
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 04 February 2025
Sec. Nutritional Epidemiology
Volume 12 - 2025 | https://doi.org/10.3389/fnut.2025.1513191
Background: The relationship between serum levels of carotenoids and endometriosis remains largely unknown. The aim of this study is to assess the association between serum levels of major carotenoids (α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin, and trans-lycopene) and the risk of endometriosis in US women.
Methods: The data were obtained from the 2001–2006 National Health and Nutrition Examination Surveys (NHANES), which included a total of 3,636 women aged 20 to 54. Serum levels of α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin, and trans-lycopene were measured using high performance liquid chromatography (HPLC) with photodiode array detection. Endometriosis was defined as self-report. Weighted multivariate logistic regression analyses were conducted to evaluate the associations of the serum levels of the major carotenoids with endometriosis risk. Additionally, restricted cubic spline (RCS) was employed to assess the possibility of nonlinear associations. Finally, subgroup analyses were utilized to estimate the influence of several covariates on the associations.
Results: Weighted multivariate logistic regression analyses showed that, after adjusting for all covariates taken into account, there was a significant association between serum lutein/zeaxanthin levels and reduced risk of endometriosis (Quartile 3 vs. Quartile 1: odds ratio [OR] = 0.62, 95% confidence interval [CI]: 0.42–0.90; Quartile 4 vs. Quartile 1: OR = 0.54, 95% CI: 0.36–0.81, P for trend = 0.001). However, no significant associations of serum levels of other carotenoids with endometriosis were found in multivariable-adjusted models that included all covariates. RCS analysis did not reveal any non-linear relationships. Subgroup analyses indicated that the inverse association between serum lutein/zeaxanthin levels and reduced endometriosis risk was significant only in individuals under 40 years of age, in both White and non-White populations, in smokers, and among those who had ever used oral contraceptives.
Conclusion: Serum lutein/zeaxanthin levels may offer protective effects against endometriosis in specific subpopulations. Further prospective research is necessary to validate these findings.
Endometriosis is a chronic inflammatory condition characterized by the implantation and growth of endometrial-like tissue outside the uterine cavity, usually in the pelvis. It’s estimated that endometriosis affects between 2 and 10% of women in the general population (1). Endometriosis most frequently presents with symptoms such as chronic pelvic pain, pain during or after sex, excessive bleeding, and infertility (1). Other associated symptoms include constipation, bloating, diarrhea, fatigue, depression, anxiety, and problems with the urinary system (2). The effects of endometriosis on physical health, emotional stability, and productivity are profound, causing a considerable strain on patients’ quality of life, as well as economic and social hardships for families and society (3). At present, no curative treatments are available. While the exact aetiology of endometriosis remains largely unknown, neurological, immunological, and hormoral factors are considered important contributors to the pathogenesis and development of endometriosis (1). In addition, evidence from clinical studies has suggested that nutritional factors including vitamin D, lipids, and gut microbiota may be implicated in the risk of developing endometriosis (4, 5).
Carotenoids are a class of bioactive red, orange, and yellow compounds produced by a variety of plants, fungi, and bacteria. More than 1,100 naturally occurring carotenoids have been identified, with around 50 being included in human diets and capable of being absorbed and processed by the body (6). Despite the variety, six carotenoids, specifically β-carotene, β-cryptoxanthin, ɑ-carotene, lycopene, lutein, and zeaxanthin, comprise more than 95% of the carotenoids present in the bloodstream (7). Carotenoids play important roles in biological function, including antioxidant, anti-inflammatory, and anti-carcinogenic activity (8). Numerous studies have suggested a strong inverse association between carotenoid levels and the risk of type 2 diabetes, stroke, coronary heart disease, asthma, and certain cancers, including lung and breast cancer (9). In addition, several studies have been undertaken to evaluate the association between carotenoids and endometriosis risk. Some studies have suggested a positive relationship. For instance, Trabert and colleagues found a suggestion of enhanced risk of endometriosis with increased β-carotene consumption (fourth quartile vs. lowest: odds ratio [OR] 1.6, 95% confidence interval [CI]: 1.0–2.5) (10). However, using data from The Nurses’ Health Study II (NHS II), Harris et al. (11) revealed that only intake of β-cryptoxanthin but not other cateroids (ɑ-carotene, β-carotene, lutein, zeaxanthin and lycopene) was associated with reduced risk of endometriosis. The findings from current studies are still inconclusive; further research is necessary, particularly studies examining the association between circulating carotenes levels and the risk of endometriosis.
Using national survey data, we evaluated the associations between serum levels of major carotenoids (ɑ-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin, and trans-lycopene) and the risk of endometriosis in a representative sample of U.S. women. We also sought to investigate whether several factors such as smoking status and oral contraceptive use would influence the associations.
We conducted a cross-sectional study using data from the National Health and Nutrition Examination Survey (NHANES) conducted by the Centers for Disease Control and Prevention for the years 2001–2002, 2003–2004 and 2005–2006. The analysis is restricted to these years as endometriosis and serum carotenoids information is available exclusively for this period. NHANES is a continuous and annual survey which evaluates a representative sample of non-institutionalised US civilians (12). Participants are selected by a complex, multistage probability design. The data collection process involved conducting interviews, distributing questionnaires, performing examinations, and analyzing biological samples in the laboratory. Comprehensive information about NHANES can be found online. The National Center for Health Statistics institutional review board approved the NHANES study protocol. Written informed consent was obtained from all NHANES participants. Our analysis did not require additional approval from the Institutional Research Board (IRB). The data used in this study is publicly accessible.1 Figure 1 shows the flow chart of study participants.
The definition of endometriosis included any self-reported diagnosis, irrespective of location, severity, or confirmation through laparoscopy. The reproductive health questionnaire assessed prior endometriosis diagnoses in women aged 20–54 by asking, “Has a doctor or other health professional ever told you that you had endometriosis?”
Following an overnight fast, venous blood from participants was taken using vacutainer tubes with red or royal blue tops, handled by a phlebotomist trained by NHANES (13). To ensure more accurate results, the serum was kept away from sunlight and other forms of full-spectrum radiation (14). Serum concentrations of ɑ-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin, and trans-lycopene were measured using high performance liquid chromatography (HPLC) with photodiode array detection (15). The data were expressed in μg/dL. Details on the laboratory procedures for serum carotenoids measurements are available in the NHANES Laboratory/Medical Technologists Procedures Manual (16).
