
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 03 February 2025
Sec. Clinical Nutrition
Volume 12 - 2025 | https://doi.org/10.3389/fnut.2025.1508381
 De-Jiang Lin1†
De-Jiang Lin1† Dong-Xia Hu1†
Dong-Xia Hu1† Qing-Ting Wu1†
Qing-Ting Wu1† Lin-Gui Huang1
Lin-Gui Huang1 Zi-Han Lin1
Zi-Han Lin1 Jia-Ting Xu1
Jia-Ting Xu1 Xing-Xiang He1*
Xing-Xiang He1* Lei Wu1,2*
Lei Wu1,2*Background and aims: Metabolic Syndrome (MS) is a cluster of metabolic abnormalities closely associated with hypertension, diabetes, hyperlipidemia, obesity, etc. Our previous research indicated that fecal microbiota transplantation (FMT) could improve MS, but the factors influencing the efficacy of washed microbiota transplantation (WMT) in treating MS patients remain unclear. The objective of this study is to analyze the influencing factors of WMT in treating MS patients.
Methods: The clinical data and influencing factors related to MS patients were collected retrospectively. Not only the changes in body mass index [BMI = weight (kg)/height (m)2], blood glucose, blood lipids, and blood pressure were analyzed, but also the influencing factors of WMT in treating MS patients were carried out based on Logistic Regression. The 16S rRNA gene amplicon sequencing was performed on fecal samples before and after WMT treatment.
Results: A total of 210 patients were included, including 68 patients in the WMT group and 142 patients in the drug treatment (DT) group. WMT had a significant improvement and ASCVD downregulation effect on MS patients, and 42.65% of MS patients removed the label of MS after WMT treatment. Independent influencing factors for treating MS patients through WMT include age <60 years old, high smoking index, infection, single donor selection, single-course WMT treatment, and having hypertension, diabetes, or obesity. WMT treated MS patients by maintaining the balance of gut microbiota.
Conclusions: WMT has a significant effect in improving MS and downregulating ASCVD risk stratification. The therapeutic effect of WMT on MS patients is closely related to their age, smoking index, infection, chronic disease status, donor type, and WMT courses. Therefore, we can improve the efficacy of WMT by reducing independent influencing factors that affect gut microbiota homeostasis.
Metabolic Syndrome (MS) is a group of clinical syndromes combined with obesity, hyperglycemia (diabetes or impaired glucose regulation), dyslipidemia (hyperglycemia and/or low HDL-c hyperemia) and hypertension, which increases the risk of type 2 diabetes mellitus by fivefold and cardiovascular disease by threefold (1). In addition, MS is closely associated with non-alcoholic fatty liver disease (NAFLD), chronic kidney disease, polycystic ovary syndrome, cancers such as liver cancer, colorectal cancer and kidney cancer (2–5). The two main potential risk factors of MS are obesity and insulin resistance, while aggravating factors include physical inactivity, aging, endocrine and genetic factors, characterized by high incidence rate, diverse clinical manifestations, complex mechanisms, and difficult treatment (1, 2). The latest data shows the prevalence of MS in the population aged 20 and above is as high as 31.1% in China, and the global prevalence is still rising, becoming a major global health hazard (6, 7).
Beyond changing lifestyle and dietary structure, the current main methods for treating MS include strengthening aerobic exercise and drug treatment (8). Unlike traditional drugs, fecal microbiota transplantation (FMT) is a new treatment method utilizing healthy microbiota to replace imbalanced microbiota in patients, thereby restoring normal gut microbiota function and maintaining various neurological and metabolic functions (9–11). Numerous studies had confirmed the close correlation between gut microbiota and MS, suggesting that improving gut microbiota could reduce insulin resistance, enhance fat utilization, promote the absorption of blood pressure regulating substances and restore the microbial biological environment (12–14). Washed microbiota transplantation (WMT) is a microbial community transplantation method similar to FMT, which increases the process of washing and filtering microbial communities. Compared with FMT, the bacterial solution of WMT is prepared by the intelligent microbial isolation system (GenFMTer), which is filtered through a multi-level filtration system to screen for adverse inflammatory factors that cause human inflammation, resulting in a safer and more effective bacterial solution (15).
Our previous research had shown that WMT had a significant improvement effect on MS patients by restoring their gut microbiota homeostasis (16), laying the foundation for the clinical application of FMT to treat MS patients. In addition, our study also confirmed that WMT did not further increase the blood lipids, blood glucose, and blood pressure of non-metabolic syndrome patients, thereby reducing the interference factors in the study (16). However, there were a number of MS patients who could not remove the label of MS after WMT treatment, and the factors that affect the efficacy of WMT in treating MS patients were still unknown. To treat MS patients effectively, we tried to explore the influencing factors of WMT in treating MS patients in the Department of Gastroenterology at the First Affiliated Hospital of Guangdong Pharmaceutical University. Therefore, we conducted a retrospective trial to collect clinical data of MS patients receiving WMT or drug treatment.
This study included MS patients who completed 1–4 courses of WMT treatment or ordinary treatment in the First Affiliated Hospital of Guangdong Pharmaceutical University from January 2017 to December 2023 during the first WMT. Inclusion criteria were patients aged 18–80 with informed consent and voluntary acceptance of WMT. Exclusion criteria were pregnant women and patients who changed their hypoglycemic, antihypertensive, or lipid-lowering medications during the observation period. The study was approved by the Ethics Committee of the First Affiliated Hospital of Guangdong Pharmaceutical University, adhering to the Helsinki Declaration (No.2021-13). Among the enrolled participants, 210 met the requirements of this study and provided written informed consent to participate.
The diagnostic criteria for MS in this study refer to the Chinese Guidelines for the Prevention and Treatment of Type 2 Diabetes (2020 Edition) (17) and the diagnostic criteria for metabolic syndrome of the Diabetes Society of the Chinese Medical Association (18). Metabolic syndrome was diagnosed with three or more of the following: (1) BMI ≥ 25 kg/m2. (2) Hyperglycemia: fasting blood glucose ≥6.1 mmol/L or blood glucose ≥7.8 mmol/L 2 h after glucose load and (or) those who have been diagnosed with diabetes and received treatment. (3) Hypertension: Blood pressure ≥ 130/85 mmHg (1 mmHg = 0.133 kPa) and (or) diagnosed with hypertension and receiving treatment. (4) Fasting triglycerides (TG) ≥ 1.70 mmol/L. (5) Fasting HDL-c < 1.04 mmol/L. The 210 patients included in this study were divided into WMT group and DT group based on whether they received WMT treatment. There were a total of 68 patients in the WMT group, and 142 patients in the DT group who were hospitalized at the same time but did not receive WMT treatment or take medication such as microbial preparations and probiotics.
In accordance with the Chinese Guidelines for the Prevention of Cardiovascular Diseases (2017 Edition) (19), ASCVD risk stratification was carried out according to the baseline and blood lipid status, which were classified extremely high risk group, high risk group, medium risk group, and low risk group. Subsequently, patients were divided into the single-course WMT group, double-course WMT group, and multi-course WMT group (three or more courses) for WMT. After receiving 1–4 rounds of WMT or drug treatment and completing follow-up, statistical analysis and evaluation of height, weight, blood glucose, blood lipids, and blood pressure results were conducted for all patients.
The WMT program adhered to the Nanjing Consensus on the Methodology of Washed Microbial Transplantation (20). All healthy donors aged between 18 and 25 must undergo rigorous counseling, psychological and physical examinations, biochemical tests, and screening for infectious diseases. The specific fecal preparation procedures can refer to the Nanjing Consensus (20) and our previous research. Relevant indicators were collected before completing each course of treatment, including baseline values, values after the first course (single-course), values after the second course (double-course), and values after the third or more courses (multi-course).
