
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 07 January 2025
Sec. Clinical Nutrition
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1520639
This article is part of the Research Topic Assessment of Nutritional Status in Chronic Diseases View all 21 articles
Purpose: This study aimed to investigate the association between serum calcium levels and periodontitis in a U.S. adult population, using data from the National Health and Nutrition Examination Survey (NHANES) 2009–2014.
Method: Data were analyzed from 8,601 participants aged over 30 years, who were categorized based on the presence or absence of periodontitis. Serum calcium levels were measured using standardized NHANES protocols, and periodontitis status was determined through clinical oral examinations. To assess the relationship between calcium levels and periodontitis, multivariate logistic regression models were applied across three levels of adjustment. Additionally, trend tests and subgroup analyses were conducted to explore associations across different demographic and clinical subgroups. A smoothing curve fitting and threshold effect analysis were also performed to examine potential nonlinear relationships.
Results: After adjusting multiple covariates, participants in the highest quartile of serum calcium showed an 18% reduced risk of periodontitis compared to those in the lowest quartile (OR = 0.82, 95% CI: 0.71–0.95, p = 0.0083; p for trend = 0.0057). The association remained stable across various subgroups. Smoothing curve fitting indicated a nonlinear negative correlation between calcium levels and periodontitis, though without a significant inflection point at 2.48 mmol/L (p = 0.094).
Conclusion: Elevated serum calcium levels appear to be associated with a lower risk of periodontitis in adults. These findings suggest that adequate calcium intake may play a role in periodontitis prevention, providing valuable insight for clinical guidance on nutritional and preventive strategies in periodontal health.
Periodontitis, a chronic inflammatory disease of the periodontium, leads to the progressive destruction of the supporting structures of teeth, including the gingiva, periodontal ligament, and alveolar bone (1–3). It is among the most prevalent dental diseases globally, with its occurrence varying significantly across different age groups, regions, and socioeconomic backgrounds (4–6). Clinically, periodontitis presents with symptoms such as gum swelling, bleeding, pocket formation, and, in severe cases, tooth loss. Despite advances in diagnostic techniques and treatment approaches, the current management of periodontitis remains challenging, particularly in terms of early detection, effective long-term treatment, and sustainable prevention (7–9). There are limitations in the existing diagnostic markers and therapeutic options, which emphasize the need for continued research to better understand the pathophysiology of periodontitis and explore novel preventative and therapeutic strategies (10, 11).
Calcium, an essential mineral in the human body, plays a critical role in numerous physiological processes, including bone mineralization and structural integrity, signal transduction in muscle contraction and neurotransmitter release, enzymatic activation as a cofactor in processes like blood clotting and metabolic regulation (12–14). Calcium deficiency can lead to various health issues, such as osteoporosis, muscle cramps, and cardiovascular complications, reflecting its importance in maintaining overall health (15–17). Previous epidemiological evidence suggested that calcium may also play a significant role in periodontal health (18–20). Studies indicate that adequate calcium levels might be protective against periodontitis, although the underlying mechanisms remain unclear (21, 22). A review highlighted the role of calcium and vitamin D supplementation as a non-surgical adjunctive treatment for periodontal disease, with an emphasis on female patients. Vitamin D facilitates calcium-phosphate homeostasis and bone metabolism, essential for regenerative periodontal therapies (23). This potential link between calcium and periodontitis has attracted research interest as it offers a promising avenue for exploring preventative measures against this disease.
The National Health and Nutrition Examination Survey (NHANES) database, a comprehensive resource for epidemiological studies, provides valuable data on various health conditions and nutritional factors among the U.S. population (24). The purpose of this study is to investigate the relationship between calcium intake and periodontitis using NHANES data, with the aim of offering insights that could contribute to the clinical diagnosis, prevention, and treatment of periodontitis. This study seeks to provide a scientific basis for further exploration of calcium’s role in periodontal health and guide future clinical approaches to managing periodontitis effectively.
This study analyzed data from the NHANES database collected between 2009 and 2014. NHANES is a nationally representative, stratified, large-scale cross-sectional survey, conducted in biennial cycles to evaluate the health and nutritional status of both adult and pediatric populations in the United States. The database includes comprehensive modules on demographic characteristics, dietary intake, physical examinations, laboratory measurements, and questionnaires (24). For additional information, please refer to the NHANES website: https://www.cdc.gov/nchs/nhanes/index.htm.
In this study, data were initially collected from 30,468 individuals. Of these, 15,912 participants under the age of 30 were excluded. Additionally, 3,873 individuals were removed due to unavailable periodontal data, while another 542 were excluded due to missing calcium information. A further 1,540 participants were omitted due to incomplete data on other covariates. Ultimately, 8,601 participants met the inclusion criteria, comprising 4,647 individuals without periodontitis and 3,954 with periodontitis. Detailed information can be found in Figure 1.
