
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 11 February 2025
Sec. Nutrition and Microbes
Volume 12 - 2025 | https://doi.org/10.3389/fnut.2025.1532313
 Lei Wang1†
Lei Wang1† Junjun Wu1†
Junjun Wu1† Ziwen Jiang2
Ziwen Jiang2 Chao Wang3
Chao Wang3 Fuxiang Lin1
Fuxiang Lin1 Yuxiang Zhong1
Yuxiang Zhong1 Pengpeng Zhao1
Pengpeng Zhao1 Wei Wei1
Wei Wei1 Jianhua Huang1
Jianhua Huang1 Zhanping Xu1*
Zhanping Xu1*Background: The dietary index for gut microbiota (DI-GM) reflects dietary patterns that support gut microbial health and may influence kidney stone (KS) risk. The role of DI-GM and its mediation by diabetes in KS pathogenesis remains unclear.
Objective: To investigate the association between DI-GM and KS prevalence, assess the mediation effect of diabetes, and explore subgroup-specific effects and underlying mechanisms.
Methods: A cross-sectional analysis of NHANES (2007–2018) data was conducted using a stratified, multistage probability sampling design. A total of 19,841 participants were included in the final analysis. Data entry and statistical analysis were performed using Empower version 4.2 (X&Y Solutions, Inc., Boston, MA, United States) and R version 3.4.3 (R Foundation). Multivariable logistic regression was employed to assess the association between DI-GM and KS prevalence, with statistical significance set at p < 0.05. Mediation analysis evaluated diabetes’s contribution to this relationship, and subgroup analyses were conducted based on sex, race/ethnicity, and alcohol consumption.
Results: Higher DI-GM scores were associated with lower KS prevalence (adjusted OR: 0.78, 95% CI: 0.65–0.92 per SD increase). Diabetes mediated 9.27% of this relationship. Subgroup analyses revealed stronger protective effects among females, non-Hispanic Black individuals, and heavy drinkers. Mechanistically, DI-GM may reduce KS risk through gut microbial modulation of oxalate metabolism, urinary citrate excretion, and systemic inflammation.
Conclusion and recommendations: Higher DI-GM scores are associated with reduced KS prevalence, partially mediated by diabetes. These findings highlight the role of dietary interventions targeting gut microbiota in KS prevention and call for longitudinal studies to confirm these results and develop tailored dietary strategies.
Kidney stone (KS) is one of the most common urinary system illnesses, with incidence rates increasing worldwide (1–3). The prevalence of KS varies significantly by region, ranging from 1 to 19% in Asia, about 4% in South America, and 5 to 10% in Europe (1, 4). These differences may be linked to dietary habits, genetic factors, and environmental influences that affect KS rates across diverse geographical areas (5). KS can cause severe complications, including intense pain, urinary obstruction, hematuria, and infection, which may lead to renal dysfunction and impair quality of life (6). It also has a high recurrence rate, with nearly 50% of individuals experiencing a relapse (7), which increases the risk of chronic kidney disease and necessitates substantial healthcare resources (8, 9). In the United States, for example, the annual cost of KS treatment exceeds $2 billion, highlighting its considerable economic impact (10).
Globally, non-communicable diseases (NCDs) account for 74% of all deaths annually, according to the World Health Organization (WHO). Conditions such as cardiovascular diseases, cancers, chronic respiratory diseases, and diabetes share common risk factors with KS, including poor dietary habits, obesity, and metabolic dysfunction (11). The Global Burden of Diseases (GBD) study highlights the rising prevalence of KS in middle-and high-SDI (Socio-Demographic Index) countries, emphasizing the role of dietary and metabolic factors (12). In China, a nationwide survey reported a prevalence of 5.8%, with higher rates in rural and southern regions, while in the United States, KS affects approximately 2.4% of the adult population, incurring significant healthcare costs (1, 10).
Dietary factors play a critical role in KS development and recurrence (13), as they directly influence urine composition, pH, and gut microbiota, all of which affect KS formation (14, 15). Gut microbiota imbalances can increase the production of stone-forming components, heightening KS risk. Consequently, optimizing diet to regulate gut microbiota is a promising preventive strategy. In 2016, Kase et al. (16) introduced the dietary index of the gut microbiota (DI-GM), a tool designed to evaluate diet quality in relation to gut health. This index considers key dietary components, including fiber, polyunsaturated fatty acids, and sugars, which either support beneficial bacteria or suppress pathogenic ones. By quantifying the effects of these dietary elements, the DI-GM categorizes them into beneficial and unfavorable groups. The overall score, calculated through a specific formula, reflects the diet’s favorability for gut microbiota, with higher scores indicating a more gut-friendly diet. The validity of this scoring system has been demonstrated through biomarkers such as microbial diversity indices and short-chain fatty acid (SCFA) production, which reliably indicate dietary contributions to gut flora diversity.
Diabetes is another critical factor associated with KS, potentially due to hyperglycemia, metabolic dysregulation, and renal impairment (17, 18). Diabetic patients often exhibit elevated uric acid levels, a known risk factor for chronic kidney disease and KS (19, 20). Urate stones are particularly prevalent in diabetic individuals, likely due to insulin resistance and associated metabolic dysfunction (21). However, the mediating role of diabetes in the relationship between DI-GM and KS remains unclear (22, 23).
This study leverages data from the US National Health and Nutrition Examination Survey (NHANES) (2007–2018) to explore the potential association between DI-GM and KS and examine diabetes as a mediating factor. By investigating the interactions among dietary patterns, gut microbiota, and diabetes, this research aims to provide insights into KS prevention and management strategies while aligning with global health priorities identified by WHO, CDC, and GBD studies.
The data were sourced from NHANES (2007–2018), a nationwide survey representative of the U.S. population. Data were collected through household interviews and mobile examination centers, covering dietary intake, health conditions, and lifestyle information.
The source population comprised participants from the NHANES database, which aims to assess the health and nutritional status of individuals across various age groups and ethnicities in the United States.
Based on inclusion and exclusion criteria, a total of 19,841 eligible participants were included in the final analysis: exclusions included pregnant women, individuals with missing data (e.g., poverty income ratio, alcohol consumption, and BMI), and those with unclear KS status.
The dependent variable in this study is the presence of kidney stones (KS), determined based on participants’ responses to the question, “Has a doctor ever diagnosed you with kidney stones?” A “yes” response indicates the presence of KS, while a “no” response indicates its absence.
