney disease (3). The pathophysiology of MASLD is complex, with insulin resistance playing a central role that leads to increased hepatic uptake and accumulation of fatty acids (4). Genetic predispositions and environmental factors often intensify this process (5), driving the progression from simple steatosis to more advanced liver diseases. Therefore, targeted interventions for MASLD have become a pressing necessity.
The global food system today is a primary driver of climate change, generating approximately 26% of all greenhouse gas emissions from human activities, consuming about 70% of the world’s freshwater, and using nearly 40% of available land (6, 7). In the meanwhile, dietary factors rank among the top three risk factors for global diseases in both men and women (8). The interconnected challenges of climate change and diet-related disease burdens underscore the pressing need for a more sustainable food system that provides a nutritious diet meeting essential nutrient requirements. To address these problems, the EAT-Lancet Commission recommended a sustainably produced, calorie-optimized planetary health diet in 2019 (7). This diet emphasizes primarily plant-based foods, limited animal-based products, unsaturated fats in place of saturated fats, and restricts refined grains, processed foods, and added sugars. Adoption of this dietary pattern is believed to help prevent premature deaths and reduce the incidence of diseases, including cardiovascular disease and type 2 diabetes (9). A worldwide transition to this diet could mitigate the dietary impact on global greenhouse gas emissions, land use, and freshwater consumption (10). Based on this planetary health diet, the Planetary Health Diet Index (PHDI) was proposed by Marchioni et al. in 2021 (11). The PDHI consists of the scores of two types of food components: adequacy components (foods to be consumed more) and moderation components (foods to be consumed less). It has been associated with improved overall dietary quality and a lower carbon footprint Adherence to the PHDI was also linked to reduced risk of various diseases, such as metabolic syndrome (12), cardiovascular disease (13), and asthma (14). However, the role of this sustainable dietary index in managing MASLD remains unclear.
Although several dietary patterns, such as the Mediterranean Diet (MD) (15, 16), Plant-based diets (PBDs) (17), and Healthy Eating Index (HEI) (18) have been reported to correlate with MASLD risk, these indices do not simultaneously consider the impact of diet on both human health and the environment. This oversight may result in missed opportunities for addressing the broader implications of dietary choices on global sustainability and public health. In contrast, the PHDI provides a comprehensive, scientifically grounded framework that addresses the challenges of modern dietary patterns while considering both environmental and health concerns. Therefore, exploring the role of the PHDI in the prevention and management of MASLD is of considerable importance.
This study aims to investigate the association between the PHDI and MASLD. By analyzing a large-scale population-based cohort, we seek to clarify the potential role of the PHDI in the prevention and management of MASLD, thereby providing a scientific foundation for clinical practice. Additionally, our findings will contribute to the promotion of sustainable dietary patterns, aligning individual health with environmental sustainability.
2 Methods
2.1 Survey description
NHANES is conducted biennially to obtain a representative sample of the U.S. civilian population not residing in institutions. Utilizing a multistage probability sampling method, NHANES tracks health and nutritional trends over time. The study received ethical approval from the National Center for Health Statistics (NCHS) Ethics Review Board, and all participants provided informed consent. All procedures complied with applicable guidelines and regulations.
2.2 Study population
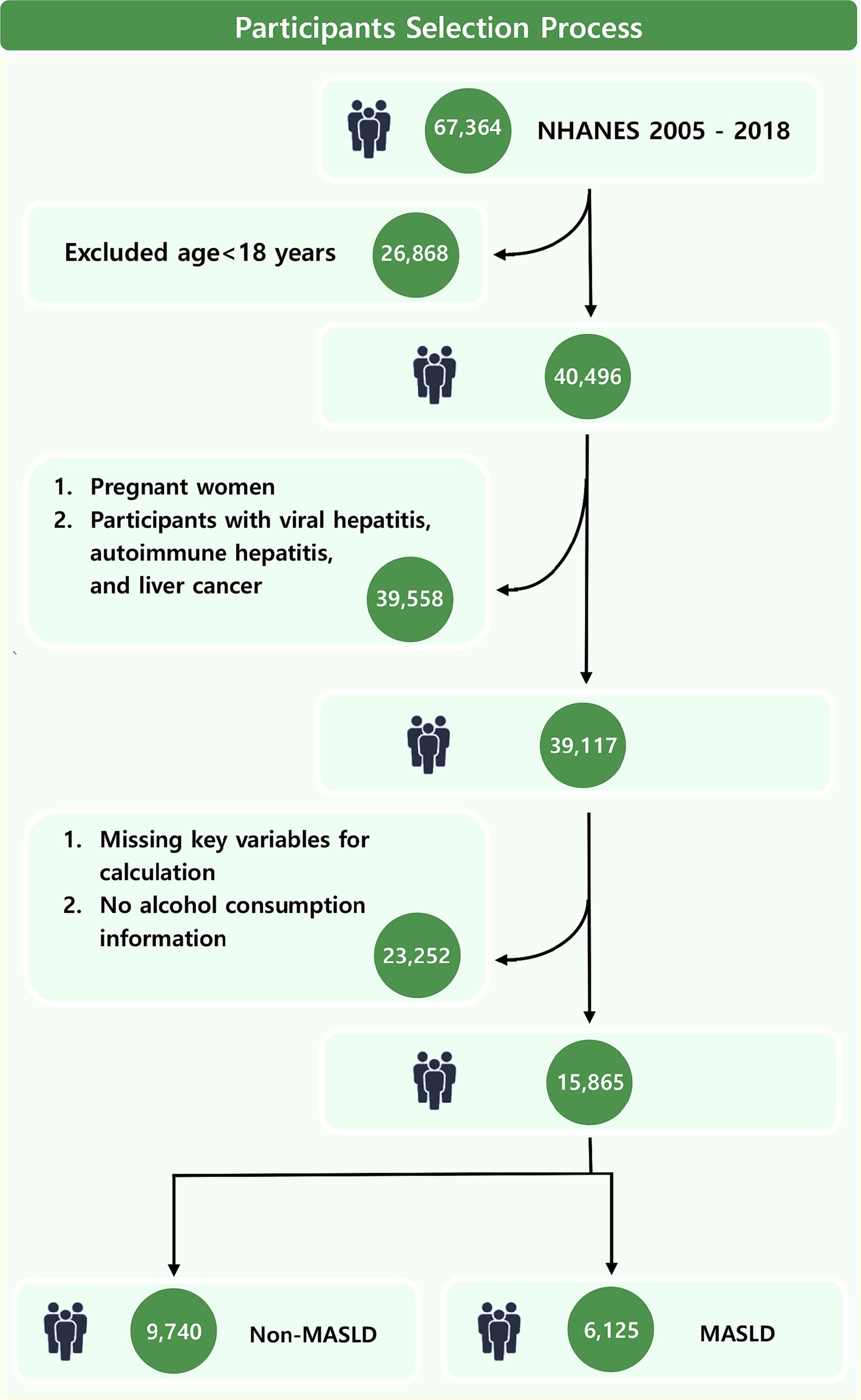
This study included a cohort of 67,364 NHANES participants from 2005 to 2018. Rigorous exclusion criteria were implemented to enhance result accuracy. Initially, individuals under 18 years old were excluded. Subsequently, participants who were pregnant or accompanied with viral hepatitis, autoimmune hepatitis, and liver cancer were removed. Finally, participants with missing information on alcohol or key variables for calculating PHDI and fatty liver index (FLI) were excluded.
2.3 Dietary data
Trained interviewers employed the United States Department of Agriculture (USDA) Automated Multiple Pass Method to collect 24-h dietary recall data (19). Participants were instructed to recount all foods and beverages consumed the previous day, aided by measuring tools to estimate portion sizes. The second dietary recording was conducted by phone, 3–10 days after the initial face-to-face session. Dietary recall data were integrated into the Food Patterns Equivalent Database (FPED), which categorizes foods into the 37 USDA Food Pattern Components using a food composition table (20). Total energy intake was calculated as the average of 2 days of reported dietary intake.
2.4 Assessment of MASLD
Steatotic liver disease (SLD) is characterized by the use of FLI, which demonstrates both high sensitivity and specificity (21). The formula is as follows (22):
TG, triglyceride; BMI, body mass index; GGT, gamma-glutamyl transferase; WC, waist circumference.
Participants with an FLI of 60 or higher were categorized as having a high likelihood of SLD (21, 23). Although this diagnostic accuracy is slightly lower than that of biopsy and imaging, it is more suitable for large-scale population screening due to its non-invasive nature and lack of associated trauma. Alcohol intake was evaluated through a 24-h dietary recall (24). Individuals who consumed fewer than 20 grams of alcohol daily (females) or under 30 grams per day (males) were categorized as light drinkers. MASLD was defined as the presence of SLD alongside light alcohol consumption, accompanied by at least one of the cardiometabolic risk factors as previously described (25).