To mitigate the impact of confounding biases, we incorporated potential confounders as covariates in our analysis (17, 18). We took into account covariates including age, race/ethnicity (non-Hispanic White, not non-Hispanic White), education (high school graduate or less, more than high school), family poverty-to-income ratio (PIR) (below poverty [PIR < 1], at or above poverty [PIR ≥ 1]), BMI (calculated as weight in kilograms divided by height in meters squared), cigarette smoking status (ascertained by serum cotinine levels; individuals were classified as a non smoker or not current smoker if cotinine levels were < 3.0 ng/mL and as a current smoker if cotinine levels were ≥ 3.0 ng/mL), age at menarche (<12 years old, ≥12 years old), and oral contraceptive use (ever, never). The confounding factors are shown in Supplementary Figure S1.
The mean and standard error are used to describe continuous variables, while categorical variables are shown as counts and percentages. Serum levels of ɑ-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin, and trans-lycopene were sorted into quartiles, using the lowest quartile as the reference category. Weighted multivariate logistic regression analyses were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the relationship between each carotenoid and endometriosis risk. We constructed three models. Model 1 was an unadjusted model that did not include any covariates. Model 2 accounted for age and BMI, whereas Model 3 adjusted for age, BMI, race, education, PIR, cigarette smoking status, age at menarche, and oral contraceptive use. To determine whether ORs for endometriosis changed with increasing quartiles or concentrations of carotenoids, we calculated the p-value for trend (assessing dose–response effects). In addition to logistic regression analyses, restricted cubic spline regression with 3 knots at the 10th, 50th and 90th percentiles was carried out to assess potential nonlinearity in the associations between serum levels of carotenoids and endometriosis. Furthermore, we performed subgroup analyses based on age (< 40 years, 40–49 years, 50–54 years), race (non-Hispanic White, not non-Hispanic White), smoking status (non-smokers, active smokers), and oral contraceptive use (ever, never) to investigate whether these factors could influence the association between serum levels of carotenoids and the risk of endometriosis.
Statistical analyses were performed using R software version 4.1.4 (R Foundation for Statistical Computing, Vienna, Austria) and the complex survey design of NHANES was taken into account. Restricted cubic spline regression was done using DataAnalyst.2 Throughout the analysis, two-sided tests were utilized, and a p-value of less than 0.05 was regarded as indicative of statistical significance.
Our study included 3,636 participants, with 263 diagnosed with endometriosis. Table 1 shows the participant characteristics based on the presence or absence of endometriosis. Participants with endometriosis were significantly older, with a mean age of 40.3 years, compared to those without endometriosis, who had a mean age of 35.2 years (p < 0.001). Additionally, significant differences were observed in race/ethnicity, smoking status, and oral contraceptive use between endometriosis patients and those without the condition (Table 1).
Table 2 shows the results for analyses on the associations between serum levels of the major carotenoids and endometriosis risk. After controlling for confounding factors (Model 3), we found that serum lutein/zeaxanthin levels were significantly associated with lower risk of endometriosis (Quartile 3 vs. Quartile 1: OR = 0.62, 95% CI: 0.42–0.90; Quartile 4 vs. Quartile 1: OR = 0.54, 95% CI: 0.36–0.81, P for trend = 0.001). We did not observe any significant associations of serum levels of ɑ-carotene, β-carotene, β-cryptoxanthin, and trans-lycopene with the risk of endometriosis (Table 2). Although serum levels of β-cryptoxanthin appeared to be statistically significantly associated with decreased endometriosis risk in Model 1 (unadjusted) and Model 2 (adjustment for age and BMI), no significant associations were identified in Model 3 when all confounders were taken into account (Table 2).
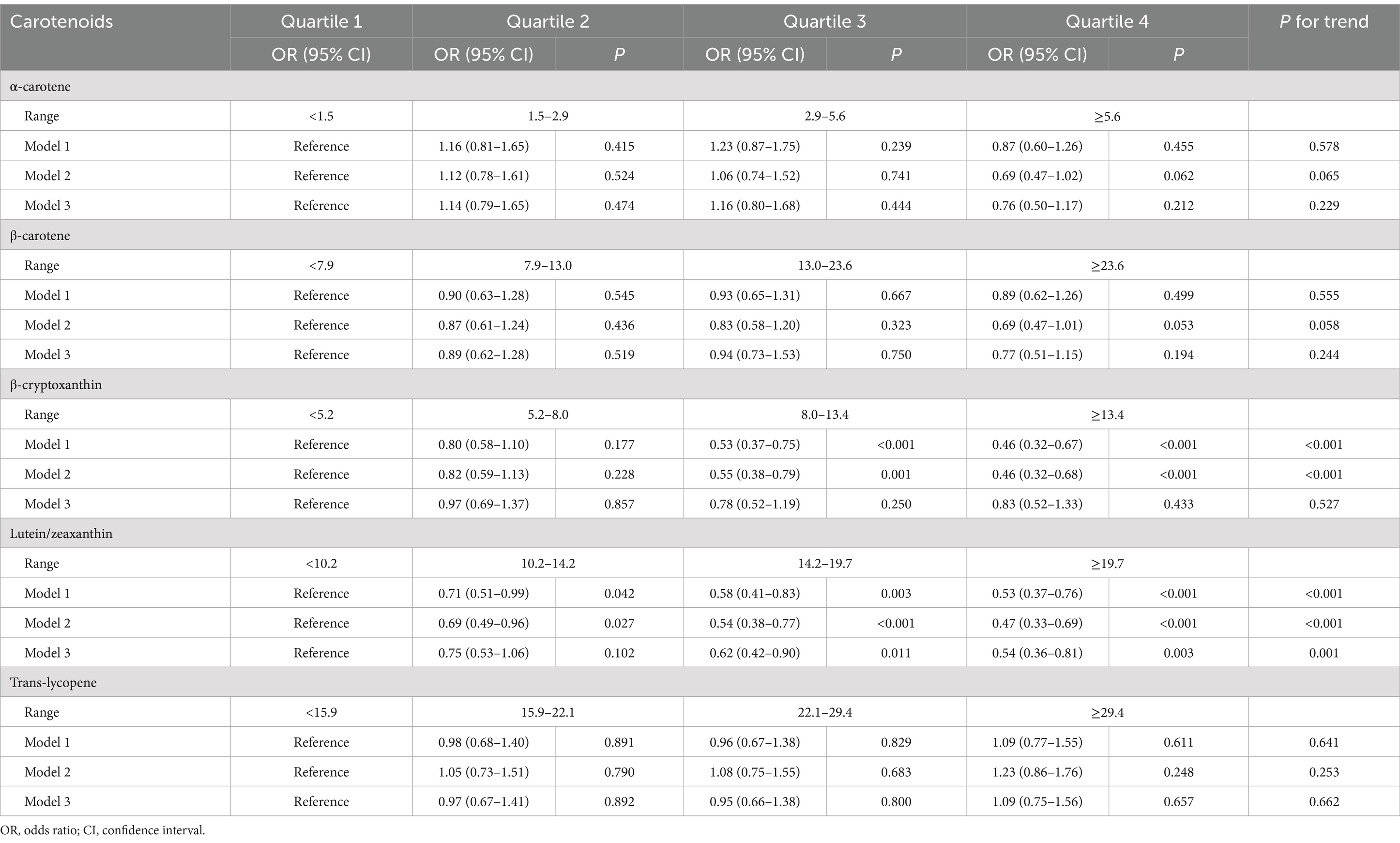
Table 2. Logistic regression analysis for association between the quartile of serum carotenoids, relative to quartile 1, and endometriosis risk.