The DI-GM score is based on the intake of 14 specific foods or nutrients. Avocado, broccoli, chickpeas, coffee, cranberries, fermented dairy products, dietary fiber, green tea, soy, and whole grains are classified as beneficial components, while red meat, processed meat, refined grains, and high-fat diets (≥40% of energy from fat) are considered detrimental components (9). Dietary components are categorized based on sex-specific medians: for beneficial components, intake equal to or above the median scores 1 point, while intake below the median scores 0 points; for detrimental components, intake below the median scores 1 point, while intake equal to or above the median scores 0 points. The total score is the sum of all component scores, ranging from 0 to 13, with higher scores indicating dietary patterns more favorable to gut microbiota health (16).
To control for confounding effects, we included a range of covariates in the analysis. Demographic covariates included age (as a continuous variable in years), gender (male or female), and race/ethnicity (Non-Hispanic White, Non-Hispanic Black, Mexican American, and other racial/ethnic groups). Socioeconomic covariates included the poverty income ratio (PIR, a continuous variable), educational level (categorized as less than high school, high school or GED, and above high school), and marital status (married/living with partner, single, or never married). Lifestyle factors included smoking status (categorized as never smoker, former smoker, and current smoker) and alcohol consumption (categorized as never drinker, low drinker, and heavy drinker). Health-related covariates included hypertension status (yes or no), body mass index (BMI, as a continuous variable in kg/m2), serum calcium level (as a continuous variable in mg/dL), and estimated glomerular filtration rate (eGFR, as a continuous variable in mL/min/1.73 m2). Additionally, diabetes status was included as a clinical covariate, categorized as diabetic, prediabetic, or non-diabetic. These covariates were selected based on their established association with kidney stone formation and gut microbiota health and were included in the multivariable models to minimize confounding bias.
In this study, diabetes was defined by the following criteria: fasting plasma glucose (FPG) ≥7.0 mmol/L (126 mg/dL) or HbA1c ≥6.5% (24). Additionally, the response to the question “Has a doctor ever diagnosed you with diabetes?” was used to confirm diabetes status, with “yes” indicating diabetes and “no” indicating non-diabetes. In Table 1, we categorized diabetes status into three groups: “diabetic,” “prediabetic,” and “non-diabetic.” For statistical analyses in other figures, the “prediabetic” and “non-diabetic” groups were combined to facilitate the assessment of the relationship between diabetes, DI-GM index, and KS.
Participants with complete records in the NHANES database and valid KS status and DI-GM scores.
Individuals with unknown KS status or incomplete data (e.g., missing BMI or PIR).
NHANES employed a stratified, multistage probability sampling design to ensure data representativeness and accuracy. The final sample size comprised 19,841 participants.
Data collection was conducted through standardized questionnaires (household interviews) and physical examinations at mobile examination centers. Dietary data were collected using 24-h dietary recall and were used to calculate DI-GM scores.
Data were collected by NHANES staff and subjected to quality control procedures to ensure accuracy. All measurements were calibrated and validated using standardized protocols.
Statistical analyses in this study followed CDC guidelines. Continuous variables are expressed as mean ± standard deviation and categorical variables as percentages. Normal data were analyzed by ANOVA, non-normal data by the Kruskal–Wallis test, and categorical data by chi-square test. A multivariable logistic regression model evaluated the association between DI-GM and KS, providing odds ratio (OR) with 95% confidence intervals (95% CI). Model 1 had no covariate adjustments; Model 2 adjusted for sex, age, and race; Model 3 further adjusted for education, marital status, smoking, drinking, hypertension, PIR, calcium, eGFR, and BMI. Subgroup analyses used multivariable regression, and mediation analysis assessed diabetes as a mediator for DI-GM and KS. A p-value <0.05 was considered significant. Analyses were conducted using Empower version 4.2 (X&Y Solutions, Inc., Boston, MA, United States) and R version 3.4.3 (R Foundation) (see Figure 1).
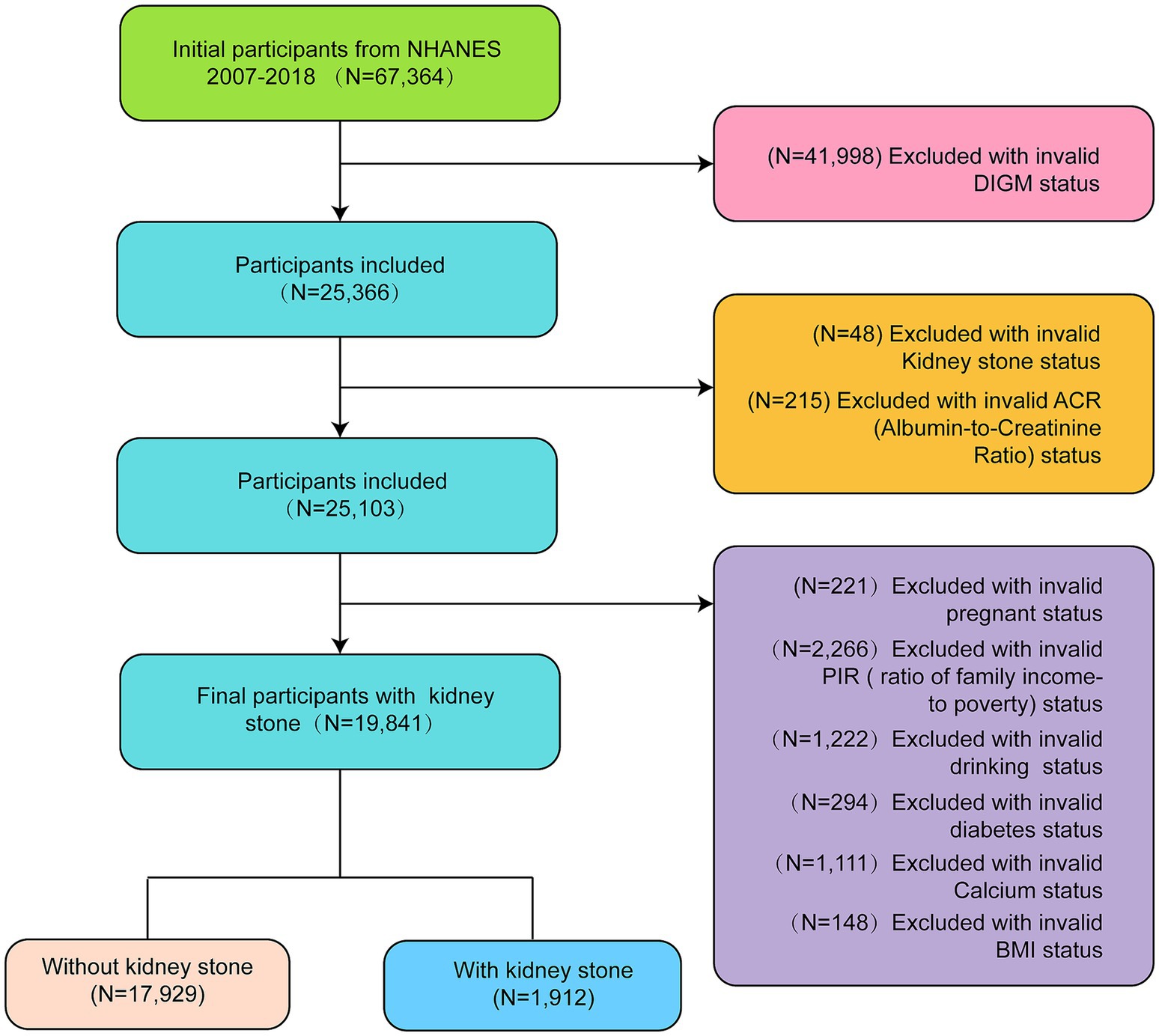
Figure 1. Flowchart of the selection of participants from NHANES 2007–2018 based on DI-GM (dietary index for gut microbiota).