2.5 Calculation of PHDI
Compared to the MD, PBDs, and the HEI, the PHDI primarily focuses on promoting both human health and planetary sustainability, with enhanced global applicability and scoring flexibility. The calculation of PHDI was performed through dietaryindex package (26). Thirty-five FPED components are originally reported in volumetric measures (cup-equivalents, ounce-equivalents, and teaspoon-equivalents), and were subsequently converted into grams and computed the average intake over 2 days for each FPED component. Then we chose total energy intake from individual food intake and 15 components (whole grains, starchy vegetables, nonstarchy vegetables, whole fruits (excludes fruit juice), dairy products, red and processed meats, poultry, eggs, fish, nuts and seeds, nonsoy legumes, soy products, unsaturated fatty acids, saturated fatty acids, and added sugar) from the converted FPED components to calculate PHDI in dietaryindex package. Each food group received a score ranging from 0 (minimum) to 10 (maximum) except nonsoy legumes and soy product, which were scored on a 0 to 5 scale, based on daily consumption levels that reflect the greatest health benefits of each food group (13, 27). Scores were assigned proportionally for intakes between the minimum and maximum levels, a method consistent with other dietary indices (28). The possible range of the total score of PHDI is 0 to 140 points.
2.6 Covariates
Covariates were identified through a comprehensive review of the literature and clinical expertise. These variables included sociodemographic characteristics such as age, sex, race, education level, family poverty income ratio (PIR), marital status, and smoking history. Clinical indicators, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total cholesterol, were also considered. Furthermore, diabetes, hypertension, and cardiovascular outcomes were evaluated. The classifications of race, PIR, education level, and BMI, as well as the definitions of smoking status, diabetes, hypertension, and cardiovascular outcomes, are detailed in Supplementary Data Sheet 1.
2.7 Statistical analysis
Continuous variables are reported as mean (standard deviation), while categorical variables are presented as frequency (percentage). PHDI values were stratified into quintiles (Q1-Q5) for further analysis, with the lowest quintile (Q1) serving as the reference. Weighted multivariable logistic regression models were carried out to compute odds ratio (OR) and their corresponding 95% confidence intervals (CI) for measuring the association between the PHDI and MASLD. Three logistic regression models were constructed: Model 1 (unadjusted), Model 2 (adjusted for age, gender, and race), and Model 3 (adjusted for age, gender, race, education, PIR, marital status, smoking, ALT, AST, diabetes, hypertension, cardiovascular outcomes, and total cholesterol). Missing data were addressed using multiple imputation techniques (29). Restricted cubic spline (RCS) analysis was employed to investigate potential nonlinear associations between PHDI and MASLD. A threshold analysis was conducted to identify possible inflection points. Interaction and subgroup analyses assessed whether the relationships were influenced by factors such as age, gender, race, education, marital status, BMI, hypertension, diabetes, and cardiovascular outcomes. A mediation analysis was performed to assess the indirect impact of PHDI on MASLD, with BMI and waist circumference serving as mediators. Weighted quantile sum (WQS) regression was utilized to investigate the effect of individual nutrients within the PHDI on MASLD.
To verify the robustness of our findings, several sensitivity analyses were conducted. First, we examined the association between the PHDI and MASLD for the period from 2011 to 2018 to evaluate its consistency across different timeframes. Next, we tested the PHDI-MASLD association using unweighted data to assess the impact of sample weighting on our results, ensuring that our findings are not biased by weighted estimates. Third, we repeated the analysis with unimputed data, excluding observations with missing variables to explore the association within different population subsets. Finally, we utilized the first day diet data to validate the PHDI-MASLD association, ensuring that the observed relationship is not affected by the averaging method of the two-day data.
All data analyses were performed using R software (version 4.4.0)1. Statistical significance was defined by a p-value of less than 0.05.
3 Results
3.1 Baseline characteristics
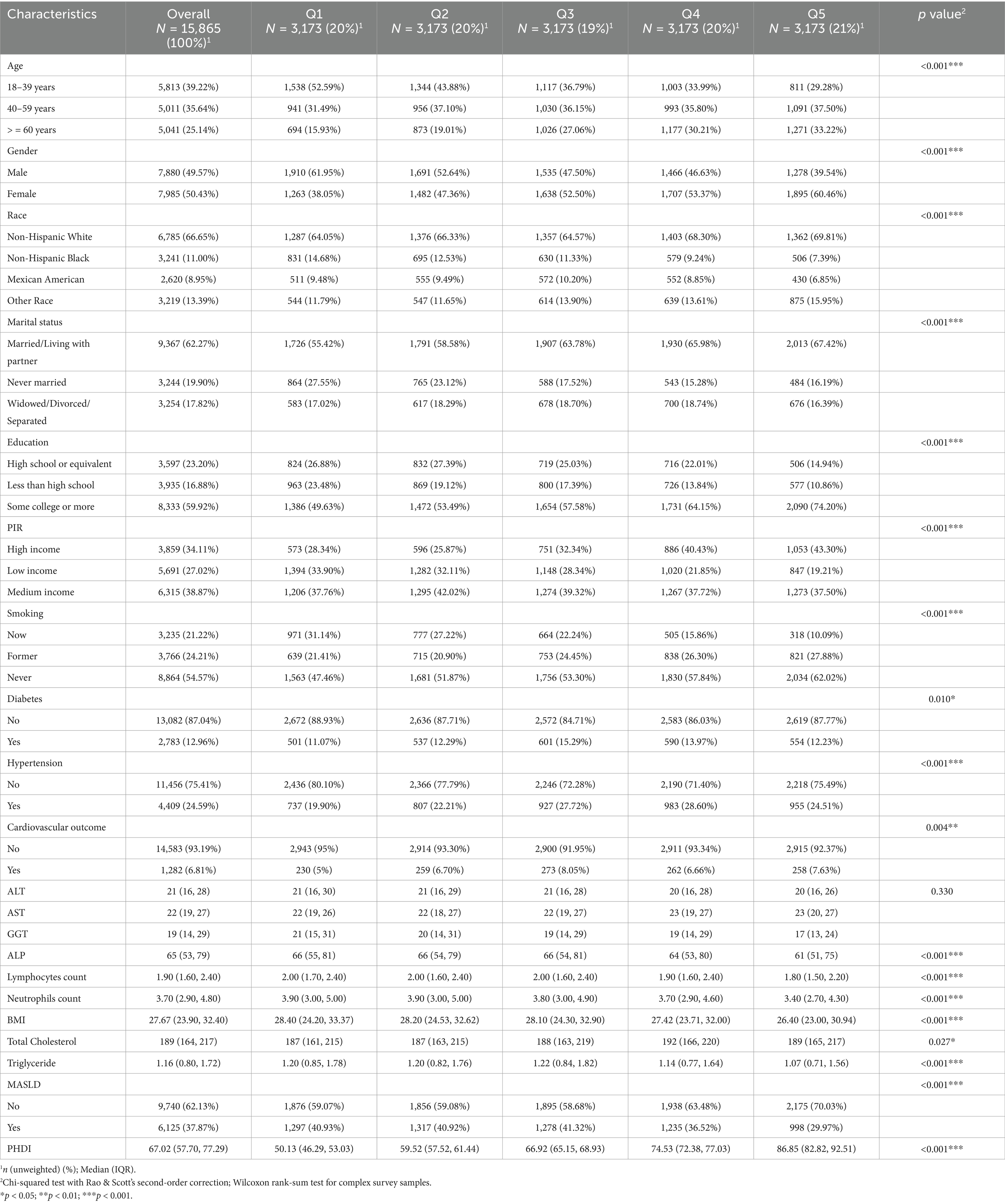
This study included 15,865 adults (Figure 1) with a median PHDI score of 66.75. Participants in the highest quintile (Q5) of PHDI were predominantly aged 40–59 years, female, married or living with partner, with higher educational attainment and income levels, and a lower prevalence of smoking (all p < 0.001) (Table 1). Additionally, individuals in the highest quintile demonstrated significantly lower levels of BMI, lymphocytes count, neutrophils count, and triglycerides (all p < 0.001). A total of 6,125 participants (38.61%) were diagnosed with MASLD. In contrast, MASLD patients tended to be male, possess lower educational qualifications, and exhibit higher values of BMI, lymphocytes count, neutrophils count, and triglycerides (all p < 0.001) (Supplementary Table S1). Notably, the median PHDI value in MASLD patients was significantly lower than in non-MASLD participants (65.61 vs. 68.09, p < 0.001). Furthermore, the prevalence of MASLD was significantly lower among participants in the highest PHDI quintile compared to those in the lowest quintile (Q1) (29.97% vs. 40.93, p < 0.001), emphasizing the need for further investigation into the protective effects of PHDI.