Next, we conducted restricted cubic spline analyses to evaluate the potential nonlinear relationship between serum levels of these carotenoids and endometriosis, but no statistically significant associations were found (Figure 2).
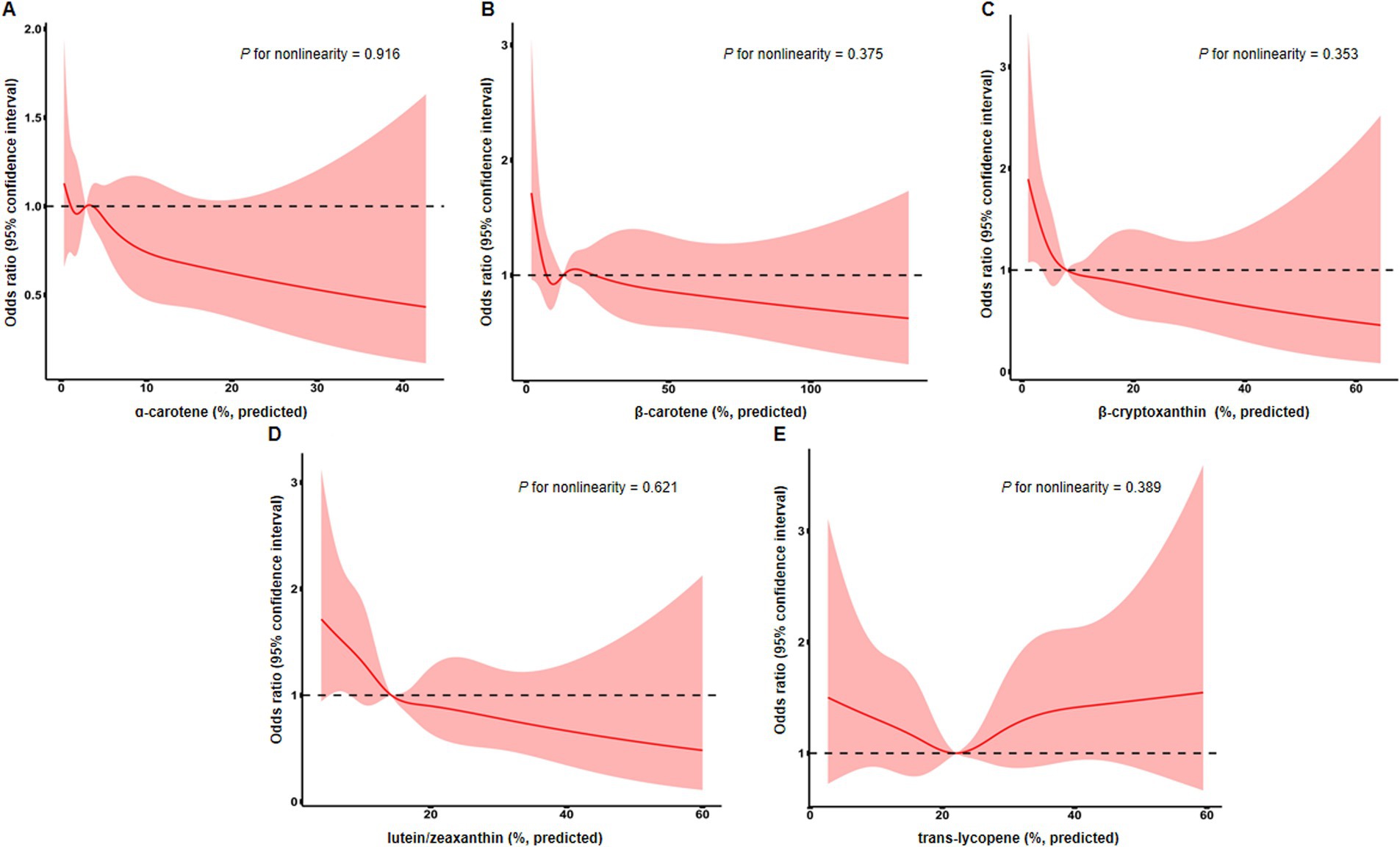
Figure 2. Restricted cubic spline analysis evaluating associations of serum levels of α-carotene (A), β-carotene (B), β-cryptoxanthin (C), lutein/zeaxanthin (D), and trans-lycopene (E) with the risk of endometriosis. Line represents multivariable-adjusted odds ratio, and shaded area represents 95% confidence interval. Models were adjusted for age, BMI, race, education, PIR, cigarette smoking status, age at menarche, and oral contraceptive use.
Furthermore, we carried out subgroup analyses to assess whether the associations of serum levels of these carotenoids with endometriosis would be affected by factors including age, race/ethnicity, smoking status, and oral contraceptive use. We took these factors into account in subgroup analyses in that they showed difference between endometriosis patients and those without the condition (Table 1). The results indicated that serum levels of lutein/zeaxanthin were associated with a reduced risk of endometriosis in individuals under 40 years old, White and non-White populations, smokers, and those with a history of oral contraceptive use (Tables 3–6). Subgroup analyses did not identify any associations of serum levels of ɑ-carotene, β-carotene, β-cryptoxanthin, and trans-lycopene with endometriosis (Tables 3–6).