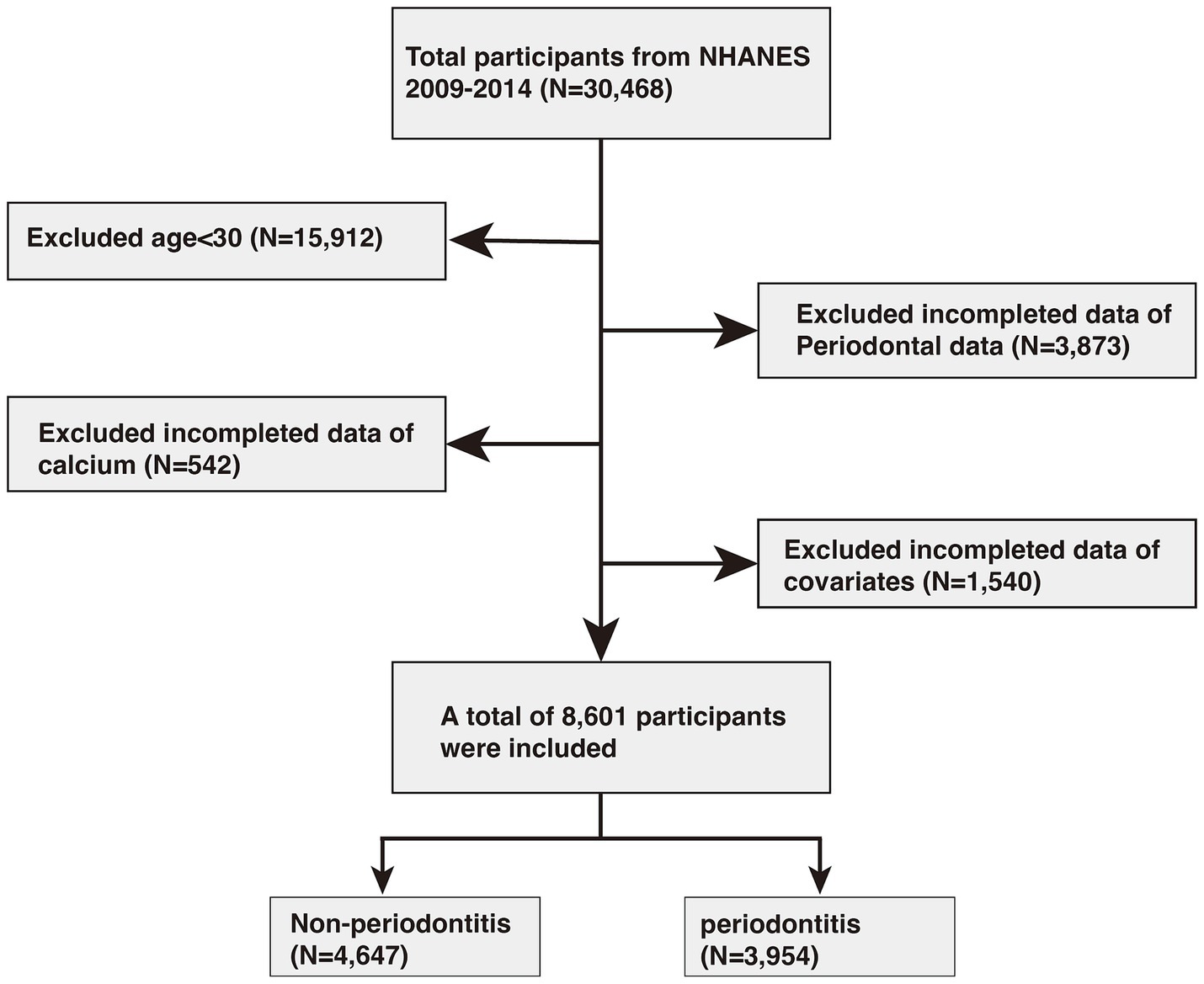
Figure 1. Flowchart of participants selection. NHANES, National Health and Nutrition Examination Survey.
In the NHANES, serum calcium level was measured through biochemical analysis of serum samples collected from participants. Blood samples were typically drawn at the NHANES Mobile Examination Center (MEC) to maintain standardization and ensure high data quality. Calcium measurement is performed using indirect (or diluted) I.S.E. (ion selective electrode) methodology of the DxC800 system to measure calcium concentration in serum. To ensure data accuracy and reliability, NHANES implements rigorous quality control measures, including regular calibration, repeated testing, and verification procedures. For further details on the laboratory methods and quality control processes, the NHANES website provides comprehensive documentation.
The primary outcome of this study was the presence of moderate to severe periodontitis. Periodontitis was diagnosed using oral examination data gathered by licensed dentists at the mobile examination center (MEC). To ensure data reliability, dental examiners underwent comprehensive training and calibration, with continuous monitoring and periodic recalibration to maintain data quality standards. The periodontal assessment followed the Full-Mouth Periodontal Examination (FMPE) protocol, measuring attachment loss (AL) and probing pocket depth (PPD) at six specific sites on each tooth, excluding the third molars. AL represents the distance from the cemento-enamel junction to the sulcus base, while probing PPD is the depth from the gingival margin to the sulcus base. Healthy or mild periodontitis were considered as case control group and moderate or severe periodontitis were identify as case group (25, 26). Periodontitis classification adhered to the CDC/AAP criteria, as outlined in Supplementary Table 1 (27).
