
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 29 January 2025
Sec. Clinical Nutrition
Volume 12 - 2025 | https://doi.org/10.3389/fnut.2025.1521882
This article is part of the Research Topic Eating Behavior and Chronic Diseases: Research Evidence from Population Studies, Volume II View all 8 articles
Background and objective: The Oxidative Balance Score (OBS) has been linked to various chronic diseases; however, its association with gallstone prevalence remains underexplored. This study aimed to investigate the relationship between OBS and gallstone risk.
Methods: This cross-sectional study utilized data from the National Health and Nutrition Examination Survey (NHANES) conducted from 2017 to March 2020. Weighted logistic regression models were applied to examine the association between OBS and the prevalence of gallstones, complemented by subgroup and sensitivity analyses. Restricted cubic spline (RCS) was used to investigate the nonlinear association between OBS and the prevalence of gallstones.
Results: A total of 5,382 participants were included, among whom 592 reported a history of gallstones. After adjusting for confounding factors, a significant negative association was observed between OBS and gallstone prevalence (quartile 4 vs. quartile 1: odds ratio [OR] 0.63, 95% confidence interval [CI] 0.43–0.90, p = 0.019). The RCS analysis further supported a negative linear relationship between OBS and gallstone risk (nonlinear p = 0.149). The findings of the subgroup analyses exhibited considerable consistency.
Conclusion: This study identified a significant negative linear association between OBS and gallstone risk, suggesting that higher OBS levels are associated with a reduced likelihood of gallstone formation.
Gallstones are solid deposits that form in the gallbladder and are primarily classified into cholesterol, pigment, or mixed gallstones, with cholesterol gallstones being the most prevalent type (1). While many individuals with gallstones remain asymptomatic, over 20% experience symptoms, which vary depending on the location of the stones (2, 3). Gallstones are a common cause of biliary colic, whereas stones in the bile duct frequently present with right upper quadrant or epigastric pain, often due to acute ductal dilation secondary to obstruction. Pain relief may occur if the stones pass into the duodenum or re-enter the dilated bile duct. However, obstruction of the common bile duct may trigger secondary infections, potentially leading to acute obstructive suppurative cholangitis—a life-threatening condition. Without prompt medical intervention, this can result in severe acid–base and electrolyte imbalances, septic shock, multi-organ failure, and even death (4, 5). Gallstone prevalence varies significantly across populations and is influenced by factors such as age, gender, ethnicity, and lifestyle choices, including smoking, alcohol consumption, and physical activity levels (6–9). Globally, studies estimate that gallstones affect approximately 11.2% of the adult population in South America, whereas prevalence rates as low as 5.1% have been reported in parts of Asia (10). These differences highlight the influence of demographics and lifestyle on gallstone risk (11). Furthermore, the economic burden of gallstone disease is substantial, encompassing costs associated with diagnosis, treatment, and surgical interventions, which collectively amount to billions of dollars annually in healthcare expenses (12).
Gallstone formation is a multifactorial process influenced by bile composition imbalances, gallbladder motility disorders, and dietary factors. Oxidative stress plays a pivotal role in various physiological processes, such as signal transduction and immune response, under normal conditions (13, 14). However, in pathological states, excessive production of reactive oxygen species (ROS) can overwhelm the body’s antioxidant defenses, leading to inflammation and cellular damage, which are key contributors to gallstone development (15–17). As such, understanding oxidative balance is crucial for elucidating the etiology of gallstones and developing early prevention strategies.
Oxidative balance refers to the equilibrium between pro-oxidants and antioxidants in the body, which influences numerous biological processes. Endogenous antioxidants, such as glutathione and superoxide dismutase, together with exogenous antioxidants like vitamins C and E, as well as β-carotene, play essential roles in mitigating oxidative stress (18). Conversely, pro-oxidants, including free radicals, can amplify cellular damage when their levels surpass the neutralizing capacity of antioxidants. The Oxidative Balance Score (OBS), a composite measure that integrates 16 dietary and 4 lifestyle factors, represents a valuable tool for assessing oxidative balance. By accounting for various components that affect oxidative stress and antioxidant levels, OBS provides a holistic and dynamic evaluation compared to individual antioxidant biomarkers (19). This multidimensional approach considers both the relative contributions of dietary and biochemical antioxidants and the influence of daily lifestyle habits on oxidative status, offering a more comprehensive reflection of an individual’s antioxidant capacity. Notably, a higher OBS signifies greater systemic antioxidant capacity. Emerging evidence suggests that higher OBS values may exert a protective effect against the development of chronic diseases (20–23), underscoring the potential of OBS as a predictive marker for health outcomes.
Oxidative stress has been suggested to play a role in gallstone formation (24, 25). However, the specific relationship between the OBS and gallstone risk remains poorly understood. Elucidating this association is crucial for identifying potential preventive strategies and therapeutic targets. Therefore, the objective of this study was to evaluate the association between OBS and gallstone prevalence using data from the National Health and Nutrition Examination Survey (NHANES) conducted from 2017 to March 2020. Through a comprehensive analysis, this study aimed to provide valuable insights into gallstone prevention by highlighting the potential protective effects of dietary antioxidants and healthy lifestyle habits.
NHANES is a stratified, multistage sampling survey designed to assess the health and nutritional status of individuals across the United States. It aims to evaluate health and nutritional profiles in both adults and children, employing a stratified, multistage probability sampling design to recruit a representative sample of the civilian, noninstitutionalized U.S. population. The sampling framework segments the nation into various strata based on demographic and geographic characteristics. Within each stratum, clusters are identified, and a random sample of households is selected. The survey protocol was approved by the National Center for Health Statistics Institutional Review Board (NCHS IRB), and all participants provided informed written consent prior to participation. For this study, data were obtained from NHANES conducted between 2017 and March 2020, encompassing a total of 15,560 participants. Participants with missing data in key variables were excluded during screening, including individuals with more than four missing values in the OBS assessment components and those lacking data on gallstone status. After excluding participants with incomplete covariate data, a final sample of 5,382 individuals was included in the analysis (refer to Figure 1).
