- 1Health Management Center, The Third Affiliated Hospital of Soochow University, Changzhou, China
- 2Department of Endocrinology, The Third Affiliated Hospital of Soochow University, Changzhou, China
Background: This research seeks to explore the link between the oxidative balance score (OBS) and sarcopenia in American adults with Metabolic Syndrome (MetS) using data from a national, population-based survey.
Methods: The study included 3,625 participants diagnosed with Metabolic Syndrome, all aged 20 years and above, derived from NHANES datasets spanning 1999–2006 and 2011–2018. OBS evaluation was based on 16 dietary and 4 lifestyle elements. MetS diagnosis followed the NCEP-ATP III guidelines, while sarcopenia identification was based on FNIH standards. We employed multivariate logistic regression analyses to delve into the connections between OBS and sarcopenia within the MetS cohort.
Results: Sarcopenia was found in 17.46% of the participants. In models adjusted for all variables, OBS, dietary OBS, and lifestyle OBS each showed a significant inverse relationship with sarcopenia among MetS individuals [OBS: OR = 0.959, 95%CI: (0.948, 0.982), P trend = 0.0005; dietary OBS: OR = 0.963, 95%CI: (0.939, 0.989), P trend = 0.0055; lifestyle OBS: OR = 0.860, 95%CI: (0.787, 0.939), P trend = 0.0011]. Higher scores in OBS were consistently linked with a decreased incidence of sarcopenia (all P for trend <0.05). Restricted cubic spline analysis confirmed that these relationships were linear. The impact of age was significant, with OBS benefits only observed in those aged 40 and older.
Conclusions: Maintaining a diet and lifestyle rich in antioxidants is both independently and collectively linked with a lower occurrence of sarcopenia in individuals with MetS. These results bolster the proposition of developing OBS-centered preventive strategies for sarcopenia in MetS patients, particularly those aged 40 years and older.
1 Introduction
Sarcopenia is a common condition affecting skeletal muscle associated with aging, marked by a steady, extensive reduction in muscle mass and strength (1, 2). It represents a critical aspect of elderly frailty and significantly affects elderly health and wellbeing (3). The occurrence of sarcopenia varies according to its defined criteria, gender, and age, impacting about 10–16% of the elderly population (4). Furthermore, sarcopenia's incidence is notably higher among individuals with chronic non-communicable diseases such as cardiovascular disease (CVD), liver disorders, chronic obstructive pulmonary disease, chronic kidney disease, and cancer (5–7). On its own, sarcopenia is associated with various adverse health effects, including cognitive decline, fractures, falls, hospitalizations, and heightened mortality risk (6, 8). Though primarily seen as a condition of old age, emerging research points to its significant relevance in younger adults as well (1, 9).
Metabolic syndrome (MetS) encompasses a set of cardiometabolic risk factors that include insulin resistance, central obesity, dyslipidemia, and hypertension (10). MetS poses a significant global public health issue, affecting roughly a quarter of the global adult population and imposing substantial economic costs annually (11, 12). While not a disease in itself, extensive epidemiological data indicate that MetS is closely linked to various severe health conditions, such as CVD, cancer, chronic kidney disease, and osteoarthritic disorders (13–15). There is growing evidence that MetS is closely related to the onset of sarcopenia, which is becoming a focal point of scientific inquiry (16). Clinical research suggests that sarcopenia related to MetS might be connected to poor clinical outcomes (17, 18).
Oxidative stress serves as a common pathogenic factor for both MetS and sarcopenia, potentially mediating the interaction between muscle degradation and metabolic imbalance (19). The oxidative balance score (OBS) is a novel metric for gauging an individual's combined exposure to pro-oxidants and antioxidants. OBS offers a refined evaluation of an individual's intricate oxidative balance by incorporating both dietary and lifestyle sources of antioxidants and pro-oxidants (20). Prior observational research has linked OBS with reduced muscle mass (21–23). Nevertheless, the protective role of OBS against sarcopenia development within the MetS group, especially its potential benefit for early-onset sarcopenia (20–39 years of age) remains under-researched. Exploring this relationship could have significant public health benefits, as controlling OBS might help prevent sarcopenia in the MetS demographic and alleviate its associated health burdens.
This research employed data from the National Health and Nutrition Examination Survey (NHANES), a nationally representative, population-based cross-sectional study, to explore how OBS influences the development of sarcopenia among adults with Metabolic Syndrome (MetS). These insights suggest that maintaining an antioxidant-rich diet and lifestyle could help thwart sarcopenia in individuals with MetS.
2 Materials and methods
2.1 Study design and population
NHANES is the principal survey conducted by the National Center for Health and Statistics (NCHS) to thoroughly evaluate the health and nutritional status of non-institutionalized U.S. children and adults and to generate vital health data. Since 1999, NHANES has operated biennially, randomly selecting about 5,000 representative samples annually from across the U.S., making it a continuous, serial cross-sectional study with a sophisticated, multistage, probability sampling framework. NHANES involves demographic and health questionnaires and a series of physical exams. All NHANES protocols are approved by the NCHS Ethics Review Board, and all participants provided written informed consent.
We included 15,350 MetS participants from eight cycles of NHANES spanning 1999–2006 and 2011–2018, and subsequently excluded those under 20 years old (n = 3,395), those unable to be diagnosed with sarcopenia (n = 5,767), those lacking OBS data (n = 2,263), and those missing other covariates (n = 300). Ultimately, 3,625 eligible adults with MetS were included in the study (Figure 1).
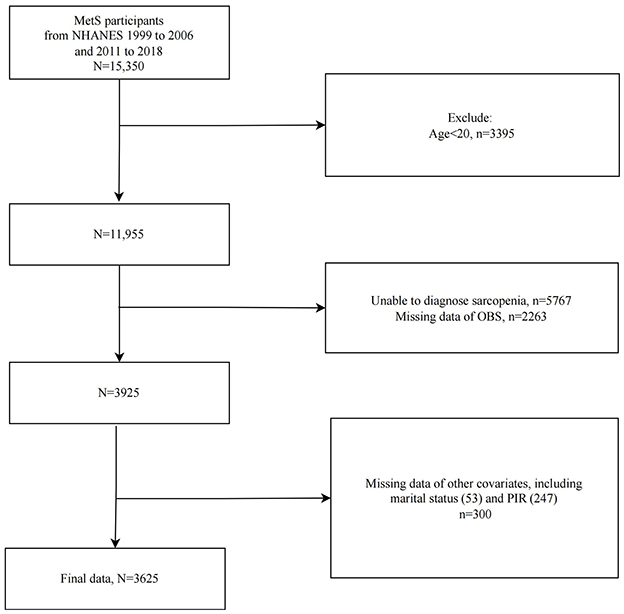
Figure 1. Flowchart of study population selection, NHANES 1999–2006 and 2011–2018. MetS, Metabolic Syndrome; OBS, oxidative balance score.
2.2 Evaluation of OBS
We utilized a validated method from prior research for evaluating the Oxidative Balance Score (OBS) that have been published in the literature (21, 24). In brief, the OBS incorporates 16 dietary elements and 4 lifestyle factors. The dietary OBS extensively measures intake of dietary fiber, carotenoids, riboflavin, niacin, vitamin B6, total folate, vitamin B12, vitamin C, vitamin E, calcium, magnesium, zinc, copper, selenium, total fat, and iron. Lifestyle OBS factors include physical activity, body mass index (BMI), alcohol intake, and serum cotinine levels. Dietary data was sourced from the average of two 24-h dietary recalls, consisting of an initial in-person dietary interview at a mobile examination center and a subsequent telephone follow-up 3–10 days later. Physical activity was quantified using metabolic equivalents (25), calculated from the frequency and duration of activities related to work, leisure, and transport, along with their respective MET values. BMI was calculated by dividing weight in kilograms by the square of height in meters. Serum cotinine, a primary nicotine metabolite, indicated smoking exposure. Antioxidants were scored as 2, 1, or 0 based on gender-specific benchmarks from high to low, while pro-oxidants were scored inversely. Alcohol consumption categories on the dietary recall were scored differently for men and women; those consuming over 30/15 g/d received a score of 0, while 0–30/0–15 g/d and no alcohol intake were scored 1 and 2, respectively (Supplementary Table S1). Each participant's overall OBS, dietary OBS, and lifestyle OBS scores were computed based on these criteria.
