
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 25 February 2025
Sec. Nutritional Epidemiology
Volume 12 - 2025 | https://doi.org/10.3389/fnut.2025.1533514
This article is part of the Research Topic Objective Dietary Assessment in Nutrition Epidemiology Studies - Volume II View all 20 articles
Background: Asthma is associated with an increased risk of cardiovascular mortality, potentially influenced by dietary phosphorus intake through its effects on inflammation and oxidative stress.
Methods: Data from 7,539 asthma patients in the National Health and Nutrition Examination Survey (NHANES) 1999–2018 cohort were analyzed using weighted Cox proportional hazards models to estimate hazard ratios (HR) and 95% confidence intervals (CI). Kaplan-Meier survival curves and a nomogram were used to assess survival probabilities and individualized risk, while restricted cubic spline (RCS) analysis evaluated non-linear dose-response relationships. Sensitivity analyses were conducted to test the robustness of the findings.
Results: Higher dietary phosphorus intake was associated with reduced cardiovascular mortality (HR: 0.43; 95% CI: 0.22–0.85 for the highest vs. lowest quartile; p for trend = 0.018). Kaplan-Meier curves showed improved survival with increasing phosphorus intake, a result consistently supported by subgroup analyses. RCS analysis confirmed a non-linear dose-response relationship, identifying a threshold at 1,861.52 mg/day, below which higher phosphorus intake was significantly associated with lower cardiovascular mortality. However, above this threshold, the protective effect diminished. Sensitivity analyses further validated these results.
Conclusion: Elevated dietary phosphorus intake is associated with reduced cardiovascular mortality in asthma patients, suggesting its potential as a dietary intervention.
Asthma is a chronic inflammatory disease that affects millions of people worldwide, contributing significantly to morbidity and mortality. In addition to respiratory symptoms, asthma has systemic effects, particularly its association with cardiovascular disease (CVD). This connection is believed to result from systemic inflammation, oxidative stress, and autonomic dysfunction, which collectively exacerbate vascular damage and endothelial dysfunction, thus increasing cardiovascular risk (1–3).
The health outcomes of asthma patients are influenced by various dietary and lifestyle factors. Diets high in saturated fats and low in fruits and vegetables may worsen airway inflammation, while obesity and physical inactivity contribute to heightened systemic inflammation and cardiovascular risk (4, 5). Excessive sodium intake has also been linked to oxidative stress and impaired vascular function (6, 7). These findings underscore the importance of identifying dietary components that can help mitigate these risks, especially in asthma patients who already face chronic inflammation and comorbid conditions.
Dietary phosphorus has attracted attention for its potential role in regulating vascular function and inflammation. Adequate phosphorus intake has been associated with improved cardiovascular health, including lower blood pressure, possibly through its effects on vascular smooth muscle cell function and endothelium-dependent vasodilation (8, 9). Additionally, phosphorus intake has been linked to reduced levels of inflammatory markers, such as C-reactive protein (CRP), suggesting a role in modulating systemic inflammation (10, 11).
Phosphorus may also provide indirect benefits for airway function in asthma patients. By supporting adenosine triphosphate (ATP) production, it enhances cellular energy metabolism, which could help stabilize airway smooth muscle and reduce bronchospasms during exacerbations (8, 12). Its role in maintaining acid-base balance may also improve the airway microenvironment and reduce the activation of inflammatory mediators, contributing to better respiratory health (13, 14).
Although research has explored the effects of various dietary factors on asthma and cardiovascular health, limited attention has been given to the specific role of dietary phosphorus. This study aims to evaluate the relationship between dietary phosphorus intake and cardiovascular mortality in asthma patients, addressing this important gap and providing insights that could inform dietary interventions.
The National Health and Nutrition Examination Survey (NHANES), conducted by the Centers for Disease Control and Prevention (CDC), is a large-scale program designed to assess the health and nutritional status of the U.S. population. By integrating interviews, physical examinations, and laboratory testing, NHANES gathers comprehensive data on key health indicators, including dietary patterns, medical conditions, and environmental exposures (15–17). Utilizing a nationally representative sample, the survey provides valuable insights into health trends, identifies risk factors, and supports evidence-based public health policymaking. This study adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines, ensuring methodological rigor and ethical compliance. The study protocol was approved by the Institutional Review Board (IRB) of the National Center for Health Statistics (NCHS).
Data for this study were derived from NHANES between 1999 and 2018, starting with an initial sample of 71,916 participants. A stepwise selection process was applied based on inclusion and exclusion criteria. First, individuals without a diagnosis of asthma were excluded (N = 58,143), leaving 13,773 participants identified as having asthma. Subsequently, 5,787 participants with missing mortality data were excluded, resulting in 7,986 eligible participants. An additional 447 participants lacking data of phosphorus intake were removed, reducing the sample size to 7,539. The selection process is illustrated in Figure 1.