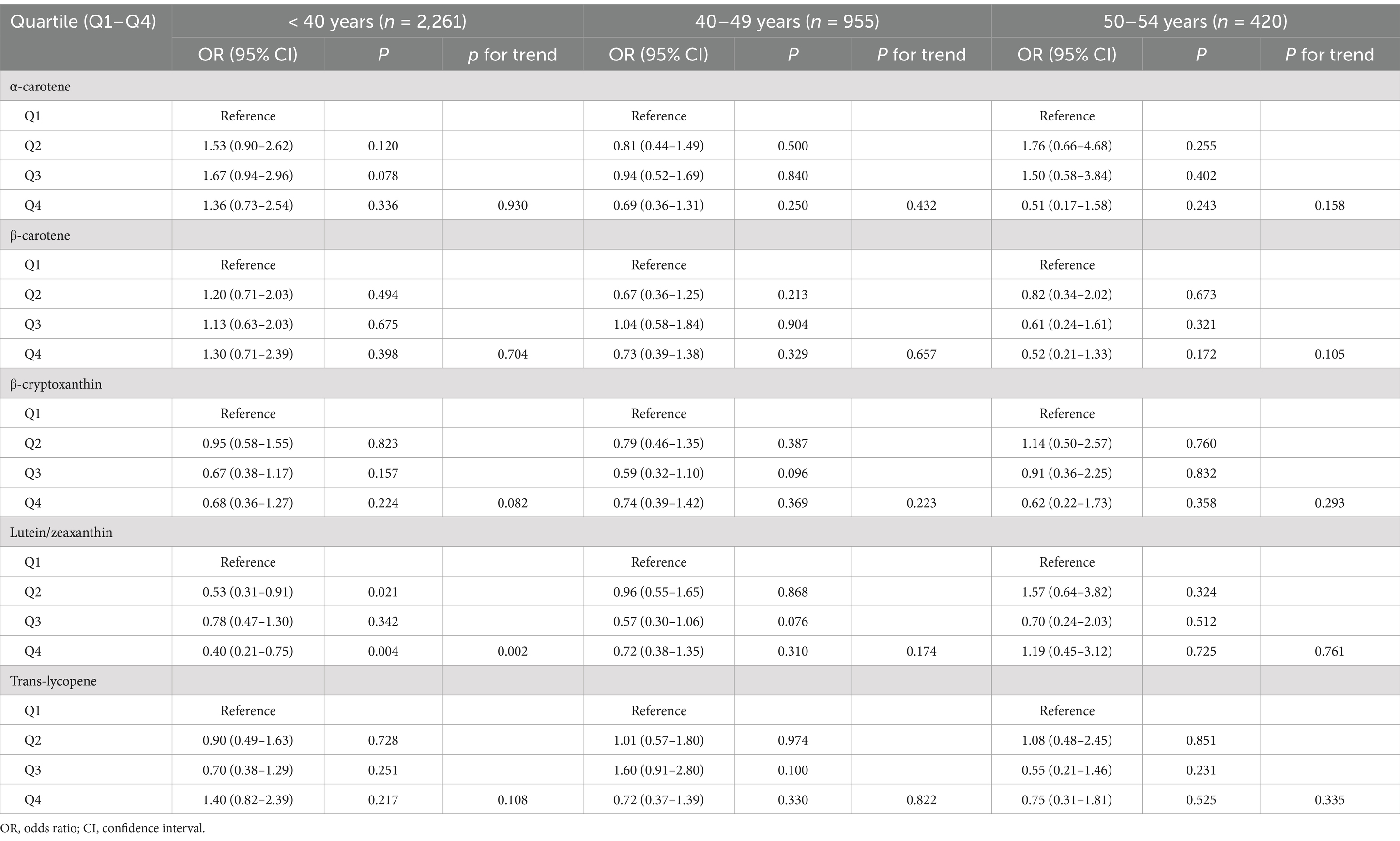
Table 3. Association between serum levels of major carotenoids and endometriosis in subgroup analysis according to age.
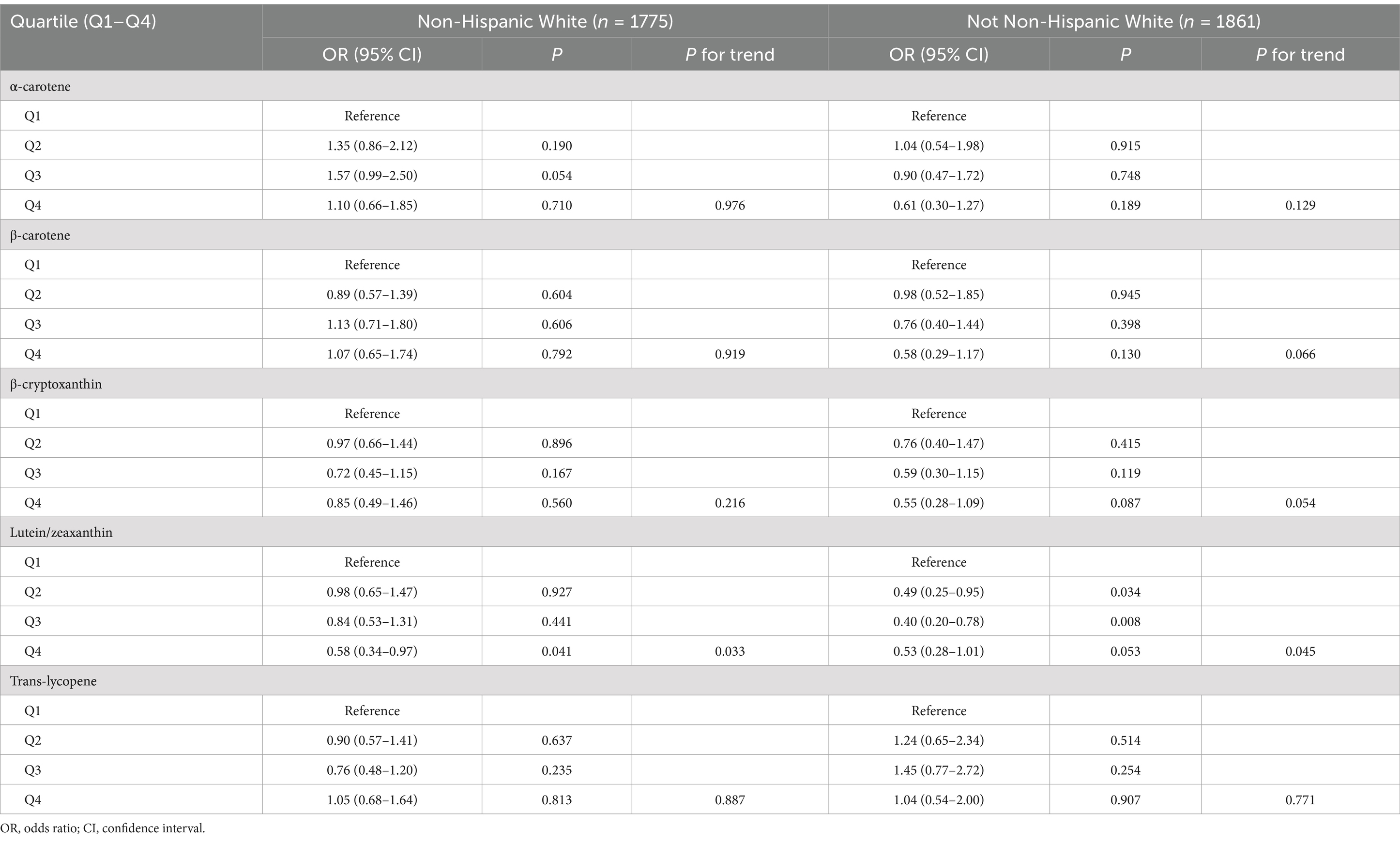
Table 4. Association between serum levels of major carotenoids and endometriosis in subgroup analysis according to ethnicity/race.