3.2 Association between PHDI and MASLD
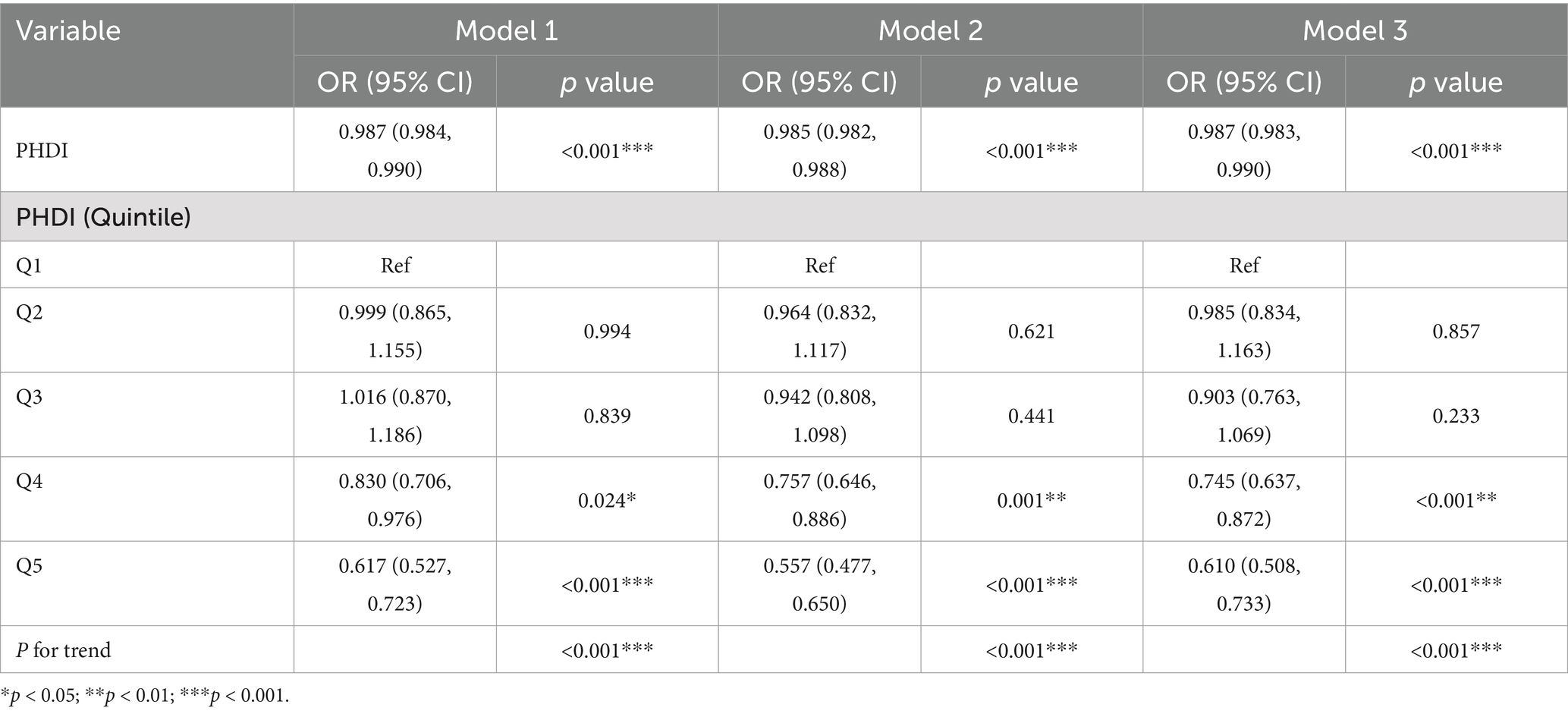
After adjusting for vital variables, including age, gender, race, education, PIR, marital status, smoking, ALT, AST, diabetes, hypertension, cardiovascular outcomes, and total cholesterol in Model 3, a significant inverse association was observed between PHDI and MASLD risk (OR = 0.987, 95% CI 0.983–0.990, p < 0.001) (Table 2). Accordingly, the highest quintile of PHDI was associated with a 39% reduction in MASLD risk compared to the lowest quintile (ORQ5 vs. Q1 = 0.610, 95% CI 0.508–0.733, p < 0.001). In addition, a significant decreasing tendency for MASLD was observed when PHDI quintile increase (p < 0.001). We then compared the association between PHDI and other well-known dietary indices, such as the Dietary Approaches to Stop Hypertension Index (DASHI), Alternative Healthy Eating Index (AHEI) (30), and the Alternate Mediterranean Diet (AMED) Score (18), with MASLD risk (Supplementary Table S2). After addressing missing values for the other dietary indices, PHDI showed a similarly significant negative association with MASLD risk, comparable to the other indices. However, adherence to PHDI also promotes planetary sustainability, highlighting the unique advantage of this index.
We further examined the associations between individual PHDI components and MASLD risk (Supplementary Table S3). The analysis revealed the highest quintile of scores for whole grains (ORQ5 vs. Q1 = 0.829; 95% CI, 0.692–0.993; p = 0.041), non-starchy vegetables (ORQ5 vs. Q1 = 0.669; 95% CI, 0.554–0.808; p < 0.001), whole fruits (excluding fruit juice) (ORQ5 vs. Q1 = 0.755; 95% CI, 0.652–0.875; p < 0.001), nuts and seeds (ORQ5 vs. Q1 = 0.760; 95% CI, 0.610–0.946; p = 0.014), and soy products (ORQ5 vs. Q1 = 0.710; 95% CI, 0.534–0.944; p = 0.019) were inversely correlated with MASLD risk. Interestingly, the highest quintile of the unsaturated fatty acids score was positively associated with MASLD risk (ORQ5 vs. Q1 = 1.638, 95% CI 1.356–1.978, p < 0.001). Moreover, the effect of increasing PHDI on reducing MASLD risk was greater than that of nearly every individual PHDI component, highlighting the superiority of this composite index in predicting MASLD.
3.3 RCS and threshold analysis of PHDI and MASLD
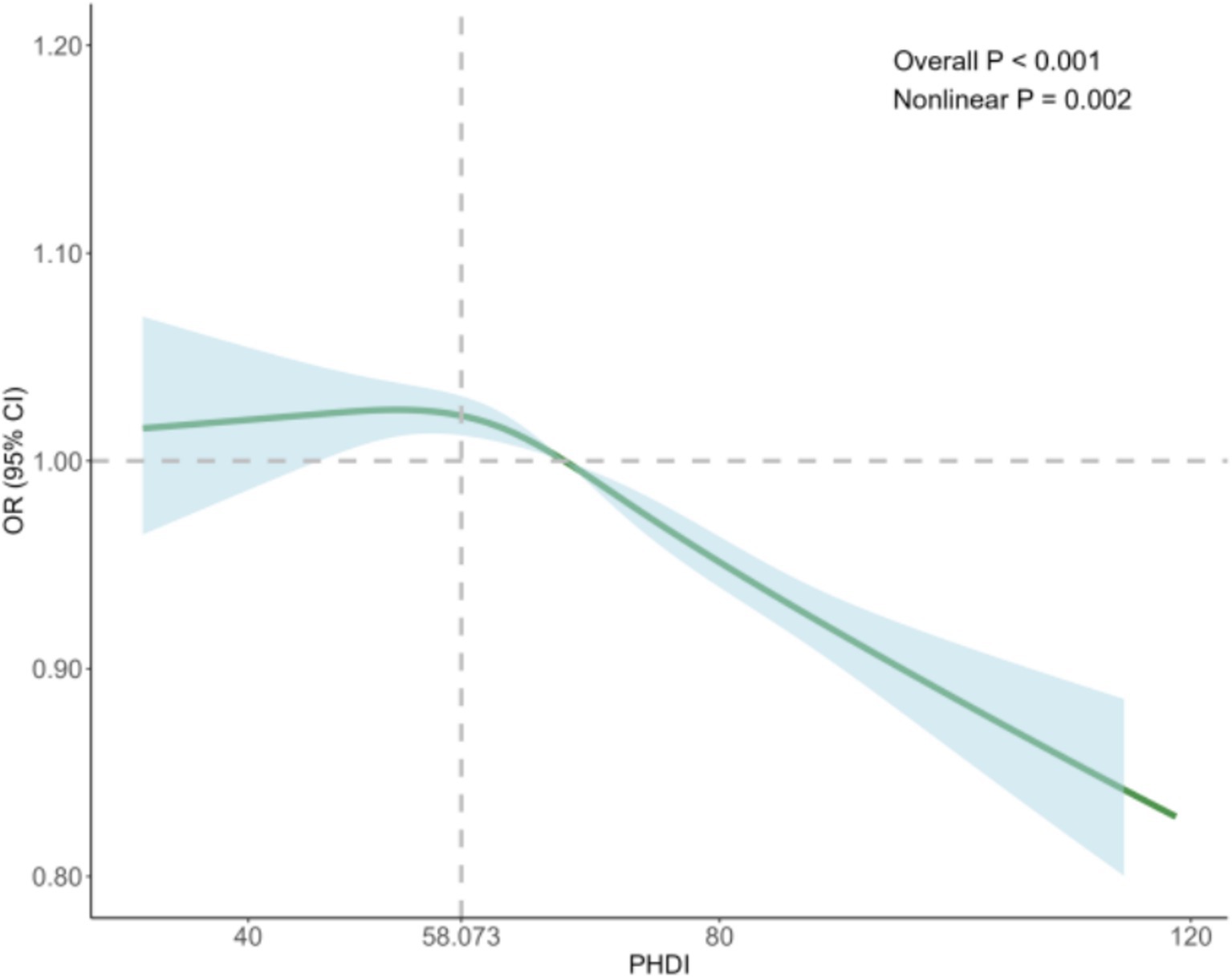
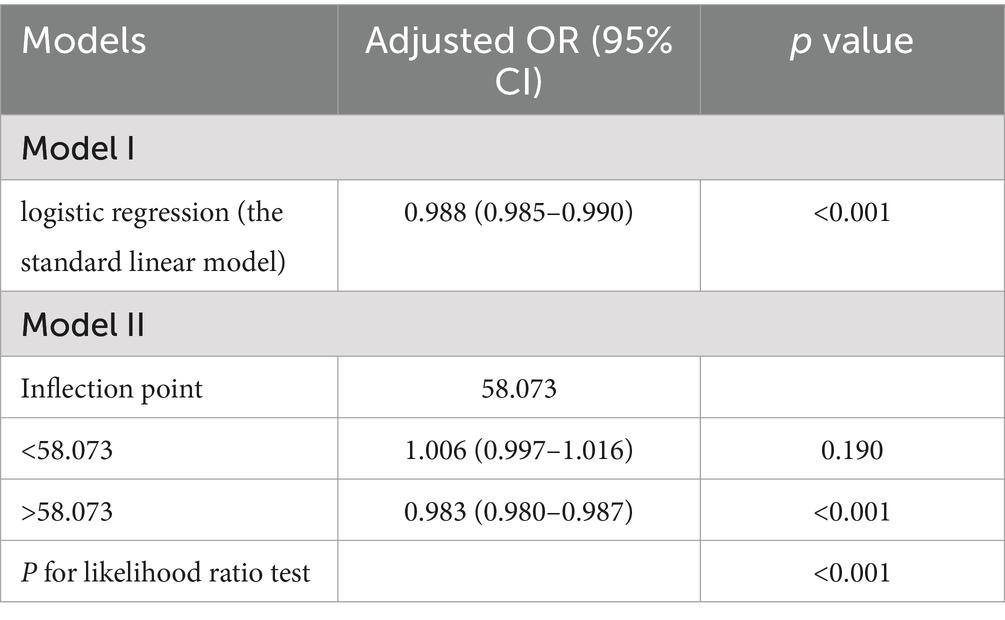
To better understand the relationship between the PHDI and the risk of MASLD, we conducted RCS analysis, revealing a non-linear association. After adjusting for key covariates in Model 3, clear non-linear trends were observed (non-linear p = 0.002) (Figure 2). Threshold analysis indicated an inflection point at 58.073. When the PHDI exceeds this point, each unit increase in PHDI correlates with a 1.7% reduction in MASLD risk (OR = 0.983, 95% CI: 0.980–0.987, p < 0.001). Conversely, when the PHDI is below 58.073, each unit increase does not significantly impact MASLD risk (OR = 1.006, 95% CI: 0.997–1.016, p = 0.190) (Table 3). These findings underline the potential clinical utility of targeting PHDI values above 58.073 to optimize MASLD prevention, highlighting the importance of dietary interventions in reducing MASLD risk.