2.3 Diagnosis of MetS
The National Cholesterol Education Program-Adult Treatment Panel III guidelines for diagnosing MetS are well-established in NHANES-based research (24, 26). MetS was identified through a combination of central obesity, elevated blood sugar, abnormal blood lipid levels, and hypertension. It was defined by meeting three or more of the following criteria: 1. waist circumference (WC) of ≥102 cm for men or ≥88 cm for women; 2. serum triglycerides (TG) levels of ≥150 mg/dL; 3. serum high-density lipoprotein cholesterol (HDL-C) levels of < 40 mg/dL in men or < 50 mg/dL in women; 4. fasting blood glucose (FBG) of ≥100 mg/dL or on diabetes medication; 5. blood pressure (BP) of ≥130/85 mm Hg. Data regarding serum TG, HDL-C, and FBG were extracted from NHANES biochemical profiles, while blood pressure readings were acquired from three consecutive tests conducted by trained staff at the mobile examination centers.
2.4 Assessment of sarcopenia
We utilized the diagnostic standards from the Foundation for the National Institutes of Health (FNIH) Sarcopenia Project for defining sarcopenia in adults, a method frequently employed in prior NHANES studies (21, 23, 27). Appendicular lean mass (ALM), measured by dual-energy X-ray absorptiometry (DXA), served to evaluate muscle mass in participants. NHANES provided DXA body composition data from 1999–2006 to 2011–2018. Notably, during NHANES 2011–2018, DXA assessments were available only for individuals aged 8–59 years, while in NHANES 1999–2006, they were accessible for individuals aged 60 years and above (DXA was performed for all ≥8 years of age from 1999 to 2004, and for those aged 8–69 years during 2005–2006) (28). As per NHANES protocol, individual's ineligible for DXA exams due to being taller than 192.5 cm, heavier than 136.4 kg, or pregnant were excluded. The ratio of ALM to BMI was applied to identify sarcopenia, defined as < 0.789 in men or < 0.521 in women (29). Early-onset sarcopenia was characterized as sarcopenia diagnosed in individuals aged 20–39 years (30).
2.5 Covariates
Several potential covariates were included based on earlier studies, covering age, sex (male/female), racial/ethnic background (Mexican American/non-Hispanic White/non-Hispanic Black/other Hispanic/other race), educational attainment (< high school/high school/>high school), income-poverty ratio (PIR), marital status (single/non-single), and daily caloric intake (31). Demographic details were sourced from the NHANES demographic files. Daily caloric intake (kcal/day) was obtained from self-reported dietary recalls conducted in person at mobile examination centers.
2.6 Statistical analysis
We properly adjusted all analyses in line with the NHANES Analytic Guidelines to reflect the intricate design of the NHANES study. Data manipulation and statistical evaluations were conducted using EmpowerStats (X&Y Solutions, Inc., Boston, MA) and R software (version 4.2.3). A two-tailed P-value below 0.05 was deemed to indicate statistical significance. In the initial analysis, participants were categorized based on their sarcopenia status. Continuous measures were reported as mean ± standard error and assessed using a weighted t-test, while categorical data were presented as number (percentage) and evaluated using a weighted chi-square test. To examine the distinct relationship of OBS with sarcopenia in MetS subjects, we developed several multivariate logistic regression models, deriving odds ratios (OR) and 95% confidence intervals (CI). The preliminary models were unadjusted, Model 1 incorporated adjustments for age, sex, race, PIR, educational level, and marital status, and Model 2 further included adjustments for dietary energy consumption beyond Model 1. Restricted cubic spline (RCS) and curve smoothing techniques were applied to investigate possible dose-response relationships of OBS with sarcopenia incidence among MetS individuals, utilizing the rms package. Stratified analyses were conducted to assess the consistency of this relationship across different subgroups and to identify potential modifiers through interaction tests. For key effect modifiers, corresponding multivariate logistic regression and RCS analyses were performed within their subgroups. In the sensitivity checks, we employed an alternative widely recognized MetS diagnostic criterion, the International Diabetes Federation (IDF), to confirm the robustness of our findings (25).
3 Results
3.1 Baseline characteristics
The study included 3,625 MetS participants, averaging 48.905 years old, 51.264% male, and 75.363% non-Hispanic White. Sarcopenia was diagnosed in 633 individuals, representing a prevalence of 17.46%. Compared to those without sarcopenia, those diagnosed with the condition tended to be older, had lower PIR and energy consumption, and were more likely to be Mexican American/Other Hispanic/Other ethnicity and have ≤ high school education. Notably, those with sarcopenia had significantly lower OBS, dietary OBS, and lifestyle OBS (all P trend < 0.001; Table 1).
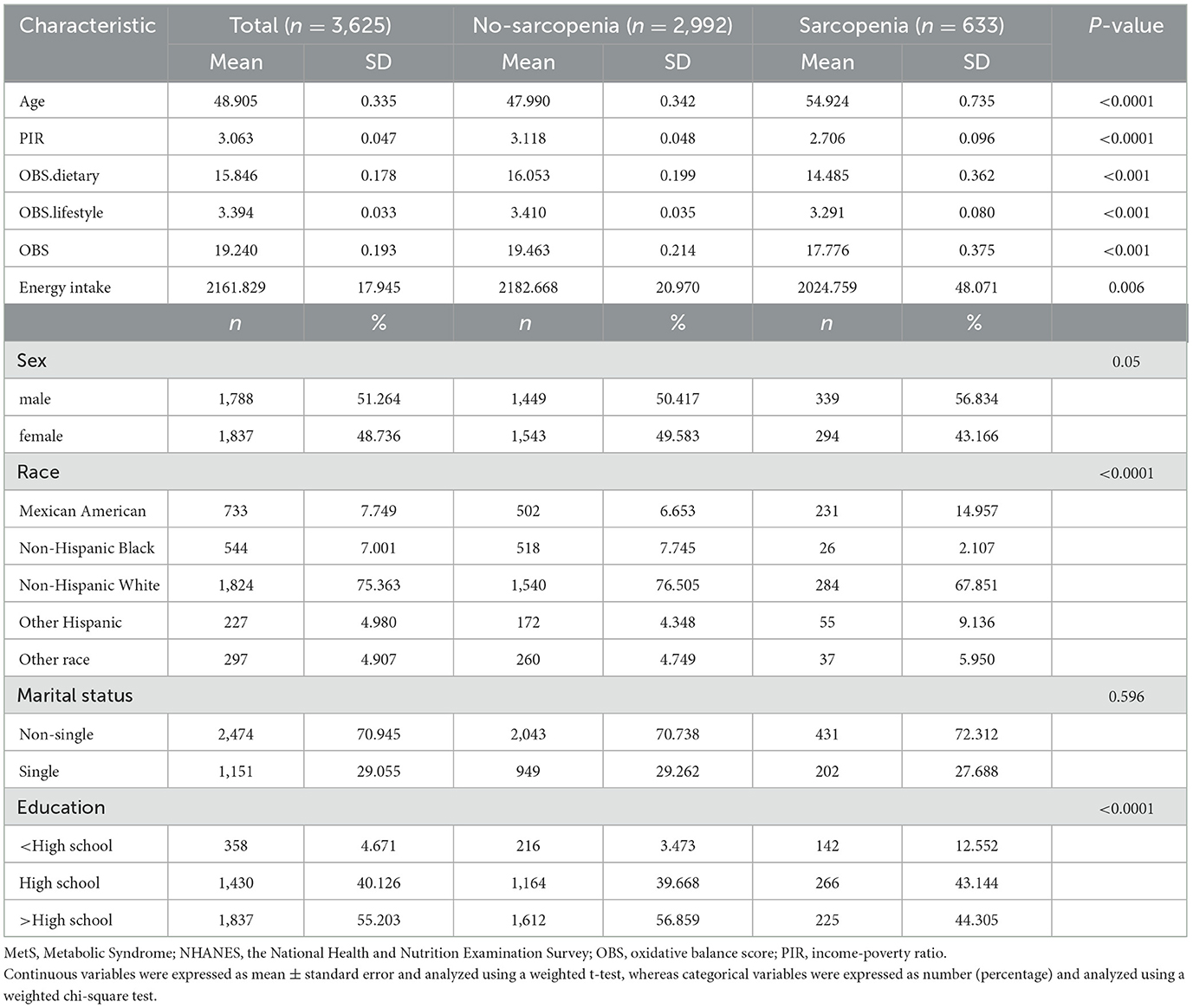
Table 1. Baseline analysis according to sarcopenia status among MetS participants, NHANES 1999–2006 and 2011–2018.