We obtained data from the NHANES website for the period from March 2017 to March 2020. This dataset encompasses various information about the study participants, including age, gender, race, marital status, education level, poverty-income ratio, smoking status, and serum total cholesterol levels. Furthermore, we collected essential data necessary for the assessment of diabetes and hypertension, including blood pressure, blood glucose, glycated hemoglobin levels, and other relevant indicators. In terms of dietary and lifestyle factors, we gathered multiple data points, including dietary intake levels of fiber, β-carotene, vitamins B6, B12, C, and E, riboflavin, folate, niacin, calcium, magnesium, zinc, copper, and selenium. Additionally, we assessed iron intake, total fat intake, cotinine levels, and alcohol consumption. Physical activity levels and body mass index (BMI) were also incorporated into the evaluation. Together, these dietary and lifestyle data points constitute a comprehensive dataset for the assessment of health outcomes.
Following established research protocols, the OBS was calculated using 16 nutrient components and 4 lifestyle factors, comprising 5 pro-oxidative and 15 antioxidative components (26). Antioxidative components included dietary intake levels of fiber, β-carotene, vitamins B6, B12, C, and E, riboflavin, folate, niacin, calcium, magnesium, zinc, copper, and selenium, as well as physical activity levels. In contrast, pro-oxidative components consisted of iron intake, total fat intake, smoking status, alcohol consumption, and obesity (BMI). Dietary intake data were averaged across two recorded days. Smoking status was evaluated via cotinine levels, while physical activity levels were assessed using methods established in prior studies (27). As for alcohol consumption, based on previous research, participants were categorized into non-drinkers, light drinkers, and heavy drinkers, receiving scores of 2, 1, and 0, respectively (26). All other factors, excluding alcohol intake, were stratified into tertiles by gender. For antioxidative components, participants in the highest tertile received 0 points, the middle tertile received 1 point, and the lowest tertile received 2 points. For pro-oxidative components, scoring was reversed, with participants in the highest tertile receiving 2 points. The OBS was computed as the sum of scores for all components, with a higher OBS indicating greater overall exposure to antioxidants. Additional details regarding the scoring algorithm are presented in Supplementary Table S1.
The presence of gallstones was determined using responses from the Medical Conditions questionnaire. Participants were asked the question: “Has a doctor or other health professional ever informed you that you had gallstones?” Those who answered “yes” were classified as having gallstones, whereas participants who responded “no” were categorized as gallstone-free.
This study accounted for various covariates, including age, race, marital status, education level, PIR, sugar intake, caffeine intake, water intake, diabetes, and hypertension. Additional details regarding the assessment of diabetes and hypertension are provided in Supplementary Table S2.
We conducted a detailed summary of baseline variables. For continuous data that were not normally distributed, we described these variables using medians and interquartile ranges (IQRs), while categorical variables were reported as counts and weighted percentages. To assess the relationship between the OBS and the presence of gallstones, we analyzed OBS in two forms: as a continuous variable and as a quartile-categorized variable. Differences in continuous variables across different OBS quartiles were evaluated using the Wilcoxon rank-sum test, whereas differences in categorical variables were assessed using the chi-square test.
Additionally, weighted logistic regression analyses were performed to explore the association between OBS, in both its continuous and quartile forms, and gallstone occurrence. Interaction analyses were conducted to investigate potential subgroup interactions, and Restricted cubic spline (RCS) curves were employed to examine the nonlinear associations between OBS and gallstones risk. The OBS was further categorized into dietary and lifestyle components, with each component analyzed using weighted logistic regression, complemented by RCS curves for visual representation. To ensure the robustness of our results, sensitivity analyses were conducted by sequentially removing each OBS component from the model. All statistical analyses were two-sided, with a significance level set at p < 0.05. All analyses were performed using R version 4.3.1, with “WTDR2DPP” used as a weighting variable.
In this study, a total of 5,382 adults were analyzed, of whom 592 were identified as gallstone patients (Table 1). Significant differences in most baseline characteristics were observed between the gallstone and non-gallstone groups. However, no significant differences were found in key parameters such as total cholesterol intake (p = 0.369), total sugar intake (p = 0.149), total caffeine intake (p = 0.777), and education level (p = 0.589). Demographic analysis revealed that gallstone patients were predominantly women (73.50% vs. 26.50%) and tended to be older than those without gallstones. Additionally, individuals with gallstones experienced a higher prevalence of unfavorable marital status (24.54% vs. 17.13%) and had lower income levels. Ethnic analysis showed that non-Hispanic White individuals were significantly more likely to have gallstones (p = 0.032). Furthermore, gallstones were notably more prevalent among individuals with a higher BMI (32.9 vs. 29.42). Lifestyle factors also differed significantly between the two groups. Gallstone patients had lower total water intake than non-gallstone individuals (p = 0.036) and exhibited longer periods of sedentary behavior (p = 0.020), higher rates of smoking exposure, and an increased prevalence of diabetes and hypertension (p < 0.001 for all). Importantly, patients with gallstones exhibited substantially lower OBS compared to those without gallstones (p = 0.013). Additional details on baseline characteristics stratified by OBS quartiles are provided in Supplementary Table S3.
A negative correlation was consistently observed between the OBS and gallstone risk across all models. As shown in Table 2, after adjusting for covariates, participants in the highest OBS quartile experienced an approximately 40% reduction in gallstone risk compared to those in the lowest quartile (95% confidence interval [CI]: 0.43–0.90), with similar trends observed in Models 1 and 2. When analyzed as a continuous variable, the OBS did not demonstrate a significant association with gallstone risk in Model 3. Figure 2 depicted a negative linear relationship between the OBS and gallstone prevalence, with a nonlinear p-value of 0.149.