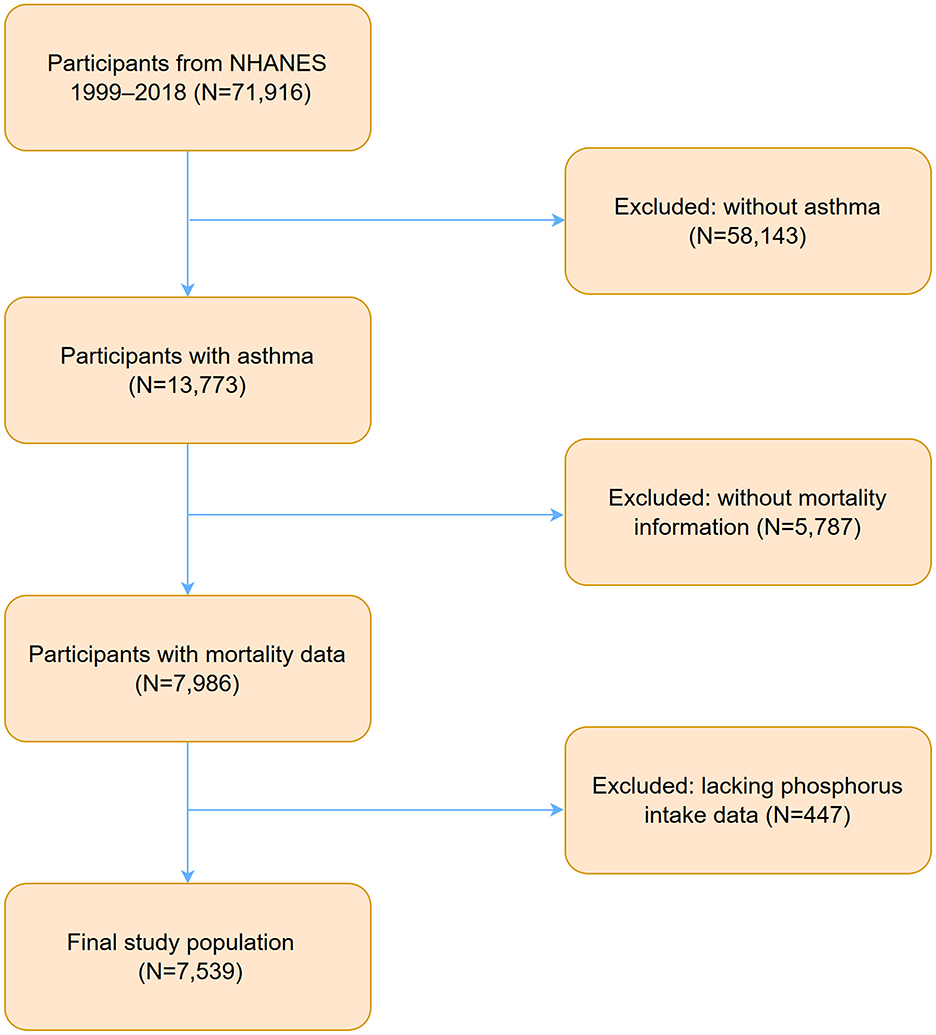
Figure 1. Flowchart of participants selection and exclusion from NHANES 1999–2018. NHANES, National Health and Nutrition Examination Survey.
Asthma status was obtained from the questionnaire data in the NHANES dataset. The definition of asthma was based on the question, “Has a doctor or other health professional ever diagnosed you with asthma?” Participants who responded “Yes” were classified as having asthma. To ensure consistency in data collection, interviewers received extensive training, and the data underwent rigorous verification processes to minimize errors and biases, in line with the quality assurance standards established by NCHS. Prior research has validated the accuracy of self-reported asthma, demonstrating the reliability of this method (18, 19).
Dietary phosphorus intake was assessed using two 24 h dietary recall interviews, based on the Food and Nutrient Database for Dietary Studies provided by the United States Department of Agriculture Available at: CDC NHANES Operations Manual. Additionally, 24 h supplement usage was recorded. The average phosphorus intake was calculated by combining dietary and supplement sources across the 2 days. Notably, data collection methods varied by survey cycle: during 1999–2002, only one 24 h dietary recall was conducted, while the 2003–2006 cycles included two recall interviews but lacked supplement usage data.
Mortality data were sourced from the 2019 Public-Use Linked Mortality Files, provided by the National Death Index (NDI) through NCHS, Division of Analysis and Epidemiology (Hyattsville, Maryland; created April 28, 2022). Following data cleaning, the variable “MORTSTAT” indicates the final mortality status, with values limited to “0” and “1,” representing presumed alive and deceased, respectively. Among deceased individuals, the variable “UCOD_LEADING” identifies the underlying leading cause of death, where a value of “001” denotes diseases of the heart as the primary cause. Other values correspond to alternative causes of death or presumed alive status. Further information is available at the CDC NCHS Mortality Data Linkage.
Demographic data, including age, gender, race, and body mass index (BMI), were extracted from NHANES. These data were collected through in-person interviews using validated, standardized questionnaires conducted by trained interviewers to ensure accuracy and consistency. All demographic data underwent multiple verification processes in accordance with the quality control standards established by NCHS (15). Laboratory data, including serum phosphorus and serum calcium levels, were obtained from certified laboratories. These analyses followed rigorous quality control protocols outlined by CDC, which include regular instrument calibration, the use of standard reference materials, and inter-laboratory precision testing to ensure reliability and standardization (20). Questionnaire data were used to assess smoking status, hypertension, hyperlipidemia, diabetes, chronic heart failure (CHF), coronary heart disease (CHD), renal impairment, and malignancy. These data were collected through structured, standardized interviews developed and tested by experts to ensure validity. Interviewers received extensive training to maintain consistency, and the data underwent multiple verification layers to minimize errors and biases, adhering to NCHS quality assurance guidelines (21–24). Other dietary factors, including potential confounding variables such as sodium, calcium, magnesium, total energy, and saturated fat, were assessed using two 24 h dietary recall interviews. These analyses were conducted utilizing the Food and Nutrient Database for Dietary Studies provided by the United States Department of Agriculture. Rigorous quality control procedures, including data calibration and consistency checks, were implemented to ensure the accuracy and reliability of these dietary assessments (25).
All statistical analyses were performed using weighted methods to ensure representativeness of the NHANES cohort. Weighted analyses were conducted using the average of WTDRD1 and WTDR2D weights from the two 24 h dietary recalls in NHANES. For combined data from the 1999–2000 and 2001–2002 cycles, a four-year weight (WTMEC4YR) was applied. Sampling weights for the 1999–2018 period were calculated as follows: for 1999–2002, weights were derived as 2/10 × WTDR4YR, while for 2003–2018, weights were computed as 1/10 × the average of WTDRD1 and WTDR2D. Power calculations were not conducted a priori, as the study utilized publicly available data. The large sample size enhances statistical power by reducing variability and increasing the ability to detect meaningful effects. Additionally, the use of NHANES-provided sample weights and adjustments for the stratified multistage sampling design ensures that the estimates are nationally representative, thereby strengthening the validity of the results. This approach is consistent with previous NHANES-based studies that have produced meaningful findings without the need for a priori power analyses (26–28).