All NHANES data were approved by the NCHS Research Ethics Review Board, and all participants signed informed consent forms. The data are publicly available and do not involve personal privacy or additional ethical approval.
Of the 19,841 participants analyzed, 1,912 (9.64%) were identified with KS. A distinct profile emerged for those with KS compared to those without. Males were more frequently affected (55.23%) than females, with significant racial differences observed, as the prevalence of KS was higher among non-Hispanic Black individuals. Marital status was also associated with KS presence, with a larger proportion of affected participants being married or living with a partner. Regarding health conditions, those with KS had higher rates of diabetes mellitus (25.99%) and hypertension (49.79%). Lifestyle factors also varied, with a higher proportion of KS patients being current smokers (20.55%) and heavy drinkers (59.78%). Clinically, participants with KS were older on average (55.12 ± 16.24 years) and had a higher mean BMI (30.57 ± 6.87 kg/m2) compared to those without KS. Moreover, mean serum calcium levels were lower, while estimated glomerular filtration rates (eGFR) were also reduced among participants with KS (93.74 ± 16.27 mL/min/1.73 m2). Analysis of dietary indices related to gut microbiota revealed a slightly lower mean score in the “beneficial to gut microbiota” category among those with KS (2.23 ± 1.41), though no significant differences were found in the “Unbeneficial to gut microbiota” category (see Table 1).
Table 2 revealed a statistically significant association between the DI-GM and KS prevalence, even after adjusting for various confounding factors. Participants with a DI-GM score of 6 or higher had a 17% lower prevalence of KS compared to those with a score of 0–3 (Model 3: OR = 0.83, 95% CI: 0.72–0.95, p = 0.007), with a significant trend of decreasing KS risk as DI-GM scores increased (p-trend <0.001). The “beneficial to gut microbiota” component of DI-GM was also independently associated with a lower KS risk (Model 3: OR = 0.94, 95% CI: 0.90–0.97, p < 0.001). Additionally, the analysis indicated that diabetes was associated with a higher prevalence of KS, with diabetic individuals having a 46% higher likelihood of developing KS compared to non-diabetic participants (Model 3: OR = 1.46, 95% CI: 1.29–1.65, p < 0.001).
Table 3 shows that both the “beneficial to gut microbiota” and “unfavorable to gut microbiota” components of the DI-GM were significantly associated with diabetes prevalence. Specifically, the “beneficial to gut microbiota” component showed a borderline association with reduced diabetes risk after full adjustment for confounding factors (Model 3: OR = 0.97, 95% CI: 0.94–1.00, p = 0.0285). The “unfavorable to gut microbiota” component demonstrated a stronger inverse association, where a higher score was associated with a significantly lower diabetes prevalence (Model 3: OR = 0.90, 95% CI: 0.86–0.93, p < 0.0001).
The results of the RCS demonstrated a nonlinear correlation between DI-GM and the prevalence of KS (Figure 2).
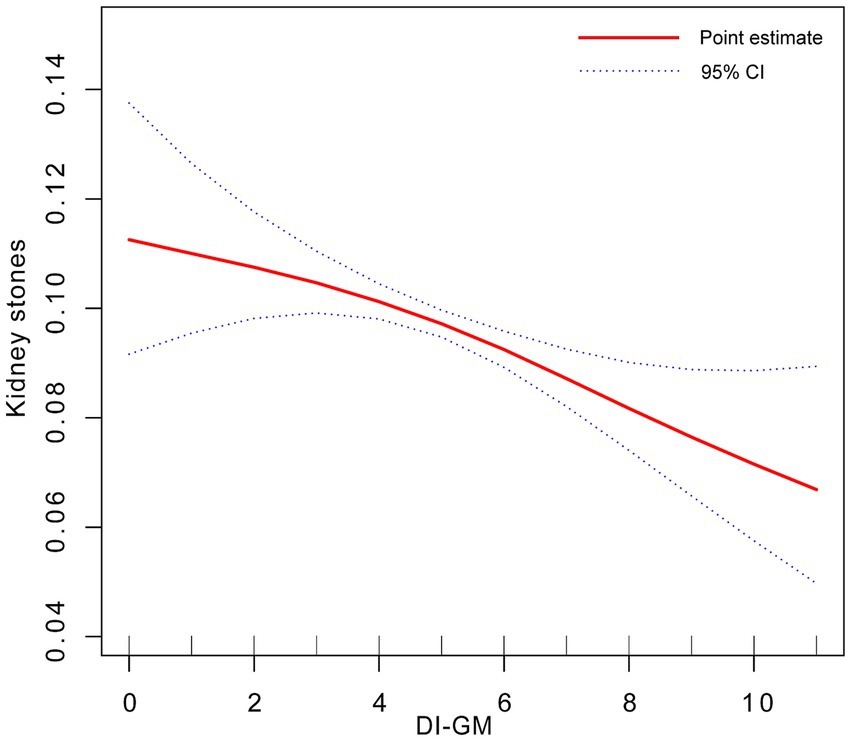
Figure 2. Dose-response relationship between DI-GM and KS. Adjusted for gender + race + age + education level + marital status + smoking status + drinking status + hypertension + PIR + serum calcium + eGRF + BMI.
