
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 09 April 2025
Sec. Nutritional Epidemiology
Volume 12 - 2025 | https://doi.org/10.3389/fnut.2025.1527522
Background: Colorectal cancer (CRC) is a major global health issue, with rising incidence and mortality rates. Dietary factors, especially whole grains consumption, are critical in determining CRC risk. Understanding CRC deaths and disability-adjusted life years (DALYs) related to low whole grains diets is important for prevention. The purpose of the study is to investigate temporal and geographic trends in CRC deaths and DALYs attributable to diet low in whole grains at the global, regional, and national levels from 1990 to 2021.
Methods: The data on CRC burden attributable to diet low in whole grains from 1990 to 2021 were extracted from the Global Burden of Diseases (GBD) 2021 database. We described the CRC burden attributable to diet low in whole grains across various years, genders, age groups (5-year age groups from 25 to 94 years and 95+ years), different Socio-demographic Index (SDI) regions and countries. To illustrate the temporal trends in the burden of CRC, we calculated the estimated annual percentage change (EAPC) from 1990 to 2021.
Results: From 1990 to 2021, the global number of CRC deaths attributable to diet low in whole grains increased from 101,813 (95% UI: 42,588 to 151,170) to 186,257 (95% UI: 76,127 to 284,803), representing a 82.94% growth. Similarly, the number of DALYs increased from 2,540,867 (95% UI: 1,050,794 to 3,754,416) to 4,327,219 (95% UI: 1,754,865 to 6,578,232), representing a 70.30% growth. However, both the age-standardized mortality rate (ASMR) and age-standardized DALY rate (ASDR) exhibited a decline, with an EAPC of −0.82 (95% CI: −0.85 to −0.78) and − 0.84 (95% CI: −0.87 to −0.81), respectively. The disease burden is heavier in high SDI and high-middle SDI regions. However, between 1990 and 2021, the only region where both ASMR and ASDR increased was low-middle SDI, while in all other regions, they showed a declining trend. In 2021, East Asia had the highest number of CRC deaths and DALYs attributable to diet low in whole grains at the regional level, followed by Western Europe and High-income North America. Additionally, the burden is greater among males and the elderly. Between 1990 and 2021, the number of CRC deaths attributable to diet low in whole grains rose by 102.13% among males and by 63.20% among females. Generally, both the global age-specific mortality rate and the DALYs rate tend to increase with age. SDI demonstrates a nonlinear “S”-shaped correlation with both ASMR and ASDR of CRC attributable to diet low in whole grains. In 2021, the EAPC in ASMR of CRC attributable to diet low in whole grains was negatively associated with SDI (R = −0.402, p < 0.001), reaching the highest EAPC at approximately SDI of 0.51 and the lowest at 0.85. Similarly, the correlation between EAPC in ASDR and SDI in 2021 exhibited a similar pattern.
Conclusion: Despite a decline in the ASMR and ASDR of CRC attributable to diet low in whole grains from 1990 to 2021 globally, the absolute number of cases continues to increase, with a particularly notable burden observed in High-middle and High SDI regions, as well as among males and the elderly population. It is imperative to intensify efforts in CRC prevention and health education, specifically targeting these high-risk groups to raise public awareness and consumption of whole grains. Furthermore, screening initiatives should be intensified among these demographics to address the elevated risk of CRC mortality due to insufficient whole grains consumption.
Colorectal cancer (CRC) refers to cancer that occur in the colon or rectum, which ranks as the third most commonly diagnosed cancer and holds the second position in terms of cancer-related death globally. Its incidence and mortality account for 9.6 and 9.3% of new cancer cases and deaths worldwide, respectively (1). According to the predictions of human disease progression, the global burden of CRC is expected to increase to 3.2 million new cases and 1.6 million deaths by 2040 (2).
Today, highly developed countries remain high-risk areas, such as Australia, New Zealand, Canada and others. The incidence of CRC is about three fold higher in high and very high human development index (HDI) countries compared to low and medium HDI countries (3). The mortality rate of early-onset colorectal cancer (below 50 years of age) is also on the rise. In the 1990s, CRC ranked as the fourth leading cause of cancer-related death among both men and women under 50 in the United States, however, it has since risen to become the leading cause of cancer death in men and the second leading cause in women within this age group (4).
In 2022, China saw 517,000 new cases of colorectal cancer, accounting for 10% of all newly diagnosed malignant tumors and ranking second in the incidence spectrum (5). Obesity, physical inactivity, poor diets, alcohol drinking and smoking are the causes of the high incidence of CRC in highly developed countries (6). With the Westernization of lifestyles, the incidence and mortality of CRC are rising rapidly and the burden is increasing in many less-developed countries, especially in Eastern Europe, Asia and South America (3, 7). CRC, as a malignant tumor that poses a serious threat to human health, necessitates the formulation and implementation of prevention strategies to alleviate the disease burden at both the societal and individual levels.
CRC is a multifactorial disease. Its onset involves the complex interplay of various factors such as genetics, environment, dietary habit and lifestyle (8, 9). Numerous studies have demonstrated that diets low in whole grains is associated with an increased risk of CRC (10–12). Whole grains, such as brown rice, oats, and whole wheat bread, retain more nutrients compared to refined grains (like white rice and white flour products), including B vitamins, magnesium, iron, and dietary fiber. The dietary fiber in whole grains can promote intestinal motility, increase stool volume, dilute carcinogens, affect bile acid metabolism, and enhance the production of short-chain fatty acids, thereby reducing the risk of CRC (13, 14). Polyphenols, as another pivotal functional compound in whole grains, can exert an inhibitory effect on colorectal cancer by influencing the colonic microbiota (15) and the inflammatory process (16). A prospective study has found that for additional 30 g/day of whole grains intake, the risk of CRC can be reduced by approximately 17% (17).
However, the epidemiological research studies of CRC burden attributable to diet low in whole grains at the global, regional, and national levels remains unclear. To fill this critical gap in the literature, our objective was to investigate the global, regional, national burden of CRC deaths and disability-adjusted life-years (DALYs) attributable to diet low in whole grains between 1990 and 2021.
All data were from the GBD 2021 through their official website at https://GHDx.healthdata.org/GBD-2021/GBD-results-tool. The GBD is a publicly accessible database, that provides estimates of 371 diseases and injuries burden in 204 countries and regions from 1990 to 2021, including incidence, prevalence, mortality, Disability-Adjusted Life Years (DALYs), Years Lived with Disability (YLDs), Years of Life Lost (YLLs) and healthy life expectancy (HALE) (18). We obtained the CRC deaths, DALYs, age-standardized mortality rates (ASMR), and age-standardized DALY rates (ASDR) due to a diet low in whole grains from GBD 2021. Based on the extracted data, we described the CRC burden attributable to diet low in whole grains across various years, genders, age groups (5-year age groups from 25 to 94 years and 95+ years), different SDI regions and countries.
Whole grains intake is defined as the average daily consumption (in grams per day) of whole grains and their products, which encompass breakfast cereals, breads, rice, biscuits, muffins, tortillas, pancakes, pasta, and other related sources. A diet low in whole grains is defined as an average daily consumption is less than 140-160 g/d (19). In GBD 2021, CRC is defined as C18–C21.9, D01.0–D01.3, D12–D12.9, D37.3–D37.5. As a comprehensive indicator of socio-demographic development, the Socio-Demographic Index (SDI) is derived from an extensive assessment that includes the total fertility rate of females younger than 25 years old, average education level of those aged 15 years and older, per capita income distribution, ranged from 0 to 1 (18). It exhibits a strong correlation with indicators related to healthy life years and disease burden, making it a valuable tool for evaluating the health development status and disease burden across countries and regions. The SDI values divide all countries and regions into five levels: low SDI, low-middle SDI, middle SDI, high-middle SDI, and high SDI. DALY is an indicator used to measure the burden of disease. It quantifies the comprehensive loss to population health caused by illness, disability, and premature death by combining the years of premature mortality and years lived with disability (18, 20).
The burden of CRC attributable to diet low in whole grains was quantified based on the number of deaths, DALYs, ASMR, and ASDR. ASR a classical epidemiological method used to standardize the incidence or mortality rate of a certain health event across different countries or regions, as well as over various time periods. This method allows for rate data from different regions and populations to be compared in a comparable manner. ASR is calculated as follows:
where ai is the age-specific rate (i denotes the ith age class) and wi is the population count (or weight) corresponding to the specific age group i in the selected standard population. All rates are expressed as per 100,000 people. Additionally, estimated Annual Percentage Change (EAPC) is often used to analyze the trends of indicators such as ASMR or ASDR from 1990 to 2021, which is calculated as EAPC = 100(exp(β)-1) (21). By calculating EAPC, we can know the annual percentage change of ASR over a period of time, thereby judging the trend of disease burden in different countries and regions. For instance, if both the EAPC and its 95% confidence interval are>0, it signifies an annual increase in ASR. Conversely, if both the EAPC and its 95% confidence interval are<0, which indicates a decrease in ASR. What’s more, the ASR would be regarded as stable if the 95% CI contained 0. Finally, to identify the factors influencing EAPC, we conducted a Pearson correlation analysis to examine the correlation between ASMR or ASDR in 1990, SDI in 2021 and EAPC at the national level. All statistical analyses and data visualization were performed using R software (version 4.4.1) (22).