The study collected data from MS patients before (baseline) and after treatment, and compared the partial clinical efficacy between the WMT group and the DT group using the difference before and after treatment as the improvement value. Mainly including BMI indicators: weight (kg), height (m), weight/height2 = BMI (kg/m2). Blood glucose indicators: fasting blood glucose (FBG, mmol/L), fasting insulin (FI, mU/mL), and the insulin resistance value (HOMA-IR, insulin resistance value = fasting blood glucose * fasting insulin/22.5). Blood lipid indexes: total cholesterol (TC, mmol/L), triglyceride (TG, mmol/L), low density lipoprotein cholesterol (LDL-c, mmol/L), high density lipoprotein cholesterol (HDL-c, mmol/L). Blood pressure indicators: systolic blood pressure (SBP, mmHg) at admission, and diastolic blood pressure (DBP, mmHg) at admission. And various influencing factors: age, gender, smoking and alcohol history, medication and infection status, chronic disease, pathways of WMT, selection of donor type and number of WMT courses. Smoking index = number of cigarettes smoked/day × Years of smoking (smoking index < 200 represents mild smoking, while smoking index 200 ≥ moderate to severe smoking). The middle digestive tract is defined as microbiota transplantation through a jejunal tube, while the lower digestive tract is microbiota transplantation through a colon tube. Adverse events (AEs): diarrhea, fever, fatigue, nausea, abdominal pain, etc.
The fecal samples from 5 MS patients who excluded independent influencing factors and 5 donors were collected before and after WMT for sequencing. After collection, all samples were stored at −80°C until DNA was extracted. DNA quality and concentration were checked by NanoDrop™ 2000 (Thermo Fisher Scientific, Wilmington, DE, USA) (21). Primers 338F (50-ACTCCTACGGGAGGCAGCAG-30) and 806R (50-GGACTACHVGGGTWTCTAAT-30) were used to amplify bacterial 16S rRNA gene fragments (V3-V4) from extracted DNA. The PCR products were subjected to agarose gel electrophoresis to determine the size of the amplicon. The constructed library was quantified by Qubit and Q-PCR, and the NovaSeq6000 (Illumina, San Diego, CA, USA) sequencing platform was used for onboard sequencing until the library was qualified (22).
From all the sample data split from plane data and amputation of barcode and primer sequences after the use of FLASH software (version 1.2.11, http://ccb.jhu.edu/software/FLASH/) to splice the sample reads, raw tags were obtained (23). Then, fastp software version 0.23.1 (Shenzhen Hypros, Shenzen, China) was used to obtain high-quality clean tags (24). Finally, clean tags were compared with the database to detect and remove chimeras, so as to obtain the effective tags (25). The DADA2 Variants in QIIME2 were used to obtain the final ASV variants and the feature list of the variant. The resulting ASVs were then compared with the database by the classify-sklearn module in QIIME2 software version 2.0 (QIIME 2 development team, https://docs.qiime2.org) to obtain species information for each ASV (26). The representative sequences of ASVs using the classification sklearn (Naive Bayes) algorithm were analyzed, obtaining the relative abundance of ASVs at the genus level.
Statistical analysis was performed using SPSS 22.0 (IBM Corp, Armonk, NY, USA) and Prism 8 (GraphPad, San Diego, CA, USA). The Categorical variables were analyzed by Chi-squared test or Fisher exact test. For comparison of continuous variables between two independent groups, unpaired Student's t-test (Normal distribution) and Mann Whitney test (non-Normal distribution) could be used. The paired data were compared by paired Student's t-test (Normal distribution) and Wilcoxon signed rank test (non-Normal distribution). Logistic regression analysis is used to summarize the main factors that affect therapeutic efficacy. Two-tailed p-values < 0.05 were considered statistically significant.
A total of 210 patients met the inclusion criteria for WMT treatment or ordinary treatment at the First Affiliated Hospital of Guangdong Pharmaceutical University from January 2017 to December 2023, including 126 males (60.00%) and 84 females (40.00%), with an average age of 60.12 ± 11.66 years. Due to different patient compliance, each WMT treatment might not be completed as scheduled. The average number of microbiota transplants is 2.68 ± 1.22, which is close to 3 times. This study calculated the time interval of selected patients, expressed in days as the median (25%−75%). The blood test results of the patient before the first treatment were the baseline values, with a treatment interval of 36 days (32–46 days) in single-course WMT, 95 days (76.75–104.25 days) in double-course WMT, 192 days (152.75–212.50 days) in multi-course WMT, and 42 days (36.42–50.48 days) in the drug treatment (DT) group.
The comparison of demography and clinical characteristics between the WMT and the DT group is shown in Table 1. Due to different compliance, not all patients had complete data, so the number of patients in each group was different for each indicator. There was no significant difference in age, gender, medical history, and laboratory indicators between the WMT group and DT group in MS patients, which reduces the interfering factors for our study of efficacy differences between treatment groups. Compared to MS patients, the various indicators of the donor, including BMI, blood glucose, blood lipid, and blood pressure, were healthier.
Figure 1 and Supplementary Table S1 show the impact of WMT on BMI, blood glucose, blood lipids and blood pressure in MS patients in the WMT group. The results showed that single-course treatment had a significant reducing effect on BMI (from 26.61 ± 4.67 to 26.05 ± 4.61, p = 0.007), FBG (from 6.36 ± 2.37 to 5.83 ± 1.76, p = 0.038), TG (from 3.20 ± 4.04 to 2.34 ± 1.87, p = 0.013), HDL-c (from 0.99 ± 0.25 to 1.10 ± 0.45, p = 0.029), SBP (from 133.12 ± 12.01 to 125.78 ± 12.56, p < 0.001) and DBP (from 82.78 ± 9.69 to 77.81 ± 9.40, p = 0.002). At the same time, the double-course treatment also showed a significant reducing effect on BMI (from 26.44 ± 4.30 to 25.74 ± 4.28, p = 0.033) and SBP (from 132.49 ± 11.82 to 125.64 ± 11.64, p = 0.006), DBP (from 82.56 ± 9.65 to 79.18 ± 8.23, p = 0.037), while the multi-course treatment only had a significant reducing effect on SBP (from 129.82 ± 12.25 to 118.68 ± 10.51, p = 0.010) and DBP (from 82.05 ± 9.96 to 75.18 ± 10.05, p = 0.024).
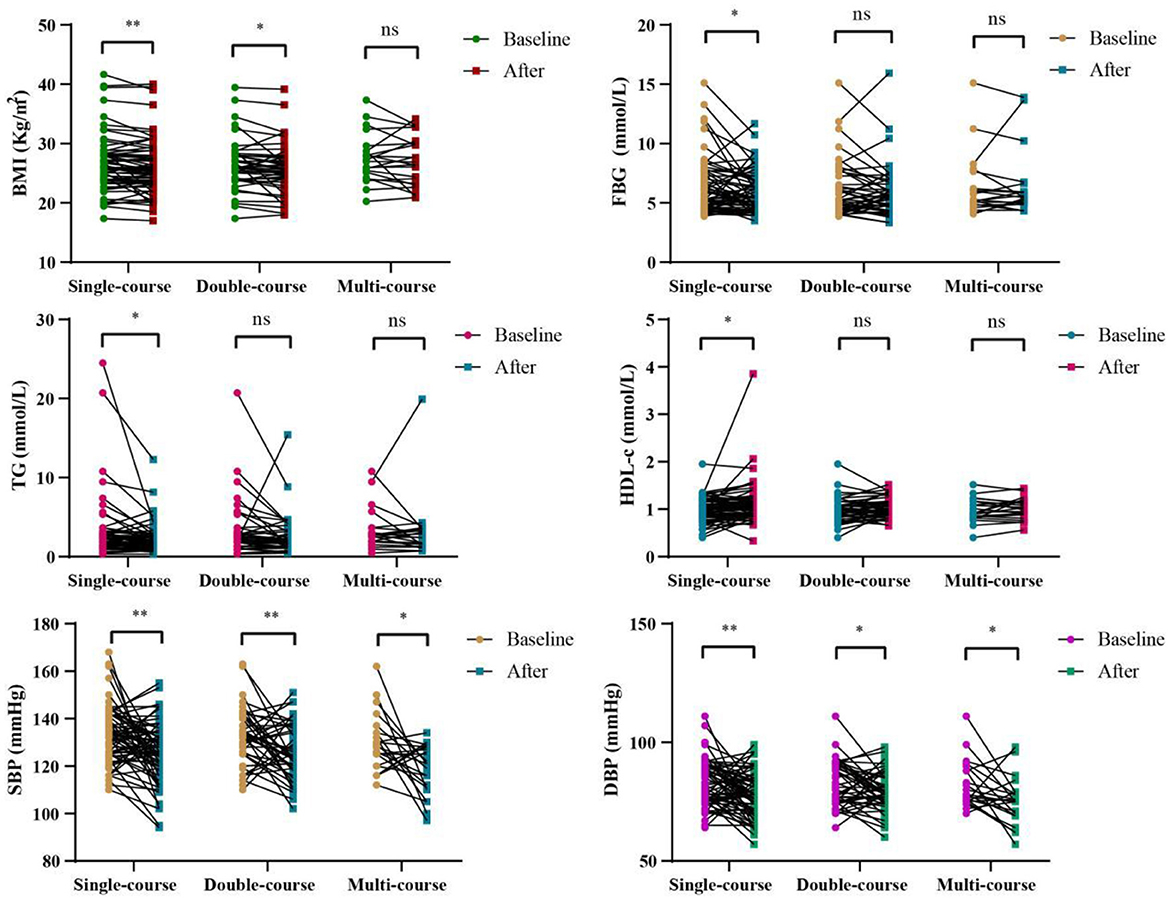
Figure 1. Changes of BMI, FBG, TG, HDL-c, SBP, DBP in different courses of in the WMT group; *indicates p < 0.05, **indicates p < 0.01; ns, not significant. All data can be found on Supplementary Table S1.