This study accounted for several potential confounding variables, including age, gender, race, education level, marital status, family poverty-to-income ratio (PIR), smoking status, alcohol, hypertension, diabetes, body mass index (BMI), total protein (TP), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), serum uric acid, and serum creatinine (Scr). PIR was categorized into three levels: low (≤1), middle (1–4), and high (≥4). Participants were identified as smokers if they reported having smoked at least 100 cigarettes in their lifetime. Alcohol drinkers were defined as those who consumed at least 12 alcoholic beverages in the past year. Hypertension status was confirmed if participants responded “Yes” to having been told they had high blood pressure. Diabetes status was classified as diabetic, non-diabetic, or borderline based on responses to whether a doctor had ever diagnosed them with diabetes. Detailed information regarding laboratory data collection methods is available on the NHANES website.
Statistical analyses in this study adhered to CDC guidelines, incorporating appropriate NHANES sample weights and adjusting for the complex multistage survey design. Participant characteristics, stratified by periodontitis status, were compared using chi-square tests for categorical variables and t-tests for continuous variables. To assess the association between calcium levels and periodontitis, three multivariate logistic regression models were constructed. Model 1 included no covariate adjustments. Model 2 adjusted for age, gender, and race, while Model 3 included a more comprehensive adjustment for factors such as age, sex, race, educational level, marital status, PIR, smoking status, alcohol intake, hypertension, diabetes, BMI, TC, TG, HDL-C, ALT, AST, BUN, SUA, and SCr. Serum calcium levels were further divided into quartiles to apply trend tests, analyzing linear trends in the association between calcium levels and periodontitis. Subgroup analyses were conducted using stratification variables, including age, gender, race, education, marital status, PIR, smoking, alcohol, hypertension, and diabetes, with interaction tests to verify the consistency of associations across these subgroups. Additionally, smoothing curve fitting was applied to explore potential nonlinear relationships between calcium levels and periodontitis. All statistical procedures were performed using R Studio (version 4.3)1 and EmpowerStats software (version 2.0)2 (see text footnote 1). A p-value <0.05 was regarded as statistically significant.
A total of 8,601 participants over the age of 30 were included in the study, of whom 3,954 were diagnosed with periodontitis. Table 1 presents participant characteristics categorized by periodontal status. Compared to those without periodontitis, individuals in the case group showed higher distributions of variables such as age, TP, TG, AST, BUN, SUA, and SCr, along with lower distributions of TC and HDL-C. Additionally, BMI, ALT, and calcium levels did not significantly differ between the periodontitis and control groups. Factors associated with a higher likelihood of periodontitis included being male, non-Hispanic White, having a higher education level, being married or living with a partner, belonging to the middle-income group, smoking, and alcohol consumption.
As shown in Table 2, the study results demonstrated an inverse association between serum calcium levels and periodontitis risk. When serum calcium levels were categorized into quartiles, both minimally and fully adjusted models indicated a negative correlation with periodontitis (p < 0.05). Specifically, individuals in the highest quartile of calcium had an 18% lower risk of periodontitis compared to those in the lowest quartile (OR = 0.82, 95% CI: 0.71–0.95, p = 0.0083; p for trend = 0.0057).
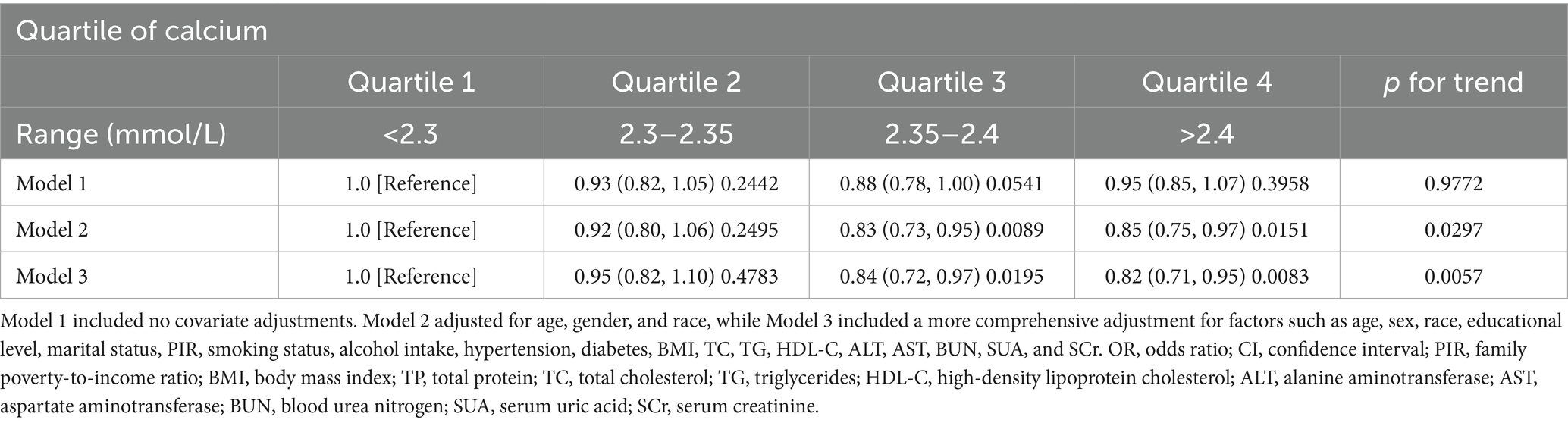
Table 2. OR (95% CI) and p-value for periodontitis risk across serum calcium level quartiles in NHANES 2009–2014.