Additionally, we examined the non-linear relationships between PHDI components and MASLD risk, finding significant associations with non-starchy vegetables (non-linear p = 0.003), whole fruits (excludes fruit juice) (non-linear p = 0.002), red and processed meats (non-linear p = 0.021), soy products (non-linear p = 0.006), unsaturated fatty acids (non-linear p < 0.001), saturated fatty acids (non-linear p < 0.001), and added sugar (non-linear p < 0.001) (Supplementary Figure S1). Threshold analyses of these components are detailed in Supplementary Table S4.
3.4 Subgroup analysis
To further investigate the association between PHDI and MASLD across diverse populations, we stratified the MASLD cohort based on age, gender, race, education level, PIR, marital status, smoking status, BMI, diabetes, hypertension, and cardiovascular outcomes. After adjusting for key variables (Model 3), stratified analysis indicated that a higher PHDI quintile was significantly associated with a reduced MASLD risk among participants identified as non-Hispanic White (p < 0.001), Mexican American (p = 0.012), or other races (p = 0.007); former (p = 0.010) or never smokers (p < 0.001); individuals with at least a college education (p < 0.001) or less than high school education (p = 0.048); those with median (p < 0.001) or high income (p = 0.002); those who were married/living with a partner (p < 0.001) or widowed/divorced/separated (p = 0.033); and participants without hypertension (p < 0.001) (Figure 3). Notably, individuals across all age groups (all p < 0.05), genders (all p < 0.001), and those with or without diabetes (all p < 0.001) or cardiovascular outcomes (all p < 0.05) benefited from increases in PHDI. Additionally, interaction effects were observed between PHDI and MASLD for gender (p < 0.001), education level (p = 0.014), and marital status (p < 0.001). These results suggest that females, individuals with higher education levels, and those married/living with a partner may be more sensitive to the MASLD risk reduction associated with higher PHDI.
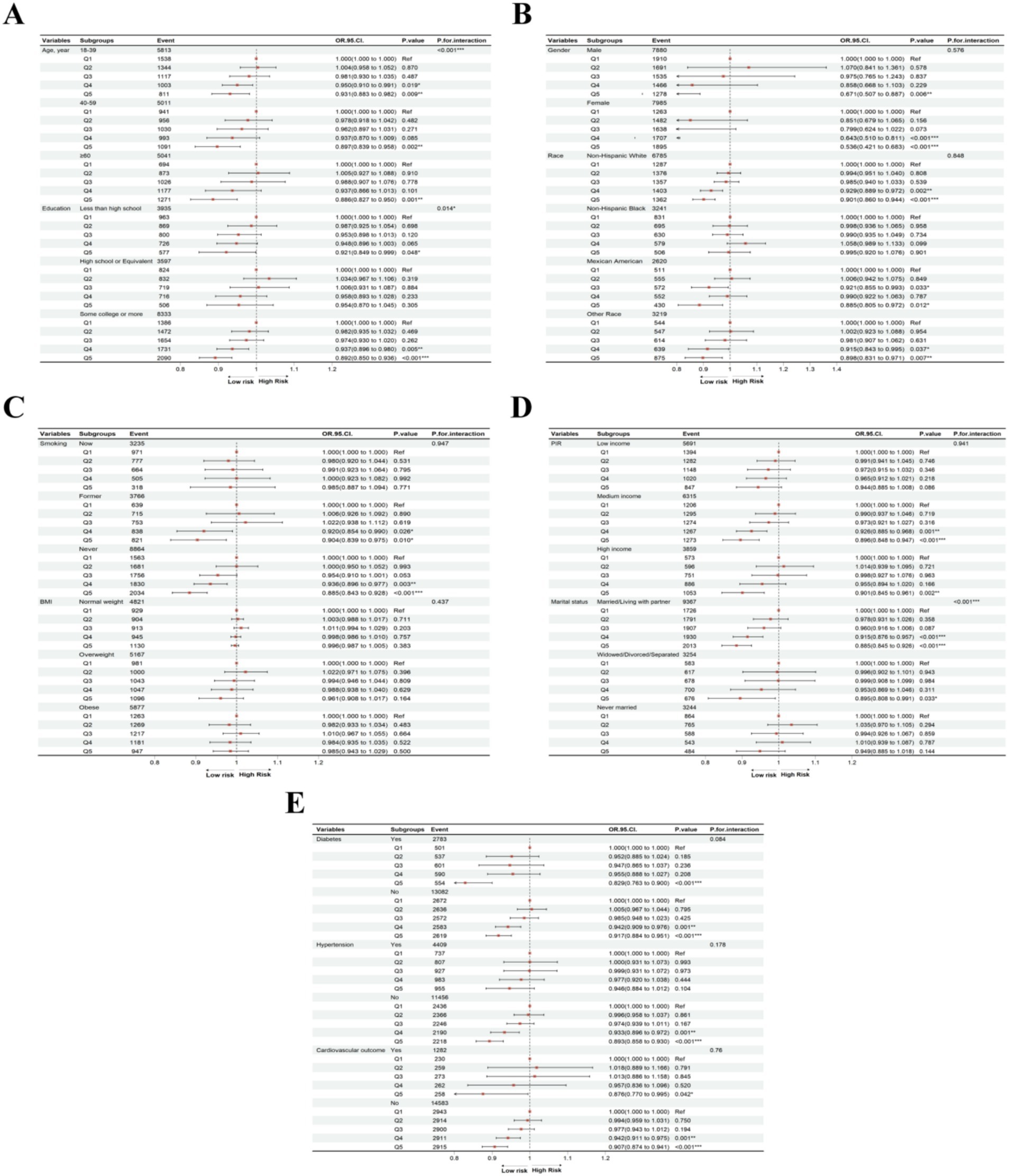
Figure 3. Subgroup analyses of association between PHDI and MASLD in (A) age and education, (B) gender and race, (C) smoking status and BMI, (D) PIR and marital status, (E) diabetes, hypertension, and cardiovascular outcome. “*”, p < 0.05; “**”, p < 0.01; “***”, p < 0.001.
