- 1Nutrition and Food Security Research Center, Department of Community Nutrition, School of Nutrition and Food Science, Isfahan University of Medical Sciences, Isfahan, Iran
- 2Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran
- 3Research Center for Food Hygiene and Safety, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
Background: The association between tomato/lycopene intake and blood levels of lycopene with the risk of specific cancers were assessed in previous meta-analyses; however, no study evaluated the risk of overall cancer incidence/mortality. Therefore, the present systematic review and dose–response meta-analysis aimed to summarize available findings from prospective studies to examine the association between tomato/lycopene intake and lycopene levels with the risk of total and specific cancers and cancer-related mortality.
Methods: A comprehensive literature search was done using Scopus, PubMed, ISI Web of Science, and Google Scholar until July 2023.
Results: In total, 121 prospective studies were included in the systematic review and 119 in the meta-analysis. During the follow-up period of 2–32 years, a total of 108,574 cancer cases and 10,375 deaths occurred. High intakes and high levels of lycopene compared to low amounts were, respectively, associated with 5% (Pooled RR: 0.95, 95% CI: 0.92–0.98, I2 = 26.4%, p = 0.002) and 11% (Pooled RR: 0.89, 95% CI: 0.84–0.95, I2 = 15.0%, p < 0.001) reduction in overall cancer risk. Also, each 10 μg/dL increase in blood levels of lycopene was associated with a 5% lower risk of overall cancer. Moreover, we found a linear inverse association between dietary lycopene intake and prostate cancer risk (Pooled RR 0.99, 95% CI 0.97–1.00, I2 = 0, p = 0.045). Regarding cancer mortality, negative relationships were found with total tomato intake (Pooled RR: 0.89, 95% CI: 0.85–0.93, I2 = 65.7%, p < 0.001), lycopene intake (Pooled RR: 0.84, 95% CI: 0.81–0.86, I2 = 86.5%, p < 0.001) and lycopene levels (Pooled RR 0.76, 95% CI: 0.60–0.98, I2 = 70.9%, p = 0.031). Also, an inverse association was observed between blood lycopene levels and lung cancer mortality (Pooled RR: 0.65, 95% CI: 0.45–0.94, I2 = 0, p = 0.022).
Conclusion: Our findings show that dietary intake and blood levels of lycopene are associated with a lower risk of cancer and death due to cancer.
Clinical trial registration: CRD42023432400.
Introduction
Diet has a potential role in the etiology of cancer, therefore dietary factors are responsible for 5–10% of cancer incidence (1–3). Based on the current evidence, fruit and vegetable intake may protect against cancer incidence and mortality (4, 5). The nutrient content of these food groups, such as fiber, vitamin C, and other antioxidants such as carotenoids and polyphenols, might explain the protective effect. Recently, the association between tomato intake and cancer risk received significant attention. Tomato contains different carotenoids, including β-carotene, lycopene, and lutein. Lycopene is a 40-carbon red pigment with antioxidant properties that is extracted from watermelon, apricot, and other red fruits and vegetables in addition to tomatoes. However, it is estimated that more than 80% of lycopene intake is from tomatoes and their products.
Several studies have shown that tomato intake is associated with a reduced risk of cancer and cancer progression. However, it is not clear that the beneficial effect is medicated by lycopene or other nutrients available in tomatoes. Experimental studies revealed that lycopene may have anticancer properties by regulating gene expression, modulating hormone and immune activity, and also stimulating the clearance of carcinogens (3). Despite the mentioned mechanisms, findings from observational studies on the associations of tomato and lycopene with cancer risk and mortality are conflicting (6–136). Some studies reported that dietary intake of lycopene or tomato was inversely associated with cancer risk (11, 13, 14, 42), while other studies indicated this inverse association for tomato or lycopene only. Also, there are inconsistent results between dietary and serum levels of lycopene in relation to cancer risk. In addition, a large number of studies found a null association between tomato and lycopene intake and risk of cancer incidence/mortality.
Although there are several meta-analyses in this area, we found no meta-analysis that considered all the exposures (tomato intake, dietary and blood levels of lycopene) together and the risk of cancer incidence/mortality. We found four meta-analyses on prostate cancer (137–140), one for pancreatic cancer (141), one for gastric cancer (142), two for breast cancer (143, 144), and one for ovarian cancer (145). It should be noted that findings from these meta-analyses are inconsistent, and there is no summary evidence for other types of cancers. Therefore, performing a meta-analysis considering all types of cancers is necessary. In addition, the dose–response analyses were not determined in some previous meta-analyses. Taken together, the current systematic review and dose–response meta-analysis were done to determine the associations of tomato intake and dietary/blood levels of lycopene with the risk of total and specific cancers and their mortality by summarizing available findings from prospective cohort studies.
Methods
The current study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) (146). The protocol for this systematic review was registered on PROSPERO with the code CRD42023432400.
Data source and search strategy
We searched databases, including PubMed, Scopus, and Web of Science, up to July 2023 to identify prospective studies that examined the association between dietary intake of tomato and lycopene and blood levels of lycopene with the risk of total and specific cancers or their mortality. The terms used in the search strategy are presented in Supplementary Table 1. No restrictions in language and time were considered. All results were included in Endnote software, and duplicate papers were removed. Eligible publications were selected based on the inclusion and exclusion criteria by two investigators (AB and KF). To maximize the search, we reviewed reference lists of selected articles and also previous systematic reviews. In addition, a manual search was done in Google Scholar using “tomato” and “lycopene” keywords separately with “cancer” to find any missing articles. The first 300 relevancy-ranked papers of this search engine were screened.
Inclusion criteria
Articles were considered for inclusion if they (1) were prospective in design, (2) evaluated the association between dietary intake of lycopene or tomato, or blood levels of lycopene with risk of cancer or cancer-related mortality, (3) were performed on adults (≥ 18 y), (4) those studies that reported odds ratio (OR) or risk ratio (RR) or hazard ratio (HR) along with 95% confidence intervals (CIs) for the association between tomato/lycopene and cancer risk and mortality. If the results of 1 dataset were published in >1 article, we chose the one with the most significant number of cases or more extended follow-up period.
Exclusion criteria
We excluded studies if they were case–control or cross-sectional in design, letters, review articles, editorials, and poster abstracts. Moreover, studies that investigated the combination association of tomatoes and other vegetables with cancer were excluded. In addition, studies with insufficient data and those that were done on critically ill patients were not included. Moreover, we excluded studies that evaluated lycopene supplementation in relation to cancer risk. Those studies that considered specific types of tomato products, such as tomato sauce, rather than raw tomato or total tomato intake, were excluded as well.
Data extraction
Two independent reviewers (AB and KF) extracted the following data from each eligible study: first author’s name, year of publication, country, participant’s age and gender, sample size, follow-up duration, cohort name, methods used for assessment of exposures (tomato and lycopene intake and blood levels of lycopene) and outcome (cancer incidence), covariates used for adjustment, and any reported effect sizes (ES) and corresponding 95% CIs for the association between dietary intake of tomato/lycopene with risk of total and specific cancers and their mortality.
Quality assessment
Two researchers (AB and KF) independently assessed the quality of all included studies using the Newcastle Ottawa Scale (NOS) (147). According to this scale, a maximum of 9 points would be awarded to each study according to the following parameters: 4 points for selection of participants, 2 points for comparability, and 3 points for the assessment of outcomes. Studies achieving a total score of ≥7 (median score of studies included in the current meta-analysis) were considered high-quality studies.
Statistical analysis
We included the RR of cancer and cancer mortality reported for the comparison between the highest and lowest intakes of lycopene and tomato and the highest and lowest circulating levels of lycopene in the meta-analysis. However, some studies reported RRs of cancer risk per 1 standard deviation (SD) increment in exposure levels. To include such studies in the meta-analysis, we converted the per SD increment risk estimates to the relative risks for the comparison of the top versus bottom quartile using the method suggested by Danesh et al. (148) in which the log risk estimates reported for the comparison are equivalent to 2.54 times the log risk estimates for a 1 SD increase. This method assumes that the exposure is a normally distributed variable and that the association with the disease risk is log-linear. Moreover, in the populations where the prevalence of cancer was ≥10%, we converted reported ORs and HRs to RRs before meta-analysis.
Since the between-study heterogeneity was low in most analyses, we used a fixed-effects model to calculate the overall effect estimates of cancer risk and mortality. In addition to the fixed model, we performed the overall analyses using a random-effects model. This model considers different sources of uncertainties, including within- (sampling or estimation) and between-studies heterogeneity (149). However, since random-effects models tend to give disproportionally more weight to smaller studies, mainly when the outcome is binary (e.g., cancer or death), fixed-effects models may present more reliable results compared with the random-effects models (150). Cochran’s Q test and the I2 statistic were used to assess heterogeneity among included studies. I2 values of >50%, or p < 0.10 for the Q-test, were considered as significant heterogeneity. To identify possible sources of heterogeneity, subgroup analyses were performed based on pre-defined variables including duration of follow-up (≥10 vs. <10 years), sample size (≥10,000 vs. <10,000 participants), geographical location (US vs. non-US countries), methods used for the assessment of exposures (FFQ vs. other tools) and outcome (medical records or pathological methods vs. self-reported data), study quality (high vs. low), adjustments for important confounders including energy intake and BMI (adjusted vs. not-adjusted), and tissue levels of lycopene (serum vs. plasma). We selected the variables based on their effects on the findings of our meta-analysis (i.e., follow-up duration, sample size, etc.) and the importance of results in their subgroups (i.e., geographical location, study quality, etc.). We used the formal tests of Egger and Begg to detect potential publication bias. Moreover, a sensitivity analysis using a random-effects model was performed to examine the dependency of overall risk estimates on each study.
In addition to the highest versus lowest comparison, we assessed the linear and non-linear dose–response associations between tomato/lycopene intakes, serum levels of lycopene, and cancer risk. For the linear dose–response analysis, the generalized least squares trend (GLST) estimation method, described by Greenland and Longnecker (151) and Orsini et al. (152), was used. First, we estimated study-specific slopes, and then these slopes were combined to obtain an overall average slope. We combined the study-specific slopes using random- or fixed-effects models. In the GLST method, the distribution of cases, the total number of participants, and the effect sizes with the variance estimates for ≥3 quantitative categories of exposure were required. The following information was required in this method for each study: distribution of total participants and cancer cases, RRs of cancer risk or mortality across categories of exposures, and the median or mean amount of serum or dietary tomato/lycopene in each category. In studies that reported the amount of exposure as ranges in each category, we estimated the midpoint by calculating the mean of the lower and upper bound. For open-ended categories, we considered the length of the category the same as an adjacent interval. For studies with reported raw tomato consumption as serving/day, we converted it to gr/day using the serving size (in grams) presented in the studies. For studies that did not report the amount of serving size, the standard serving size of 180 grams was used for this conversion. The non-linear dose–response relationship was also assessed using the restricted cubic splines with 3 knots at percentiles of 10, 50, and 90% of the distribution. The correlation within each set of provided risk estimates was considered, and the study-specific estimates were combined using a one-stage linear mixed-effects meta-analysis. The significance for nonlinearity was calculated by null hypothesis testing, in which the coefficient of the second spline was considered equal to zero. All statistical analyses were done using Stata software, version 17 (Stata Corp, College Station, TX). p-values were considered significant at the level of <0.05.
Results
Search results
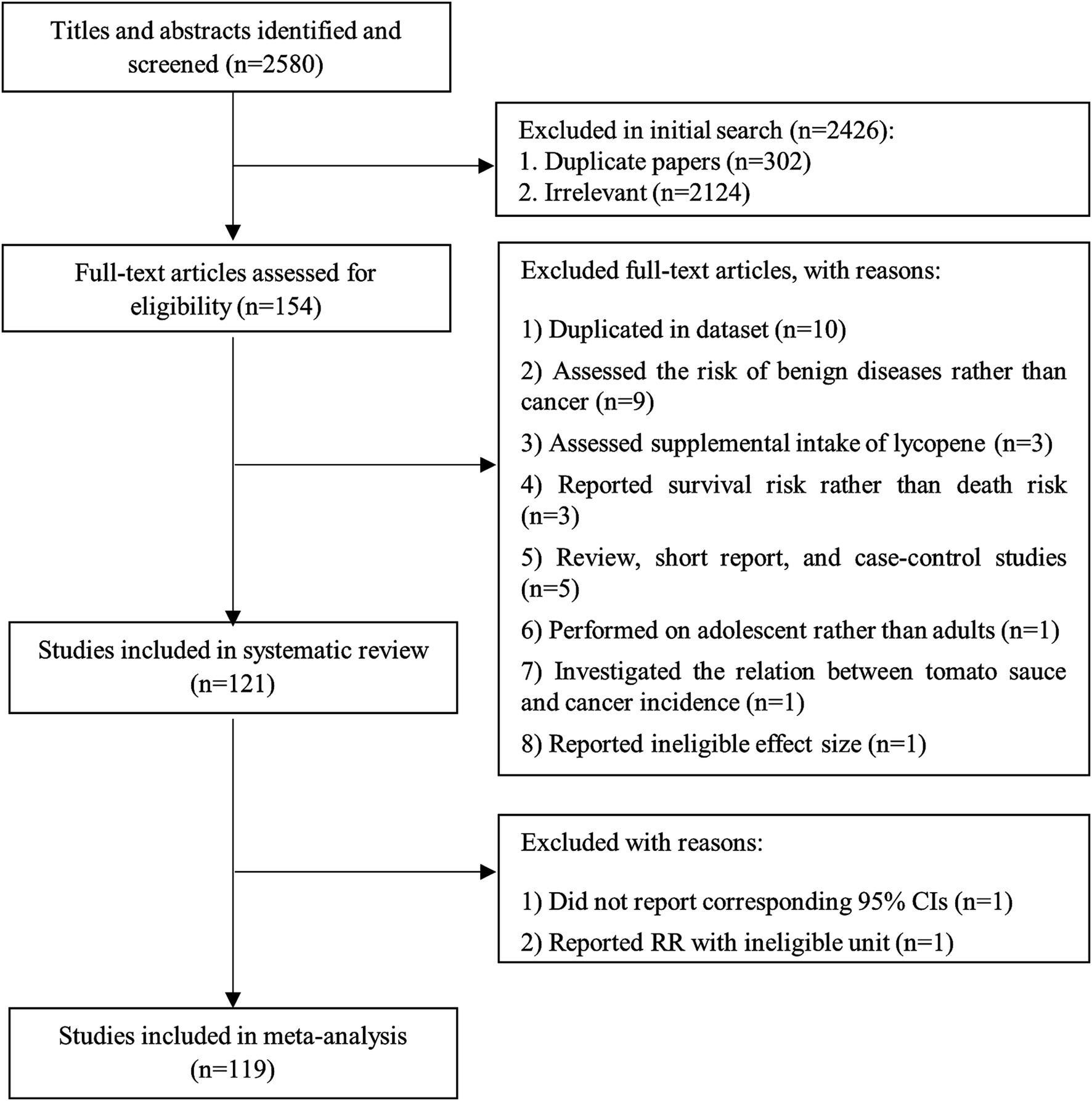
We found a total of 2,580 papers in the online databases. After excluding duplicate papers (n = 302), 2,278 articles remained for the title and abstract review. Accordingly, 2,124 papers were considered unrelated, and 154 articles were included in the full-text assessment. Of the 154 articles, nine studies were excluded because the risk of benign diseases was assessed rather than cancer risk (153–161). We also excluded three studies that reported survival risk instead of death risk (111, 162, 163). Three studies assessed the lycopene supplementation in relation to cancer risk and, therefore, were excluded (164–166). One study performed on children and adolescents was excluded as well (167). Moreover, in one study, the relationship between the consumption of tomato sauce and cancer incidence was investigated. Therefore, it was excluded (168). In addition, one study was excluded because of reporting correlation coefficient rather than RR (169). Furthermore, three studies with a case–control design (170–172), one review article (173), and one short report (174) were excluded. We found three publications which were conducted on Health Professional Follow-up Study (HPFS) (37, 128, 129), four papers on Nurses’ Health Study (NHS) (76, 130–132), two papers on Comprehensive Health Examination Program (CHEP) (113, 133), two articles on Japan Collaborative Cohort Study (JACC) (105, 134), two publications on the Prostate, Lung, Colorectal, and Ovarian trial (PLCO) (17, 135), and two on The European Prospective Investigation into Cancer and Nutrition (EPIC) (70, 136). With respect to these articles assessed similar exposure and outcome variables, we included only the one with the highest quality or with the most significant number of cases for each dataset (17, 37, 70, 76, 105, 113) and excluded the duplicated papers (128–136). Also, we found a pooled analysis of 10 datasets (121). All studies included in the pooled analysis, except the data from the NHS (9), were different. To avoid double-counting data, we excluded the study of Fairfield et al. (9) containing data from the NHS, and therefore, we included the pooled analysis. Finally, after these exclusions, 121 articles containing prospective studies were included in the current systematic review. Figure 1 summarizes the process of study selection.