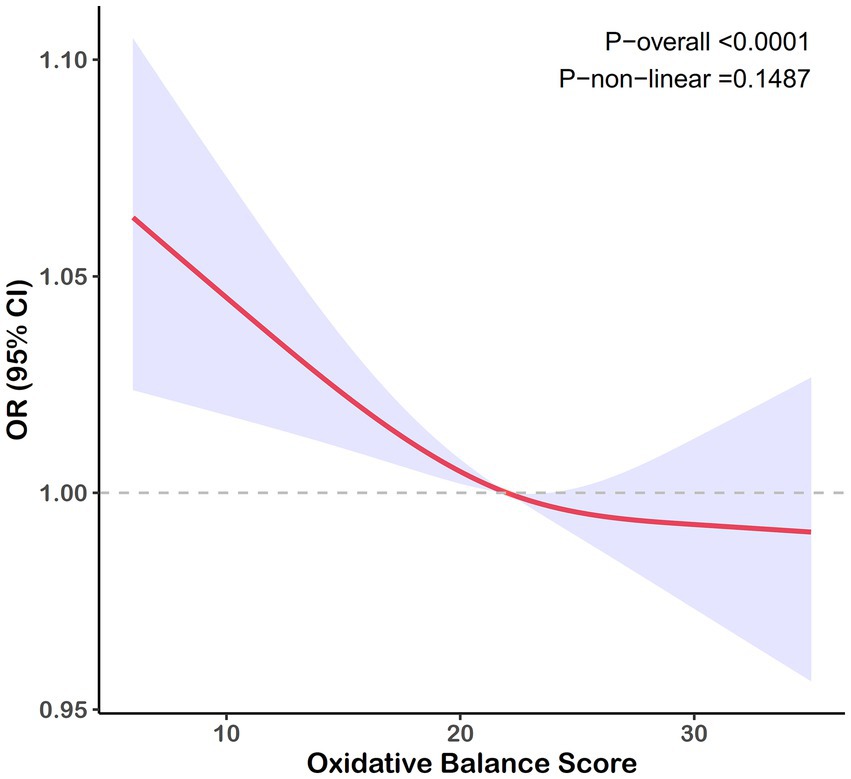
Figure 2. RCS curve for the association between the OBS and the risk of gallstones. Lines represent odds ratios, and areas represent 95% confidence intervals. The model was adjusted for age, race, marital, poverty income ratio, education, total sugars, total caffe, total water, diabetes, and hypertension.
We analyzed the association between overall OBS and gallstone risk by separating OBS into dietary and lifestyle components, as shown in Supplementary Table S4. A significant inverse association was observed between dietary OBS and gallstone prevalence in models 1 and 2. However, in Model 3, after comprehensive adjustments, this association weakened; while individuals in the third dietary OBS quartile (Q3) had a significantly lower risk of gallstones compared to those in the lowest quartile ([OR]: 0.58, 95% CI: 0.38–0.89, p = 0.019), the association between dietary OBS as a continuous variable and gallstone risk became non-significant ([OR]: 0.97, 95% CI: 0.95–1.00, p = 0.094). Similarly, lifestyle OBS showed a significant inverse association with gallstone prevalence in all models when analyzed as a continuous variable ([OR]: 0.88, 95% CI: 0.79–0.99, p = 0.042 in Model 3). Furthermore, individuals in the highest lifestyle OBS quartile (Q4) exhibited a significantly reduced risk of gallstones in Model 3 ([OR]: 0.60, 95% CI: 0.37–0.96, p = 0.046). Supplementary Figures S1, S2 demonstrate a consistent, negative trend between both dietary and lifestyle OBS components and gallstone prevalence, with nonlinear p-values of 0.151 and 0.680, respectively.
Subgroup analyses and interaction tests, stratified by age, marital status, race, education, diabetes, and hypertension status, were conducted to assess variations in the association between OBS and gallstone risk (Figure 3). No statistically significant interactions were found (P for interaction > 0.05). However, stratified analyses showed some variations in effect size. The inverse association was strongest among younger participants (20–40 years: OR = 0.72, 95% CI: 0.57–0.90; 40–65 years: OR = 0.83, 95% CI: 0.72–0.95) but weakened in those aged ≥65 years (OR = 0.90, 95% CI: 0.76–1.06, P for interaction = 0.915). Married or partnered individuals exhibited a significant inverse association (OR = 0.81, 95% CI: 0.71–0.92), while no significant association was observed in other marital groups. Mexican Americans showed the strongest negative association by race (OR = 0.73, 95% CI: 0.56–0.96), followed by other groups with weaker or non-significant associations. Participants with education above high school demonstrated a significant association (OR = 0.79, 95% CI: 0.69–0.90), whereas the association was weaker in those with lower education levels. Diabetes did not significantly modify the association (P for interaction = 0.710), though the association was stronger in individuals without diabetes (OR = 0.80, 95% CI: 0.70–0.90) compared to those with diabetes (OR = 0.92, 95% CI: 0.78–1.09). Despite minor variations, the overall inverse association between OBS and gallstone risk remained consistent across most subgroups. Sensitivity analyses further confirmed the robustness of these findings, with stable associations across various OBS components (Supplementary Table S5).