3.5 WQS and mediation analysis
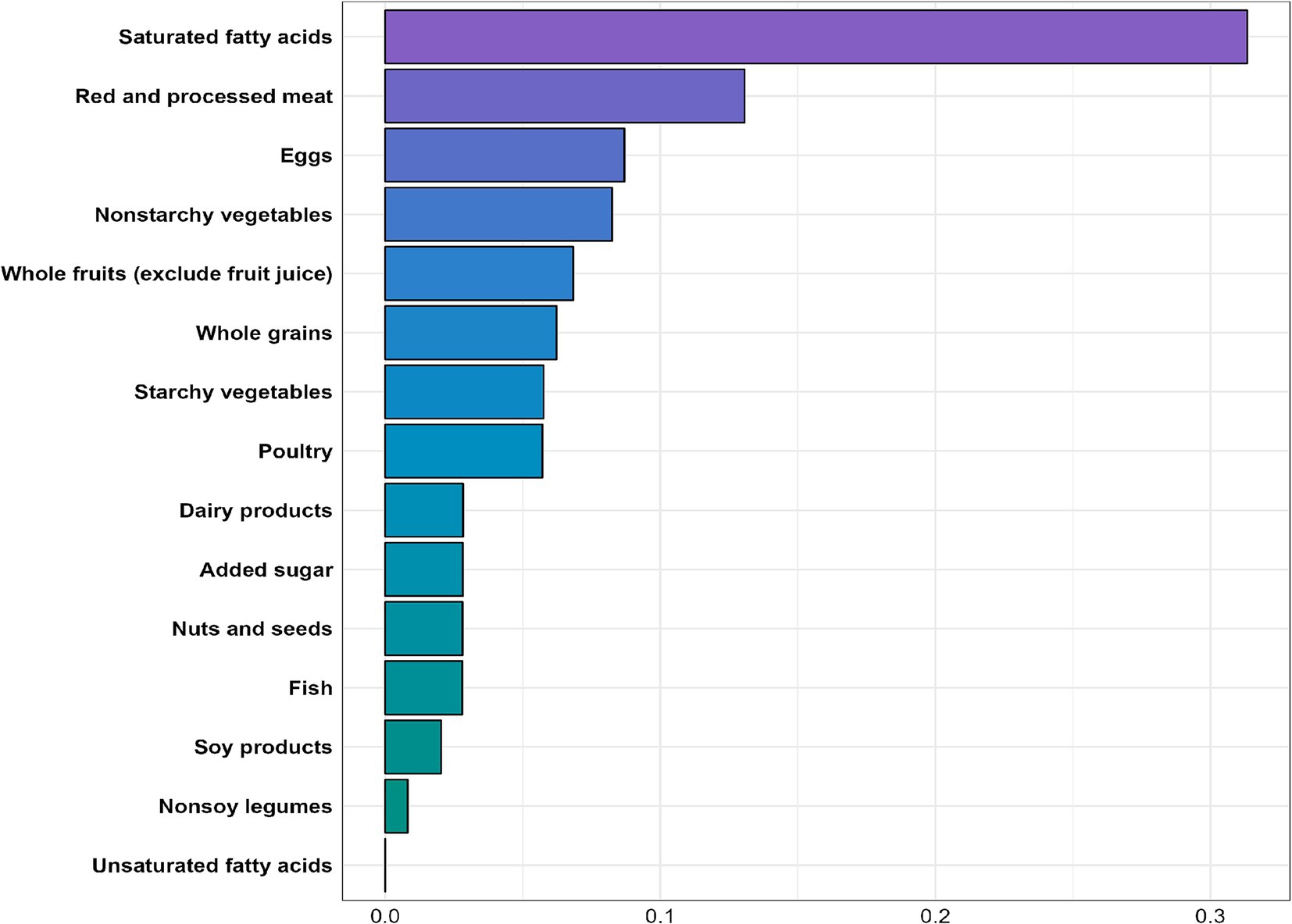
The WQS regression analysis indicated an inverse association between the WQS index and MASLD risk (OR = 0.394, 95% CI: 0.318–0.487, p < 0.001). As shown in Figure 4, nearly all nutrient scores were inversely related to MASLD, with saturated fatty acids (weight = 0.313) identified as the most significant contributor to MASLD risk, followed by red and processed meats and eggs (weights = 0.131 and 0.087, respectively).
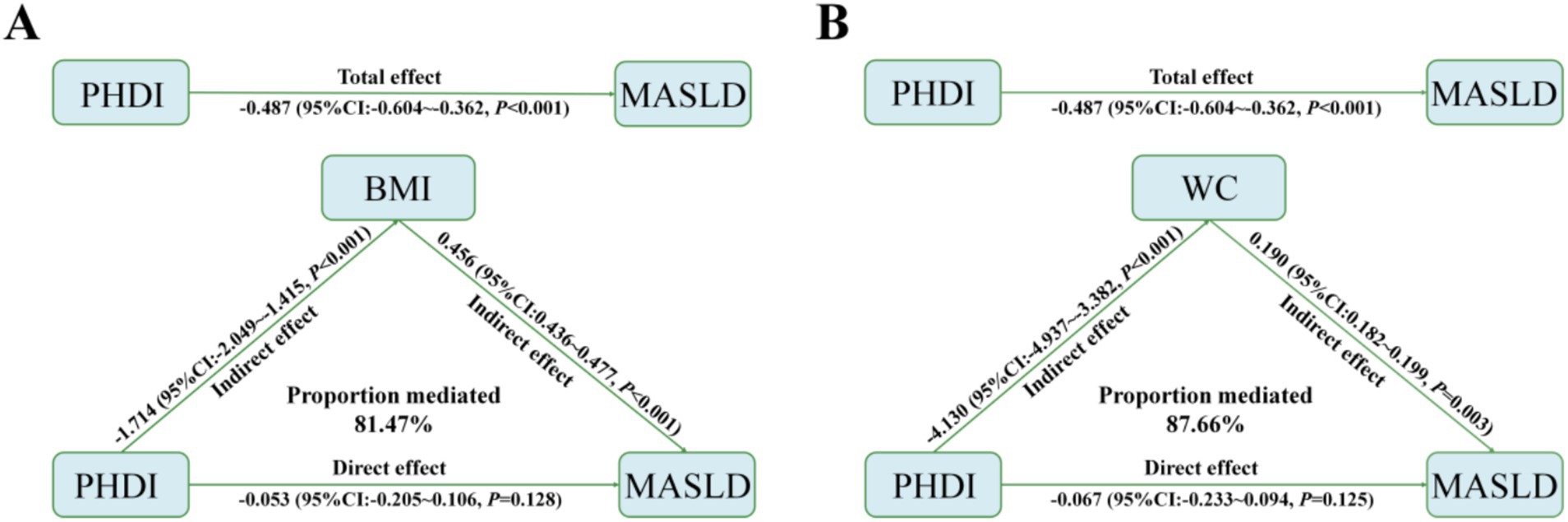
Mediation analysis was conducted to explore the mediating effects of BMI and WC on the relationship between PHDI and MASLD. Specifically, the highest PHDI quintile was inversely associated with BMI (estimate = −1.714, 95% CI: −2.049 to −1.415, p < 0.001) and WC (estimate = −4.130, 95% CI: −4.937 to −3.382, p < 0.001). Both BMI and WC were positively associated with MASLD risk (OR = 0.456, 95% CI: 0.436–0.477, p < 0.001; OR = 0.190, 95% CI: 0.182–0.199, p = 0.003, respectively) (Figure 5). Ultimately, 81.47 and 87.66% of the association between the highest PHDI quintile and MASLD risk was mediated by BMI and WC, respectively. Similar results were obtained when PHDI was analyzed as a continuous variable (Supplementary Figure S2), underscoring the critical roles of these indicators in MASLD risk.
3.6 Sensitivity analysis
To robustly validate our findings, we conducted a series of sensitivity analyses. First, a consistent association between PHDI and MASLD was observed in earlier NHANES cycles from 2011 to 2018 (ORQ5 vs. Q1 = 0.565, 95% CI 0.436–0.731, p < 0.001, Supplementary Table S5). Second, analysis of unweighted data confirmed a significant association between PHDI and MASLD (ORQ5 vs. Q1 = 0.617, 95% CI 0.549–0.694, p < 0.001, Supplementary Table S6). Third, excluding participants with missing covariate data rather than using imputation yielded similar results (ORQ5 vs. Q1 = 0.564, 95% CI 0.457–0.697, p < 0.001, Supplementary Table S7). Finally, the inverse association between PHDI and MASLD persisted when we considered only the dietary data from the first day (ORQ5 vs. Q1 = 0.540, 95% CI 0.439–0.663, p < 0.001, Supplementary Table S8).
4 Discussion
In this study, we identified a significant inverse relationship between levels of the PHDI and the risk of MASLD. Participants in the highest quintile of PHDI scores exhibited an approximately 39% reduction in MASLD risk compared to those in the lowest quintile. We also observed a non-linear relationship between PHDI and MASLD, with an inflection point around 58.073. Notably, individuals who were women, had higher education levels, or were married/living with a partner showed an even greater reduction in MASLD risk with increased PHDI. These findings remained robust under various sensitivity analyses, highlighting the potential of adhering to a high PHDI as a strategy to mitigate MASLD risk.