From 1990 to 2021, the global number of CRC deaths attributable to diet low in whole grains increased from 101,813 (95% UI: 42,588 to 151,170) to 186,257 (95% UI: 76,127 to 284,803), representing a 82.94% growth. Similarly, the number of DALYs increased from 2,540,867 (95% UI: 1,050,794 to 3,754,416) to 4,327,219 (95% UI: 1,754,865 to 6,578,232), representing a 70.30% growth. However, the ASMR decreased from 2.79 per 100,000 population (95% UI: 1.17 to 4.15) to 2.21 per 100,000 population (95% UI: 0.91 to 3.38), with an EAPC of −0.82 (95% CI: −0.85 to −0.78). Similarly, the ASDR declined from 63.47 per 100,000 people (95% UI: 26.35 to 93.84) to 50.19 per 100,000 population (95% UI: 20.37 to 76.30), with an EAPC of −0.84 (95% CI: −0.87 to −0.81) (Table 1).
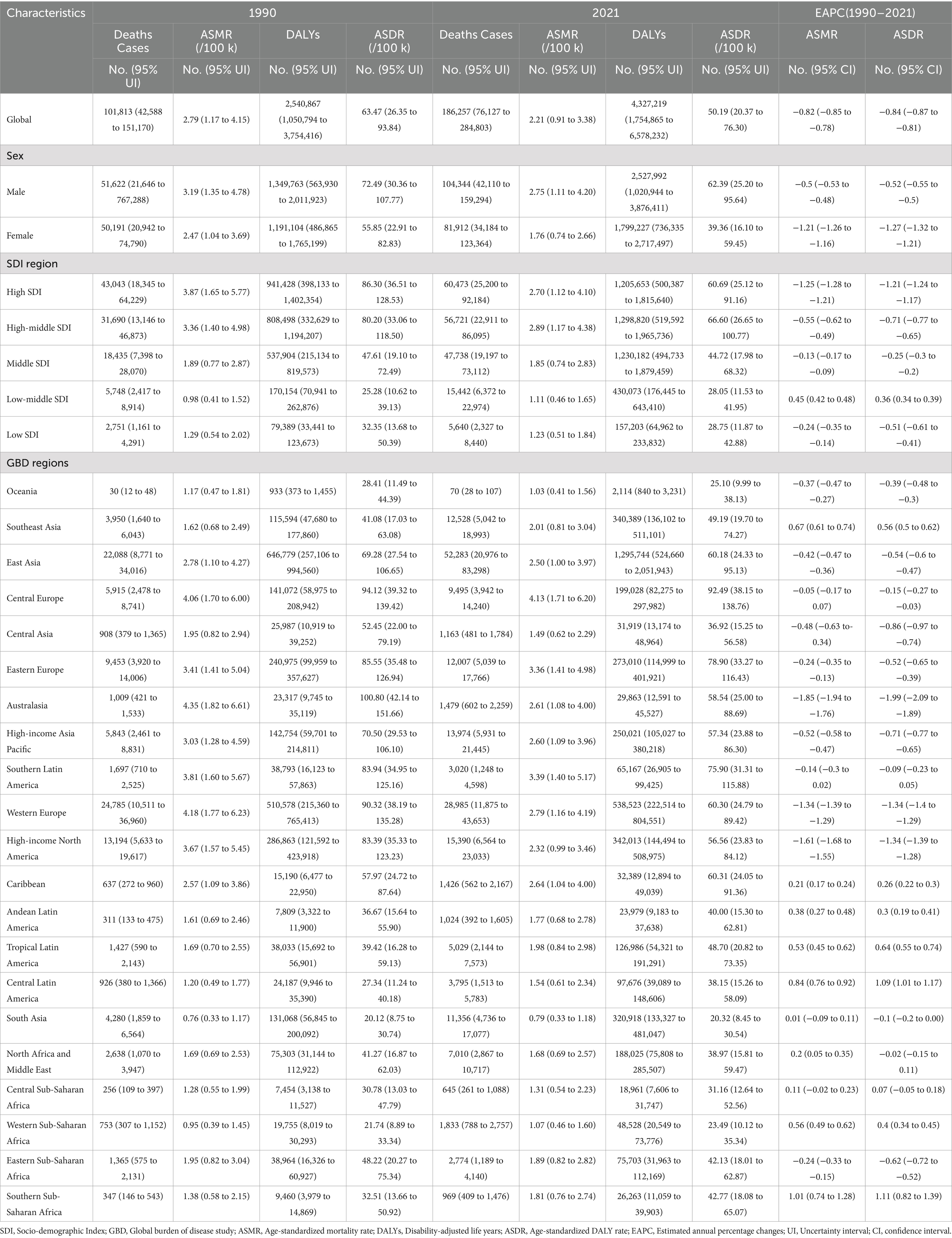
Table 1. Burden of CRC attributable to diet low in whole grains in Global, SDI regions, and GBD regions.
At the SDI regional level, the number of CRC deaths attributable to diet low in whole grains was highest in high SDI regions and lowest in low SDI regions in both 1990 and 2021. In 1990, the number of CRC DALYs attributable to diet low in whole grains was highest in high SDI regions, at 941,428 (95% UI: 398,133 to 1,402,354). While in 2021, the highest number of DALYs was observed in high-middle SDI regions, at 1,298,820 (95% UI: 519,592 to 1,965,736). At the same time, high-middle SDI regions had the greatest ASMR [2.89 (95% UI: 1.17 to 4.38)] and ASDR [66.60 (95% UI: 26.65 to 100.77)] per 100,000, respectively. Between 1990 and 2021, the only region where both ASMR and ASDR increased was Low-middle SDI, while they declined in all other regions (Table 1; Figures 1A,B).

Figure 1. Number and rate of colorectal cancer deaths (A) and DALYs (B) attributable to diet low in whole grains from 1990 to 2019 by SDI region. The bars represent the number of colorectal cancer deaths (A) and DALYs (B) attributable to diet low in whole grains from 1990 to 2019 in the SDI regions. The line represents ASMR (A) and ASDR (B) (per 100,000) attributable to diet low in whole grains from 1990 to 2019 in the global and SDI regions. ASMR, Age-standardized mortality rate; ASDR, Age-standardized DALY rate; DALYs, Disability-adjusted life years; SDI, Socio-demographic index.
At the regional level, East Asia had the highest number of CRC deaths [52,283 (95% UI: 20,976 to 83,298)] and DALYs [1,295,744 (95% UI: 524,660 to 2,051,943)] attributable to diet low in whole grains in 2021, followed by Western Europe and High-income North America. Also in 2021, South Asia had the lowest ASMR [0.79 (95% UI: 0.33 to 1.18)] and ASDR [20.32 (95% UI: 8.45 to 30.54)], while Central Europe had the highest ASMR [4.13 (95% UI: 1.71 to 6.20)] and ASDR [92.49 (95% UI: 38.15 to 138.76)]. Australasia showed the largest decrease in the ASMR and ASDR of CRC attributable to diet low in whole grains, with an EAPC of −1.85 (95% CI: −1.94 to −1.76) in ASMR and an EAPC of −1.99 (95% CI: −2.09 to −1.89) in ASDR. While, Southern Sub-Saharan Africa showed the largest increase with an EAPC of 1.01 (95% CI: 0.74 to 1.28) in ASMR and an EAPC of 1.11 (95% CI: 0.82 to 1.39) in ASDR (Table 1).
At the national level, China had the highest number of CRC deaths [49,991 (95% UI: 20,100 to 79,929)] and DALYs [1,241,928 (95% UI: 503,165 to 1,978,508)] attributable to diet low in whole grains in 2021. The number of deaths increased by 134.37% compared to 1990, and the DALYs increased by 98.73% compared to 1990. The United States of America followed closely, with 13,474 (95% UI: 5,747 to 20,241) and 303,637 (95% UI: 129,374 to 454,027), respectively. In contrast, Niue, Tokelau, Nauru, Tuvalu and Cook Islands showed the lowest level. Also in 2021, the highest ASMR was found in Uruguay [5.18 (95% UI: 2.09 to 7.73)], followed by Hungary, Bulgaria, Monaco, Slovakia, Poland, Croatia, Greenland and Barbados, with an ASMR of over 4 per 100,000 population. Meanwhile, Hungary had the highest ASDR, at 113.76 (95% UI: 47.61 to 173.37) in 2021, followed by Bulgaria, Uruguay, Slovakia, Greenland and Monaco, with an ASDR of over 100 per 100,000 population (Supplementary Table S1; Figures 2A,B). Notably, Lesotho had the highest increases in ASMR and ASDR from 1990 to 2021, with EAPCs of 2.77 (95% CI: 2.37 to 3.17) and 3.00 (95% CI: 2.56 to 3.44), followed by Cabo Verde. In contrast, Austria experienced the fastest decline in ASMR from 1990 to 2021, with an EAPC of −2.32 (95% CI: −2.37 to −2.27), followed by Israel, Maldives, Singapore and Germany. Maldives experienced the fastest decline in ASDR, with an EAPC of −2.52 (95% CI: −2.65 to −2.38), followed by Austria, Singapore, Israel and Luxembourg (Supplementary Table S1; Figures 2C,D).