Overview of the included studies
Supplementary Tables 2, 3 illustrate the characteristics of included studies in the current systematic review and meta-analysis. The sample size of included studies ranged between 102 and 521,911 participants, resulting in a total sample size of 4,598,358 subjects aged 18–104 years. During follow-up periods ranging from 2 to 32 years, a total of 108,574 cancer cases and 10,375 deaths due to cancer were recognized. Out of 121 articles, 66 were conducted in the US, 54 in non-US countries, and one in both US and non-US countries. Dietary intakes of lycopene and tomato were assessed using FFQ in 59 articles, a researcher-made questionnaire in 3 publications, dietary history in 3 papers, food recall in 2 papers, food record in 2 papers, and both researcher-made questionnaire and food recall in one study. In terms of cancer assessment, 28 papers used self-reported data, 84 articles used data from medical records, two papers used both medical records and self-reported data, and other studies used pathological or histological findings for cancer diagnosis. Among the included studies, different confounding variables, including energy intake (n = 52), BMI, smoking, and age, were adjusted. The NOS scores of the included studies ranged between 5 and 9. We considered the score of 7 as the median for a total score of NOS; 88 articles had a score of ≥7, defined as high-quality studies (Supplementary Tables 4, 5).
Findings from the systematic review
From 46 articles on dietary lycopene and overall cancer risk, four papers found an inverse association, and others illustrated no significant association. Of the 19 papers on total tomato consumption and overall cancer risk, four indicated an inverse association, but others found no significant association. Two articles illustrated an inverse association between blood levels of lycopene and total cancer risk (n = 43). In the case of cancer mortality, one study indicated a protective association between lycopene intake and cancer mortality. Such a protective association was also found in two studies for total tomato intake and three papers for blood levels of lycopene; however, others showed no significant association.
Findings from the meta-analysis
Of the 121 articles in the systematic review, 119 papers with complete data were included in the current meta-analysis. One study that reported RRs without corresponding 95% CIs was not included (127). Moreover, the study of Fujii et al. (112) was excluded because they reported an RR of cancer mortality per 25% increase in serum lycopene. Since the conversion of this unit to other usual units was impossible, we excluded this study from the meta-analysis. Some included papers reported RRs of different cancers from one dataset. To avoid double-counting data, we first merged the RRs to calculate an overall RR of cancer for that dataset. Then, the pooled RR was included in the primary meta-analysis. Accordingly, we merged the effect sizes of 3 papers from the NHS (8, 15, 36), five papers from the Women’s Health Initiative Study (WHI) (7, 13, 32, 108, 109), five publications from the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (ATBC) (14, 20, 23, 26, 123), two papers from the PLCO (17, 33), two papers from the HPFS (21, 37), two articles from the Multiethnic Cohort Study (MEC) (28, 29), six articles from the Netherlands Cohort Study (NLCS) (30, 55, 56, 64, 65, 125), three papers from the NLCS (67, 68, 120), five publications from the National Breast Screening Study (NBSS) (24, 57, 59, 61, 62), two papers from the Singapore Chinese Health Study (SCHS) (35, 63), and two articles from both NHS and HPFS (16, 22) to calculate overall RRs of cancer.
Total tomato intake and cancer
Overall cancer
Nineteen papers (11, 17, 31, 40, 42–49, 52, 60, 63, 66, 67, 69, 124) with a total of 1,120,154 participants and 30,009 cases were included in this association. Overall RR for this relation, comparing the highest with the lowest intake of tomato, was 1.01 (95% CI: 0.97–1.05, p = 0.687), indicating no significant association between total tomato intake and overall risk of cancer (Table 1). Also, there was evidence of significant heterogeneity between the studies (I2 = 61.0%, p < 0.001).
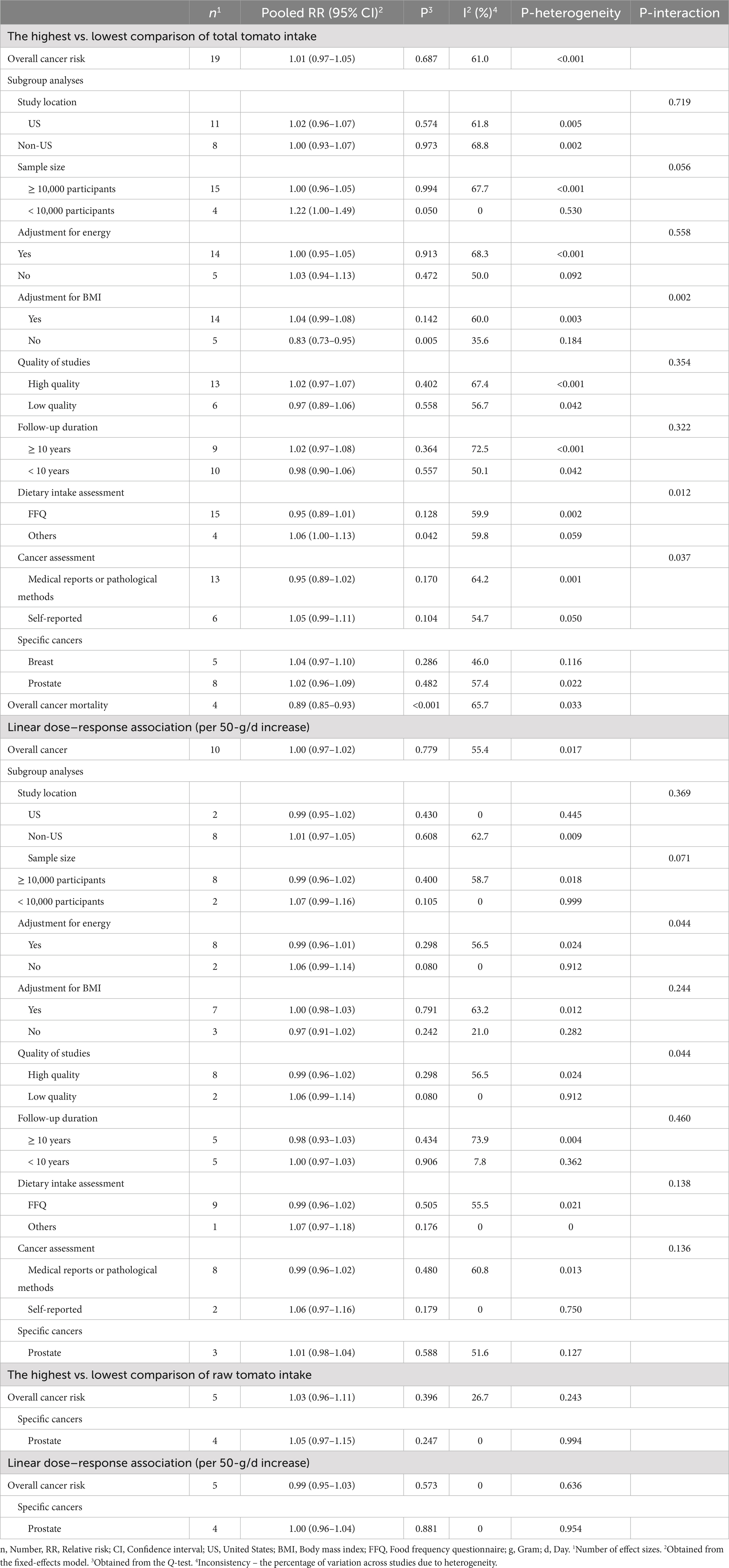
Table 1. Summary risk estimates for the association between tomato intake with cancer risk and mortality in adults.
Eight (17, 40, 44, 46, 48, 49, 52, 63) and 12 articles (17, 40, 41, 44, 46, 48, 49, 52, 63, 67, 68, 120) with sufficient data were recognized for inclusion in the non-linear and linear dose–response analysis, respectively. We found no significant association between a 50-g/d increase in total tomato intake and overall risk of cancer (Pooled RR: 1.00, 95% CI: 0.97–1.02, p = 0.779; Table 1). Moreover, there was no evidence of a non-linear association in this regard (P for nonlinearity = 0.618; Figure 2A).
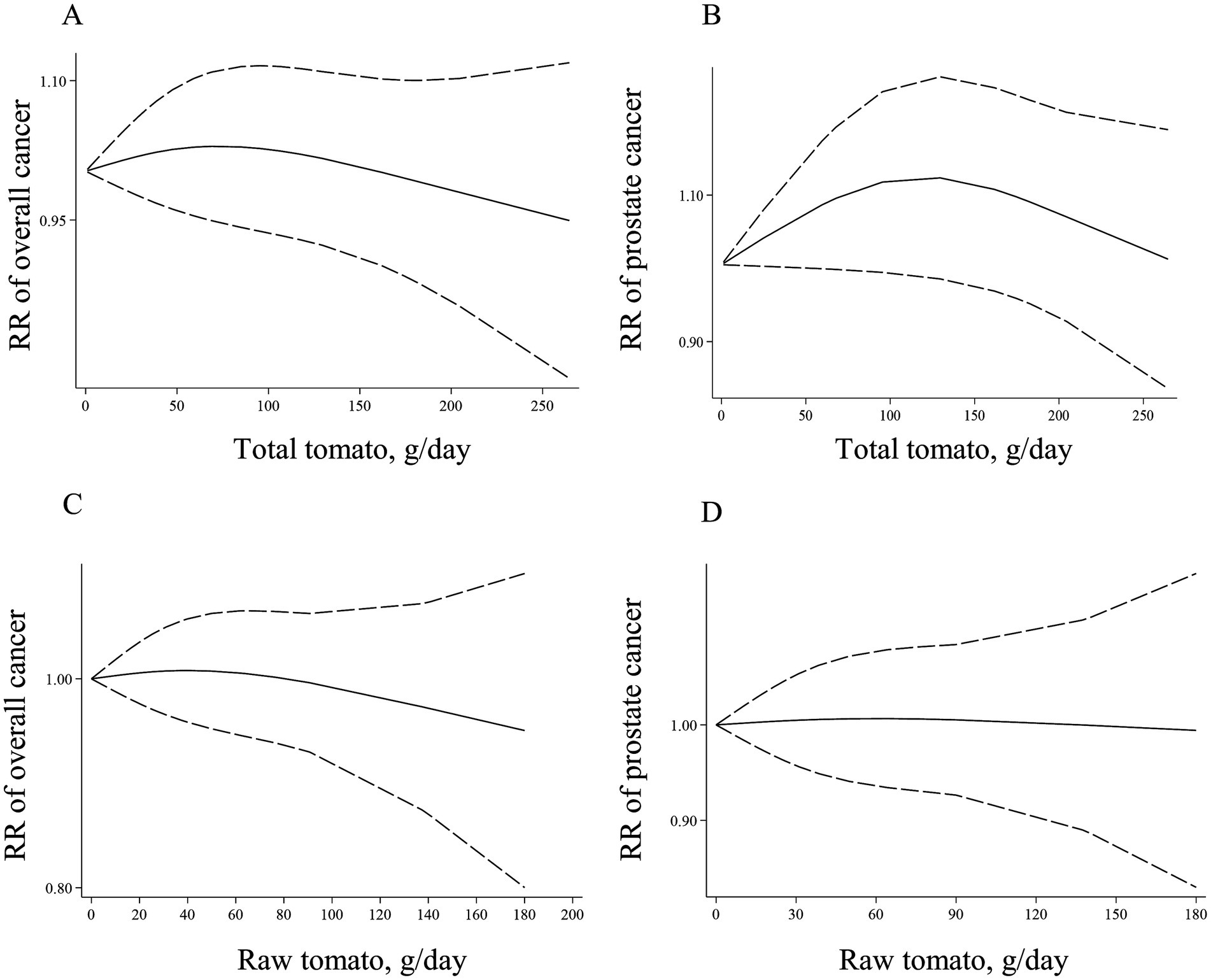
Figure 2. Non-linear dose–response associations of total tomato consumption with risk of overall (A) and prostate cancer (B), and non-linear dose–response associations of raw tomato consumption with risk of overall (C), and prostate cancer (D) in adults aged ≥18 years. The solid lines indicate the spline model. The dashed lines present the 95% CI. RR: relative risk.
Specific cancers
Overall, combining five articles on breast cancer (44, 48, 66, 69, 124) and eight publications on prostate cancer (11, 17, 31, 40, 45, 47, 49, 67), comparing the highest and lowest intakes of total tomato, presented an overall RR of 1.04 (95% CI: 0.97–1.10, I2 = 46.0%, p = 0.286) for breast cancer and 1.02 (95% CI: 0.96–1.09, I2 = 57.4%, p = 0.482) for prostate cancer that both were statistically non-significant (Table 1).
Regarding prostate cancer, three publications (17, 40, 49) had sufficient data to perform non-linear and linear dose–response analysis. We found no linear (Table 1) and non-linear (Figure 2B) associations for this cancer (P for nonlinearity = 0.157).
Cancer mortality
Four articles (38, 39, 50, 51) with a total sample size of 249,308 and 8,863 cancer deaths were included. Summary RR of cancer mortality, comparing the highest and lowest intakes of total tomato, was 0.89 (95% CI: 0.85–0.93, I2 = 65.7%, p < 0.001), indicating a significant inverse association (Table 1).
Raw tomato intake and cancer
Overall cancer
Six papers (6, 10, 17, 31, 33, 44) with a total of 285,840 participants and 8,429 cases were included in this association. The summary effect size for the risk of total cancer comparing the highest with the lowest intakes of raw tomato was 1.03 (95% CI: 0.96–1.11; I2 = 26.7%, p = 0.396), indicating a non-significant positive association (Table 1).
Six articles (6, 10, 17, 31, 33, 44) with sufficient data were included in the linear and non-linear dose–response analyses. We found no linear (Table 1) and non-linear (Figure 2C) associations for the overall cancer risk (P for nonlinearity = 0.777).