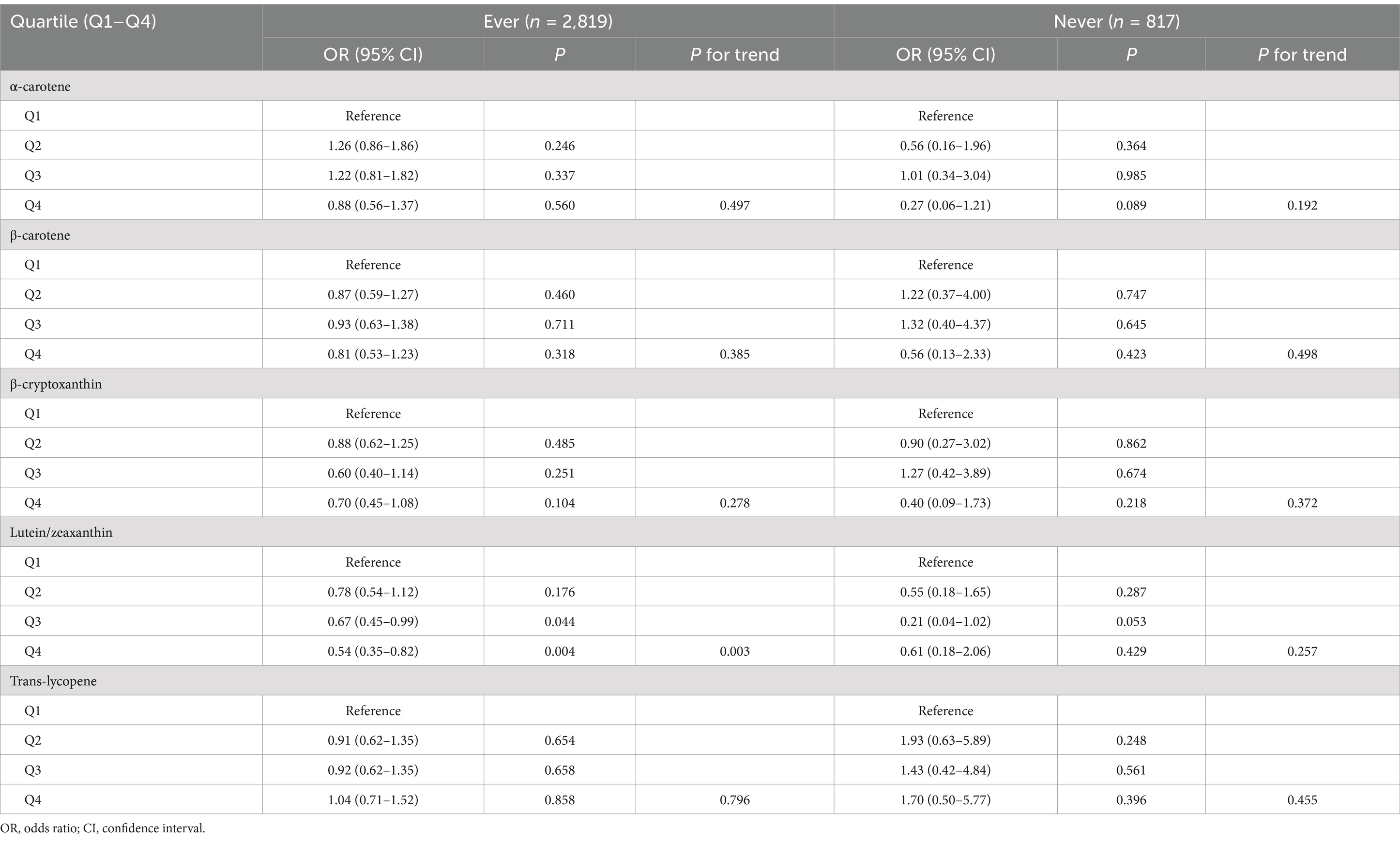
Table 5. Association between serum levels of major carotenoids and endometriosis in subgroup analysis according to oral contraceptive use.