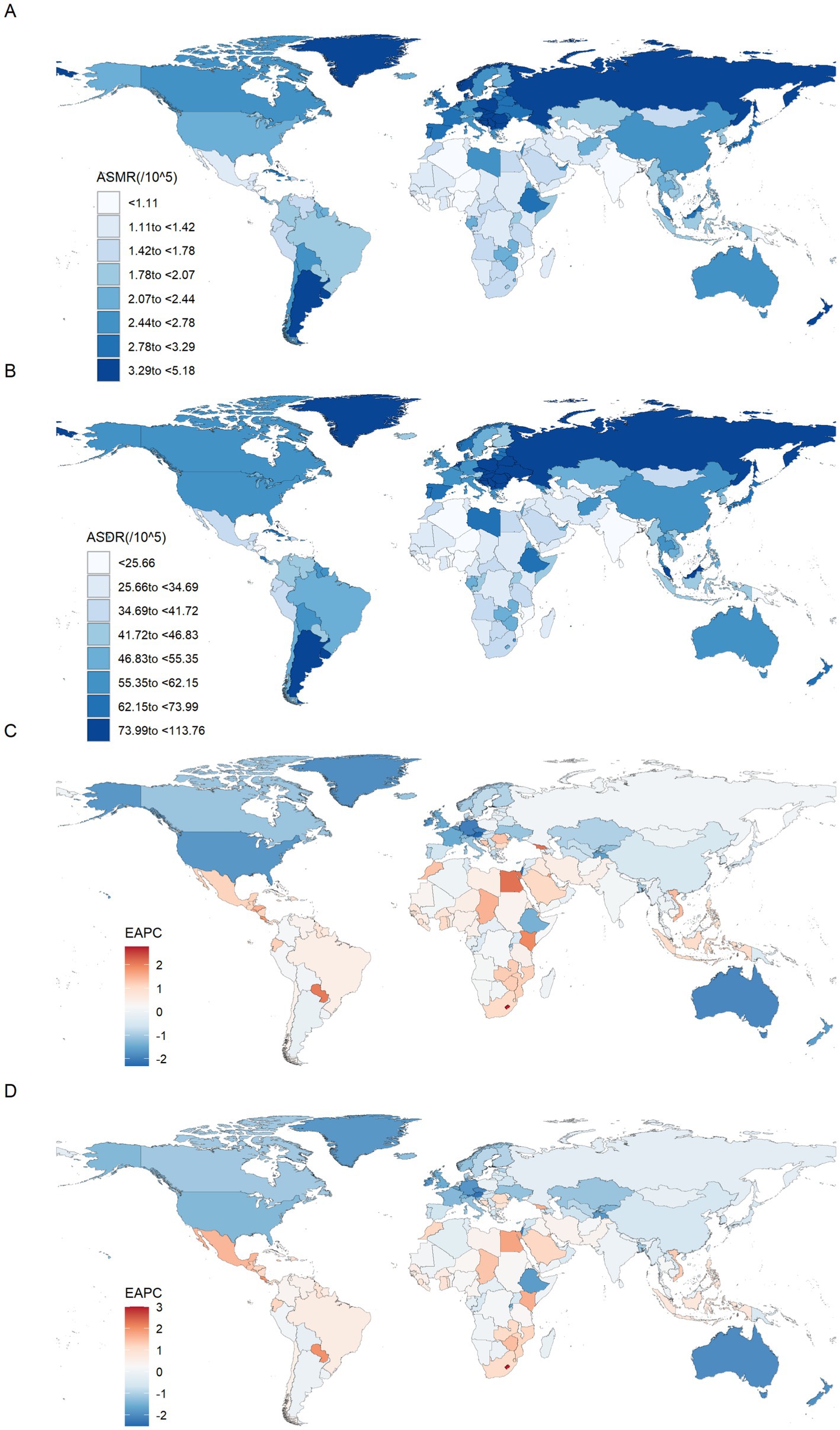
Figure 2. The spatial distribution of the colorectal cancer ASMR (A) and ASDR (B) attributable to diet low in whole grains in 2021, and the EAPC in colorectal cancer ASMR (C) and ASDR (D) attributable to diet low in whole grains. ASMR, Age-standardized mortality rate; ASDR, Age-standardized DALY rate; EAPC, Estimated annual percentage changes.
In 2021, the number of CRC deaths attributable to diet low in whole grains globally was predominantly concentrated in the 70–74 age group, while DALYs were mainly concentrated in the 65–69 age group. In the case of individuals under the age of 60, the greatest numbers of deaths and DALYs are observed in the middle SDI regions. For those between the ages of 60 and 75, the greatest numbers are seen in high-middle SDI regions. Finally, for individuals aged 75 and above, the greatest numbers are observed in high SDI regions. In general, the global age-specific mortality rate and the DALYs rate increase with age. However, in the low and middle SDI regions, the age-specific mortality and DALY rates exhibit a decline at ages above 90. In the low-middle SDI region, the age-specific DALYs rate demonstrates a fluctuating decline when age reaches 70 and above (Figures 3A,B). From 1990 to 2021, the age-specific mortality rate and DALYs rate for all age groups globally exhibited a downward trend, with the largest decline observed in the 35–39 age group. In the high SDI regions, the age-specific mortality rate for the 25–94 age group exhibited a decline, whereas the rate for those over 95 remained stable. The age-specific DALYs rate exhibited a decline across all age groups. In the high-middle SDI regions, the age-specific mortality rate and DALYs rate remained stable for the 80–84 and over 90 age groups, while an increase was observed in the 85–89 age group. For the remaining age groups, both the age-specific mortality rate and DALYs rate exhibited a downward trend. In the middle SDI regions, the age-specific mortality rate increased among individuals over 75 and decreased among those aged 25–69. Similarly, the age-specific DALYs rate exhibited an increase among individuals over 70 and a decline among those aged 25 to 64. In the Low-middle SDI regions, the age-specific mortality rate and DALYs rate exhibited a downward trend among individuals aged 25–34, while they showed an upward trend among those over 40. In the Low SDI regions, the age-specific mortality rate and DALYs rate declined among individuals aged 25–79, but increased among those over 80 (Figures 4A,B).
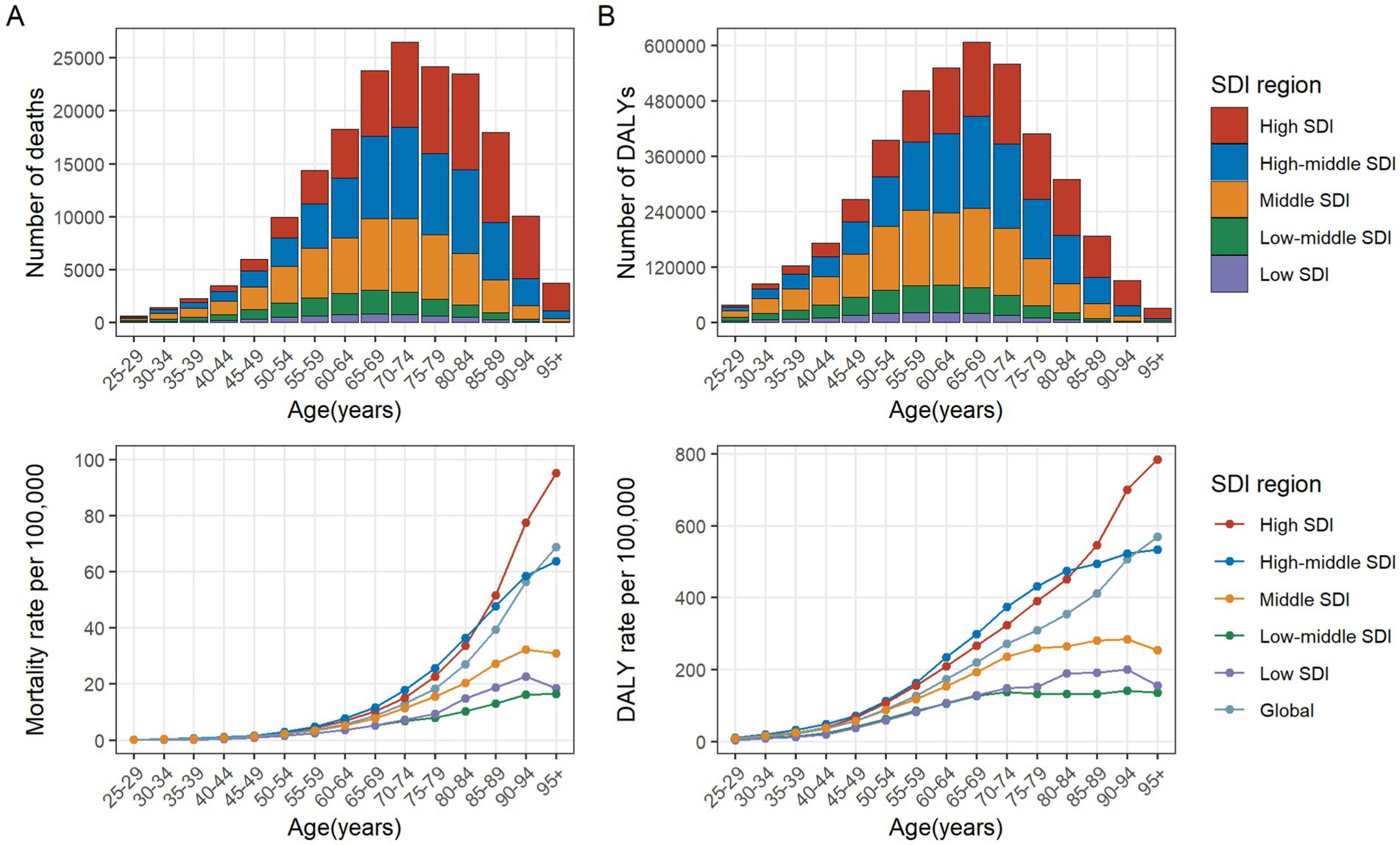
Figure 3. Number and rate of colorectal cancer deaths (A) and DALYs (B) attributable to diet low in whole grains by age group and SDI region in 2021. The bars represent the number of colorectal cancer deaths (A) and DALYs (B) attributable to diet low in whole grains among different age groups in the SDI regions in 2021. The line represents the rates of mortality (A) and DALYs (B) of colorectal cancer due to diet low in whole grains among different age groups in the global and SDI regions in 2021. DALYs, Disability-adjusted life years; SDI, Socio-demographic index.