Covariates with ≤ 20% missing data were addressed using multiple imputation, while those with more than 20% missing data were excluded to ensure accurate and reliable estimates (see Supplementary Table S1). Outliers were managed using winsorization, with the 5th and 95th percentiles as cutoff thresholds. The normality of continuous variables was assessed with the Kolmogorov-Smirnov test. All continuous variables were found to be non-normally distributed and are presented as medians with interquartile ranges (IQRs). Categorical variables are reported as frequencies and percentages. Statistical comparisons were made using the Wilcoxon rank-sum test for continuous variables and the Chi-square test for categorical variables.
Cox proportional hazards regression models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI). Trend tests across the three models were conducted to assess the association between dietary phosphorus intake and cardiovascular mortality in asthma patients. Model 1 adjusted for demographic factors, including age, gender, race, and BMI. Model 2 further adjusted for comorbidities such as smoking status, hypertension, hyperlipidemia, CHD, CHF, diabetes, renal impairment, and malignancy. Model 3 included additional adjustments for dietary and biochemical covariates, including sodium, calcium, magnesium, total energy, unsaturated fat, serum calcium, and serum phosphorus. Kaplan-Meier (KM) survival curves were generated to compare survival outcomes, with differences evaluated using the Log-rank test.
Subgroup analyses were performed to assess heterogeneity and interactions, stratified by age (< 60 vs. ≥60 years), gender, race (Non-Hispanic White vs. others), BMI (< 25 vs. ≥25 kg/m2), smoking status (ever-smokers vs. never-smokers), and the presence of comorbidities. Analyses of non-linear relationships and model performance were conducted based on Model 3. Restricted cubic spline (RCS) analysis was employed to explore non-linear relationships, while threshold effect analysis identified inflection points in the dose-mortality relationship.
Sensitivity analyses were conducted to ensure the robustness of the findings. First, phosphorus intake was examined both as a continuous variable and in quartiles, using both categorical and continuous formats. Second, models with progressively adjusted covariates (Model 1, Model 2, and Model 3) were evaluated. Third, subgroup analyses were conducted by stratifying participants based on age, gender, BMI, and the presence of comorbidities. Finally, after excluding all participants with missing data, the analyses were repeated to validate the results.
All analyses were performed using R (version 4.4.2) and Decisionlinnc software (version 1.1.1.5). A significance threshold of p < 0.05 was adopted, in line with its widespread use in statistical analysis as the standard for determining statistically significant results (29).
Table 1 reveals the baseline characteristics. Cardiovascular mortality was significantly associated with older age (median 68.00 years vs. median 41.00 years, p < 0.001) and higher median BMI (29.48 kg/m2 vs. 28.29 kg/m2, p = 0.039). Gender and racial distribution did not differ significantly between groups (p > 0.05). Smoking history was more prevalent in the cardiovascular mortality group (60.15% vs. 50.04%, p = 0.021). Cardiovascular mortality was also significantly associated with a higher prevalence of comorbidities, including hypertension (66.47% vs. 32.59%, p < 0.001), hyperlipidemia (58.28% vs. 35.21%, p < 0.001), diabetes (30.44% vs. 9.71%, p < 0.001), coronary heart disease (CHD) (18.76% vs. 3.92%, p < 0.001), chronic heart failure (CHF) (23.47% vs. 3.68%, p < 0.001), and malignancy (23.15% vs. 10.56%, p < 0.001). Although renal impairment was more prevalent (6.64% vs. 3.67%), the difference did not reach statistical significance (p = 0.039). Dietary intake in the cardiovascular mortality group was characterized by significantly lower phosphorus intake (median 1,029.80 mg vs. median 1,217.00 mg, p < 0.001), calcium intake (median 716.00 mg vs. median 901.00 mg, p < 0.001), and sodium intake (median 2,637.50 mg vs. median 3,120.00 mg, p < 0.001). Total energy intake (median 1,586.00 kcal vs. median 1,932.73 kcal, p < 0.001) and unsaturated fat intake (median 20.28 g vs. median 23.51 g, p < 0.001) were also significantly lower. Serum phosphorus (median 3.49 mg/dL vs. median 4.07 mg/dL, p < 0.001) and serum calcium (median 9.40 mg/dL vs. median 9.70 mg/dL, p < 0.001) levels were significantly lower in the cardiovascular mortality group.
The Cox proportional hazards regression analysis showed a significant inverse association between dietary phosphorus intake and cardiovascular mortality among asthma patients (Table 2). As a continuous variable, higher dietary phosphorus intake was consistently linked to lower cardiovascular mortality across all models (Model 1: p = 0.007; Model 2: p = 0.022; Model 3: p = 0.043). When analyzed by quartiles, participants in the highest quartile (Q4, ≥ 1518.69 mg/day) had significantly lower risks of cardiovascular mortality compared to those in the lowest quartile (Q1, ≤ 765.50 mg/day), with hazard ratios (HR) of 0.36 (95% CI: 0.23–0.57, p < 0.001) in Model 1, 0.39 (95% CI: 0.24–0.64, p < 0.001) in Model 2, and 0.28 (95% CI: 0.09–0.40, p = 0.003) in Model 3. Trend analysis across quartiles further supported a significant dose-response relationship in all models (p for trend < 0.001 in Model 1, < 0.001 in Model 2, and 0.013 in Model 3). Participants were categorized into higher and lower phosphorus intake groups based on the median intake, and their survival curves were compared using Kaplan-Meier analysis (Figure 2). The group with higher phosphorus intake (HPI) demonstrated significantly better survival rates compared to the lower phosphorus intake (LPI) group (p < 0.001), with a hazard ratio (HR) of 0.52 (95% CI: 0.40–0.68).