3.2 Multivariate logistic regression analysis
When OBS was considered as a continuous variable, in the initial analysis and Model 1, OBS was found to significantly inversely correlate with sarcopenia incidence among MetS patients [crude model: OR = 0.965, 95%CI: (0.948, 0.982), P trend = 0.0001; Model 1: OR = 0.966, 95%CI: (0.949, 0.984), P trend =0.0003]. When adjusting for all variables in Model 2, OBS continued to show an inverse association with sarcopenia among these participants, each additional unit of OBS will reduce the incidence rate of sarcopenia in MetS patients by 4.1% [OR: 0.96 (0.95, 0.97), P < 0.001]. When OBS was considered as a categorical variable, the incidence of sarcopenia markedly decreased with higher quartiles of OBS (P trend = 0.0026). Relative to the baseline group (Q1), belonging to Q3 and Q4 of OBS corresponded with a substantially reduced incidence of sarcopenia [Q3: OR = 0.659, 95% CI: (0.458, 0.949), P trend = 0.0270; Q4: OR = 0.494, 95% CI: (0.314, 0.776), P trend = 0.0028]. Both dietary OBS and lifestyle OBS showed significant and inverse correlations with sarcopenia odds in MetS individuals. Each increment in dietary OBS and lifestyle OBS was linked to a 3.7 and 14% decrease in sarcopenia odds, respectively. Higher levels of dietary OBS and lifestyle OBS were each associated with a notably lower sarcopenia prevalence (P trend = 0.0091 and 0.0093, respectively), with Q4 levels of dietary OBS and lifestyle OBS relative to the baseline group showing reductions in sarcopenia prevalence of 51.4 and 40.4%, respectively (Table 2).
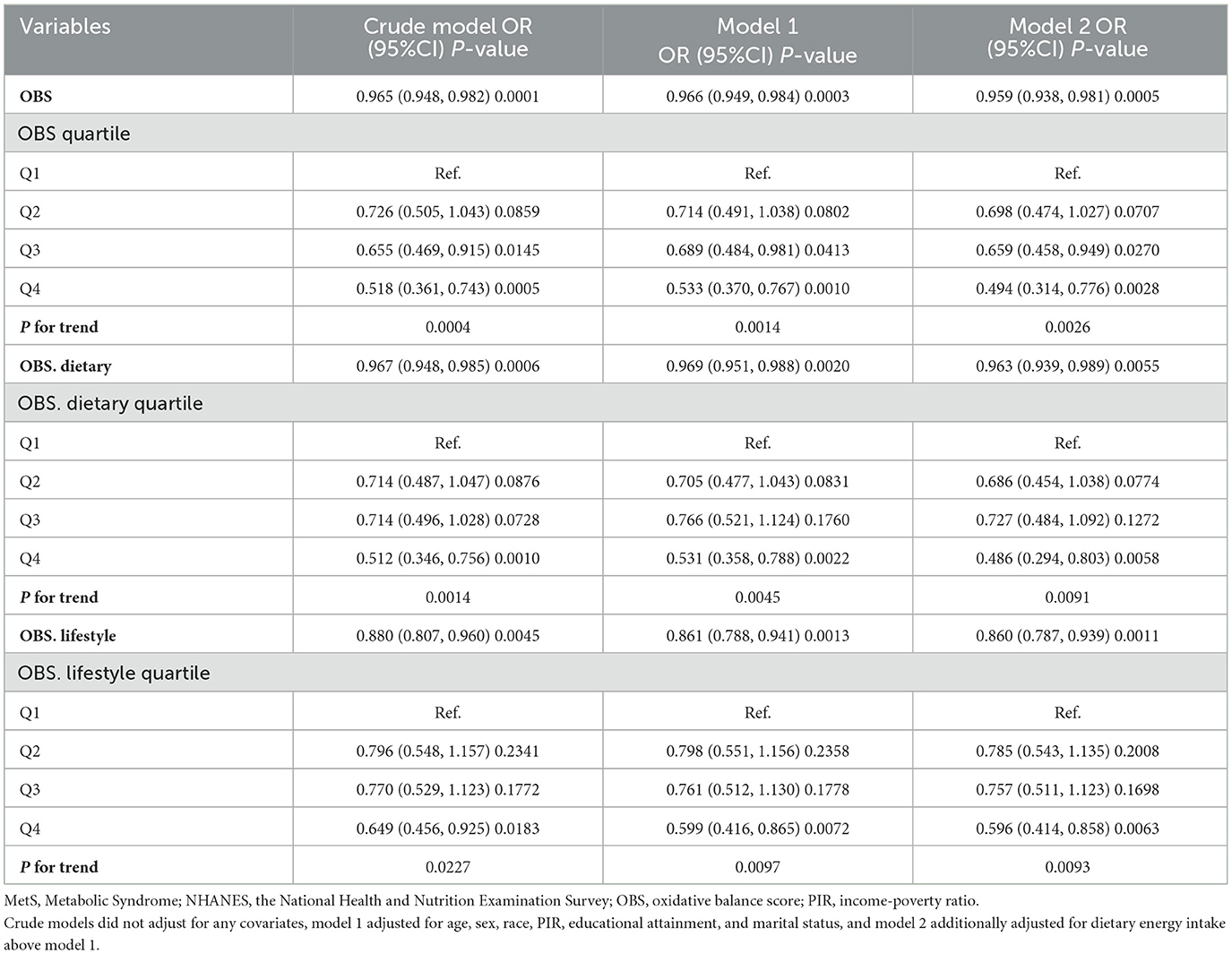
Table 2. Association of OBS, dietary OBS, and lifestyle OBS with prevalence of sarcopenia in MetS populations, NHANES 1999–2006 and 2011–2018.
3.3 RCS analysis
RCS analysis with curve smoothing indicated a clear inverse linear relationship between OBS and sarcopenia occurrence in MetS subjects (P trend for overall = 0.003, P trend for non-linear = 0.6907; Figure 2A). Similarly, dietary OBS (Figure 2B) and lifestyle OBS (Figure 2C) both had a negative dose-response association with the odds of sarcopenia in MetS individuals (P trend for non-linear = 0.2916 and 0.0504, respectively).
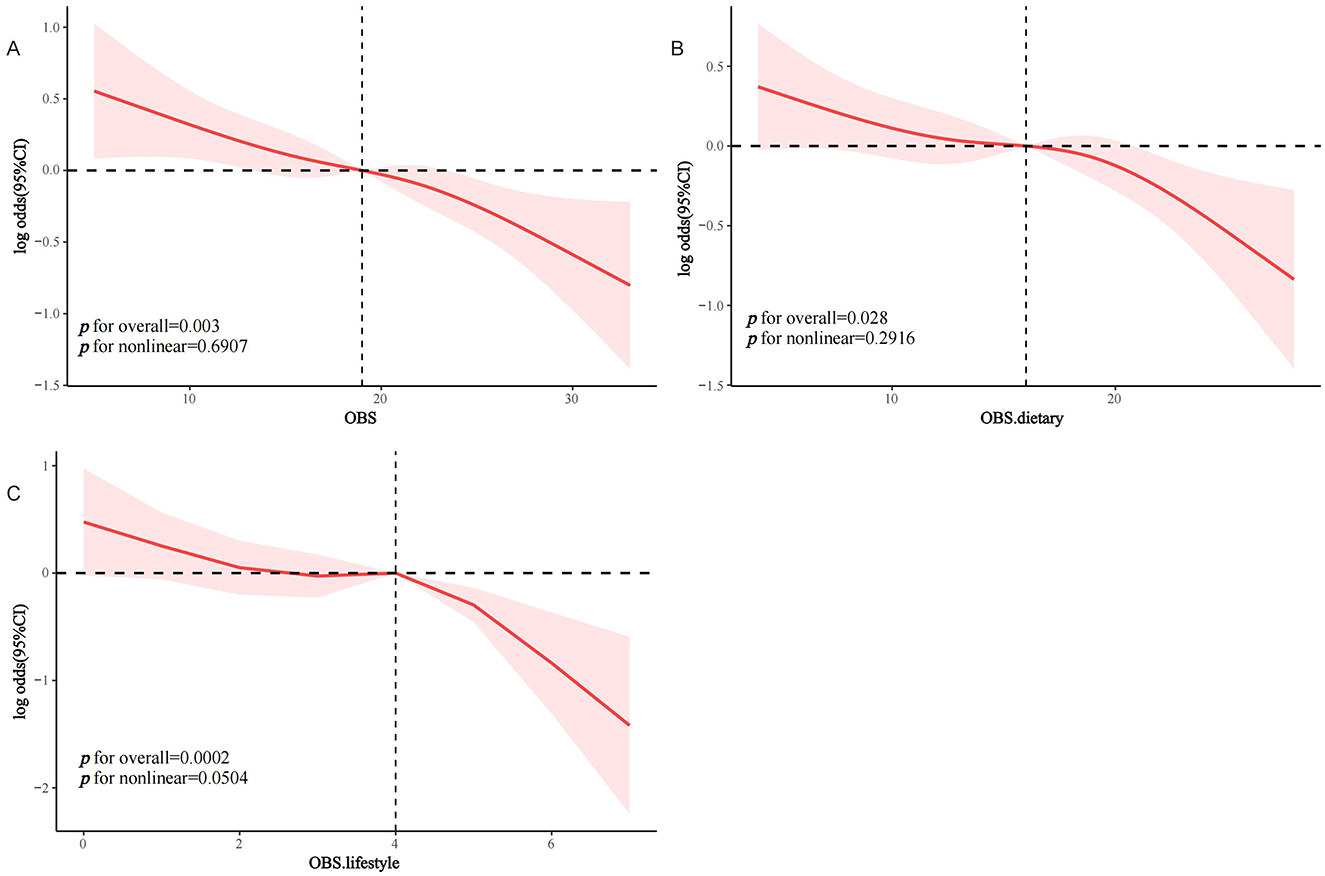
Figure 2. RCS analysis of the association between OBS and the prevalence of sarcopenia in the MetS population. (A) OBS; (B) dietary OBS; (C) lifestyle OBS. MetS, Metabolic Syndrome; OBS, oxidative balance score.