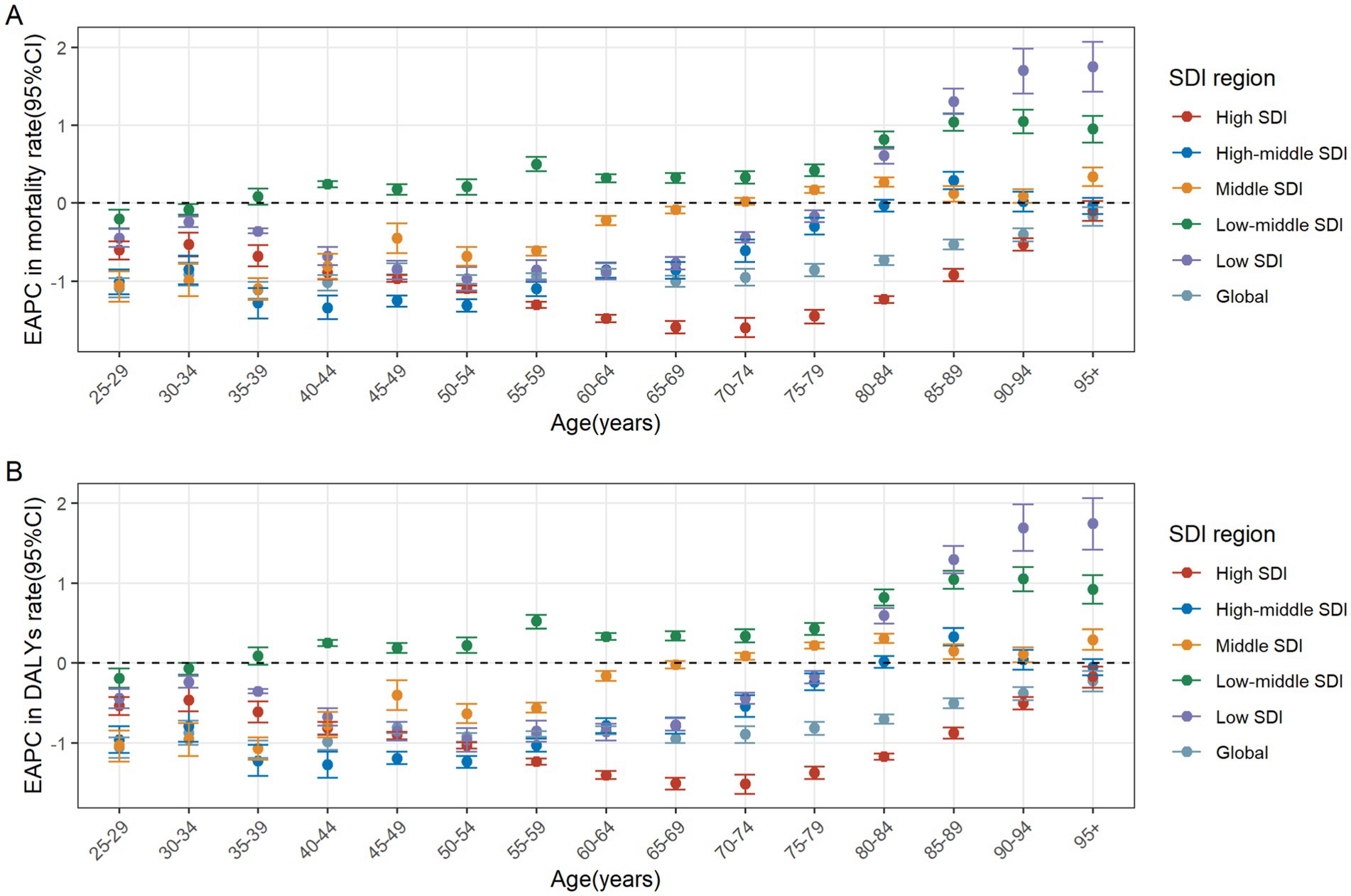
Figure 4. The age distribution of the trends in colorectal cancer mortality rate (A) and DALYs rate (B) attributable to diet low in whole grains from 1990 to 2021 by SDI region. DALYs, Disability-adjusted life years; EAPC, Estimated annual percentage changes; CI, confidence interval; SDI, Socio-demographic index.
From 1990 to 2021, the number of male deaths increased from 51,622 (95% UI: 21,646 to 767,288) to 104,344 (95% UI: 42,110 to 159,294), representing a growth rate of 102.13%. Similarly, the number of female deaths increased from 50,191 (95% UI: 20,942 to 74,790) to 81,912 (95% UI: 34,184 to 123,364), representing a 63.20% rise (Table 1). From 1990 to 2021, the average annual decrease in ASMR of CRC attributable to diet low in whole grains was −0.5(95% CI: −0.53 to −0.48) for males, and the average annual decrease was −1.21 (95% CI: −1.26 to −1.16) for females (Table 1). In 2021, globally, the number of CRC deaths and DALYs attributable to diet low in whole grains was consistently higher among males than females in the 25–84 age group, with the reverse pattern observed for those aged 85 and above. The peak number of deaths was reached among males aged 70–74 and females aged 80–84 (Figure 5A). The number of DALYs follows a normal distribution, peaking in the 65–69 age group for both males and females (Figure 5B). The age-specific mortality rate and DALYs rate per 100,000 cases of CRC attributable to diet low in whole grains exhibit an upward trend with advancing age, and consistently demonstrate higher values in males compared to females in 2021 (Figures 5A,B). Among males, from 1990 to 2021, the age-specific mortality rate and DALYs rate declined in the 25–89 age group, while remaining stable in the 90+ age group. The most significant decline was observed in the 35–39 age group (Figures 6A,B). In the case of females, a decline was observed in the age-specific mortality rate and DALYs rate across all age groups, with the most notable decrease also occurring in the 35–39 age group (Figures 6A,B).
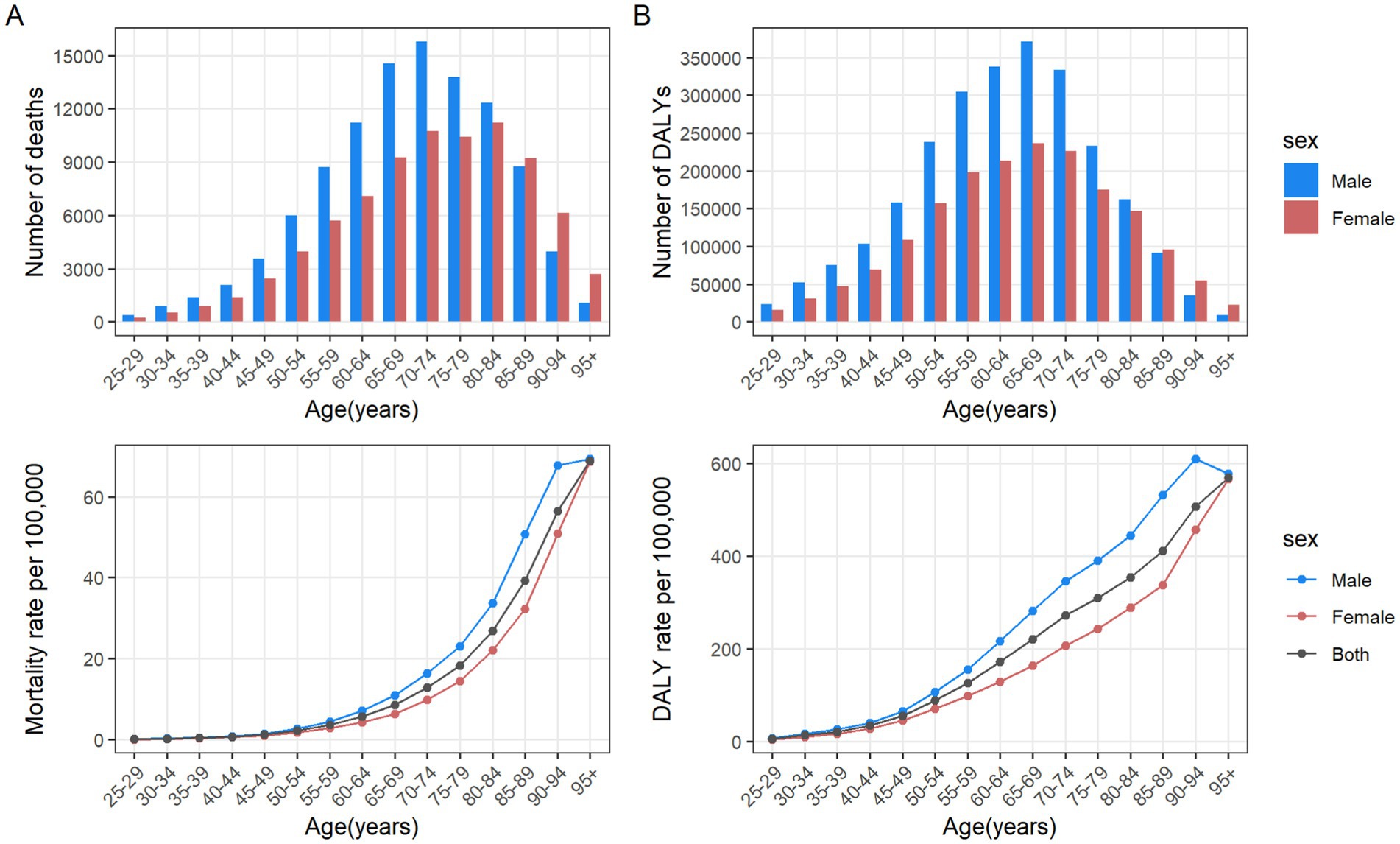
Figure 5. Number and rate of colorectal cancer deaths (A) and DALYs (B) attributable to diet low in whole grains by age group and sex in the global in 2021. The bars represent the number of colorectal cancer deaths (A) and DALYs (B) attributable to diet low in whole grains among different age groups and genders. The line represents the rates of mortality (A) and DALYs (B) of colorectal cancer due to diet low in whole grains among different age groups and genders. DALYs, Disability-adjusted life years.