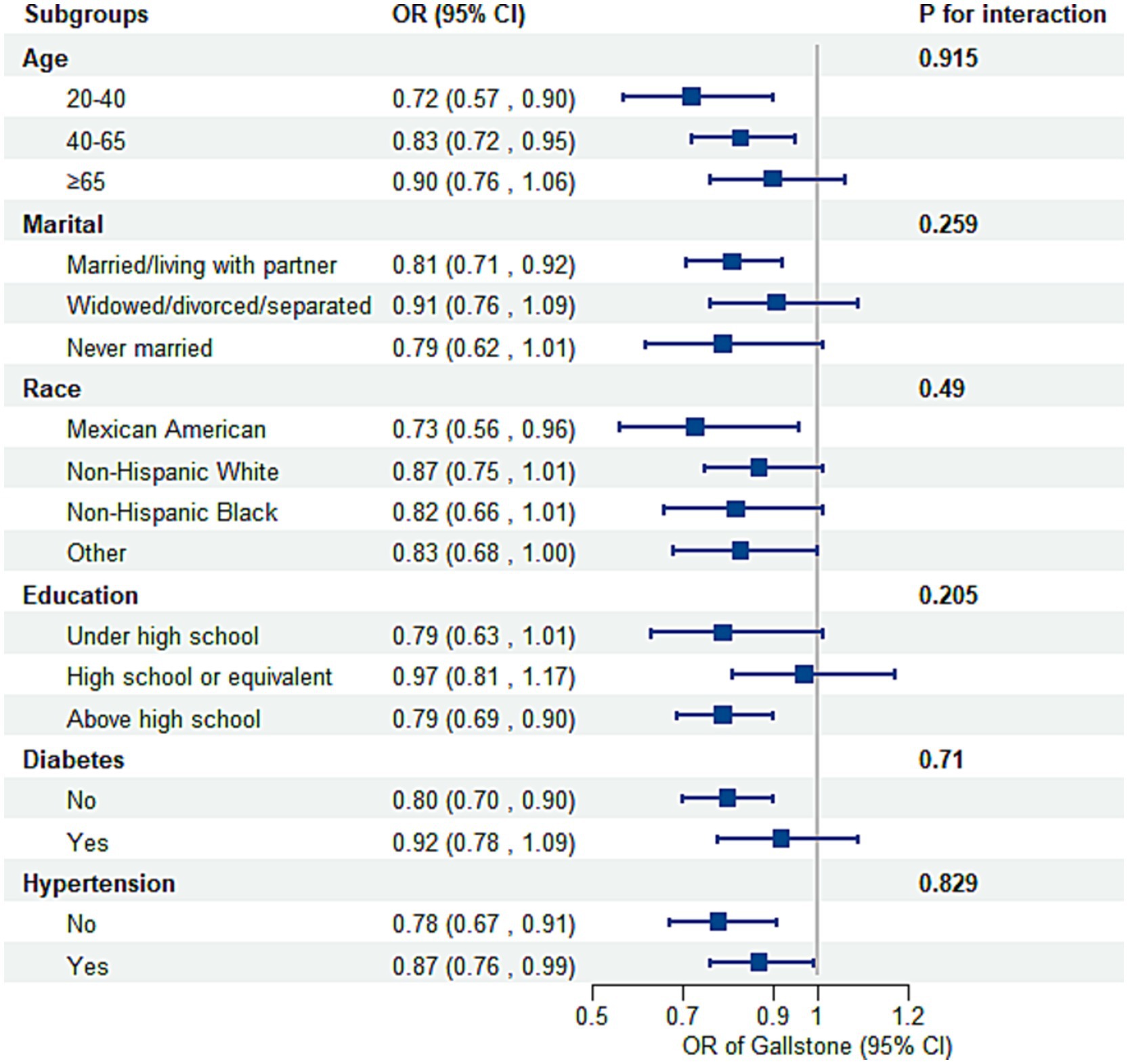
Figure 3. Subgroup analysis of the association between the OBS and the risk of gallstones. Adjusted for age, race, marital, poverty income ratio, education, total sugars, total caffe, total water, diabetes, and hypertension. OR, odds ratio; CI, confidence interval.
This study analyzed a cohort of 5,382 adults from the NHANES database (2017 to March 2020) to evaluate the association between the OBS and gallstone prevalence. Logistic regression and trend analyses identified a significant inverse association between OBS and gallstone prevalence, with higher OBS values linked to a reduced risk of gallstones. Findings from dose–response analyses indicated a significant linear negative correlation between OBS and gallstone prevalence. When OBS was divided into dietary and lifestyle components, the results demonstrated an intriguing imbalance: lifestyle OBS exhibited a stronger and more consistent inverse association with gallstone prevalence, whereas dietary OBS showed a weaker and attenuated association, particularly in higher quantiles. Overall, our results indicated that enhancing OBS through dietary intake and lifestyle modifications may contributed to a reduced prevalence of gallstones.
The pathogenesis of gallstones is a complex and multifactorial process involving various factors such as environment, diet, and metabolism (28–30). Mechanistically, the formation of gallstones is typically related to the imbalance of bile components, specifically the dysregulation of cholesterol, bile salts, and phospholipid ratios within bile. This imbalance may lead to cholesterol supersaturation, resulting in the formation of solid crystals in the gallbladder. Additionally, gallbladder dysmotility, such as reduced gallbladder emptying capacity, can lead to bile stasis, creating favorable conditions for stone formation. Oxidative stress and inflammation are significant promoting factors in the formation of gallstones. Studies have shown that in gallstone patients, the gallbladder mucosa often exhibits elevated levels of oxidative stress, which can induce the oxidation of bilirubin and the polymerization of free radicals through the action of ROS, ultimately leading to the deposition of insoluble macromolecular polymers that promote stone formation (31, 32). Furthermore, oxidative stress can exacerbate the inflammatory response in the gallbladder. During the inflammatory process, the activation of immune cells such as neutrophils not only further triggers the accumulation of cholesterol and calcium crystals but may also damage gallbladder endothelial cells, affecting normal gallbladder function, such as reduced bile emptying capacity, thus exacerbating bile stasis and forming a vicious cycle (24, 25). Our research results indicate that high OBS has a protective effect on the development of gallstones, which is consistent with current understanding of the role of oxidative stress in the pathogenesis of gallstones.
Simultaneously, environmental factors and dietary habits, such as high-fat diets, low fiber intake, and adverse lifestyle choices (e.g., smoking and lack of exercise), indirectly influence the risk of gallstone formation by altering bile metabolism and oxidative stress levels in the body (8). A large-scale prospective cohort study indicates that adherence to the Alternative Mediterranean Diet score (aMED) and the 2015 Healthy Eating Index (HEI-2015) dietary patterns can reduce the risk of gallstone disease by 10% (33). Regarding dietary factors, some studies indicate that dietary fiber (34, 35), carotenoids (36), B vitamins (37), vitamin C (38, 39), vitamin E (40), magnesium (41), zinc (42), and copper (43) may help reduce the formation of gallstones. In contrast, the intake of pro-oxidants represented by fats is associated with the occurrence of gallstones (44). The role of lifestyle factors in the incidence of gallstones has been extensively studied. Evidence suggests that obesity and behaviors associated with smoking and alcohol consumption significantly elevate the risk of gallstone formation (45, 46). In obese individuals, adipocytes play a critical role by promoting cholesterol crystallization and gallstone formation through the secretion of leptin (47). Leptin exerts multifaceted effects on gallbladder physiology, including alterations in bile volume, sodium and pH regulation, as well as modulation of inflammatory cytokine-related and ion transport-related genes (48). Furthermore, obesity is frequently associated with insulin resistance, which dysregulates hepatic metabolism. Specifically, hepatic insulin resistance leads to the activation of the forkhead transcription factor (FoxO1), which promotes the overexpression of bile cholesterol transport proteins Abcg5 and Abcg8, resulting in excessive cholesterol secretion into bile. Simultaneously, it suppresses bile acid synthase (e.g., Cyp7b1) expression and interferes with the farnesoid X receptor (FXR) pathway, thus altering the bile salt composition in a manner that favors gallstone formation (49). Obesity also induces systemic inflammation similar to the effects of smoking and alcohol consumption, triggering heightened oxidative stress in the gallbladder, which damages endothelial cells and impairs gallbladder motility (50, 51). This hypocontraction further exacerbates bile stasis, facilitating gallstone development. Physical activity, on the other hand, has been shown to mitigate oxidative stress by reducing free radical levels, thereby preventing the onset of various chronic diseases (52). However, compared to lifestyle factors, dietary antioxidants alone appear to have a relatively weaker impact on mitigating oxidative damage. Studies indicate that a high level of dietary antioxidant intake does not consistently lead to a pronounced reduction in oxidative stress, even act as pro-oxidants (53). Our results corroborate this finding: the association between dietary OBS and gallstone risk was only significant at the Q2 level after controlling for relevant covariates. In contrast, lifestyle OBS demonstrated a more robust and significant negative association with gallstone risk. Interestingly, both dietary OBS and lifestyle OBS exhibited their strongest negative associations with gallstones at the Q2 level. However, RCS analysis did not reveal a non-linear relationship. Importantly, a combined OBS, integrating both dietary and lifestyle factors, demonstrated the strongest and most consistent protective association with gallstones. Thus, we propose that an integrated OBS combining dietary antioxidants and healthy lifestyle behaviors may offer superior preventive and monitoring capabilities for gallstones.