Specific cancers
Overall, combining four articles (6, 10, 17, 31) on prostate cancer, we found no significant association when comparing the highest and the lowest categories of raw tomato intake (RR: 1.05, 95% CI: 0.97–1.15, p = 0.247) (Table 1).
Four studies (6, 10, 17, 31) with sufficient data were included in linear and non-linear dose–response analyses. We found no significant linear association between each 50-g/d increase in raw tomato intake and risk of prostate cancer (Table 1). Moreover, there was no evidence of non-linear association in this regard (P for nonlinearity = 0.978; Figure 2D).
Dietary lycopene intake and cancer
Overall cancer
Forty-six articles (7, 8, 10, 12–30, 32, 33, 35–37, 53–66, 121–123, 125, 126) with a total of 2,687,842 subjects and 49,617 cases were included in this association. The summary effect size for the risk of total cancer comparing the highest with the lowest intakes of lycopene was 0.95 (95% CI: 0.92–0.98; I2 = 26.4%, p = 0.002), indicating a significant inverse association (Table 2).
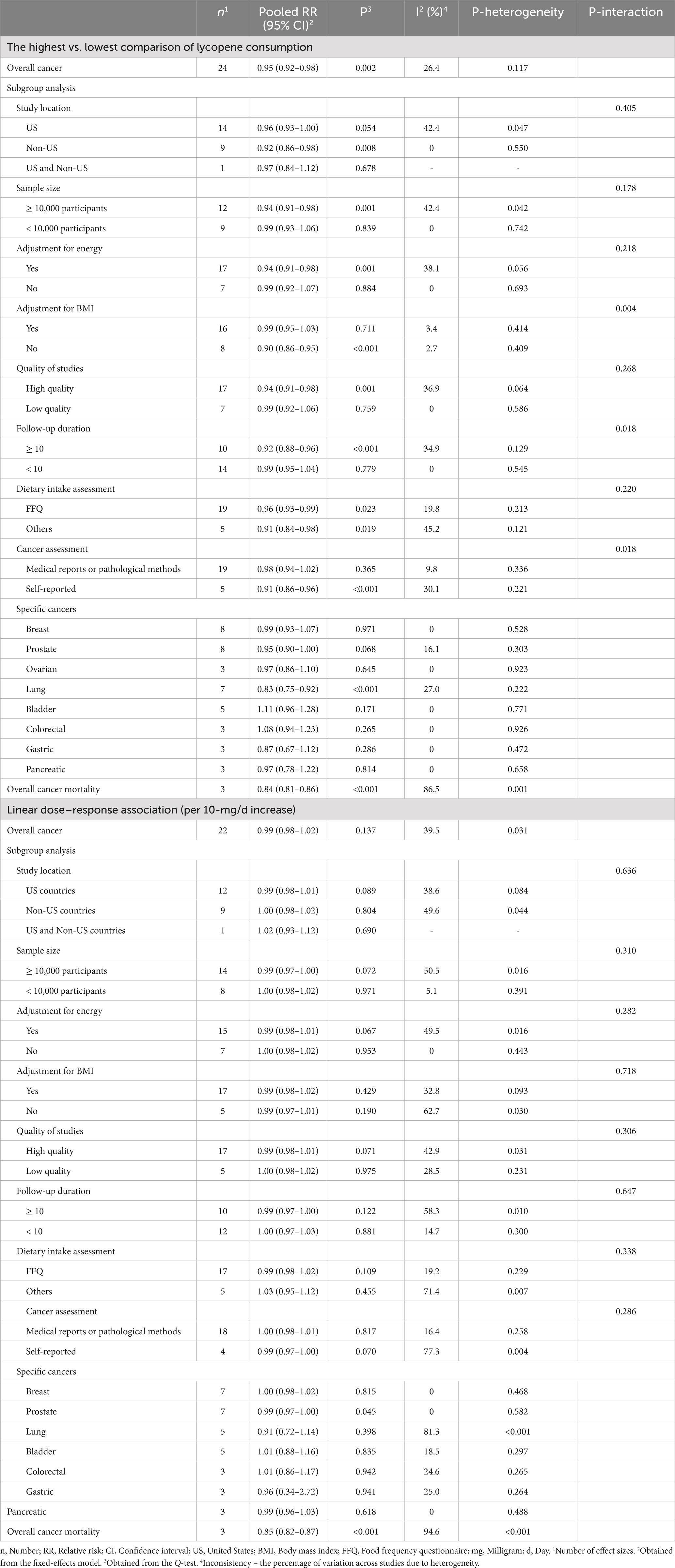
Table 2. Summary risk estimates for the association between dietary intake of lycopene with cancer risk and mortality in adults.
Forty-one (7, 8, 12–21, 23–30, 32, 33, 35, 37, 53–59, 61–66, 122, 123, 125, 126) and 42 papers (7, 8, 12–21, 23–30, 32, 33, 35, 37, 53–59, 61–66, 121–123, 125, 126) with sufficient data were included in the non-linear and linear dose–response analyses, respectively. There was no significant association between a 10-mg/d increase in lycopene intake and overall risk of cancer (Pooled RR: 0.99, 95% CI: 0.98–1.02, I2 = 39.5%, p = 0.137; Table 2). Moreover, the non-linear dose–response analysis indicated no non-linear relation between dietary lycopene intake and overall risk of cancer (P for nonlinearity = 0.166; Figure 3A).
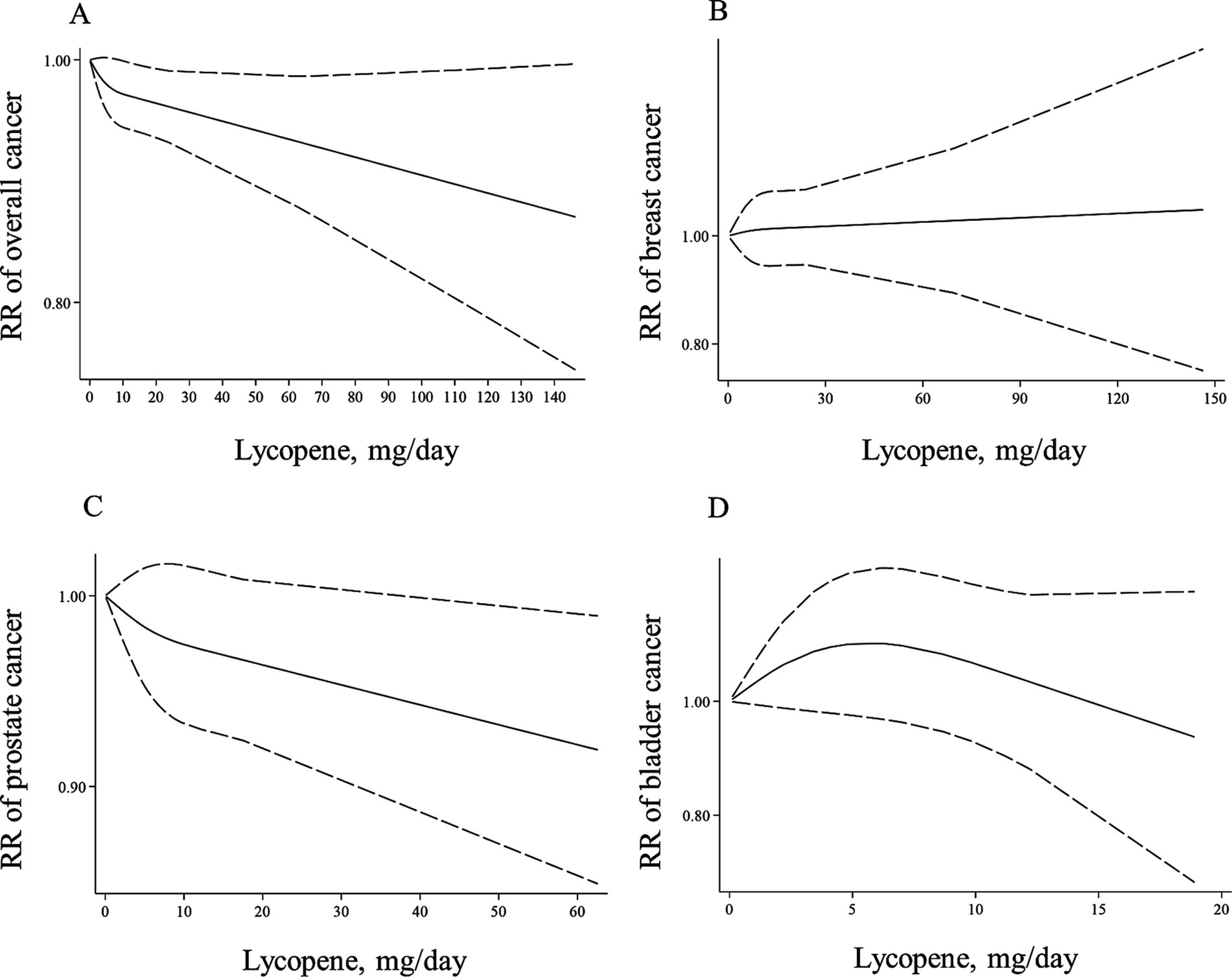
Figure 3. Non-linear dose–response associations of lycopene consumption with risk of overall (A), breast (B), prostate (C), and bladder cancer (D) in adults aged ≥18 years. The solid lines indicate the spline model. The dashed lines present the 95% CI. RR: relative risk.
Specific cancers
Combing eight studies for breast cancer (7, 19, 27, 36, 61, 66, 122, 126), eight studies for prostate cancer (10, 17, 29, 30, 37, 53, 54, 58), and three studies for ovarian cancer (24, 32, 121), we found no significant association when comparing the highest and the lowest categories of lycopene intake (RR for breast cancer: 0.99, 95% CI: 0.93–1.07, p = 0.971, RR for prostate cancer: 0.95, 95% CI: 0.90–1.00, p = 0.068, and RR for ovarian cancer: 0.97, 95% CI: 0.86–1.10, p = 0.645) (Table 2). In addition, we found no significant association for bladder, colorectal, gastric, and pancreatic cancers. However, a significant inverse association was observed between lycopene intake and risk of lung cancer (Pooled RR: 0.83, 95% CI: 0.75–0.92, I2 = 27.0%, p < 0.001) (14, 22, 25, 35, 59, 60, 64) (Table 2).
In the case of dose–response analysis, except for prostate cancer (Pooled RR: 0.99, 95% CI: 0.97–1.00, I2 = 0, p = 0.045; Table 2), we found no significant linear association between dietary lycopene intake and risk of breast, lung, bladder, colorectal, gastric, and pancreatic cancers (Table 2). Also, there was no evidence of a non-linear association for these cancers except for lung cancer, where a non-linear association was found (Figures 3B–D, 4A–C). For this association, the risk of lung cancer decreased from zero to 5 mg/d of lycopene intake, whereas the risk started to rise at approximately 10 mg/d intake (Figure 4D).
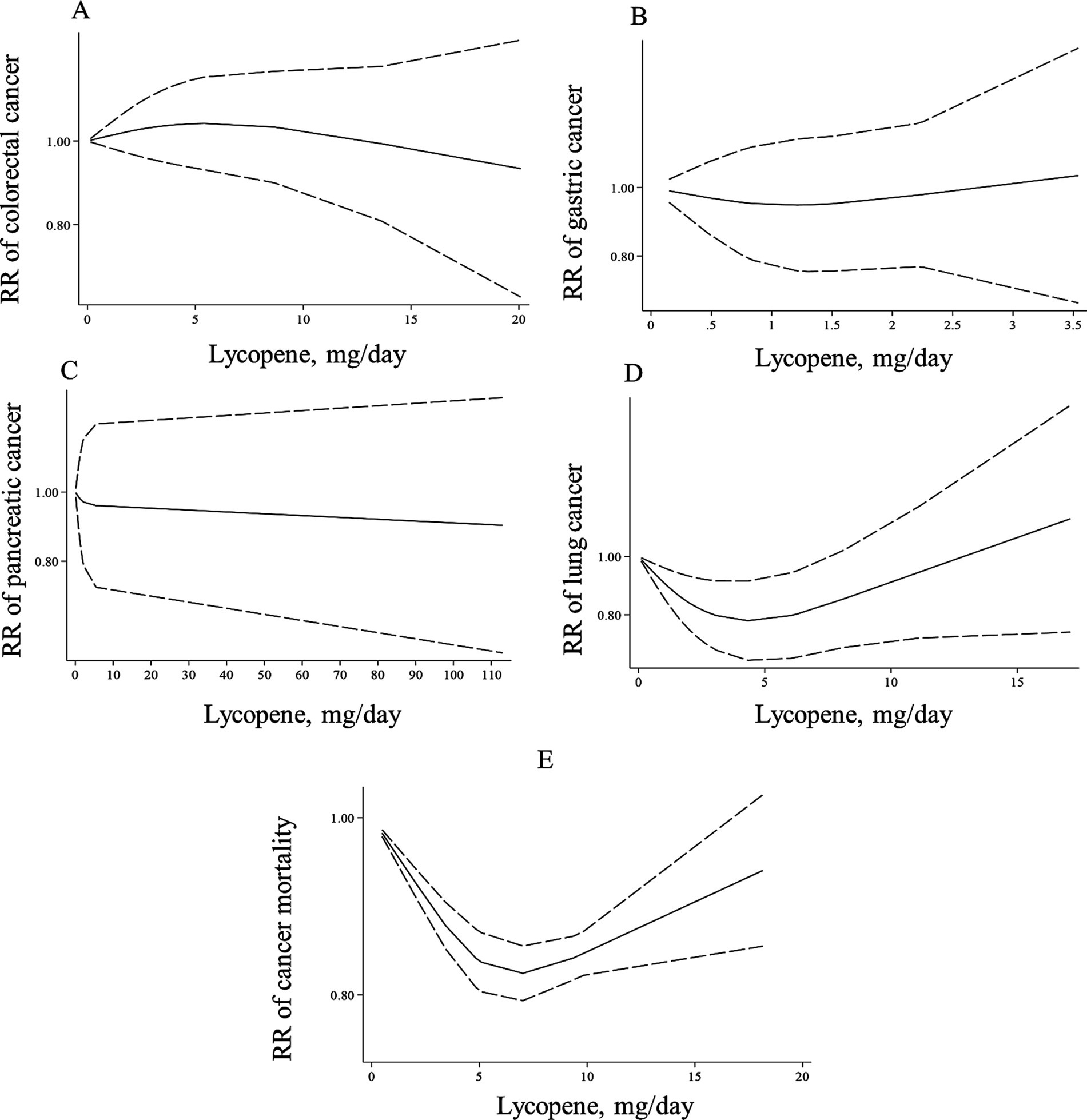
Figure 4. Non-linear dose–response associations of lycopene consumption with risk of colorectal (A), gastric (B), pancreatic (C), lung cancer (D), and cancer mortality (E) in adults aged ≥18 years. The solid lines indicate the spline model. The dashed lines present the 95% CI. RR: relative risk.