Figure 3 presents a subgroup analysis of the association between DI-GM scores and KS prevalence across various demographic and health-related factors. The analysis shows that the protective association between DI-GM and KS is consistent across most subgroups, including age, sex, race, education, marital status, BMI, hypertension status, serum calcium levels, and eGFR categories (p for interaction >0.05). Drinking status is the only factor that significantly modified the association (p = 0.004). Among heavy drinkers, a higher DI-GM score was associated with a reduced risk of KS (OR = 0.92, 95% CI: 0.88–0.95), while no significant association was observed between never-drinkers or moderate-drinkers.
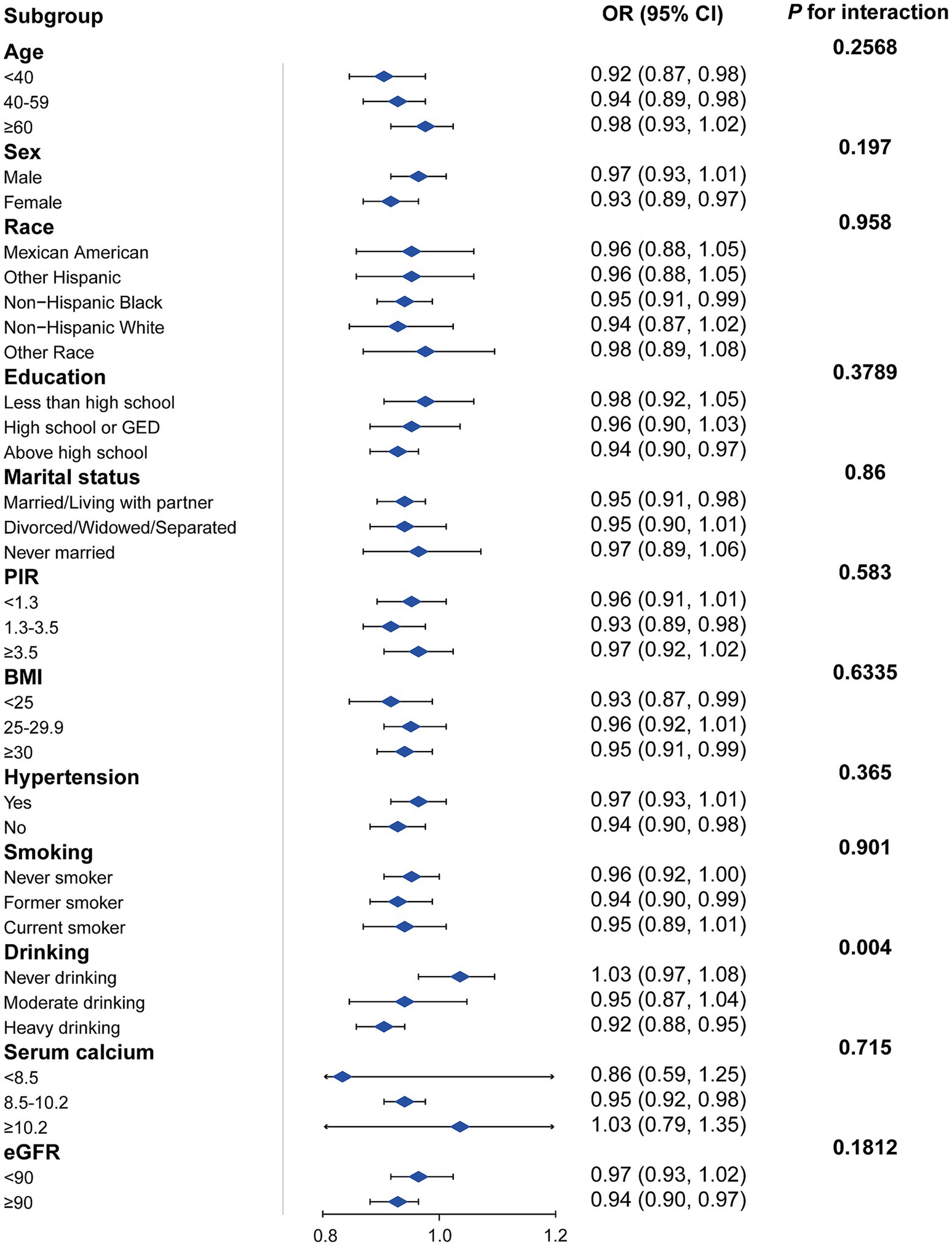
Figure 3. Subgroup analysis of the association between DI-GM and KS. Adjusted for age + sex + race + education + marital status + poverty income ratio + hypertension + BMI + smoking status+ drinking + serum calcium + eGFR. The strata variable was not included when stratifying by itself. PIR, poverty income ratio; eGRF, estimated glomerular filtration rates; DI-GM, dietary index for gut microbiota, BMI, body mass index.
The outcome of the mediation analysis shows that diabetes partially mediates the relationship between DI-GM and KS prevalence. In Model 3, the total effect of DI-GM on KS was significant (Estimate = −0.009489, p < 0.0001). The mediation effect through diabetes was also significant (Estimate = −0.000903, p = 0.0020), accounting for 9.27% of the total effect. The direct effect of DI-GM remained significant after adjusting for diabetes (Estimate = −0.008633, p < 0.0001) (see Table 4 and Figure 4).