Subgroup analysis revealed heterogeneity in the association between OBS and gallstones across different subgroups. Notably, the association between OBS and gallstones became non-significant in populations aged 65 years or older. This could be attributed to the fact that older adults often experience heightened baseline oxidative stress. Aging is inherently accompanied by increased ROS production due to mitochondrial dysfunction, cellular senescence, and a decline in antioxidant defense mechanisms (54). Furthermore, multiple metabolic comorbidities common in older adults contribute additional sources of oxidative stress, which may overshadow the protective effects of OBS. Consequently, the oxidative stress background in elderly individuals might exceed the protective threshold of OBS, diminishing observable associations with gallstone risk. Additionally, gallstone formation in the elderly may be more influenced by non-oxidative stress-related mechanisms. For instance, slowed bile flow due to declining gallbladder motility and increased cholesterol saturation are significant age-related risk factors for gallstones (55). Moreover, although older adults may report higher antioxidant intake (e.g., through dietary supplements), issues related to absorption efficiency and metabolic limitations may reduce the actual antioxidant efficacy, thereby contributing to the non-significant association observed in this group (56). No significant association between OBS and gallstones was observed in populations with unfavorable marital statuses, such as widowed, divorced, or single individuals. This may be partially explained by the role of psychological and behavioral factors in mediating oxidative stress. Married or cohabitating individuals often demonstrate more stable lifestyle habits, such as healthier diets, more regular routines, and greater social support, all of which are associated with lower oxidative stress and potentially higher OBS scores. Conversely, single individuals or those in unhealthy marriages are more likely to experience heightened psychosocial stress, which has been shown to strongly correlate with elevated oxidative stress levels (57). This increased psychosocial stress may counteract the protective effects of a theoretically higher OBS. Additionally, these individuals often engage in less favorable health behaviors, such as poor dietary habits, inadequate physical activity, smoking, and alcohol consumption, which further exacerbate cholesterol metabolism disorders or bile stasis, masking the protective role of OBS. For instance, evidence indicates that smoking and high-fat diets significantly elevate gallstone risk, and these behaviors tend to be more prevalent in single or widowed individuals. The subgroup analysis of education levels similarly revealed discrepancies. The negative association between OBS and gallstones was significant only in populations with higher education levels. This could reflect disparities in health resource accessibility and health literacy. Individuals with lower educational attainment may have limited access to health-promoting resources and knowledge, leading to difficulties in adopting and sustaining health behaviors aligned with higher OBS scores (e.g., balanced diets and regular exercise). Furthermore, individuals with lower education levels are often subjected to greater life stressors, including economic disadvantages, which are associated with elevated oxidative stress. These factors collectively attenuate the potential protective effect of OBS in this group. In diabetic populations, no significant association between OBS and gallstones was observed. This may be explained by the inherently high baseline oxidative stress levels associated with diabetes, driven by chronic hyperglycemia, insulin resistance, and elevated ROS production (58). Such oxidative damage may diminish or even negate the protective effect of OBS. Furthermore, diabetes is known to disrupt bile secretion and metabolism, such as through impaired insulin-mediated bile signaling, leading to bile composition changes that increase gallstone risk (59). These complex physiological mechanisms likely act independently of oxidative stress and may overshadow the protective role of OBS. Interestingly, ethnicity-based analysis revealed a significant negative association between OBS and gallstones in Mexican Americans, a population at moderate risk of oxidative stress due to a combination of genetic and environmental factors (60). This finding may appear contradictory to the lack of significant associations observed in other high oxidative stress populations, such as older adults and diabetic patients. A plausible explanation lies in genetic predisposition. For instance, the ApoE E4 allele, which is more prevalent in Mexican Americans, confers susceptibility to gallstones. In individuals with this genetic background, the protective effect of OBS may remain within an “effective range,” counteracting moderate oxidative stress levels. Additionally, the high prevalence of sedentary behavior and negative emotional states in Mexican Americans may enhance the manifestation of OBS effects. In contrast, in populations with excessively elevated oxidative stress levels (e.g., diabetic and elderly individuals), the oxidative stress burden may surpass the capacity of OBS to confer protection, reducing its effectiveness in preventing gallstones. Although no non-linear association was observed between OBS and gallstones in our analysis, the findings from subgroup analyses suggest that the interaction of oxidative stress and gallstone risk is highly context-specific. Unlike other chronic diseases, the relationship between OBS and gallstones appears more complex, influenced by genetic, metabolic, and behavioral factors. Future research should aim to investigate the dose–response relationship of OBS under varying oxidative stress conditions, particularly in high-risk subpopulations such as diabetic and elderly patients. Refining and optimizing OBS evaluation systems to account for interindividual variability may help enhance its protective effects and improve preventive strategies for gallstones in diverse populations.