Subgroup analysis was performed to evaluate the consistency of the relationship between serum calcium levels and periodontitis across different subgroups. Our results indicated that the association between calcium levels and periodontitis did not vary by subgroup. As shown in Figure 2, none of the factors—such as age, gender, race, education level, marital status, PIR, smoking, alcohol consumption, hypertension, or diabetes—affected the inverse relationship between calcium and periodontitis (all p for interaction >0.05).
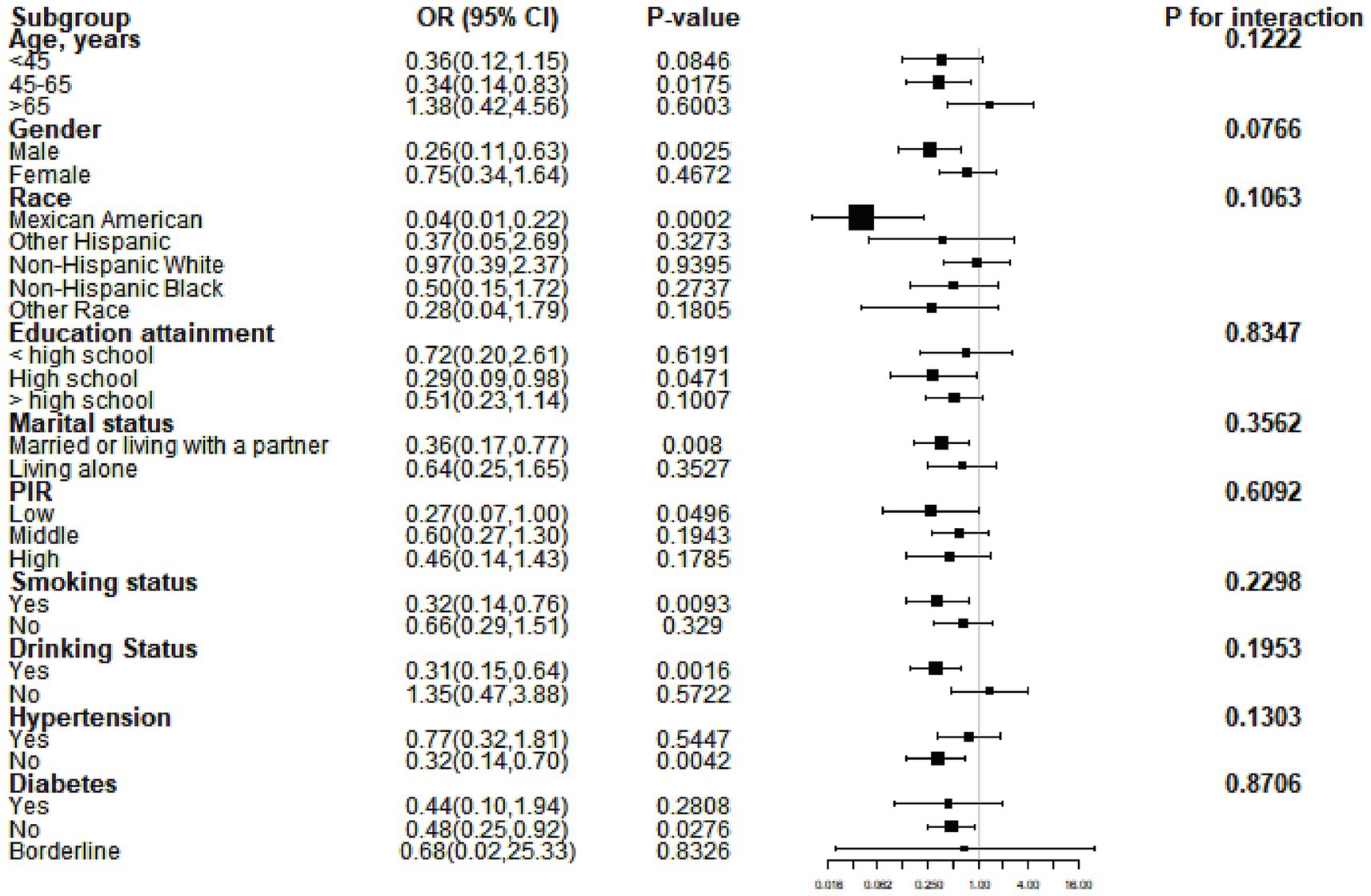
Figure 2. Subgroup analysis results based on model 3. OR, odds ratio; CI, confidence interval; PIR, poverty-to-income ratio.