Cancer mortality
The summary RR for cancer mortality risk when comparing the highest with the lowest lycopene consumption was 0.84 (95% CI: 0.81–0.86, p < 0.001), which indicates a significant inverse association (34, 38, 39). Moreover, a significant between-study heterogeneity was observed (I2 = 86.5%, p = 0.001; Table 2). Also, there was evidence of linear dose–response association in which a 10-mg/d increase in lycopene intake was associated with a 15% risk reduction in cancer mortality (Pooled RR: 0.85, 95% CI: 0.82–0.87, I2 = 94.6%, p < 0.001; Table 2). Moreover, a non-linear relation was observed in this regard (P for nonlinearity <0.001; Figure 4E) in such a way that the mortality risk reduced from zero to a lycopene intake of 7 mg/d; nonetheless, the risk began to rise at a dosage of 10 mg/d.
Blood levels of lycopene and cancer
Overall cancer
Forty-three articles (66, 71–103, 106–110, 117–119) with a total number of 92,356 subjects and 21,707 cases that examined the association between blood levels of lycopene and cancer risk were included in the meta-analysis. The summary RR for the risk of total cancer comparing the highest with the lowest levels of lycopene was 0.89 (95% CI: 0.84–0.95, p < 0.001), indicating a significant inverse association with no evidence of significant heterogeneity between studies (I2 = 15.0%, p = 0.204; Table 3).
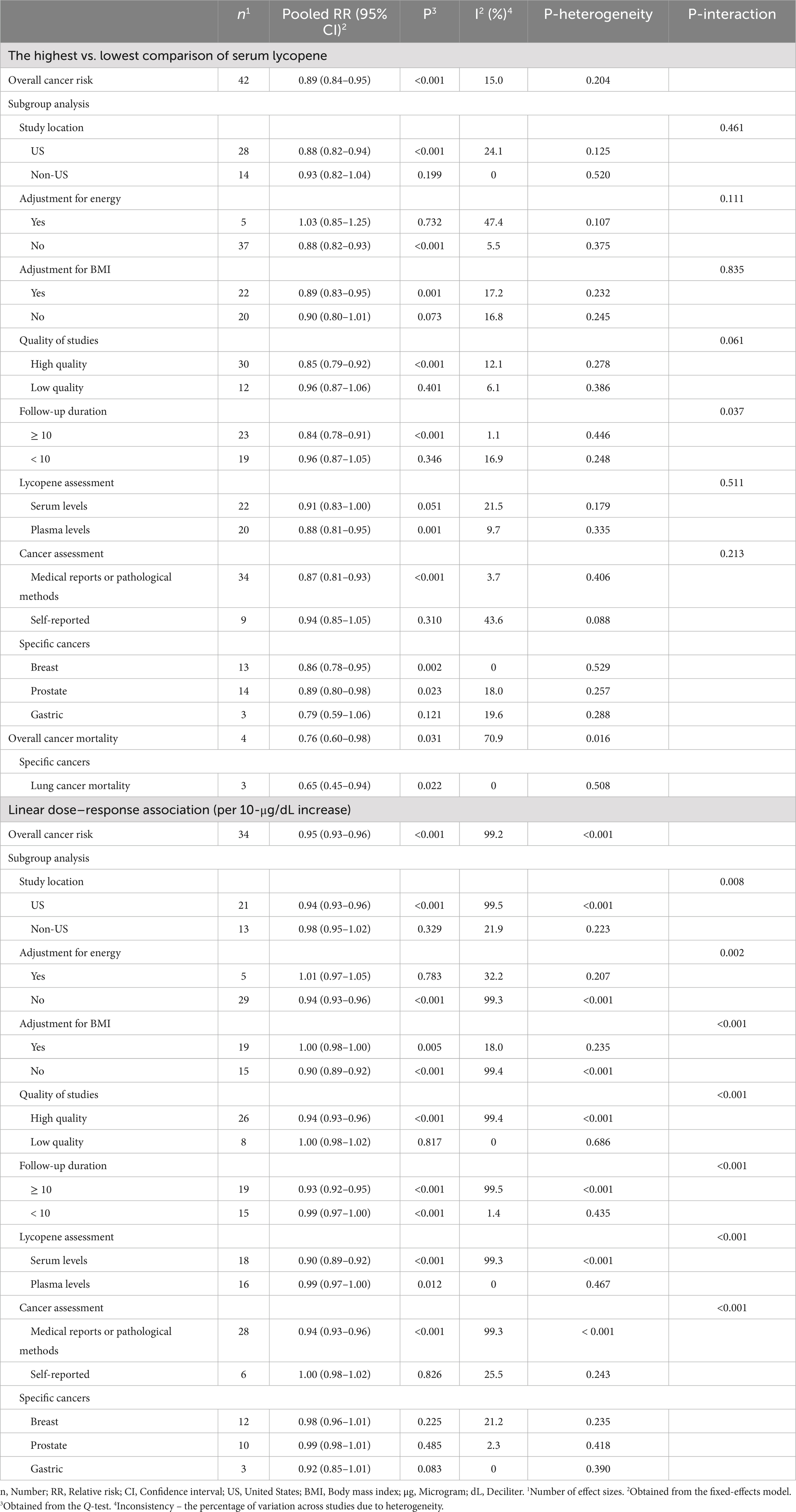
Table 3. Summary risk estimates for the association between blood levels of lycopene with cancer risk and mortality in adults.
Thirty-four (66, 70–72, 74–87, 89, 91, 94–99, 101, 102, 107–110, 118, 119) and 35 papers (66, 70–72, 74–87, 89, 91, 94–99, 101, 102, 107–110, 117–119) with sufficient data were included in the non-linear and linear dose–response analyses, respectively. In the linear analysis, each 10 μg/dL increase in blood levels of lycopene was associated with a 5% lower risk of total cancer (Pooled RR: 0.95, 95% CI: 0.93–0.96, p < 0.001; Table 3). Also, we found evidence of a non-linear association in this regard (P for nonlinearity <0.001), in which the risk of total cancer decreased continuously until 50 μg/dL of lycopene levels, and then, the risk reduction slowed down at the higher dosages (Figure 5A).
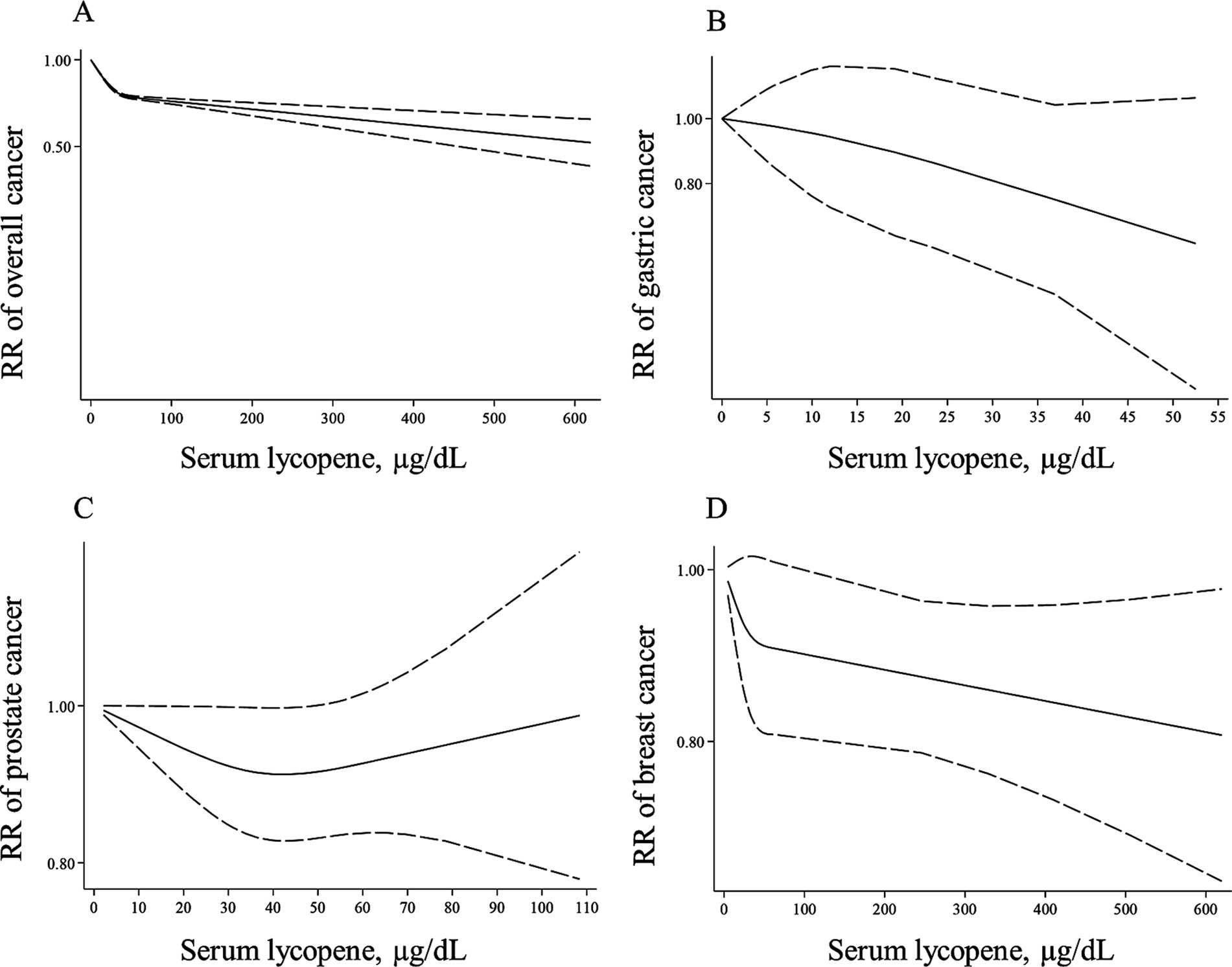
Figure 5. Non-linear dose–response associations of lycopene levels with risk of overall (A), gastric (B), prostate (C), and breast cancer (D) in adults aged ≥18 years. The solid lines indicate the spline model. The dashed lines present the 95% CI. RR: relative risk.
Specific cancers
Blood levels of lycopene in relation to breast cancer were examined in 13 studies (66, 70, 74–76, 84, 93, 97–99, 106, 108, 118) with 24,599 participants and 9,061 cases. A significant inverse association was found when comparing the highest with the lowest levels of lycopene (Pooled RR: 0.86, 95% CI: 0.78–0.95, I2 = 0%, p = 0.002). In terms of prostate cancer, we also observed a significant inverse association by comparing the highest with the lowest lycopene concentrations (Pooled RR: 0.89, 95% CI: 0.80–0.98, p = 0.023) with no evidence of heterogeneity between studies (I2 = 18.0%, p = 0.257; Table 3). Additionally, by comparing the highest vs. lowest lycopene concentrations, no significant association was found regarding gastric cancer (Pooled RR: 0.79, 95% CI: 0.59–1.06, I2 = 19.6%, p = 0.121).
Three articles for gastric cancer (94, 102, 119) and 10 publications on prostate cancer (72, 77–79, 82, 83, 86, 87, 95, 110) with required data were included in the dose–response analyses. We found no significant associations between a 10-μg/dL increase in blood levels of lycopene and the risk of these two cancers (Table 3). In terms of breast cancer, 11 (66, 70, 74–76, 84, 97–99, 108, 118) and 12 papers (66, 70, 74–76, 84, 97–99, 108, 117, 118) had sufficient data for the non-linear and linear dose–response analyses, respectively. A non-significant inverse association was also observed for a 10-μg/dL elevate in lycopene levels and risk of breast cancer (Pooled RR: 0.98, 95% CI: 0.96–1.01, p = 0.225, Table 3). Regarding the non-linear dose–response analysis, no evidence of nonlinearity was observed for gastric, prostate, and breast cancers (P for nonlinearity > 0.10; Figures 5B–D).
Cancer mortality
The association between lycopene levels and overall cancer mortality was examined in 4 articles (104, 113, 114, 116), which enrolled 19,178 participants and 887 cases. We found an inverse significant association for cancer death, comparing the highest with the lowest concentration of lycopene (Pooled RR: 0.76, 95% CI: 0.60–0.98, I2 = 70.9%, p = 0.031). In terms of lung cancer mortality, such a significant association was also observed (Pooled RR: 0.65, 95% CI: 0.45–0.94, I2 = 0%, p = 0.022; Table 3). Data for other types of cancers were not sufficient for a meta-analysis. Also, we had insufficient data to perform the dose–response analyses.
Sensitivity analyses, publication bias, and subgroup analyses
In the sensitivity analyses based on a fixed-effects model, the summary RRs obtained in the current meta-analysis were not driven by single studies. Based on Begg’s linear regression test, we found publication bias for the association between blood levels of lycopene and overall cancer risk (0.022) and between a 10-μg/dL increase in lycopene levels and overall (p < 0.001) and prostate cancer risk (p = 0.032). However, the application of the trim-and-fill method did not alter the pooled RRs, indicating that the results were not affected by the publication bias.
In the subgroup analyses, we found that the observed heterogeneity was explained by study location, sample size, adjustment for BMI, the tools used for dietary assessment and cancer diagnosis, follow-up duration, and quality of studies. Subgroup analyses for the association between total tomato intake and overall cancer risk, comparing the highest with the lowest tomato intake, revealed a significant inverse association between total tomato intake and cancer risk in studies that did not adjust for BMI (Table 1). For the association between dietary intake of lycopene and total cancer risk, significant interactions were found in terms of follow-up durations, adjustments for BMI, and methods used for cancer assessment (Table 2). A significant inverse association was found between lycopene intake and overall cancer risk in studies that were performed in non-US countries and those with high quality. In terms of blood levels of lycopene and total cancer risk, when comparing the highest with the lowest levels of lycopene, we found a significant inverse association between high-quality studies and those that were conducted in the US (Table 3).
Overall findings based on a random-effects model
When we performed all analyses based on a random-effects model, our findings on cancer incidence remained unchanged (Supplementary Table 6). However, all significant inverse associations obtained for total tomato/lycopene intakes and blood levels of lycopene with cancer mortality became non-significant.
Discussion
In this systematic review and meta-analysis, we found that higher levels of dietary and blood lycopene were, respectively, associated with 5 and 11% lower risk of overall cancer. In the dose–response analysis, each 10-μg/dL increase in blood levels of lycopene was associated with a 5% lower risk of overall cancer. Moreover, higher lycopene intakes/levels were negatively associated with lung, breast, and prostate cancers. Also, the association between lycopene intake and lung and prostate cancers was dose-dependent. For cancer mortality, higher total tomato/lycopene intakes and higher levels of blood lycopene were associated with a lower risk of overall cancer mortality. In the case of dietary lycopene, this association was dose-dependent based on the dose–response analyses.
The potential health benefits of tomatoes on cancer risk have been investigated in previous studies. However, the evidence seems to be conflicting (11, 44, 45, 49). In the present meta-analysis of prospective studies, no significant association was observed between total/raw tomato intake and risk of overall cancer and also breast and prostate cancers based on comparing the highest with the lowest intakes of total/raw tomato. In agreement with our findings, in a meta-analysis by Luo et al. (175), total tomato consumption was not associated with the risk of prostate cancer. Moreover, in two previous meta-analyses (138, 140), no significant association was observed between raw tomato intake and prostate cancer risk. However, Xu et al. (137) and Rowles et al. (138) indicated that total tomato intake was inversely associated with prostate cancer risk. It should be noted that Xu et al. (137) and Rowles et al. (138) combined effect sizes from case–control studies with those obtained from prospective studies. This difference might explain the disparity in the previous findings. In terms of breast cancer, a recent meta-analysis indicated no significant relationship with total tomato intake (144).