Figure 2 and Supplementary Table S2 show the comparison of improvement values for various indicators under different conditions during the single-course of treatment in the WMT group. Regarding blood glucose indicators and BMI in Figure 2A, the WMT plus drug (WMT-D) group showed superior effects to the WMT without drug (WMT-ND) group in reducing FBG, while the WMT-ND group was superior in reducing BMI, HbA1c, FI, and HOMA-IR. For blood lipid indicators in Figure 2B, the WMT-D group had a better improvement effect than the WMT-ND group on TC, TG, LDL-c, and HDL-c. However, there was not much difference between the two groups in terms of blood pressure reduction in Figure 2C. In our study, even though some indicators of the WMT-D group were superior to those of the WMT-ND group, there was no statistically significant difference between the two groups as a whole, indicating that WMT can improve indicators such as blood glucose, blood lipids and blood pressure in MS patients.
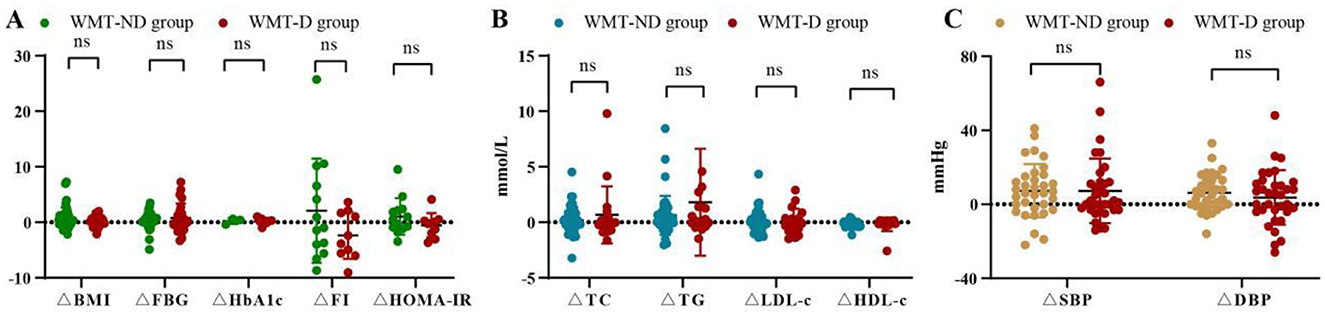
Figure 2. Comparison of improvement values for various indicators during the single-course of treatment in the WMT group. ΔBMI refers to the improvement value in BMI, and so on. WMT-ND group represents WMT without drug treatment and WMT-D group represents WMT plus drug treatment. (A) Comparison of blood glucose indicators and BMI improvement values between two groups. (B) Comparison of blood lipid indicators and BMI improvement values between two groups. (C) Comparison of blood pressure indicators and BMI improvement values between two groups; ns, not significant. All data can be found on Supplementary Table S2.
As shown in Figure 3 and Supplementary Table S3, compared to the DT group, the WMT group had greater advantages in reducing blood glucose, blood pressure, lipid, and weight, such as FBG improvement value (0.53 ± 1.99 vs. −0.41 ± 2.57, p = 0.012), TG improvement value (0.96 ± 2.97 vs. 0.02 ± 1.67, p = 0.006), SBP improvement value (7.34 ± 15.86 vs. 1.97 ± 18.00, p = 0.037), DBP improvement value (4.97 ± 12.52 vs. 0.99 ± 13.74, p = 0.045), and BMI improvement value (0.56 ± 1.64 vs. 0.12 ± 1.33, p = 0.047).
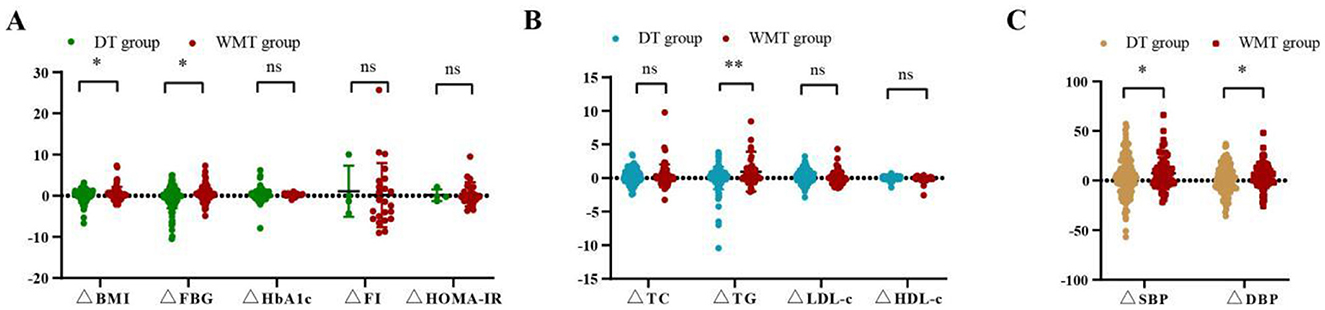
Figure 3. Comparison of improvement values for various indicators between the DT group and WMT group. (A) Comparison of blood glucose indicators and BMI improvement values between two groups. (B) Comparison of blood lipid indicators and BMI improvement values between two groups. (C) Comparison of blood pressure indicators and BMI improvement values between two groups; *indicates p < 0.05, **indicates p < 0.01; ns, not significant. All data can be found on Supplementary Table S3.
As shown in Table 2, WMT had the effect of treating MS patients as non-MS patients (p = 0.011), with 29 (42.65%) MS patients improving to non-MS patients in the WMT group. Therefore, WMT treatment was superior to drug therapy in treating MS patients effectively.