MASLD is the most prevalent chronic liver disease, posing a significant burden due to its hepatic and extrahepatic complications. Lifestyle modifications—particularly dietary interventions—remain central to MASLD management and the improvement of insulin sensitivity (31). Prior research has established that adherence to the Mediterranean diet can substantially lower MASLD risk and its associated complications (15, 32). Similarly, plant-based diets have demonstrated protective effects against MASLD, whereas high-fat diets have been linked to its development (17, 33). Consistent with these reports, our study suggests that a higher PHDI, reflecting increased intake of plant-based and nutrient-dense foods, is linked to a reduced MASLD risk.
Beyond individual health benefits, prior investigations indicate that diets aligned with planetary health principles can reduce mortality and lower incidences of cardiovascular disease, cancer, and diabetes (34–37). In parallel, they also confer environmental co-benefits, such as reduced greenhouse gas emissions (GHGE), when compared with other healthy eating indices (e.g., HEI-2015 or DASHI) (38, 39). Adopting the planetary health diet in all counties may decrease the global water footprint by 12% (39). Our results, demonstrating an inverse association between PHDI and MASLD risk, further support the notion that planetary health-oriented diets benefit both public health and ecological sustainability.
In the present study, PHDI is composed of the scores of 15 food components categorized as adequacy (higher intake yields higher scores) and moderation (lower intake yields higher scores). A deeper look at each component showed that elevated quintiles of whole grains, non-starchy vegetables, whole fruits (excluding fruit juice), nuts and seeds, and soy products were associated with lower MASLD risk. Conversely, higher intake of saturated fatty acids correlated significantly with increased MASLD risk, aligning with prior literature (40–42).
Interestingly, a higher score for unsaturated fatty acids—an adequacy component of the PHDI—was associated with an increased risk of MASLD. The role of unsaturated fatty acids, which include monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs), in MASLD remains controversial. While MUFAs may reduce saturated fatty acid-induced lipotoxicity in hepatocytes (43), a diet rich in MUFAs can increase the incidence of macrovesicular steatosis and hepatic triglyceride levels, thereby elevating the risk of MASLD (44). PUFAs, on the other hand, may exacerbate cholesterol-induced mitochondrial damage hepatocytes (45), and intake of PUFAs could contribute to increased BMI, insulin resistance, and the expression of lipogenesis-related genes in the liver (46). Our findings support the harmful effect of high dietary intake of unsaturated fatty acids, as the highest quintile of unsaturated fatty acid scores was associated with a 63.8% increased in MASLD risk. Additionally, potential confounders, such as physical activity and other metabolic factors, may have influenced the observed association. Further research is needed to elucidate the specific roles of different types of unsaturated fatty acids in MASLD pathogenesis.
In our WQS regression analysis, we found that among the 15 components of the PHDI, saturated fatty acids had the strongest association with MASLD development, with a weight of 0.313—significantly higher than that of other components, consistent with previous studies (47–49). The most common saturated fatty acids include palmitic acid, lauric acid, myristic acid, and stearic acid (50), which play contradictory roles in MASLD. Excessive dietary intake of palmitic acid and lauric acid may promote insulin resistance (51), which is also correlated with the consumption of added sugar (52), contributing to hepatic steatosis and inflammation (53). Additionally, palmitic acid can enhance the expression of various inflammatory mediators, such as interleukin-32 (IL-32) and chemokine CCL20, which are involved in chronic liver inflammation (54). In contrast, dietary stearic acid may offer protective effects for hepatocytes and attenuates liver injury (55, 56). In our study, reducing the dietary intake of saturated fatty acids resulted in the most significant decrease in MASLD risk in our study, with a 47.5% reduction observed in the highest quintile of saturated fatty acid scores compared to the lowest quintile. Furthermore, added sugar was found to have a similar contribution to MASLD risk as nuts, seeds, and fish. This may be attributed to the complexity of dietary patterns, in which multiple food components interact, leading to individual components showing similar associations with MASLD risk.
Furthermore, mediation analysis indicated that the effect of PHDI on MASLD risk was largely mediated by BMI and WC, both of which are well-established independent risk factors for MASLD (57–59). Increased BMI is associated with elevated blood lipid levels, such as TG and low-density lipoprotein cholesterol (LDL-C), which mediate the relationship between BMI and MASLD by promoting lipid deposition in hepatocytes (58). Similarly, women with a WC greater than 78.5 cm had a 1.54-fold increased risk of MASLD compared to those with a WC below 78.5 cm, while men with a WC greater than 81 cm had a 1.44-fold increased risk compared to those with a WC below 81 cm (59). Our findings also suggest that a higher PHDI value was associated with lower BMI and WC levels, thereby contributing to a reduced MASLD risk. These findings may offer insights into the mechanism by which PHDI influences MASLD.
From a public health perspective, integrating PHDI-based guidelines into existing dietary recommendations could offer both metabolic and environmental advantages. Strategies might include reducing intakes of saturated fatty acids, red and processed meats, and added sugars, while prioritizing whole grains, legumes, vegetables, fruits, and other nutrient-dense, plant-based foods. Such guidelines could be reinforced by policies that promote sustainable farming practices, reduce food waste, and increase the availability of locally sourced plant-based foods. By aligning individual health goals with global sustainability objectives, PHDI-oriented interventions hold promise for dual benefits at both the population and planetary levels.
4.1 Strengths and limitations
A major strength of this study is its large sample size, enabling comprehensive adjustment for multiple potential confounders in assessing the relationship between PHDI and MASLD. Additionally, our various sensitivity analyses support the robustness of the findings. However, several limitations should be acknowledged. First, MASLD was identified using the Fatty Liver Index (FLI), which, while practical for large-scale research, is not as definitive as liver biopsy. Second, the cross-sectional nature of our design precludes causal inferences, and unmeasured factors such as other dietary behaviors or genetic predisposition may still confound our results. Third, relying on only 2 days of 24-h dietary recall may not fully represent habitual long-term intake. Lastly, the NHANES dataset focuses on the U.S. population; thus, our findings might not be fully generalizable to regions with different dietary patterns, food availability, and cultural practices. Future longitudinal or interventional studies in diverse populations are warranted to confirm the applicability of the PHDI and to further elucidate the causal mechanisms linking dietary patterns to MASLD risk.
5 Conclusion
In summary, this study demonstrates that adherence to the PHDI is associated with a reduced risk of MASLD, with BMI and WC acting as partial mediators. Saturated fatty acids emerged as the most influential component in PHDI’s impact on MASLD risk. These findings provide valuable insights for MASLD management and support efforts toward environmental sustainability. Further prospective and intervention studies are warranted to validate these results. Incorporating PHDI-based recommendations into national dietary guidelines could play a crucial role in MASLD prevention and support broader sustainability initiatives.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XQ: Conceptualization, Writing – original draft. SS: Conceptualization, Writing – original draft. NJ: Data curation, Writing – review & editing. DL: Methodology, Writing – review & editing. YF: Methodology, Writing – review & editing. GY: Methodology, Writing – review & editing. BX: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82260573); National Major Special Science and Technology Project (2017ZX10203207); the Youth Science Foundation of Guangxi Medical University (GXMUYSF202321); and Self-funded Scientific Research Project of the Health and Family Planning Commission of Guangxi Zhuang Autonomous Region (Z-A20240412).