After adjusting for all covariates, a smoothed curve fitting was used to visually depict the relationship between serum calcium levels and periodontitis. This curve (Figure 3) showed a nonlinear inverse correlation between calcium levels and periodontitis. Threshold effect analysis further confirmed this nonlinear negative association (OR = 0.43, 95% CI = 0.24–0.78, p = 0.0057), although no significant inflection point was found at 2.48, with p-value of Logarithm likelihood ratio test = 0.094, as presented in Table 3.
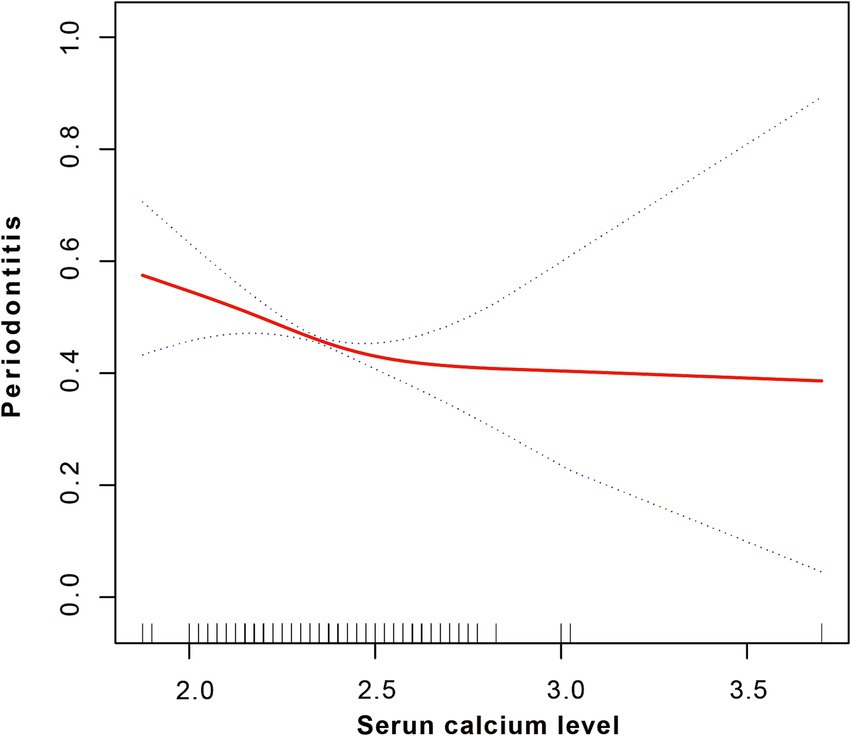
Figure 3. Smoothed fitting curve indicating a non-linear negative correlation between serum calcium level and periodontitis.
In this study, we investigated the association between serum calcium levels and periodontitis in a U.S. adult population, utilizing data from the NHANES 2009–2014. Our findings demonstrated a significant inverse association, with higher serum calcium levels linked to a reduced risk of periodontitis. This relationship persisted across multiple subgroups and adjustment models, and the nonlinear analysis further supported this inverse association without identifying a critical threshold point. These results suggest that maintaining adequate calcium levels could play a role in periodontitis prevention and inform both diagnostic and therapeutic strategies for periodontal health.
Previous epidemiological studies have explored the relationship between calcium levels and periodontal disease, with several studies supporting the potential protective role of calcium against periodontitis (18). For example, a study found that individuals taking vitamin D and calcium supplements showed trends of improved periodontal health, including shallower probing depths, less attachment loss, and reduced alveolar crest height loss, compared to non-supplement users (19). Research discovered that higher intakes of calcium, casein, and whey protein were associated with a lower likelihood of severe periodontitis, while vitamin D intake alone showed no significant association, highlighting the potential preventive role of calcium and protein-rich foods in periodontal health. Studies have shown that calcium supplementation may lower the risk of periodontal disease progression (28). Calcium and vitamin D supplementation had a modest positive effect on periodontal health over 1 year, though consistent dental care improved periodontal parameters in all participants, highlighting the need for further randomized trials (20). Research by Adegboye et al. and others has reported that individuals with higher calcium intake had a lower prevalence of periodontal disease among Danish adults, emphasizing calcium’s influence on maintaining periodontal health (28, 29). A systematic review explores the salivary ionomic profile in periodontitis compared to healthy controls. Findings indicate elevated levels of sodium and potassium in periodontitis patients, with calcium, copper, and manganese also showing increased trends in some studies (30). These findings contribute to a growing body of literature suggesting that calcium is a modifiable factor in periodontal disease risk.