3.4 Stratified analysis
Interaction assessments revealed that the correlations between OBS, dietary OBS, and lifestyle OBS with sarcopenia in the MetS group were consistent across various subgroups. Yet, age proved to be a significant modifying factor (OBS: P for interaction = 0.019; dietary OBS: P for interaction = 0.038; lifestyle OBS: P trend = 0.011). Notably, the relationships of all OBS variants with sarcopenia in those with MetS were evident only in participants aged 40 years and above, with no connections found in younger onset sarcopenia (Figures 3A–C). Subsequent multivariate regression analyses across age groups (20–39 and ≥40 years) also reflected that OBS, dietary OBS, and lifestyle OBS did not correlate with the occurrence of sarcopenia in younger MetS individuals when all confounders were accounted for (all P trend > 0.05; Supplementary Table S2). However, in individuals aged 40 years and older, OBS, dietary OBS, and lifestyle OBS all showed a negative association with sarcopenia within the MetS group [OBS: OR = 0.964, 95% CI: (0.942, 0.987), P trend = 0.003; dietary OBS: OR = 0.967, 95% CI: (0.942, 0.993), P trend = 0.0012; lifestyle OBS: OR = 0.873, 95% CI: (0.797,0.957), P trend = 0.0041], with higher levels of each indicating significantly lower sarcopenia rates (P for trend 0.0060, 0.0162, and 0.0156, respectively). At the fourth quartile (compared to the first), OBS, dietary OBS, and lifestyle OBS were linked with 49.1, 49.5, and 42.4% decreased odds of sarcopenia, respectively (Table 3). Additional RCS analysis revealed that while OBS, dietary OBS, and lifestyle OBS showed no link with the prevalence of early-onset sarcopenia in MetS, they were all significantly and linearly correlated with sarcopenia in individuals aged 40 years or older (P trend for non-linear 0.3516, 0.2181, and 0.756, respectively; Figures 4A–C).
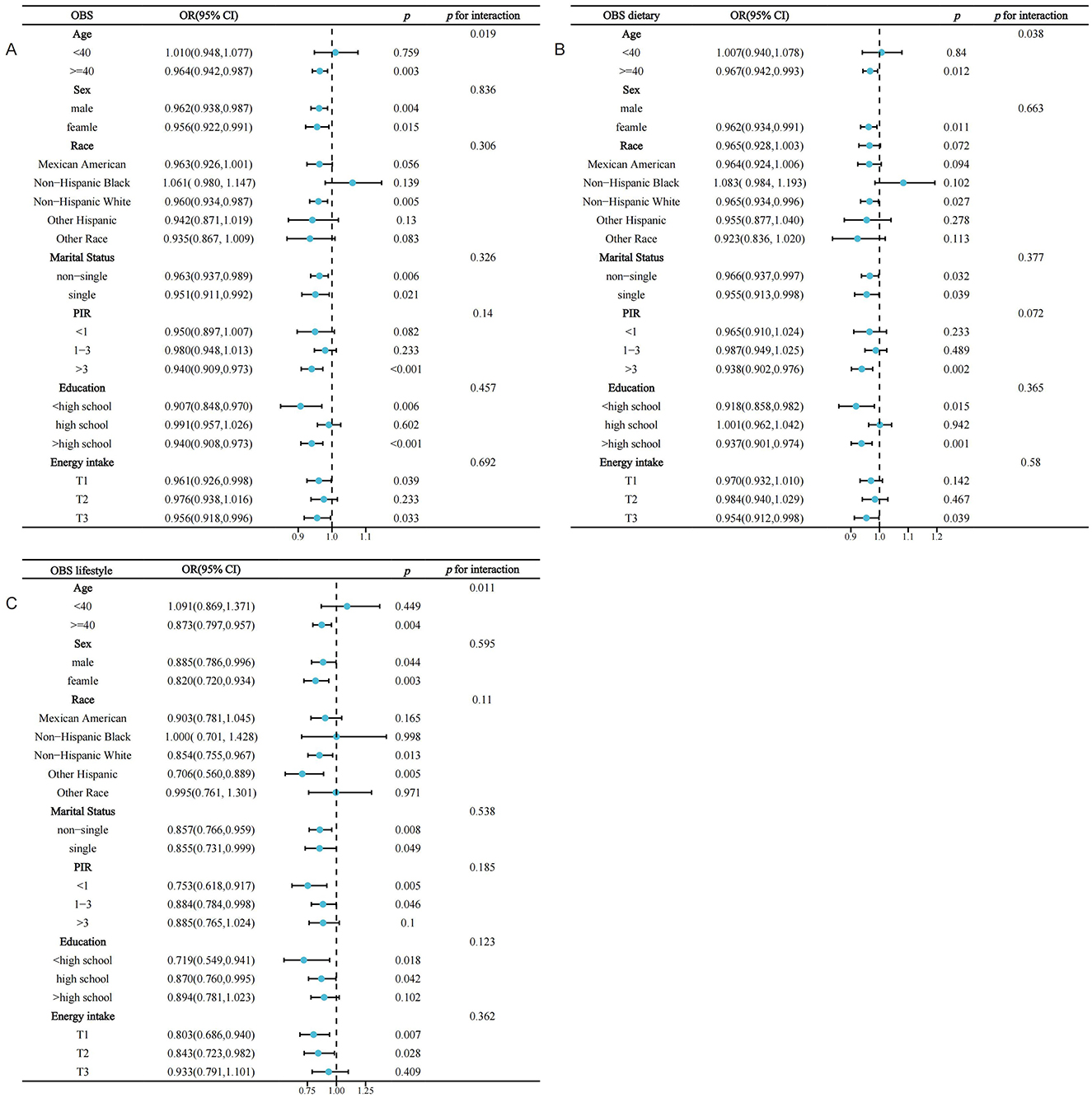
Figure 3. Stratified analysis of the association between OBS and the prevalence of sarcopenia in the MetS population based on included covariates. MetS, Metabolic Syndrome; OBS, oxidative balance score; PIR, income-poverty ratio. (A) OBS; (B) dietary OBS; (C) lifestyle OBS.