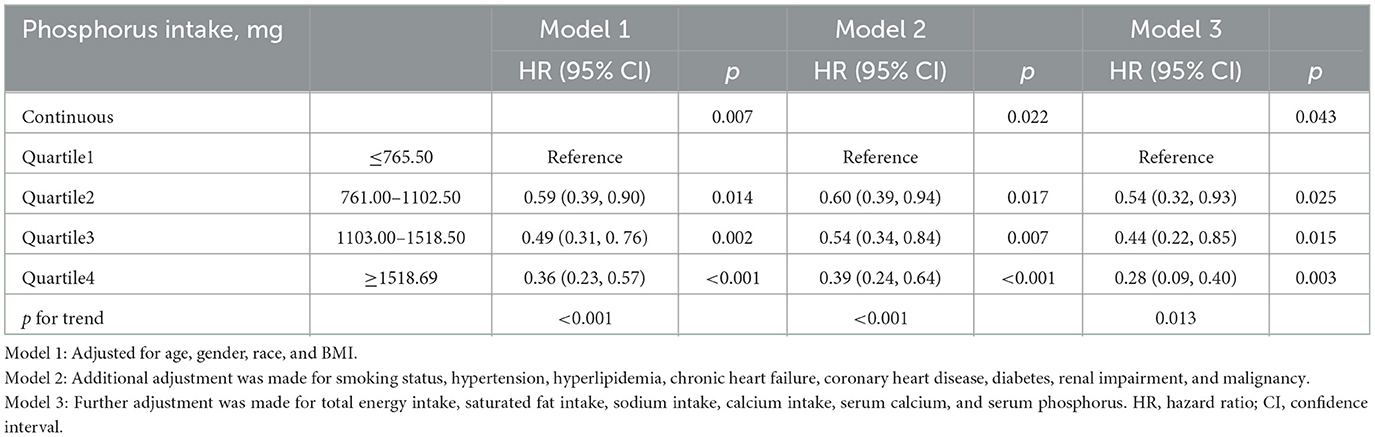
Table 2. Association between dietary phosphorus intake and cardiovascular mortality in asthma patients.
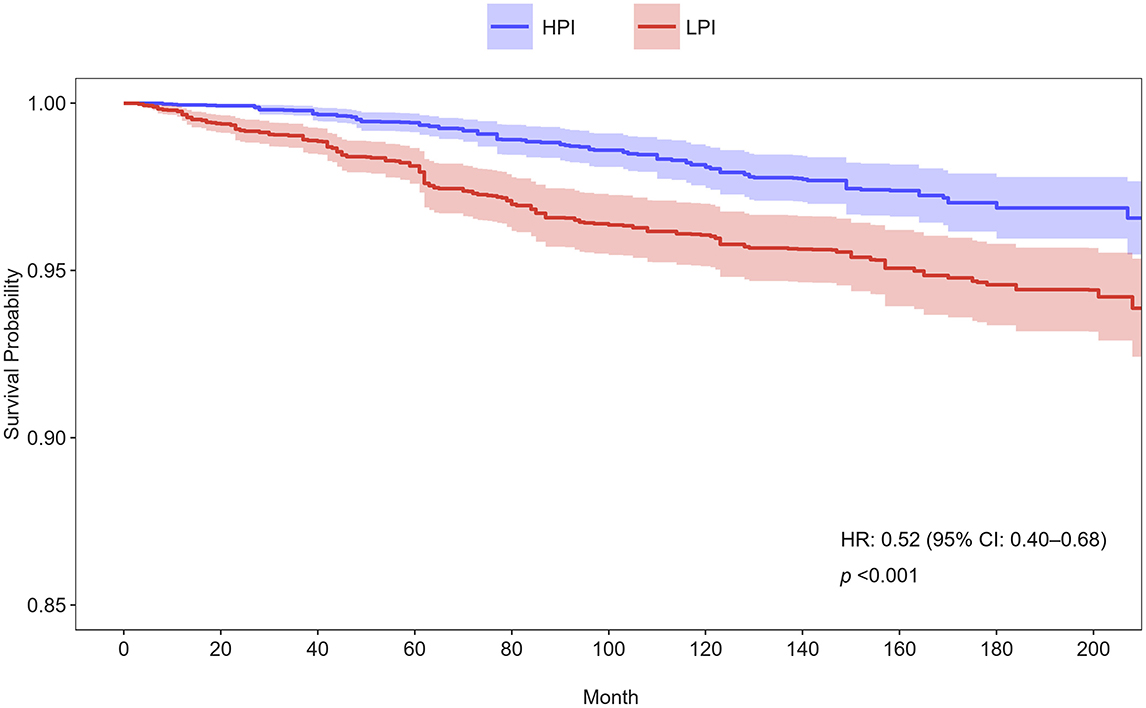
Figure 2. Kaplan-Meier survival curves for cardiovascular mortality stratified by phosphorus intake groups in Model 3. Participants were divided into higher and lower phosphorus intake groups based on the median intake. HPI, higher phosphorus intake; LPI, low phosphorus intake; HR, hazard ratio; CI, confidence interval.
Figure 3 presents the subgroup analyses of the association between dietary phosphorus intake and cardiovascular mortality, stratified by various demographic and clinical characteristics. Higher dietary phosphorus intake was consistently associated with a protective effect across all subgroups, with hazard ratios (HR) below 1. The interaction analysis revealed no significant differences across the subgroups (all p for interaction >0.05), indicating that the association between phosphorus intake and cardiovascular mortality did not vary significantly based on these factors.