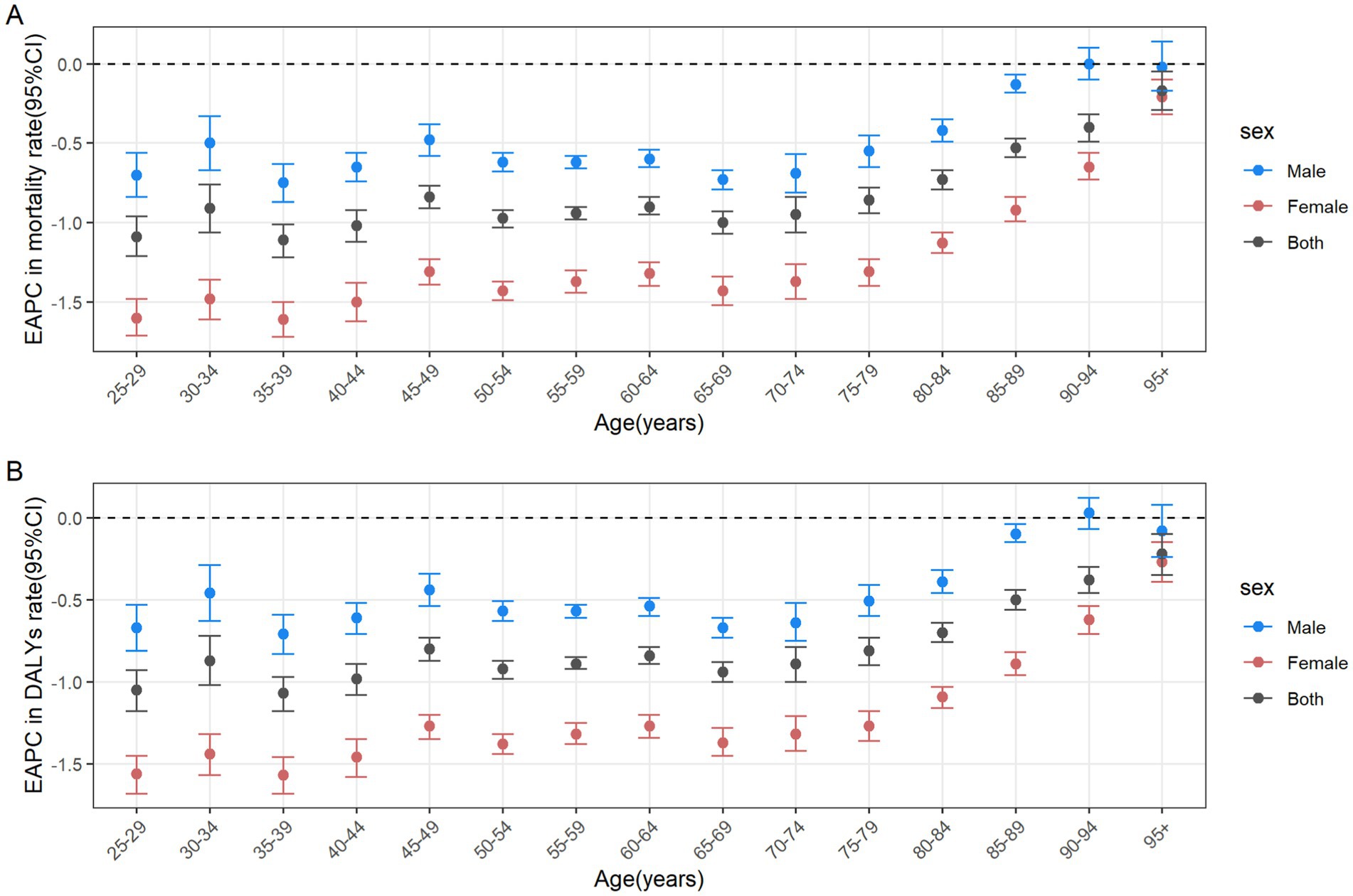
Figure 6. The age distribution of the trends in colorectal cancer mortality rate (A) and DALYs rate (B) attributable to diet low in whole grains from 1990 to 2021 among different genders. DALYs, Disability-adjusted life years; EAPC, Estimated annual percentage changes; CI, confidence interval.
In general, there was a nonlinear “S”-shaped correlation between SDI and the ASMR and ASDR of CRC attributable to diet low in whole grains. When SDI was between 0.39 and 0.74, both ASMR and ASDR showed an increasing trend, peaking at an SDI of approximately 0.74. This suggested that the greatest burden of death and disability due to CRC occurs at middle to high levels of SDI. Conversely, when SDI was greater than 0.74, both ASMR and ASDR decreased significantly. Among the different regions, Central Europe had the highest ASMR and ASDR of CRC attributable to diet low in whole grains, while South Asia had the lowest (Figures 7A,B).
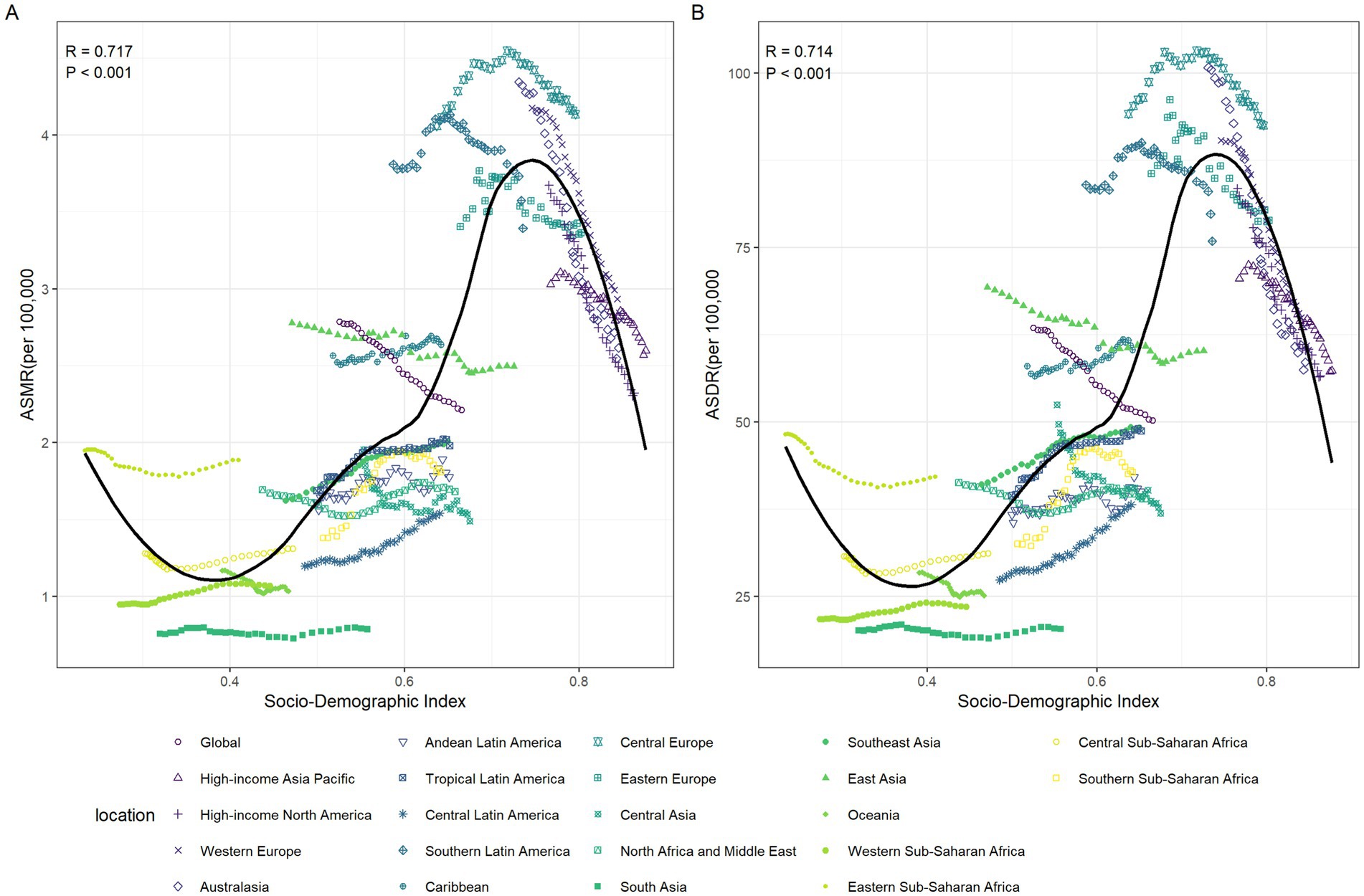
Figure 7. The relationships between the SDI and the colorectal cancer burdens attributable to diet low in whole grains among the 21 GBD regions between 1990 and 2021. The association between colorectal cancer attributable to diet low in whole grains ASMR and SDI among 21 GBD regions (A). The association between colorectal cancer attributable to diet low in whole grains ASDR and SDI among 21 GBD regions (B). ASMR, Age-standardized mortality rate; ASDR, Age-standardized DALY rate; SDI, Socio-demographic index; GBD: global burden of disease.