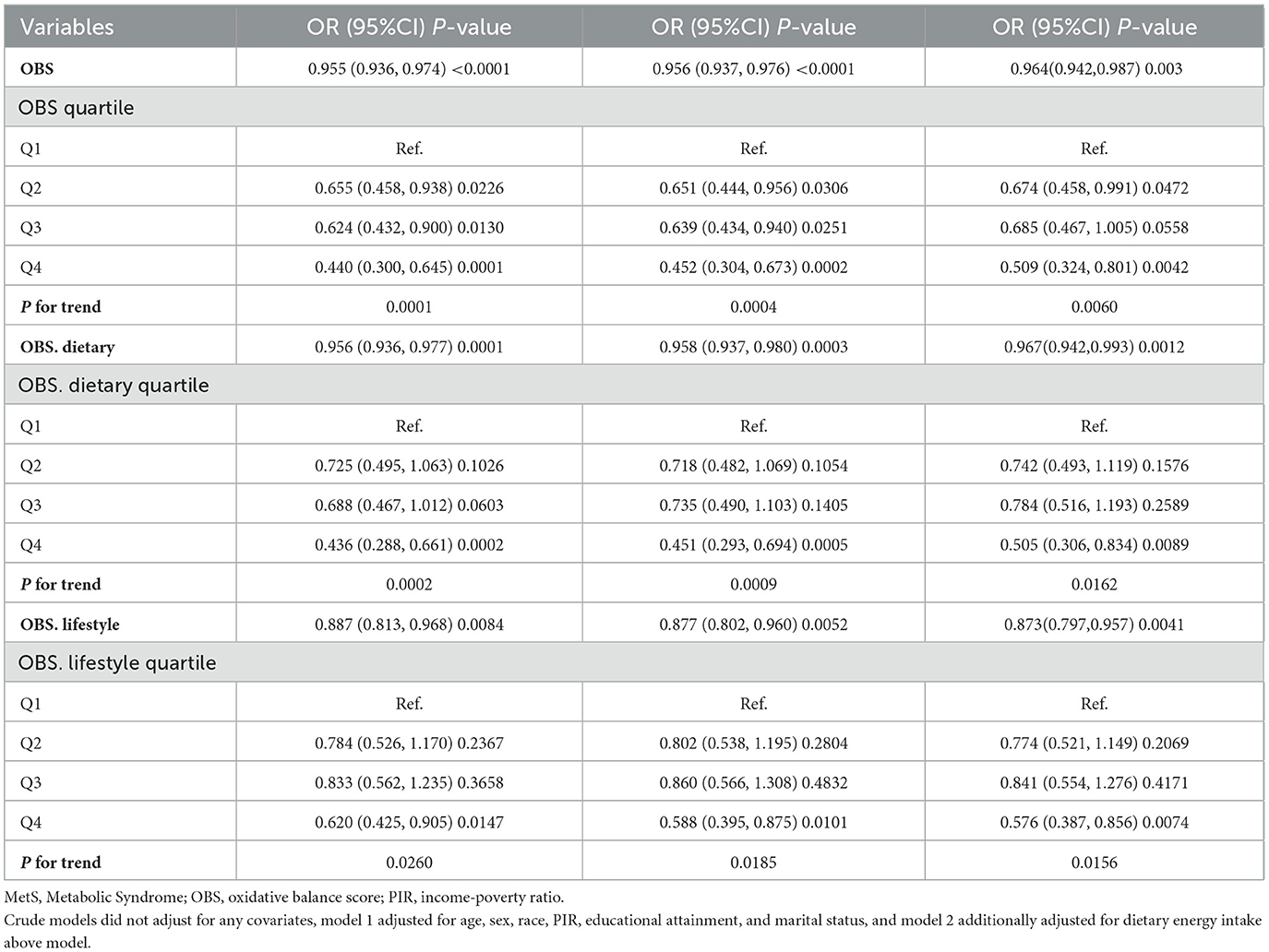
Table 3. Associations of OBS, dietary OBS, and lifestyle OBS with sarcopenia in people ≥40 years of age with MetS.
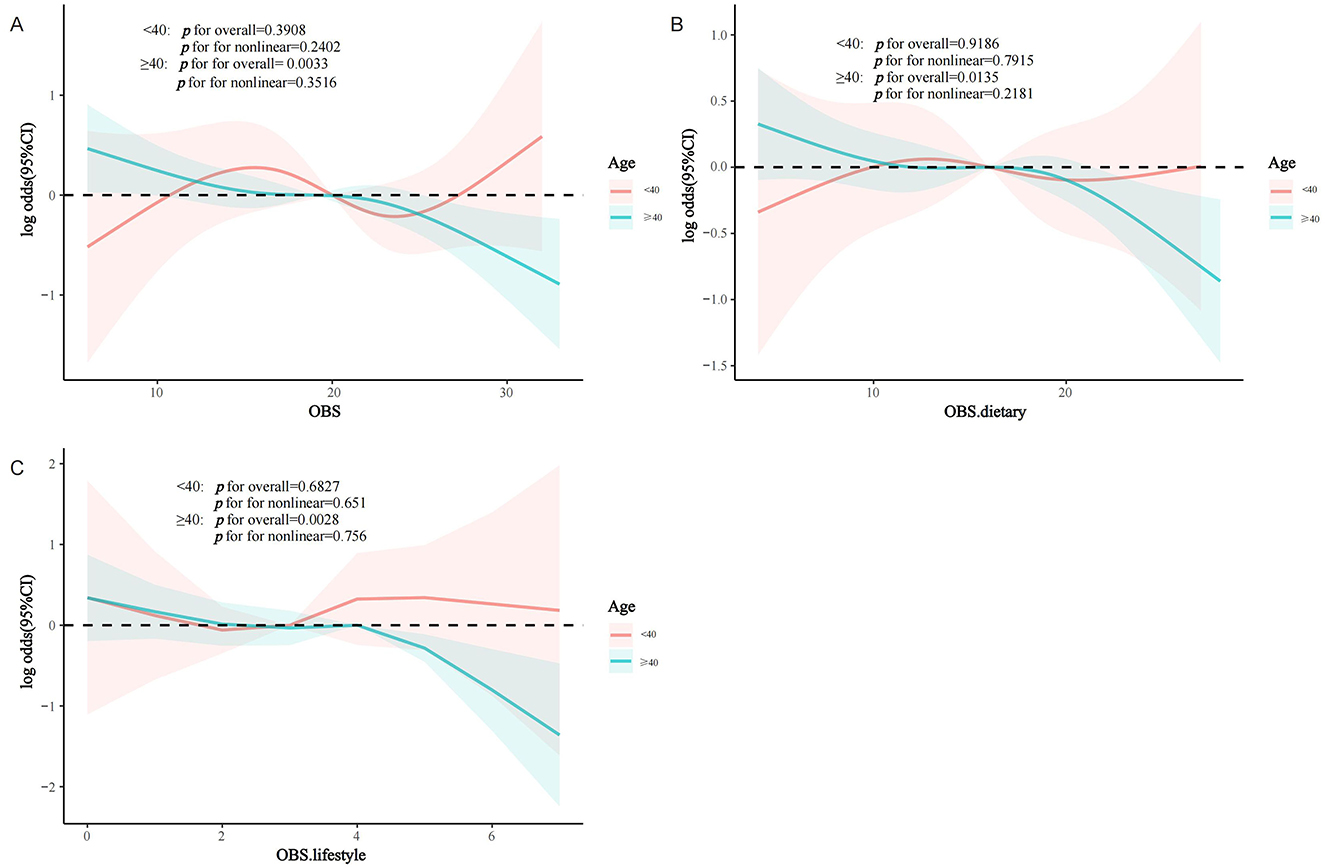
Figure 4. RCS analysis according to age (<40 years/≥40 years) subgroups. (A) OBS; (B) dietary OBS; (C) lifestyle OBS. MetS, Metabolic Syndrome; OBS, oxidative balance score.
3.5 Sensitivity analysis
In the sensitivity analysis, we found that similar results were obtained using the IDF criteria for diagnosing MetS, indicating the stability of the conclusions. After controlling for potential confounders, OBS, dietary OBS, and lifestyle OBS each showed an inverse relationship with the incidence of sarcopenia within the MetS demographic [OBS: OR = 0.959, 95% CI: (0.940, 0.979), P trend = 0.0001; dietary OBS: OR = 0.967, 95% CI: (0.945, 0.989), P trend = 0.0039; lifestyle OBS: OR = 0.828, 95% CI: (0.760, 0.902), P trend < 0.0001]. Elevated levels of OBS, dietary OBS, and lifestyle OBS were linked to a substantially reduced occurrence of sarcopenia (all P for trend < 0.05; Supplementary Table S3).