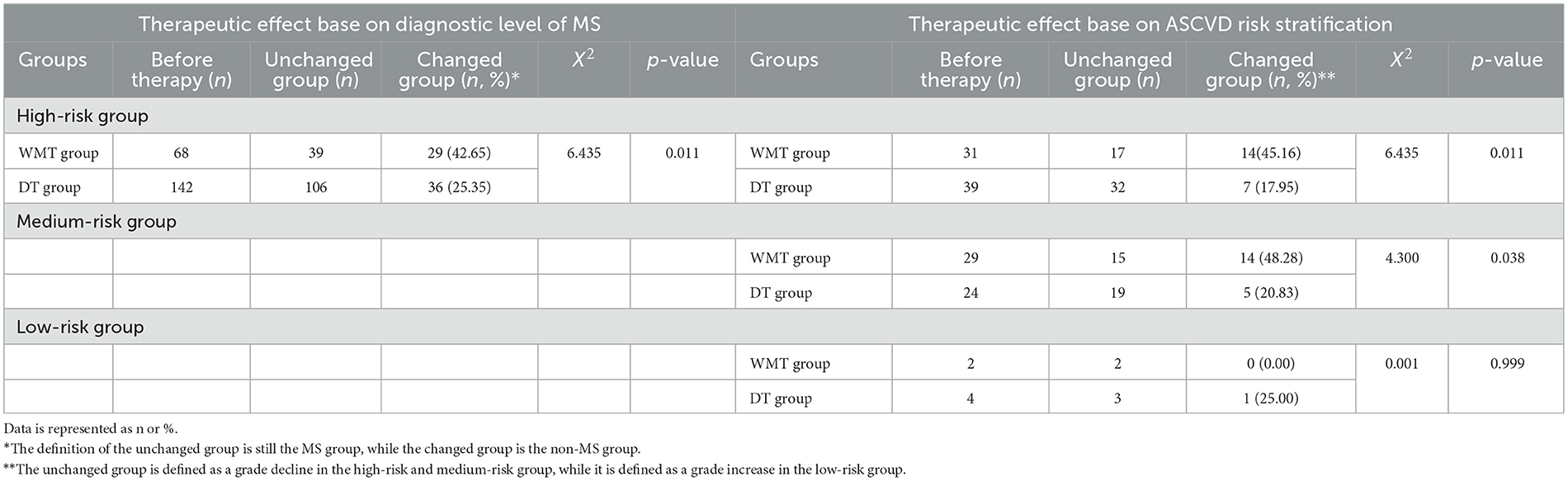
Table 2. Clinical comprehensive efficacy evaluation based on diagnostic level of MS and ASCVD risk stratification.
In addition, according to ASCVD risk stratification (19), MS patients were divided into extremely high-risk group, high-risk group, medium-risk group and low-risk group. Acute coronary syndrome, stable coronary heart diseases, ischemic cardiomyopathy, peripheral atherosclerosis and ischemic stroke were included in the extremely high-risk group. This group of patients was not reassigned after WMT treatment and is not listed in Table 2.
In the high-risk group, 14 (45.16%) patients experienced a grade decline, which reduced high-risk ASCVD stratification from 45.59% to 25.00% in the WMT group (p = 0.011). In the medium-risk group, 14 (48.28%) patients experienced a grade decline, which reduced medium-risk ASCVD stratification from 42.65% to 22.06% (p = 0.038). In the low-risk group, this change was not statistically significant, indicating that WMT treatment did not increase the risk of ASCVD. In conclusion, WMT has a significant ASCVD downgrade effect on high-risk and medium-risk MS patients.
Among the completed WMT treatments, the total adverse reaction rate was 2.96%. Only one patient experienced fever with a maximum body temperature of 37.6°C, along with diarrhea after treatment completion. All symptoms were alleviated after < 3 days of symptomatic treatment.
This study included 135 MS patients in the WMT group, including 68 patients who completed a single course of WMT, 45 patients who completed a dual course of WMT, and 22 patients who completed multiple courses of WMT. Among the 135 MS patients, 55 patients recovered to non-MS patients after treatment, with a success rate of 40.74%.
It showed the influencing factors that may affect the efficacy of WMT in treating MS patients in Tables 3, 4, including antibiotics and immunosuppressants use, infection with pathogenic bacteria or viruses during observation, hypertension, diabetes, hyperlipemia, obesity, other chronic diseases, the pathways of WMT, the selection of donor type and number of WMT courses. As shown in Table 4, in both the MS group and non-MS group formed after WMT treatment, the MS group had a significantly higher proportion of age < 60 years old, smoking index ≥200, using of antibiotics or immunosuppressants during observation, infection with pathogenic bacteria or viruses during observation, having hypertension, diabetes, obesity, single donor selection and single-course WMT treatment compared to the non-MS group (p < 0.050), suggesting the main factors affecting the efficacy of WMT in treating MS patients. However, there was not currently statistical significance in gender, alcohol consumption, having hyperlipidemia, suffering from other chronic diseases and pathways of WMT.
Based on the logistic regression analysis in Table 5, not only the age < 60 years old (OR = 3.525, p = 0.023), smoking index ≥200 (OR = 11.705, p = 0.034), infection of pathogenic bacteria or viruses during observation (OR = 8.783, p = 0.037), single donor selection (OR = 3.003, p = 0.026) and single-course WMT (OR = 2.721, p = 0.041), but also suffering from hypertension (OR = 3.375, p = 0.026), diabetes (OR = 3.928, p = 0.008), and obesity (OR = 2.945, p = 0.038) were independent influencing factors. As shown in Figure 4A, the area under the AUC curve of some independent influencing factors was >0.700, including smoking index (X4), area under AUC curve = 0.706 (0.621–0.792), infection with pathogenic bacteria or viruses during observation (X7), area under AUC curve = 0.719 (0.634–0.803), having obesity (X11), area under AUC curve = 0.712 (0.621–0.803), selection of donor type (X14), and area under AUC curve = 0.712 (0.624–0.796), indicating that the model had a certain degree of predictability. However, some independent influencing factors had an area under the AUC curve < 0.700 in Figure 4B.
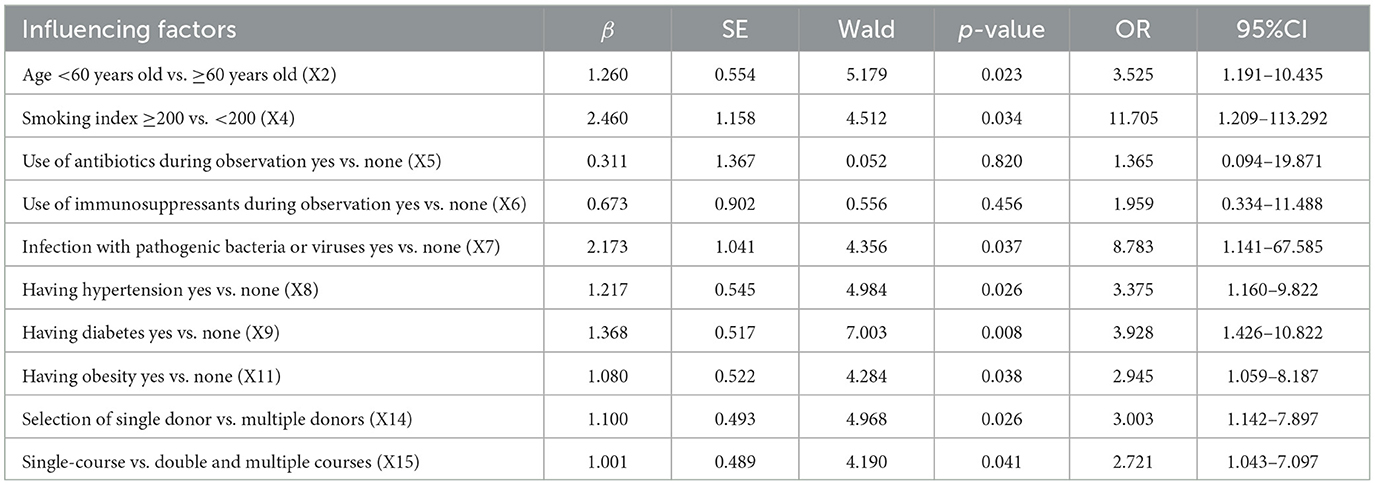
Table 5. Logistic regression analysis of the influencing factors of WMT on the efficacy of MS patients.