The EAPC in ASMR and ASMR in 1990 showed a significant negative correlation (R = −0.612, p < 0.001) (Figure 8A), as well as the EAPC in ASDR and ASDR in 1990 (R = −0.591, p < 0.001) (Figure 8B). In 2021, the EAPC in ASMR of CRC attributable to diet low in whole grains was negatively associated with SDI (R = −0.402, p < 0.001), reaching the highest EAPC at approximately SDI of 0.51 and the lowest at 0.85 (Figure 8C). Similarly, the correlation between EAPC in ASDR and SDI in 2021 exhibited a similar pattern (Figure 8D).
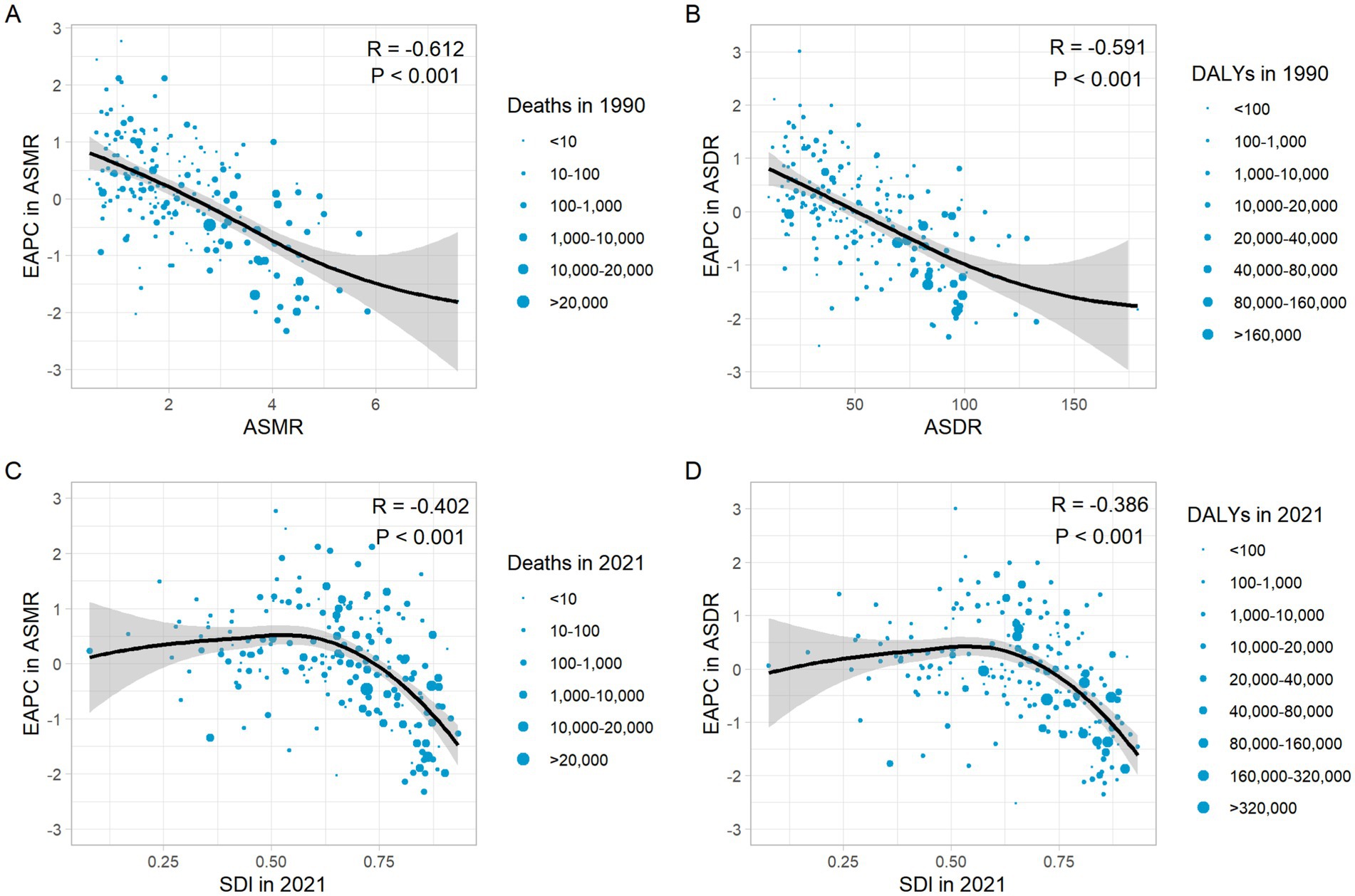
Figure 8. The correlation between EAPC in ASMR and ASMR in 1990 (A). The correlation between EAPC in ASDR and ASDR in 1990 (B). The correlation between EAPC in ASMR and SDI in 2021 (C). The correlation between EAPC in ASDR and SDI in 2021 (D). ASMR, Age-standardized mortality rate; ASDR, Age-standardized DALY rate; DALYs, Disability-adjusted life years; SDI, Socio-demographic index.
Dietary risk factors have a significant impact on population health and are among the primary risk factors contributing to global diseases and deaths. The GBD 2017 Diet Collaborators revealed that inadequate whole grains intake is one of the leading dietary risk factors for the increase in global DALYs (23). In this study, we conducted an analysis of global, regional, and national spatio-temporal trends in mortality and DALY of CRC attributable to diet low in whole grains.
It was found that from 1990 to 2021, the ASMR and ASDR of CRC attributable to diet low in whole grains declined globally, with a faster decline observed in females compared to males. However, the absolute number of deaths and DALYs increased annually by 82.94 and 70.30% respectively, this increase posing a persistent health challenge. Population growth and changes in age structure may account for this increase. In addition, among people aged 25–84 years, the global burden of CRC deaths and DALYs attributable to diet low in whole grains was significantly higher in males than in females, in contrast to the situation among people aged 85 years and older.
This burden was closely related to socioeconomic development, and notable differences exist in the spatial distribution of CRC burden across different countries and regions. Over the past three decades, the burden of CRC attributable to diet low in whole grains has been relatively high in high, high-middle, and middle SDI regions, especially in East Asia, Western Europe, and High-income North America. However, their ASMR and ASDR have shown a declining trend, with the most significant decline observed in high SDI regions. This may be attributed to a multitude of factors. For example, cultural practices and dietary habits unique to these regions play a crucial role. In East Asia, the high consumption of processed meats and refined grains, coupled with a lower intake of whole grains, likely contributes to the elevated CRC burden (24–26). Additionally, these variations could be influenced by socio-economic and demographic factors, including access to healthcare, smoking rates, alcohol consumption, and levels of physical activity (27). Despite initially higher CRC burdens, high SDI regions may benefit from advancements in healthcare, which have led to observed declines in ASMR and ASDR. In contrast, some less developed regions, including Southern Sub-Saharan Africa, Central Latin America and Western Sub-Saharan Africa, had maintained a relatively low number of deaths and DALYs of CRC attributable to diet low in whole grains. Yet, the ASMR and ASDR in these regions were gradually increasing. In 2021, Uruguay had the highest ASMR, while Bangladesh had the lowest. China ranked 132nd among the 204 countries and regions. In addition, a significant negative correlation was observed between the EAPC in ASMR and the ASMR in 1990. This highlights that even countries with initially lower mortality rates may experience a rapid increase in mortality over time. These findings have important clinical implications, raising public awareness of the importance of increasing whole grains consumption and providing a basis for policymakers to formulate targeted dietary strategies to more effectively address the issue of CRC.
Whole grain foods encompass a wide array of potential cancer-fighting compounds, including antioxidants, trace minerals, phytates, phenolic acids, phytoestrogens and fiber (28). Many studies of whole grains consumption found a negative association between CRC risk and whole grains intake (29–31). However, the mechanism underlying the reduced risk of CRC associated with increased whole grains consumption remains unclear. In intestinal diseases, the primary components of whole grains that exert effects are dietary fiber and polyphenols (32). Dietary fiber has been demonstrated to promote intestinal motility and increases the frequency of bowel movements, thereby diluting and reducing toxins and harmful substances in the intestine (6). A meta-analysis of 25 prospective studies found that for every 10 g/day increase in dietary fiber intake, there is a 10% reduction in the incidence of colorectal cancer (17). Whole grains polyphenols possess antioxidant and anti-inflammatory properties, capable of scavenging free radicals in the body and inhibiting the proliferation of tumor cells (33, 34). Moreover, there may be a dose–response relationship between the inhibitory effects of these substances on colon cancer cells (35). The more whole grains consumed, the more dietary fiber, polyphenols, and other plant-based active ingredients from whole grains are ingested. When these components accumulate to a certain level in the human body, they can more effectively exert their anticancer effects.