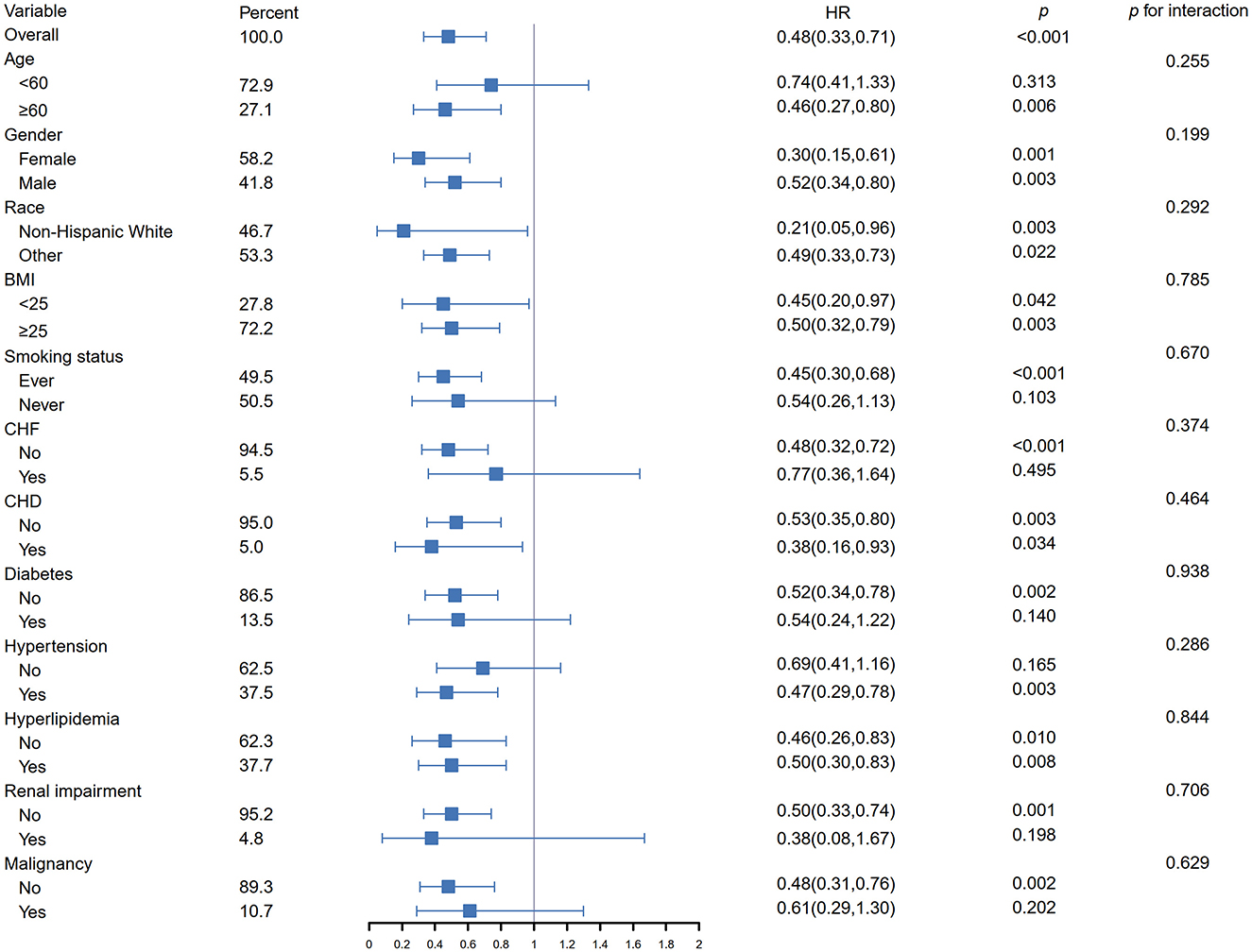
Figure 3. Subgroup analysis of the association between dietary phosphorus intake and cardiovascular mortality in asthma patients. HR, hazard ratio; CI, confidence interval; BMI, body mass index; CHF, chronic heart failure; CHD, coronary heart disease.
The relationship between phosphorus intake and cardiovascular mortality in asthma exhibited an inverted J-shaped curve. The RCS analysis in Model 3 confirmed a nonlinear association (p for nonlinearity = 0.011), indicating a protective effect of phosphorus intake below a specific threshold (see Figure 4). Table 3 shows that a threshold effect was identified for phosphorus intake < 1,861.52 mg/day, where higher phosphorus intake was associated with a significantly reduced risk of cardiovascular mortality (HR: 0.39; 95% CI: 0.16–0.58). However, for phosphorus intake exceeding this threshold, although phosphorus intake appeared to be a harmful factor associated with increased cardiovascular mortality (HR: 1.74; 95% CI: 0.67–4.51), the association was not statistically significant.