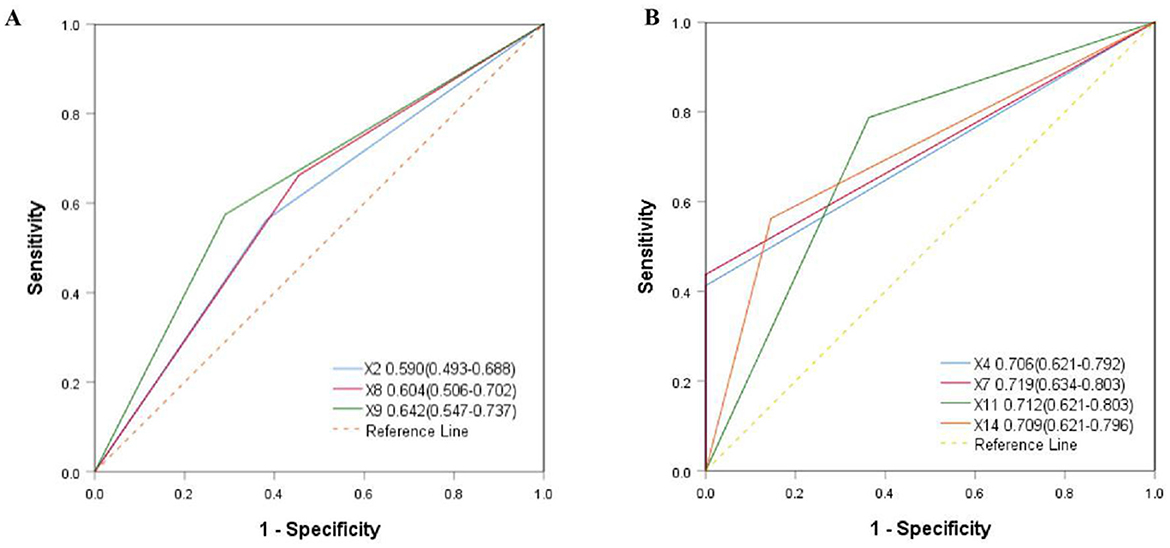
Figure 4. Comparison of area under AUC aurve for independent influencing factors. (A) X4 = smoking index, X7 = infection with bacteria or viruses during observation, X11 = having obesity, X14 = selection of donor type. (B) X2 = age, X8 = having hypertension, X9 = having diabetes.
We analyzed the gut microbiota composition of MS patients who excluded independent influencing factors before and after WMT and donors. At the phylum level, the gut microbiota mainly included Firmicutes, Fusobacteriota, Bacteroidota, Actinobacteriota, and Proteobacteria. At the phylum level, the relative abundance of Firmicutes, Bacteroidota, and Actinobacteriota increased after WMT. The relative abundance of Fusobacteriota and Proteobacteria decreased (Figure 5A). At the family level, the relative abundance of Bacteroideaceae, Lachnospiraceae, Bifidobacteriaceae, and Ruminococcaceae increased, with a great increase of Bifidobacteriaceae in MS patients after WMT and donors, while the relative abundance of Enterococcaceae and Fusobacteriaceae decreased, with a great decrease of Enterococcaceae in MS patients after WMT and donors (Figure 5B). At the genus level, the relative abundance of Bifidobacterium, Bacteroides, Faecalibacterium, and Prevotella increased after WMT. The relative abundance of Enterococcus, Fusobacterium, Escherichia–Shigella, and Klebsiella reduced (Figure 5C).
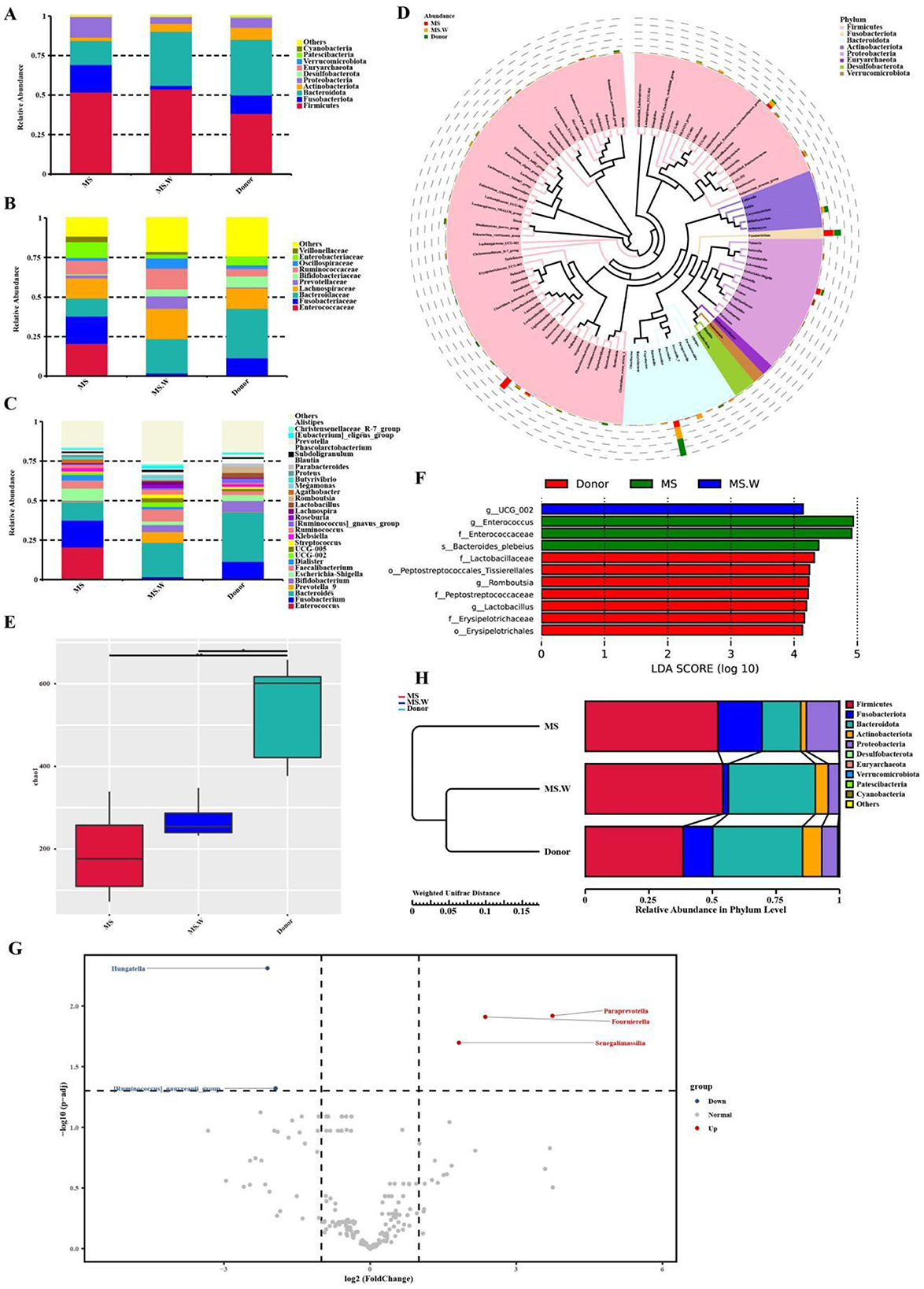
Figure 5. The composition of gut microbiota before and after WMT. (A) The composition of the top ten phyla of gut microbiota. (B) The composition of gut microbiota in the top ten families. (C) The composition of the gut microbiota of the top 30 genera. (D) The phylogenetic relationships of the top 100 genera of gut microbiota. (E) Chao1 index α diversity analysis. (F) LEfSe analysis of the MS patients and donors. (G) Analysis of metastatic lesions before and after WMT in MS patients. (H) Comparison of the abundance of the top five microbiota at the phylum level in MS patients before and after WMT, as well as in donors. MS: in the MS group before WMT. MS.W: in the MS group after WMT. d1-5: the first donor to the fifth donor. *indicates p < 0.05, **indicates p < 0.01.
The phylogenetic relationships of the top 100 gut microbiota at the genus level were analyzed, with the top seven being Bacteroides, Fusobacterium, Bifidobacterium, Escherichia–Shigella, Faecalibacterium, Enterococcus, and Prevotella (Figure 5D). Among them, not only could WMT increase the relative abundance of beneficial bacteria such as Bifidobacterium and Bacteroidetes, playing the basic functions of beneficial bacteria, but also reduce the relative abundance of pathogenic bacteria such as Fusobacterium and Escherichia-Shigella. WMT increased gut microbiota α diversity, such as Chao1 index (Figure 5E), revealing that WMT could significantly improve the diversity of gut microbiota in MS patients.
LEfSe analysis was performed on MS patients before and after WMT and donors to identify the biomarkers with statistical differences between groups. It was found that Enterococcus was the distinct species before WMT, while UCG-002 was the distinct species after WMT in MS patients, and the distinct species was Lactobacillaceae in donors (Figure 5F). The species with significant differences between the MS patients before and after WMT were identified by Metastat. We found that it could still significantly increase the relative abundance of Paraprevotella, Fournierella, and Senegalimassilia compared to baseline WMT, with a great reduction of the relative abundance of Hungatella and Ruminococcus at the genus level (Figure 5G).