This study has several strengths. First, the analysis utilized a standardized and weighted database, enhancing the reliability and generalizability of the findings. Second, the adoption of a straightforward composite index, such as the OBS, provided an efficient method for assessing oxidative balance at the population level. Third, rigorous control of confounding factors and the inclusion of stratified analyses allowed for a detailed examination of the relationship between OBS and gallstone prevalence across various subgroups. However, several limitations should be noted. First, as a cross-sectional study, it cannot establish a causal relationship between OBS and gallstone prevalence. Second, the dietary data used to calculate OBS were derived from two 24-h dietary recall surveys, which are subject to recall bias and may not reliably reflect long-term dietary patterns. Additionally, the diagnosis of gallstones relied on self-reported data, increasing the risk of underreporting or misclassification. Lastly, the study primarily focused on American participants, which may limit the applicability of the findings to other populations.
This study identifies a significant inverse association between the OBS and gallstone prevalence, with varying effects across subgroups. These findings highlight the role of oxidative balance in influencing gallstone risk and underscore the complexity of its relationship with population-specific factors.
Publicly available datasets were analyzed in this study. This data can be found at: https://wwwn.cdc.gov/nchs/nhanes/.
The studies involving humans were approved by the National Center for Health Statistics Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
TX: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. ZC: Data curation, Investigation, Validation, Writing – review & editing. JY: Conceptualization, Supervision, Validation, Writing – review & editing. TY: Software, Validation, Writing – review & editing. KW: Formal analysis, Funding acquisition, Project administration, Software, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by the following grant: Jiangxi Provincial Natural Science Foundation Key Project 20242BAB26143 and Jiangxi Province Key Laboratory of Molecular Medicine (No. 2024SSY06231).
The data utilized in this study were obtained from the NHANES. We extend our gratitude to all the staff members and participants of NHANES for their invaluable contributions. We also thank Bullet Edits Limited for providing linguistic editing and proofreading services for this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1521882/full#supplementary-material
1. Littlefield, A, and Lenahan, C. Cholelithiasis: presentation and management. J Midwifery Womens Health. (2019) 64:289–97. doi: 10.1111/jmwh.12959
2. Lammert, F, Gurusamy, K, Ko, CW, Miquel, JF, Méndez-Sánchez, N, Portincasa, P, et al. Gallstones. Nat Rev Dis Primers. (2016) 2:16024. doi: 10.1038/nrdp.2016.24
3. Innes, K, Hudson, J, Banister, K, Croal, B, Ramsay, C, Ahmed, I, et al. Core outcome set for symptomatic uncomplicated gallstone disease. Br J Surg. (2022) 109:539–44. doi: 10.1093/bjs/znac095
4. Vorob'ev, LP, Shestakov, VA, and Vovk, EI. Acute suppurative cholangitis. Klin Med (Mosk). (1985) 63:139–43.
5. Dow, RW, and Lindenauer, SM. Acute obstructive suppurative cholangitis. Ann Surg. (1969) 169:272–6. doi: 10.1097/00000658-196902000-00015
6. Shabanzadeh, DM, Sørensen, LT, and Jørgensen, T. A prediction rule for risk stratification of incidentally discovered gallstones: results from a large cohort study. Gastroenterology. (2016) 150:156–67.e1. doi: 10.1053/j.gastro.2015.09.002
7. Di Ciaula, A, Garruti, G, Frühbeck, G, De Angelis, M, de Bari, O, Wang, DQ, et al. The role of diet in the pathogenesis of cholesterol gallstones. Curr Med Chem. (2019) 26:3620–38. doi: 10.2174/0929867324666170530080636
8. Wirth, J, Joshi, AD, Song, M, Lee, DH, Tabung, FK, Fung, TT, et al. A healthy lifestyle pattern and the risk of symptomatic gallstone disease: results from 2 prospective cohort studies. Am J Clin Nutr. (2020) 112:586–94. doi: 10.1093/ajcn/nqaa154
9. Unalp-Arida, A, and Ruhl, CE. Increasing gallstone disease prevalence and associations with gallbladder and biliary tract mortality in the US. Hepatology. (2023) 77:1882–95. doi: 10.1097/HEP.0000000000000264
10. Wang, X, Yu, W, Jiang, G, Li, H, Li, S, Xie, L, et al. Global epidemiology of gallstones in the 21st century: a systematic review and Meta-analysis. Clin Gastroenterol Hepatol. (2024) 22:1586–95. doi: 10.1016/j.cgh.2024.01.051
11. Yang, T, Zhong, J, Zhang, R, Xiao, F, Wang, Y, Tao, H, et al. Different types and numbers metabolic abnormalities and risk of gallbladder stone disease in adults. Front Nutr. (2024) 11:1443575. doi: 10.3389/fnut.2024.1443575
12. Everhart, JE, and Ruhl, CE. Burden of digestive diseases in the United States part III: liver, biliary tract, and pancreas. Gastroenterology. (2009) 136:1134–44. doi: 10.1053/j.gastro.2009.02.038
13. Sies, H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. (2015) 4:180–3. doi: 10.1016/j.redox.2015.01.002
14. Jomova, K, Raptova, R, Alomar, SY, Alwasel, SH, Nepovimova, E, Kuca, K, et al. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch Toxicol. (2023) 97:2499–574. doi: 10.1007/s00204-023-03562-9