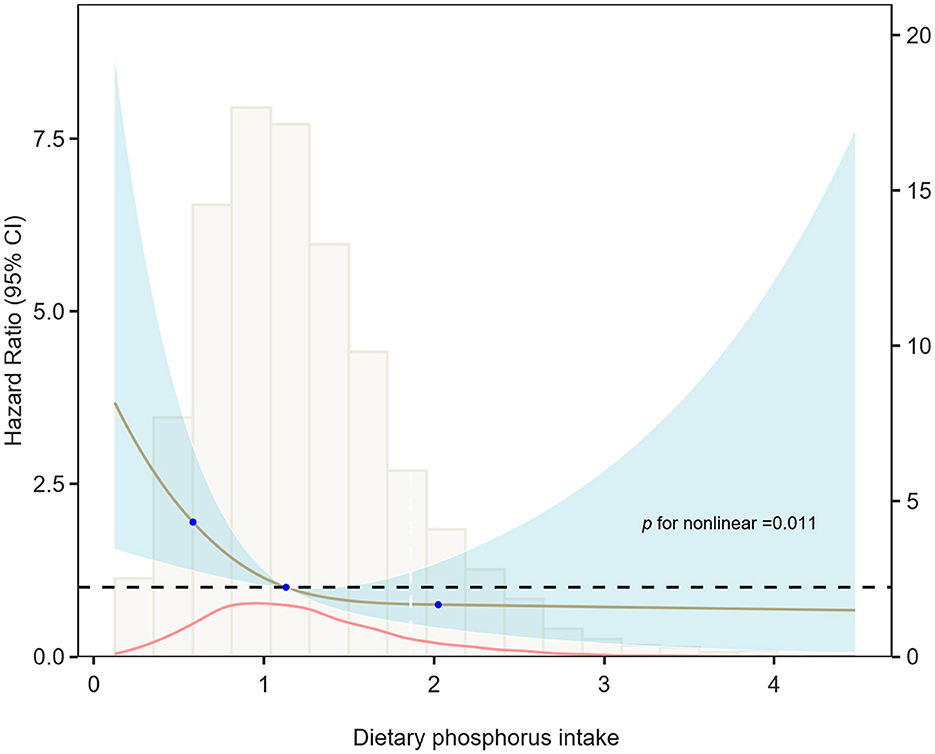
Figure 4. Restricted cubic spline of the relationship between dietary phosphorus intake and cardiovascular mortality risk in Model 3. The relationship between dietary phosphorus intake and cardiovascular mortality risk in asthma patients exhibited an inverted J-shaped curve, with a statistically significant non-linear association (p = 0.011).
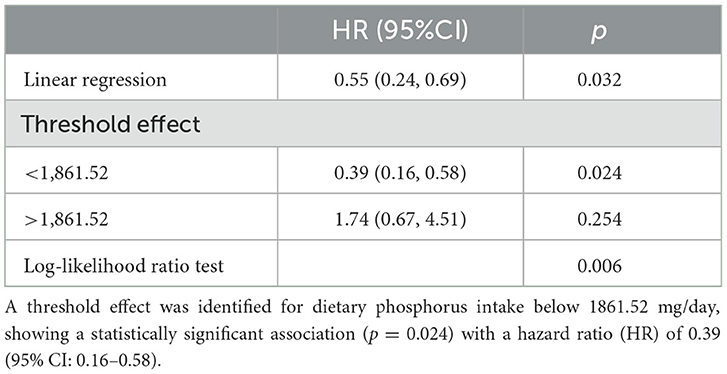
Table 3. The threshold and saturation effect of dietary phosphorus intake on cardiovascular mortality.
Sensitivity analyses were conducted using multiple approaches. Phosphorus intake was analyzed as a continuous variable, a quartile-based categorical variable, and as a continuous variable within quartiles. The relationship between phosphorus intake and mortality was assessed using several adjustment models, including unadjusted, demographic-adjusted, and fully adjusted models. Subgroup analyses were performed by stratifying key covariates. After excluding all participants with missing data, the analyses were repeated, yielding consistent results (as detailed in Supplementary Tables S2 and Supplementary Figures S1–S3). These comprehensive analyses reinforced the robustness and reliability of the findings.
This study found a significant inverse association between dietary phosphorus intake and cardiovascular mortality in asthma patients, likely due to phosphorus's role in modulating inflammation and oxidative stress. Phosphorus helps reduce systemic inflammation by downregulating pro-inflammatory cytokines, such as interleukin-6 and tumor necrosis factor-α, while lowering C-reactive protein (CRP). This effect is particularly beneficial for asthma patients, where chronic inflammation contributes to endothelial dysfunction and increases cardiovascular risk (1, 2, 9). Moreover, phosphorus mitigates oxidative stress by enhancing antioxidant enzymes like superoxide dismutase and glutathione peroxidase, thereby reducing reactive oxygen species (ROS). This effect is especially important for asthma patients on long-term corticosteroid therapy, which can increase oxidative stress (8). Phosphorus metabolism is also crucial for maintaining calcium-phosphorus homeostasis, which is vital for both vascular and skeletal health. In asthma patients, chronic inflammation and corticosteroid use can disrupt this balance, potentially leading to arterial calcification and vascular damage. Adequate phosphorus intake supports stable mineral metabolism, thus reducing cardiovascular risks associated with these imbalances (30, 31). Additionally, natural phosphorus sources, such as whole grains, legumes, and dairy products, offer essential nutrients like fiber, antioxidants, and minerals that promote cardiovascular health (11, 32).
Subgroup analyses consistently demonstrated that dietary phosphorus reduces cardiovascular mortality across different age groups, genders, and comorbidities. These benefits are likely attributable to phosphorus's role in reducing inflammation, enhancing antioxidant activity, and improving endothelial function through nitric oxide synthesis and vascular relaxation (1, 2, 8–10). Furthermore, natural phosphorus sources contribute to better cholesterol metabolism, improved insulin sensitivity, and reduced vascular inflammation, which can benefit individuals with hypertension or metabolic syndrome (11, 32, 33). These findings underscore dietary phosphorus as an important modifiable factor for cardiovascular health.
Although serum phosphorus levels are associated with cardiovascular risk, dietary phosphorus intake does not directly reflect serum phosphorus concentrations. Elevated serum phosphorus is linked to vascular calcification and endothelial dysfunction, both of which contribute to cardiovascular mortality (3, 34). However, dietary phosphorus intake is influenced by the body's regulatory mechanisms and renal function, making it a better indicator of long-term exposure, particularly in non-chronic kidney disease (CKD) populations. A non-linear, inverted J-shaped relationship was observed between dietary phosphorus intake and cardiovascular mortality, with excessive phosphorus intake found to be harmful, consistent with previous studies (11, 35). Importantly, appropriate phosphorus intake may offer a protective effect against cardiovascular mortality, particularly in specific populations such as asthma patients. These results highlight the need for balanced phosphorus intake, especially in populations at risk of cardiovascular disease. Further research is required to determine optimal phosphorus intake levels for cardiovascular protection.