In conclusion, compared to baseline, the relative abundance of Firmicutes, Bacteroidota, and Actinobacteriota increased after WMT, while the relative abundance of Fusobacteriota and Proteobacteria decreased at the phylum level, maintaining the balance of gut microbiota (Figure 5H). In addition, the proportion of Firmicutes/Bacteroidota decreased after WMT and in the donor group. The relative abundance of gut microbiota in MS patients after WMT gradually tended toward healthy donors, suggesting that maintaining the homeostasis of gut microbiota might be a new approach for treating MS patients.
As far as we know, our previous clinical study was the first to investigate the impact of WMT on MS patients in China, suggesting that the regulation of gut microbiota might be a new approach for treating MS (16). Therefore, WMT had been applied to treat numerous MS patients with gastrointestinal diseases. In this study, we found that WMT had a significant effect in removing the label of MS and downregulating ASCVD risk stratification. In addition, the therapeutic effect of WMT on MS patients was closely related to their age, smoking index, infection, chronic disease status, donor type and WMT courses based on Logistic Regression.
FMT is the process of transferring fecal bacteria extracted from healthy individuals to the patients' intestines through various optimization methods, thereby restoring intestinal microbiota homeostasis and providing effective treatment for a range of diseases both inside and outside the intestine. Scientific research has demonstrated the potential roles of gut microbiota in many diseases. In real-world clinical treatments, FMT has successfully treated various diseases, such as clostridium difficile infection (CDI) (27, 28) and inflammatory bowel disease (IBD) (29). However, FMT is not limited to digestive system diseases, it has also been proven to be a potential therapy for improving lipid (30, 31), blood glucose levels (32), MS (33, 34), obesity (35), sleep disorders (36) and hyperlipidemia (37) in recent years. Our early studies had shown that WMT significantly improves indicators such as FBG, TG, TC, LDL-c, SBP, and BMI in MS patients. In this study, the WMT group was more likely to remove the label of MS compared to the DT group based on improvements in aspects such as blood lipids, blood glucose and blood pressure.
Vrieze et al. (38) found that insulin sensitivity and the diversity of gut microbiota significantly increased in MS patients after transplanting healthy gut microbiota to for 6 weeks. The ratio of Firmicutes to Bacteroidota was commonly used to correlate changes in microbiota composition with obesity and type 2 diabetes mellitus (T2DM) phenotypes. Patients with obesity and T2DM had more Firmicutes than Bacteroidota in their intestines, while healthy people had fewer Firmicutes and more Bacteroidota (39, 40). Correspondingly, in this study, we not only observed an increase in gut microbial diversity and beneficial bacteria (such as Bifidobacterium and Bacteroidetes), but also a decrease in the proportion of Firmicutes/Bacteroidetes after WMT.
We believed that the improvement of gut microbiota might be key to treating MS patients with WMT. Therefore, the survival activity and diversity of gut microbiota after transplantation were crucial for the efficacy of WMT. Lange et al. found that the abuse of antibiotics could transform the gut microbiota into a long-term dysbiosis, potentially promoting the development and deterioration of diseases (41). de Oliveira et al. found that the diarrhea after 2019 corona virus disease (COVID-19) was related to the decrease of the abundance and diversity, as well as dysfunction of gut microbiota (42). In our study, the infection with pathogenic bacteria or viruses and the use of antibiotics significantly reduced the efficacy of WMT, suggesting minimizing pathogenic bacterial or viral infection and avoiding antibiotics after WMT. Zhang et al. (43) showed that the success of treating for ulcerative colitis (UC) with FMT was correlated with donor selection, believing that donor-recipient matching strategy was more effective in increasing the abundance of gut microbiota. Chehri et al. (44) showed that multiple courses could continuously improve the effectiveness of FMT in treating CDI. Similar to the study above, our study indicated that multiple donors and multiple courses could improve the efficacy of WMT, which suggested that the number of donors and frequency of WMT should be appropriately increased to achieve better efficacy.
The efficacy of WMT was also associated with patient age, lifestyle and dietary habits (45, 46). Coman and Vodnar (45) found that supplementing prebiotics or transplanting fecal microbiota could significantly improve the gut microbiota of elderly people and extend their health lifespan. In our study, individuals over 60 years old showed improved efficacy after WMT, likely due to significant gut microbiota improvements and high medical compliance. Moreover, a large number of studies had confirmed that smoking could cause oxidative stress, vascular inflammation, platelet coagulation, vascular dysfunction and dysregulate blood lipid levels, all of which could harm the cardiovascular system (47, 48). Consistent with this, our study supported that MS patients who are addicted to smoking had poorer efficacy, which was more difficult to remove the label of MS after WMT. As is well known, the homeostasis of the gut microbiota was closely related to good sleep time and exercise (49, 50). Therefore, we recommend that patients maintain regular schedules and exercise habits, while also advising them to quit smoking immediately.
To our knowledge, MS was a symptom of one or more high-risk factors such as centripetal obesity, hypertension, hyperglycemia and dyslipidemia (51, 52). Numerous studies had shown that patients with T2DM and obesity had metabolic disorders and chronic inflammatory states, accompanied by disturbances in gut microbiota (53, 54). On the contrary, if the gut microbiota was imbalanced for a long time, the abnormal situation of blood lipids, blood glucose and blood pressure would further worsen. Vich vila et al. believed that the use of hypoglycemic drugs such as Metformin could reduce the richness and diversity of gut microbiota (55). Xiong et al. showed that the pharmacokinetics and metabolism of antihypertensive drugs might be influenced by the gut microbiota (56). In our study, MS patients with one or more diseases such as hypertension, diabetes and obesity often took oral antidiabetic drugs or antihypertensive drugs for a long time, which made it more difficult to remove the label of MS. Therefore, we suggested that MS patients should strengthen monitoring of blood pressure, blood glucose and weight, as well as have a light diet, exercise reasonably, control weight and take medication reasonably.
It was known that many factors affect the efficacy of FMT, including the amount of transplanting feces, sample handling, injection method and colonization resistance (57, 58). Our WMT process was based on the standard of the Nanjing Consensus (20), which used automatic purification machines for repeated centrifugation and filtration to prepare washed microbial suspensions, thereby reducing differences in fecal volume and sample processing, as well as adverse reactions after transplantation. Due to the small sample size, further exploration was needed on the factors that affect the efficacy of WMT. However, the impact and influencing factors of gut microbiota on MS were still in its early stages, while the data on the impact and influencing factors of WMT on MS patients was limited. Our early research was the first large-scale retrospective study in China, indicating that WMT had a significant improvement effect on blood lipids, blood glucose, and blood pressure in MS patients (16). In this study, we further explored the factors influencing the efficacy of WMT in treating MS patients, laying the foundation for subsequent research on the effects of environmental factors (59), gut microbiota (60, 61) and metabolic biomarkers (62, 63) on metabolic diseases.
Our data had several limitations. Firstly, our study focused on single center data with a relatively low number of MS patients returning for evaluation of WMT efficacy. Therefore, more data was needed to confirm the factors influencing the efficacy of WMT in treating MS patients. Secondly, our study mainly focused on the analysis of clinical data and gut microbiota structure. A great deal of data such as the dietary structure, exercise frequency, sleep time, sample processing methods, and time of transplantation had not been evaluated yet. Therefore, more data should to be collected to further evaluate the factors that affect the efficacy of WMT. Thirdly, we did not evaluate the confusion factors between the main diseases for WMT treatment and MS. Although our research suggested that WMT had a significant effect in restoring MS to non-MS, and its therapeutic effect was closely related to patient age, smoking index, infection, chronic disease status, donor type and WMT course, the large scale prospective studies were needed to further approve our conclusions. In the future, we would continue to follow up all patients in this study and plan to conduct a large sample prospective study to verify the impact of WMT on MS patients and the influencing factors of WMT efficacy.