4 Discussion
In a nationwide, population-based sectional analysis, our findings demonstrated for the first time that a dietary and lifestyle-based oxidative balance metric, OBS, was inversely correlated with sarcopenia among the MetS population, exhibiting dose-response relationships. In the fully adjusted model, for every 1-unit increase in OBS, the risk of sarcopenia in MetS patients was reduced by 4.1% (OR = 0.959, 95%CI: 0.938–0.981, P = 0.0005). Compared to Q1, participants with OBS at Q3 and Q4 had a significantly lower prevalence of sarcopenia (OR 0.659 and 0.494, respectively; p for trend = 0.026). Age emerged as a critical modifier in these correlations, manifesting only in individuals aged 40 years and above (OR = 0.964, P = 0.003), while there was no link between OBS and early-onset sarcopenia within the MetS demographic (P > 0.05). These results indicate that managing external oxidative stress may play a crucial role in the development of sarcopenia in people with MetS, highlighting the importance of monitoring both dietary and lifestyle sources of antioxidants and pro-oxidants in medical settings. Our research underlines that maintaining a diet and lifestyle rich in antioxidants, evaluated through OBS, is instrumental in preventing sarcopenia among those with MetS, particularly in older adults.
OBS effectively gauges a person's external oxidative equilibrium by encompassing dietary and lifestyle interactions with well-known pro-oxidants and antioxidants. Since the initial proposition of OBS (involving intake assessments of dietary vitamin C, beta carotene, and iron), ongoing studies have refined the components and methodologies for OBS evaluation (32). Substantial clinical studies have proposed their respective components and assessment criteria for OBS, and more than 20 types of OBS are currently represented (20). In this investigation, we utilized the OBS computation method previously adopted in studies leveraging the NHANES dataset, incorporating 16 dietary OBS components and 4 prevalent lifestyle OBS elements (8, 21, 23, 24, 33). Extensive practical research has shown that OBS is linked with the onset and prognosis of various conditions, encompassing a range of cardiometabolic diseases such as cardiovascular disease (CVD) (34), non-alcoholic fatty liver disease (NAFLD) (33), chronic kidney disease (21), metabolic syndrome (MetS) (24), and type 2 diabetes mellitus (35). Additionally, multiple studies have explored the relationship between OBS and sarcopenia across the general populace. A recent case-control study from Iran revealed that OBS significantly inversely correlated with the probability of sarcopenia in older adults (22). A sectional analysis utilizing NHANES data from 2011 to 2018 also demonstrated a negative correlation between OBS and sarcopenia in adults aged 20–59 years [OR = 0.942, 95% CI: (0.920, 0.964), P trend < 0.001] (23). Another sectional analysis from NHANES indicated that OBS was significantly inversely related to low muscle mass in the middle-aged demographic (OR = 0.96, P trend < 0.0001) (21). Our results, for the first time, indicate that OBS, dietary OBS, and lifestyle OBS all inversely relate to sarcopenia in the MetS group, offering the latest population-level clinical evidence supporting the benefit of higher OBS for preventing sarcopenia in individuals with MetS. It is important to note that previous investigations employing NHANES data from only 2011–2018 (during which DXA scans were performed on individuals aged 8–59 years) imply that their findings were applicable primarily to the non-elderly population (under 60 years). Our study, on the other hand, comprehensively included data from NHANES 1999–2006 and 2011–2018, providing a more comprehensive age range of participants and making the conclusions more generalizable. Interestingly, whereas a previous study showed a non-linear correlation between OBS and sarcopenia in the general population aged 20–59 years (21), our RCS findings illustrate a dose-response relationship between all OBS and sarcopenia in the MetS population.
Mounting research indicates that consuming certain antioxidants can help prevent sarcopenia (36). Foods rich in antioxidants like dietary fiber, micronutrients, vitamins, and polyphenols are beneficial in managing sarcopenia (34, 37). Regular consumption of antioxidant-rich vitamins such as C and E is crucial for neutralizing reactive oxygen species and correcting cellular redox imbalances, potentially reducing sarcopenia by alleviating oxidative stress (33). The role of dietary antioxidants in the diet or as supplements is increasingly recognized as a significant area for sarcopenia research, though definitive benefits in sarcopenia are yet to be established (38). Beyond diet, lifestyle factors that influence antioxidant and pro-oxidant levels also significantly impact sarcopenia progression. Numerous epidemiological studies have identified sedentary lifestyles as primary contributors to sarcopenia. Engaging in suitable physical activities is essential for preventing sarcopenia and enhancing functional outcomes and life quality in affected individuals (39). Several observational studies have noted that excessive alcohol consumption might correlate with lower muscle mass and decreased grip strength, although results are not consistent (40, 41). The link between BMI and sarcopenia also remains debated (42, 43). Moreover, smoking is a major risk factor for developing sarcopenia, yet the relationship between serum cotinine levels, a marker for smoking exposure, and sarcopenia has not been extensively explored (44). While individual antioxidants or pro-oxidants have been identified as potentially relevant to sarcopenia development, research into how redox balance within one's diet and lifestyle affects sarcopenia is still limited. Our research indicates that the OBS, a comprehensive external oxidative stress assessment tool, correlates with sarcopenia in MetS individuals and could inform future prevention strategies.
Oxidative stress is a shared pathophysiological hallmark of MetS and sarcopenia and has an important role in disease development (16, 19). Individuals with comorbid sarcopenia tend to have poorer clinical outcomes than those with MetS alone. A prospective cohort study that included 749 patients with resectable gastric cancer demonstrated a worse prognosis in populations with comorbid preoperative MetS and sarcopenia regardless of whether they were compared to normal, MetS-alone, or sarcopenia-alone patients (17). A recent longitudinal study utilizing NHANES data revealed that the simultaneous presence of MetS and sarcopenia correlated with significantly elevated risks of all-cause and CVD mortality within the general population (45). Therefore, not only do MetS and sarcopenia share potential biological and clinical connections, but the dual occurrence of sarcopenia in those with MetS may profoundly impact clinical outcomes and escalate overall health burdens, particularly as sarcopenia might also contribute to other health issues, including CVD. Identifying risk determinants for sarcopenia in individuals with MetS could aid in its prevention, thus enhancing overall public health outcomes, with our results supporting the beneficial role of an antioxidant-enriched diet and lifestyle in mitigating sarcopenia.
Accumulating experimental evidence suggests that oxidative stress has a key role in the pathogenesis of sarcopenia. Excessive reactive oxygen species (ROS) have important effects on the regulation of muscle protein synthesis and catabolism and on mitochondrial function (46). ROS can directly damage mitochondrial DNA and weaken mitochondrial function, leading to impaired energy metabolism and functional decline in muscle cells (47). Oxidative stress also inhibits the expression of genes involved in mitochondrial biosynthesis, which reduces mitochondrial quantity and quality and exacerbates the decline in muscle function (48). Notably, oxidative stress can crosstalk with inflammatory signaling pathways, which in turn promotes the production and release of multiple inflammatory factors, disrupting the dynamic balance of muscle proteins and accelerating muscle atrophy (49). Interestingly, oxidative stress also affects the structure and function of the neuromuscular junction, depriving the muscle of innervation and consequently atrophy (50). In summary, oxidative stress affects the structure and function of muscle tissue through multiple pathways and is one of the key factors in the pathogenesis of sarcopenia.
Several observational clinical studies have shown that higher OBS is associated with lower levels of oxidative stress markers in individuals. A cross-sectional analysis from Korea showed that OBS was negatively associated with serum γ-glutamyltransferase levels among adults (51). Another cross-sectional analysis from Japan showed that OBS was negatively associated with levels of an oxidative stress marker, urinary 8-hydroxydeoxyguanosine, in adults (52). In a retrospective study from the US, Kong et al. showed that OBS was negatively associated with levels of oxidative stress markers (F2-isoprostanes) and systemic inflammatory markers [C-reactive protein (CRP)] in individuals (53). Another cross-sectional analysis similarly showed that higher OBS was associated with lower serum high-sensitivity-CRP levels and reduced systemic inflammation in middle-aged and older Japanese (54). These significant associations suggest that OBS may modulate the body's intrinsic levels of oxidative stress and inflammation, which may partially explain the negative correlation between OBS and sarcopenia.
Age markedly affected the link between OBS and sarcopenia among MetS patients, with the advantageous impact of OBS on this condition manifesting only in individuals aged 40 and above, suggesting no protective benefit against sarcopenia emerging at younger ages. Sarcopenia, characterized by an age-related gradual decline in muscle mass and function, remains under-researched in younger demographics. Earlier NHANES-based research indicated that factors like obstructive sleep apnea and intake levels of dietary vitamins B1 and B2 are positively and inversely related to early-onset sarcopenia, respectively (30, 55). Recent epidemiological research indicates that sarcopenia might affect one in 10 young adults, potentially linked to MetS, lack of physical activity, genetic and prenatal influences, vitamin D deficiency, hormonal imbalances, and other variables (9). Although current studies do not delve into the foundational mechanisms, our results imply that prevention strategies for MetS-related early-onset sarcopenia may not derive substantial benefits from an antioxidant-focused diet and lifestyle. Further exploration and intervention in other modifiable risk elements are necessary for this specific group.