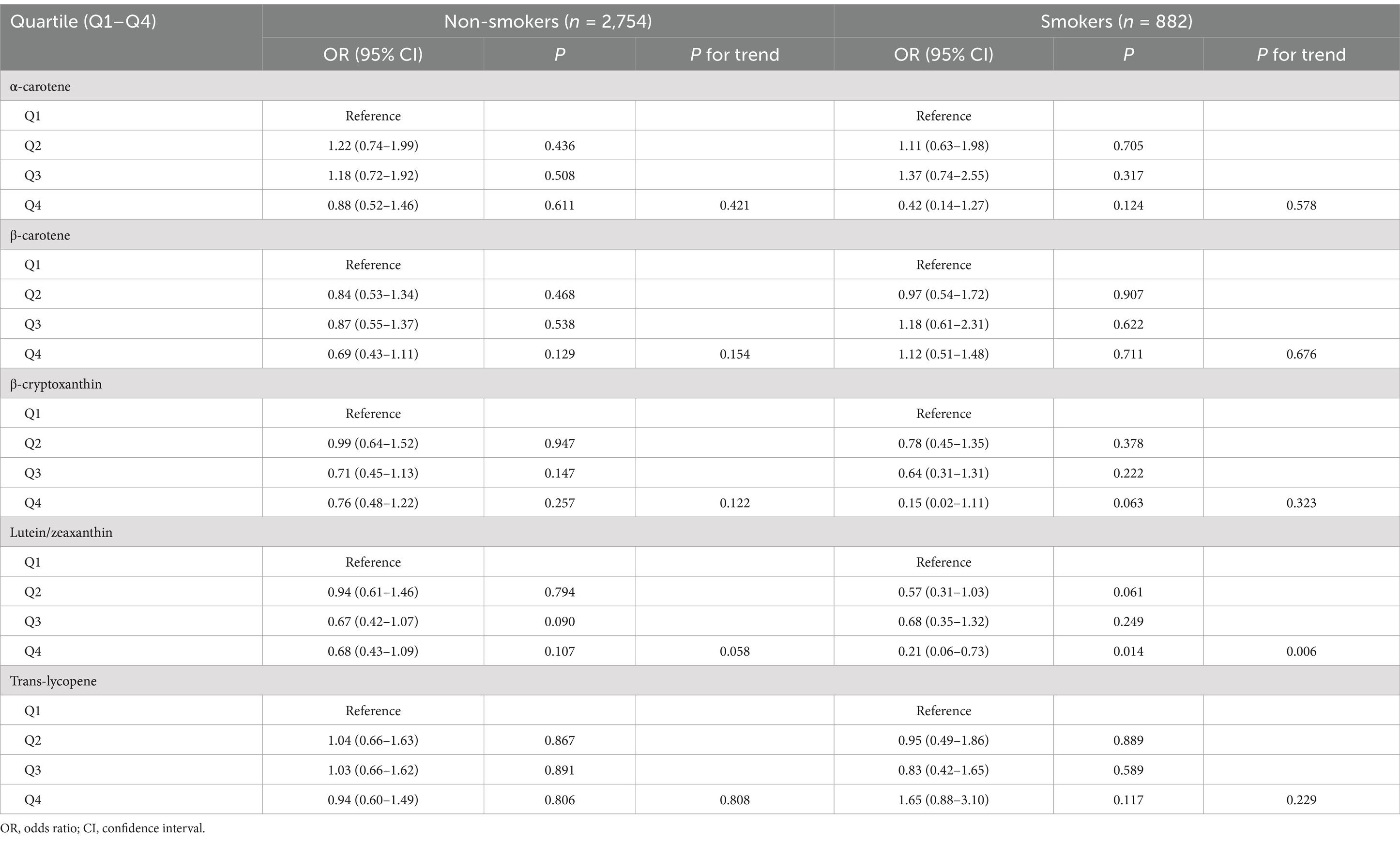
Table 6. Association between serum levels of major carotenoids and endometriosis in subgroup analysis according to smoking status.
In this population-based study, we found that higher serum levels of lutein/zeaxanthin were associated with a reduced risk of endometriosis. This protective effect was significant only in individuals under 40 years of age, in both White and non-White populations, among smokers, and in those who had ever used oral contraceptives. No non-linear associations were observed between serum lutein/zeaxanthin levels and endometriosis risk. Furthermore, no associations were found between serum levels of ɑ-carotene, β-carotene, β-cryptoxanthin, or trans-lycopene and the risk of endometriosis.
When comparing our results with previous studies, it is important to highlight the differences in study design and measurement methods. While some studies have examined the relationship between dietary carotenoid intake and endometriosis risk (10, 11), our study is one of the few to assess circulating carotenoid levels as a more direct measure of exposure. This distinction is crucial because circulating carotenoids reflect actual bioavailability and metabolic processing, rather than relying on self-reported dietary intake, which is subject to recall bias and inaccuracies. Our findings align with those of Harris et al. (11), who found a reduced risk of endometriosis associated with beta-cryptoxanthin intake, although our study did not identify a significant relationship between beta-cryptoxanthin and endometriosis risk. Our findings are also consistent with those of studies showing that lutein and zeaxanthin played a protective role against other female-related diseases, such as benign breast disease and breast cancer (19, 20). Conversely, Trabert et al. (10) reported an increased risk of endometriosis associated with higher β-carotene intake. These contrasting results may reflect the variability in carotenoid absorption, metabolism, and bioactivity across individuals, as well as differences in study populations, methodologies, and sample sizes. Our study, by using serum carotenoid measurements, overcomes some of these limitations and provides a more accurate reflection of carotenoid status in the body.
The inverse association between serum lutein/zeaxanthin levels and endometriosis risk may be attributed to several mechanisms. First, lutein and zeaxanthin are potent antioxidants, and their elevated serum levels may help to mitigate oxidative stress, which is known to play a crucial role in the pathogenesis and progression of endometriosis (21, 22). Elevated oxidative stress markers such as HSP70b’ and malondialdehyde, have been observed in patients with endometriosis compared to control groups (23–25), which can promote inflammation and lesion growth. By neutralizing ROS, lutein and zeaxanthin may reduce oxidative damage and inflammatory signaling, thereby lowering the risk of lesion formation and progression. Second, lutein and zeaxanthin are known to have anti-inflammatory effects. Chronic inflammation is a hallmark of endometriosis, driven by elevated cytokine levels and immune cell infiltration in the pelvic cavity (26). These carotenoids can downregulate the expression of pro-inflammatory cytokines such as IL-6 and IL-1β (27, 28), which are heavily implicated in the inflammatory milieu of endometriosis. Their modulation of immune responses may dampen the chronic inflammatory state, reducing tissue invasion and adhesion formation. Third, lutein and zeaxanthin may influence estrogen metabolism (29, 30). As endometriosis is an estrogen-dependent condition, carotenoids have been shown to modulate estrogen receptor activity (31). By impacting the estrogenic environment, lutein and zeaxanthin may limit the estrogen-driven proliferation of endometrial cells, thus inhibiting the growth of endometriotic lesions.