WMT has a significant effect in removing the label of MS and downregulating ASCVD risk stratification. The therapeutic effect of WMT on MS patients is closely related to their age, smoking index, infection, chronic disease status, donor type, and WMT courses. Therefore, we can improve the efficacy of WMT by reducing independent influencing factors that affect gut microbiota homeostasis.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Ethics Committee (No. 2021-13) in accordance with the Declaration of Helsinki at the First Affiliated Hospital of Guangdong Pharmaceutical University, Guangzhou, China. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
D-JL: Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft. D-XH: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft. Q-TW: Data curation, Investigation, Methodology, Software, Writing – original draft. L-GH: Data curation, Investigation, Methodology, Software, Writing – original draft. Z-HL: Data curation, Investigation, Software, Writing – review & editing. J-TX: Data curation, Investigation, Software, Writing – review & editing. X-XH: Conceptualization, Funding acquisition, Resources, Writing – review & editing. LW: Conceptualization, Funding acquisition, Investigation, Resources, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Key-Area Research and Development Program of Guangdong Province (No. 2022B1111070006) and the China postdoctoral science foundation (No. 2023M740782).
We sincerely thank all patients in the study and all funding agencies that supported the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1508381/full#supplementary-material
1. O'Neill S, O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. (2015) 16:1–2. doi: 10.1111/obr.12229
2. Michelotti A, de Scordilli M, Palmero L, Guardascione M, Masala M, Roncato R, et al. NAFLD–related hepatocarcinoma: the malignant side of metabolic syndrome. Cells. (2021) 10:2034. doi: 10.3390/cells10082034
3. Scurt FG, Ganz MJ, Herzog C, Bose K, Mertens PR, Chatzikyrkou C. Association of metabolic syndrome and chronic kidney disease. Obes Rev. (2024). 25:e13649. doi: 10.1111/obr.13649
4. Pasquali R. Metabolic syndrome in polycystic ovary syndrome. Front Horm Res. (2018) 49:114–30. doi: 10.1159/000485995
5. Mili N, Paschou SA, Goulis DG, Dimopoulos MA, Lambrinoudaki I, Psaltopoulou T. Obesity, metabolic syndrome, and cancer: pathophysiological and therapeutic associations. Endocrine. (2021) 74:478–97. doi: 10.1007/s12020-021-02884-x
6. Ni Q, Li HL. Endocrinology and metabolic diseases committee of integrated traditional Chinese and Western medicine branch of Chinese medical doctor association. Guidelines for the diagnosis and treatment of metabolic syndrome combined with disease and syndrome. World Tradit Chin Med. (2023) 22:3157–66.
7. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. (2018) 20:12. doi: 10.1007/s11906-018-0812-z
8. Chen Y, Xu W, Zhang W, Tong R, Yuan A, Li Z, et al. Plasma metabolic fingerprints for large–scale screening and personalized risk stratification of metabolic syndrome. Cell Rep Med. (2023) 4:101109. doi: 10.1016/j.xcrm.2023.101109
9. Wagh A, Stone NJ. Treatment of metabolic syndrome. Expert Rev Cardiovasc Ther. (2004) 2:213–28. doi: 10.1586/14779072.2.2.213
10. Bafeta A, Yavchitz A, Riveros C, Batista R, Ravaud P. Methods and reporting studies assessing fecal microbiota transplantation. Ann Internal Med. (2017) 167:34–9. doi: 10.7326/M16-2810
11. Wang PX, Deng XR, Zhang CH, Yuan HJ. Gut microbiota and metabolic syndrome. Chin Med J. (2020) 133:808–16. doi: 10.1097/CM9.0000000000000696
12. Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest. (2019) 129:4050–7. doi: 10.1172/JCI129194
13. Gérard P. Gut microbiota and obesity. Cell Mol Life Sci. (2016) 73:147–62. doi: 10.1007/s00018-015-2061-5
14. Kc D, Sumner R, Lippmann S. Gut microbiota and health. Postgrad Med. (2020) 132:274. doi: 10.1080/00325481.2019.1662711
15. Zhang T, Lu GC, Zhao Z, Liu YF, Shen Q, Li P, et al. Washed microbiota transplantation vs. manual fecal microbiota transplantation: clinical findings, animal studies and in vitro screening. Protein Cell. (2020) 11:251–66. doi: 10.1007/s13238-019-00684-8
16. Wu L, Lu XJ, Lin DJ, Chen WJ, Xue XY, Liu T, et al. Washed microbiota transplantation improves patients with metabolic syndrome in South China. Front Cell Infect Microbiol. (2022) 12:1044957. doi: 10.3389/fcimb.2022.1044957
17. Chinese Diabetes S. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition) (Part 1). Chin J Endocrinol Metab. (2021) 37:311–98. doi: 10.3760/cma.j.cn311282-20210304-00142
18. Metabolic Syndrome Research Collaboration Group of Diabetes Branch of Chinese Medical Association. Suggestions of diabetes branch of Chinese medical association onmetabolic syndrome. Chin J Diab. (2018) 12:156–161.
19. Chinese Cardiovascular Disease Prevention Guidelines Writing Group Editorial Editorial Board of Chinese Journal of Cardiovascular Diseases. Chinese Guidelines for the prevention of cardiovascular diseases (2017) Chin. J. Cardiol. (2018) 46:10–25. doi: 10.3760/cma.j.issn.0253-3758.2018.01.004
20. Nanjing consensus on methodology of washed microbiota transplantation. Chin Med J. (2020) 133:2330–2. doi: 10.1097/CM9.0000000000000954
21. Ranjan R, Rani A, Metwally A, McGee HS, Perkins DL. Analysis of the microbiome: advantages of whole genome shotgun versus 16S amplicon sequencing. Biochem Biophys Res Commun. (2016) 469:967–77. doi: 10.1016/j.bbrc.2015.12.083
22. Shahi SK, Zarei K, Guseva NV, Mangalam AK. Microbiota analysis using two–step PCR and next–generation 16S rRNA Gene Sequencing. J Vis Exp. (2019) 15:10.3791/59980. doi: 10.3791/59980-v
23. Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. (2011) 27:2957–63. doi: 10.1093/bioinformatics/btr507
24. Chen S, Zhou Y, Chen Y, Gu J. Fastp: an ultra–fast all–in–one FASTQ preprocessor. Bioinformatics. (2018) 34:i884–90. doi: 10.1093/bioinformatics/bty560
25. Haas BJ, Gevers D, Earl AM, Feldgarden M, Ward DV, Giannoukos G, et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454–pyrosequenced PCR amplicons. Genome Res. (2011) 21:494–504. doi: 10.1101/gr.112730.110
26. Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. (2019) 37:852–7. doi: 10.1038/s41587-019-0209-9
27. Kao D, Roach B, Silva M, Beck P, Rioux K, Kaplan GG, et al. Effect of oral capsule vs. colonoscopy–delivered fecal microbiota transplantation on recurrent clostridium diffificile infection: a randomized clinical trial. JAMA. (2017) 318:1985–93. doi: 10.1001/jama.2017.17077
28. Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for clostridium diffificile infection: systematic review and meta–analysis. Am J Gastroenterol. (2013) 108:500–8. doi: 10.1038/ajg.2013.59
29. El–Salhy M, Hatlebakk JG, Gilja OH, Kristoffersen AB, Hausken T, et al. Effificacy of fecal microbiota transplantation for patients with irritable bowel syndrome in a randomized, double–blind, placebo–controlled study. Gut. (2020) 69:859–67. doi: 10.1136/gutjnl-2019-319630
30. Liang F, Lu X, Deng Z, Zhong H–J, Zhang W, Li Q, et al. Effect of washed microbiota transplantation on patients with dyslipidemia in South China. Front Endocrinol. (2022) 13:827107. doi: 10.3389/fendo.2022.827107