Acknowledgments
We sincerely thank all the staff members who contributed to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1534604/full#supplementary-material
Footnotes
References
1. Xiao, J, Wang, F, Yuan, Y, Gao, J, Xiao, L, Yan, C, et al. Epidemiology of liver diseases: global disease burden and forecasted research trends. Sci China Life Sci. (2024) 68:541–57. doi: 10.1007/s11427-024-2722-2
2. Zheng, H, Sechi, LA, Navarese, EP, Casu, G, and Vidili, G. Metabolic dysfunction-associated steatotic liver disease and cardiovascular risk: a comprehensive review. Cardiovasc Diabetol. (2024) 23:346. doi: 10.1186/s12933-024-02434-5
3. Chan, KE, Ong, EYH, Chung, CH, Ong, CEY, Koh, B, Tan, DJH, et al. Longitudinal outcomes associated with metabolic dysfunction-associated Steatotic liver disease: a Meta-analysis of 129 studies. Clin Gastroenterol Hepatol. (2024) 22:488–498.e14. doi: 10.1016/j.cgh.2023.09.018
4. Bo, T, Gao, L, Yao, Z, Shao, S, Wang, X, Proud, CG, et al. Hepatic selective insulin resistance at the intersection of insulin signaling and metabolic dysfunction-associated steatotic liver disease. Cell Metab. (2024) 36:947–68. doi: 10.1016/j.cmet.2024.04.006
5. Rosso, C, Caviglia, GP, Birolo, G, Armandi, A, Pennisi, G, Pelusi, S, et al. Impact of PNPLA3 rs738409 polymorphism on the development of liver-related events in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. (2023) 21:3314–3321.e3. doi: 10.1016/j.cgh.2023.04.024
6. Poore, J, and Nemecek, T. Reducing food's environmental impacts through producers and consumers. Science. (2018) 360:987–92. doi: 10.1126/science.aaq0216
7. Willett, W, Rockström, J, Loken, B, Springmann, M, Lang, T, Vermeulen, S, et al. Food in the Anthropocene: the EAT-lancet commission on healthy diets from sustainable food systems. Lancet. (2019) 393:447–92. doi: 10.1016/S0140-6736(18)31788-4
8. GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1223–49. doi: 10.1016/S0140-6736(20)30752-2
9. Willett, WC, Hu, FB, Rimm, EB, and Stampfer, MJ. Building better guidelines for healthy and sustainable diets. Am J Clin Nutr. (2021) 114:401–4. doi: 10.1093/ajcn/nqab079
10. Springmann, M, Wiebe, K, Mason-D'Croz, D, Sulser, TB, Rayner, M, and Scarborough, P. Health and nutritional aspects of sustainable diet strategies and their association with environmental impacts: a global modelling analysis with country-level detail. Lancet Planet Health. (2018) 2:e451–61. doi: 10.1016/S2542-5196(18)30206-7
11. Cacau, LT, De Carli, E, de Carvalho, AM, et al. Development and validation of an index based on EAT-lancet recommendations: the planetary health diet index. Nutrients. (2021) 13:1698. doi: 10.3390/nu13051698
12. Shojaei, S, Dehnavi, Z, Irankhah, K, Fatemi, SF, and Sobhani, SR. Adherence to the planetary health diet index and metabolic syndrome: cross-sectional results from the PERSIAN cohort study. BMC Public Health. (2024) 24:2988. doi: 10.1186/s12889-024-20484-y
13. Sotos-Prieto, M, Ortolá, R, Maroto-Rodriguez, J, Carballo-Casla, A, Kales, SN, and Rodríguez-Artalejo, F. Association between planetary health diet and cardiovascular disease: a prospective study from the UK biobank. Eur J Prev Cardiol. (2024) 29:zwae282. doi: 10.1093/eurjpc/zwae282
14. Rodrigues, M, Padrão, P, Castro Mendes, F, Moreira, A, and Moreira, P. The planetary health diet and its association with asthma and airway inflammation in school-aged children. Nutrients. (2024) 16:2241. doi: 10.3390/nu16142241
15. Lee, JY, Kim, S, Lee, Y, Kwon, YJ, and Lee, JW. Higher adherence to the Mediterranean diet is associated with a lower risk of Steatotic, alcohol-related, and metabolic dysfunction-associated Steatotic liver disease: a retrospective analysis. Nutrients. (2024) 16:3551. doi: 10.3390/nu16203551
16. Zeng, XF, Varady, KA, Wang, XD, Targher, G, Byrne, CD, Tayyem, R, et al. The role of dietary modification in the prevention and management of metabolic dysfunction-associated fatty liver disease: an international multidisciplinary expert consensus. Metab Clin Exp. (2024) 161:156028. doi: 10.1016/j.metabol.2024.156028
17. Castelnuovo, G, Perez-Diaz-Del-Campo, N, Rosso, C, Armandi, A, Caviglia, GP, and Bugianesi, E. A healthful plant-based diet as an alternative dietary approach in the management of metabolic dysfunction-associated Steatotic liver disease. Nutrients. (2024) 16:2027. doi: 10.3390/nu16132027
18. Huang, X, Gan, D, Fan, Y, Fu, Q, He, C, Liu, W, et al. The associations between healthy eating patterns and risk of metabolic dysfunction-associated Steatotic liver disease: a case-control study. Nutrients. (2024) 16:1956. doi: 10.3390/nu16121956
19. Steinfeldt, L, Anand, J, and Murayi, T. Food reporting patterns in the USDA automated multiple-pass method. Procedia Food Sci. (2013) 2:145–56. doi: 10.1016/j.profoo.2013.04.022
20. Food surveys research group, Beltsville human nutrition research center, Agricultural Research Service, U.S Department of Agriculture. Food Patterns Equivalents Database 2017-2018: Methodology and User Guide Amended for Use with WWEIA, NHANES 2017-march 2020 Prepandemic. Beltsville, Maryland: Food surveys research group, Beltsville human nutrition research center, Agricultural Research Service, U.S Department of Agriculture (2023).
21. Min, Y, Wei, X, Wei, Z, Song, G, Zhao, X, and Lei, Y. Prognostic effect of triglyceride glucose-related parameters on all-cause and cardiovascular mortality in the United States adults with metabolic dysfunction-associated steatotic liver disease. Cardiovasc Diabetol. (2024) 23:188. doi: 10.1186/s12933-024-02287-y
22. Bedogni, G, Bellentani, S, Miglioli, L, Masutti, F, Passalacqua, M, Castiglione, A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. (2006) 6:33. doi: 10.1186/1471-230X-6-33
23. Park, J, Kim, G, Kim, BS, Han, KD, Kwon, SY, Park, SH, et al. The associations of hepatic steatosis and fibrosis using fatty liver index and BARD score with cardiovascular outcomes and mortality in patients with new-onset type 2 diabetes: a nationwide cohort study. Cardiovasc Diabetol. (2022) 21:53. doi: 10.1186/s12933-022-01483-y
24. Moshfegh, AJ, Rhodes, DG, Baer, DJ, Murayi, T, Clemens, JC, Rumpler, WV, et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. (2008) 88:324–32. doi: 10.1093/ajcn/88.2.324
25. Lee, CM, Yoon, EL, Kim, M, Kang, BK, Cho, S, Nah, EH, et al. Prevalence, distribution, and hepatic fibrosis burden of the different subtypes of steatotic liver disease in primary care settings. Hepatology. (2024) 79:1393–400. doi: 10.1097/HEP.0000000000000664
26. Zhan, JJ, Hodge, RA, Dunlop, AL, Lee, MM, Bui, L, Liang, D, et al. Dietaryindex: a user-friendly and versatile R package for standardizing dietary pattern analysis in epidemiological and clinical studies. Am J Clin Nutr. (2024) 120:1165–74. doi: 10.1016/j.ajcnut.2024.08.021
27. Bui, LP, Pham, TT, Wang, F, Chai, B, Sun, Q, Hu, FB, et al. Planetary health diet index and risk of total and cause-specific mortality in three prospective cohorts. Am J Clin Nutr. (2024) 120:80–91. doi: 10.1016/j.ajcnut.2024.03.019
28. Kesse-Guyot, E, Rebouillat, P, Brunin, J, Langevin, B, Allès, B, Touvier, M, et al. Environmental and nutritional analysis of the EAT-lancet diet at the individual level: insights from the NutriNet-Santé study. J Clean Prod. (2021) 296:126555. doi: 10.1016/j.jclepro.2021.126555
29. Muntner, P, Hardy, ST, Fine, LJ, Jaeger, BC, Wozniak, G, Levitan, EB, et al. Trends in blood pressure control among US adults with hypertension, 1999-2000 to 2017-2018. JAMA. (2020) 324:1190–200. doi: 10.1001/jama.2020.14545
30. Xu, M, Zhan, Y, Gao, G, Zhu, L, Wu, T, and Xin, G. Associations of five dietary indices with metabolic dysfunction-associated steatotic liver disease and liver fibrosis among the United States population. Front Nutr. (2024) 11:1446694. doi: 10.3389/fnut.2024.1446694
31. EASL-EASD-EASO clinical practice guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. (2024) 81:492–542. doi: 10.1016/j.jhep.2024.04.031
32. Jamil, A, Chivese, T, Elshaikh, U, and Sendall, M. Efficacy of the Mediterranean diet in treating metabolic dysfunction-associated steatotic liver disease (MASLD) in children and adolescents: a systematic review and meta-analysis. BMC Public Health. (2024) 24:2701. doi: 10.1186/s12889-024-19378-w
33. Szudzik, M, Hutsch, T, Chabowski, D, Zajdel, M, and Ufnal, M. Normal caloric intake with high-fat diet induces metabolic dysfunction-associated steatotic liver disease and dyslipidemia without obesity in rats. Sci Rep. (2024) 14:22796. doi: 10.1038/s41598-024-74193-y