In contrast with tomato, we found that dietary lycopene intake was inversely associated with the risk of overall cancer and also lung cancer. It seems that an interaction between lycopene and other constituents in tomatoes results in a non-significant association between tomato intake and cancer risk. In addition, different cooking or processing methods may affect the properties of tomatoes. Recent studies have shown that cooked tomato has higher antioxidants compared to raw tomato. These antioxidants, such as FruHis, may help lycopene for its anticancer properties. Therefore, different findings on tomato and lycopene intake may be explained by the effects of processing methods on tomato properties.
Although we found no significant association between dietary intake of lycopene and the risk of prostate cancer, there was evidence of a linear link in this regard. However, by comparing the highest vs. the lowest levels of circulating lycopene, a negative association was observed. In line with our findings, three prior meta-analyses (139, 176, 177) indicated a significant inverse relationship between circulating lycopene and prostate cancer risk. However, Wang et al. (176) found a non-linear association between lycopene intake and risk of prostate cancer. Of the reasons explaining the discrepancy, one could be missing some eligible papers in the previous meta-analysis (37, 53). Also, the effect sizes from various observational studies (i.e., case–control, cross-sectional, cohort) were combined in the previous meta-analysis. In contrast to the meta-analyses mentioned above, a previous meta-analysis in 2013 (140) found no significant association between circulating levels of lycopene and prostate cancer risk. This difference is due to the lack of two eligible studies that were not included in the 2013 meta-analysis (88, 92). Additionally, the results of the present investigation did not support the inverse association between dietary and blood levels of lycopene and other types of cancers, including breast, ovarian, colorectal, gastric, and pancreatic cancer. These findings were in line with previous meta-analyses (141–143, 145, 178).
Lycopene intake in a usual diet is negligible, and it is difficult to investigate its association with health outcomes. Among the studies included in this meta-analysis, lycopene intake varied between 0.1 and 146.3 mg/day, which helped us to examine the relationship between lycopene intake and cancer risk at different levels of intake. However, due to the low dosage of lycopene in a diet, its estimation through dietary questionnaires is challenging. Thus, we evaluated blood levels of lycopene, which are the best indicators of lycopene intake. In most associations evaluated in the current meta-analysis, our findings regarding dietary lycopene intake were in line with those obtained for its blood levels. However, we found some differences in the risk of breast and prostate cancers that were inversely associated with blood lycopene levels but not dietary levels. These differences might be due to the low power of dietary questionnaires to estimate accurate dietary intakes. In addition, we found a significant inverse association for lung cancer risk in relation to dietary lycopene but not blood lycopene. Therefore, our findings on lung cancer should be considered with caution. Further studies are needed in this regard.
In the current study, total tomato intake was associated with a reduced risk of cancer mortality. However, this association with cancer incidence was not significant. This difference might be due to the duration of follow-up required for occurring outcomes. For survival studies, a short follow-up duration might be adequate for the incidence of cancer death. However, in prospective studies on healthy individuals, a long follow-up period is required for cancer incidence. Therefore, follow-up duration in the included studies on cancer incidence might be insufficient for cancer incidence. Future studies should consider this issue.
Some potential mechanisms could explain the cancer-protective effects of lycopene. Lycopene, as an antioxidant, exerts anticancer properties by inhibiting the production of insulin-like growth factor 1 and angiogenesis, promoting apoptosis and differentiation, and also protecting DNA and macromolecules from oxidation and carcinogens (179). Recent reports suggest that lycopene can suppress the proliferation of prostate cancer cells through the activation of peroxisome proliferator-activated receptor γ (PPARγ), liver X receptor α (LXRα), and ATP-binding cassette transporter ABCA1 (180). Additionally, lycopene could alleviate the prostate cancer risk by modulating the growth genes like cyclin-dependent protein kinase 7 (CDK7), B-cell lymphoma 2 (BCL2), epidermal growth factor receptor (EGFR), and insulin-like growth factor 1 (IGF-1) receptor (181).
In the present meta-analysis, we identified significant publication bias regarding the associations between blood levels of lycopene and the risk of overall cancer as well as prostate cancer. However, when we applied the trim-and-fill method, this publication bias was mitigated. This technique involved estimating and incorporating findings from potentially missing studies into the meta-analysis. By doing so, we created a hypothetical symmetry and assessed the overall effect size under conditions free from publication bias. Ultimately, this approach demonstrated that our results were not influenced by publication bias.
Strengths and weaknesses of this study
The present meta-analysis has some strengths. First, including prospective studies with a large number of participants and cancer cases allowed us to quantitatively investigate the association between tomato/lycopene intake and blood levels of lycopene with cancer risk and mortality. Second, linear and non-linear dose–response analyses were performed to reach compelling evidence for the quantitative evaluation of relationships. Third, due to the prospective design of included studies, the effect of selection and recall bias is negligible.
However, our findings should be interpreted by considering some limitations. Although the included studies had controlled their analyses for potential confounders, the role of residual or unmeasured confounders, like dietary intakes of other food groups or nutrients, cannot be ruled out. Additionally, in some included studies, the confounding effects of important variables such as energy intake and BMI were not taken into account. Moreover, a number of studies in this review did not have sufficient data to be included in the dose–response meta-analyses. Also, because of the limited number of studies, we were not able to assess the relationship between exposures to other types of cancers like endometrial, hepatocellular, renal, head and neck, and skin cancers. Different approaches that were used for the assessment of exposures and outcomes among included studies are other limitations of this meta-analysis. However, subgroup analysis was performed to control for these differences. Lastly, most studies evaluated tomato and lycopene intakes based on a single measurement at the baseline of the study, and dietary changes during the follow-up were not considered.
Conclusion
In conclusion, lycopene (both dietary intake and blood levels) was inversely linked with overall cancer risk. There was also evidence of a linear relationship in the case of lycopene levels so that each 10 μg/dL increase in blood levels of lycopene was associated with a 5% lower risk of overall cancer. In terms of specific cancers, we found a linear inverse association between lycopene consumption and prostate cancer risk and a significant inverse association between blood lycopene levels and the risk of breast and prostate cancers. Regarding cancer mortality, total tomato/lycopene intakes and blood levels of lycopene were associated with a lower risk of cancer mortality. Also, the association between dietary lycopene and cancer mortality was non-linear, so the highest risk reduction was observed in the dosages between 5 and 8 mg/day. As most of the included studies were conducted in Western countries, the generalizability of findings to the worldwide population should be done with caution. Thus, further studies are warranted to affirm our findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AB: Formal analysis, Methodology, Writing – original draft. KF: Data curation, Writing – original draft. GA: Conceptualization, Writing – review & editing. OS: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Isfahan University of Medical Sciences, Isfahan, Iran (ID: 2402307) and Omid Sadeghi was funded by a grant from the Isfahan University of Medical Sciences, Isfahan, Iran (code: 1402100).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1516048/full#supplementary-material
Abbreviations
RR, Risk ratio; OR, Odds ratio; HR, Hazard ratio; CI, Confidence interval; HPFS, Health professional follow-up study; NHS, Nurses’ health study; CHEP, Comprehensive health examination program; JACC, Japan collaborative cohort study; PLCO, Prostate, lung, colorectal, and ovarian trial; EPIC, European prospective investigation into cancer and nutrition; PRISMA, Preferred reporting items for systematic reviews and meta-analyses; NOS, Newcastle Ottawa scale; BMI, Body mass index; FFQ, Food frequency questionnaire.
References
1. Islami, F, Goding Sauer, A, Miller, KD, Siegel, RL, Fedewa, SA, Jacobs, EJ, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. (2018) 68:31–54. doi: 10.3322/caac.21440
2. Parkin, DM, Boyd, L, and Walker, LC. 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. (2011) 105:S77–81. doi: 10.1038/bjc.2011.489
3. Zhang, FF, Cudhea, F, Shan, Z, Michaud, DS, Imamura, F, Eom, H, et al. Preventable cancer burden associated with poor diet in the United States. JNCI Cancer. Spectrum. (2019) 3:pkz 034. doi: 10.1093/jncics/pkz034
4. Zhao, Z, Yin, Z, and Zhao, Q. Red and processed meat consumption and gastric cancer risk: a systematic review and meta-analysis. Oncotarget. (2017) 8:30563–75. doi: 10.18632/oncotarget.15699
5. Fiolet, T, Srour, B, Sellem, L, Kesse-Guyot, E, Allès, B, Méjean, C, et al. Consumption of ultra-processed foods and cancer risk: results from NutriNet-Santé prospective cohort. BMJ. (2018) 360:k322. doi: 10.1136/bmj.k322
6. Ambrosini, GL, de Klerk, NH, Fritschi, L, Mackerras, D, and Musk, B. Fruit, vegetable, vitamin a intakes, and prostate cancer risk. Prostate Cancer Prostatic Dis. (2008) 11:61–6. doi: 10.1038/sj.pcan.4500979
7. Cui, Y, Shikany, JM, Liu, S, Shagufta, Y, and Rohan, TE. Selected antioxidants and risk of hormone receptor-defined invasive breast cancers among postmenopausal women in the Women's Health Initiative observational study. Am J Clin Nutr. (2008) 87:1009–18. doi: 10.1093/ajcn/87.4.1009
8. Cui, X, Rosner, B, Willett, WC, and Hankinson, SE. Antioxidant intake and risk of endometrial cancer: results from the Nurses' health study. Int J Cancer. (2011) 128:1169–78. doi: 10.1002/ijc.25425
9. Fairfield, KM, Hankinson, SE, Rosner, BA, Hunter, DJ, Colditz, GA, and Willett, WC. Risk of ovarian carcinoma and consumption of vitamins a, C, and E and specific carotenoids: a prospective analysis. Cancer. (2001) 92:2318–26. doi: 10.1002/1097-0142(20011101)92:9<2318::AID-CNCR1578>3.0.CO;2-7
10. Fraser, GE, Jacobsen, BK, Knutsen, SF, Mashchak, A, and Lloren, JI. Tomato consumption and intake of lycopene as predictors of the incidence of prostate cancer: the Adventist health Study-2. CCC. (2020) 31:341–51. doi: 10.1007/s10552-020-01279-z
11. Giovannucci, E, Ascherio, A, Rimm, EB, Stampfer, MJ, Colditz, GA, and Willett, WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst. (1995) 87:1767–76. doi: 10.1093/jnci/87.23.1767
12. Han, X, Li, J, Brasky, TM, Xun, P, Stevens, J, White, E, et al. Antioxidant intake and pancreatic cancer risk: the vitamins and lifestyle (VITAL) study. Cancer. (2013) 119:1314–20. doi: 10.1002/cncr.27936
13. Ho, WJ, Simon, MS, Yildiz, VO, Shikany, JM, Kato, I, Beebe-Dimmer, JL, et al. Antioxidant micronutrients and the risk of renal cell carcinoma in the Women's Health Initiative cohort. Cancer. (2015) 121:580–8. doi: 10.1002/cncr.29091
14. Holick, CN, Michaud, DS, Stolzenberg-Solomon, R, Mayne, ST, Pietinen, P, Taylor, PR, et al. Dietary carotenoids, serum beta-carotene, and retinol and risk of lung cancer in the alpha-tocopherol, beta-carotene cohort study. Am J Epidemiol. (2002) 156:536–47. doi: 10.1093/aje/kwf072
15. Holick, CN, De Vivo, I, Feskanich, D, Giovannucci, E, Stampfer, M, and Michaud, DS. Intake of fruits and vegetables, carotenoids, folate, and vitamins a, C, E and risk of bladder cancer among women (United States). Cancer Causes Control. (2005) 16:1135–45. doi: 10.1007/s10552-005-0337-z
16. Kim, J, Park, MK, Li, WQ, Qureshi, AA, and Cho, E. Association of vitamin A intake with cutaneous squamous cell carcinoma risk in the United States. JAMA Dermatol. (2019) 155:1260–8. doi: 10.1001/jamadermatol.2019.1937
17. Kirsh, VA, Mayne, ST, Peters, U, Chatterjee, N, Leitzmann, MF, Dixon, LB, et al. A prospective study of lycopene and tomato product intake and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. (2006) 15:92–8. doi: 10.1158/1055-9965.EPI-05-0563
18. Larsson, SC, Bergkvist, L, Näslund, I, Rutegård, J, and Wolk, A. Vitamin a, retinol, and carotenoids and the risk of gastric cancer: a prospective cohort study. Am J Clin Nutr. (2007) 85:497–503. doi: 10.1093/ajcn/85.2.497
19. Larsson, SC, Bergkvist, L, and Wolk, A. Dietary carotenoids and risk of hormone receptor-defined breast cancer in a prospective cohort of Swedish women. Eur J Cancer. (2010) 46:1079–85. doi: 10.1016/j.ejca.2010.01.004
20. Malila, N, Virtamo, J, Virtanen, M, Pietinen, P, Albanes, D, and Teppo, L. Dietary and serum alpha-tocopherol, beta-carotene and retinol, and risk for colorectal cancer in male smokers. Eur J Clin Nutr. (2002) 56:615–21. doi: 10.1038/sj.ejcn.1601366
21. Michaud, DS, Spiegelman, D, Clinton, SK, Rimm, EB, Willett, WC, and Giovannucci, EL. Fruit and vegetable intake and incidence of bladder cancer in a male prospective cohort. J Natl Cancer Inst. (1999) 91:605–13. doi: 10.1093/jnci/91.7.605
22. Michaud, DS, Feskanich, D, Rimm, EB, Colditz, GA, Speizer, FE, Willett, WC, et al. Intake of specific carotenoids and risk of lung cancer in 2 prospective US cohorts. Am J Clin Nutr. (2000) 72:990–7. doi: 10.1093/ajcn/72.4.990
23. Michaud, DS, Pietinen, P, Taylor, PR, Virtanen, M, Virtamo, J, and Albanes, D. Intakes of fruits and vegetables, carotenoids and vitamins a, E, C in relation to the risk of bladder cancer in the ATBC cohort study. Br J Cancer. (2002) 87:960–5. doi: 10.1038/sj.bjc.6600604
24. Navarro Silvera, SA, Jain, M, Howe, GR, Miller, AB, and Rohan, TE. Carotenoid, vitamin a, vitamin C, and vitamin E intake and risk of ovarian cancer: a prospective cohort study. Cancer Epidemiol. Biomarkers Prev. (2006) 15:395–7. doi: 10.1158/1055-9965.EPI-05-0835
25. Neuhouser, ML, Patterson, RE, Thornquist, MD, Omenn, GS, King, IB, and Goodman, GE. Fruits and vegetables are associated with lower lung cancer risk only in the placebo arm of the β-carotene and retinol efficacy trial (CARET). Cancer Epidemiol Biomarkers Prev. (2003) 12:350–8.