31. Chen ML, Yi L, Zhang Y, Zhou X, Ran L, Yang J, et al. Resveratrol attenuates trimethylamine–N–oxide (TMAO)–induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. mBio. (2016) 7:e02210–5. doi: 10.1128/mBio.02210-15
32. Wu L, Li MQ, Xie YT, Zhang Q, Lu XJ, Liu T, et al. Washed microbiota transplantation improves patients with high blood glucose in South China. Front Endocrinol. (2022) 13:985636. doi: 10.3389/fendo.2022.985636
33. Peng J, Xiao X, Hu M, Zhang X. Interaction between gut microbiome and cardiovascular disease. Life Sci. (2018) 214:153–7. doi: 10.1016/j.lfs.2018.10.063
34. Aron–Wisnewsky J, Clement K, Nieuwdorp M. Fecal microbiota transplantation: a future therapeutic option for obesity/diabetes? Curr Diabetes Rep. (2019) 19:51. doi: 10.1007/s11892-019-1180-z
35. Lin DJ, Hu DX, Song YL, He XX, Wu L. Long–term efficacy of washed microbiota transplantation in overweight patients. Eur J Clin Invest. (2024) 10:e14260. doi: 10.1111/eci.14260
36. He HX, Li MQ, Qiu YF, Wu ZQ, Wu L. Washed microbiota transplantation improves sleep quality in patients with sleep disorder by the gut–brain axis. Front Neurosci. (2024) 18:1415167. doi: 10.3389/fnins.2024.1415167
37. Liang F, Song Y, Lin D, He H, Xu J, et al. Washed microbiota transplantation is associated with improved lipid profiles: long–term efficacy and safety in an observational cohort from South China. Clin Transl Gastroenterol. (2024) 15:e00735. doi: 10.14309/ctg.0000000000000735
38. Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. (2012) 143:913–6.e7. doi: 10.1053/j.gastro.2012.06.031
39. Louis S, Tappu RM, Damms–Machado A, Huson DH, Bischoff SC. Characterization of the gut microbial community of obese patients following a weight–loss intervention using whole metagenome shotgun sequencing. PLoS ONE. (2016) 11:e0149564. doi: 10.1371/journal.pone.0149564
40. Zhou Z, Sun B, Yu D, Zhu C. Gut microbiota: an important player in type 2 diabetes mellitus. Front Cell Infect Microbiol. (2022) 12:834485. doi: 10.3389/fcimb.2022.834485
41. Lange K, Buerger M, Stallmach A, Bruns T. Effects of antibiotics on gut microbiota. Dig Dis. (2016) 34:260–8. doi: 10.1159/000443360
42. de Oliveira GLV, Oliveira CNS, Pinzan CF, de Salis LVV, Cardoso CRB. Microbiota modulation of the gut–lung axis in COVID−19. Front Immunol. (2021) 12:635471. doi: 10.3389/fimmu.2021.635471
43. Zhang B, Yang L, Ning H, Cao M, Chen Z, Chen Q, et al. A matching strategy to guide donor selection for ulcerative colitis in fecal microbiota transplantation: meta–analysis and analytic hierarchy process. Microbiol Spectr. (2023) 11:e0215921. doi: 10.1128/spectrum.02159-21
44. Chehri M, Christensen AH, Halkjær SI, Günther S, Petersen AM, Helms M, et al. Case series of successful treatment with Fecal Microbiota Transplant (FMT) oral capsules mixed from multiple donors even in patients previously treated with FMT enemas for recurrent clostridium diffificile infection. Medicine. (2018) 97:e11706. doi: 10.1097/MD.0000000000011706
45. Coman V, Vodnar DC. Gut microbiota and old age: modulating factors and interventions for healthy longevity. Exp Gerontol. (2020). 141:111095. doi: 10.1016/j.exger.2020.111095
46. Clancy AK, Lee C, Hamblin H, Gunaratne AW, LeBusque A, Beck EJ, et al. Dietary intakes of recipients of faecal microbiota transplantation: an observational pilot study. Nutrients. (2021) 13:1487. doi: 10.3390/nu13051487
47. Siasos G, Tsigkou V, Kokkou E, Oikonomou E, Vavuranakis M, Vlachopoulos C, et al. Smoking and atherosclerosis: mechanisms of disease and new therapeutic approaches. Curr Med Chem. (2014) 21:3936–48. doi: 10.2174/092986732134141015161539
48. Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. (2004) 43:1731–7. doi: 10.1016/j.jacc.2003.12.047
49. Matenchuk BA, Mandhane PJ, Kozyrskyj AL. Sleep, circadian rhythm, and gut microbiota. Sleep Med Rev. (2020) 53:101340. doi: 10.1016/j.smrv.2020.101340
50. Aragón-Vela J, Solis-Urra P, Ruiz-Ojeda FJ, Álvarez-Mercado AI, Olivares-Arancibia J, Plaza-Diaz J. Impact of exercise on gut microbiota in obesity. Nutrients. (2021) 13:3999. doi: 10.3390/nu13113999
52. Sherling DH, Perumareddi P, Hennekens CH. Metabolic syndrome. J Cardiovasc Pharmacol Ther. (2017) 22:365–7. doi: 10.1177/1074248416686187
53. Sharma S, Tripathi P. Gut microbiome and type 2 diabetes: where we are and where to go? J Nutr Biochem. (2019) 63:101–8. doi: 10.1016/j.jnutbio.2018.10.003
54. Yang G, Wei J, Liu P, Zhang Q, Tian Y, Hou G, et al. Role of the gut microbiota in type 2 diabetes and related diseases. Metabolism. (2021). 117:154712. doi: 10.1016/j.metabol.2021.154712
55. Vich Vila A, Collij V, Sanna S, Sinha T, Imhann F, Bourgonje AR, et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat Commun. (2020) 11:362. doi: 10.1038/s41467-019-14177-z
56. Xiong Y, Xiong Y, Zhu P, Wang Y, Yang H, Zhou R, et al. The role of gut microbiota in hypertension pathogenesis and the efficacy of antihypertensive drugs. Curr Hypertens Rep. (2021) 23:40. doi: 10.1007/s11906-021-01157-2
57. Porcari S, Benech N, Valles–Colomer M, Segata N, Gasbarrini A, Cammarota G, et al. Key determinants of success in fecal microbiota transplantation: from microbiome to clinic. Cell Host Microbe. (2023) 31:712–33. doi: 10.1016/j.chom.2023.03.020
58. Kim S, Covington A, Pamer EG. The intestinal microbiota: antibiotics, colonization resistance, and enteric pathogens. Immunol Rev. (2017) 279:90–105. doi: 10.1111/imr.12563
59. Wu L, Xie X, Zhang J, Ding Y, Wu Q. Bacterial diversity and community in regional water microbiota between different towns in world's longevity township Jiaoling, China. Diversity. (2021) 13:361. doi: 10.3390/d13080361
60. Wu L, Xie X, Li Y, Liang T, Zhong H, Ma J, et al. Metagenomics–based analysis of the age–related cumulative effect of antibiotic resistance genes in gut microbiota. Antibiotics. (2021) 10:1006. doi: 10.3390/antibiotics10081006
61. Wu L, Xie X, Li Y, Liang T, Zhong H, Yang L, et al. Gut microbiota as an antioxidant system in centenarians associated with high antioxidant activities of gut–resident Lactobacillus. NPJ Biofilms Microbiomes. (2022) 8:102. doi: 10.1038/s41522-022-00366-0
62. Wu L, Xie X, Liang T, Ma J, Yang L, Yang J, et al. Integrated multi–omics for novel aging biomarkers and antiaging targets. Biomolecules. (2021) 12:39. doi: 10.3390/biom12010039
Keywords: fecal microbiota transplantation, washed microbiota transplantation, metabolic syndrome, logistic regression, influencing factors
Citation: Lin D-J, Hu D-X, Wu Q-T, Huang L-G, Lin Z-H, Xu J-T, He X-X and Wu L (2025) Analysis of influencing factors of washed microbiota transplantation in treating patients with metabolic syndrome. Front. Nutr. 12:1508381. doi: 10.3389/fnut.2025.1508381
Received: 09 October 2024; Accepted: 13 January 2025;
Published: 03 February 2025.
Edited by:
Lubia Velázquez López, Instituto Mexicano del Seguro Social, MexicoReviewed by:
Jenny Vilchis-Gil, Hospital Infantil de México Federico Gómez, MexicoCopyright © 2025 Lin, Hu, Wu, Huang, Lin, Xu, He and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xing-Xiang He, aGV4aW5neGlhbmdAZ2RwdS5lZHUuY24=; Lei Wu, d3VsZWlnZGltQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.