The Dietary Guidelines for Americans (2020–2025) recommend a daily intake of six ounce-equivalents of grains, with a minimum of half of this quantity derived from whole grains sources (36). Nevertheless, the entire American population, regardless of age, continues to fall short of the recommended intake of at least one serving of whole grains per day (37). This is primarily due to the gradual premiumization of consumption trends in developed countries, where there is a preference for high-protein, high-nutrition animal-based products. While the burden is concentrated mainly in high-SDI countries, these countries have seen an increase in whole grains consumption in recent decades, primarily attributed to the formulation of relevant dietary guidelines (38, 39).
As a populous country, China also has the highest number of CRC deaths and DALYs attributable to diet low in whole grains (40). In China, although grains have historically been regarded as a traditional dietary staple, there has been a notable decline in the consumption of whole grains among the Chinese population since 1982. Conversely, there has been a steady increase in the consumption of refined grains during the same period (41). Statistical data indicates that the mean daily consumption of whole grains among Chinese residents in 2018 was only 20.1 grams, a figure that falls significantly below the recommended range of 50–150 grams as outlined in the Dietary Guidelines for Chinese Residents (42). The primary factors contributing to this situation are the rapid economic development and changes in dietary patterns, especially the increased consumption of processed foods and fast food, which has resulted in a notable decline in the consumption of traditional whole grains diets. This phenomenon is particularly prominent in developing countries. In some less developed countries in Africa, the Middle East, Latin America and Southeast Asia, the intake of whole grains is deemed to be adequate. However, it is concerning that the ASMR and ASDR in these countries are showing an upward trend year by year, which is closely related to the lagging medical conditions in these regions. It is therefore recommended that policymakers in regions such as Africa, the Middle East, Latin America and Southeast Asia should prioritize the promotion of innovation and advancement in medical technology. At the present time, the consumption of whole grains is more influenced by traditional food consumption habits in specific countries or regions than by adherence to dietary recommendations for whole grains or consumers’ in-depth understanding of the health benefits of whole grains (43). In light of the aforementioned considerations, it is imperative that strategies designed to promote whole grains diets take into account the local cultural context and dietary habits in order to ensure effective implementation.
In 2021, the global burden of CRC attributable to diet low in whole grains was higher among individuals aged 50 to 94, and the age-specific mortality rate and DALYs rate increased with age. This may be due to the decline in general physical condition with age, leading to difficulties in treatment and health management. Additionally, older adults generally have lower whole grains intake compared to younger people due to smaller appetite and weaker digestive function. Therefore, prevention and treatment efforts should be strengthened for the elderly population. It is important to note that due to factors such as being close to the average life expectancy, tolerance for surgical interventions, and the threat of other diseases, elderly individuals should not blindly pursue survival time but should instead prioritize their quality of life.
The findings of our research indicate that the age-specific mortality rate and DALYs rate of CRC attributable to diet low in whole grains are higher in males than in females across all age groups. The precise aetiology of this gender disparity remains unclear; however, genetic and environmental factors are considered to be crucial (44). Compared to females, males typically have diets that encompass a higher prevalence of cancer risk factors, including alcohol consumption, tobacco use, and a higher intake of red meat. Conversely, females tend to engage in less smoking and drinking and consume a larger quantity of fruits and vegetables (45, 46). Studies have also shown that the difference in the incidence and mortality rates of CRC between the sexes is attributed to the role of female estrogens, and relevant epidemiological research results indicate that estrogen replacement therapy can prevent CRC (47, 48). Therefore, we recommend strengthening CRC prevention education and advocating for healthy diets among males, and encouraging females to continue maintaining healthy eating habits, in order to reduce CRC incidence and mortality rates.
To effectively address the escalating burden of colorectal cancer (CRC) attributable to insufficient whole grains intake, a multifaceted and approach tailored to regions with varying levels of development must be implemented. In High SDI and High-middle SDI regions, efforts must involve raising public awareness of the health benefits of whole grains to foster increased daily consumption. Additionally, there is a need to develop scientifically sound and practical dietary guidelines and policies to support these initiatives. Specifically, since processed food consumption is typically higher in these regions, policies should prioritize reducing the intake of such foods and promoting healthier alternatives, such as whole grains. For less developed countries, it is imperative to strengthen healthcare systems, particularly by enhancing early screening and diagnostic capabilities for CRC, to ensure that patients receive timely and effective treatment. Additionally, it is crucial to enhance society’s awareness and acceptance of healthy lifestyles through education and media campaigns, with special attention and guidance given to elderly populations and males.
There are several limitations in this study. First, the data for this study were derived from the GBD 2021. Data collected from low-income countries may vary considerably in accuracy and completeness, potentially impacting the reliability of our findings in these regions. Although the GBD 2021 encompasses a wide range of countries over an extensive time period, it relies on complex estimation and predictive models, thereby introducing a degree of uncertainty. Second, regarding the statistical methodology, the EAPC calculation assumes a linear trend in the data, which may not accurately capture complex or non-linear changes over time, especially when examining long-term health outcome trends. Third, the study, constrained by the limitations of the GBD database, does not account for other dietary factors such as red meat and alcohol consumption, which may interact with whole grains intake and influence colorectal cancer risk. This omission could lead to underestimations or overestimations of the burden attributable to diet low in whole grains.
Despite a decline in the ASMR and ASDR of CRC attributable to diet low in whole grains from 1990 to 2021 on a global scale, the absolute number of cases continues to increase. It is noteworthy that High-middle SDI regions have the highest ASDR and ASMR of CRC attributable to diet low in whole grains in 2021. Furthermore, Low-middle SDI regions are the only ones where both the ASMR and ASDR have shown an upward trend over the past three decades. The study also revealed significant age- and gender-based disparities, indicating that older adults and males may be at an elevated risk of CRC mortality due to insufficient whole grains consumption.
In summary, this finding not only prompts a profound reflection on the current status of CRC prevention and control globally but also serves as a clear guidance for the formulation and implementation of future health promotion strategies. To effectively address this public health challenge, comprehensive measures and precisely targeted interventions are required. Specifically, longitudinal studies should be conducted to assess the impact of increased whole grains consumption on CRC incidence and mortality. In terms of public health interventions, several strategies can be considered to boost whole grains intake. These include public education campaigns to raise awareness about the benefits of whole grains, subsidies for whole grains products to make them more affordable, and changes to food labeling regulations to highlight whole grains content. By adopting these and other innovative approaches, we can work together to mitigate the burden of CRC related to inadequate whole grains consumption.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
YM: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. JN: Data curation, Writing – original draft, Writing – review & editing. PM: Data curation, Writing – review & editing. YC: Data curation, Funding acquisition, Supervision, Writing – review & editing. XG: Funding acquisition, Project administration, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the National Natural Science Foundation of China (82405390 and 82305230); Three-Year Action Plan for Further Accelerating the Inheritance, Innovation, and Development of Traditional Chinese Medicine in Shanghai-Construction of the Shanghai Traditional Chinese Medicine Specialty Alliance for Mixed Hemorrhoids(ZY(2021-2023)-0302); Future Plan for Traditional Chinese Medicine Inheritance and Development of Shanghai Municipal Hospital of Traditional Chinese Medicine (WLJH2021ZY-MZY024 and WLJH2021ZY-GZS003); Shanghai Municipal Science Commission Project (23Y11920800).
Thanks to the Global Burden of Disease study collaborations.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1527522/full#supplementary-material
ASDR, Age-standardized DALY rate; ASMR, Age-standardized mortality rate; CRC, Colorectal cancer; CI, Confidence interval; DALYs, Disability-adjusted life-years; EAPC, Estimated annual percentage change; GBD, Global Burden of Diseases; HALE, Health life expectancy; HDI, Human development index; SDI, Socio-demographic Index; UI, Uncertainty interval; YLD, Years Lived with disability; YLL, Years of Life Lost.