26. Nouraie, M, Pietinen, P, Kamangar, F, Dawsey, SM, Abnet, CC, Albanes, D, et al. Fruits, vegetables, and antioxidants and risk of gastric cancer among male smokers. Cancer Epidemiol Biomarkers Prev. (2005) 14:2087–92. doi: 10.1158/1055-9965.EPI-05-0038
27. Pantavos, A, Ruiter, R, Feskens, EF, De Keyser, CE, Hofman, A, Stricker, BH, et al. Total dietary antioxidant capacity, individual antioxidant intake and breast cancer risk: the Rotterdam study. Int J Cancer. (2015) 136:2178–86. doi: 10.1002/ijc.29249
28. Park, SY, Nomura, AM, Murphy, SP, Wilkens, LR, Henderson, BE, and Kolonel, LN. Carotenoid intake and colorectal cancer risk: the multiethnic cohort study. J Epidemiol. (2009) 19:63–71. doi: 10.2188/jea.JE20080078
29. Park, SY, Haiman, CA, Cheng, I, Park, SL, Wilkens, LR, Kolonel, LN, et al. Racial/ethnic differences in lifestyle-related factors and prostate cancer risk: the multiethnic cohort study. CCC. (2015) 26:1507–15. doi: 10.1007/s10552-015-0644-y
30. Schuurman, AG, Goldbohm, RA, Brants, HA, and van den Brandt, PA. A prospective cohort study on intake of retinol, vitamins C and E, and carotenoids and prostate cancer risk (Netherlands). CCC. (2002) 13:573–82. doi: 10.1023/A:1016332208339
31. Stram, DO, Hankin, JH, Wilkens, LR, Park, S, Henderson, BE, Nomura, AM, et al. Prostate cancer incidence and intake of fruits, vegetables and related micronutrients: the multiethnic cohort study* (United States). CCC. (2006) 17:1193–207. doi: 10.1007/s10552-006-0064-0
32. Thomson, CA, Neuhouser, ML, Shikany, JM, Caan, BJ, Monk, BJ, Mossavar-Rahmani, Y, et al. The role of antioxidants and vitamin a in ovarian cancer: results from the women's health initiative. Nutr Cancer. (2008) 60:710–9. doi: 10.1080/01635580802233983
33. Xu, X, Xie, B, Li, S, Wang, S, Xia, D, and Meng, H. Association of dietary tomato intake with bladder cancer risk in a prospective cohort of 101, 683 individuals with 12.5 years of follow-up. Aging. (2021) 13:17629–37. doi: 10.18632/aging.203252
34. Xu, X, Li, S, and Zhu, Y. Dietary intake of tomato and lycopene and risk of all-cause and cause-specific mortality: results from a prospective study. Front Nutr. (2021) 8:684859. doi: 10.3389/fnut.2021.684859
35. Yuan, JM, Stram, DO, Arakawa, K, Lee, HP, and Yu, MC. Dietary cryptoxanthin and reduced risk of lung cancer: the Singapore Chinese health study. Cancer Epidemiol Biomarkers Prev. (2003) 12:890–8.
36. Zhang, S, Hunter, DJ, Forman, MR, Rosner, BA, Speizer, FE, Colditz, GA, et al. Dietary carotenoids and vitamins a, C, and E and risk of breast cancer. J Natl Cancer Inst. (1999) 91:547–56. doi: 10.1093/jnci/91.6.547
37. Zu, K, Mucci, L, Rosner, BA, Clinton, SK, Loda, M, Stampfer, MJ, et al. Dietary lycopene, angiogenesis, and prostate cancer: a prospective study in the prostate-specific antigen era. J Natl Cancer Inst. (2014) 106:djt430. doi: 10.1093/jnci/djt430
38. Mazidi, M, Ferns, GA, and Banach, M. A high consumption of tomato and lycopene is associated with a lower risk of cancer mortality: results from a multi-ethnic cohort. Public Health Nutr. (2020) 23:1569–75. doi: 10.1017/S1368980019003227
39. Wang, Y, Jacobs, EJ, Newton, CC, and McCullough, ML. Lycopene, tomato products and prostate cancer-specific mortality among men diagnosed with nonmetastatic prostate cancer in the Cancer prevention study II nutrition cohort. Int J Cancer. (2016) 138:2846–55. doi: 10.1002/ijc.30027
40. Diallo, A, Deschasaux, M, Galan, P, Hercberg, S, Zelek, L, Latino-Martel, P, et al. Associations between fruit, vegetable and legume intakes and prostate cancer risk: results from the prospective Supplémentation en Vitamines et Minéraux Antioxydants (SU.VI.MAX) cohort. Br J Nutr. (2016) 115:1579–85. doi: 10.1017/S0007114516000520
41. Dunneram, Y, Greenwood, DC, and Cade, JE. Diet and risk of breast, endometrial and ovarian cancer: UK Women's cohort study. Br J Nutr. (2019) 122:564–74. doi: 10.1017/S0007114518003665
42. Kiani, F, Knutsen, S, Singh, P, Ursin, G, and Fraser, G. Dietary risk factors for ovarian cancer: the Adventist health study (United States). CCC. (2006) 17:137–46. doi: 10.1007/s10552-005-5383-z
43. Lin, J, Zhang, SM, Wu, K, Willett, WC, Fuchs, CS, and Giovannucci, E. Flavonoid intake and colorectal cancer risk in men and women. Am J Epidemiol. (2006) 164:644–51. doi: 10.1093/aje/kwj296
44. Masala, G, Assedi, M, Bendinelli, B, Ermini, I, Sieri, S, Grioni, S, et al. Fruit and vegetables consumption and breast cancer risk: the EPIC Italy study. Breast Cancer Res Treat. (2012) 132:1127–36. doi: 10.1007/s10549-011-1939-7
45. Mills, PK, Beeson, WL, Phillips, RL, and Fraser, GE. Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer. (1989) 64:598–604. doi: 10.1002/1097-0142(19890801)64:3<598::AID-CNCR2820640306>3.0.CO;2-6
46. Nöthlings, U, Wilkens, LR, Murphy, SP, Hankin, JH, Henderson, BE, and Kolonel, LN. Vegetable intake and pancreatic cancer risk: the multiethnic cohort study. Am J Epidemiol. (2007) 165:138–47. doi: 10.1093/aje/kwj366
47. Perez-Cornago, A, Travis, RC, Appleby, PN, Tsilidis, KK, Tjønneland, A, Olsen, A, et al. Fruit and vegetable intake and prostate cancer risk in the European prospective investigation into Cancer and nutrition (EPIC). Int J Cancer. (2017) 141:287–97. doi: 10.1002/ijc.30741
48. Suzuki, R, Iwasaki, M, Hara, A, Inoue, M, Sasazuki, S, Sawada, N, et al. Fruit and vegetable intake and breast cancer risk defined by estrogen and progesterone receptor status: the Japan public health center-based prospective study. CCC. (2013) 24:2117–28. doi: 10.1007/s10552-013-0289-7
49. Takachi, R, Inoue, M, Sawada, N, Iwasaki, M, Sasazuki, S, Ishihara, J, et al. Fruits and vegetables in relation to prostate cancer in Japanese men: the Japan public health center-based prospective study. Nutr Cancer. (2010) 62:30–9. doi: 10.1080/01635580903191502
50. Colditz, GA, Branch, LG, Lipnick, RJ, Willett, WC, Rosner, B, Posner, BM, et al. Increased green and yellow vegetable intake and lowered cancer deaths in an elderly population. Am J Clin Nutr. (1985) 41:32–6. doi: 10.1093/ajcn/41.1.32
51. Sakauchi, F, Mori, M, Washio, M, Watanabe, Y, Ozasa, K, Hayashi, K, et al. Dietary habits and risk of urothelial cancer death in a large-scale cohort study (JACC study) in Japan. Nutr Cancer. (2004) 50:33–9. doi: 10.1207/s15327914nc5001_5
52. Takata, Y, Xiang, YB, Yang, G, Honglan, L, Jing, G, Cai, H, et al. Intakes of fruits, vegetables, and related vitamins and lung cancer risk: results from the Shanghai men's health study (2002-2009). Nutr Cancer. (2013) 65:51–61. doi: 10.1080/01635581.2013.741757
53. Kristal, AR, Arnold, KB, Neuhouser, ML, Goodman, P, Platz, EA, Albanes, D, et al. Diet, supplement use, and prostate cancer risk: results from the prostate cancer prevention trial. Am J Epidemiol. (2010) 172:566–77. doi: 10.1093/aje/kwq148
54. Agalliu, I, Kirsh, VA, Kreiger, N, Soskolne, CL, and Rohan, TE. Oxidative balance score and risk of prostate cancer: results from a case-cohort study. Cancer Epidemiol. (2011) 35:353–61. doi: 10.1016/j.canep.2010.11.002
55. Botterweck, AA, van den Brandt, PA, and Goldbohm, RA. Vitamins, carotenoids, dietary fiber, and the risk of gastric carcinoma: results from a prospective study after 6.3 years of follow-up. Cancer. (2000) 88:737–48. doi: 10.1002/(SICI)1097-0142(20000215)88:4<737::AID-CNCR2>3.0.CO;2-H
56. de Munter, L, Maasland, DH, van den Brandt, PA, Kremer, B, and Schouten, LJ. Vitamin and carotenoid intake and risk of head-neck cancer subtypes in the Netherlands cohort study. Am J Clin Nutr. (2015) 102:420–32. doi: 10.3945/ajcn.114.106096
57. Jain, MG, Rohan, TE, Howe, GR, and Miller, AB. A cohort study of nutritional factors and endometrial cancer. Eur J Epidemiol. (2000) 16:899–905. doi: 10.1023/A:1011012621990
58. Lane, JA, Oliver, SE, Appleby, PN, Lentjes, MA, Emmett, P, Kuh, D, et al. Prostate cancer risk related to foods, food groups, macronutrients and micronutrients derived from the UK dietary cohort consortium food diaries. Eur J Clin Nutr. (2017) 71:274–83. doi: 10.1038/ejcn.2016.162
59. Rohan, TE, Jain, M, Howe, GR, and Miller, AB. A cohort study of dietary carotenoids and lung cancer risk in women (Canada). CCC. (2002) 13:231–7. doi: 10.1023/A:1015048619413
60. Steinmetz, KA, Potter, JD, and Folsom, AR. Vegetables, fruit, and lung cancer in the Iowa Women's health study. Cancer Res. (1993) 53:536–43.
61. Terry, P, Jain, M, Miller, AB, Howe, GR, and Rohan, TE. Dietary carotenoids and risk of breast cancer. Am J Clin Nutr. (2002) 76:883–8. doi: 10.1093/ajcn/76.4.883
62. Terry, P, Jain, M, Miller, AB, Howe, GR, and Rohan, TE. Dietary carotenoid intake and colorectal cancer risk. Nutr Cancer. (2002) 42:167–72. doi: 10.1207/S15327914NC422_3
63. Thomas, CE, Luu, HN, Wang, R, Adams-Haduch, J, Jin, A, Koh, WP, et al. Association between dietary tomato intake and the risk of hepatocellular carcinoma: the Singapore Chinese health study. Cancer Epidemiol Biomarkers Prev. (2020) 29:1430–5. doi: 10.1158/1055-9965.EPI-20-0051
64. Voorrips, LE, Goldbohm, RA, Brants, HA, van Poppel, GA, Sturmans, F, Hermus, RJ, et al. A prospective cohort study on antioxidant and folate intake and male lung cancer risk. Cancer Epidemiol Biomarkers Prev. (2000) 9:357–65.
65. Zeegers, MP, Goldbohm, RA, and van den Brandt, PA. Are retinol, vitamin C, vitamin E, folate and carotenoids intake associated with bladder cancer risk? Results from the Netherlands cohort study. Br J Cancer. (2001) 85:977–83. doi: 10.1054/bjoc.2001.1968
66. Sesso, HD, Buring, JE, Zhang, SM, Norkus, EP, and Gaziano, JM. Dietary and plasma lycopene and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev. (2005) 14:1074–81. doi: 10.1158/1055-9965.EPI-04-0683
67. Schuurman, AG, Goldbohm, RA, Dorant, E, and van den Brandt, PA. Vegetable and fruit consumption and prostate cancer risk: a cohort study in the Netherlands. Cancer Epidemiol Biomarkers Prev. (1998) 7:673–80.
68. Maasland, DH, van den Brandt, PA, Kremer, B, Goldbohm, RA, and Schouten, LJ. Consumption of vegetables and fruits and risk of subtypes of head–neck cancer in the Netherlands cohort study. Int J Cancer. (2015) 136:E396–409. doi: 10.1002/ijc.29219
69. Boggs, DA, Palmer, JR, Wise, LA, Spiegelman, D, Stampfer, MJ, Adams-Campbell, LL, et al. Fruit and vegetable intake in relation to risk of breast cancer in the black Women's health study. Am J Epidemiol. (2010) 172:1268–79. doi: 10.1093/aje/kwq293
70. Bakker, MF, Peeters, PH, Klaasen, VM, Bueno-de-Mesquita, HB, Jansen, EH, Ros, MM, et al. Plasma carotenoids, vitamin C, tocopherols, and retinol and the risk of breast cancer in the European prospective investigation into Cancer and nutrition cohort. Am J Clin Nutr. (2016) 103:454–64. doi: 10.3945/ajcn.114.101659
71. Batieha, AM, Armenian, HK, Norkus, EP, Morris, JS, Spate, VE, and Comstock, GW. Serum micronutrients and the subsequent risk of cervical cancer in a population-based nested case-control study. Cancer Epidemiol Biomarkers Prev. (1993) 2:335–9.
72. Beilby, J, Ambrosini, GL, Rossi, E, de Klerk, NH, and Musk, AW. Serum levels of folate, lycopene, β-carotene, retinol and vitamin E and prostate cancer risk. Eur J Clin Nutr. (2010) 64:1235–8. doi: 10.1038/ejcn.2010.124
73. Breslow, RA, Alberg, AJ, Helzlsouer, KJ, Bush, TL, Comstock, GW, Norkus, EP, et al. Serological precursors of Cancer: malignant melanoma, basal and squamous cell skin Cancer, and Prediagnostic levels of retinol, β-carotene, lycopene, α-tocopherol, and selenium. Cancer Epidemiol. Biomarkers Prev. (1995) 4:837–42.
74. Dorgan, JF, Sowell, A, Swanson, CA, Potischman, N, Miller, R, Schussler, N, et al. Relationships of serum carotenoids, retinol, alpha-tocopherol, and selenium with breast cancer risk: results from a prospective study in Columbia, Missouri (United States). CCC. (1998) 9:89–97. doi: 10.1023/A:1008857521992
75. Dorjgochoo, T, Gao, YT, Chow, WH, Shu, XO, Li, H, Yang, G, et al. Plasma carotenoids, tocopherols, retinol and breast cancer risk: results from the Shanghai women health study (SWHS). Breast Cancer Res Treat. (2009) 117:381–9. doi: 10.1007/s10549-008-0270-4
76. Eliassen, AH, Liao, X, Rosner, B, Tamimi, RM, Tworoger, SS, and Hankinson, SE. Plasma carotenoids and risk of breast cancer over 20 y of follow-up. Am J Clin Nutr. (2015) 101:1197–205. doi: 10.3945/ajcn.114.105080
77. Gann, PH, Ma, J, Giovannucci, E, Willett, W, Sacks, FM, Hennekens, CH, et al. Lower prostate cancer risk in men with elevated plasma lycopene levels: results of a prospective analysis. Cancer Res. (1999) 59:1225–30.