Our study assessed factors that may modify the relationship between serum carotenoid levels and endometriosis risk. Subgroup analyses suggested that elevated serum levels of lutein and zeaxanthin may offer a protective effect against the development of endometriosis, particularly in individuals under 40 years of age. One possible explanation is that younger women with endometriosis may experience higher levels of oxidative stress and inflammation (21), making them more sensitive to the antioxidative and anti-inflammatory properties of lutein and zeaxanthin. Furthermore, the role of lutein and zeaxanthin in modulating estrogenic pathways might be more pronounced in younger women, given the hormone-driven nature of endometriosis (1). Future studies focusing on age-specific mechanisms of these carotenoids could help clarify the observed age-related effects in the present study. The protective association was consistent across both White and non-White populations, highlighting the potential for broad applicability across different ethnic groups. Moreover, the effect was particularly pronounced in active smokers and participants who had ever used oral contraceptives, suggesting that these subgroups may experience unique oxidative stressors or hormonal influences that amplify the benefit of lutein and zeaxanthin’s antioxidative properties. These carotenoids are known to mitigate oxidative stress and inflammation, both of which play central roles in the pathophysiology of endometriosis. Therefore, modulation of oxidative pathways and inflammation by lutein and zeaxanthin may represent a key mechanism in reducing the risk of disease onset, particularly in higher-risk populations, such as women of reproductive age (1), smokers (32, 33), and those with specific hormonal exposures (34).
In terms of clinical implications, our findings raise the possibility that dietary recommendations or supplementation with lutein/zeaxanthin-rich foods could serve as a complementary approach to reduce the risk of endometriosis. Foods such as dark leafy greens, corn, eggs, and certain fruits are rich in lutein and zeaxanthin. However, while our results are promising, it is important to note that dietary interventions should be considered as part of a broader strategy that includes medical management, particularly since endometriosis is a multifactorial condition involving both genetic and environmental factors (1). Further clinical trials are needed to assess whether increasing lutein/zeaxanthin intake can indeed reduce the incidence or severity of endometriosis.
Our study has several limitations that should be considered when interpreting the findings. First, while we conducted a cross-sectional analysis on the data from the NHANES 2001–2006 cycles, the study’s design limited the ability to establish a temporal relationship between serum carotenoid levels and endometriosis risk, preventing causal inferences. Second, endometriosis diagnosis in NHANES is self-reported, which may introduce recall bias and lead to potential misclassification of cases. Additionally, the serum carotenoid levels were measured at a single point in time, failing to account for potential fluctuations over the course of the disease. Finally, unmeasured confounders, such as environmental exposures and genetic predispositions, could have influenced both carotenoid levels and endometriosis risk, and while we adjusted for several known covariates, residual confounding may still be present.
In summary, this study found an inverse association between serum lutein/zeaxanthin levels and the risk of endometriosis, with a particularly pronounced protective effect in individuals under 40 years of age, as well as in both White and non-White populations, smokers, and those who had ever used oral contraceptives. However, no significant associations were found between serum levels of ɑ-carotene, β-carotene, β-cryptoxanthin, or trans-lycopene and endometriosis risk. Future research with a prospective design and a focus on establishing causality is warranted to validate these findings.
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/.
This study utilized publicly available data from the National Health and Nutrition Examination Survey (NHANES), which is conducted by the National Center for Health Statistics (NCHS), a part of the Centers for Disease Control and Prevention (CDC). NHANES data collection protocols were approved by the NCHS Research Ethics Review Board, and all participants provided informed consent. As this analysis was based on de-identified secondary data, additional ethical approval was not required.
JH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The author would like to thank the participants of the National Health and Nutrition Examination Survey for their valuable contribution.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that Gen AI was used in the creation of this manuscript. During the preparation of this work the author used ChatGPT in order to check spelling and grammar. After using this tool, the author reviewed and edited the content as needed and take full responsibility for the content of the publication.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1513191/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Confounding factors taken into account in the analyses.
1. Horne, AW, and Missmer, SA. Pathophysiology, diagnosis, and management of endometriosis. BMJ. (2022) 379:e070750. doi: 10.1136/bmj-2022-070750
2. Saunders, PTK, Whitaker, LHR, and Horne, AW. Endometriosis: improvements and challenges in diagnosis and symptom management. Cell Rep Med. (2024) 5:101596. doi: 10.1016/j.xcrm.2024.101596
3. Sarria-Santamera, A, Orazumbekova, B, Terzic, M, Issanov, A, Chaowen, C, and Asúnsolo-Del-Barco, A. Systematic review and Meta-analysis of incidence and prevalence of endometriosis. Healthcare (Basel). (2020) 9:29. doi: 10.3390/healthcare9010029
4. Osmanlıoğlu, Ş, and Sanlier, N. The relationship between endometriosis and diet. Hum Fertil (Camb). (2023) 26:649–64. doi: 10.1080/14647273.2021.1995900
5. Barnard, ND, Holtz, DN, Schmidt, N, Kolipaka, S, Hata, E, Sutton, M, et al. Nutrition in the prevention and treatment of endometriosis: a review. Front Nutr. (2023) 10:1089891. doi: 10.3389/fnut.2023.1089891
6. Bohn, T, Balbuena, E, Ulus, H, Iddir, M, Wang, G, Crook, N, et al. Carotenoids in health as studied by omics-related endpoints. Adv Nutr. (2023) 14:1538–78. doi: 10.1016/j.advnut.2023.09.002
7. Mueller, L, and Boehm, V. Antioxidant activity of β-carotene compounds in different in vitro assays. Molecules. (2011) 16:1055–69. doi: 10.3390/molecules16021055
8. Saini, RK, Prasad, P, Lokesh, V, Shang, X, Shin, J, Keum, YS, et al. Carotenoids: dietary sources, extraction, encapsulation, bioavailability, and health benefits-a review of recent advancements. Antioxidants (Basel). (2022) 11:795. doi: 10.3390/antiox11040795
9. Udensi, J, Loughman, J, Loskutova, E, and Byrne, HJ. Raman spectroscopy of carotenoid compounds for clinical applications-a review. Molecules. (2022) 27:9017. doi: 10.3390/molecules27249017
10. Trabert, B, Peters, U, De Roos, AJ, Scholes, D, and Holt, VL. Diet and risk of endometriosis in a population-based case-control study. Br J Nutr. (2011) 105:459–67. doi: 10.1017/S0007114510003661
11. Harris, HR, Eke, AC, Chavarro, JE, and Missmer, SA. Fruit and vegetable consumption and risk of endometriosis. Hum Reprod. (2018) 33:715–27. doi: 10.1093/humrep/dey014
12. National Center for Health Statistics. NHANES survey methods and analytic guidelines. Available at: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx#sample-design (Accessed September 14, 2024).