1. Bray, F, Laversanne, M, Sung, H, Ferlay, J, Siegel, RL, Soerjomataram, I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Morgan, E, Arnold, M, Gini, A, Lorenzoni, V, Cabasag, CJ, Laversanne, M, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. (2023) 72:338–44. doi: 10.1136/gutjnl-2022-327736
3. Arnold, M, Abnet, CC, Neale, RE, Vignat, J, Giovannucci, EL, McGlynn, KA, et al. Global burden of 5 major types of gastrointestinal Cancer. Gastroenterology. (2020) 159:335–349.e15. doi: 10.1053/j.gastro.2020.02.068
4. Siegel, RL, Giaquinto, AN, and Jemal, A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
5. Han, B, Zheng, R, Zeng, H, Wang, S, Sun, K, Chen, R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. (2024) 4:47–53. doi: 10.1016/j.jncc.2024.01.006
6. Keum, N, and Giovannucci, E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. (2019) 16:713–32. doi: 10.1038/s41575-019-0189-8
7. Arnold, M, Sierra, MS, Laversanne, M, Soerjomataram, I, Jemal, A, and Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. (2017) 66:683–91. doi: 10.1136/gutjnl-2015-310912
8. Cho, YA, Lee, J, Oh, JH, Chang, HJ, Sohn, DK, Shin, A, et al. Genetic risk score, combined lifestyle factors and risk of colorectal Cancer. Cancer Res Treat. (2019) 51:1033–40. doi: 10.4143/crt.2018.447
9. Bodén, S, Zheng, R, Ribbenstedt, A, Landberg, R, Harlid, S, Vidman, L, et al. Dietary patterns, untargeted metabolite profiles and their association with colorectal cancer risk. Sci Rep. (2024) 14:2244. doi: 10.1038/s41598-023-50567-6
10. Vieira, AR, Abar, L, Chan, DSM, Vingeliene, S, Polemiti, E, Stevens, C, et al. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR continuous update project. Ann Oncol. (2017) 28:1788–802. doi: 10.1093/annonc/mdx171
11. Tabung, FK, Brown, LS, and Fung, TT. Dietary patterns and colorectal Cancer risk: a review of 17 years of evidence (2000-2016). Curr Colorectal Cancer Rep. (2017) 13:440–54. doi: 10.1007/s11888-017-0390-5
12. Tullio, V, Gasperi, V, Catani, MV, and Savini, I. The impact of whole grain intake on gastrointestinal tumors: a focus on colorectal, gastric, and esophageal cancers. Nutrients. (2020) 13:81. doi: 10.3390/nu13010081
13. Mehta, M, and Shike, M. Diet and physical activity in the prevention of colorectal cancer. J Natl Compr Cancer Netw. (2014) 12:1721–6. doi: 10.6004/jnccn.2014.0174
14. Pan, P, Yu, J, and Wang, LS. Colon Cancer: what we eat. Surg Oncol Clin N Am. (2018) 27:243–67. doi: 10.1016/j.soc.2017.11.002
15. Fernández-Navarro, T, Salazar, N, Gutiérrez-Díaz, I, Sánchez, B, Rúas-Madiedo, P, de Los Reyes-Gavilán, CG, et al. Bioactive compounds from regular diet and faecal microbial metabolites. Eur J Nutr. (2018) 57:487–97. doi: 10.1007/s00394-016-1332-8
16. Owczarek, K, and Lewandowska, U. The impact of dietary polyphenols on COX-2 expression in colorectal Cancer. Nutr Cancer. (2017) 69:1105–18. doi: 10.1080/01635581.2017.1367940
17. Aune, D, Chan, DS, Lau, R, Vieira, R, Greenwood, DC, Kampman, E, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. (2011) 343:d6617. doi: 10.1136/bmj.d6617
18. GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403:2133–61. doi: 10.1016/S0140-6736(24)00757-8
19. Deng, Y, Wei, B, Zhai, Z, Zheng, Y, Yao, J, Wang, S, et al. Dietary risk-related colorectal Cancer burden: estimates from 1990 to 2019. Front Nutr. (2021) 8:690663. doi: 10.3389/fnut.2021.690663
20. GBD 2019 Demographics Collaborators. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950-2019: a comprehensive demographic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1160–203. doi: 10.1016/S0140-6736(20)30977-6
21. Hankey, BF, Ries, LA, Kosary, CL, Feuer, EJ, Merrill, RM, Clegg, LX, et al. Partitioning linear trends in age-adjusted rates. Cancer Causes Control. (2000) 11:31–5. doi: 10.1023/a:1008953201688
22. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing (2023).
23. GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2019) 393:1958–72. doi: 10.1016/S0140-6736(19)30041-8
24. Nam, KC, Jo, C, and Lee, M. Meat products and consumption culture in the east. Meat Sci. (2010) 86:95–102. doi: 10.1016/j.meatsci.2010.04.026
25. Tan, B, and Zhai, X. The background, development status and its Prospect of the industry of whole grain in China. Sci Technol Cereals Oils Foods. (2024) 32:13. doi: 10.16210/j.cnki.1007-7561.2024.01.001
26. Bail, J, Meneses, K, and Demark-Wahnefried, W. Nutritional status and diet in Cancer prevention. Semin Oncol Nurs. (2016) 32:206–14. doi: 10.1016/j.soncn.2016.05.004
27. Chu, AHY, Lin, K, Croker, H, Kefyalew, S, Markozannes, G, Tsilidis, KK, et al. Dietary-lifestyle patterns and colorectal Cancer risk: global Cancer update Programme (CUP global) systematic literature review. Am J Clin Nutr. (2025) 11:S0002-9165 (25)00014-0. doi: 10.1016/j.ajcnut.2025.01.014
28. Miller, HE, Rigelhof, F, Marquart, L, Prakash, A, and Kanter, M. Antioxidant content of whole grain breakfast cereals, fruits and vegetables. J Am Coll Nutr. (2000) 19:312S–9S. doi: 10.1080/07315724.2000.10718966
29. Schwingshackl, L, Schwedhelm, C, Hoffmann, G, Knüppel, S, Laure Preterre, A, Iqbal, K, et al. Food groups and risk of colorectal cancer. Int J Cancer. (2018) 142:1748–58. doi: 10.1002/ijc.31198
30. Cho, HJ, Woo, HD, Park, S, Choi, WJ, Kim, JH, Kweon, SS, et al. Gastric and colorectal cancer incidence attributable to dietary factors in Korea. J Gastrointest Oncol. (2024) 15:963–73. doi: 10.21037/jgo-24-10
31. Su, J, Liang, Y, and He, X. The global burden and trends analysis of early-onset colorectal cancer attributable to dietary risk factors in 204 countries and territories, 1990-2019: a secondary analysis for the global burden of disease study 2019. Front Nutr. (2024) 11:1384352. doi: 10.3389/fnut.2024.1384352
32. Wei, X, Wang, J, Wang, Y, Zhao, Y, Long, Y, Tan, B, et al. Dietary fiber and polyphenols from whole grains: effects on the gut and health improvements. Food Funct. (2024) 15:4682–702. doi: 10.1039/d4fo00715h
33. Abdal Dayem, A, Choi, HY, Yang, GM, Kim, K, Saha, SK, and Cho, SG. The anti-Cancer effect of polyphenols against breast Cancer and Cancer stem cells: molecular mechanisms. Nutrients. (2016) 8:581. doi: 10.3390/nu8090581
34. Zhang, H, and Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci. (2016) 8:33–42. doi: 10.1016/j.cofs.2016.02.002
35. Reynolds, A, Mann, J, Cummings, J, Winter, N, Mete, E, and Te Morenga, L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. (2019) 393:434–45. doi: 10.1016/S0140-6736(18)31809-9
36. US Department of Health and Human Services and US Department of Agriculture. Dietary guidelines for Americans 2015–2020. 8th ed. (2015). Available at: https://health.gov/dietaryguidelines/2015/guidelines/
37. De Leon, A, Burnett, DJ, Rust, B, Lyly, M, and Keim, NL. Initial implicit association between whole grains and taste does not predict consumption of whole grains in low-whole grain consumers: a pilot randomized controlled trial. Front Nutr. (2024) 11:1408256. doi: 10.3389/fnut.2024.1408256
38. Smith, TA, Lin, BH, and Guthrie, J. School meal nutrition standards reduce disparities across income and race/ethnicity. Am J Prev Med. (2024) 67:249–57. doi: 10.1016/j.amepre.2024.03.012
39. Toups, KE. Global approaches to promoting whole grain consumption. Nutr Rev. (2020) 78:54–60. doi: 10.1093/nutrit/nuz067
40. Sharma, R, and Rakshit, B. Spatial and temporal patterns of colorectal cancer in Asia, 1990-2019. Int J Clin Oncol. (2023) 28:255–67. doi: 10.1007/s10147-022-02274-x
41. Yu, D, Zhao, L, and Zhao, W. Status and trends in consumption of grains and dietary fiber among Chinese adults (1982-2015). Nutr Rev. (2020) 78:43–53. doi: 10.1093/nutrit/nuz075
42. CNS. Dietary Guidelines for Chinese Residents in Beijing. Acta Nutrimenta Sinica. (2022) 2022:521–2. doi: 10.13325/j.cnki.acta.nutr.sin.2022.06.019
43. Miller, KB. Review of whole grain and dietary fiber recommendations and intake levels in different countries. Nutr Rev. (2020) 78:29–36. doi: 10.1093/nutrit/nuz052
44. La Vecchia, M, Sala, G, Sculco, M, Aspesi, A, and Dianzani, I. Genetics, diet, microbiota, and metabolome: partners in crime for colon carcinogenesis. Clin Exp Med. (2024) 24:248. doi: 10.1007/s10238-024-01505-x
45. Frank, SM, Jaacks, LM, Batis, C, Vanderlee, L, and Taillie, LS. Patterns of red and processed meat consumption across North America: a nationally representative cross-sectional comparison of dietary recalls from Canada, Mexico, and the United States. Int J Environ Res Public Health. (2021) 18:357. doi: 10.3390/ijerph18010357
46. Conti, L, Del Cornò, M, and Gessani, S. Revisiting the impact of lifestyle on colorectal cancer risk in a gender perspective. Crit Rev Oncol Hematol. (2020) 145:102834. doi: 10.1016/j.critrevonc.2019.102834
47. Maingi, JW, Tang, S, Liu, S, Ngenya, W, and Bao, E. Targeting estrogen receptors in colorectal cancer. Mol Biol Rep. (2020) 47:4087–91. doi: 10.1007/s11033-020-05414-6
Keywords: colorectal cancer, diet low in whole grains, death, disability-adjusted life years, global burden of disease colorectal cancer, global burden of disease
Citation: Ma Y, Ni J, Mei P, Chen Y and Guo X (2025) The burden of colorectal cancer attributable to diet low in whole grains from 1990 to 2021: a global, regional and national analysis. Front. Nutr. 12:1527522. doi: 10.3389/fnut.2025.1527522
Received: 13 November 2024; Accepted: 25 March 2025;
Published: 09 April 2025.
Edited by:
Zeinab Ghorbani, Guilan University of Medical Sciences, IranReviewed by:
Nazanin Moslehi, Shahid Beheshti University of Medical Sciences, IranCopyright © 2025 Ma, Ni, Mei, Chen and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Chen, NDk4MzU3NjMyQHFxLmNvbQ==; Xiutian Guo, Z3VveGl1dGlhbkAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.