78. Gill, JK, Franke, AA, Steven Morris, J, Cooney, RV, Wilkens, LR, Le Marchand, L, et al. Association of selenium, tocopherols, carotenoids, retinol, and 15-isoprostane F2t in serum or urine with prostate cancer risk: the multiethnic cohort. Cancer Causes Control. (2009) 20:1161–71. doi: 10.1007/s10552-009-9304-4
79. Goodman, GE, Schaffer, S, Omenn, GS, Chen, C, and King, I. The association between lung and prostate cancer risk, and serum micronutrients: results and lessons learned from β-carotene and retinol efficacy trial. Cancer Epidemiol Biomarkers Prev. (2003) 12:518–26.
80. Helzlsouer, KJ, Comstock, GW, and Morris, JS. Selenium, lycopene, α-tocopherol, α-carotene, retinol, and subsequent bladder Cancer. Cancer Res. (1989) 49:6144–8.
81. Helzlsouer, KJ, Alberg, AJ, Norkus, EP, Morris, JS, Hoffman, SC, and Comstock, GW. Prospective study of serum micronutrients and ovarian cancer. J Natl Cancer Inst. (1996) 88:32–7. doi: 10.1093/jnci/88.1.32
82. Hsing, AW, Comstock, GW, Abbey, H, and Polk, BF. Serologic precursors of cancer. Retinol, carotenoids, and tocopherol and risk of prostate cancer. J Natl Cancer Inst. (1990) 82:941–6. doi: 10.1093/jnci/82.11.941
83. Huang, HY, Alberg, AJ, Norkus, EP, Hoffman, SC, Comstock, GW, and Helzlsouer, KJ. Prospective study of antioxidant micronutrients in the blood and the risk of developing prostate cancer. Am J Epidemiol. (2003) 157:335–44. doi: 10.1093/aje/kwf210
84. Hultén, K, Van Kappel, AL, Winkvist, A, Kaaks, R, Hallmans, G, Lenner, P, et al. Carotenoids, alpha-tocopherols, and retinol in plasma and breast cancer risk in northern Sweden. CCC. (2001) 12:529–37. doi: 10.1023/A:1011271222153
85. Jeurnink, SM, Ros, MM, Leenders, M, van Duijnhoven, FJ, Siersema, PD, Jansen, EH, et al. Plasma carotenoids, vitamin C, retinol and tocopherols levels and pancreatic cancer risk within the European prospective investigation into Cancer and nutrition: a nested case-control study: plasma micronutrients and pancreatic cancer risk. Int J Cancer. (2015) 136:E665–76. doi: 10.1002/ijc.29175
86. Key, TJ, Appleby, PN, Allen, NE, Travis, RC, Roddam, AW, Jenab, M, et al. Plasma carotenoids, retinol, and tocopherols and the risk of prostate cancer in the European prospective investigation into Cancer and nutrition study. Am J Clin Nutr. (2007) 86:672–81. doi: 10.1093/ajcn/86.3.672
87. Kristal, AR, Till, C, Platz, EA, Song, X, King, IB, Neuhouser, ML, et al. Serum lycopene concentration and prostate cancer risk: results from the prostate Cancer prevention trial. Cancer Epidemiol Biomarkers Prev. (2011) 20:638–46. doi: 10.1158/1055-9965.EPI-10-1221
88. Lee, SA, Cai, Q, Franke, AA, Steinwandel, M, Wu, J, Wen, W, et al. Associations of subtype and isomeric plasma carotenoids with prostate Cancer risk in low-income African and European Americans. Cancer Epidemiol Biomarkers Prev. (2021) 30:1846–57. doi: 10.1158/1055-9965.EPI-20-1785
89. Leenders, M, Leufkens, AM, Siersema, PD, van Duijnhoven, FJ, Vrieling, A, Hulshof, PJ, et al. Plasma and dietary carotenoids and vitamins a, C and E and risk of colon and rectal cancer in the European prospective investigation into Cancer and nutrition. Int J Cancer. (2014) 135:2930–9. doi: 10.1002/ijc.28938
90. Nomura, AM, Stemmermann, GN, Lee, J, and Craft, NE. Serum micronutrients and prostate cancer in Japanese Americans in Hawaii. Cancer Epidemiol Biomarkers Prev. (1997) 6:487–91.
91. Nomura, AM, Lee, J, Stemmermann, GN, and Franke, AA. Serum vitamins and the subsequent risk of bladder cancer. J Urol. (2003) 170:1146–50. doi: 10.1097/01.ju.0000086040.24795.ad
92. Nordström, T, Van Blarigan, EL, Ngo, V, Roy, R, Weinberg, V, Song, X, et al. Associations between circulating carotenoids, genomic instability and the risk of high-grade prostate cancer. Prostate. (2016) 76:339–48. doi: 10.1002/pros.23125
93. Peng, C, Gao, C, Lu, D, Rosner, BA, Zeleznik, O, Hankinson, SE, et al. Circulating carotenoids and breast cancer among high-risk individuals. Am J Clin Nutr. (2021) 113:525–33. doi: 10.1093/ajcn/nqaa316
94. Persson, C, Sasazuki, S, Inoue, M, Kurahashi, N, Iwasaki, M, Miura, T, et al. Plasma levels of carotenoids, retinol and tocopherol and the risk of gastric cancer in Japan: a nested case-control study. Carcinogenesis. (2008) 29:1042–8. doi: 10.1093/carcin/bgn072
95. Peters, U, Leitzmann, MF, Chatterjee, N, Wang, Y, Albanes, D, Gelmann, EP, et al. Serum lycopene, other carotenoids, and prostate cancer risk: a nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. (2007) 16:962–8. doi: 10.1158/1055-9965.EPI-06-0861
96. Ros, MM, Bueno-de-Mesquita, HB, Kampman, E, Aben, KK, Büchner, FL, Jansen, EH, et al. Plasma carotenoids and vitamin C concentrations and risk of urothelial cell carcinoma in the European prospective investigation into Cancer and nutrition. Am J Clin Nutr. (2012) 96:902–10. doi: 10.3945/ajcn.111.032920
97. Sato, R, Helzlsouer, KJ, Alberg, AJ, Hoffman, SC, Norkus, EP, and Comstock, GW. Prospective study of carotenoids, tocopherols, and retinoid concentrations and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev. (2002) 11:451–7.
98. Sisti, JS, Lindström, S, Kraft, P, Tamimi, RM, Rosner, BA, Wu, T, et al. Premenopausal plasma carotenoids, fluorescent oxidation products, and subsequent breast cancer risk in the nurses' health studies. Breast Cancer Res Treat. (2015) 151:415–25. doi: 10.1007/s10549-015-3391-6
99. Wang, Y, Gapstur, SM, Gaudet, MM, Furtado, JD, Campos, H, and McCullough, ML. Plasma carotenoids and breast cancer risk in the Cancer prevention study II nutrition cohort. Cancer Causes Control. (2015) 26:1233–44. doi: 10.1007/s10552-015-0614-4
100. Wu, K, Erdman, JW Jr, Schwartz, SJ, Platz, EA, Leitzmann, M, Clinton, SK, et al. Plasma and dietary carotenoids, and the risk of prostate cancer: a nested case-control study. Cancer Epidemiol Biomarkers Prev. (2004) 13:260–9. doi: 10.1158/1055-9965.EPI-03-0012
101. Yu, MW, Chiu, YH, Chiang, YC, Chen, CH, Lee, TH, Santella, RM, et al. Plasma carotenoids, glutathione S-transferase M1 and T1 genetic polymorphisms, and risk of hepatocellular carcinoma: independent and interactive effects. Am J Epidemiol. (1999) 149:621–9. doi: 10.1093/oxfordjournals.aje.a009862
102. Yuan, JM, Ross, RK, Gao, YT, Qu, YH, Chu, XD, and Yu, MC. Prediagnostic levels of serum micronutrients in relation to risk of gastric cancer in Shanghai, China. Cancer Epidemiol Biomarkers Prev. (2004) 13:1772–80. doi: 10.1158/1055-9965.1772.13.11
103. Yuan, JM, Gao, YT, Ong, CN, Ross, RK, and Yu, MC. Prediagnostic level of serum retinol in relation to reduced risk of hepatocellular carcinoma. J Natl Cancer Inst. (2006) 98:482–90. doi: 10.1093/jnci/djj104
104. Ito, Y, Kurata, M, Hioki, R, Suzuki, K, Ochiai, J, and Aoki, K. Cancer mortality and serum levels of carotenoids, retinol, and tocopherol: a population-based follow-up study of inhabitants of a rural area of Japan. APJCP. (2005) 6:10–5.
105. Ito, Y, Wakai, K, Suzuki, K, Ozasa, K, Watanabe, Y, Seki, N, et al. Lung cancer mortality and serum levels of carotenoids, retinol, tocopherols, and folic acid in men and women: a case-control study nested in the JACC study. J Epidemiol. (2005) 15:S140–9. doi: 10.2188/jea.15.S140
106. Toniolo, P, Van Kappel, AL, Akhmedkhanov, A, Ferrari, P, Kato, I, Shore, RE, et al. Serum carotenoids and breast cancer. Am J Epidemiol. (2001) 153:1142–7. doi: 10.1093/aje/153.12.1142
107. Dorgan, JF, Boakye, NA, Fears, TR, Schleicher, RL, Helsel, W, Anderson, C, et al. Serum carotenoids and alpha-tocopherol and risk of nonmelanoma skin cancer. Cancer Epidemiol Biomarkers Prev. (2004) 13:1276–82. doi: 10.1158/1055-9965.1276.13.8
108. Kabat, GC, Kim, M, Adams-Campbell, LL, Caan, BJ, Chlebowski, RT, Neuhouser, ML, et al. Longitudinal study of serum carotenoid, retinol, and tocopherol concentrations in relation to breast cancer risk among postmenopausal women. Am J Clin Nutr. (2009) 90:162–9. doi: 10.3945/ajcn.2009.27568
109. Kabat, GC, Kim, MY, Sarto, GE, Shikany, JM, and Rohan, TE. Repeated measurements of serum carotenoid, retinol and tocopherol levels in relation to colorectal cancer risk in the Women's Health Initiative. Eur J Clin Nutr. (2012) 66:549–54. doi: 10.1038/ejcn.2011.207
110. Karppi, J, Kurl, S, Nurmi, T, Rissanen, TH, Pukkala, E, and Nyyssönen, K. Serum lycopene and the risk of cancer: the Kuopio Ischaemic heart disease risk factor (KIHD) study. Ann Epidemiol. (2009) 19:512–8. doi: 10.1016/j.annepidem.2009.03.017
111. Cooney, RV, Chai, W, Franke, AA, Wilkens, LR, Kolonel, LN, and Le Marchand, L. C-reactive protein, lipid-soluble micronutrients, and survival in colorectal cancer patients. Cancer Epidemiol. Biomarkers Prev. (2013) 22:1278–88. doi: 10.1158/1055-9965.EPI-13-0199
112. Fujii, R, Tsuboi, Y, Maeda, K, Ishihara, Y, and Suzuki, K. Analysis of repeated measurements of serum carotenoid levels and all-cause and cause-specific mortality in Japan. JAMA Netw Open. (2021) 4:e2113369. doi: 10.1001/jamanetworkopen.2021.13369
113. Ito, Y, Suzuki, K, Suzuki, S, Sasaki, R, Otani, M, and Aoki, K. Serum antioxidants and subsequent mortality rates of all causes or cancer among rural Japanese inhabitants. Int J Vitamin Nutr Res. (2002) 72:237–50. doi: 10.1024/0300-9831.72.4.237
114. Mayne, ST, Cartmel, B, Lin, H, Zheng, T, and Goodwin, WJ Jr. Low plasma lycopene concentration is associated with increased mortality in a cohort of patients with prior oral, pharynx or larynx cancers. J Am Coll Nutr. (2004) 23:34–42. doi: 10.1080/07315724.2004.10719340
115. Min, KB, and Min, JY. Serum carotenoid levels and risk of lung cancer death in US adults. Cancer Sci. (2014) 105:736–43. doi: 10.1111/cas.12405
116. Shardell, MD, Alley, DE, Hicks, GE, El-Kamary, SS, Miller, RR, Semba, RD, et al. Low-serum carotenoid concentrations and carotenoid interactions predict mortality in US adults: the third National Health and nutrition examination survey. Nutr Res. (2011) 31:178–89. doi: 10.1016/j.nutres.2011.03.003
117. Pouchieu, C, Galan, P, Ducros, V, Latino-Martel, P, Hercberg, S, and Touvier, M. Plasma carotenoids and retinol and overall and breast cancer risk: a nested case-control study. Nutr Cancer. (2014) 66:980–8. doi: 10.1080/01635581.2014.936952
118. Epplein, M, Shvetsov, YB, Wilkens, LR, Franke, AA, Cooney, RV, Le Marchand, L, et al. Plasma carotenoids, retinol, and tocopherols and postmenopausal breast cancer risk in the multiethnic cohort study: a nested case-control study. Breast Cancer Res. (2009) 11:R49. doi: 10.1186/bcr2338
119. Jenab, M, Riboli, E, Ferrari, P, Friesen, M, Sabate, J, Norat, T, et al. Plasma and dietary carotenoid, retinol and tocopherol levels and the risk of gastric adenocarcinomas in the European prospective investigation into cancer and nutrition. Br J Cancer. (2006) 95:406–15. doi: 10.1038/sj.bjc.6603266
120. Steevens, J, Schouten, LJ, Goldbohm, RA, and van den Brandt, PA. Vegetables and fruits consumption and risk of esophageal and gastric cancer subtypes in the Netherlands cohort study. Int J Cancer. (2011) 129:2681–93. doi: 10.1002/ijc.25928
121. Koushik, A, Hunter, DJ, Spiegelman, D, Anderson, KE, Buring, JE, Freudenheim, JL, et al. Intake of the major carotenoids and the risk of epithelial ovarian cancer in a pooled analysis of 10 cohort studies. Int J Cancer. (2006) 119:2148–54. doi: 10.1002/ijc.22076
122. Cho, E, Spiegelman, D, Hunter, DJ, Chen, WY, Zhang, SM, Colditz, GA, et al. Premenopausal intakes of vitamins a, C, and E, folate, and carotenoids, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. (2003) 12:713–20.
123. Stolzenberg-Solomon, RZ, Pietinen, P, Taylor, PR, Virtamo, J, and Albanes, D. Prospective study of diet and pancreatic cancer in male smokers. Am J Epidemiol. (2002) 155:783–92. doi: 10.1093/aje/155.9.783
124. Farvid, MS, Chen, WY, Rosner, BA, Tamimi, RM, Willett, WC, and Eliassen, AH. Fruit and vegetable consumption and breast cancer incidence: repeated measures over 30 years of follow-up. Int J Cancer. (2019) 144:1496–510. doi: 10.1002/ijc.31653
125. Heinen, MM, Verhage, BA, Goldbohm, RA, and van den Brandt, PA. Intake of vegetables, fruits, carotenoids and vitamins C and E and pancreatic cancer risk in the Netherlands cohort study. Int J Cancer. (2012) 130:147–58. doi: 10.1002/ijc.25989
126. Horn-Ross, PL, Hoggatt, K, West, DW, Krone, MR, Stewart, SL, Anton-Culver, H, et al. Recent diet and breast cancer risk: the California teachers study (USA). Cancer Causes Control. (2002) 13:407–15. doi: 10.1023/A:1015786030864
127. Zheng, W, Blot, WJ, Diamond, EL, Norkus, EP, Spate, V, Morris, JS, et al. Serum micronutrients and the subsequent risk of oral and pharyngeal cancer. Cancer Res. (1993) 53:795–8.