One limitation of this study is the lack of an a priori power analysis due to the predetermined sample size and the complex multistage design of the publicly available NHANES dataset. However, the robustness of the study was supported by several factors. The large sample size increases statistical power, reduces variability, and improves the likelihood of detecting meaningful effects (1, 2). Additionally, the use of NHANES sample weights and stratified sampling adjustments ensured nationally representative estimates, further enhancing the validity of the results (3, 4). Previous studies that have used NHANES data consistently affirm its reliability for epidemiological research (5–7).
Another limitation is that the study could not account for all relevant dietary and lifestyle factors, such as physical activity. The absence of these variables may introduce confounding effects, highlighting the need for further randomized controlled trials (RCTs) to better control for these factors. Future research should aim to provide a comprehensive evaluation of the influence of diet and lifestyle on asthma outcomes, offering more precise and actionable insights for clinical practice.
By utilizing NHANES data, this study benefits from a large, nationally representative sample, which enhances statistical power and reduces random error. The rigorous stratified multistage sampling design, combined with weighting adjustments, ensures that the findings are reflective of the U.S. population's diverse characteristics. These strengths emphasize the need for future large-scale, multicenter, and multinational RCTs to validate these results and provide vital evidence for the development of universally applicable clinical practice guidelines.
Dietary phosphorus intake within an appropriate range is associated with a protective effect against cardiovascular mortality in asthma patients. Future randomized trials and mechanistic studies are needed to confirm these findings and elucidate the underlying pathways.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
The studies involving humans were approved by Institutional Review Board of the National Center for Health Statistics, Centers for Disease Control and Prevention, USA. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
SY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. HC: Data curation, Formal analysis, Investigation, Writing – review & editing. CX: Data curation, Investigation, Validation, Writing – review & editing. NZ: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Fujian Provincial Health Commission Science and Technology Program, China (Grant No. 2024CX01010061).
We gratefully acknowledge the National Health and Nutrition Examination Survey (NHANES) for granting access to publicly available datasets, which were fundamental to the success of this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1533514/full#supplementary-material
1. Chonchol M, Dale R, Schrier RW, Estacio R. Serum phosphorus and cardiovascular mortality in type 2 diabetes. Am J Med. (2009) 122:380–6. doi: 10.1016/j.amjmed.2008.09.039
2. Menon MC, Ix JH. Dietary phosphorus, serum phosphorus, and cardiovascular disease. Ann N Y Acad Sci. (2013) 1301:21–6. doi: 10.1111/nyas.12283
3. Aronson D, Kapeliovich M, Hammerman H, Dragu R. The relation between serum phosphorus levels and clinical outcomes after acute myocardial infarction. PLoS ONE. (2013) 8:e58348. doi: 10.1371/journal.pone.0058348
4. Guilleminault L, Williams EJ, Scott HA, Berthon BS, Jensen M, Wood LG. Diet and asthma: is it time to adapt our message? Nutrients. (2017) 9:1227. doi: 10.3390/nu9111227
5. Peters U., Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. (2018) 141:1169–79. doi: 10.1016/j.jaci.2018.02.004
6. Ketonen J, Merasto S, Paakkari I, Mervaala EM. High sodium intake increases vascular superoxide formation and promotes atherosclerosis in apolipoprotein E-deficient mice. Blood Press. (2005) 14:373–82. doi: 10.1080/08037050500383687
7. Dewan SM, Meem SS, Proma AY, Shahriar M. Dietary salt can be crucial for food-induced vascular inflammation. Clin Pathol. (2024) doi: 10.1177/2632010X241228039
8. Elliott P, Kesteloot H, Appel LJ, Dyer AR, Ueshima H, Chan Q, et al. Dietary phosphorus and blood pressure: international study of macro- and micro-nutrients and blood pressure. Hypertension. (2008) 51:669–75. doi: 10.1161/HYPERTENSIONAHA.107.103747
9. Palomino HL, Rifkin DE, Anderson C, Criqui MH, Whooley MA, Ix JH. 24 h urine phosphorus excretion and mortality and cardiovascular events. Clin J Am Soc Nephrol. (2013) 8:1202–10. doi: 10.2215/CJN.11181012
10. Kalantar–Zadeh K. Patient education for phosphorus management in chronic kidney disease. Patient Prefer Adherence. (2013) 7:379–90. doi: 10.2147/PPA.S43486
11. Chang AR, Lazo M, Appel LJ, Gutierrez OM, Grams ME. High dietary phosphorus intake is associated with all-cause mortality: results from NHANES III. Am J Clin Nutr. (2014) 99:320–7. doi: 10.3945/ajcn.113.073148
12. Gutiérrez OM. The connection between dietary phosphorus, cardiovascular disease, and mortality: where we stand and what we need to know. Adv Nutr. (2013) 4:723–9. doi: 10.3945/an.113.004812
13. Salcedo-Betancourt JD, Moe OW. The effects of acid on calcium and phosphate metabolism. Int J Mol Sci. (2024) 25:2081. doi: 10.3390/ijms25042081
14. Ito J, Lemus H, Wu T. Serum phosphorus, serum bicarbonate, and renal function in relation to liver CYP1A2 activity. Diagnostics. (2023) 13:2996. doi: 10.3390/diagnostics13182996
15. Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat 1. (2013) 1–37.
16. Terry AL, Chiappa MM, McAllister J, Woodwell DA, Graber JE. Plan and Operations of the National Health and Nutrition Examination Survey, August 2021–August 2023. Vital Health Stat 1. (2024) 1–21.
17. Borrud L, Chiappa MM, Burt VL, Gahche J, Zipf G, Johnson CL, et al. National Health and Nutrition Examination Survey: national youth fitness survey plan, operations, and analysis, 2012. Vital Health Stat 2. (2014) 1–24.