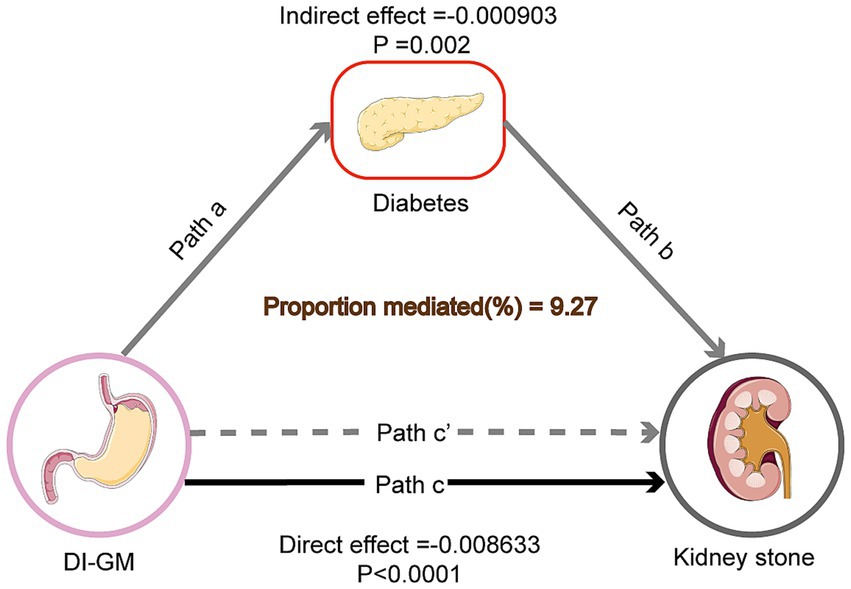
Figure 4. Mediation model of the effect of DI-GM on KS risk through diabetes. Solid arrows represent direct effects, while dashed arrows represent indirect effects through diabetes. Path coefficients (β) are shown along each arrow, indicating the strength of the relationship. DI-GM refers to the dietary index for gut microbiota, diabetes is the mediator, and KS risk is the outcome. The mediation effect of diabetes accounts for 9.27% of the total effect of DI-GM on KS risk.
This study, based on NHANES data (2007–2018), provides robust evidence linking the DI-GM to KS prevalence. Higher DI-GM scores, reflective of diets rich in fiber, whole grains, and fermented foods, were associated with a reduced risk of KS, even after adjusting for multiple confounders. Furthermore, diabetes was found to partially mediate this relationship, accounting for 9.27% of the total effect. These findings emphasize the critical role of dietary patterns that support gut microbiota health in preventing KS, highlighting an avenue for non-invasive, dietary-based preventive strategies.
This study contributes to a growing body of research exploring the role of gut microbiota in KS pathogenesis. Prior studies have highlighted the role of specific microbial taxa, such as Oxalobacter formigenes, in degrading oxalates, reducing urinary oxalate excretion, and lowering the risk of calcium oxalate stones (25). Our findings align with this mechanism but extend the scope by demonstrating the impact of overall dietary patterns on gut microbial functionality. Furthermore, while previous research has identified reduced urinary citrate as a contributing factor to KS formation, this study links gut microbiota dysbiosis, such as an increased abundance of Escherichia-Shigella, to lower citrate levels, suggesting a novel pathway connecting microbiota composition with lithogenesis (26). Unlike studies that focus on single microbial or dietary factors, this research underscores the cumulative impact of diet-induced microbial shifts on KS risk (27, 28).
The protective effect of DI-GM appeared more pronounced in subgroups such as females, non-Hispanic Black individuals, and heavy drinkers. Among heavy drinkers, the strong inverse association suggests that dietary components beneficial to gut microbiota may mitigate the adverse metabolic effects of alcohol on the kidneys. Alcohol consumption is known to disrupt gut microbiota balance, increasing intestinal permeability and systemic inflammation (29, 30). Diets rich in fiber and polyphenols, reflected in higher DI-GM scores, may counteract these disruptions by enhancing microbial diversity and short-chain fatty acid (SCFA) production, which have anti-inflammatory and renal-protective effects (31, 32). However, the absence of significant effects in non-drinkers and moderate drinkers highlights the complexity of interactions between alcohol, diet, and gut microbiota. These findings align with prior studies on alcohol’s effects on gut health and suggest the need for further investigation into subgroup-specific dietary interventions (33).
Gut microbiota impacts KS formation through several pathways. First, oxalate metabolism remains a central mechanism, as oxalate-degrading bacteria, such as Oxalobacter formigenes, reduce hyperoxaluria by breaking down dietary oxalate in the gut and limiting its intestinal absorption, which is a key risk factor for calcium oxalate stones (34, 35). Loss of these bacteria due to dysbiosis or antibiotic use is associated with increased urinary oxalate excretion and higher KS risk (36, 37). Second, microbial metabolites, such as short-chain fatty acids (SCFAs), including butyrate and acetate, not only improve gut barrier function but also modulate systemic inflammation and oxidative stress, both of which are critical in maintaining renal health and reducing stone risk (31, 38). Moreover, SCFAs help regulate metabolic pathways related to citrate excretion and urinary pH, creating a less favorable environment for stone formation (39). Lastly, gut dysbiosis promotes systemic inflammation by increasing gut permeability and endotoxin levels, such as lipopolysaccharides (LPS), impairing renal function and exacerbating metabolic conditions (22, 40). These mechanisms highlight the potential of dietary patterns that enhance gut microbial diversity and functionality to protect against KS.
The mediation analysis revealed that diabetes accounted for 9.27% of the total effect of DI-GM on KS risk. While this proportion is modest, it underscores the interconnected roles of diet, gut microbiota, and metabolic health. Diabetes exacerbates lithogenesis through hyperglycemia-induced increases in urinary calcium and reduced citrate excretion (41, 42). These mechanisms create a urinary environment conducive to stone formation, with higher calcium concentrations promoting calcium oxalate crystal aggregation and reduced citrate levels decreasing the inhibition of such crystallization. Furthermore, diabetic patients often exhibit changes in urinary pH due to impaired renal ammoniagenesis, which may further contribute to stone formation by facilitating uric acid stone precipitation (43).
In addition to metabolic alterations, diabetic patients have distinct gut microbial profiles characterized by reduced microbial diversity and an increased abundance of pro-inflammatory taxa, which exacerbate systemic inflammation and oxidative stress (44, 45). This gut dysbiosis has downstream effects on metabolic pathways relevant to kidney stone formation, such as increased intestinal permeability and the translocation of microbial endotoxins like lipopolysaccharides (LPS) into circulation. LPS triggers systemic inflammation, which has been linked to renal tubular damage, altered calcium handling, and increased oxalate excretion, thereby enhancing lithogenesis. Moreover, inflammation may directly disrupt gut-kidney axis interactions, highlighting the multifaceted role of gut microbiota in kidney stone risk (46, 47).
These findings align with prior studies demonstrating that high-fiber, low-sugar diets support beneficial bacterial taxa, such as Bifidobacterium and Lactobacillus, which improve insulin sensitivity and reduce urinary calcium excretion (21, 48). Additionally, such dietary patterns promote the production of short-chain fatty acids (SCFAs), such as butyrate, which not only improve gut barrier integrity but also have anti-inflammatory properties that may mitigate the systemic effects of diabetes on kidney stone formation. These dietary influences extend beyond diabetes itself to directly impact kidney stone risk through gut microbiota-mediated pathways (49, 50).