13. Zhang, C, Li, K, Xu, SN, Zhang, JK, Ma, MH, and Liu, Y. Higher serum carotenoid concentrations were associated with the lower risk of cancer-related death: evidence from the National Health and nutrition examination survey. Nutr Res. (2024) 126:88–98. doi: 10.1016/j.nutres.2024.03.012
14. Bruno, RR, Rosa, FC, Nahas, PC, de Branco, FMS, and de Oliveira, EP. Serum ɑ-carotene, but not other antioxidants, is positively associated with muscle strength in older adults: NHANES 2001–2002. Antioxidants (Basel). (2022) 11:2386. doi: 10.3390/antiox11122386
15. Beydoun, MA, Canas, JA, Beydoun, HA, Chen, X, Shroff, MR, and Zonderman, AB. Serum antioxidant concentrations and metabolic syndrome are associated among U.S. adolescents in recent national surveys. J Nutr. (2012) 142:1693–704. doi: 10.3945/jn.112.160416
16. Mazidi, M, Kengne, AP, Katsiki, N, Mikhailidis, DP, and Banach, M. Inverse association between serum antioxidant levels and inflammatory markers is moderated by adiposity: a report based on a large representative population sample of American adults. Br J Nutr. (2018) 120:1272–8. doi: 10.1017/S0007114518002581
17. Han, GM, Meza, JL, Soliman, GA, Islam, KM, and Watanabe-Galloway, S. Higher levels of serum lycopene are associated with reduced mortality in individuals with metabolic syndrome. Nutr Res. (2016) 36:402–7. doi: 10.1016/j.nutres.2016.01.003
18. Lee, AW, and Eata, V. Association of environmental phenols with endometriosis and uterine leiomyoma: an analysis of NHANES, 2003–2006. Reprod Toxicol. (2022) 113:30–4. doi: 10.1016/j.reprotox.2022.08.003
19. Boeke, CE, Tamimi, RM, Berkey, CS, Colditz, GA, Eliassen, AH, Malspeis, S, et al. Adolescent carotenoid intake and benign breast disease. Pediatrics. (2014) 133:e1292–8. doi: 10.1542/peds.2013-3844
20. Park, SH, Lee, J, Jung, SY, Park, S, Kang, YH, and Kim, J. Association between dietary carotenoid intake and breast cancer risk: a case-control study among Korean women. Int J Food Sci Nutr. (2024) 75:496–508. doi: 10.1080/09637486.2024.2358111
21. Ansariniya, H, Yavari, A, Javaheri, A, and Zare, F. Oxidative stress-related effects on various aspects of endometriosis. Am J Reprod Immunol. (2022) 88:e13593. doi: 10.1111/aji.13593
22. Clower, L, Fleshman, T, Geldenhuys, WJ, and Santanam, N. Targeting oxidative stress involved in endometriosis and its pain. Biomol Ther. (2022) 12:1055. doi: 10.3390/biom12081055
23. Lambrinoudaki, IV, Augoulea, A, Christodoulakos, GE, Economou, EV, Kaparos, G, Kontoravdis, A, et al. Measurable serum markers of oxidative stress response in women with endometriosis. Fertil Steril. (2009) 91:46–50. doi: 10.1016/j.fertnstert.2007.11.021
24. Matsuzaki, S, and Schubert, B. Oxidative stress status in normal ovarian cortex surrounding ovarian endometriosis. Fertil Steril. (2010) 93:2431–2. doi: 10.1016/j.fertnstert.2009.08.068
25. Nasiri, N, Moini, A, Eftekhari-Yazdi, P, Karimian, L, Salman-Yazdi, R, and Arabipoor, A. Oxidative stress statues in serum and follicular fluid of women with endometriosis. Cell J. (2017) 18:582–7. doi: 10.22074/cellj.2016.4724
26. Symons, LK, Miller, JE, Kay, VR, Marks, RM, Liblik, K, Koti, M, et al. The Immunopathophysiology of endometriosis. Trends Mol Med. (2018) 24:748–62. doi: 10.1016/j.molmed.2018.07.004
27. Chung, RWS, Leanderson, P, Lundberg, AK, and Jonasson, L. Lutein exerts anti-inflammatory effects in patients with coronary artery disease. Atherosclerosis. (2017) 262:87–93. doi: 10.1016/j.atherosclerosis.2017.05.008
28. Stringham, NT, Holmes, PV, and Stringham, JM. Effects of macular xanthophyll supplementation on brain-derived neurotrophic factor, pro-inflammatory cytokines, and cognitive performance. Physiol Behav. (2019) 211:112650. doi: 10.1016/j.physbeh.2019.112650
29. Maggio, M, de Vita, F, Lauretani, F, Bandinelli, S, Semba, RD, Bartali, B, et al. Relationship between carotenoids, retinol, and estradiol levels in older women. Nutrients. (2015) 7:6506–19. doi: 10.3390/nu7085296
30. Tamimi, RM, Hankinson, SE, Campos, H, Spiegelman, D, Zhang, S, Colditz, GA, et al. Plasma carotenoids, retinol, and tocopherols and risk of breast cancer. Am J Epidemiol. (2005) 161:153–60. doi: 10.1093/aje/kwi030
31. Hirsch, K, Atzmon, A, Danilenko, M, Levy, J, and Sharoni, Y. Lycopene and other carotenoids inhibit estrogenic activity of 17beta-estradiol and genistein in cancer cells. Breast Cancer Res Treat. (2007) 104:221–30. doi: 10.1007/s10549-006-9405-7
32. Andolf, E, Thorsell, M, and Källén, K. Caesarean section and risk for endometriosis: a prospective cohort study of Swedish registries. BJOG. (2013) 120:1061–5. doi: 10.1111/1471-0528.12236
33. Yen, CF, Kim, MR, and Lee, CL. Epidemiologic factors associated with endometriosis in East Asia. Gynecol Minim Invasive Ther. (2019) 8:4–11. doi: 10.4103/GMIT.GMIT_83_18
Keywords: endometriosis, risk, carotenoids, serum lutein/zeaxanthin levels, NHANES
Citation: Huang J (2025) Association between serum carotenoids levels and endometriosis risk: evidence from the National Health and Nutrition Examination Survey. Front. Nutr. 12:1513191. doi: 10.3389/fnut.2025.1513191
Received: 21 October 2024; Accepted: 03 January 2025;
Published: 04 February 2025.
Edited by:
Elma Izze da Silva Magalhães, Federal University of Rio Grande do Sul, BrazilReviewed by:
Samanta Catherine Ferreira, Federal University of Minas Gerais, BrazilCopyright © 2025 Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Huang, aHVhbmdqaWFuX2NobkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.