18. You J, He Y, Xu M, Qian M. Association between the C–reactive protein to albumin ratio with asthma and mortality in adult: a population–based study. Sci Rep. (2024) 14:20573. doi: 10.1038/s41598-024-71754-z
19. Xu F, Jiang H, Li F, Wen Y, Jiang P, Chen F, Feng Y. Association between the systemic inflammation response index and mortality in the asthma population. Front Med. (2024) 11:1446364. doi: 10.3389/fmed.2024.1446364
20. Gunter EW, McQuillan G. Quality control in planning and operating the laboratory component for the Third National Health and Nutrition Examination Survey. J Nutr. (1990). 11:1451–4. doi: 10.1093/jn/120.suppl_11.1451
21. Yuan M, Zhang Y, Zuo N, Lei H, Zhao X, Xu Y. Association of oxidative balance score with blood pressure, all–cause and cardiovascular disease mortality among hypertensive patients: a prospective study. J Hypertens. (2024) 43:492–503. doi: 10.1097/HJH.0000000000003931
22. Miao X, Li B, Zhu Z, Yang T. Sex differences in the association between composite dietary antioxidant index and hyperlipidemia: Insights from NHANES. PLoS ONE. (2025) 20:e0316130. doi: 10.1371/journal.pone.0316130
23. Zhu H, Yang C, Liu X, Xu X, Chen Q, Fang X, et al. Urinary albumin–to–creatinine ratio as an independent predictor of long–term mortality in atherosclerotic cardiovascular disease patients: A propensity score–matched study: UACR and Long–term Mortality in ASCVD. Am J Prev Cardiol. (2025) 21:100920. doi: 10.1016/j.ajpc.2024.100920
24. Li J, Chen XL, Ou-Yang XL, Zhang XJ, Li Y, Sun SN, et al. Association of tea consumption with all–cause/cardiovascular disease mortality in the chronic kidney disease population: an assessment of participation in the national cohort. Ren Fail. (2025) 47:2449578. doi: 10.1080/0886022X.2025.2449578
25. Ding Q, Hao T, Gao Y, Jiang S, Huang Y, Liang Y. Association between dietary choline intake and asthma and pulmonary inflammation and lung function: NHANES analysis 2009–2018. J Health Popul Nutr. (2024) 43:143. doi: 10.1186/s41043-024-00635-y
26. Stierman B, Afful J, Carroll MD, Chen TC, Davy O, Fink S, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files–Development of Files and Prevalence Estimates for Selected Health Outcomes. Natl Health Stat Report. (2021). 158:10.15620/cdc:106273. doi: 10.15620/cdc:106273
27. Chobufo MD, Singla A, Rahman EU, Michos ED, Whelton PK, Balla S. Temporal trends in atherosclerotic cardiovascular disease risk among U.S. adults. Analysis of the National Health and Nutrition Examination Survey, 1999–2018. Eur J Prev Cardiol. (2022) 29:2289–300. doi: 10.1093/eurjpc/zwac161
28. Ge J, Peng W, Lu J. Predictive value of life's crucial 9 for cardiovascular and all–cause mortality: a prospective cohort study From the NHANES 2007 to 2018. J Am Heart Assoc. (2024) 13:e036669. doi: 10.1161/JAHA.124.036669
29. Andrade C. The p value and statistical significance: misunderstandings, explanations, challenges, and alternatives. Indian J Psychol Med. (2019) 41:210–15. doi: 10.4103/IJPSYM.IJPSYM_193_19
30. Sun M, Wu X, Yu Y, Wang L, Xie D, Zhang Z, et al. Disorders of calcium and phosphorus metabolism and the proteomics/metabolomics–based research. Front Cell Dev Biol. (2020) 8:576110. doi: 10.3389/fcell.2020.576110
31. Papadopoulou A Bountouvi E and Karachaliou FE. The molecular basis of calcium and phosphorus inherited metabolic disorders. Genes. (2021) 12:734. doi: 10.3390/genes12050734
32. Gutiérrez OM, Katz R, Peralta CA, de Boer IH, Siscovick D, Wolf M, et al. Associations of socioeconomic status and processed food intake with serum phosphorus concentration in community–living adults: the Multi–Ethnic Study of Atherosclerosis (MESA). J Ren Nutr. (2012) 22:480–9. doi: 10.1053/j.jrn.2011.08.008
33. Silva AP, Fragoso A, Pinho A, Tavares N, Camacho A, Faísca M, et al. Phosphorus as an early marker of morbidity and mortality in type 2 chronic kidney disease diabetic patients. J Diabetes Complications. (2013) 27:328–32. doi: 10.1016/j.jdiacomp.2013.02.007
34. Bai W, Li J, Liu J. Serum phosphorus, cardiovascular and all–cause mortality in the general population: a meta–analysis. Clin Chim Acta. (2016) 461:76–82. doi: 10.1016/j.cca.2016.07.020
Keywords: dietary phosphorus intake, cardiovascular mortality, asthma, NHANES, nutritional epidemiology
Citation: Yang S, Chen H, Xie C and Zhang N (2025) Protective effect of dietary phosphorus intake on cardiovascular mortality in asthma: evidence from NHANES 1999–2018. Front. Nutr. 12:1533514. doi: 10.3389/fnut.2025.1533514
Received: 24 November 2024; Accepted: 11 February 2025;
Published: 25 February 2025.
Edited by:
Amanda Jane Lloyd, Aberystwyth University, United KingdomReviewed by:
Nagham Nafiz Hendi, Middle East University, JordanCopyright © 2025 Yang, Chen, Xie and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Zhang, emhhbmcubmluZ0B6c3htaG9zcGl0YWwuY29t
†ORCID: Ning Zhang orcid.org/0000-0002-0485-5028
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.