15. Kärkkäinen, J, Aspinen, S, Harju, J, Juvonen, P, Pulkki, K, and Eskelinen, M. Plasma glutathione peroxidase (GPX1) levels and oxidative stress in gallstone patients operated with two different cholecystectomy techniques: a randomized study with special reference to Cancer patients. Anticancer Res. (2017) 37:6921–7. doi: 10.21873/anticanres.12156
16. Navarro-Yepes, J, Burns, M, Anandhan, A, Khalimonchuk, O, del Razo, LM, Quintanilla-Vega, B, et al. Oxidative stress, redox signaling, and autophagy: cell death versus survival. Antioxid Redox Signal. (2014) 21:66–85. doi: 10.1089/ars.2014.5837
17. Sies, H. Oxidative eustress: the physiological role of oxidants. Sci China Life Sci. (2023) 66:1947–8. doi: 10.1007/s11427-023-2336-1
18. Jomova, K, Alomar, SY, Alwasel, SH, Nepovimova, E, Kuca, K, and Valko, M. Several lines of antioxidant defense against oxidative stress: antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch Toxicol. (2024) 98:1323–67. doi: 10.1007/s00204-024-03696-4
19. Goodman, M, Bostick, RM, Dash, C, Flanders, WD, and Mandel, JS. Hypothesis: oxidative stress score as a combined measure of pro-oxidant and antioxidant exposures. Ann Epidemiol. (2007) 17:394–9. doi: 10.1016/j.annepidem.2007.01.034
20. Wu, C, Ren, C, Song, Y, Gao, H, Pang, X, and Zhang, L. Gender-specific effects of oxidative balance score on the prevalence of diabetes in the US population from NHANES. Front Endocrinol (Lausanne). (2023) 14:1148417. doi: 10.3389/fendo.2023.1148417
21. Liu, X, Wang, Y, Liu, X, Zeng, B, Zhu, B, Zhang, Y, et al. Higher oxidative balance scores are associated with lower nonalcoholic fatty liver disease and not with fibrosis in US adults. Nutr Metab Cardiovasc Dis. (2023) 33:2488–96. doi: 10.1016/j.numecd.2023.08.004
22. Meng, F, Lu, S, Li, L, Qian, T, Zhang, C, Liu, X, et al. Different gender of oxidative balance score on the risk of rheumatoid arthritis in the US population from NHANES. Int J Rheum Dis. (2024) 27:e15237. doi: 10.1111/1756-185X.15237
23. Cao, Y, Zhou, Y, Zhong, Y, Liao, X, Chen, X, and Pi, Y. Association between oxidative balance score in adults with and without chronic kidney disease: 2011-2028 NHANES. Front Nutr. (2024) 11:1374719. doi: 10.3389/fnut.2024.1374719
24. Muñoz, LE, Boeltz, S, Bilyy, R, Schauer, C, Mahajan, A, Widulin, N, et al. Neutrophil extracellular traps initiate gallstone formation. Immunity. (2019) 51:443–50.e4. doi: 10.1016/j.immuni.2019.07.002
25. Maurer, KJ, Carey, MC, and Fox, JG. Roles of infection, inflammation, and the immune system in cholesterol gallstone formation. Gastroenterology. (2009) 136:425–40. doi: 10.1053/j.gastro.2008.12.031
26. Zhang, W, Peng, SF, Chen, L, Chen, HM, Cheng, XE, and Tang, YH. Association between the oxidative balance score and telomere length from the National Health and nutrition examination survey 1999-2002. Oxidative Med Cell Longev. (2022) 2022:1–11. doi: 10.1155/2022/1345071
27. Ussery, EN, Fulton, JE, Galuska, DA, Katzmarzyk, PT, and Carlson, SA. Joint prevalence of sitting time and leisure-time physical activity among US adults, 2015-2016. JAMA. (2018) 320:2036–8. doi: 10.1001/jama.2018.17797
28. Krawczyk, M, Wang, DQ, Portincasa, P, and Lammert, F. Dissecting the genetic heterogeneity of gallbladder stone formation. Semin Liver Dis. (2011) 31:157–72. doi: 10.1055/s-0031-1276645
29. Tsai, CJ, Leitzmann, MF, Willett, WC, and Giovannucci, EL. Prospective study of abdominal adiposity and gallstone disease in US men. Am J Clin Nutr. (2004) 80:38–44. doi: 10.1093/ajcn/80.1.38
30. Venneman, NG, and van Erpecum, KJ. Gallstone disease: primary and secondary prevention. Best Pract Res Clin Gastroenterol. (2006) 20:1063–73. doi: 10.1016/j.bpg.2006.03.008
31. Geetha, A. Evidence for oxidative stress in the gall bladder mucosa of gall stone patients. J Biochem Mol Biol Biophys. (2002) 6:427–32. doi: 10.1080/1025814021000036179
32. Sanikidze, T, and Chikvaidze, E. Role of the free radicals in mechanisms of gallstone formation: an EPR study. Radiat Prot Dosim. (2016) 172:317–24. doi: 10.1093/rpd/ncw237
33. Jin, K, Mi, N, He, W, Zhong, R, Jin, B, Liu, Z, et al. Dietary patterns, genetic predisposition, and risk of cholelithiasis: a large-scale prospective cohort study. Front Nutr. (2024) 11:1469789. doi: 10.3389/fnut.2024.1469789
34. Tehrani, AN, Saadati, S, Yari, Z, Salehpour, A, Sadeghi, A, Daftari, G, et al. Dietary fiber intake and risk of gallstone: a case-control study. BMC Gastroenterol. (2023) 23:119. doi: 10.1186/s12876-023-02752-0
35. Tseng, M, Everhart, JE, and Sandler, RS. Dietary intake and gallbladder disease: a review. Public Health Nutr. (1999) 2:161–72. doi: 10.1017/S136898009900021X
36. Worthington, HV, Hunt, LP, McCloy, RF, Maclennan, I, and Braganza, JM. A pilot study of antioxidant intake in patients with cholesterol gallstones. Nutrition. (1997) 13:118–27. doi: 10.1016/S0899-9007(96)00385-1
37. Wang, L, Li, X, Montazeri, A, MacFarlane, AJ, Momoli, F, Duthie, S, et al. Phenome-wide association study of genetically predicted B vitamins and homocysteine biomarkers with multiple health and disease outcomes: analysis of the UK biobank. Am J Clin Nutr. (2023) 117:564–75. doi: 10.1016/j.ajcnut.2023.01.005
38. Walcher, T, Haenle, MM, Kron, M, Hay, B, Mason, RA, Walcher, D, et al. Vitamin C supplement use may protect against gallstones: an observational study on a randomly selected population. BMC Gastroenterol. (2009) 9:74. doi: 10.1186/1471-230X-9-74