The direct effect of DI-GM on kidney stones, which constitutes a larger proportion of the total effect, highlights the importance of dietary quality in directly modulating gut microbiota composition and kidney stone risk, independent of diabetes.
This study’s strengths include its use of a large, nationally representative dataset and robust statistical analyses, which enhance its reliability and generalizability within the U.S. population. However, several limitations must be acknowledged. First, while the DI-GM is a validated tool for assessing the impact of diet on gut microbiota, it provides an indirect estimation rather than a direct measurement of microbial composition, highlighting the need for future studies to incorporate microbiome sequencing technologies. Second, the NHANES dataset lacks detailed information on specific kidney stone types (e.g., calcium oxalate, uric acid stones), comprehensive antibiotic use data (e.g., frequency, duration, and type), and urinary factors critical to kidney stone risks, such as oxalate and citrate levels. These urinary metabolites play essential roles in kidney stone formation: oxalate is a key component of calcium oxalate stones, while citrate acts as an inhibitor of crystal aggregation. The absence of such data limits the ability to fully assess the mechanistic pathways linking diet, gut microbiota, and kidney stone risk. Antibiotic use, in particular, is known to disrupt gut microbial taxa, including oxalate-degrading bacteria, potentially influencing stone risk. Lastly, while diabetes was included as a mediating variable, future research should explore additional mediators, such as systemic inflammation, oxalate metabolism, and other urinary factors, to better understand the complex pathways linking diet, gut microbiota, and kidney stones. Future studies should also examine diverse populations and leverage multi-omics approaches to provide a more nuanced understanding of these relationships.
Higher DI-GM scores, reflecting diets rich in fiber, whole grains, fruits, vegetables, and fermented foods, are associated with a reduced risk of KS, partially mediated by diabetes. These findings highlight the importance of dietary strategies targeting gut microbiota health in KS prevention. Specifically, increasing the intake of fiber-rich foods (e.g., legumes, whole grains, leafy greens), reducing added sugars and processed foods, and incorporating probiotics or fermented foods (e.g., yogurt, kefir, sauerkraut) may enhance gut microbial diversity and functionality (51). For high-risk populations, such as individuals with diabetes or recurrent KS, targeted interventions like personalized nutrition plans and supplementation with oxalate-degrading probiotics (Oxalobacter formigenes) warrant further exploration (52). Future research should focus on developing practical dietary recommendations and microbial therapies to address KS risk effectively.
This study provides compelling evidence that dietary patterns promoting gut microbiota health, as reflected by higher DI-GM scores, are associated with a reduced risk of KS. Compared to prior research focusing on individual dietary components or microbial taxa, this study emphasizes the broader impact of cumulative dietary patterns on gut microbiota and metabolic health. These findings suggest that promoting diets aligned with higher DI-GM scores could serve as a cost-effective, non-invasive strategy to prevent KS, particularly in populations at high risk for diabetes and metabolic disorders. Future research should focus on translating these findings into practical dietary recommendations and developing targeted interventions to address this significant public health challenge.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the National Center for Health Statistics (NCHS). The study uses publicly available data from the National Health and Nutrition Examination Survey (NHANES), which is conducted by the National Center for Health Statistics (NCHS). All NHANES protocols were reviewed and approved by the NCHS Research Ethics Review Board (ERB), and informed consent was obtained from all participants. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
LW: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. JW: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. ZJ: Formal analysis, Writing – review & editing. CW: Visualization, Writing – original draft. FL: Data curation, Investigation, Writing – review & editing. YZ: Data curation, Investigation, Writing – review & editing. PZ: Validation, Writing – review & editing. WW: Validation, Writing – review & editing. JH: Project administration, Writing – review & editing. ZX: Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the 2025 Annual Guangdong Provincial Administration of Traditional Chinese Medicine Scientific Research Project (NO. 20252043).
Our team would like to thank all the staff and all the subjects for their participation in the NHANES data collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
NHANES, National Health and Nutrition Examination Survey; KS, Kidney stone; DI-GM, Dietary index for gut microbiota; OR, Odds ratio; 95% CI, 95% confidence interval.
1. Zeng, G, Mai, Z, Xia, S, Wang, Z, Zhang, K, Wang, L, et al. Prevalence of kidney stones in China: an ultrasonography based cross-sectional study. BJU Int. (2017) 120:109–16. doi: 10.1111/bju.13828
2. Wang, W, Fan, J, Huang, G, Li, J, Zhu, X, Tian, Y, et al. Prevalence of kidney stones in mainland China: a systematic review. Sci Rep. (2017) 7:41630. doi: 10.1038/srep41630
3. Scales, CD, Smith, AC, Hanley, JM, and Saigal, CS. Prevalence of kidney stones in the United States. Eur Urol. (2012) 62:160–5. doi: 10.1016/j.eururo.2012.03.052