The protective role of calcium against periodontitis can be attributed to several key biological mechanisms. First, calcium is essential for bone mineralization and structural integrity, directly influencing the alveolar bone that supports teeth (31, 32). Sufficient calcium levels are crucial in maintaining bone density, preventing resorption, and reducing susceptibility to bone loss, which is a primary characteristic of periodontitis (33, 34). Additionally, calcium plays a role in the mineralization of dental tissues, enhancing periodontal stability (33, 34). Beyond structural support, calcium also plays a critical role in cellular signaling and immune response regulation (35). It acts as a secondary messenger in various immune cells, influencing the activity of leukocytes and macrophages that are involved in inflammation and immune response (35). Adequate calcium levels help stabilize these cell membranes, potentially mitigating exaggerated inflammatory responses in the periodontal tissue, which could otherwise lead to tissue breakdown and disease progression (35). Calcium’s involvement in reducing inflammatory cytokines, such as IL-1β and TNF-α, may further decrease periodontal tissue inflammation, thereby limiting disease progression and supporting tissue healing (31, 36). A study focused on the efficacy of calcium hydroxide (Ca[OH]2) as an intracanal medication in reducing endotoxin levels in infected root canals, a condition linked to periodontal health. The findings reveal that Ca[OH]2 significantly decreased lipopolysaccharide levels, especially when combined with antimicrobial agents like chlorhexidine. The paper highlights its role in modulating inflammatory mediators, emphasizing its potential relevance to systemic effects linked to periodontitis (37). Finally, calcium may aid in vascular health, as calcium-dependent signaling is crucial for vascular tone and endothelial function, potentially affecting the blood supply and healing capacity of periodontal tissues (38).
Considering current evidence, calcium intake appears to have potential applications in periodontitis prevention, particularly in at-risk populations. Increasing dietary calcium intake or considering supplementation in individuals with low serum calcium levels could be beneficial in maintaining periodontal health. Given the positive outcomes observed in this study, further investigation is warranted to evaluate calcium’s role in periodontal health across diverse populations and to explore its potential as a preventive measure in clinical practice.
This study has several notable strengths. A primary strength is the use of data from the NHANES database, a large, nationally representative survey that ensures robust and generalizable findings across diverse demographic groups. Additionally, the study design included a thorough adjustment for potential confounders, such as lifestyle factors (smoking, alcohol use), demographic variables (age, gender, income), and health conditions (hypertension, diabetes), which strengthens the reliability of the observed associations. The use of advanced statistical techniques, including nonlinear analysis and threshold effect analysis, allowed for a nuanced understanding of the calcium-periodontitis relationship, providing insights that extend beyond a simple linear association.
However, there are also limitations to consider. The cross-sectional nature of the study limits the ability to draw causal inferences between serum calcium levels and periodontitis risk. Longitudinal studies would be required to determine whether calcium deficiency directly contributes to periodontitis development over time. Another limitation is the reliance on self-reported data for some variables, such as smoking and alcohol use, which may introduce recall bias. Additionally, unmeasured confounders, such as dietary habits and genetic predispositions, could influence both serum calcium levels and periodontitis risk, potentially impacting the observed association. Lastly, while this study highlights the potential importance of calcium in periodontal health, the exact optimal serum calcium levels and the role of dietary calcium intake versus serum calcium concentration remain to be fully clarified. Future research should aim to address these limitations through well-designed, longitudinal studies with precise measurement of dietary calcium intake and more detailed biochemical assessments.
This study found an inverse relationship between serum calcium levels and periodontitis risk, suggesting that adequate calcium may help protect against periodontal disease. Increasing calcium intake could benefit individuals at higher risk, particularly those with low serum calcium. While promising, further research is needed to confirm causality and determine optimal calcium levels for periodontal health, potentially informing future preventive and therapeutic strategies.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The study protocol received approval from the ethical review board of the National Center for Health Statistics, and informed consent was obtained from all participants.
HC: Conceptualization, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. MW: Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. MD: Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. SW: Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. HZ: Conceptualization, Funding acquisition, Software, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by Jilin Provincial Department of Science and Technology (No: 20230204011YY).