39. Gustafsson, U, Wang, FH, Axelson, M, Kallner, A, Sahlin, S, and Einarsson, K. The effect of vitamin C in high doses on plasma and biliary lipid composition in patients with cholesterol gallstones: prolongation of the nucleation time. Eur J Clin Investig. (1997) 27:387–91. doi: 10.1046/j.1365-2362.1997.1240670.x
40. Waniek, S, di Giuseppe, R, Esatbeyoglu, T, Ratjen, I, Enderle, J, Jacobs, G, et al. Association of Circulating Vitamin E (α- and γ-tocopherol) levels with gallstone disease. Nutrients. (2018) 10:133. doi: 10.3390/nu10020133
41. Du, W, Yan, C, Wang, Y, Song, C, Li, Y, Tian, Z, et al. Association between dietary magnesium intake and gallstones: the mediating role of atherogenic index of plasma. Lipids Health Dis. (2024) 23:82. doi: 10.1186/s12944-024-02074-4
42. Cikim, G, Hatipoglu, HS, and Susam, S. Evaluation of homocysteine, vitamin, and trace element levels in women with gallstones. J Trace Elem Med Biol. (2023) 78:127177. doi: 10.1016/j.jtemb.2023.127177
43. Yan, S, Yu, L, Fang, S, and Gu, C. The association between intakes of dietary trace minerals and gallstone disease: a cross-sectional study from National Health and nutrition examination survey 2017 to 2018. Medicine (Baltimore). (2024) 103:e37741. doi: 10.1097/MD.0000000000037741
44. Kubica, K, and Balbus, J. A computer study of the risk of cholesterol gallstone associated with obesity and normal weight. Sci Rep. (2021) 11:8868. doi: 10.1038/s41598-021-88249-w
45. Katsika, D, Tuvblad, C, Einarsson, C, Lichtenstein, P, and Marschall, HU. Body mass index, alcohol, tobacco and symptomatic gallstone disease: a Swedish twin study. J Intern Med. (2007) 262:581–7. doi: 10.1111/j.1365-2796.2007.01860.x
46. Yuan, S, Gill, D, Giovannucci, EL, and Larsson, SC. Obesity, type 2 diabetes, lifestyle factors, and risk of gallstone disease: a Mendelian randomization investigation. Clin Gastroenterol Hepatol. (2022) 20:e529–37. doi: 10.1016/j.cgh.2020.12.034
47. Hyogo, H, Roy, S, and Cohen, DE. Restoration of gallstone susceptibility by leptin in C57BL/6J Ob/Ob mice. J Lipid Res. (2003) 44:1232–40. doi: 10.1194/jlr.M300029-JLR200
48. Swartz-Basile, DA, Lu, D, Basile, DP, Graewin, SJ, Al-Azzawi, H, Kiely, JM, et al. Leptin regulates gallbladder genes related to absorption and secretion. Am J Physiol Gastrointest Liver Physiol. (2007) 293:G84–90. doi: 10.1152/ajpgi.00389.2006
49. Biddinger, SB, Haas, JT, Yu, BB, Bezy, O, Jing, E, Zhang, W, et al. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat Med. (2008) 14:778–82. doi: 10.1038/nm1785
50. Balan, AI, Halațiu, VB, and Scridon, A. Oxidative stress, inflammation, and mitochondrial dysfunction: a link between obesity and atrial fibrillation. Antioxidants (Basel). (2024) 13:117. doi: 10.3390/antiox13010117
51. Bondia-Pons, I, Ryan, L, and Martinez, JA. Oxidative stress and inflammation interactions in human obesity. J Physiol Biochem. (2012) 68:701–11. doi: 10.1007/s13105-012-0154-2
52. Kruk, J, Aboul-Enein, BH, Duchnik, E, and Marchlewicz, M. Antioxidative properties of phenolic compounds and their effect on oxidative stress induced by severe physical exercise. J Physiol Sci. (2022) 72:19. doi: 10.1186/s12576-022-00845-1
53. Chaudhary, P, Janmeda, P, Docea, AO, Yeskaliyeva, B, Abdull Razis, AF, Modu, B, et al. Oxidative stress, free radicals and antioxidants: potential crosstalk in the pathophysiology of human diseases. Front Chem. (2023) 11:1158198. doi: 10.3389/fchem.2023.1158198
54. Yang, J, Luo, J, Tian, X, Zhao, Y, Li, Y, and Wu, X. Progress in understanding oxidative stress, aging, and aging-related diseases. Antioxidants (Basel). (2024) 13:394. doi: 10.3390/antiox13040394
55. Di Francesco, V, Zamboni, M, Dioli, A, Zoico, E, Mazzali, G, Omizzolo, F, et al. Delayed postprandial gastric emptying and impaired gallbladder contraction together with elevated cholecystokinin and peptide YY serum levels sustain satiety and inhibit hunger in healthy elderly persons. J Gerontol A Biol Sci Med Sci. (2005) 60:1581–5. doi: 10.1093/gerona/60.12.1581
56. Holt, PR. Intestinal malabsorption in the elderly. Dig Dis. (2007) 25:144–50. doi: 10.1159/000099479
57. Correia, AS, Cardoso, A, and Vale, N. Oxidative stress in depression: the link with the stress response, neuroinflammation, serotonin, neurogenesis and synaptic plasticity. Antioxidants (Basel). (2023) 12:470. doi: 10.3390/antiox12020470
58. González, P, Lozano, P, Ros, G, and Solano, F. Hyperglycemia and oxidative stress: an integral, updated and critical overview of their metabolic interconnections. Int J Mol Sci. (2023) 24:9352. doi: 10.3390/ijms24119352
59. Hou, Y, Zhai, X, Wang, X, Wu, Y, Wang, H, Qin, Y, et al. Research progress on the relationship between bile acid metabolism and type 2 diabetes mellitus. Diabetol Metab Syndr. (2023) 15:235. doi: 10.1186/s13098-023-01207-6
Keywords: oxidative balance score, gallstone, NHANES, retrospective study, cross-sectional study
Citation: Xiong T, Chen Z, Yi J, Yu T and Wang K (2025) Higher levels of oxidative balance score linked to lower risk of gallstones: findings from the 2017–2020 National Health and Nutrition Examination Survey. Front. Nutr. 12:1521882. doi: 10.3389/fnut.2025.1521882
Received: 03 November 2024; Accepted: 10 January 2025;
Published: 29 January 2025.
Edited by:
Fei Xu, Nanjing Municipal Center for Disease Control and Prevention, ChinaReviewed by:
Long Yang, First Affiliated Hospital of Xinjiang Medical University, ChinaCopyright © 2025 Xiong, Chen, Yi, Yu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai Wang, bmRlZnkwNzAyMUBuY3UuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.