128. Giovannucci, E, Rimm, EB, Liu, Y, Stampfer, MJ, and Willett, WC. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst. (2002) 94:391–8. doi: 10.1093/jnci/94.5.391
129. Graff, RE, Pettersson, A, Lis, RT, Ahearn, TU, Markt, SC, Wilson, KM, et al. Dietary lycopene intake and risk of prostate cancer defined by ERG protein expression. Am J Clin Nutr. (2016) 103:851–60. doi: 10.3945/ajcn.115.118703
130. Tamimi, RM, Hankinson, SE, Campos, H, Spiegelman, D, Zhang, S, Colditz, GA, et al. Plasma carotenoids, retinol, and tocopherols and risk of breast cancer. Am J Epidemiol. (2005) 161:153–60. doi: 10.1093/aje/kwi030
131. Tamimi, RM, Colditz, GA, and Hankinson, SE. Circulating carotenoids, mammographic density, and subsequent risk of breast cancer. Cancer Res. (2009) 69:9323–9. doi: 10.1158/0008-5472.CAN-09-1018
132. Peng, C, Zeleznik, OA, Shutta, KH, Rosner, BA, Kraft, P, Clish, CB, et al. A metabolomics analysis of circulating carotenoids and breast Cancer risk. Cancer Epidemiol Biomarkers Prev. (2022) 31:85–96. doi: 10.1158/1055-9965.EPI-21-0837
133. Ito, Y, Suzuki, S, Yagyu, K, Sasaki, R, Suzuki, K, and Aoki, K. Relationship between serum carotenoid levels and cancer death rates in the residents, living in a rural area of Hokkaido, Japan. J Epidemiol. (1997) 7:1–8. doi: 10.2188/jea.7.1
134. Ito, Y, Wakai, K, Suzuki, K, Tamakoshi, A, Seki, N, Ando, M, et al. Serum carotenoids and mortality from lung cancer: a case-control study nested in the Japan collaborative cohort (JACC) study. Cancer Sci. (2003) 94:57–63. doi: 10.1111/j.1349-7006.2003.tb01352.x
135. Kirsh, VA, Peters, U, Mayne, ST, Subar, AF, Chatterjee, N, Johnson, CC, et al. Prospective study of fruit and vegetable intake and risk of prostate cancer. J Natl Cancer Inst. (2007) 99:1200–9. doi: 10.1093/jnci/djm065
136. Maillard, V, Kuriki, K, Lefebvre, B, Boutron-Ruault, MC, Lenoir, GM, Joulin, V, et al. Serum carotenoid, tocopherol and retinol concentrations and breast cancer risk in the E3N-EPIC study. Int J Cancer. (2010) 127:1188–96. doi: 10.1002/ijc.25138
137. Xu, X, Li, J, Wang, X, Wang, S, Meng, S, Zhu, Y, et al. Tomato consumption and prostate cancer risk: a systematic review and meta-analysis. Sci Rep. (2016) 6:37091. doi: 10.1038/srep37091
138. Rowles, JL 3rd, Ranard, KM, Applegate, CC, Jeon, S, An, R, and Erdman, JW Jr. Processed and raw tomato consumption and risk of prostate cancer: a systematic review and dose-response meta-analysis. Prostate Cancer Prostatic Dis. (2018) 21:319–36. doi: 10.1038/s41391-017-0005-x
139. Chen, P, Zhang, W, Wang, X, Zhao, K, Negi, DS, Zhuo, L, et al. Lycopene and risk of prostate Cancer: a systematic review and meta-analysis. Medicine. (2015) 94:e1260. doi: 10.1097/MD.0000000000001260
140. Chen, J, Song, Y, and Zhang, L. Lycopene/tomato consumption and the risk of prostate cancer: a systematic review and meta-analysis of prospective studies. J Nutr Sci Vitaminol. (2013) 59:213–23. doi: 10.3177/jnsv.59.213
141. Chen, J, Jiang, W, Shao, L, Zhong, D, Wu, Y, and Cai, J. Association between intake of antioxidants and pancreatic cancer risk: a meta-analysis. Int J Food Sci Nutr. (2016) 67:744–53. doi: 10.1080/09637486.2016.1197892
142. Yang, T, Yang, X, Wang, X, Wang, Y, and Song, Z. The role of tomato products and lycopene in the prevention of gastric cancer: a meta-analysis of epidemiologic studies. Med Hypotheses. (2013) 80:383–8. doi: 10.1016/j.mehy.2013.01.005
143. Hu, F, Wang Yi, B, Zhang, W, Liang, J, Lin, C, Li, D, et al. Carotenoids and breast cancer risk: a meta-analysis and meta-regression. Breast Cancer Res Treat. (2012) 131:239–53. doi: 10.1007/s10549-011-1723-8
144. Farvid, MS, Barnett, JB, and Spence, ND. Fruit and vegetable consumption and incident breast cancer: a systematic review and meta-analysis of prospective studies. Br J Cancer. (2021) 125:284–98. doi: 10.1038/s41416-021-01373-2
145. Li, X, and Xu, J. Meta-analysis of the association between dietary lycopene intake and ovarian cancer risk in postmenopausal women. Sci Rep. (2014) 4:4885. doi: 10.1038/srep04885
146. Page, MJ, and Moher, D. Evaluations of the uptake and impact of the preferred reporting items for systematic reviews and Meta-analyses (PRISMA) statement and extensions: a scoping review. Syst Rev. (2017) 6:1–14. doi: 10.1186/s13643-017-0663-8
147. Stang, A . Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
148. Danesh, J, Collins, R, Appleby, P, and Peto, R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. (1998) 279:1477–82. doi: 10.1001/jama.279.18.1477
149. Der Simonian, R, and Laird, N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. (2015) 45:139–45. doi: 10.1016/j.cct.2015.09.002
150. Borenstein, M, Hedges, LV, Higgins, JP, and Rothstein, HR. Fixed-effect versus random-effects models. Intr Meta-Anal. (2009) 77:85. doi: 10.1002/9780470743386.ch13
151. Greenland, S, and Longnecker, MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237
152. Orsini, N, Bellocco, R, and Greenland, S. Generalized least squares for trend estimation of summarized dose–response data. Stata J. (2006) 6:40–57. doi: 10.1177/1536867X0600600103
153. Boeke, CE, Tamimi, RM, Berkey, CS, Colditz, GA, Eliassen, AH, Malspeis, S, et al. Adolescent carotenoid intake and benign breast disease. Pediatrics. (2014) 133:e1292–8. doi: 10.1542/peds.2013-3844
154. Chan, JM, Holick, CN, Leitzmann, MF, Rimm, EB, Willett, WC, Stampfer, MJ, et al. Diet after diagnosis and the risk of prostate cancer progression, recurrence, and death (United States). CCC. (2006) 17:199–208. doi: 10.1007/s10552-005-0413-4
155. Cohen, K, Liu, Y, Luo, J, Appleton, CM, and Colditz, GA. Plasma carotenoids and the risk of premalignant breast disease in women aged 50 and younger: a nested case–control study. Breast Cancer Res Treat. (2017) 162:571–80. doi: 10.1007/s10549-017-4152-5
156. Ford, ES, Li, C, Cunningham, TJ, and Croft, JB. Associations between antioxidants and all-cause mortality among US adults with obstructive lung function. Br J Nutr. (2014) 112:1662–73. doi: 10.1017/S0007114514002669
157. Fujii, T, Takatsuka, N, Nagata, C, Matsumoto, K, Oki, A, Furuta, R, et al. Association between carotenoids and outcome of cervical intraepithelial neoplasia: a prospective cohort study. Int J Clin Oncol. (2013) 18:1091–101. doi: 10.1007/s10147-012-0486-5
158. Kristal, AR, Arnold, KB, Schenk, JM, Neuhouser, ML, Goodman, P, Penson, DF, et al. Dietary patterns, supplement use, and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am J Epidemiol. (2008) 167:925–34. doi: 10.1093/aje/kwm389
159. Richman, EL, Carroll, PR, and Chan, JM. Vegetable and fruit intake after diagnosis and risk of prostate cancer progression. Int J Cancer. (2012) 131:201–10. doi: 10.1002/ijc.26348
160. Jung, S, Wu, K, Giovannucci, E, Spiegelman, D, Willett, WC, and Smith-Warner, SA. Carotenoid intake and risk of colorectal adenomas in a cohort of male health professionals. CCC. (2013) 24:705–17. doi: 10.1007/s10552-013-0151-y
161. Terry, KL, Missmer, SA, Hankinson, SE, Willett, WC, and De Vivo, I. Lycopene and other carotenoid intake in relation to risk of uterine leiomyomata. Am J Obstet Gynecol. (2008) 198:37.e1–8. doi: 10.1016/j.ajog.2007.05.033
162. Sakhi, AK, Bøhn, SK, Smeland, S, Thoresen, M, Smedshaug, GB, Tausjø, J, et al. Postradiotherapy plasma lutein, alpha-carotene, and beta-carotene are positively associated with survival in patients with head and neck squamous cell carcinoma. Nutr Cancer. (2010) 62:322–8. doi: 10.1080/01635580903441188
163. Van Blarigan, EL, Ma, J, Kenfield, SA, Stampfer, MJ, Sesso, HD, Giovannucci, EL, et al. Plasma antioxidants, genetic variation in SOD2, CAT, GPX1, GPX4, and prostate cancer survival. Cancer Epidemiol Biomarkers Prev. (2014) 23:1037–46. doi: 10.1158/1055-9965.EPI-13-0670
164. Greenlee, H, Kwan, ML, Kushi, LH, Song, J, Castillo, A, Weltzien, E, et al. Antioxidant supplement use after breast cancer diagnosis and mortality in the life after Cancer epidemiology (LACE) cohort. Cancer. (2012) 118:2048–58. doi: 10.1002/cncr.26526
165. Satia, JA, Littman, A, Slatore, CG, Galanko, JA, and White, E. Long-term use of β-carotene, retinol, lycopene, and lutein supplements and lung cancer risk: results from the vitamins and lifestyle (vital) study. Am J Epidemiol. (2009) 169:815–28. doi: 10.1093/aje/kwn409
166. Chen, F, Du, M, Blumberg, JB, Chui, KKH, Ruan, M, Rogers, G, et al. Association among dietary supplement use, nutrient intake, and mortality among U.S. Adults Annal Int Med. (2019) 170:604–13. doi: 10.7326/M18-2478
167. Lan, T, Park, Y, Colditz, GA, Liu, J, Wang, M, Wu, K, et al. Adolescent plant product intake in relation to later prostate Cancer risk and mortality in the NIH-AARP diet and health study. J Nutr. (2021) 151:3223–31. doi: 10.1093/jn/nxab241
168. Giovannucci, E, Liu, Y, Platz, EA, Stampfer, MJ, and Willett, WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. (2007) 121:1571–8. doi: 10.1002/ijc.22788
169. Nomura, AM, Ziegler, RG, Stemmermann, GN, Chyou, PH, and Craft, NE. Serum micronutrients and upper aerodigestive tract cancer. Cancer Epidemiol Biomarkers Prev. (1997) 6:407–12.
170. Er, V, Lane, JA, Martin, RM, Emmett, P, Gilbert, R, Avery, KN, et al. Adherence to dietary and lifestyle recommendations and prostate cancer risk in the prostate testing for cancer and treatment (Protec T) trial. Cancer Epidemiol Biomarkers Prev. (2014) 23:2066–77. doi: 10.1158/1055-9965.EPI-14-0322
171. Salem, S, Salahi, M, Mohseni, M, Ahmadi, H, Mehrsai, A, Jahani, Y, et al. Major dietary factors and prostate cancer risk: a prospective multicenter case-control study. Nutr Cancer. (2011) 63:21–7. doi: 10.1080/01635581.2010.516875
172. Freudenheim, JL, Marshall, JR, Vena, JE, Laughlin, R, Brasure, JR, Swanson, MK, et al. Premenopausal breast cancer risk and intake of vegetables, fruits, and related nutrients. J Natl Cancer Inst. (1996) 88:340–8. doi: 10.1093/jnci/88.6.340
173. Key, TJ . Nutrition, hormones and prostate cancer risk: results from the European prospective investigation into cancer and nutrition. Recent Results Cancer Res. (2014) 22:39–46. doi: 10.1007/978-3-642-45195-9_4
174. Leung, EY, Crozier, JE, Talwar, D, O'Reilly, DS, McKee, RF, Horgan, PG, et al. Vitamin antioxidants, lipid peroxidation, tumour stage, the systemic inflammatory response and survival in patients with colorectal cancer. Int J Cancer. (2008) 123:2460–4. doi: 10.1002/ijc.23811
175. Luo, J, Ke, D, and He, Q. Dietary tomato consumption and the risk of prostate Cancer: a Meta-analysis. Front Nutr. (2021) 8:625185. doi: 10.3389/fnut.2021.625185
176. Wang, Y, Cui, R, Xiao, Y, Fang, J, and Xu, Q. Effect of carotene and lycopene on the risk of prostate Cancer: a systematic review and dose-response Meta-analysis of observational studies. PloS One. (2015) 10:e0137427. doi: 10.1371/journal.pone.0137427
177. Rowles, J, Ranard, K, Smith, J, An, R, and Erdman, J. Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. (2017) 20:361–77. doi: 10.1038/pcan.2017.25
178. Wang, X, Yang, HH, Liu, Y, Zhou, Q, and Chen, ZH. Lycopene consumption and risk of colorectal Cancer: a meta-analysis of observational studies. Nutr Cancer. (2016) 68:1083–96. doi: 10.1080/01635581.2016.1206579
179. Rackley, JD, Clark, PE, and Hall, MC. Complementary and alternative medicine for advanced prostate cancer. Urol Clin. (2006) 33:237–46. doi: 10.1016/j.ucl.2005.12.007
180. Yang, C-M, Lu, Y-L, Chen, H-Y, and Hu, M-L. Lycopene and the LXRα agonist T0901317 synergistically inhibit the proliferation of androgen-independent prostate cancer cells via the PPARγ-LXRα-ABCA1 pathway. J Nutr Biochem. (2012) 23:1155–62. doi: 10.1016/j.jnutbio.2011.06.009
Keywords: LYCOPENE, tomato, Cancer, mortality, meta-analysis
Citation: Balali A, Fathzadeh K, Askari G and Sadeghi O (2025) Dietary intake of tomato and lycopene, blood levels of lycopene, and risk of total and specific cancers in adults: a systematic review and dose–response meta-analysis of prospective cohort studies. Front. Nutr. 12:1516048. doi: 10.3389/fnut.2025.1516048
Edited by:
Marwan El Ghoch, University of Modena and Reggio Emilia, ItalyReviewed by:
Sankar Devarajan, University of Arkansas at Pine Bluff, United StatesJohn Erdman, University of Illinois at Urbana-Champaign, United States
Copyright © 2025 Balali, Fathzadeh, Askari and Sadeghi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Omid Sadeghi, by5zYWRlZ2hpQG51dHIubXVpLmFjLmly; b21pZHNhZGVnaGk2OUB5YWhvby5jb20=
†These authors share first authorship
 Arghavan Balali
Arghavan Balali Kimia Fathzadeh2†
Kimia Fathzadeh2† Gholamreza Askari
Gholamreza Askari Omid Sadeghi
Omid Sadeghi