4. López, M, and Hoppe, B. History, epidemiology and regional diversities of urolithiasis. Pediatr Nephrol. (2010) 25:49–59. doi: 10.1007/s00467-008-0960-5
5. Konjengbam, H, and Meitei, SY. Association of kidney stone disease with dietary factors: a review. Anthropol Rev. (2020) 83:65–73. doi: 10.2478/anre-2020-0005
6. Sigurjonsdottir, VK, Runolfsdottir, HL, Indridason, OS, Palsson, R, and Edvardsson, VO. Impact of nephrolithiasis on kidney function. BMC Nephrol. (2015) 16:149. doi: 10.1186/s12882-015-0126-1
7. Wang, K, Ge, J, Han, W, Wang, D, Zhao, Y, Shen, Y, et al. Risk factors for kidney stone disease recurrence: a comprehensive meta-analysis. BMC Urol. (2022) 22:62. doi: 10.1186/s12894-022-01017-4
8. El-Zoghby, ZM, Lieske, JC, Foley, RN, Bergstralh, EJ, Li, X, Melton, LJI, et al. Urolithiasis and the risk of ESRD. Clin J Am Soc Nephrol. (2012) 7:1409–15. doi: 10.2215/CJN.03210312
9. Antonelli, JA, Maalouf, NM, Pearle, MS, and Lotan, Y. Use of the National Health and Nutrition Examination Survey to calculate the impact of obesity and diabetes on cost and prevalence of urolithiasis in 2030. Eur Urol. (2014) 66:724–9. doi: 10.1016/j.eururo.2014.06.036
10. Hill, AJ, Basourakos, SP, Lewicki, P, Wu, X, Arenas-Gallo, C, Chuang, D, et al. Incidence of kidney stones in the United States: the continuous National Health and Nutrition Examination Survey. J Urol. (2022) 207:851–6. doi: 10.1097/JU.0000000000002331
11. Singh, P, Harris, PC, Sas, DJ, and Lieske, JC. The genetics of kidney stone disease and nephrocalcinosis. Nat Rev Nephrol. (2022) 18:224–40. doi: 10.1038/s41581-021-00513-4
12. Vos, T, Lim, SS, Abbafati, C, Abbas, KM, Abbasi, M, Abbasifard, M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
13. Mao, W, Zhang, H, Xu, Z, Geng, J, Zhang, Z, Wu, J, et al. Relationship between urine specific gravity and the prevalence rate of kidney stone. Transl Androl Urol. (2021) 10:184–18194. doi: 10.21037/tau-20-929
14. Lemberger, U, Pjevac, P, Hausmann, B, Berry, D, Moser, D, Jahrreis, V, et al. The microbiome of kidney stones and urine of patients with nephrolithiasis. Urolithiasis. (2023) 51:27. doi: 10.1007/s00240-022-01403-5
15. Ferraro, PM, Taylor, EN, Gambaro, G, and Curhan, GC. Dietary and lifestyle risk factors associated with incident kidney stones in men and women. J Urol. (2017) 198:858–63. doi: 10.1016/j.juro.2017.03.124
16. Kase, BE, Liese, AD, Zhang, J, Murphy, EA, Zhao, L, and Steck, SE. The development and evaluation of a literature-based dietary index for gut microbiota. Nutrients. (2024) 16:1045. doi: 10.3390/nu16071045
17. Weiner, DE, Tighiouart, H, Elsayed, EF, Griffith, JL, Salem, DN, and Levey, AS. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. (2008) 19:1204–11. doi: 10.1681/ASN.2007101075
18. Obermayr, RP, Temml, C, Gutjahr, G, Knechtelsdorfer, M, Oberbauer, R, and Klauser-Braun, R. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol. (2008) 19:2407–13. doi: 10.1681/ASN.2008010080
19. Rule, AD, Bergstralh, EJ, Melton, LJI, Li, X, Weaver, AL, and Lieske, JC. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol. (2009) 4:804–11. doi: 10.2215/CJN.05811108
20. Saucier, NA, Sinha, MK, Liang, KV, Krambeck, AE, Weaver, AL, Bergstralh, EJ, et al. Risk factors for CKD in persons with kidney stones: a case-control study in Olmsted County, Minnesota. Am J Kidney Dis. (2010) 55:61–8. doi: 10.1053/j.ajkd.2009.08.008
21. Akoudad, S, Szklo, M, MA, MA, Fulop, T, Anderson, CA, Coresh, J, et al. Correlates of kidney stone disease differ by race in a multi-ethnic middle-aged population: the ARIC study. Prev Med. (2010) 51:416–20. doi: 10.1016/j.ypmed.2010.08.011
22. Larsen, N, Vogensen, FK, van den Berg, FWJ, Nielsen, DS, Andreasen, AS, Pedersen, BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. (2010) 5:e9085. doi: 10.1371/journal.pone.0009085
23. Sanz, Y, Olivares, M, Moya-Pérez, Á, and Agostoni, C. Understanding the role of gut microbiome in metabolic disease risk. Pediatr Res. (2015) 77:236–44. doi: 10.1038/pr.2014.170
24. International Diabetes Federation Guideline Development Group. Global guideline for type 2 diabetes. Diabetes Res Clin Pract. (2014) 104:1–52. doi: 10.1016/j.diabres.2012.10.001
25. Mehta, M, Goldfarb, DS, and Nazzal, L. The role of the microbiome in kidney stone formation. Int J Surg. (2016) 36:607–12. doi: 10.1016/j.ijsu.2016.11.024
26. Ticinesi, A, Nouvenne, A, Chiussi, G, Castaldo, G, Guerra, A, and Meschi, T. Calcium oxalate nephrolithiasis and gut microbiota: not just a gut-kidney axis. A nutritional perspective. Nutrients. (2020) 12:548. doi: 10.3390/nu12020548
27. Sorensen, MD, Hsi, RS, Chi, T, Shara, N, Wactawski-Wende, J, Kahn, AJ, et al. Dietary intake of fiber, fruit and vegetables decreases the risk of incident kidney stones in women: a women’s health initiative report. J Urol. (2014) 192:1694–9. doi: 10.1016/j.juro.2014.05.086
28. Gupta, SK, Baghel, MS, Bhuyan, C, Ravishankar, B, Ashok, BK, and Patil, PD. Evaluation of anti-urolithiatic activity of Pashanabhedadi Ghrita against experimentally induced renal calculi in rats. Ayu. (2012) 33:429–34. doi: 10.4103/0974-8520.108860
29. Gill, VJS, Soni, S, Shringarpure, M, Anusheel, BS, Yadav, NK, Patel, A, et al. Gut microbiota interventions for the management of obesity: a literature review. Cureus. (2022) 14:e29317. doi: 10.7759/cureus.29317
30. Meyer, KA, and Bennett, BJ. Diet and gut microbial function in metabolic and cardiovascular disease risk. Curr Diab Rep. (2016) 16:93. doi: 10.1007/s11892-016-0791-x
31. Qin, Z, Zhao, J, Geng, J, Chang, K, Liao, R, and Su, B. Higher triglyceride-glucose index is associated with increased likelihood of kidney stones. Front Endocrinol. (2021) 12:774567. doi: 10.3389/fendo.2021.774567