Our research possesses several notable strengths. It is a comprehensive, population-wide study with a robust sample size and diverse ethnic participation, which enhances the extrapolation of the findings. OBS serves as an integrated measure of oxidative balance, previously well-validated in NHANES-affiliated research, and it offers a more precise reflection of a person's total exposure compared to isolated antioxidants.
4.1 Limitations
Nonetheless, our study faces certain limitations. Firstly, its cross-sectional nature precludes causal inference and may be vulnerable to underlying confounding. The cross-sectional design prevents us from determining the temporal sequence of the relationship between OBS and sarcopenia. Prospective detailed cohort studies are essential to confirm the long-term relationships of OBS with sarcopenia in the MetS group. In addition, intervention studies could be designed to clarify potential causal associations. Secondly, while the sarcopenia diagnosis aligns with prior NHANES studies and FNIH standards, it did not permit evaluation of muscle functionality, such as strength or mobility, due to data limitations within NHANES. Further validation of these findings using well-supported diagnostic criteria for sarcopenia is needed. Moreover, the roles of additional factors such as genetic influences, prenatal conditions, and hormonal disorders require further clarification. Although we used an OBS assessment method validated in NHANES, differences in OBS components across studies may lead to limited extrapolation of results and warrant standardization of scoring systems. Assessment of OBS components relies mostly on self-report and may be affected by recall bias; future studies may use more objective assessment instruments to improve the accuracy of OBS scale.
5 Conclusions
In a nationwide sectional study, OBS, dietary OBS, and lifestyle OBS each demonstrated significant inverse correlations with sarcopenia prevalence among those with MetS, exhibiting dose-response relationships. Our fully adjusted regression data suggest that each point increase in OBS may reduce the prevalence of sarcopenia in the MetS population by 4.1%, and that OBS in the highest quartile (compared to Q1) may have prevented 50.6% of sarcopenia occurrences. Age markedly impacted these relationships, with the beneficial effects of OBS manifesting in individuals aged 40 years and older. Therefore, we suggest that sarcopenia prevention strategies for patients with MetS should begin in middle age (≥40 years) to enhance OBS scores by improving diet (e.g., increasing antioxidant nutrient intake) and lifestyle (e.g., increasing physical activity, quitting smoking and restricting alcohol). These outcomes indicate that an antioxidant-rich diet and lifestyle in line with OBS metrics might aid in preventing sarcopenia within the MetS demographic, underscoring the need for future rigorously designed longitudinal cohort studies.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: https://www.cdc.gov/nchs/nhanes/.
Ethics statement
The studies involving humans were approved by National Center for Health and Statistics (NCHS) Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
MW: Methodology, Writing – original draft. HS: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This investigation was funded by the Changzhou Science and Technology program (Grant No. 20210162) and the Jiangsu Social Sciences Applied Research scheme (Grant No. 20SYB-111).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1529140/full#supplementary-material
References
1. Ahn SH, Lee JH, Lee JW. Inverse association between triglyceride glucose index and muscle mass in Korean adults: 2008–2011 KNHANES. Lipids Health Dis. (2020) 19:243. doi: 10.1186/s12944-020-01414-4
2. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9
3. Papadopoulou SK. Sarcopenia: a contemporary health problem among older adult populations. Nutrients. (2020) 12:1293. doi: 10.3390/nu12051293
4. Wu C, Ren C, Song Y, Gao H, Pang X, Zhang L. Gender-specific effects of oxidative balance score on the prevalence of diabetes in the US population from NHANES. Front Endocrinol. (2023) 14:1148417. doi: 10.3389/fendo.2023.1148417
5. Damluji AA, Alfaraidhy M, AlHajri N, Rohant NN, Kumar M, Al Malouf C, et al. Sarcopenia and cardiovascular diseases. Circulation. (2023) 147:1534–53. doi: 10.1161/CIRCULATIONAHA.123.064071
6. Sepulveda-Loyola W, Osadnik C, Phu S, Morita AA, Duque G, Probst VS. Diagnosis, prevalence, and clinical impact of sarcopenia in COPD: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2020) 11:1164–76. doi: 10.1002/jcsm.12600
7. Tandon P, Montano-Loza AJ, Lai JC, Dasarathy S, Merli M. Sarcopenia and frailty in decompensated cirrhosis. J Hepatol. (2021) 75:S147–S62. doi: 10.1016/j.jhep.2021.01.025
8. Xu J, Wan CS, Ktoris K, Reijnierse EM, Maier AB. Sarcopenia is associated with mortality in adults: a systematic review and meta-analysis. Gerontology. (2022) 68:361–76. doi: 10.1159/000517099
9. Jung HN, Jung CH, Hwang YC. Sarcopenia in youth. Metabolism. (2023) 144:155557. doi: 10.1016/j.metabol.2023.155557
10. Fahed G, Aoun L, Bou Zerdan M, Allam S, Bou Zerdan M, Bouferraa Y, et al. Metabolic syndrome: updates on pathophysiology and management in 2021. Int J Mol Sci. (2022) 23:786. doi: 10.3390/ijms23020786
11. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. (2018) 20:12. doi: 10.1007/s11906-018-0812-z
12. Xu H, Li X, Adams H, Kubena K, Guo S. Etiology of metabolic syndrome and dietary intervention. Int J Mol Sci. (2018) 20:128. doi: 10.3390/ijms20010128
13. Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. (2012) 35:2402–11. doi: 10.2337/dc12-0336
14. Sebastian SA, Padda I, Johal G. Cardiovascular-kidney-metabolic (CKM) syndrome: a state-of-the-art review. Curr Probl Cardiol. (2024) 49:102344. doi: 10.1016/j.cpcardiol.2023.102344
15. Sampath SJP, Venkatesan V, Ghosh S, Kotikalapudi N. Obesity, metabolic syndrome, and osteoarthritis-an updated review. Curr Obes Rep. (2023) 12:308–31. doi: 10.1007/s13679-023-00520-5
16. Nishikawa H, Asai A, Fukunishi S, Nishiguchi S, Higuchi K. Metabolic syndrome and sarcopenia. Nutrients. (2021) 13:3519. doi: 10.3390/nu13103519
17. Xu LB, Zhang HH, Shi MM, Huang ZX, Zhang WT, Chen XD, et al. Metabolic syndrome-related sarcopenia is associated with worse prognosis in patients with gastric cancer: a prospective study. Eur J Surg Oncol. (2020) 46:2262–9. doi: 10.1016/j.ejso.2020.07.032
18. Buchmann N, Nikolov J, Spira D, Demuth I, Steinhagen-Thiessen E, Eckardt R, et al. Identifying sarcopenia in metabolic syndrome: data from the berlin aging study II. J Gerontol A Biol Sci Med Sci. (2016) 71:265–72. doi: 10.1093/gerona/glv089
19. Rubio-Ruiz ME, Guarner-Lans V, Perez-Torres I, Soto ME. Mechanisms underlying metabolic syndrome-related sarcopenia and possible therapeutic measures. Int J Mol Sci. (2019) 20:647. doi: 10.3390/ijms20030647
20. Hernandez-Ruiz A, Garcia-Villanova B, Guerra-Hernandez E, Amiano P, Ruiz-Canela M, Molina-Montes E. A review of a priori defined oxidative balance scores relative to their components and impact on health outcomes. Nutrients. (2019) 11:774. doi: 10.3390/nu11040774
21. Cao Y, Zhou Y, Zhong Y, Liao X, Chen X, Pi Y. Association between oxidative balance score in adults with and without chronic kidney disease: 2011–2028 NHANES. Front Nutr. (2024) 11:1374719. doi: 10.3389/fnut.2024.1374719
22. Mahmoodi M, Shateri Z, Nazari SA, Nouri M, Nasimi N, Sohrabi Z, et al. Association between oxidative balance score and sarcopenia in older adults. Sci Rep. (2024) 14:5362. doi: 10.1038/s41598-024-56103-4