34. Liu, F, Si, C, Chen, L, Peng, Y, Wang, P, Wang, X, et al. EAT-lancet diet pattern, genetic predisposition, inflammatory biomarkers, and risk of lung Cancer incidence and mortality. Mol Nutr Food Res. (2024) 68:e2400448. doi: 10.1002/mnfr.202400448
35. Pitt, S, Kałuża, J, Widenfalk, A, Åkesson, A, and Wolk, A. Adherence to the EAT-lancet diet in relation to mortality and exposure to food contaminants in population-based cohorts of Swedish men and women. Environ Int. (2024) 184:108495. doi: 10.1016/j.envint.2024.108495
36. Cahill, LE, and Hummel, SL. Eating to prevent both heart and planetary failure: the EAT-lancet diet. JACC Heart Fail. (2024) 12:1209–11. doi: 10.1016/j.jchf.2024.04.012
37. Lin, X, Wang, S, and Huang, J. The association between the EAT-lancet diet and diabetes: a systematic review. Nutrients. (2023) 15:4462. doi: 10.3390/nu15204462
38. Frank, SM, Jaacks, LM, Meyer, K, Rose, D, Adair, LS, Avery, CL, et al. Dietary quality and dietary greenhouse gas emissions in the USA: a comparison of the planetary health diet index, healthy eating index-2015, and dietary approaches to stop hypertension. Int J Behav Nutr Phys Act. (2024) 21:36. doi: 10.1186/s12966-024-01581-y
39. Tuninetti, M, Ridolfi, L, and Laio, F. Compliance with EAT-lancet dietary guidelines would reduce global water footprint but increase it for 40% of the world population. Nat Food. (2022) 3:143–51. doi: 10.1038/s43016-021-00452-0
40. Notarnicola, M, Tutino, V, De Nunzio, V, et al. Daily Orange consumption reduces hepatic steatosis prevalence in patients with metabolic dysfunction-associated Steatotic liver disease: exploratory outcomes of a randomized clinical trial. Nutrients. (2024) 16:3191. doi: 10.3390/nu16183191
41. Tung, YC, Liang, ZR, Chou, SF, Ho, CT, Kuo, YL, Cheng, KC, et al. Fermented soy paste alleviates lipid accumulation in the liver by regulating the AMPK pathway and modulating gut microbiota in high-fat-diet-fed rats. J Agric Food Chem. (2020) 68:9345–57. doi: 10.1021/acs.jafc.0c02919
42. Meex, RCR, and Blaak, EE. Mitochondrial dysfunction is a key pathway that links saturated fat intake to the development and progression of NAFLD. Mol Nutr Food Res. (2021) 65:e1900942. doi: 10.1002/mnfr.201900942
43. Chen, X, Li, L, Liu, X, Luo, R, Liao, G, Li, L, et al. Oleic acid protects saturated fatty acid mediated lipotoxicity in hepatocytes and rat of non-alcoholic steatohepatitis. Life Sci. (2018) 203:291–304. doi: 10.1016/j.lfs.2018.04.022
44. Xu, F, Albadry, M, Döding, A, Chen, X, Dirsch, O, Schulze-Späte, U, et al. The effects of saturated and unsaturated fatty acids on MASLD: a Mendelian randomization analysis and in vivo experiment. Eur J Nutr. (2024) 64:52. doi: 10.1007/s00394-024-03560-2
45. Henkel, J, Alfine, E, Saín, J, Jöhrens, K, Weber, D, Castro, J, et al. Soybean oil-derived poly-unsaturated fatty acids enhance liver damage in NAFLD induced by dietary cholesterol. Nutrients. (2018) 10:1326. doi: 10.3390/nu10091326
46. Hao, L, Chen, CY, Nie, YH, Kaliannan, K, and Kang, JX. Differential interventional effects of Omega-6 and Omega-3 polyunsaturated fatty acids on high fat diet-induced obesity and hepatic pathology. Int J Mol Sci. (2023) 24:7261. doi: 10.3390/ijms242417261
47. Gan, L, Xiang, W, Xie, B, and Yu, L. Molecular mechanisms of fatty liver in obesity. Front Med. (2015) 9:275–87. doi: 10.1007/s11684-015-0410-2
48. Mells, JE, Fu, PP, Kumar, P, Smith, T, Karpen, SJ, and Anania, FA. Saturated fat and cholesterol are critical to inducing murine metabolic syndrome with robust nonalcoholic steatohepatitis. J Nutr Biochem. (2015) 26:285–92. doi: 10.1016/j.jnutbio.2014.11.002
49. Monserrat-Mesquida, M, Quetglas-Llabrés, MM, Bouzas, C, Pastor, O, Ugarriza, L, Llompart, I, et al. Plasma fatty acid composition, oxidative and inflammatory status, and adherence to the Mediterranean diet of patients with non-alcoholic fatty liver disease. Antioxidants. (2023) 12:1554. doi: 10.3390/antiox12081554
50. Li, B, Leung, JCK, Chan, LYY, Yiu, WH, and Tang, SCW. A global perspective on the crosstalk between saturated fatty acids and toll-like receptor 4 in the etiology of inflammation and insulin resistance. Prog Lipid Res. (2020) 77:101020. doi: 10.1016/j.plipres.2019.101020
51. Saraswathi, V, Kumar, N, Gopal, T, Bhatt, S, Ai, W, Ma, C, et al. Lauric acid versus palmitic acid: effects on adipose tissue inflammation, insulin resistance, and non-alcoholic fatty liver disease in obesity. Biology. (2020) 9:346. doi: 10.3390/biology9110346
52. Wang, J, Light, K, Henderson, M, O’Loughlin, J, Mathieu, ME, Paradis, G, et al. Consumption of added sugars from liquid but not solid sources predicts impaired glucose homeostasis and insulin resistance among youth at risk of obesity. J Nutr. (2014) 144:81–6. doi: 10.3945/jn.113.182519
53. Li, Y, Lu, Z, Ru, JH, Lopes-Virella, MF, Lyons, TJ, and Huang, Y. Saturated fatty acid combined with lipopolysaccharide stimulates a strong inflammatory response in hepatocytes in vivo and in vitro. Am J Physiol Endocrinol Metab. (2018) 315:E745–e757. doi: 10.1152/ajpendo.00015.2018
54. Schilcher, K, Dayoub, R, Kubitza, M, Riepl, J, Klein, K, Buechler, C, et al. Saturated fat-mediated upregulation of IL-32 and CCL20 in hepatocytes contributes to higher expression of these fibrosis-driving molecules in MASLD. Int J Mol Sci. (2023) 24:3222. doi: 10.3390/ijms241713222
55. Nie, W, Xu, F, Zhou, K, Yang, X, Zhou, H, and Xu, B. Stearic acid prevent alcohol-induced liver damage by regulating the gut microbiota. Food Res Int. (2022) 155:111095. doi: 10.1016/j.foodres.2022.111095
56. Pan, PH, Lin, SY, Ou, YC, Chen, WY, Chuang, YH, Yen, YJ, et al. Stearic acid attenuates cholestasis-induced liver injury. Biochem Biophys Res Commun. (2010) 391:1537–42. doi: 10.1016/j.bbrc.2009.12.119
57. Li, L, Liu, DW, Yan, HY, Wang, ZY, Zhao, SH, and Wang, B. Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes Rev. (2016) 17:510–9. doi: 10.1111/obr.12407
58. Lu, S, Xie, Q, Kuang, M, Hu, C, Li, X, Yang, H, et al. Lipid metabolism, BMI and the risk of nonalcoholic fatty liver disease in the general population: evidence from a mediation analysis. J Transl Med. (2023) 21:192. doi: 10.1186/s12967-023-04047-0
59. Lee, JH, Jeon, S, Lee, HS, and Kwon, YJ. Cutoff points of waist circumference for predicting incident non-alcoholic fatty liver disease in middle-aged and older Korean adults. Nutrients. (2022) 14:994. doi: 10.3390/nu14142994
Glossary
MASLD - Metabolic Dysfunction-Associated Steatotic Liver Disease
NAFLD - Nonalcoholic Fatty Liver Disease
MASH - Metabolic Dysfunction-Associated Steatohepatitis
HCC - Hepatocellular Carcinoma
PHDI - Planetary Health Diet Index
MD - Mediterranean Diet
PBDs - Plant-Based Diets
HEI - Healthy Eating Index
NCHS - National Center for Health Statistics
FLI - Fatty Liver Index
USDA - United States Department of Agriculture
FPED - Food Patterns Equivalent Database
SLD - Steatotic Liver Disease
TG - Triglyceride
BMI - Body Mass Index
GGT - Gamma-Glutamyl Transferase
WC - Waist Circumference
PIR - Poverty Income Ratio
ALT - Alanine Aminotransferase
AST - Aspartate Aminotransferase
OR - Odds Ratio
CI - Confidence Intervals
RCS - Restricted Cubic Spline
WQS - Weighted Quantile Sum
HEI-2015 - Healthy Eating Index-2015
DASHI - Dietary Approaches to Stop Hypertension
AHEI - Alternative Healthy Eating Index
AMED - Alternate Mediterranean Diet
MUFAs - Monounsaturated Fatty Acids
PUFAs - Polyunsaturated Fatty Acids
GHGE - Greenhouse Gas Emissions
IL-32 - Interleukin-32
LDL-C - Low-Density Lipoprotein Cholesterol
Keywords: dietary pattern, planetary health diet, metabolic dysfunction-associated steatotic liver disease, epidemiology, sustainable diet
Citation: Qiu X, Shen S, Jiang N, Lu D, Feng Y, Yang G and Xiang B (2025) Adherence to the planetary health diet index and metabolic dysfunction-associated steatotic liver disease: a cross-sectional study. Front. Nutr. 12:1534604. doi: 10.3389/fnut.2025.1534604
Edited by:
Joan Sabate, Loma Linda University, United StatesReviewed by:
Nazanin Abbaspour, Loma Linda University, United StatesAndrew Berardy, United States Military Academy West Point, United States
Copyright © 2025 Qiu, Shen, Jiang, Lu, Feng, Yang and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bangde Xiang, eGlhbmdiYW5nZGVAZ3htdS5lZHUuY24=
†These authors have contributed equally to this work
 Xin Qiu
Xin Qiu Shuang Shen
Shuang Shen Nizhen Jiang3
Nizhen Jiang3