The authors extend their gratitude to the participants of the NHANES database.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1520639/full#supplementary-material
1. Kinane, DF, and Bornstein, MM. Introduction to the diagnostics in periodontology and implant dentistry issue. Periodontol. (2000) 95:7–9. doi: 10.1111/prd.12596
2. Heitz-Mayfield, LJA. Conventional diagnostic criteria for periodontal diseases (plaque-induced gingivitis and periodontitis). Periodontol. (2000) 95:10–9. doi: 10.1111/prd.12579
3. Savage, A, Eaton, KA, Moles, DR, and Needleman, I. A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J Clin Periodontol. (2009) 36:458–67. doi: 10.1111/j.1600-051X.2009.01408.x
4. Tibúrcio-Machado, CS, Michelon, C, Zanatta, FB, Gomes, MS, Marin, JA, and Bier, CA. The global prevalence of apical periodontitis: a systematic review and meta-analysis. Int Endod J. (2021) 54:712–35. doi: 10.1111/iej.13467
5. Kocher, T, König, J, Borgnakke, WS, Pink, C, and Meisel, P. Periodontal complications of hyperglycemia/diabetes mellitus: epidemiologic complexity and clinical challenge. Periodontol. (2000) 78:59–97. doi: 10.1111/prd.12235
6. Petersen, PE, Bourgeois, D, Ogawa, H, Estupinan-Day, S, and Ndiaye, C. The global burden of oral diseases and risks to oral health. Bull World Health Organ. (2005) 83:661–9. doi: 10.1590/S0042-96862005000900011
7. Deas, DE, and Mealey, BL. Response of chronic and aggressive periodontitis to treatment. Periodontol. (2000) 53:154–66. doi: 10.1111/j.1600-0757.2009.00334.x
8. Herrera, D, Sanz, M, Kebschull, M, Jepsen, S, Sculean, A, Berglundh, T, et al. Treatment of stage IV periodontitis: the EFP S3 level clinical practice guideline. J Clin Periodontol. (2022) 49:4–71. doi: 10.1111/jcpe.13639
9. Sanz, M, Herrera, D, Kebschull, M, Chapple, I, Jepsen, S, Beglundh, T, et al. Treatment of stage I-III periodontitis-the EFP S3 level clinical practice guideline. J Clin Periodontol. (2020) 47:4–60. doi: 10.1111/jcpe.13290
10. Slots, J. Periodontitis: facts, fallacies and the future. Periodontol. (2000) 75:7–23. doi: 10.1111/prd.12221
11. Jepsen, K, Sculean, A, and Jepsen, S. Complications and treatment errors related to regenerative periodontal surgery. Periodontol. (2000) 92:120–34. doi: 10.1111/prd.12504
12. Terrell, K, Choi, S, and Choi, S. Calcium's role and signaling in aging muscle, cellular senescence, and mineral interactions. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms242317034
13. Proudfoot, D. Calcium signaling and tissue calcification. Cold Spring Harb Perspect Biol. (2019) 11:11. doi: 10.1101/cshperspect.a035303
14. Ciosek, Ż, Kot, K, Kosik-Bogacka, D, Łanocha-Arendarczyk, N, and Rotter, I. The effects of calcium, magnesium, phosphorus, fluoride, and Lead on bone tissue. Biomol Ther. (2021) 11:11. doi: 10.3390/biom11040506
15. Brini, M, and Carafoli, E. Calcium pumps in health and disease. Physiol Rev. (2009) 89:1341–78. doi: 10.1152/physrev.00032.2008
16. Carafoli, E. The Symposia on calcium binding proteins and calcium function in health and disease: an historical account, and an appraisal of their role in spreading the calcium message. Cell Calcium. (2005) 37:279–81. doi: 10.1016/j.ceca.2005.01.001
17. Meier, C, and Kränzlin, ME. Calcium supplementation, osteoporosis and cardiovascular disease. Swiss Med Wkly. (2011) 141:w13260. doi: 10.4414/smw.2011.13260
18. Nascimento, GG, Leite, FRM, Gonzalez-Chica, DA, Peres, KG, and Peres, MA. Dietary vitamin D and calcium and periodontitis: a population-based study. Front Nutr. (2022) 9:1016763. doi: 10.3389/fnut.2022.1016763
19. Miley, DD, Garcia, MN, Hildebolt, CF, Shannon, WD, Couture, RA, Anderson Spearie, CL, et al. Cross-sectional study of vitamin D and calcium supplementation effects on chronic periodontitis. J Periodontol. (2009) 80:1433–9. doi: 10.1902/jop.2009.090077
20. Garcia, MN, Hildebolt, CF, Miley, DD, Dixon, DA, Couture, RA, Spearie, CL, et al. One-year effects of vitamin D and calcium supplementation on chronic periodontitis. J Periodontol. (2011) 82:25–32. doi: 10.1902/jop.2010.100207
21. Hildebolt, CF. Effect of vitamin D and calcium on periodontitis. J Periodontol. (2005) 76:1576–87. doi: 10.1902/jop.2005.76.9.1576
22. Nishida, M, Grossi, SG, Dunford, RG, Ho, AW, Trevisan, M, and Genco, RJ. Calcium and the risk for periodontal disease. J Periodontol. (2000) 71:1057–66. doi: 10.1902/jop.2000.71.7.1057