23. Xu W, Mu D, Wang Y, Wang Y, Wang C, Zhang X. Association between oxidative balance score and sarcopenia in US adults: NHANES 2011–2018. Front Nutr. (2024) 11:1342113. doi: 10.3389/fnut.2024.1342113
24. Lu Y, Wang M, Bao J, Chen D, Jiang H. Association between oxidative balance score and metabolic syndrome and its components in US adults: a cross-sectional study from NHANES 2011–2018. Front Nutr. (2024) 11:1375060. doi: 10.3389/fnut.2024.1375060
25. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. (2006) 23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x
26. Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C, American Heart A, et al. Definition of metabolic syndrome: report of the national heart, lung, and blood institute/American Heart Association conference on scientific issues related to definition. Circulation. (2004) 109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6
27. Chen W, Shi S, Jiang Y, Chen K, Liao Y, Huang R, et al. Association of sarcopenia with ideal cardiovascular health metrics among US adults: a cross-sectional study of NHANES data from 2011 to 2018. BMJ Open. (2022) 12:e061789. doi: 10.1136/bmjopen-2022-061789
28. Murdock DJ, Wu N, Grimsby JS, Calle RA, Donahue S, Glass DJ, et al. The prevalence of low muscle mass associated with obesity in the USA. Skelet Muscle. (2022) 12:26. doi: 10.1186/s13395-022-00309-5
29. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. (2014) 69:547–58. doi: 10.1093/gerona/glu010
30. Tao X, Niu R, Lu W, Zeng X, Sun X, Liu C. Obstructive sleep apnea (OSA) is associated with increased risk of early-onset sarcopenia and sarcopenic obesity: results from NHANES 2015–2018. Int J Obes. (2024) 48:891–9. doi: 10.1038/s41366-024-01493-8
31. Xu Z, Lei X, Chu W, Weng L, Chen C, Ye R. Oxidative balance score was negatively associated with the risk of metabolic syndrome, metabolic syndrome severity, and all-cause mortality of patients with metabolic syndrome. Front Endocrinol. (2023) 14:1233145. doi: 10.3389/fendo.2023.1233145
32. Van Hoydonck PG, Temme EH, Schouten EG. A dietary oxidative balance score of vitamin C, beta-carotene and iron intakes and mortality risk in male smoking Belgians. J Nutr. (2002) 132:756–61. doi: 10.1093/jn/132.4.756
33. Liu S, Zhang L, Li S. Advances in nutritional supplementation for sarcopenia management. Front Nutr. (2023) 10:1189522. doi: 10.3389/fnut.2023.1189522
34. Cailleaux PE, Dechelotte P, Coeffier M. Novel dietary strategies to manage sarcopenia. Curr Opin Clin Nutr Metab Care. (2024) 27:234–43. doi: 10.1097/MCO.0000000000001023
35. Liu Y, Chen M. Dietary and lifestyle oxidative balance scores are independently and jointly associated with nonalcoholic fatty liver disease: a 20 years nationally representative cross-sectional study. Front Nutr. (2023) 10:1276940. doi: 10.3389/fnut.2023.1276940
36. Cho MR, Lee S, Song SK. A review of sarcopenia pathophysiology, diagnosis, treatment and future direction. J Korean Med Sci. (2022) 37:e146. doi: 10.3346/jkms.2022.37.e146
37. van Dronkelaar C, van Velzen A, Abdelrazek M, van der Steen A, Weijs PJM, Tieland M. Minerals and sarcopenia; the role of calcium, iron, magnesium, phosphorus, potassium, selenium, sodium, and zinc on muscle mass, muscle strength, and physical performance in older adults: a systematic review. J Am Med Dir Assoc. (2018) 19:6–11.e3. doi: 10.1016/j.jamda.2017.05.026
38. Fougere B, van Kan GA, Vellas B, Cesari M. Redox systems, antioxidants and sarcopenia. Curr Protein Pept Sci. (2018) 19:643–8. doi: 10.2174/1389203718666170317120040
39. Marzetti E, Calvani R, Tosato M, Cesari M, Di Bari M, Cherubini A, et al. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin Exp Res. (2017) 29:35–42. doi: 10.1007/s40520-016-0705-4
40. Zhai J, Ma B, Qin J, Lyu Q, Khatun P, Liang R, et al. Alcohol consumption patterns and the risk of sarcopenia: a population-based cross-sectional study among chinese women and men from Henan province. BMC Public Health. (2022) 22:1894. doi: 10.1186/s12889-022-14275-6
41. Hong SH, Bae YJ. Association between alcohol consumption and the risk of sarcopenia: a systematic review and meta-analysis. Nutrients. (2022) 14:3266. doi: 10.3390/nu14163266
42. Hashim NNA, Mat S, Myint PK, Kioh SH, Delibegovic M, Chin AV, et al. Increased body mass index is associated with sarcopenia and related outcomes. Eur J Clin Invest. (2023) 53:e13874. doi: 10.1111/eci.13874
43. Curtis M, Swan L, Fox R, Warters A, O'Sullivan M. Associations between body mass index and probable sarcopenia in community-dwelling older adults. Nutrients. (2023) 15:1505. doi: 10.3390/nu15061505
44. Steffl M, Bohannon RW, Petr M, Kohlikova E, Holmerova I. Relation between cigarette smoking and sarcopenia: meta-analysis. Physiol Res. (2015) 64:419–26. doi: 10.33549/physiolres.932802
45. Huang W, Deng S, Liu S, Ma Q, Cao L, Liu L, et al. Association of metabolic syndrome and sarcopenia with all-cause and cardiovascular mortality: a prospective cohort study based on the NHANES. Front Endocrinol. (2024) 15:1346669. doi: 10.3389/fendo.2024.1346669
46. Zhang H, Qi G, Wang K, Yang J, Shen Y, Yang X, et al. Oxidative stress: roles in skeletal muscle atrophy. Biochem Pharmacol. (2023) 214:115664. doi: 10.1016/j.bcp.2023.115664
47. Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ, et al. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol. (2013) 45:2288–301. doi: 10.1016/j.biocel.2013.06.024
48. Migliavacca E, Tay SKH, Patel HP, Sonntag T, Civiletto G, McFarlane C, et al. Mitochondrial oxidative capacity and NAD(+) biosynthesis are reduced in human sarcopenia across ethnicities. Nat Commun. (2019) 10:5808. doi: 10.1038/s41467-019-13694-1
49. Meng SJ, Yu LJ. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. (2010) 11:1509–26. doi: 10.3390/ijms11041509
50. Xu H, Brown JL, Bhaskaran S, Van Remmen H. Reactive oxygen species in the pathogenesis of sarcopenia. Free Radic Biol Med. (2025) 227:446–58. doi: 10.1016/j.freeradbiomed.2024.11.046
51. Cho AR, Kwon YJ, Lim HJ, Lee HS, Kim S, Shim JY, et al. Oxidative balance score and serum γ-glutamyltransferase level among Korean adults: a nationwide population-based study. Eur J Nutr. (2018) 57:1237–44. doi: 10.1007/s00394-017-1407-1
52. Nanri H, Hara M, Nishida Y, Shimanoe C, Li YS, Kasai H, et al. The association between oxidative balance score and urinary levels of 8-hydroxydeoxyguanosine among Japanese adults. Nutrients. (2023) 15:4533. doi: 10.3390/nu15214533
53. Kong SY, Bostick RM, Flanders WD, McClellan WM, Thyagarajan B, Gross MD, et al. Oxidative balance score, colorectal adenoma, and markers of oxidative stress and inflammation. Cancer Epidemiol Biomarkers Prev. (2014) 23:545–54. doi: 10.1158/1055-9965.EPI-13-0619
54. Nanri H, Hara M, Nishida Y, Shimanoe C, Higaki Y, Tanaka K. Association between oxidative balance score and inflammatory markers in middle-aged and older Japanese people. Am J Hum Biol. (2024) 36:e24059. doi: 10.1002/ajhb.24059
Keywords: oxidative balance score, oxidative stress, sarcopenia, metabolic syndrome, NHANES
Citation: Wang M and Shi H (2025) Oxidative balance score is independently associated with reduced prevalence of sarcopenia among US adults with metabolic syndrome. Front. Nutr. 12:1529140. doi: 10.3389/fnut.2025.1529140
Received: 16 November 2024; Accepted: 24 March 2025;
Published: 08 April 2025.
Edited by:
Mateusz Maciejczyk, Medical University of Bialystok, PolandReviewed by:
Owen Kelly, Sam Houston State University, United StatesRaquel Evelyn Horowitz, Brooklyn Hospital Center, United States
Copyright © 2025 Wang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huan Shi, bHl3MTEyMjg4QDE2My5jb20=
 Miaohong Wang
Miaohong Wang Huan Shi
Huan Shi