32. Duan, Q, Huang, H, Zhang, S, Wang, Y, Lu, D, Wan, L, et al. Association between composite dietary antioxidant index and kidney stone prevalence in adults: data from National Health and Nutrition Examination Survey (NHANES, 2007–2018). Front Nutr. (2024) 11:1389714. doi: 10.3389/fnut.2024.1389714
33. Schneider, KM, Mohs, A, Kilic, K, Candels, LS, Elfers, C, Bennek, E, et al. Intestinal microbiota protects against MCD diet-induced steatohepatitis. Int J Mol Sci. (2019) 20:308. doi: 10.3390/ijms20020308
34. Tavasoli, S, Alebouyeh, M, Naji, M, Majd, GS, Nashtaei, MS, Broumandnia, N, et al. Association of intestinal oxalate-degrading bacteria with recurrent calcium kidney stone formation and hyperoxaluria: a case-control study. BJU Int. (2020) 125:133–43. doi: 10.1111/bju.14840
35. Nazzal, L, Francois, F, Henderson, N, Liu, M, Li, H, Koh, H, et al. Effect of antibiotic treatment on Oxalobacter formigenes colonization of the gut microbiome and urinary oxalate excretion. Sci Rep. (2021) 11:16428. doi: 10.1038/s41598-021-95992-7
36. Suryavanshi, MV, Bhute, SS, Jadhav, SD, Bhatia, MS, Gune, RP, and Shouche, YS. Hyperoxaluria leads to dysbiosis and drives selective enrichment of oxalate metabolizing bacterial species in recurrent kidney stone endures. Sci Rep. (2016) 6:34712. doi: 10.1038/srep34712
37. Xie, J, Huang, J, Huang, X, Peng, J, Yu, Z, Yuan, Y, et al. Profiling the urinary microbiome in men with calcium-based kidney stones. BMC Microbiol. (2020) 20:41. doi: 10.1186/s12866-020-01734-6
38. Denburg, MR, Koepsell, K, Lee, J-J, Gerber, J, Bittinger, K, and Tasian, GE. Perturbations of the gut microbiome and metabolome in children with calcium oxalate kidney stone disease. J Am Soc Nephrol. (2020) 31:1358–69. doi: 10.1681/ASN.2019101131
39. Ticinesi, A, Milani, C, Guerra, A, Allegri, F, Lauretani, F, Nouvenne, A, et al. Understanding the gut-kidney axis in nephrolithiasis: an analysis of the gut microbiota composition and functionality of stone formers. Gut. (2018) 67:2097–106. doi: 10.1136/gutjnl-2017-315734
40. Kanbay, M, Onal, EM, Afsar, B, Dagel, T, Yerlikaya, A, Covic, A, et al. The crosstalk of gut microbiota and chronic kidney disease: role of inflammation, proteinuria, hypertension, and diabetes mellitus. Int Urol Nephrol. (2018) 50:1453–66. doi: 10.1007/s11255-018-1873-2
41. Ping, H, Lu, N, Wang, M, Lu, J, Liu, Y, Qiao, L, et al. New-onset metabolic risk factors and the incidence of kidney stones: a prospective cohort study. BJU Int. (2019) 124:1028–33. doi: 10.1111/bju.14805
42. Khalili, P, Jamali, Z, Sadeghi, T, Esmaeili-nadimi, A, Mohamadi, M, Moghadam-Ahmadi, A, et al. Risk factors of kidney stone disease: a cross-sectional study in the southeast of Iran. BMC Urol. (2021) 21:141. doi: 10.1186/s12894-021-00905-5
43. Maalouf, NM, Cameron, MA, Moe, OW, and Sakhaee, K. Metabolic basis for low urine pH in type 2 diabetes. Clin J Am Soc Nephrol. (2010) 5:1277–81. doi: 10.2215/CJN.08331109
44. Gao, H, Lin, J, Xiong, F, Yu, Z, Pan, S, and Huang, Y. Urinary microbial and metabolomic profiles in kidney stone disease. Front Cell Infect Microbiol. (2022) 12:953392. doi: 10.3389/fcimb.2022.953392
45. Chen, L, Zhang, J, Shen, K, Zhu, Y, Zhang, J, Pan, J, et al. Kidney stones are associated with metabolic syndrome in a health screening population: a cross-sectional study. Transl Androl Urol. (2023) 12:967–96976. doi: 10.21037/tau-23-51
46. Huazano-García, A, Silva-Adame, MB, Vázquez-Martínez, J, Gastelum-Arellanez, A, Sánchez-Segura, L, and López, MG. Highly branched neo-fructans (agavins) attenuate metabolic endotoxemia and low-grade inflammation in association with gut microbiota modulation on high-fat diet-fed mice. Foods. (2020) 9:1792. doi: 10.3390/foods9121792
47. Tokuhara, D. Role of the gut microbiota in regulating non-alcoholic fatty liver disease in children and adolescents. Front Nutr. (2021) 8:700058. doi: 10.3389/fnut.2021.700058
48. Spivacow, F, Valle, ED, Boailchuk, J, Allo, PM, and Pailler, M. (2021). Metabolic risk factors in patients with kidney stones with and without type 2 diabetes. Preprints. Available at: doi: 10.21203/rs.3.rs-855238/v1. [Epub ahead of preprint]
49. Cardesa-Salzmann, TM, Simon, A, and Graf, N. Antibiotics in early life and childhood pre-B-ALL. Reasons to analyze a possible new piece in the puzzle. Discov Oncol. (2022) 13:5. doi: 10.1007/s12672-022-00465-6
50. Buscarinu, MC, Fornasiero, A, Romano, S, Ferraldeschi, M, Mechelli, R, Reniè, R, et al. The contribution of gut barrier changes to multiple sclerosis pathophysiology. Front Immunol. (2019) 10:1916. doi: 10.3389/fimmu.2019.01916
51. Soliman, NR, Effat, BAM, Mehanna, NS, Tawfik, NF, and Ibrahim, MK. Activity of probiotics from food origin for oxalate degradation. Arch Microbiol. (2021) 203:5017–28. doi: 10.1007/s00203-021-02484-3
Keywords: kidney stone, NHANES, microbiota, diabetes, cross-sectional study
Citation: Wang L, Wu J, Jiang Z, Wang C, Lin F, Zhong Y, Zhao P, Wei W, Huang J and Xu Z (2025) Dietary index for gut microbiota and its protective role against kidney stones: evidence of diabetes as a mediator from NHANES cross-sectional data. Front. Nutr. 12:1532313. doi: 10.3389/fnut.2025.1532313
Received: 21 November 2024; Accepted: 30 January 2025;
Published: 11 February 2025.
Edited by:
Bowen Li, Southwest University, ChinaReviewed by:
Ziye Huang, The Second Affiliated Hospital of Kunming Medical University, ChinaCopyright © 2025 Wang, Wu, Jiang, Wang, Lin, Zhong, Zhao, Wei, Huang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhanping Xu, cXdlMTY1NzVAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.