23. Sllamniku Dalipi, Z, and Dragidella, F. Calcium and vitamin D supplementation as non-surgical treatment for periodontal disease with a focus on female patients. Dent J. (2022) 10:10. doi: 10.3390/dj10070120
24. Paulose-Ram, R, Graber, JE, Woodwell, D, and Ahluwalia, N. The National Health and nutrition examination survey (NHANES), 2021-2022: adapting data collection in a COVID-19 environment. Am J Public Health. (2021) 111:2149–56. doi: 10.2105/AJPH.2021.306517
25. Eke, PI, Thornton-Evans, GO, Wei, L, Borgnakke, WS, Dye, BA, and Genco, RJ. Periodontitis in US adults: National Health and nutrition examination survey 2009-2014. J Am Dent Assoc. (2018) 149:576–88.e6. doi: 10.1016/j.adaj.2018.04.023
26. Wu, Q, Zhang, S, and Cao, R. Association between magnesium depletion score and periodontitis in US adults: results from NHANES 2009-2014. BMC Oral Health. (2024) 24:1274. doi: 10.1186/s12903-024-05048-1
27. Eke, PI, Page, RC, Wei, L, Thornton-Evans, G, and Genco, RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. (2012) 83:1449–54. doi: 10.1902/jop.2012.110664
28. Adegboye, AR, Boucher, BJ, Kongstad, J, Fiehn, NE, Christensen, LB, and Heitmann, BL. Calcium, vitamin D, casein and whey protein intakes and periodontitis among Danish adults. Public Health Nutr. (2016) 19:503–10. doi: 10.1017/S1368980015001202
29. Adegboye, AR, Christensen, LB, Holm-Pedersen, P, Avlund, K, Boucher, BJ, and Heitmann, BL. Intake of dairy products in relation to periodontitis in older Danish adults. Nutrients. (2012) 4:1219–29. doi: 10.3390/nu4091219
30. Baima, G, Iaderosa, G, Corana, M, Romano, F, Citterio, F, Giacomino, A, et al. Macro and trace elements signature of periodontitis in saliva: a systematic review with quality assessment of ionomics studies. J Periodontal Res. (2022) 57:30–40. doi: 10.1111/jre.12956
31. Ku, SK, Cho, HR, Sung, YS, Kang, SJ, and Lee, YJ. Effects of calcium gluconate on experimental periodontitis and alveolar bone loss in rats. Basic Clin Pharmacol Toxicol. (2011) 108:241–50. doi: 10.1111/j.1742-7843.2010.00646.x
32. Park, SI, Kang, SJ, Han, CH, Kim, JW, Song, CH, Lee, SN, et al. The effects of topical application of Polycal (a 2:98 (g/g) mixture of Polycan and calcium gluconate) on experimental periodontitis and alveolar bone loss in rats. Molecules. (2016) 21:527. doi: 10.3390/molecules21040527
33. Gil-Montoya, JA, Garrido-Martínez, M, Barrios-Rodríguez, R, Ramos-García, P, Lenouvel, D, Montes-Castillo, C, et al. Association between low bone mineral density and periodontitis in generally healthy perimenopausal women. J Periodontol. (2021) 92:95–103. doi: 10.1002/JPER.20-0029
34. Takahashi, O, Yoshihara, A, Nakamura, K, and Miyazaki, H. Association between periodontitis and systemic bone mineral density in Japanese community-dwelling postmenopausal women. J Dent. (2012) 40:304–11. doi: 10.1016/j.jdent.2012.01.005
35. Hasiakos, S, Gwack, Y, Kang, M, and Nishimura, I. Calcium signaling in T cells and chronic inflammatory disorders of the Oral cavity. J Dent Res. (2021) 100:693–9. doi: 10.1177/0022034521990652
36. Tenkumo, T, Rojas-Sánchez, L, Vanegas Sáenz, JR, Ogawa, T, Miyashita, M, Yoda, N, et al. Reduction of inflammation in a chronic periodontitis model in rats by TNF-α gene silencing with a topically applied siRNA-loaded calcium phosphate paste. Acta Biomater. (2020) 105:263–79. doi: 10.1016/j.actbio.2020.01.031
37. Sadaf, D, and Ahmad, MZ. Calcium hydroxide (ca[oh](2)) as an INTRACANAL medication may significantly reduce endotoxins level from infected teeth. J Evid Based Dent Pract. (2021) 21:101616. doi: 10.1016/j.jebdp.2021.101616
Keywords: serum calcium, periodontitis, NHANES, cross-sectional study, epidemiology, nutrition
Citation: Cao H, Wang M, Duan M, Wang S and Zhang H (2025) Association of serum calcium level with periodontitis: a cross-sectional study from NHANES 2009–2014. Front. Nutr. 11:1520639. doi: 10.3389/fnut.2024.1520639
Received: 31 October 2024; Accepted: 23 December 2024;
Published: 07 January 2025.
Edited by:
Mariacristina Siotto, IRCCS Don Carlo Gnocchi Firenze, ItalyReviewed by:
Sadaf Dabeer, Emory University, United StatesCopyright © 2025 Cao, Wang, Duan, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyang Zhang, aGFpeWFuZzgzMzlAamx1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.